1. Introduction
Unlike nett values of CO2 exchange flux between the atmosphere and the land/ocean, the absolute each-way values are not accurately known, yet they are key to understanding the carbon cycle and to estimating the effects of anthropogenic CO2 fossil fuel emissions, (CO2ff) (IPCC (Ed.) 2013; IPCC(Ed) 2021). The exchange flow is caused by diurnal and seasonal variations which, as the earth spins on its axis and orbits the sun, alternately cause absorption and emission, arising from cyclical diurnal and seasonal changes in seawater solubility, photosynthesis, plant decay and respiration. This complicated pattern of both positive and negative fluxes implies that while absorption is occurring at one geographical location, there is simultaneous emission at another. This two-way exchange flow is estimated at approximately 210GtC in each direction per year, easily exceeding the one way CO2ff despite its rise from 2.5 GtC in 1960 to 9 GtC in 2021 (Friedlingstein et al., 2022). However, although CO2ff is smaller than exchange flow, it is still significant when compared to the imbalance in the exchange flux. In the 1970s box models were developed (e.g. Oeschger et al., 1975) to describe the carbon cycle; however, their popularity waned as General Circulation Models (GCMs) became common which, although requiring greater computer resources, could create gridded global maps of the carbon-related processes.
Symbol Table and Acronyms
| A, Aabs, As
|
Relative, absolute standard, and specific 14C activity |
| A14[], R14[] |
14C/C ratio: Atmosphere, Reservoir |
| AF |
Airborne Fraction |
| AFF[], |
Atmospheric Fossil Fuel content |
| AFL[], ANL[] |
Atmospheric Fossil Level, Natural (non-fossil) Level (0-1) |
| B |
Listed Annual Bomb Yield (MegaTonnes) |
| C |
Atmospheric carbon mass GtC |
| CO2ff |
Anthropogenic fossil fuel CO2 emissions |
| F14C |
14C Carbon Flux |
| Fa , Fe , Fi
|
Atmospheric CO2 flux: Anthropogenic, exiting, going in |
| GCM |
General Circulation Models |
| GtC |
Gigatonnes Carbon: equals 109 tonnes of carbon |
| IPCC |
Intergovernmental Panel on Climate Change |
| ke
|
Atmospheric CO2 Exchange rate |
| MT, Mi
|
Mass of Mixture and Mass of portion i |
| R, R14
|
Isotopic ratio, 14C/C ratio |
| RCO2 |
Relative Reservoir Size |
| RT, Ri
|
Ratio of a specific molecule in mixture, T and portion i |
| Yb |
Bomb Yield in megatons |
| αt |
Removal time (years) |
| β |
Rel. proportion of CO2ff mixing into atmosphere |
| Δ14C |
An offset age & fractionation corrected ratio of 14C/C † |
| Δ14Cinit |
Initial value of Δ14C at start of iteration |
| ΔC |
Change in C at each iteration |
| ΔT |
Change in time at each iteration |
| δ |
Isotopic ratio relative to a standard |
| δ13C |
An offset measure of 13C/C ratio relative to a standard |
| δ13CF , δ13CN
|
δ13C: For Fossil CO2, Natural (non-fossil CO2) |
|
δ13Cinit, δ13Cff
|
The value of δ13C : Initial, fossil fuels |
| δ13CM, δ13CW
|
δ13C for a Measurement, for Wood |
| δ14C |
An offset measure of 14C/C ratio relative to a standard |
| σT, σ1, σ2
|
Standard deviation of fit of time series: Total, 1, 2 |
| (t) |
Denotes a function value at time, t |
| [i] |
Denotes the value at each iteration, i |
| Bold-shaded indicates the seven internal optimized parameters; † 14C/12C ratio rel. to hypothetical value of atmos 14C in 1950 (See Stuiver & Polach, 1977). |
Undoubtedly GCMs have a significant role to play in modeling the complexity of the global carbon cycle. However, there are advantages in adopting a strategy of top-down simplification using box models, rather than adopting the open-ended system of micro-accounting in the GCMs. This is because simpler models by their very nature are "better testable" (Popper, 1934) and are more likely to shed light on errors in formulation, focusing on the most significant factors. This paper describes a 2-box model that derives 14C and 13C isotopic ratios given anthropogenic emissions and CO2 mixing ratio. The major differences between this and previous box models are; a) its use of atmospheric CO2 level as an input, not an output, and b) its use of absolute gross flow, not nett flow. This model accurately calculates isotopic 14CO2 and 13CO2 time-series spanning some 200 years (including the bomb pulse). Yet according to conventional wisdom the model should not work. It is widely known that the carbonate ions form a buffer solution limiting the absorption rate of CO2, this effect being known as the Revelle factor. It is commonly held that the 13C and 14C isotopic forms of carbon bypass seawater carbonate chemistry, exhibiting very different absorption properties as compared to 12C. (Tans 1993, Joos 1994, Harvey 2000), although this was never proven. This study demonstrates this assumption is invalid, since it uses the same residence time and reservoir mixing properties for all isotopes and yet produces accurate results. For a full discussion see section 7.1.
1.1. Radiocarbon
The global isotopic abundance of 13C and 14C present within atmospheric CO2 is accurately known, having been measured in samples from tree rings (Stuiver & Quay 1981), ice cores (Rubino et al., 2013), and the atmosphere (Stuiver et al., 1998; D6). While 13C is radioactively stable and forms around one percent of atmospheric carbon, 14C undergoes radioactive decay with a half-life of 5700 ± 30 years (Kutschera 2013), comprising about 1 part in 1012. There is virtually no presence of 14C in fossil fuel since it has all radioactively decayed. The 14C/12C ratio decreased between 1800 and 1960 because of the lack of 14C in fossil fuel emissions. Then in 1960 atmospheric nuclear weapons testing caused a sudden doubling of the absolute level, also known as 14C activity. Since 1975 it has decreased (D6), partially due to washout from the exchange flow which contains a somewhat lower level of 14C but also because of dilution arising from fossil fuels CO2 input, which is 14C depleted (Levin, 2010). This process is known as "Suess" dilution (Suess 1955). Turning now to 13C, its presence in fossil fuels is reduced when compared with the atmosphere, but only by around 2% (Stuiver and Polach, 1977); this small reduction arising from fractionation, i.e. because the larger less mobile isotopic CO2 molecules are less likely to become embedded in leaves and fossil carbon. Measurements show that the atmospheric 13C/12C ratio has decreased during the past 200-year period (D4), due to dilution by fossil fuel carbon which contains the lower level of 13C and washout from the exchange flow.(see Discussion)
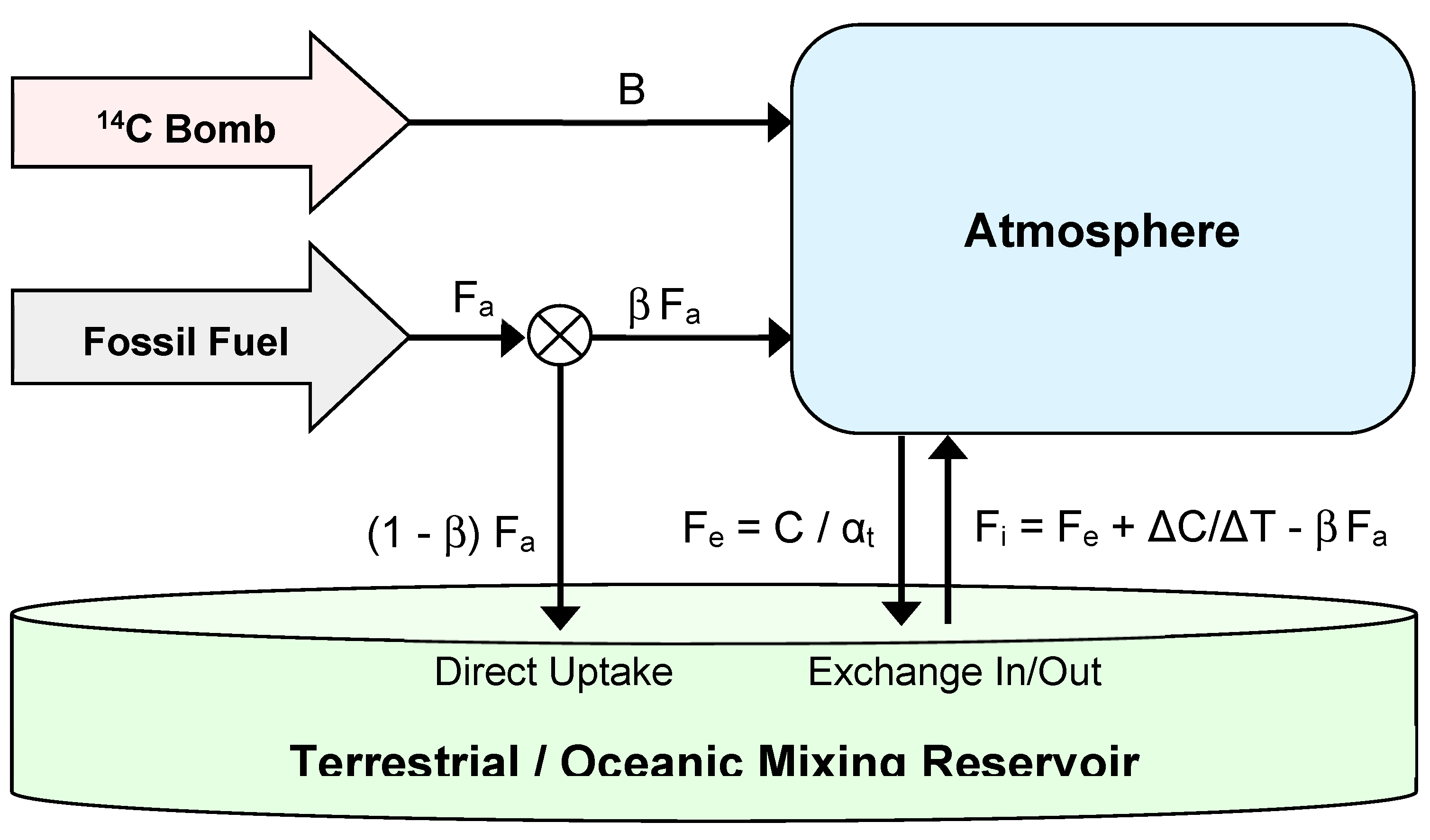
2. CO2 Absolute Flow Finite Reservoir Model
The model describes the movement of CO
2 and its carbon isotopes between atmosphere and terrestrial / oceanic mixing reservoir. See
Figure 1. The size of this mixing reservoir is an "effective" value, corresponding to the combined effect of the ocean and terrestrial storage. The idea of effective values is commonplace in many fields of engineering, science and economics. Economic inflation represents, in a single figure, the average of many different price increases on many items. Productivity measures the effective value created by each hour of work. Effective resistance in electronic engineering or hydraulics gives the overall resistance of a complex system to current or fluid flow. The assumption in each of these examples is that a single value can account for some property which is distributed throughout a complex system. The elegance of this method is its ability, unlike other methods, to determine the effective values without requiring a detailed audit of the underlying constituent components.
