1. Introduction
As one of the important strategic metals, nickel is widely used in the stainless steel and new material industries owing to its superior mechanical strength, high chemical stability, good ductility, and strong corrosion resistance [
1]. The nickel used in the stainless steel industry accounts for approximately 66.2% of the total consumption of nickel worldwide, resulting in dramatically increased demand for nickel in recent years [
2]. In nickel production, most metal nickel was produced from nickel sulfide ores, and laterite nickel ore only accounts for about 40% due to its complex mineralogy, low nickel and iron grades, and the high treatment cost [
3]. However, with the diminution of high-grade nickel sulfide ores, recovering nickel from laterite nickel ore as an alternative of nickel sulphide ores is drawing increasing attention since laterite nickel ore constitutes 72.2% of the world’s land-based nickel reserves [
4,
5,
6]. Therefore, an economical method is urgently needed for recovering nickel from laterite nickel ore.
Nickel in laterite nickel ore existed mainly as silicate, indicating that simple physical method is far from extracting nickel from the ore [
7]. The current process approach for enriching ferronickel from laterite nickel ore is a pyrometallurgical process, such as rotary kiln electric furnace process and blast furnace process [
8]. The pyrometallurgical process has incomparable advantages in the quality of the ferronickel concentrate [
9], nevertheless, it also suffers from the high energy consumption, high demand for raw materials, and high volume of the waste stream [
10,
11]. To achieve the carbon peak and neutrality targets, and make full use of laterite nickel ore, the research of new technology with low carbon emission and reduced energy consumption becomes the top priority [
12,
13]. Considering on the low grade of nickel and iron in laterite nickel ore, the solid-state reduction roasting combined with magnetic separation to remove the magnesium silicates and unreduced oxides can be a promising method to produce a ferronickel concentrate from laterite nickel ore [
14].
Herein, in this study, high-grade ferronickel concentrates were prepared by a carbothermal reduction and magnetic separation method from low-grade laterite nickel ore. The main influencing factors that could significantly influence the formation of ferronickel concentrate were investigated, including carbon ratio, types and amounts of additives, and grinding time. This work provides a promising approach for making full utilization of abundant low-grade laterite nickel ore with low energy consumption, which is of great significance in maintaining the nickel supply.
2. Materials and Methods
2.1. Raw materials
2.1.1. Laterite nickel ore
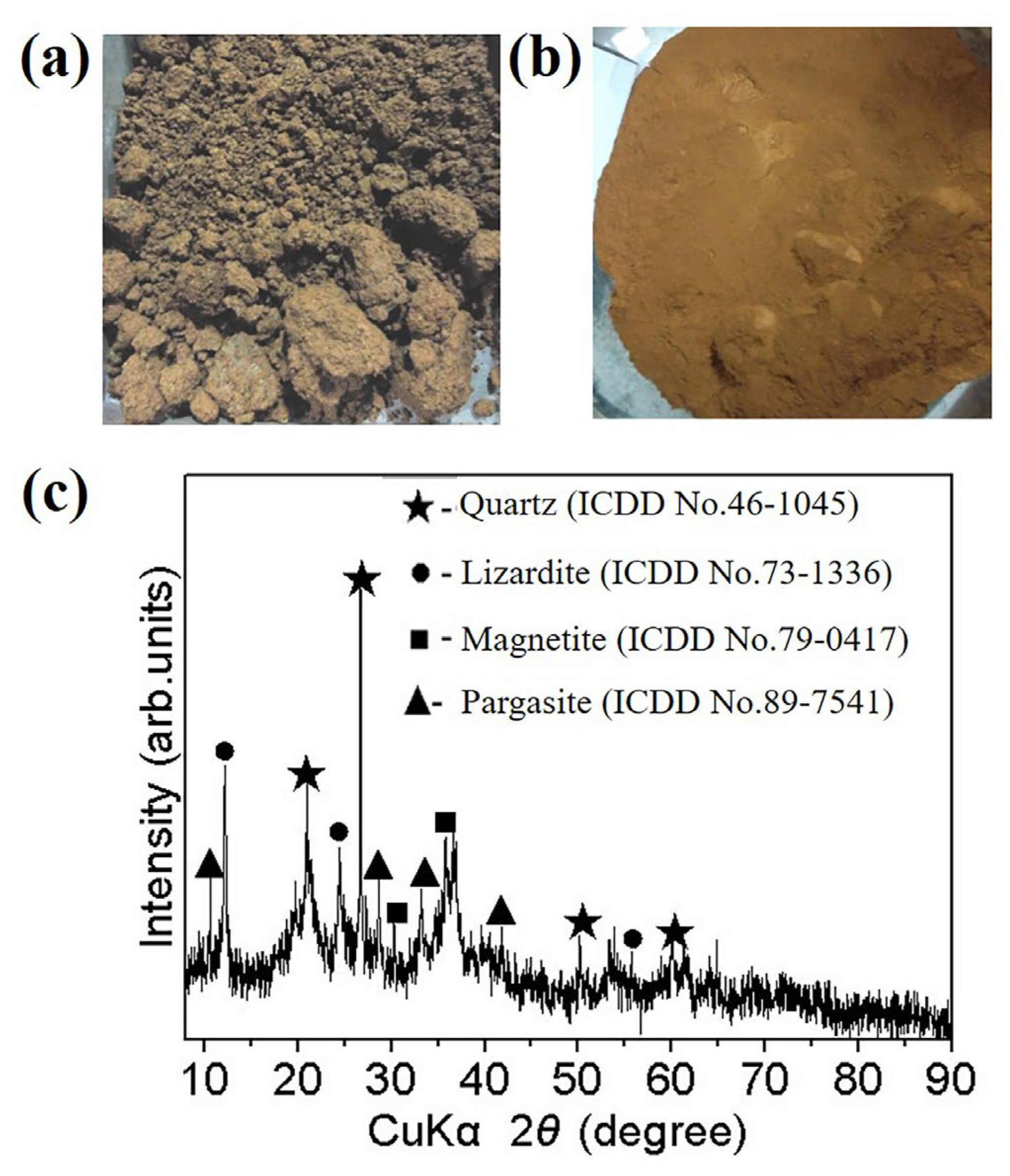
The laterite nickel ore used in this work was bought from Sulawesi, Indonesia. As displayed in
