Submitted:
20 October 2023
Posted:
21 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
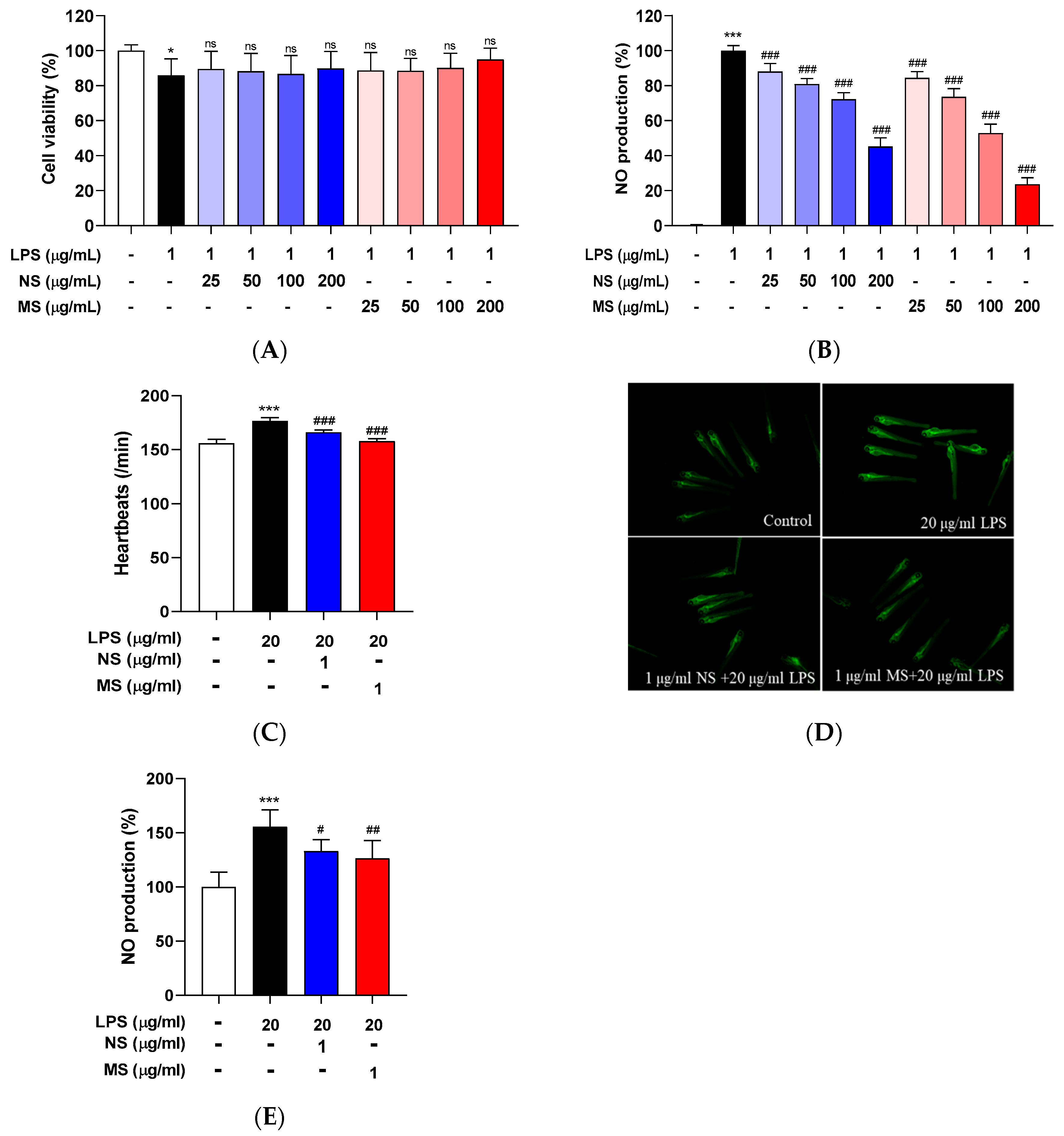
2.1. Carotenoid Extract from Natural Seawater (NS) and Magma Seawater (MS) Inhibits Lipopolysaccharide (LPS)-Induced Toxicity and Nitric Oxide Production
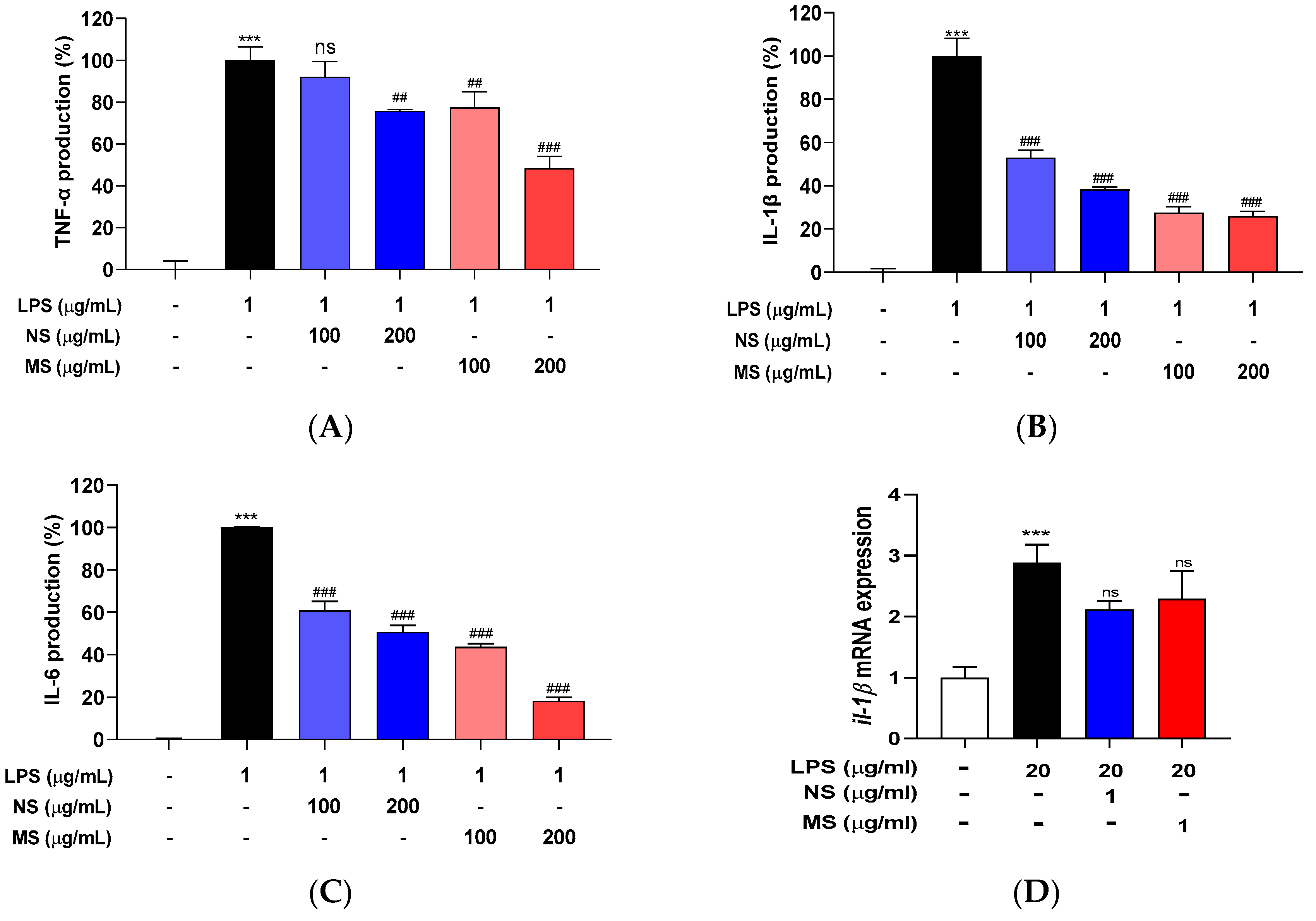
2.2. Carotenoid Extract from Natural (NS) and Magma Seawater (MS) Inhibits Lipopolysaccharide (LPS)-Induced Pro-Inflammatory Cytokines Secretion
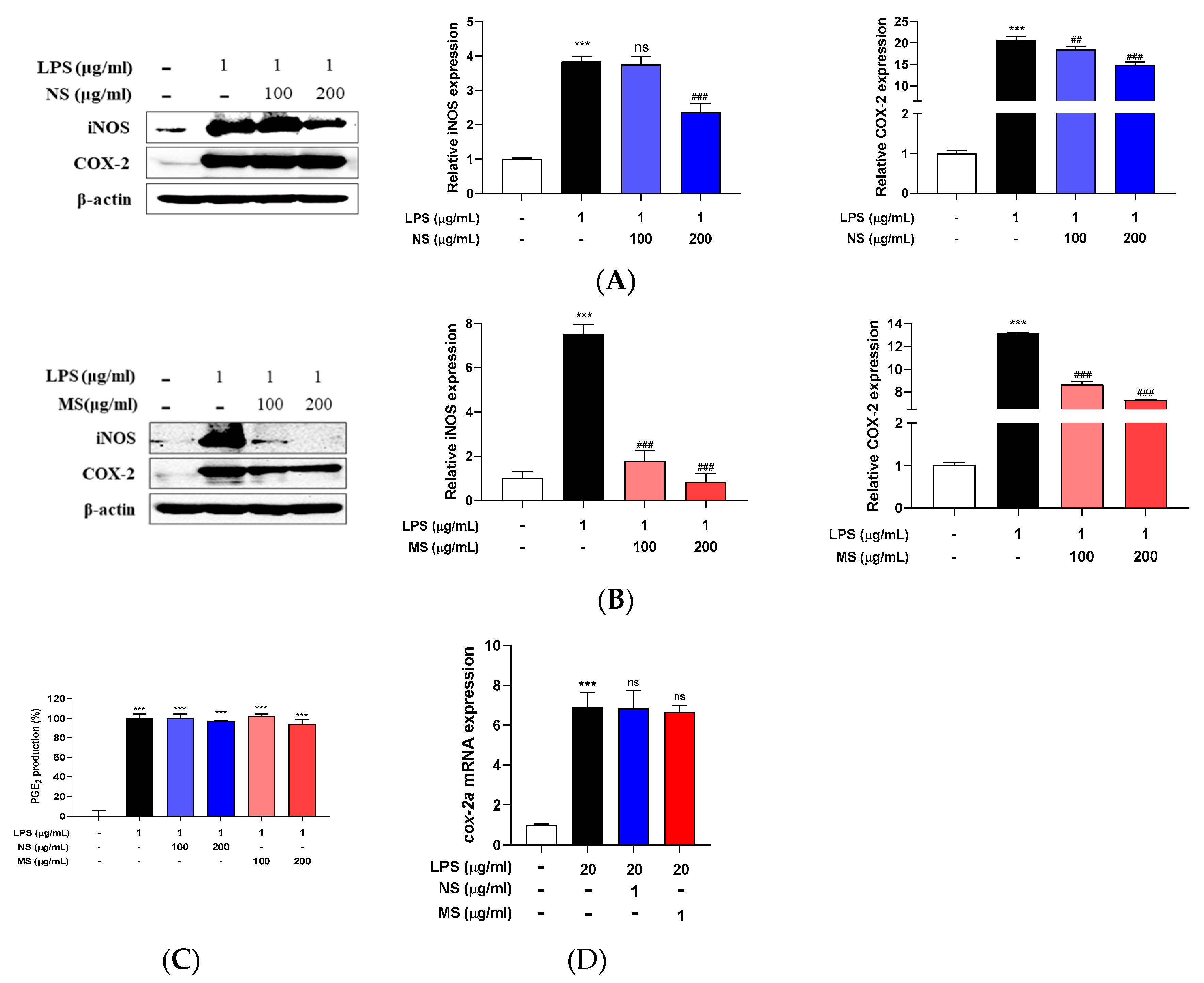
2.3. Carotenoid Extract from Natural Seawater (NS) and Magma Seawater (MS) Inhibits Lipopolysaccharide (LPS)-Induced Inflammatory Mediators
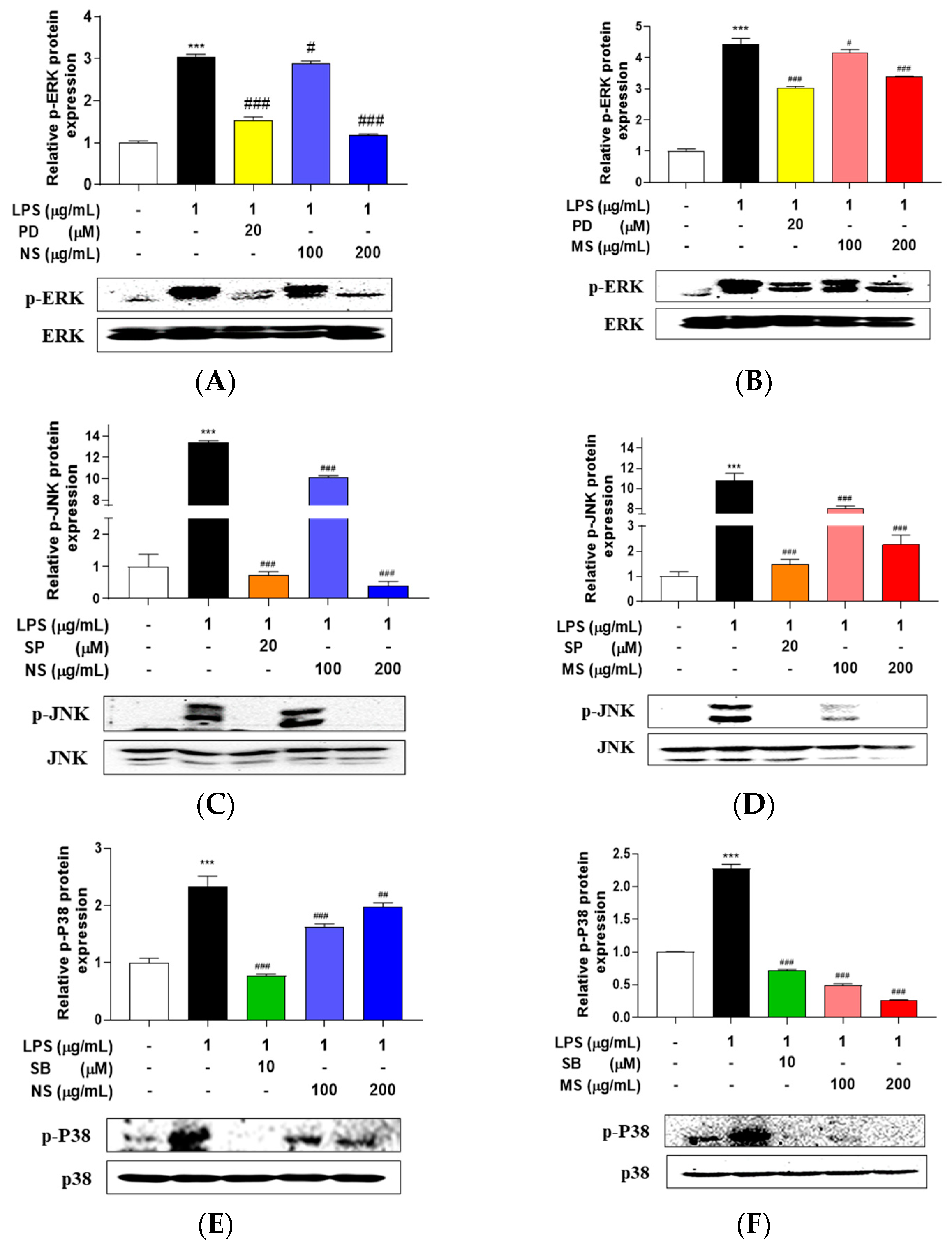
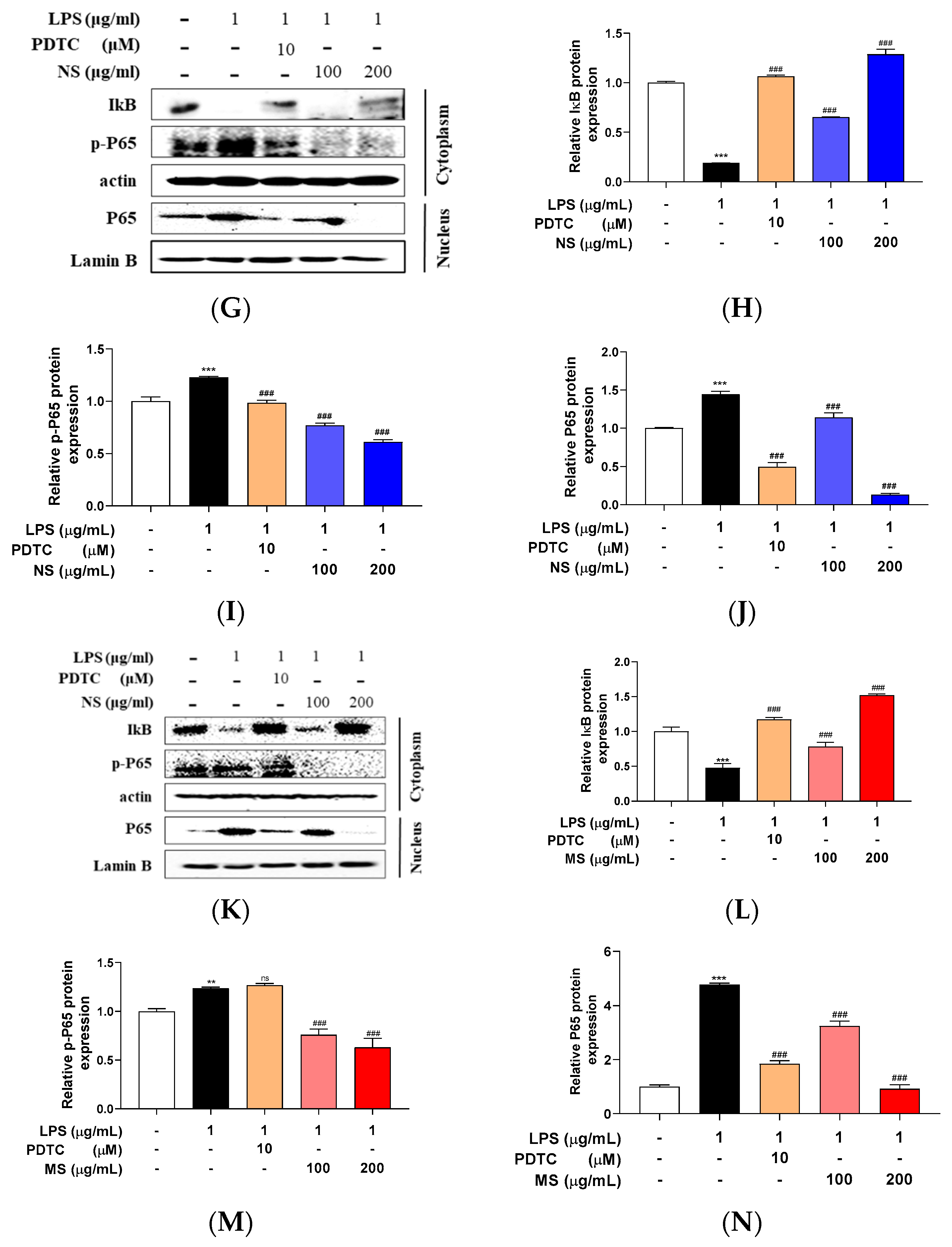
2.4. Carotenoid Extract from Natural Seawater (NS) and Magma Seawater (MS) Suppress Lipopolysaccharide (LPS)-Stimulated Mitogen Activated Protein Kinases (MAPKs) and Nuclear Factor (NF)-kB Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Microalgal Culture
4.2. Carotenoid Extract And Analysis the Content
4.3. Measurement of Cytotoxicity
4.4. Measurement of and Nitric Oxide (NO), Prostaglandin E2 (PGE2) and Pro-Inflammatory Cytokines of RAW 264.7 Cells
4.5. Western Blots
4.6. Zebrafish Maintenance and Embryo Harvesting
4.7. Extract Treatment on Zebrafish Embryos
4.8. Heartbeat Measurement
4.9. NO Measurement of the Embryos
4.10. RT-qPCR
4.11. Statistics
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Jo, W. S.; Choi, Y. J.; Kim, H. J.; Nam, B. H.; Hong, S. H.; Lee, G. A.; Lee, S. W.; Seo, S. Y.; Jeong, M. H. Anti-inflammatory effect of microalgal extracts from Tetraselmis suecica. Food Science and Biotechnology 2010, 19, 1519–1528. [Google Scholar] [CrossRef]
- Moriya, J. Critical roles of inflammation in atherosclerosis. Journal of cardiology 2019, 73, 22–27. [Google Scholar] [CrossRef]