The two boxes in the model are considered well-mixed. At each iteration, the new isotopic concentration within the reservoir and atmosphere caused by the incoming
13C and
14C is calculated. We now explain the ideas behind the model's formulation. Using a similar manner and notation as Tans et al.,(1993) and Cawley (2011) we have
where C is the atmospheric carbon mass, F
a is the anthropogenic flux, F
i, is the flux in and F
e is the flux exiting the atmosphere. Many authors e.g. Oeschger (1975), assume a pre-industrial equilibrium but their method is not suitable for our purposes since it relies on the calculation of a net flux; it is essential that F
i and F
e are kept separate because we will need the separate fluxes to calculate the isotopic flows. We first need an expression which connects the atmospheric CO
2 mass C, to the outflow, F
e an obvious choice being
Absolute inflow, as opposed to outflow, can then be calculated by balancing the budget; if the atmosphere is growing by an amount
ΔC in time
ΔT it must be receiving the outflow plus the growth amount minus the inflow from anthropogenic input, which is anthropogenic output, F
a, times the proportion which arrives in the atmosphere, β , hence we obtain
Equation (1) satisfies the obvious requirement that the exit flux F
e , is zero when CO
2 mass C is zero. A further requirement, satisfied by equation(2) is that when F
i - F
e is zero the atmospheric growth
ΔC/ΔT, equals the anthropogenic input, F
a. These requirements distinguish this absolute flow description from other descriptions such as provided by Oeschger (1975) and Cawley (2011). This formulation leads to a radically different solution from conventional box models. It avoids the necessity to include allowance for seawater carbonate chemistry, because the outflow behaviour is lumped into a single constant,
ke which is empirically derived. Similarly the inflow behaviour, being calculated by balancing the budget, eliminates the need to provide a bottom-up audit of CO
2 release from the ocean or land due to outgassing, respiration, decay and combustion. Once outflow is derived, inflow can be defined from the known anthropogenic input and CO
2 measurements. We assume the constant k
e applies equally to the calculation of outflow for all three carbon species
14CO
2,
13CO
2 and
12CO
2 (see Discussion).
The model estimates the nuclear bomb yield of
14C each year, B
14[i], by using a bomb yield multiplication factor, Yb, this being one of the seven optimisation parameters. The bomb yield in megatons was obtained from records of atomic weapons atmospheric test detonations [D5] and was converted using Yb. A simple time delay of one year was used to allow for atmospheric mixing. The initial values i.e. δ
13C
init, Δ
14C
init, are parameters within the model and determine the initial level of the curves in
Figure 3 and
Figure 4, while δ
13C
FF determine the curve slopes in
Figure 4 and
Figure 5.
2.1. Mass-Balance and Isotopic Dilution
If a number of gases are mixed but do not react, and each component gas, i, has a mass M
i, then from the law of conservation of mass (also known as Mass Balance)
If within each component gas there is a specific molecule present in a ratio to its mass, R
i, then, since that specific molecule is conserved, the resulting mixture (e.g. paint mixing) having mass M
T has a ratio R
T given by
Equation (3) applies to the situation of mixing of isotopic gases. For practical reasons associated with historical measurements of radioactivity, studies of isotopic mixtures normally utilize the ratio of the sample activity, A
S to a standard sample activity A
abs, this ratio being known as the relative specific activity, A. Its value is then offset by one to provide the radioactivity scale, δ written (see Strenstrom 2011) in the form
In this scale, although δ increases linearly with A
s, its value is offset to be zero at the value of the absolute standard (often chosen to approximate background level), as for example in the cases of δ C and δ C. Applying (4) and substituting A
Si for each component R
i of the mixture, leads after some algebraic manipulation, to
The similarity with Eq.(3) shows that although δ is defined by an offset ratio scale we can still directly apply equation(3) to calculate the resulting value of δ
T for the mixture. The above scheme forms the basis for the system of equations A
14[], R
14[], A
FF[] and R
FF[] shown in the implementation section below.
2.2. Fractionation
The model generates values of δ
14C but comparison is required with measured values of Δ
14C, which incorporates a fractionation correction to "translate the measured activity to the activity the sample would have had if it had been wood" (Strenstrom 2011) . The correction formulae, a function of δ
13C, may be derived from equations by Strenstrom 2011 using equations 25, 28, 38, or Stuiver & Polach 1977 p356, 360,
Table 1 , where A
SN is the normalised specific sample activity, A
s is the specific activity of the atmosphere or reservoir, A
abs is the absolute specific standard, δ
13C
M is the
13C sample measurement , and δ
13C
W is the
13C standard for wood, giving
Eliminating A
S , A
abs and A
SN gives
The fractionation correction was applied to δ
14C, with δ
13C for wood as -25‰ (the standard), and δ
13C
M being taken from the model. The correction is quite small, Δ
14C is around 10‰ less than δ
14C at the peak of the bomb pulse ( δ
14C ~ 800‰) decreasing to 0.03‰ (δ
14C ~0).
2.3. Age Correction
The 14C half-life of 5700± 30 years (Kutschera, W., 2013), which translates to a radioactive decay of around 2% over the period from 1820 to 2020. However, we know the steady production of stratospheric 14C roughly balances the amount of 14C decay since the two are in approximate equilibrium. Therefore neither 14C decay nor natural stratospheric 14C production were included in the model.
2.4. Implementation
The implementation processes several calculations at each iteration. The following 3 equations are based upon . The amount of CO2 in the atmosphere is C, its removal time is αt, the atmosphere outflow is Fe , the inflow is Fi , and the amount of CO2 initially in the reservoir is R, with the square brackets '[i]' referring to the value at iteration i, hence the iteration relationships (using equations 1 and 2) become:-
The ratio of 14C / C in the atmosphere is denoted by A14 and for the reservoir by R14. The new amount is given by the previous amount added to the input amounts with the output amount being subtracted, all divided by the total mass, see equation 5. If necessary we include the annual 14C production due to atomic weapons bomb input, B14. Hence
Similarly, the relative atmospheric and reservoir fossil fuel content AFF and RFF, are calculated on a scale of 0 to 1 using the values at the previous iteration levels, where A refers to the atmosphere, and R the reservoir and subscripts FL means fossil fuel level, and NL means natural non-fossil level,
AFF[i] = (AFF [i-1].C[i-1] + Fi[i-1].RFF [i-1] - Fe[i-1].AFF[i-1] + β Fa [i-1]) / C [i]
RFF[i] = (RFF [i-1].R [i-1] - Fi[i-1].RFF [i-1] + Fe[i-1].AFF[i-1] + (1-β ) Fa [i-1]) / R [i]
AFL[i] = C[i] AFF[i], ANL[i] = C[i] - AFL[i]
RFL[i] = RFF[i] R[i], RNL[i] = R[i] - RFL[i]
The offset isotopic ratiometric measure for 13C is δ13C and that for 14C is Δ14C. These are calculated from the iterative series by:-
Initial Conditions: Fi[0] = Fe[0] , A14 [0] = 1 , R14 [0] = 1, AFF[0] = 0 , RFF[0] = 0
In accounting for isotopic concentration, it is not necessary to explicitly embed an attenuation factor as discussed by Stuiver & Quay (1981), or to account separately for Suess dilution (1955) because they are implicitly represented.
5. Method
The input data was selected between 1750 to 2020 from the appropriate data source listing [D1-D6] and entered into a spreadsheet. The cell formulae were set to correspond to those iteration equations given in the Appendix. The solution was found by minimizing the discrepancy between observed and calculated values of δ
13C and Δ
14C using the standard "Solver" function of Microsoft Excel. The solver system was set to use the 7 parameters shown in
Table 1 using the default solver options. For version details see appendix.
The optimisation-solution process is shown in
Figure 2 above. The standard deviations σ
1 and σ
2 between observed and predicted was determined for δ
13C and Δ
14C. The square root of this product gives the overall geometric mean standard deviation, σ. Hence
The Excel Solver was set to minimise the value of the target cell containing the overall geometric mean standard deviation by variation of the seven parameters shown in
Table 1. Only one true constant was used, atmospheric capacity, which was taken to have a value of 2.124 ppm GtC-1 as published by Ballantyne (2012). Thus the model uses a very different technique from standard modeling techniques for deriving anthropogenic and non-anthropogenic flux. It optimises the seven parameters to fit δ
13C and Δ
14C ratios, and the flux and mixing reservoir levels emerge as by-products from this process.
