Submitted:
24 October 2023
Posted:
25 October 2023
You are already at the latest version
Abstract
Keywords:
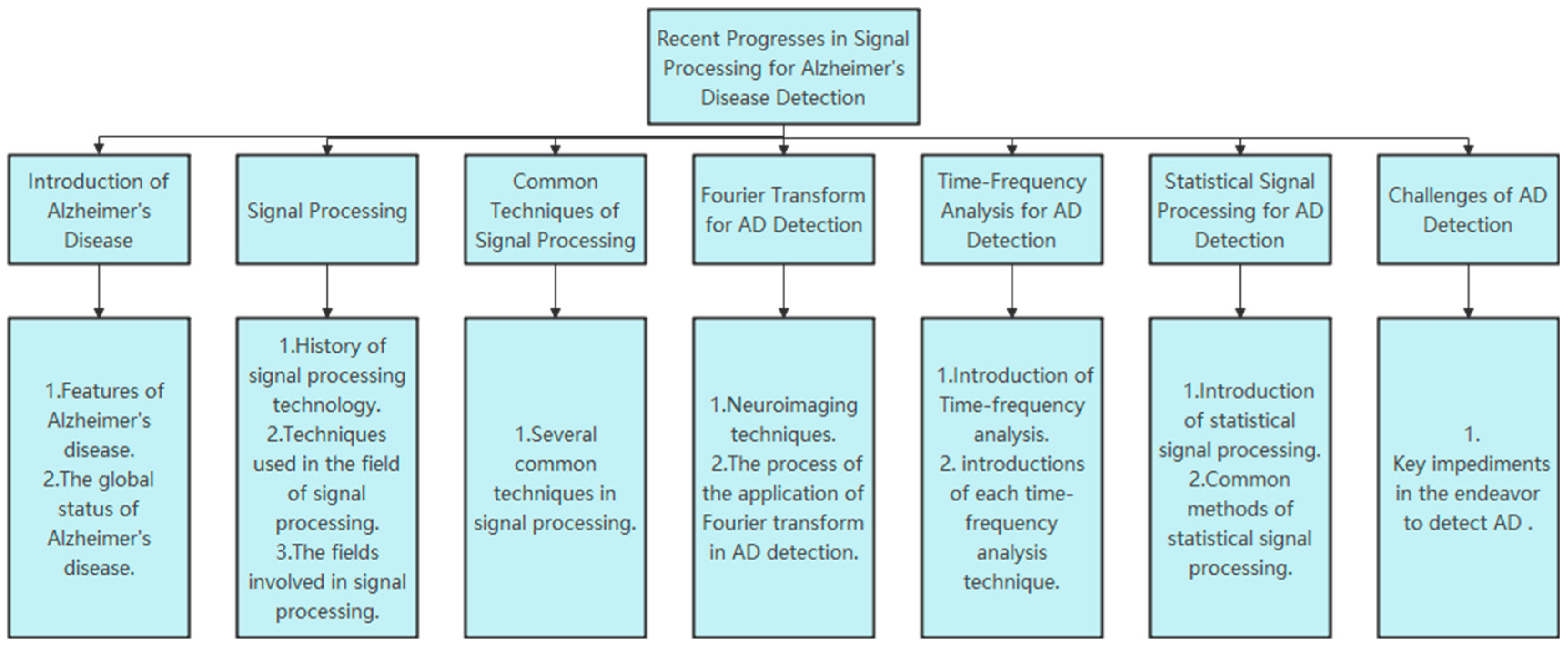
1. Introduction of Alzheimer’s Disease
2. Signal Processing
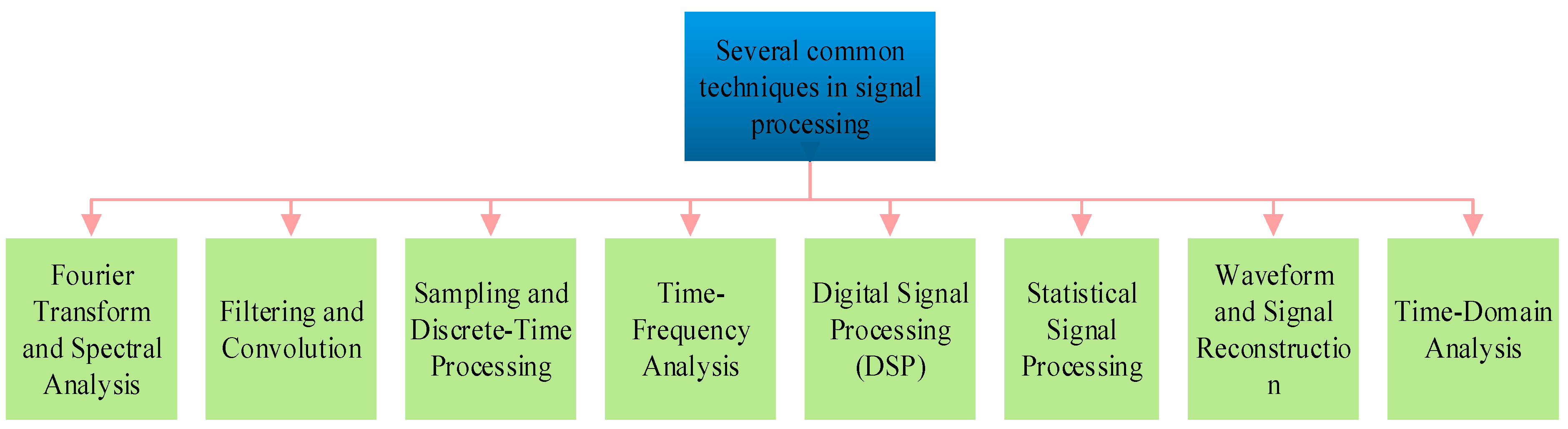
3. Common Techniques of Signal Processing
4. Fourier Transform for AD Detection
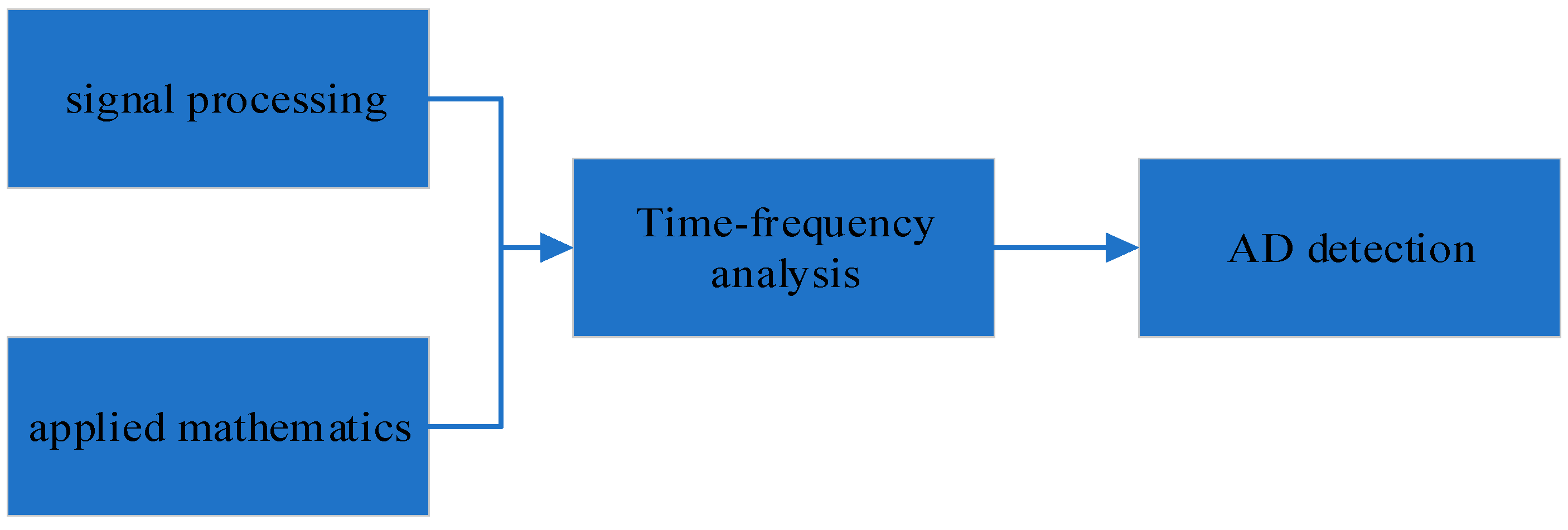
5. Time-Frequency Analysis for AD Detection
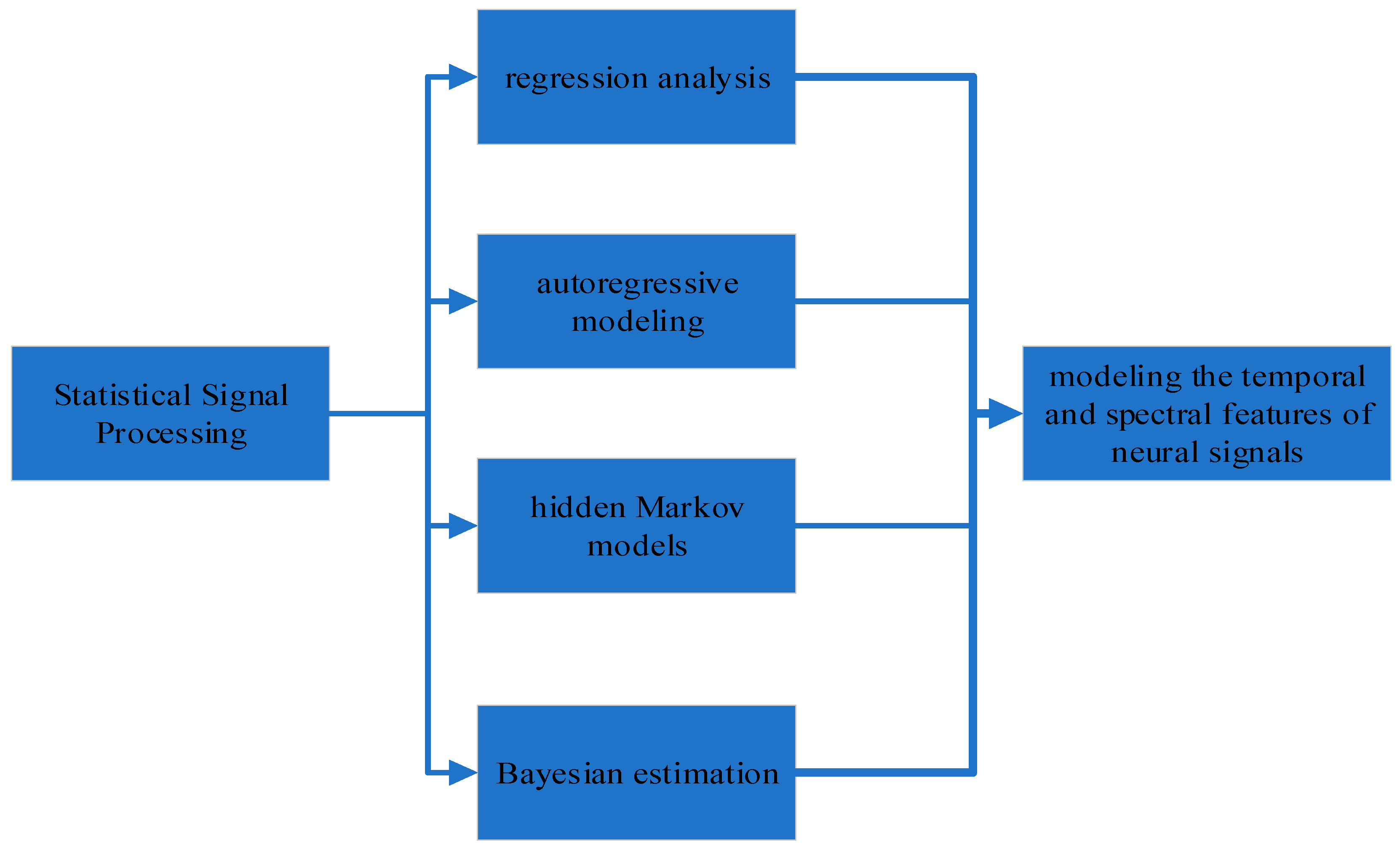
6. Statistical Signal Processing for AD Detection
7. Challenges of AD Detection
8. Conclusion
9. Future Research Directions
Funding
Data Availability Statement
Acknowledgment
Conflict of Interest
References