- Hansson, G. K. Inflammation, atherosclerosis, and coronary artery disease. The New England journal of medicine 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- KleinJan, A. Airway inflammation in asthma: Key players beyond the Th2 pathway. Current opinion in pulmonary medicine 2016, 22, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Zeremski, M.; Petrovic, L. M.; Talal, A. H. The role of chemokines as inflammatory mediators in chronic hepatitis C virus infection. Journal of viral hepatitis 2007, 14, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Liu, S. F.; Malik, A. B. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. American journal of physiology. Lung cellular and molecular physiology 2006, 290, L622–l645. [Google Scholar] [CrossRef] [PubMed]
- Zipp, F.; Aktas, O. The brain as a target of inflammation: Common pathways link inflammatory and neurodegenerative diseases. Trends in neurosciences 2006, 29, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Abdulkhaleq, L. A.; Assi, M. A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y. H.; Hezmee, M. N. M. The crucial roles of inflammatory mediators in inflammation: A review. Veterinary world 2018, 11, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M. C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative medicine and cellular longevity 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Lasry, A.; Ben-Neriah, Y. Senescence-associated inflammatory responses: Aging and cancer perspectives. Trends in immunology 2015, 36, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R. F.; Sá-Correia, I.; Valvano, M. A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS microbiology reviews 2016, 40, 480–493. [Google Scholar] [CrossRef]
- Han, Y.; Jung, H. W.; Lee, D. H.; Kwon, S. Y.; Son, K. H.; Park, Y. K. Anti-inflammatory effects of prosapogenin III from the dried roots of Liriope platyphylla in LPS-stimulated RAW264.7 cells. Journal of Asian natural products research 2013, 15, 1038–1049. [Google Scholar] [CrossRef]