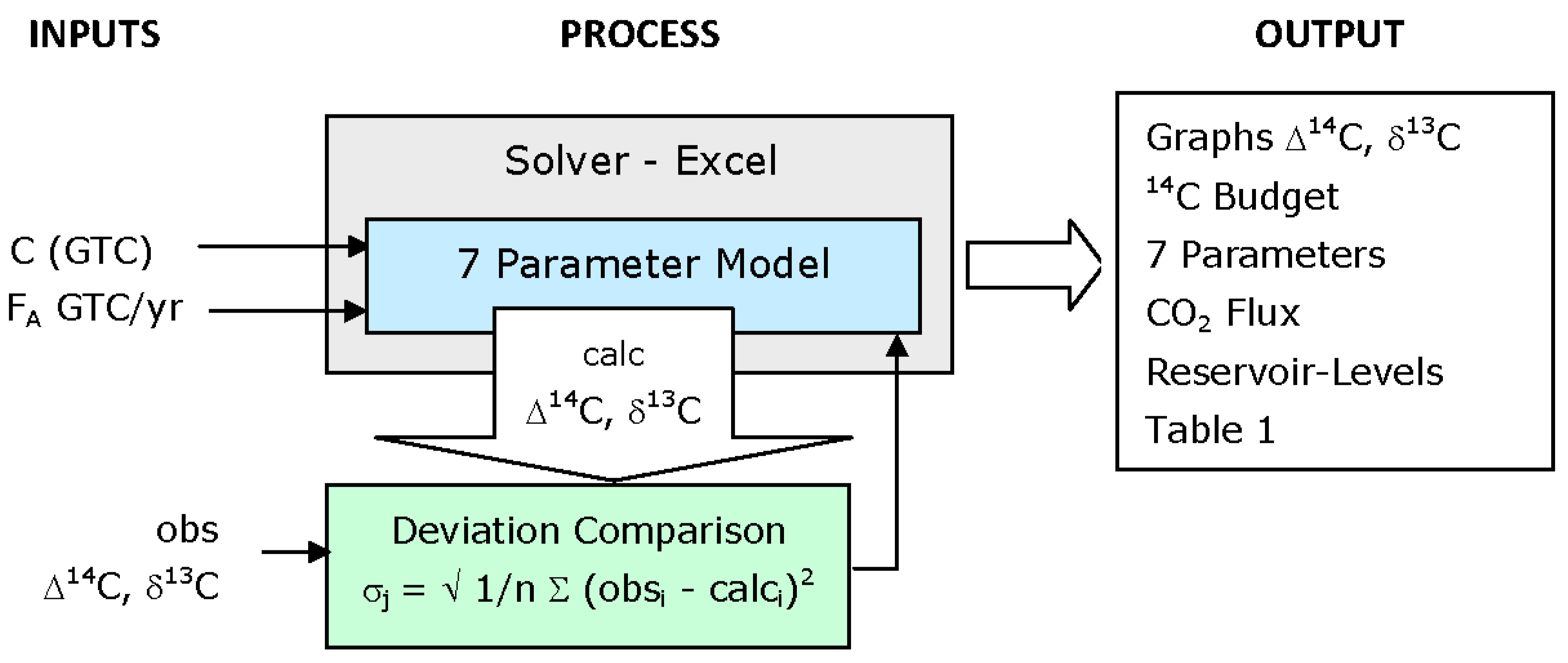
Figure 2.
Solution Optimisation. The 7 parameter model and the deviation comparison formulae are implemented as cell formulae within Microsoft Excel. The deviation comparison formulae compares the measured time-series values of atmospheric Δ14C and δ13C with the calculated values. The Add-In Solver was used to numerically minimise the standard deviation by varying the values of the 7 parameters, providing the above outputs shown below in this study.
Figure 2.
Solution Optimisation. The 7 parameter model and the deviation comparison formulae are implemented as cell formulae within Microsoft Excel. The deviation comparison formulae compares the measured time-series values of atmospheric Δ14C and δ13C with the calculated values. The Add-In Solver was used to numerically minimise the standard deviation by varying the values of the 7 parameters, providing the above outputs shown below in this study.
6. Results
The results divide into three main types; the optimised seven parameters themselves, the graphical output, and flux tables. Flux and cumulative flux are model outputs.
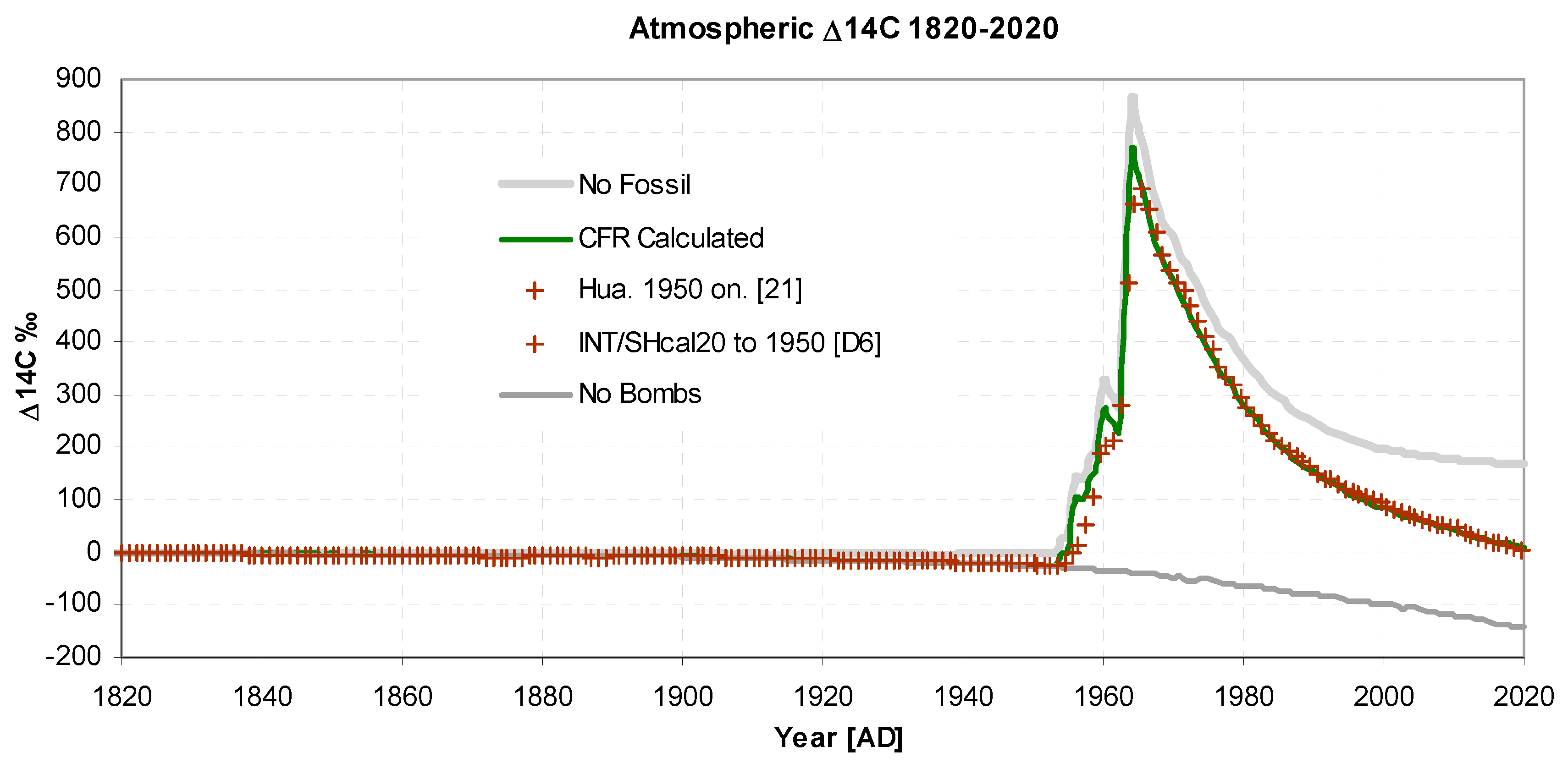
Figure 3 and
Figure 4 show actual and modelled values of ∆
14C. In total 340 observed data points were used giving a total overall standard deviation of 0.39‰. The quality of fit is excellent, with a standard deviation of 3‰. In
Figure 3 the slight decrease from 1820 to 1940 is due to Suess dilution, the magnitude of the dilution being somewhat reduced by reservoir inflow, giving excellent agreement with observations.
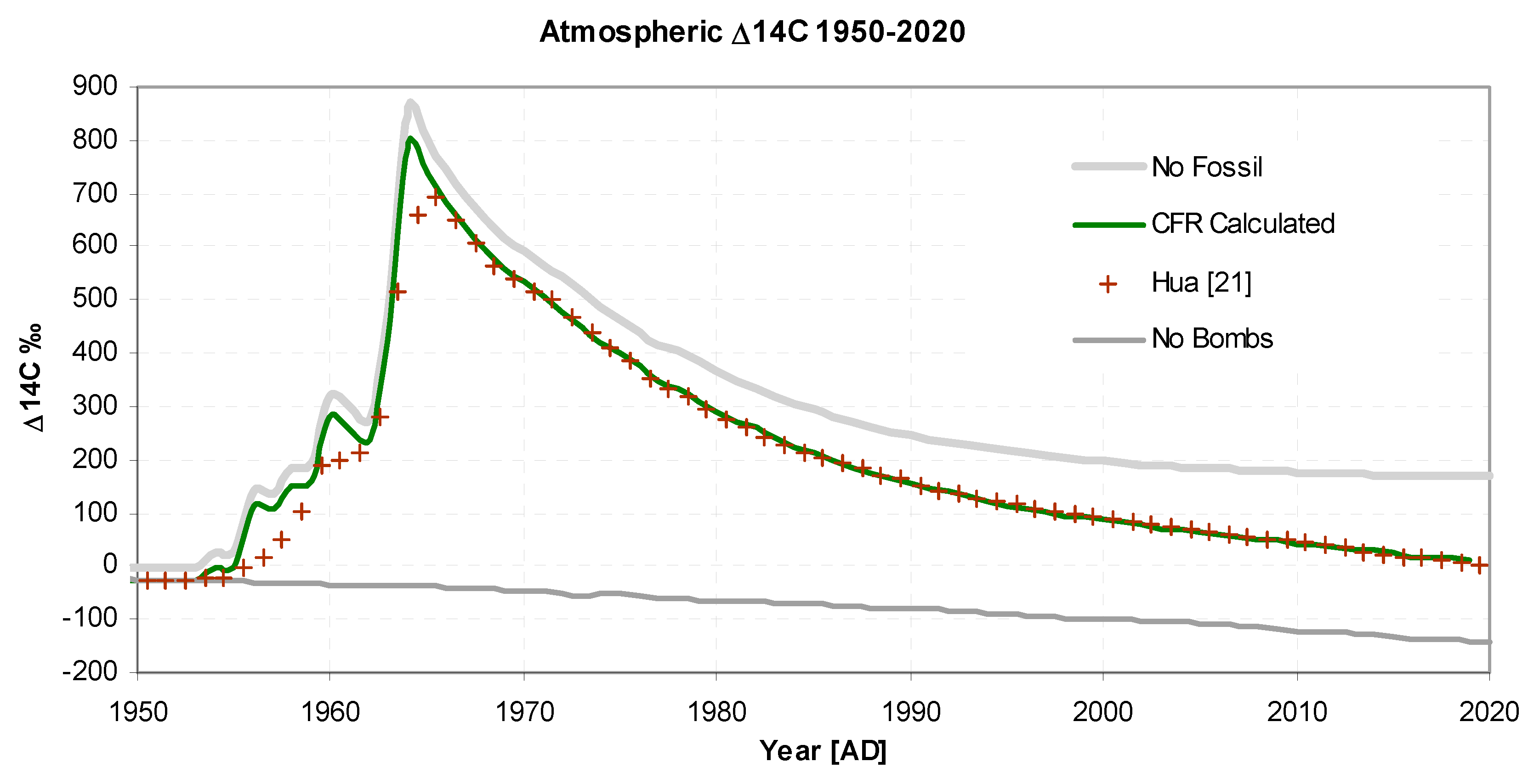
Figure 4 shows the bomb pulse plotted as ∆
14C. Initially the pulse shape is a decaying exponential but then, as it is influenced by Suess dilution and the re-emergence of
14C from the mixing reservoir, becomes more of a linear fall. Nevertheless, despite these complicating factors, the predicted and observed values are in excellent agreement.