- C. T. Zhang, J. C. T. Zhang, J. Wang, and W. Y. Wang, “Wnt signaling in synaptogenesis of Alzheimer’s disease,” Ibrain, 2023. [CrossRef]
- Y. Deng, H. Y. Deng, H. Wang, K. Gu, and P. Song, “Alzheimer’s disease with frailty: Prevalence, screening, assessment, intervention strategies and challenges,” BioScience Trends, vol. 17, pp. 283-292, 2023. [CrossRef]
- Jiang, X.; Chang, L.; Zhang, Y.-D. Classification of Alzheimer’s Disease via Eight-Layer Convolutional Neural Network with Batch Normalization and Dropout Techniques. J. Med Imaging Heal. Informatics 2020, 10, 1040–1048. [Google Scholar] [CrossRef]
- Dave, B.P.; Shah, Y.B.; Maheshwari, K.G.; Mansuri, K.A.; Prajapati, B.S.; Postwala, H.I.; Chorawala, M.R. Pathophysiological Aspects and Therapeutic Armamentarium of Alzheimer’s Disease: Recent Trends and Future Development. Cell. Mol. Neurobiol. 2023, 43, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I. “Hypothesis review: Alzheimer’s overture guidelines,” Brain Pathology, vol. 33, p. e13122, 2023. [CrossRef]
- Mattap, S.M.; Mohan, D.; McGrattan, A.M.; Allotey, P.; Stephan, B.C.; Reidpath, D.D.; Siervo, M.; Robinson, L.; Chaiyakunapruk, N. The economic burden of dementia in low- and middle-income countries (LMICs): a systematic review. BMJ Glob. Heal. 2022, 7, e007409. [Google Scholar] [CrossRef] [PubMed]
- AlSharabi, K.; Bin Salamah, Y.; Abdurraqeeb, A.M.; Aljalal, M.; Alturki, F.A. EEG Signal Processing for Alzheimer’s Disorders Using Discrete Wavelet Transform and Machine Learning Approaches. IEEE Access 2022, 10, 89781–89797. [Google Scholar] [CrossRef]
- Gafni, T.; Shlezinger, N.; Cohen, K.; Eldar, Y.C.; Poor, H.V. Federated Learning: A signal processing perspective. IEEE Signal Process. Mag. 2022, 39, 14–41. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, L.; Wei, G.; Wang, S. A novel algorithm for all pairs shortest path problem based on matrix multiplication and pulse coupled neural network. Digit. Signal Process. 2011, 21, 517–521. [Google Scholar] [CrossRef]
- G. Buttman, “The shadow of the telescope: a biography of John Herschel,” The Shadow of the Telescope, pp. 1-288, 2022.