- Xu, R.; Ma, L.; Chen, T.; Wang, J. Sophorolipid Suppresses LPS-Induced Inflammation in RAW264.7 Cells through the NF-κB Signaling Pathway. Molecules (Basel, Switzerland) 2022, 27. [Google Scholar] [CrossRef]
- Park, M. Y.; Ha, S. E.; Kim, H. H.; Bhosale, P. B.; Abusaliya, A.; Jeong, S. H.; Park, J. S.; Heo, J. D.; Kim, G. S. Scutellarein Inhibits LPS-Induced Inflammation through NF-κB/MAPKs Signaling Pathway in RAW264.7 Cells. Molecules (Basel, Switzerland) 2022, 27. [Google Scholar] [CrossRef]
- Patel, A. K.; Singhania, R. R.; Awasthi, M. K.; Varjani, S.; Bhatia, S. K.; Tsai, M. L.; Hsieh, S. L.; Chen, C. W.; Dong, C. D. Emerging prospects of macro- and microalgae as prebiotic. Microb Cell Fact 2021, 20, 112. [Google Scholar] [CrossRef] [PubMed]
- Martínez Andrade, K. A.; Lauritano, C.; Romano, G.; Ianora, A. Marine Microalgae with Anti-Cancer Properties. Mar Drugs 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Román, J.; García-Gil, S.; Rodríguez-Luna, A.; Motilva, V.; Talero, E. Anti-Inflammatory and Anticancer Effects of Microalgal Carotenoids. Mar Drugs 2021, 19. [Google Scholar] [CrossRef] [PubMed]
- Riccio, G.; Lauritano, C. Microalgae with Immunomodulatory Activities. Mar Drugs 2019, 18. [Google Scholar] [CrossRef] [PubMed]
- Hyung, J.-H.; Kim, E.-J.; Moon, S.-J.; Kang, N. S.; Park, J. Tetraselmis jejuensis sp. nov. (Chlorodendrophyceae), a Euryhaline Microalga Found in Supralittoral Tide Pools at Jeju Island, Korea. 2021, 10, 1289. [Google Scholar]
- Isdepsky, A.; Borowitzka, M. A. In-pond strain selection of euryhaline Tetraselmis sp. strains for reliable long-term outdoor culture as potential sources of biofuel and other products. Journal of Applied Phycology 2019, 31, 3359–3370. [Google Scholar] [CrossRef]
- Fon-Sing, S.; Borowitzka, M. A. Isolation and screening of euryhaline Tetraselmis spp. suitable for large-scale outdoor culture in hypersaline media for biofuels. Journal of Applied Phycology 2016, 28, 1–14. [Google Scholar] [CrossRef]