Figure 3 and
Figure 4 also show two hypothetical scenarios "no bombs" and "no fossil". The "no bombs" scenario shows how the decrease would have continued without nuclear atmospheric weapons testing, while "no fossils" show the situation without fossil fuel emissions.
Figure 3.
Atmospheric Δ14C 1820-2020. showing 130 values before 1950 [D6] and 70 values from 1968 onwards (Hua et al.. 2022). Standard deviation of observed and predicted is σ = 3.0‰, excluding values during the rising pulse from 1950 to 1968. For "no bombs" and "no fossil". See text.
Figure 3.
Atmospheric Δ14C 1820-2020. showing 130 values before 1950 [D6] and 70 values from 1968 onwards (Hua et al.. 2022). Standard deviation of observed and predicted is σ = 3.0‰, excluding values during the rising pulse from 1950 to 1968. For "no bombs" and "no fossil". See text.
Figure 4.
Atmospheric Δ14C between 1950 and 2020 showing the "bomb pulse" to 2020. For "no bombs" and "no fossil" see text.
Figure 4.
Atmospheric Δ14C between 1950 and 2020 showing the "bomb pulse" to 2020. For "no bombs" and "no fossil" see text.
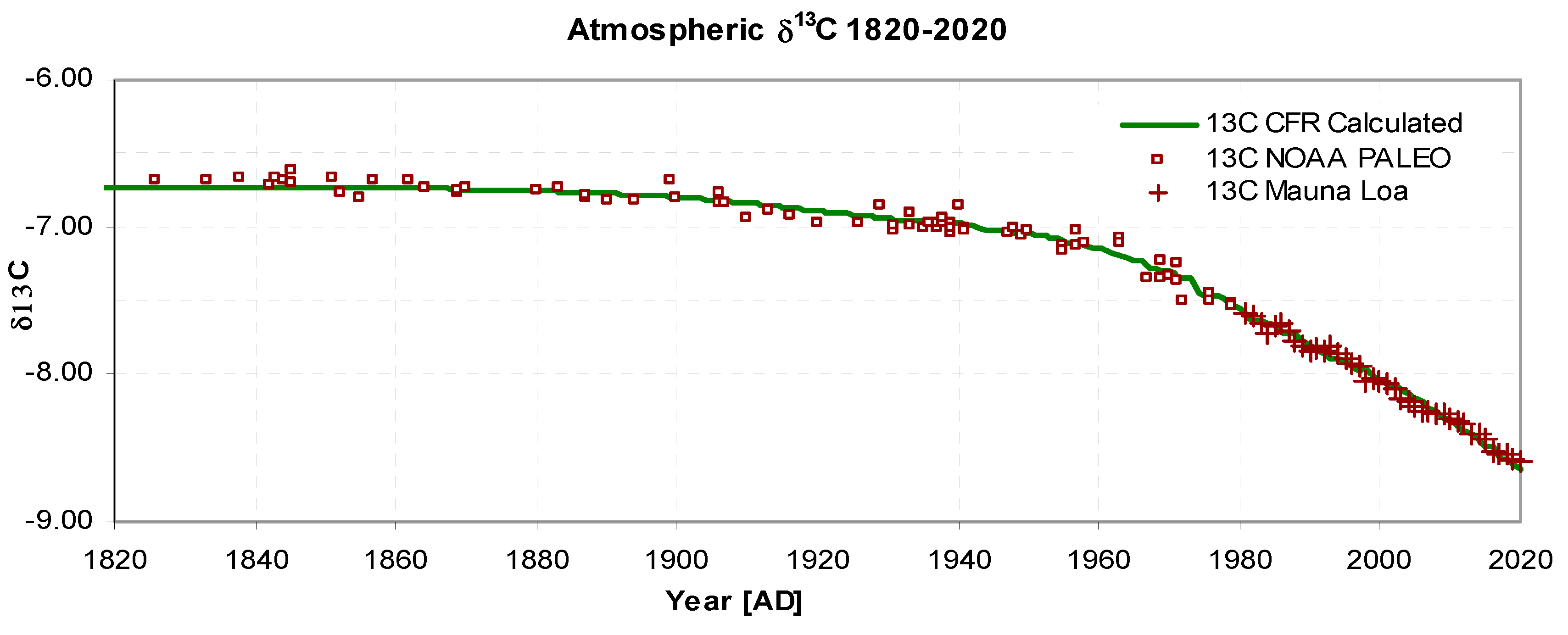
Figure 5 shows the change in δ
13C, with a standard deviation of 0.05‰. with the reduction being caused by Suess dilution (since fossil fuel emissions contains a lower level of
13C) and by re-emission of CO
2 from the reservoir. This shape is also accurately tracked by the model.
Figure 5.
Atmospheric δ13C 1820-2020. Model calculated values: solid green, Observed NOAA Paleo [D4]: squares, Mauna Loa [D2]: crosses, σ1820-2020= 0.05‰.
Figure 5.
Atmospheric δ13C 1820-2020. Model calculated values: solid green, Observed NOAA Paleo [D4]: squares, Mauna Loa [D2]: crosses, σ1820-2020= 0.05‰.
Table 1.
Solved 7 Parameter Values.
Table 1.
Solved 7 Parameter Values.
| Parameter |
Sym. |
Value |
Err. |
Notes |
| Removal time (years) |
αt
|
14.87 |
1.7 |
Compares reasonably with Revelle & Suess (1957) of approximately 10 yr, and Arnold (1957) of 10-20 yr. |
| Relative Reservoir Size |
RCO2 |
6.16 |
1.4 |
IPCC Ed., (2014) of 4250 GtC. 4250/588 = 7.2. |
| CO2ff Direct Uptake |
β |
0.56 |
0.11 |
See Discussion |
| Atomic Bomb Yield: 14C/MT |
Yb |
1.60 |
0.1 |
In mills. See Footnote |
Pre-industrial
14C ‰ |
Δ14Cinit |
-3.1 |
10 |
Broad agreement with Intcal20[D6] for 1820 of 0.7‰. Max level between 1820-2020 is 800‰. |
Pre-industrial
13C ‰ |
δ13Cinit |
-6.7 |
0.2 |
Excellent agreement with Rubino (2013) 1820 of δ13C =6.7‰. |
Fossil fuel
13C ‰ |
δ13Cff |
-20.4 |
4 |
Slightly low as compared to figures reported by Stuiver & Polach (1977) for coal of δ13C = -23 ‰. |
Nuclear Bomb Yield: The value returned is 1.6 ± 0.1 mills of 14C per MT, with a total bomb yield of 440MT. For comparison, we convert the figures to estimates of the number of 14C atoms per MT bomb yield using formulae derived from those of Svetlik (2010) and Strensom (2011). The number of carbon atoms in the atmosphere in 1950 with the CO
2 mixing level of 312.8 ppm corresponding to ACO2 = 664.43 GtC, where the molecular weight of naturally abundant carbon, MC = 12.01, and Avogadro's number N
A = 6.022×10
23, was
The
14C/C ratio R
14C can be obtained from the specific activity α = 0.226 Bq per gram,
14C half life T
1/2 = 5730 years = 1.807 10
11 s, molar weight of
14C is M
C14 = 14, and ln(2) as
The number of
14C atoms N
14C created is given by the product of the two expressions giving
Hence the number of 14C atoms per MT of bomb yield is 0.0016 x 4.6 x 10
28 = 7.4 x 10
25, comparing favourably with reported values by Hesshaimer et al. (1994) of 1 to 2 x 10
26 atoms per MT. The total bomb yield of 440MT gives a figure of 3.23 x 10
28 which compares with 6.1 x 10
28 by Naegler & Levin (2006). The discrepancy may be due to
14C which is stored but does not mix in the biosphere.