Figure 1a, the raw laterite nickel ore exhibits a texture resembling clay, with lumps and a considerable amount of moisture, making it lack of representativeness for sampling the laterite nickel ore directly. To address this, the raw laterite nickel ore was dried at 110 °C for 12 hours, resulting in a mass loss of 27.9 %. The dried sample was then subjected to a crushing process using a jaw crusher (XPC 125 × 100), followed by a thorough mixing of the crushed specimen. Subsequently, a fraction of the sample was finely ground in a vibration mill and then passed through a 0.125 mm sieve. The image of the fine ground laterite powder is shown in
Figure 1b.
To investigate the chemical composition of laterite nickel ore, the finely ground laterite powder was dried at 105 °C until reaching a constant weight. Subsequently, comprehensive chemical analysis and XRD characterization were conducted. The XRD pattern shown in
Figure 1(c) indicated that the laterite nickel ore predominantly consists of quartz (ICDD card No.46-1045), lizardite (ICDD card No.73-1336), magnetite (ICDD card No.79-0417), and pargasite (ICDD card No.89-7541). In addition, the determined chemical component of laterite powder is listed in
Table 1, revealing a low nickel content of only 1.32 wt.%, a total iron of 15.87 wt.%, alongside a remarkable abundance of magnesium oxide (15.10 wt.%) and silicon dioxide (40.46 wt.%).
2.1.2. Reductants and additives
Anthracite was employed as a reductant in this work, and its chemical composition determined by chemical analysis is shown in
Table 2. The carbon ration (n
C/n
O) was defined as the ratio of oxygen atoms in metal oxides to carbon atoms in anthracite. Furthermore, calcium hydroxide (Ca(OH)
2), calcium fluoride (CaF
2), sodium sulfate (Na
2SO
4), and boric acid (H
3BO
3) were employed to lower the melting temperature and decrease its surface tension.
2.2. Experimental methods
2.2.1. Sample preparation
The laterite nickel ore samples were prepared by using 100 g laterite nickel ore powders, anthracite, and CaF
2 as raw materials. Among them, anthracite was added with a carbon ratio of 1.2 or 1.4, and CaF
2 was added in varying amounts of 6 wt.%, 9 wt.% or 12 wt.% of the laterite nickel ore powders, they were noted as C1.2-F6, C1.2-F12, C1.4-F6, C1.4-F9 and C1.4-F12, respectively. The effect of slake lime on the carbothermal reduction of laterite nickel ore was investigated by adding the former into sample C1.2 and C1.2-F6 with a CaO/SiO
2 ratio of 1.0, the two samples were respectively noted as C1.2-SL and C1.2-F6-SL. Two other additives Na
2SO
4 and H
3BO
3 were added into C1.4-F6 or C1.4-F12 with additive amount of 1 wt.% or 2 wt.% of the laterite nickel ore powders, and the samples were noted as C1.4-F6-S1, C1.4-F12-B1 and C1.4-F12-B2, respectively. In addition, control samples were prepared by using laterite nickel ore powders and anthracite as starting materials with carbon ratios of 1.2 and 1.4, and respectively noted as C1.2 and C1.4. The detailed raw material compositions of each sample were exhibited in
Table 3.