- Ahmad, I.; Hellebust, J. A. Osmoregulation in the Extremely Euryhaline Marine Micro-Alga Chlorella autotrophica. Plant Physiol 1984, 74, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Garrity, S. D. Some Adaptations of Gastropods to Physical Stress on a Tropical Rocky Shore. Ecology 1984, 65, 559–574. [Google Scholar] [CrossRef]
- Cardoso, C.; Pereira, H.; Franca, J.; Matos, J.; Monteiro, I.; Pousão-Ferreira, P.; Gomes, A.; Barreira, L.; Varela, J.; Neng, N.; Nogueira, J. M.; Afonso, C.; Bandarra, N. M. Lipid composition and some bioactivities of 3 newly isolated microalgae (Tetraselmis sp. IMP3, Tetraselmis sp. CTP4, and Skeletonema sp.). Aquaculture International 2020, 28, 711–727. [Google Scholar] [CrossRef]
- Kabir, M. T.; Rahman, M. H.; Shah, M.; Jamiruddin, M. R.; Basak, D.; Al-Harrasi, A.; Bhatia, S.; Ashraf, G. M.; Najda, A.; El-kott, A. F.; Mohamed, H. R. H.; Al-malky, H. S.; Germoush, M. O.; Altyar, A. E.; Alwafai, E. B.; Ghaboura, N.; Abdel-Daim, M. M. Therapeutic promise of carotenoids as antioxidants and anti-inflammatory agents in neurodegenerative disorders. Biomedicine & Pharmacotherapy 2022, 146, 112610. [Google Scholar]
- Meng, F.; Lowell, C. A. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn. J Exp Med 1997, 185, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, E. M.; McGinity, C.; Wink, D. A.; McVicar, D. W. Nitric Oxide in Macrophage Immunometabolism: Hiding in Plain Sight. Metabolites 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Rostkowska-Nadolska, B.; Pośpiech, L.; Fortuna, W.; Szymaniec, S.; Miedzybrodzki, R.; Gawron, W.; Latocha, M. [RANTES expression in nasal polyps fibroblasts; spontaneously and after stimulation with lipopolisaccharides (LPS) and phytohemagglutinin (PHA)]. Otolaryngologia polska = The Polish otolaryngology 2006, 60, 143–147. [Google Scholar]