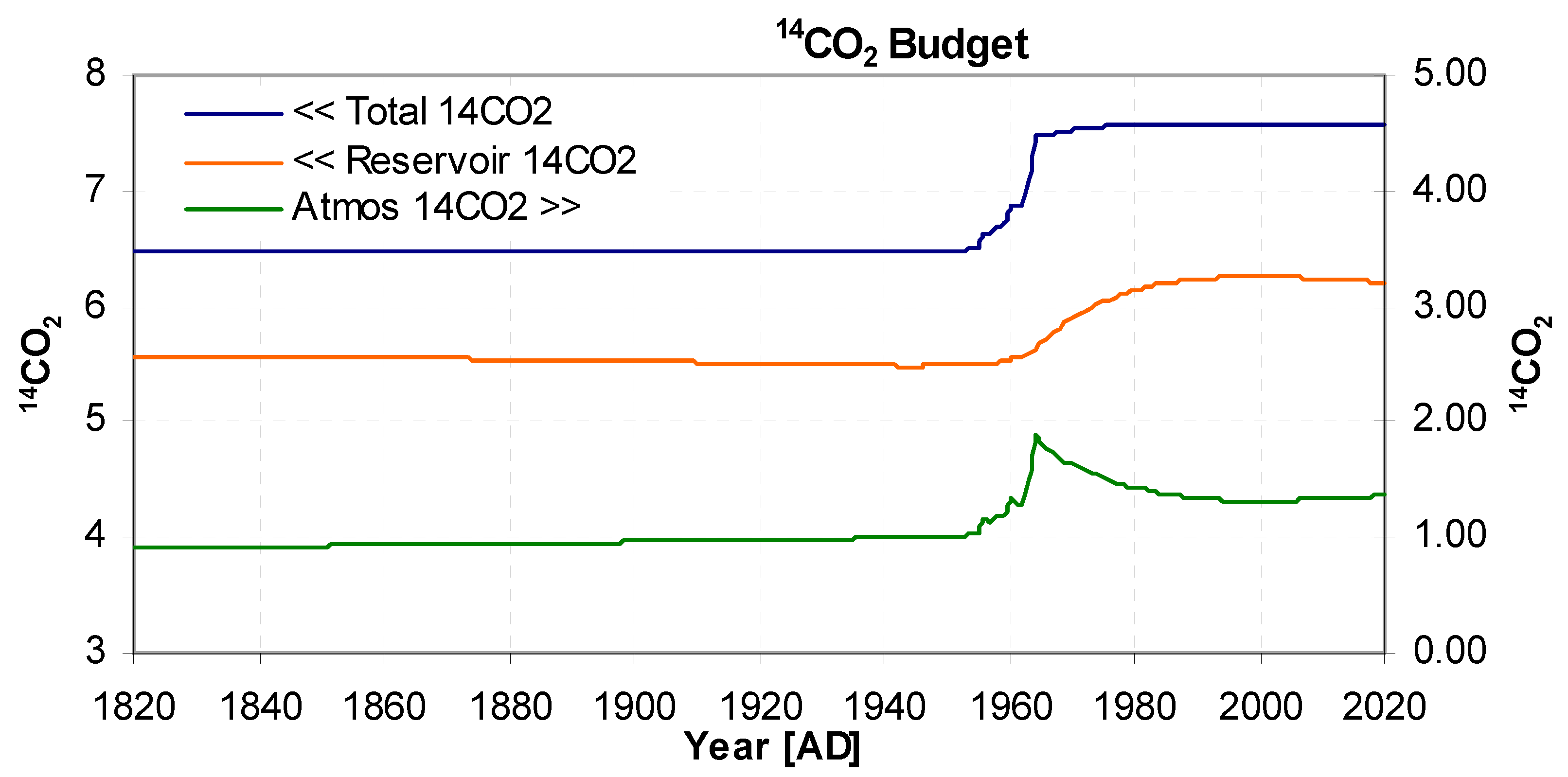
The amount of
14C, also known as activity concentration, (as opposed to Δ
14C), in the reservoir and atmosphere is shown in
Figure 6. Their total (blue) represents the amount of
14C in the system, the sudden step increase can be seen around 1960 due to atmospheric nuclear weapons testing. The atmospheric activity concentration shows the bomb pulse decaying to a minimum around 2000 but then rising slightly again, which has been experimentally reported by Svetlik (2010). The model shows the increase can be attributed to
14CO
2 re-emerging from the mixing reservoir, at the same time causing a slight drop in reservoir level.
6.2. Parameter Values
The solution of all seven parameter values, is listed in
Table 1 along with their estimated error, which was derived by varying each parameter up and down, while holding the other parameter values constant, until the overall geometric mean standard deviation, σ, approximately doubled. The error figure was the mean of the two variations. For all the parameters except fossil-fuel direct uptake, the variation by the error figure causes an insignificant change in the flux figures, however in the case of fossil-fuel direct uptake the change in non-fossil input is from -10GtC to 83GtC, implying an uncertainty in non-fossil input from -3.5% sink to 29% source.
The study reports that, over the past 270 years, 27% of CO2ff remains in the atmosphere corresponding to 13.9% of the atmospheric CO
2 content, and 88% of atmospheric growth is caused by CO2ff (see
Table 2). It is found that 44% of CO2ff is directly absorbed into terrestrial biosphere and ocean with the remainder i.e. 56%, becoming mixed into the atmosphere. A small amount, around 12% of atmospheric growth originates from natural sources although this figure had the largest uncertainty. See Discussion.
6.3. Flux and Cumulative Flux Output
Table 2 lists cumulative CO
2 flows and storage from 1750 to 2020, and 1960 to 2020, while
Figure 7 shows the flux diagrammatically.
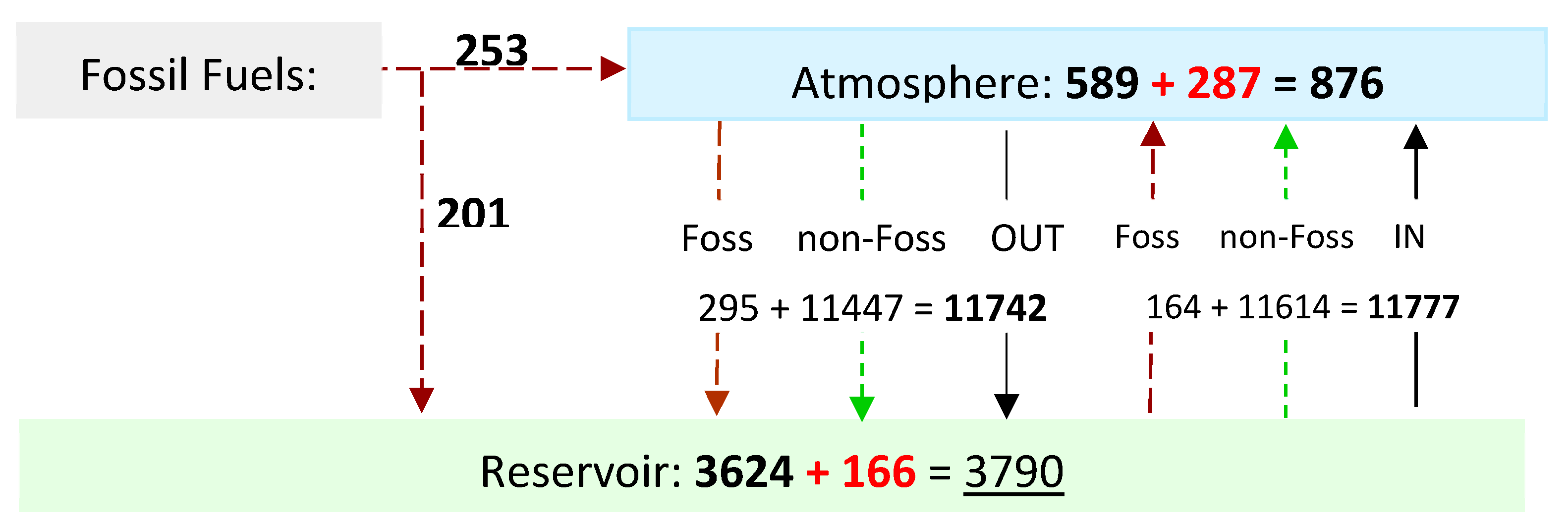
Figure 7.
Atmospheric CO
2 levels and cumulative flux 1750 to 2020. (GtC) The figures in red represent the increase over the period, underscore indicates 2020 values. The concentration of CO
2 from fossil fuel emissions (CO2ff) is higher in the atmosphere than in the reservoir, therefore the atmospheric flux OUT contains more CO2ff than the flux IN, removing fossil fuel emissions from the atmosphere. Atmospheric CO2ff = 253 - 295 + 164 = 121 (without rounding) corresponding to 13.9% of the atmosphere in 2020 (See
Table 2, rows 3 / 9).
Figure 7.
Atmospheric CO
2 levels and cumulative flux 1750 to 2020. (GtC) The figures in red represent the increase over the period, underscore indicates 2020 values. The concentration of CO
2 from fossil fuel emissions (CO2ff) is higher in the atmosphere than in the reservoir, therefore the atmospheric flux OUT contains more CO2ff than the flux IN, removing fossil fuel emissions from the atmosphere. Atmospheric CO2ff = 253 - 295 + 164 = 121 (without rounding) corresponding to 13.9% of the atmosphere in 2020 (See
Table 2, rows 3 / 9).
Table 2.
Cumulative CO2 Flow and Storage.
Table 2.
Cumulative CO2 Flow and Storage.