- Fan, Y.; Sun, J.; Shu, Y.; Zhang, Z.; Zheng, G.; Chen, W.; Zhang, J.; Gui, K.; Wang, K.; Chen, Q.; et al. Efficient Synthetic Aperture for Phaseless Fourier Ptychographic Microscopy with Hybrid Coherent and Incoherent Illumination. Laser Photon- Rev. 2022, 17. [Google Scholar] [CrossRef]
- Yu, J.; Wei, Y. Digital Signal Processing for High-Speed THz Communications. Chin. J. Electron. 2022, 31, 534–546. [Google Scholar] [CrossRef]
- Valizadeh, M.; Wolff, S.J. Convolutional Neural Network applications in additive manufacturing: A review. Adv. Ind. Manuf. Eng. 2022, 4, 100072. [Google Scholar] [CrossRef]
- Rajaby, E.; Sayedi, S.M. A structured review of sparse fast Fourier transform algorithms. Digit. Signal Process. 2022, 123, 103403. [Google Scholar] [CrossRef]
- Soori, M.; Arezoo, B.; Dastres, R. Artificial intelligence, machine learning and deep learning in advanced robotics, a review. Cogn. Robot. 2023, 3, 54–70. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Phillips, P.; Yang, J.; Yuan, T.-F. Three-Dimensional Eigenbrain for the Detection of Subjects and Brain Regions Related with Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 50, 1163–1179. [Google Scholar] [CrossRef] [PubMed]
- Vowels, M.J.; Vowels, L.M.; Wood, N.D. Spectral and cross-spectral analysis—A tutorial for psychologists and social scientists. Psychol. Methods 2023, 28, 631–650. [Google Scholar] [CrossRef] [PubMed]
- Stanković, L.; Mandic, D. Convolutional Neural Networks Demystified: A Matched Filtering Perspective-Based Tutorial. IEEE Trans. Syst. Man, Cybern. Syst. 2023, 53, 3614–3628. [Google Scholar] [CrossRef]
- Campbell, J. A. , Benton, V. De Bortoli, T. Rainforth, G. Deligiannidis, and A. Doucet, “A continuous time framework for discrete denoising models,” Advances in Neural Information Processing Systems, vol. 35, pp. 28266-28279, 2022.
- R. B. Pachori, Time-frequency analysis techniques and their applications: CRC Press, 2023.
- Ha, L.D.; Kim, K.J.; Kwon, S.J.; Chang, B.; Hwang, S. Time-Resolved Electrochemical Impedance Spectroscopy of Stochastic Nanoparticle Collision: Short Time Fourier Transform versus Continuous Wavelet Transform. Small 2023, 19, e2302158. [Google Scholar] [CrossRef] [PubMed]
- M. Khan, S. K. M. Khan, S. K. Hasnain, and M. Jamil, Digital Signal Processing: A Breadth-first Approach: CRC Press, 2022.
- X.-D. Zhang, Modern signal processing: Walter de Gruyter GmbH & Co KG, 2022.
- Dong, M.; Yan, D.; Gong, Y. Adversarial Example Devastation and Detection on Speech Recognition System by Adding Random Noise. J. Audio Eng. Soc. 2023, 71, 34–44. [Google Scholar] [CrossRef]
- Ruggeri, R.; Blake, C.; DeRose, J.; Garcia-Quintero, C.; Hadzhiyska, B.; Ishak, M.; Jeffrey, N.; Joudaki, S.; Krolewski, A.; Lange, J.U.; et al. A data compression and optimal galaxy weights scheme for Dark Energy Spectroscopic Instrument and weak lensing data sets. Mon. Not. R. Astron. Soc. 2023, 525, 3865–3878. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, C.; Zhang, B. Reconstruction of central arterial pressure waveform based on CNN-BILSTM. Biomed. Signal Process. Control. 2022, 74, 103513. [Google Scholar] [CrossRef]
- Zhou, K.; Tang, J. A wavelet neural network informed by time-domain signal preprocessing for bearing remaining useful life prediction. Appl. Math. Model. 2023, 122, 220–241. [Google Scholar] [CrossRef]
- V. Goyal, A. V. Goyal, A. Chaudhary, and R. Saxena, “Review of Wavelet Transform and its Application in Different Compression Technique,” 2022.