- Ozleyen, A.; Yilmaz, Y. B.; Tumer, T. B. Dataset on the differentiation of THP-1 monocytes to LPS inducible adherent macrophages and their capacity for NO/iNOS signaling. Data Brief 2021, 35, 106786. [Google Scholar] [CrossRef]
- Lee, Y. M.; Kim, M.; Yuk, H. J.; Kim, S. H.; Kim, D. S. Siraitia grosvenorii Residual Extract Inhibits Inflammation in RAW264.7 Macrophages and Attenuates Osteoarthritis Progression in a Rat Model. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Park, J. Y.; Pillinger, M. H.; Abramson, S. B. Prostaglandin E2 synthesis and secretion: The role of PGE2 synthases. Clin Immunol 2006, 119, 229–240. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Trepiana, J.; González-Arceo, M.; Aguirre, L.; Milton-Laskibar, I.; González, M.; Eseberri, I.; Fernández-Quintela, A.; Portillo, M. P. Anti-Obesity Effects of Microalgae. Int J Mol Sci 2019, 21. [Google Scholar] [CrossRef]
- Khavari, F.; Saidijam, M.; Taheri, M.; Nouri, F. Microalgae: Therapeutic potentials and applications. Mol Biol Rep 2021, 48, 4757–4765. [Google Scholar] [CrossRef] [PubMed]
- Zhuge, F.; Ni, Y.; Wan, C.; Liu, F.; Fu, Z. Anti-diabetic effects of astaxanthin on an STZ-induced diabetic model in rats. Endocr J 2021, 68, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M. A.; D'Orazio, N. Anti-obesity activity of the marine carotenoid fucoxanthin. Mar Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Pappalardo, I.; Santarsiero, A.; Radice, R. P.; Martelli, G.; Grassi, G.; de Oliveira, M. R.; Infantino, V.; Todisco, S. Effects of Extracts of Two Selected Strains of Haematococcus pluvialis on Adipocyte Function. 2023, 23, 1737. [Google Scholar] [CrossRef] [PubMed]
- El-Baz, F. K.; Salama, A.; Ali, S. I.; Elgohary, R. Lutein isolated from Scenedesmus obliquus microalga boosts immunity against cyclophosphamide-induced brain injury in rats. Scientific Reports 2022, 12, 22601. [Google Scholar] [CrossRef] [PubMed]