| Duration |
1750-2020 |
|
1960 -2020 |
| |
GtC |
% |
GtC |
% |
| CO2ff Delivered |
|
|
|
|
| delivered to Atmos. (β ) |
253 |
56 |
207 |
46 |
| delivered to Rsvr. (1-β ) |
201 |
44 |
165 |
36 |
| Total |
454 |
100 |
372 |
82 |
| CO2ff by Destination |
|
|
|
|
| In Atmos |
121 |
27 |
100 |
26 |
| In Rsvr |
332 |
73 |
272 |
71 |
| Total |
454 |
100 |
382 |
100 |
| Atmospheric Growth |
|
|
|
|
| due to CO2ff |
253 |
88 |
207 |
102 |
| due to non-Foss* |
35 |
12 |
-3 |
-1 |
| Total |
287 |
100 |
204 |
100 |
| Reservoir Growth |
|
|
|
|
| due to CO2ff |
332 |
|
272 |
|
| due to non-Foss* |
-166 |
|
-103 |
|
| Total |
166 |
|
164 |
|
| Atmospheric Outflow |
|
|
|
|
| CO2ff |
295 |
|
248 |
|
| non-Foss |
11447 |
|
2798 |
|
| Total |
11742 |
|
3045 |
|
| Reservoir Outflow |
|
|
|
|
| CO2ff |
164 |
|
141 |
|
| non-Foss |
11614 |
|
2902 |
|
| Total |
11777 |
|
3042 |
|
| CO2ff rel. to atmos CO2 2020† |
121/876 |
14 |
100/876 |
11.5 |
| CO2ff induced growth/Co2ff (AF) |
253/454 |
55 |
56 |
|
| Average Annual Flux from Reservoir |
43.7 |
|
50.8 |
|
| Average Annual Flux to Reservoir |
43.5 |
|
50.9 |
|
| |
|
|
|
|
6.4. Correlation of Inflow and Temperature
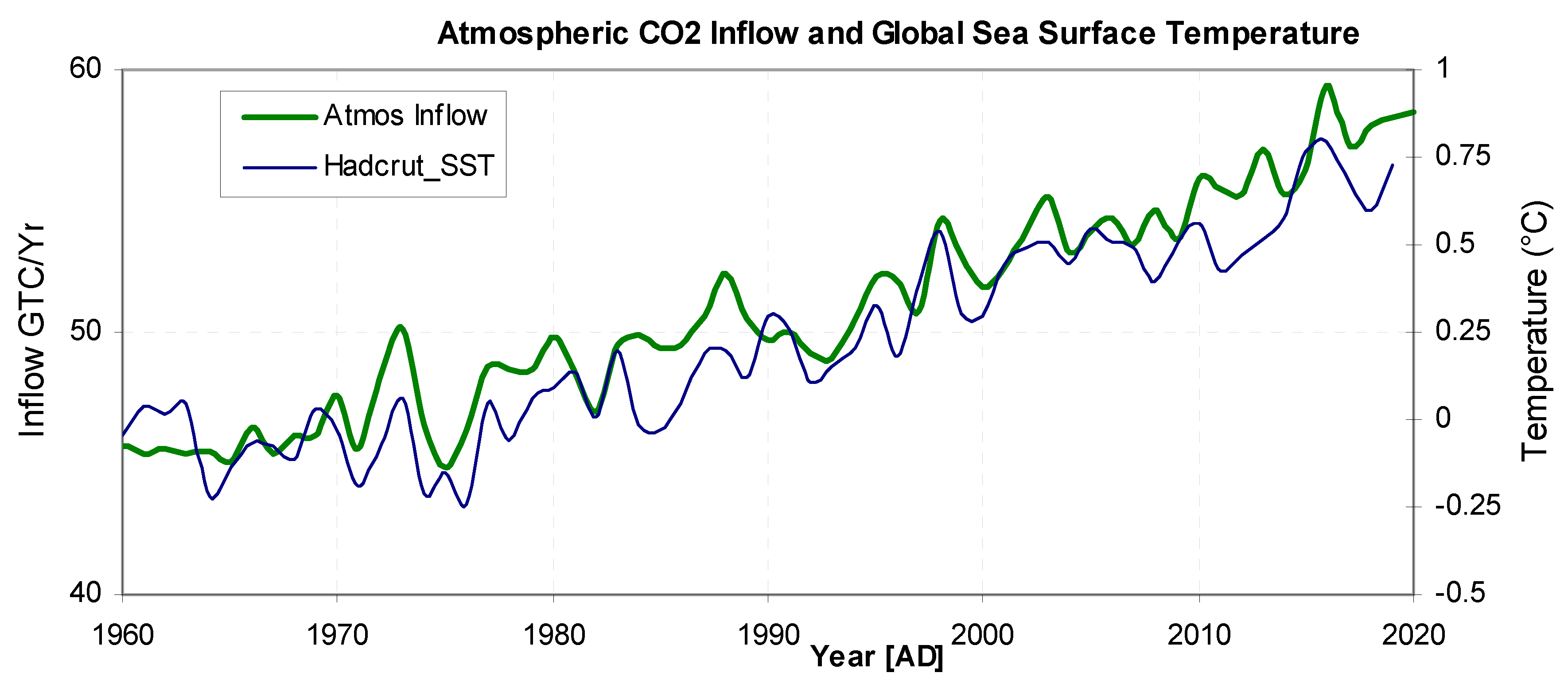
Figure 8 shows the absolute inflow (taken from the absolute model using the same 7 optimised parameters) and global temperature (D7) plotted on suitable vertical scales. The correlation coefficient is 0.94. Although correlation does not by itself imply causation, it is difficult to conceive of a mechanism whereby a global change in temperature could be caused by the rate of CO
2 inflow. The obvious temperature dependant mechanisms for CO
2 gas release in the ocean is the decrease of solubility with temperature, while on land there is a balance between productivity, respiration and decay (Melillo et al. 2011). This evidence suggests that when global temperature increases, CO
2 absolute inflow also increases although it could be conceivable that both share a common cause. The absolute global inflow should not be confused with net figures which show a net uptake of anthropogenic CO
2.
7. Discussion
This section discusses the differences between this work and that of other authors. Some authors (e.g. Levin et al. 2010) have found it necessary to propose additional industrial pollution figures for
14C in order to close the
14C budget. This paper has no such requirement, yet accurately reproduces the
14C curve. A recently published work (Graven et al. 2020) used a model originally produced by M. Heimann and R. Keeling and rewritten for Matlab, to give results for a "no bombs" and "no fossil" scenarios , in a similar way to those shown in
Figure 5 of this paper. The results shown by Graven are almost identical to those shown here further vindicating the model described and its approach. Recent work by Svetlik (2010) showed measurements of the
14C activity concentration. This model produces an output of activity concentration which is very similar to that in Svetlik's work showing that the calculations regarding
14C are in broad agreement.
7.1. Isotopic Behaviour
It is a widely held view that the various isotopes of carbon in their molecular form of CO
2 behave differently when the exchange flux interacts with sea-water, bypassing carbonate chemistry (Revelle). This was first suggested by Siegenthaler and Oeschger (1987) and has been repeated many time since. It is claimed to lead to a factor of ten difference in isotopic behaviour of
13C and
14C as compared to
12C, thus preventing a straightforward analysis of the
14C bomb pulse (e.g. Harvey 2000). The argument is refuted here. It suggests isotope separation occurs naturally on a global scale. This is unlikely since, from a thermodynamic point of view, the isotopic mixture has a lower Gibbs free energy and lower entropy, requiring an expenditure of energy to achieve separation of its constituents (Seader & Henley 2005). We present here a further argument showing why we believe the claimed bypass effect does not occur in practice. Given the carbon concentration C and the isotopic ratio R the flux F14C can be written as
The term, R.dC/dt, describes the bulk flow of carbon without isotopic variation and hence suffers from carbonate chemistry while C.dR/dt represents the isotopic flow which it is claimed bypasses seawater carbonate chemistry. We can better estimate the magnitude of the contribution of variations of either term, by dividing both sides by CR giving
A B
d(CR)/CR = dC/C + dR/R
A comparison of the local values of the relative sizes of dC/C and dR/R indicates which term dominates. Fortunately one can obtain approximate estimates of these ratios in a variety of situations, over sea and land from a number of papers (e.g. Bishcof 1960, Dai et al. 2009, Faassen et al, 2022, Leinweber et al., 2009, Olsen et al. 2004, Palonen et al. 2018, Takahashi et al. 2002). It is essential to use local estimates rather than global nett figures, because nett figures would average out differences in isotopic content. The local variation of dC/C was estimated from the above references as of the order of ≈6 ppm/400ppm (≈0.015) per hour, with variation in dR/R for 14C estimated as around ≈8‰ (≈0.008) per hour and for 13C ≈ 0.2 ‰ (≈0.0002) per hour. Hence |A| amply dominates |B|. Therefore, it is irrelevant whether or not B has an ability to bypass carbonate chemistry (Revelle factor) as claimed by many, since A is the dominant effect. Regarding the A term how does it respond to different isotopes of carbon? According to (Zeebe and Wolf 2001: Section 2.5 Page 119) referring to three isotopic forms
12C,
13C and
14C isotopic components "
There are no direct reactions between each of these compartments... Thus there are three independent systems with respect to carbon isotopes." Furthermore there is only one common subsidiary chemical process, i.e. "
chemical coupling of both systems brought about by the chemical reactions of the carbon compounds with "H+ and OH-". This is assumed here to be a relatively weak effect. Therefore in this study we consider the isotopic behaviour to be equal, although we accept there could be a small differences due to fractionation. The accuracy of the results for
Δ14C and δ13C shown in
Figure 3,
Figure 4 and
Figure 5 results amply vindicates these assumptions, despite the widely held belief to the contrary. Furthermore, the values of the 7 parameters in
Table 1 all have reasonable values, which seem extremely unlikely by chance. Therefore, regarding global absorption of atmospheric isotopic CO
2 by seawater, the argument is rejected that there is significant bypass of the Revelle factor.
7.2. Exchange Flow
As the earth daily rotates and annually orbits the sun, the terrestrial /oceanic reservoirs cyclically sucks in and blows out CO2. These are the exchange fluxes. In the model, the effective exchange flow is found to be around 50 GtC, considerably smaller than the IPCC figure of exchange flow at around 210 GtC. Consequently the exchange washout Residence Time (RT) in 1960 was around C/Fe= 689/46 ~ 15 years. Thus the residence time RT now aligns with the exponential decay of the atomic weapons pulse, without requiring the usual, but unlikely, hypothesis of unequal behaviour of isotopes. The difference in exchange flow arises because the model determines "effective values" which reflects the global average behaviour of molecular transfer, bearing in mind the mixing properties and cycle time lag (Oeschger 1975). Consider an analogy. Two isolated islands are separated by the services of a large passenger ferry. You may think the average residence time of a person on each island is given by the number of island inhabitants divided by the ferry flow. But the true average can be much less than this. How? Because the passenger ferry may carry much the same passengers back and forth, while many of the rural residents never take the ferry. Therefore the residence time is much longer because the "effective" washout flux is smaller. Similarly if the same exchange flow arises from decaying leaves and plant litter being cycled each year, the effective exchange flow is reduced, changing the residence time from around 4 years to 15.
7.3. Airborne Fraction
The Airborne Fraction, (AF) is said to be the ratio of "CO
2 remaining in the atmosphere" divided by "the anthropogenic CO
2 supplied" (Broeker 1975). It has been suggested that the reason for its constancy is related to the solution of an ordinary differential equation as applied to atmospheric CO2 flux (Cawley 2011). According to this view, if the time constant for the exponential rate of rise of CO2ff matches the time constant for the rate of depletion of CO2ff then the Airborne Fraction would be 0.5. However this paper suggests that the origin is quite different and is related to the direct uptake of CO
2 before the remainder is mixed into the atmosphere (
Figure 1).