- Özhan, O. Basic Transforms for Electrical Engineering; Springer Science and Business Media LLC: Dordrecht, GX, Netherlands, 2022; ISBN 9783030988456. [Google Scholar]
- Ebrahiminia, F.; Cichy, R.M.; Khaligh-Razavi, S.-M. A multivariate comparison of electroencephalogram and functional magnetic resonance imaging to electrocorticogram using visual object representations in humans. Front. Neurosci. 2022, 16, 983602. [Google Scholar] [CrossRef]
- Granado, M.; Collavini, S.; Baravalle, R.; Martinez, N.; Montemurro, M.A.; Rosso, O.A.; Montani, F. High-frequency oscillations in the ripple bands and amplitude information coding: Toward a biomarker of maximum entropy in the preictal signals. Chaos: Interdiscip. J. Nonlinear Sci. 2022, 32, 093151. [Google Scholar] [CrossRef]
- Liu, S.; Chen, S.; Huang, Z.; Liu, X.; Li, M.; Su, F.; Hao, X.; Ming, D. Hypofunction of directed brain network within alpha frequency band in depressive patients: a graph-theoretic analysis. Cogn. Neurodynamics 2022, 16, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Vitorino, R.; Barros, A.S.; Guedes, S.; Caixeta, D.C.; Sabino-Silva, R. Diagnostic and monitoring applications using near infrared (NIR) spectroscopy in cancer and other diseases. Photodiagnosis Photodyn. Ther. 2023, 42, 103633. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Han, X.; Ouyang, M.; Burke, A.F. Specialized deep neural networks for battery health prognostics: Opportunities and challenges. J. Energy Chem. 2023, 87, 416–438. [Google Scholar] [CrossRef]
- Lam, S.I.; Pant, M. Manning, M. Kubanski, P. Fox, S. Rajan, et al., “Time-frequency analysis using V-band radar for drone detection and classification,” in 2023 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), 2023, pp. 1-6. [CrossRef]
- Rodríguez, F.; Azcárate, I.; Vadillo, J.; Galarza, A. Forecasting intra-hour solar photovoltaic energy by assembling wavelet based time-frequency analysis with deep learning neural networks. Int. J. Electr. Power Energy Syst. 2021, 137, 107777. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Ji, G.; Dong, Z. AN IMPROVED QUALITY GUIDED PHASE UNWRAPPING METHOD AND ITS APPLICATIONS TO MRI. Prog. Electromagn. Res. 2014, 145, 273–286. [Google Scholar] [CrossRef]
- Zhang, Y.-D.; Wang, S.-H.; Yang, X.-J.; Dong, Z.-C.; Liu, G.; Phillips, P.; Yuan, T.-F. Pathological brain detection in MRI scanning by wavelet packet Tsallis entropy and fuzzy support vector machine. SpringerPlus 2015, 4, 1–16. [Google Scholar] [CrossRef]
- Allam, A.K.; Froudarakis, E.; Papageorgiou, T.D. Individualized Real-Time fMRI Neuromodulation for the Alleviation of Cranial Nerve IX and XII Neuropathy (S38.004). Neurology 2023, 100. [Google Scholar] [CrossRef]
- Siddique, M.F.; Ahmad, Z.; Ullah, N.; Kim, J. A Hybrid Deep Learning Approach: Integrating Short-Time Fourier Transform and Continuous Wavelet Transform for Improved Pipeline Leak Detection. Sensors 2023, 23, 8079. [Google Scholar] [CrossRef]
- Torres-Castilllo, J.R.; López-López, C.O.; Padilla-Castañeda, M.A. Neuromuscular disorders detection through time-frequency analysis and classification of multi-muscular EMG signals using Hilbert-Huang transform. Biomed. Signal Process. Control. 2021, 71, 103037. [Google Scholar] [CrossRef]
- Nafea, M.S.; Ismail, Z.H. Supervised Machine Learning and Deep Learning Techniques for Epileptic Seizure Recognition Using EEG Signals—A Systematic Literature Review. Bioengineering 2022, 9, 781. [Google Scholar] [CrossRef]