- Gallego, R.; Valdés, A.; Sánchez-Martínez, J. D.; Suárez-Montenegro, Z. J.; Ibáñez, E.; Cifuentes, A.; Herrero, M. Study of the potential neuroprotective effect of Dunaliella salina extract in SH-SY5Y cell model. Anal Bioanal Chem 2022, 414, 5357–5371. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Wu, H.; He, Q.; Wang, L.; Yi, X.; Feng, G.; Wu, Q.; Tao, B.; Han, D.; Hu, Q.; Xia, H.; Xu, L. Fucoxanthin alleviated atherosclerosis by regulating PI3K/AKT and TLR4/NFκB mediated pyroptosis in endothelial cells. Int Immunopharmacol 2023, 120, 110370. [Google Scholar] [CrossRef]
- El-Baz, F. K.; Hussein, R. A.; Saleh, D. O.; Abdel Jaleel, G. A. R. Zeaxanthin Isolated from Dunaliella salina Microalgae Ameliorates Age Associated Cardiac Dysfunction in Rats through Stimulation of Retinoid Receptors. Mar Drugs 2019, 17. [Google Scholar] [CrossRef]
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the Antioxidant and Anti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. 2021, 26, 4886. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef]
- Böni-Schnetzler, M.; Meier, D. T. Islet inflammation in type 2 diabetes. Semin Immunopathol 2019, 41, 501–513. [Google Scholar] [CrossRef]
- Diedisheim, M.; Carcarino, E.; Vandiedonck, C.; Roussel, R.; Gautier, J. F.; Venteclef, N. Regulation of inflammation in diabetes: From genetics to epigenomics evidence. Mol Metab 2020, 41, 101041. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J. P.; Patil, P. B.; Thakkannavar, S. S.; Pujari, V. B. Inflammation and cancer. Ann Afr Med 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Henein, M. Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. 2022, 23, 12906. [Google Scholar] [CrossRef] [PubMed]
- Perez, V. L.; Stern, M. E.; Pflugfelder, S. C. Inflammatory basis for dry eye disease flares. Exp Eye Res 2020, 201, 108294. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Kolko, M.; Melik-Parsadaniantz, S.; Messmer, E. M. Inflammation in Glaucoma: From the back to the front of the eye, and beyond. Prog Retin Eye Res 2021, 83, 100916. [Google Scholar] [CrossRef]