7.4. Direct Uptake
The presence of a direct uptake mechanism (as shown in
Figure 1) significantly improves the model's accuracy, reducing the overall standard deviation by approximately 30%. Recent studies of daytime releases of
14CO
2 in the vicinity of nuclear power plants have reported significant
14CO
2 uptake (Naegler & Levin 2006) while a study of urban grasses near a major highway reported plants stored up to 13% of fossil-fuel carbon (Lichtfouse et al, 2005; Ota et al 2016). According to Kuderer et al (2018), "
The 14C signals from such point sources are well detectable in plant samples". Therefore it seems reasonable to propose the existence of a direct uptake mechanism for CO2ff before it becomes mixed in the atmosphere. The effect of the direct uptake mechanism is to more rapidly transfer part of the rising CO2ff emissions to the reservoir, hence slightly changing the shape of the
14C and
13C curves.
7.5. 14C Bomb Pulse
Regarding the shape of the bomb pulse, it would be expected that, if there is a constant level of washout exchange flux, its shape would be a reducing exponential converging back to its original background value. However simple inspection reveals this not to be the case. See
Figure 4. This shape departs from an exponential decay because of three main reasons; a) incoming fossil fuel (free of
14C) is diluting the atmosphere, this being called "Suess Dilution" (Suess 1955), b) some of that incoming fossil fuel is also being washed out by the exchange flow and c) some of the incoming exchange flow has a higher level of
14C, because bomb
14C having accumulated in the oceans is now being re-emitted back to the atmosphere. If (a) was the sole cause of the deviation of the bomb curve shape from a pure exponential decay, the curve would be well below Δ
14C=0, bearing in mind that the atmospheric growth of 30% is attributed to fossil fuel emissions containing no
14C. The effect of washout of fossil fuel emissions (b) raises the curve; this was noticed many years ago and termed by Stuiver and Quay (1981) the "attenuation factor". However the raise is still not quite sufficient to make a perfect match. Finally effect (c) raises the tail slightly further improving the fit to the current level shown in
Figure 4.
Keeping track of all of these factors in a "back of the envelope" calculation is complex. The absolute flow model described calculates at each iteration 13C and 14C in reservoir and atmosphere (see Implementation section). It does not need to explicitly consider Suess dilution, the Stuiver attenuation factor, flow-back or the airborne factor as these emerge implicitly from the calculation.
7.6. Amount "Remaining" in the Atmosphere
The ambiguous meaning of "remaining" has led to some confusion between different authors; the situation is clarified here by extending the above island analogy further. Suppose one of the above islands, island A has an annual supply of people arriving via its Airport, increasing its population. The ferry continues to carry exactly the same number of people back and forth between island A and island B. The population of the island A increases, yet over time the ferry has carried many of the airport arrivals to island B, and has brought inhabitants back from B to A in their place. A census would report that the number of people on island A who flew in, is less than its population growth because some are living on island B. However others would say that airport arrivals caused 100% of the growth of A since without airport arrivals their population would not have grown. This illustrates the discrepancy. IPCC state "The combustion of fossil fuels and land-use change for the period 1750–2019 resulted in the release of 700 ± 75 PgC (likely range, 1 PgC = 1015 g of carbon) to the atmosphere, of which about 41% ± 11% remains in the atmosphere today (high confidence)" (IPCC 2023 p80). However the amount "remaining" of CO2ff has been found by Skrable to be 23% and in this paper to be 27%. The apparent discrepancy arises because a) a significant part of CO2ff causing the growth has been washed out and almost exactly replaced by the exchange flow, and b) because the IPCC figure includes an allowance for Land Use Change (LUC) which cannot be accounted for by the isotopic method because of its unknown isotopic signature (although LUC has significantly reduced in recent years). The alternative measure of molecular CO2 remaining relative to atmospheric CO2 content was provided by Skrable (12%), Stallinga (<10%), this paper (14%) providing reasonable agreement between the three authors.
8. Conclusion
A two box CO2 absolute flow model is described which
accurately predicts the values of Δ14C and δ13C over 200 years.
revises the view of the airborne fraction, proposing that it reflects the relative amount of CO2ff that is absorbed directly into the terrestrial/oceanic reservoir,
does not use or require a consideration of carbonate seawater chemistry (Revelle),
shows there is no practical significant difference in behaviour of different isotopes apart from fractionation
explains how exponential decay time of the bomb pulse does, after all, relate to the residence time,
resolves the conflicting calculations of how much CO2ff "remains" in the atmosphere.
The method produces annual estimates of both nett inflow and the individual exchange flows, making it possible to calculate the molecular CO2ff remaining and also to provide the amount of atmospheric growth due to CO2ff. Thus the model leads to new estimates for the period 1750 to 2020 of the partitioning of anthropogenic CO2 emissions, with 88% of growth being attributable to CO2 fossil fuel emissions, and 12% being due to net "natural" emissions from the terrestrial and oceanic source, including land use change. Relative to total CO2ff of 454 GtC, 253 GtC or 55% was accountable for the growth of the atmosphere. However, at the molecular level, because of the washout caused by exchange flow, only 27% of fossil fuel emissions remain airborne, which corresponds to 14% of the entire CO2 atmosphere. The model uniquely provides both figures thus helping to resolve any confusion regarding the meaning and effects of fossil fuel CO2 emissions.
Acknowledgments
I would like to thank my colleague Dr. Andrew Layfield (Engineering and Environmental Studies), City University, Hong Kong, for his detailed comments and proofreading. The author would like to thank and acknowledge all the data providers indicated in "Data References", without whom this work could never have been carried out. This research was self-funded and received no external funding. The author declares no conflict of interest. The author would like to thank the publisher for reducing the APC which was funded by the publisher.
Appendix A. Data References
- D1.
Institute for Atmospheric and Climate Science (IAC), CO2 Mean Global AD0 to AD2014 ftp://data.iac.ethz.ch/CMIP6/input4MIPs/UoM/GHGConc/CMIP/yr/atmos/UoM-CMIP-1-1- 0/GHGConc/gr3-GMNHSH/v20160701/ mole_fraction_of_ carbon_dioxide_in_air_input 4MIPs_GHGConcentrations_CMIP_UoM-CMIP-1-1-0_gr3-GMNHSH_0000-2014.csv
- D2.
- D3.
Global Carbon Budget: National_Carbon_Emissions_2021v0.4.xlsx Historical Budget, Global Fossil Emissions Visited 04 March 2022. Friedlingstein et al (2021),
- D4.
- D5.
UNSCEAR: United Nations Scientific Committee on the Effects of Atomic Radiation 2000 Report To The General Assembly. Volume I: Sources. Annex C: Exposures To The Public From Man-Made Sources Of Radiation 207 Sources And Effects Of Ionizing Radiation. Table 4. Annual Fission And Fusion Yields.
- D6.
Calib: INTCAL20/SGCAL20. Stuiver, M. et al, 2022 CALIB 8.2 [WWW program] at
http://calib.org, accessed 2022-3-4 Rev 8.1.0 intcal20.14c, shcal20.14c
- D7.
- D8.
hadcrut4 /data/current/time_series/ HadCRUT.4.6.0.0.monthly_ns_avg.txt, hadsst4/data/csv/ HadSST.4.0.0.0_monthly_GLOBE.csv
-
END
References
- Arnold, J.R. , 1957. The Distribution of Carbon-14 in Nature. Tellus 9, 28–32. [CrossRef]
- Ballantyne, A.P. , Alden, C.B., Miller, J.B., Tans, P.P., White, J.W.C., 2012. Increase in observed net carbon dioxide uptake by land and oceans during the past 50 years. Nature 488, 70–72. [CrossRef]
- Bischof, W. , 1960. Periodical Variations of the Atmospheric CO 2 -content in Scandinavia. Tellus 12, 216–226. [CrossRef]
- Broecker, W.S. , 1975. Climatic Change: Are We on the Brink of a Pronounced Global Warming? Science 189, 460–463. [CrossRef]
- Cawley, G.C. , 2011. On the Atmospheric Residence Time of Anthropogenically Sourced Carbon Dioxide. Energy Fuels 25, 5503–5513. [CrossRef]
- Dai, M. , Lu, Z., Zhai, W., Chen, B., Cao, Z., Zhou, K., Cai, W.-J., Chenc, C.-T.A., 2009. Diurnal variations of surface seawater pCO2 in contrasting coastal environments. Limnol. Oceanogr. 54, 735–745. [CrossRef]
- Faassen, K.A.P. , Nguyen, L.N.T., Broekema, E.R., Kers, B.A.M., Mammarella, I., Vesala, T., Pickers, P.A., Manning, A.C., Vilà-Guerau De Arellano, J., Meijer, H.A.J., Peters, W., Luijkx, I.T., 2022. Diurnal variability of atmospheric O2, CO2 and their exchange ratio above a boreal forest in southern Finland (preprint). Gases/Field Measurements / Troposphere / Physics (physical properties and processes). [CrossRef]
- Friedlingstein, P. , Jones, M.W., O’Sullivan, M., Andrew, R.M., Bakker, D.C.E., Hauck, J., Le Quéré, C., Peters, G.P., Peters, W., Pongratz, J., Sitch, S., Canadell, J.G., Ciais, P., Jackson, R.B., Alin, S.R., Anthoni, P., Bates, N.R., Becker, M., Bellouin, N., Bopp, L., Chau, T.T.T., Chevallier, F., Chini, L.P., Cronin, M., Currie, K.I., Decharme, B., Djeutchouang, L.M., Dou, X., Evans, W., Feely, R.A., Feng, L., Gasser, T., Gilfillan, D., Gkritzalis, T., Grassi, G., Gregor, L., Gruber, N., Gürses, Ö., Harris, I., Houghton, R.A., Hurtt, G.C., Iida, Y., Ilyina, T., Luijkx, I.T., Jain, A., Jones, S.D., Kato, E., Kennedy, D., Klein Goldewijk, K., Knauer, J., Korsbakken, J.I., Körtzinger, A., Landschützer, P., Lauvset, S.K., Lefèvre, N., Lienert, S., Liu, J., Marland, G., McGuire, P.C., Melton, J.R., Munro, D.R., Nabel, J.E.M.S., Nakaoka, S.-I., Niwa, Y., Ono, T., Pierrot, D., Poulter, B., Rehder, G., Resplandy, L., Robertson, E., Rödenbeck, C., Rosan, T.M., Schwinger, J., Schwingshackl, C., Séférian, R., Sutton, A.J., Sweeney, C., Tanhua, T., Tans, P.P., Tian, H., Tilbrook, B., Tubiello, F., Van Der Werf, G.R., Vuichard, N., Wada, C., Wanninkhof, R., Watson, A.J., Willis, D., Wiltshire, A.J., Yuan, W., Yue, C., Yue, X., Zaehle, S., Zeng, J., 2022. Global Carbon Budget 2021. Earth Syst. Sci. Data 14, 1917–2005. [CrossRef]
- Graven, H. , Keeling, R.F., Rogelj, J., 2020. Changes to Carbon Isotopes in Atmospheric CO 2 Over the Industrial Era and Into the Future. Global Biogeochemical Cycles 34, e2019GB006170. [CrossRef]
- Harvey, L.D.D. 2000. Global warming: the hard science, 1. publ. ed, Pearson education. Prentice Hall, Harlow Munich. Routledge, (2018) ISBN 0582-38167-3.