- Pokorny, F.B.; Schmitt, M.; Egger, M.; Bartl-Pokorny, K.D.; Zhang, D.; Schuller, B.W.; Marschik, P.B. Automatic vocalisation-based detection of fragile X syndrome and Rett syndrome. Sci. Rep. 2022, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-H.; Du, S.; Zhang, Y.; Phillips, P.; Wu, L.-N.; Chen, X.-Q.; Zhang, Y.-D. Alzheimer’s Disease Detection by Pseudo Zernike Moment and Linear Regression Classification. CNS Neurol. Disord. - Drug Targets 2017, 16, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Peel, L.; Peixoto, T.P.; De Domenico, M. Statistical inference links data and theory in network science. Nat. Commun. 2022, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, J.; Wang, S.; Dong, Z.; Phillips, P. Pathological brain detection in MRI scanning via Hu moment invariants and machine learning. J. Exp. Theor. Artif. Intell. 2016, 29, 299–312. [Google Scholar] [CrossRef]
- M. Hashemi, A. N. M. Hashemi, A. N. Vattikonda, J. Jha, V. Sip, M. M. Woodman, F. Bartolomei, et al., “Simulation-based inference for whole-brain network modeling of epilepsy using deep neural density estimators,” medRxiv, p. 2022.06. 02.22275860, 2022.
- Dara, O.A.; Lopez-Guede, J.M.; Raheem, H.I.; Rahebi, J.; Zulueta, E.; Fernandez-Gamiz, U. Alzheimer’s Disease Diagnosis Using Machine Learning: A Survey. Appl. Sci. 2023, 13, 8298. [Google Scholar] [CrossRef]
- J. 10 3705, 2023. [CrossRef]
- Wang, S.; Zhang, Y.; Liu, G.; Phillips, P.; Yuan, T.-F. Detection of Alzheimer’s Disease by Three-Dimensional Displacement Field Estimation in Structural Magnetic Resonance Imaging. J. Alzheimer’s Dis. 2016, 50, 233–248. [Google Scholar] [CrossRef]
- Fu, X.; Chu, C.; Pang, Y.; Cai, H.; Ren, Z.; Jia, L. A blood mRNA panel that differentiates Alzheimer’s disease from other dementia types. J. Neurol. 2023, 270, 2117–2127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S. Detection of Alzheimer’s disease by displacement field and machine learning. PeerJ 2015, 3, e1251. [Google Scholar] [CrossRef]
- Yu, X.; Shao, K.; Wan, K.; Li, T.; Li, Y.; Zhu, X.; Han, Y. Progress in blood biomarkers of subjective cognitive decline in preclinical Alzheimer’s disease. Chin. Med J. 2023, 136, 505–521. [Google Scholar] [CrossRef] [PubMed]
- M. T. deBettencourt, W. A. M. T. deBettencourt, W. A. Bainbridge, and M. D. Rosenberg, “Functional neuroimaging,” 2023. [CrossRef]
- Wang, S.-H.; Zhang, Y.; Li, Y.-J.; Jia, W.-J.; Liu, F.-Y.; Yang, M.-M.; Zhang, Y.-D. Single slice based detection for Alzheimer’s disease via wavelet entropy and multilayer perceptron trained by biogeography-based optimization. Multimedia Tools Appl. 2016, 77, 10393–10417. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Sui, Y.; Yang, M.; Liu, B.; Cheng, H.; Sun, J.; Jia, W.; Phillips, P.; Gorriz, J.M. Multivariate Approach for Alzheimer’s Disease Detection Using Stationary Wavelet Entropy and Predator-Prey Particle Swarm Optimization. J. Alzheimer’s Dis. 2018, 65, 855–869. [Google Scholar] [CrossRef] [PubMed]

| Techniques used in the field of signal processing | Advantages | Drawbacks |
| digital signal processing (DSP) |
|
|
| fast Fourier transform (FFT) |
|
|
| Neuroimaging technique | Advantages | Drawbacks |
| electroencephalography (EEG) | High temporal resolution: EEG is able to record brain electrical activity with a temporal resolution of milliseconds, which can capture the brain’s rapidly changing activity. Low cost and easy to operate: EEG devices are relatively inexpensive and simple to operate, allowing for a wide range of applications in laboratory and clinical Settings. Strong tolerance for movement: EEG is more tolerant of subjects’ head movements, which is suitable for situations where active participation or dynamic tasks are required. |
Limited spatial resolution: Because EEG signals are interfered with by the skull and tissues, their spatial resolution is low, making it difficult to accurately locate the source of brain activity. Subject to artifacts and noise: EEG is susceptible to eye movement, muscle activity, and other electromagnetic interference, which may produce artifacts and noise that require subsequent signal processing. Unable to directly observe brain structure: EEG can only reflect the electrical activity of the brain and cannot provide direct information about brain structure. |