- Jutley, G. S.; Sahota, K.; Sahbudin, I.; Filer, A.; Arayssi, T.; Young, S. P.; Raza, K. Relationship Between Inflammation and Metabolism in Patients With Newly Presenting Rheumatoid Arthritis. Front Immunol 2021, 12, 676105. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M. V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol 2021, 320, C375–c391. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Rao, X.; Sigdel, K. R. Regulation of Inflammation in Autoimmune Disease. J Immunol Res 2019, 2019, 7403796. [Google Scholar] [CrossRef]
- Saez, A.; Herrero-Fernandez, B.; Gomez-Bris, R.; Sánchez-Martinez, H.; Gonzalez-Granado, J. M. Pathophysiology of Inflammatory Bowel Disease: Innate Immune System. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Rodriguez, P.; Chen, H.; Erndt-Marino, J. D.; Liu, F.; Totsingan, F.; Gross, R. A.; Hahn, M. S. Impact of Select Sophorolipid Derivatives on Macrophage Polarization and Viability. ACS applied bio materials 2019, 2, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Yeom, M.; Park, J.; Lim, C.; Sur, B.; Lee, B.; Han, J. J.; Choi, H. D.; Lee, H.; Hahm, D. H. Glucosylceramide attenuates the inflammatory mediator expression in lipopolysaccharide-stimulated RAW264.7 cells. Nutrition research (New York, N.Y.) 2015, 35, 241–250. [Google Scholar] [CrossRef]
- Alam, M. B.; Chowdhury, N. S.; Sohrab, M. H.; Rana, M. S.; Hasan, C. M.; Lee, S. H. Cerevisterol Alleviates Inflammation via Suppression of MAPK/NF-κB/AP-1 and Activation of the Nrf2/HO-1 Signaling Cascade. Biomolecules 2020, 10. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Chen, S.; Hu, Y.; Zhu, Z.; Wang, Y.; Du, N.; Song, T.; Yang, Y.; Guo, A.; Wang, Y. Macrophage migration inhibitory factor facilitates prostaglandin E(2) production of astrocytes to tune inflammatory milieu following spinal cord injury. Journal of neuroinflammation 2019, 16, 85. [Google Scholar] [CrossRef]