- Hesshaimer, V. , Heimann, M., Levin, I., 1994. Radiocarbon evidence for a smaller oceanic carbon dioxide sink than previously believed. Nature 370, 201–203. [CrossRef]
- Houghton, J.T. , Intergovernmental Panel on Climate Change (Eds.), 1996. Climate change 1995: the science of climate change. Cambridge University Press, Cambridge ; New York.
- Hua, Q. , Turnbull, J.C., Santos, G.M., Rakowski, A.Z., Ancapichún, S., De Pol-Holz, R., Hammer, S., Lehman, S.J., Levin, I., Miller, J.B., Palmer, J.G., Turney, C.S.M., 2022. ATMOSPHERIC RADIOCARBON FOR THE PERIOD 1950–2019. Radiocarbon 64, 723–745. [CrossRef]
- Intergovernmental Panel On Climate Change (Ed.), 2014. Carbon and Other Biogeochemical Cycles, in: Climate Change 2013 – The Physical Science Basis. Cambridge University Press, pp. 465–570. [CrossRef]
- Intergovernmental Panel On Climate Change, 2023. Climate Change 2021 – The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, 1st ed. Cambridge University Press. [CrossRef]
- Joos, F. , 1994. Imbalance in the budget. Nature 370, 181–182. [CrossRef]
- Joos, F. , Bruno, M., Fink, R., Siegenthaler, U., Stocker, T.F., Le Quéré, C., Sarmiento, J.L., 1996. An efficient and accurate representation of complex oceanic and biospheric models of anthropogenic carbon uptake. Tellus B: Chemical and Physical Meteorology 48, 397. [CrossRef]
- Kutschera, W. , 2013. Applications of accelerator mass spectrometry. International Journal of Mass Spectrometry 349–350, 203–218. [CrossRef]
- Leinweber, A. , Gruber, N., Frenzel, H., Friederich, G. E., and Chavez, F. P., 2009, Diurnal carbon cycling in the surface ocean and lower atmosphere of Santa Monica Bay, California, Geophys. Res. Lett. 0860; 36. [Google Scholar] [CrossRef]
- Levin, I. , Naegler, T., Kromer, B., Diehl, M., Francey, R.J., Gomez-Pelaez, A.J., Steele, L.P., Wagenbach, D., Weller, R., Worthy, D.E., 2010. Observations and modelling of the global distribution and long-term trend of atmospheric 14CO2. Tellus B: Chemical and Physical Meteorology 62, 26. [CrossRef]
- Lichtfouse, E. , Lichtfouse, M., Kashgarian, M., Bol, R., 2005. 14C of grasses as an indicator of fossil fuel CO2 pollution. Environ Chem Lett 3, 78–81. [CrossRef]
- Kuderer, Matthias, Samuel Hammer, and Ingeborg Levin. “The Influence of 14CO2 Releases from Regional Nuclear Facilities at the Heidelberg 14CO2 Sampling Site (1986–2014).” Atmospheric Chemistry and Physics 18, no. 11 (June 6, 2018): 7951–59. [CrossRef]
- Melillo, J.M. , Butler, S., Johnson, J., Mohan, J., Steudler, P., Lux, H., Burrows, E., Bowles, F., Smith, R., Scott, L., Vario, C., Hill, T., Burton, A., Zhou, Y.-M., Tang, J., 2011. Soil warming, carbon–nitrogen interactions, and forest carbon budgets. Proc. Natl. Acad. Sci. U.S.A. 108, 9508–9512. [CrossRef]
- Naegler, T. , Levin, I., 2006. Closing the global radiocarbon budget 1945–2005. J. Geophys. Res. 111, D12311. [CrossRef]
- Ota, M. , Katata, G., Nagai, H., Terada, H., 2016. Impacts of C-uptake by plants on the spatial distribution of 14 C accumulated in vegetation around a nuclear facility—Application of a sophisticated land surface 14 C model to the Rokkasho reprocessing plant, Japan. Journal of Environmental Radioactivity 162–163, 189–204. [CrossRef]
- Oeschger, H. , Siegenthaler, U., Schotterer, U., Gugelmann, A., 1975. A box diffusion model to study the carbon dioxide exchange in nature. Tellus A: Dynamic Meteorology and Oceanography 27, 168. [CrossRef]
- Olsen, A. , Omar, A. M., Stuart-Menteth, A. C., and Triñanes, J. A., 2004, Diurnal variations of surface ocean pCO2 and sea-air CO2 flux evaluated using remotely sensed data, Geophys. Res. Lett., 31, L20304. [CrossRef]
- Palonen, V. , Pumpanen, J., Kulmala, L., Levin, I., Heinonsalo, J., Vesala, T., 2018. Seasonal and Diurnal Variations in Atmospheric and Soil Air 14 CO 2 in a Boreal Scots Pine Forest. Radiocarbon 60, 283–297. [CrossRef]
- Popper, Karl. 1934. Logik der Forschung [The Logic of Scientific Discovery] (2nd ed.). Reprint 1962. La lógica de la investigación científica. Tecnos, Madrid. Reprint 1992. London: Routledge. pp. 121–132. (1992) ISBN 978-84-309-0711-3.
- Revelle, R. , Suess, H.E., 1957. Carbon Dioxide Exchange Between Atmosphere and Ocean and the Question of an Increase of Atmospheric CO2 during the Past Decades. Tellus 9, 18–27. [CrossRef]
- Rubino, M. , Etheridge, D.M., Trudinger, C.M., Allison, C.E., Battle, M.O., Langenfelds, R.L., Steele, L.P., Curran, M., Bender, M., White, J.W.C., Jenk, T.M., Blunier, T., Francey, R.J., 2013. A revised 1000 year atmospheric δ 13 C-CO 2 record from Law Dome and South Pole, Antarctica: 1000 YEARS OF ATMOSPHERIC δ 13 C-CO 2. J. Geophys. Res. Atmos. 118, 8482–8499. [CrossRef]
- Seader, J.D. , Henley, E.J., Roper, D.K., 2011. Separation Process Principles with Applications using Process Simulators, 3rd ed. ed. Wiley, Somerset. ISBN 1118139623.
- Siegenthaler, U. , Oeschger, H., 1987. Biospheric CO2 emissions during the past 200 years reconstructed by deconvolution of ice core data. Tellus B: Chemical and Physical Meteorology 39, 140. [CrossRef]
- Skrable, K. , Chabot, G., French, C., 2022. World Atmospheric CO2, Its 14C Specific Activity, Non-fossil Component, Anthropogenic Fossil Component, and Emissions (1750–2018). Health Physics 122, 291–305. [CrossRef]
- Stallinga, P. , 2023. Residence Time vs. Adjustment Time of Carbon Dioxide in the Atmosphere. Entropy 25, 384. [CrossRef]
- Stenström, K, Skog, G, Georgiadou, E, Genberg, J & Mellström, A. 2011. A guide to radiocarbon units and calculations. LUNFD6(NFFR-3111)/1-17/(2011), Lund University, Nuclear Physics.
- Stuiver, M. , Polach, H.A., 1977. Discussion Reporting of 14 C Data. Radiocarbon 19, 355–363. [CrossRef]
- Stuiver, M. , Quay, P.D., 1981. Atmospheric 14C changes resulting from fossil fuel CO2 release and cosmic ray flux variability. Earth and Planetary Science Letters 53, 349–362. [CrossRef]
- Stuiver, M. , Reimer, P.J., Bard, E., Beck, J.W., Burr, G.S., Hughen, K.A., Kromer, B., McCormac, G., Van Der Plicht, J., Spurk, M., 1998. INTCAL98 Radiocarbon Age Calibration, 24,000–0 cal BP. Radiocarbon 40, 1041–1083. [CrossRef]
- Suess, H.E. , 1955. Radiocarbon Concentration in Modern Wood. Science 122, 415–417. [CrossRef]
- Svetlik, I. , Povinec, P.P., Molnár, M., Meinhardt, F., Michálek, V., Simon, J., Svingor, É., 2010. Estimation of Long-Term Trends in the Tropospheric 14 CO 2 Activity Concentration. Radiocarbon 52, 815–822. [CrossRef]
- Takahashi, H.A. , Konohira, E., Hiyama, T., Minami, M., Nakamura, T., Yoshida, N., 2002. Diurnal variation of CO2 concentration, Δ14C and δ13C in an urban forest: estimate of the anthropogenic and biogenic CO2 contributions. Tellus B: Chemical and Physical Meteorology 54, 97. [CrossRef]
- Tans, P.P. , Berry, J.A., Keeling, R.F., 1993. Oceanic 13 C/ 12 C observations: A new window on ocean CO 2 uptake. Global Biogeochemical Cycles 7, 353–368. [CrossRef]
- Tans, P. , 2022. Reminiscing On The Use And Abuse Of 14C And 13C In Atmospheric CO2. Radiocarbon 64, 747–760. [CrossRef]
- Zeebe, Richard E., and Dieter Wolf-Gladrow. 2001. CO2 in Seawater: Equilibrium, Kinetics, Isotopes. Gulf Professional Publishing, 2001. ISBN 9780444509468.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).