| functional magnetic resonance imaging (fMRI) | High spatial resolution: fMRI can provide high spatial resolution and can accurately locate the region where brain activity occurs, which plays an important role in the study of brain function regions. Non-invasive: fMRI is a non-invasive imaging technology that does not require intervention by means of surgery or inserting probes, and is more suitable for clinical and human studies. Visualizing brain structure and functional connectivity: fMRI can provide detailed three-dimensional images of brain structure and reveal interactions between brain regions through functional connectivity analysis. |
Low temporal resolution: The temporal resolution of fMRI is usually in the second level and does not capture rapid changes in brain activity. High cost and complexity: fMRI equipment and operations are relatively expensive and complex, requiring highly trained and specialized operators. Sensitivity to motion: fMRI is very sensitive to the subject’s head movements, that is, even small movements can lead to distortion of imaging results. |
| Time-frequency analysis technique | Introduction |
| Short-Time Fourier Transform (STFT) | The Short-Time Fourier Transform (STFT) is a time-frequency analysis technique used to analyze non-stationary signals. It provides a way to examine the frequency content of a signal over short and successive time intervals. The main idea behind STFT is to divide the signal into shorter segments called windows and then perform Fourier Transform on each window individually. STFT uses a sliding window function to extract short sections of the signal, which are then transformed into the frequency domain using the Fourier Transform. By applying this transformation to overlapping windows of the signal, we obtain a time-frequency representation that reveals how the frequency content of the signal evolves over time. The resulting representation is often visualized as a spectrogram, which shows the varying intensity of different frequencies at different time points. |
| Wavelet Transform | Wavelet Transform is another time-frequency analysis technique commonly used to analyze non-stationary signals. Unlike the STFT, which uses fixed-sized windows, the wavelet transform uses variable-sized windows called wavelets. These wavelets have different shapes and scales, allowing for a more flexible analysis of signal features at different resolutions. Wavelet Transform decomposes a signal into a set of wavelet coefficients at different scales and positions. This decomposition allows us to capture both localized and global frequency information of the signal. Like STFT, Wavelet Transform can also produce a time-frequency representation, but it offers better localization in time and frequency compared to STFT. Wavelet Transform is particularly useful in analyzing signals with transient or rapidly changing characteristics, as it can provide detailed information about the time-varying behavior of different frequency components. |
| Spectrogram Analysis | Spectrogram analysis refers to the visualization of the time-frequency representation of a signal using techniques like the STFT or Wavelet Transform. A spectrogram provides a 2D representation of the signal’s frequency content over time, revealing how different frequencies contribute to the signal at different time points. In practice, a spectrogram is obtained by calculating the magnitude or power of the frequency components obtained from the STFT or Wavelet Transform and displaying it as a function of time and frequency. The resulting image shows the intensity of different frequencies at different time intervals, allowing us to identify patterns, changes, and relationships between various frequency components in the signal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