- Park, C. M.; Song, Y. S. Luteolin and luteolin-7-O-glucoside inhibit lipopolysaccharide-induced inflammatory responses through modulation of NF-κB/AP-1/PI3K-Akt signaling cascades in RAW 264.7 cells. Nutrition research and practice 2013, 7, 423–429. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Zheng, F.; Yu, H.; Wei, K. Xanthatin Alleviates LPS-Induced Inflammatory Response in RAW264.7 Macrophages by Inhibiting NF-κB, MAPK and STATs Activation. Molecules (Basel, Switzerland) 2022, 27. [Google Scholar] [CrossRef]
- Patton, E. E.; Tobin, D. M. Spotlight on zebrafish: The next wave of translational research. Disease models & mechanisms 2019, 12. [Google Scholar]
- Kim, E. A.; Kang, N.; Kim, J.; Yang, H. W.; Ahn, G.; Heo, S. J. Anti-Inflammatory Effect of Turbo cornutus Viscera Ethanolic Extract against Lipopolysaccharide-Stimulated Inflammatory Response via the Regulation of the JNK/NF-kB Signaling Pathway in Murine Macrophage RAW 264.7 Cells and a Zebrafish Model: A Preliminary Study. Foods (Basel, Switzerland) 2022, 11. [Google Scholar]
- Forn-Cuní, G.; Varela, M.; Pereiro, P.; Novoa, B.; Figueras, A. Conserved gene regulation during acute inflammation between zebrafish and mammals. Sci Rep 2017, 7, 41905. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-A.; Kang, N.; Heo, S.-Y.; Oh, J.-Y.; Lee, S.-H.; Cha, S.-H.; Kim, W.-K.; Heo, S.-J. Antioxidant, Antiviral, and Anti-Inflammatory Activities of Lutein-Enriched Extract of Tetraselmis Species. 2023, 21, 369. 2023, 21, 369. [Google Scholar] [PubMed]
| Gene name | Sequence 5' - 3' |
|---|---|
| il-1β | 5` - TCAAACCCCAATCCACAGAG- 3` |
| 5` - TCACTTCACGCTCTTGGATG- 3` | |
| cox-2a | 5` - AGCCCTACTCATCCTTTGAGG - 3` |
| 5` - TCAACCTTGTCTACGTGACCATA - 3` | |
| β-actin | 5` - AATCTTGCGGTATCCACGAGACCA - 3` |
| 5` - TCTCCTTCTGCATCCTGTCAGCAA - 3` |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