2.2.2. Carbothermal reduction and magnetic separation
Laterite nickel ore, anthracite and CaF
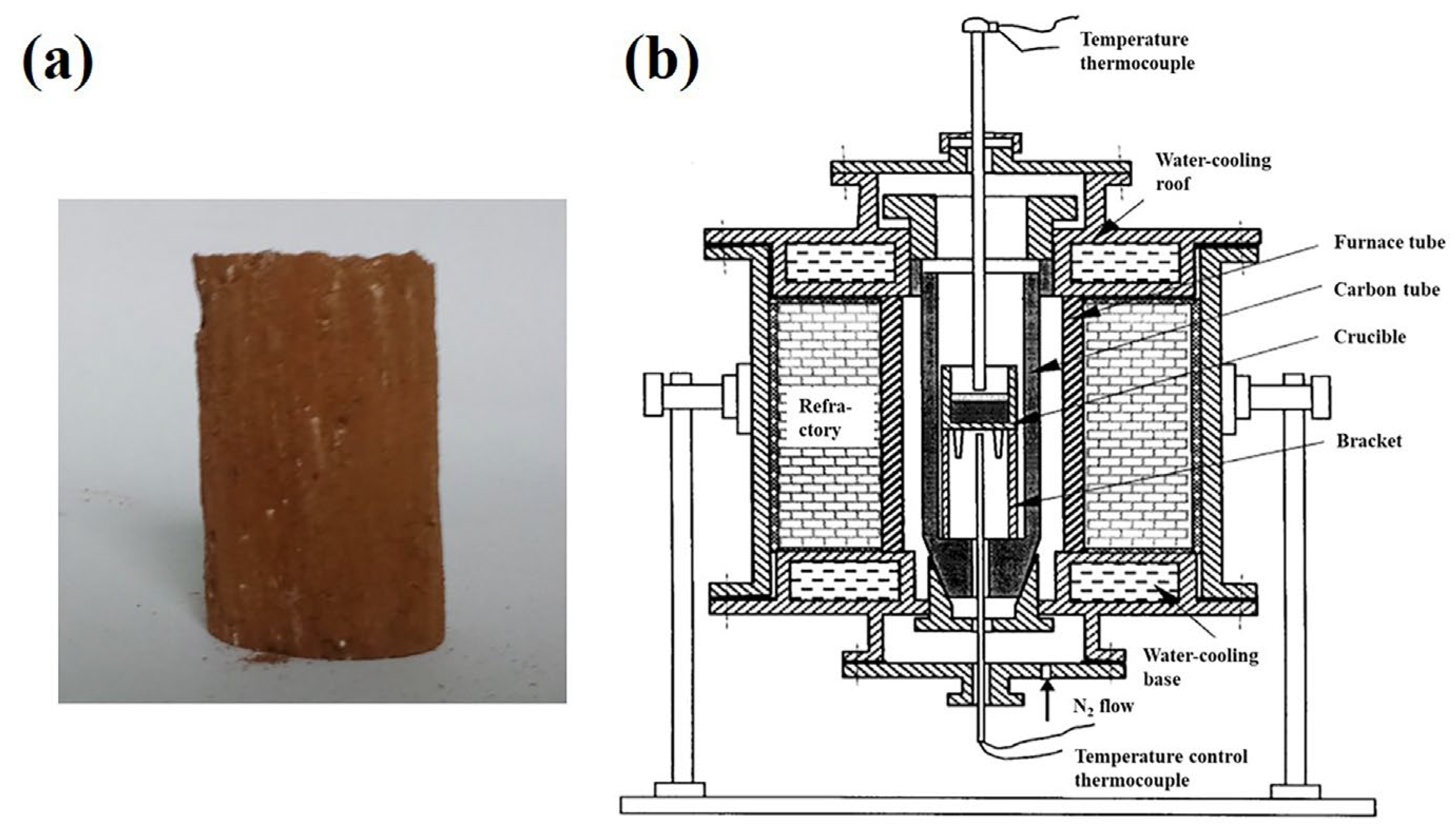
2 were firstly mixed homogeneously, followed by the addition of water in a quantity of 10 wt.% of the mixture. Subsequently, the mixtures were compressed into cylindrical specimens (φ 20 × 30 mm, as depicted in
Figure 2a). Then the samples underwent a drying in an oven at 110 °C overnight before being placed in a graphite crucible and heated to 1250 °C and soaked for 15 min under a N
2 atmosphere in a 25-kW high temperature carbon tube furnace (
Figure 2b) with a heating rate of 120 °C /min. After the carbothermal reduction experiment, the samples were cooled to room temperature in the N
2 atmosphere, and the microstructures of the samples were characterized using a field emission scanning electron microscope (FESEM, Novo 400, FEI Co., USA). Then the samples were finely ground and underwent a magnetic separation before a chemical analysis to examine the iron and nickel content.
3. Results and discussion
3.1. The morphology of as-prepared ferronickel concentrates
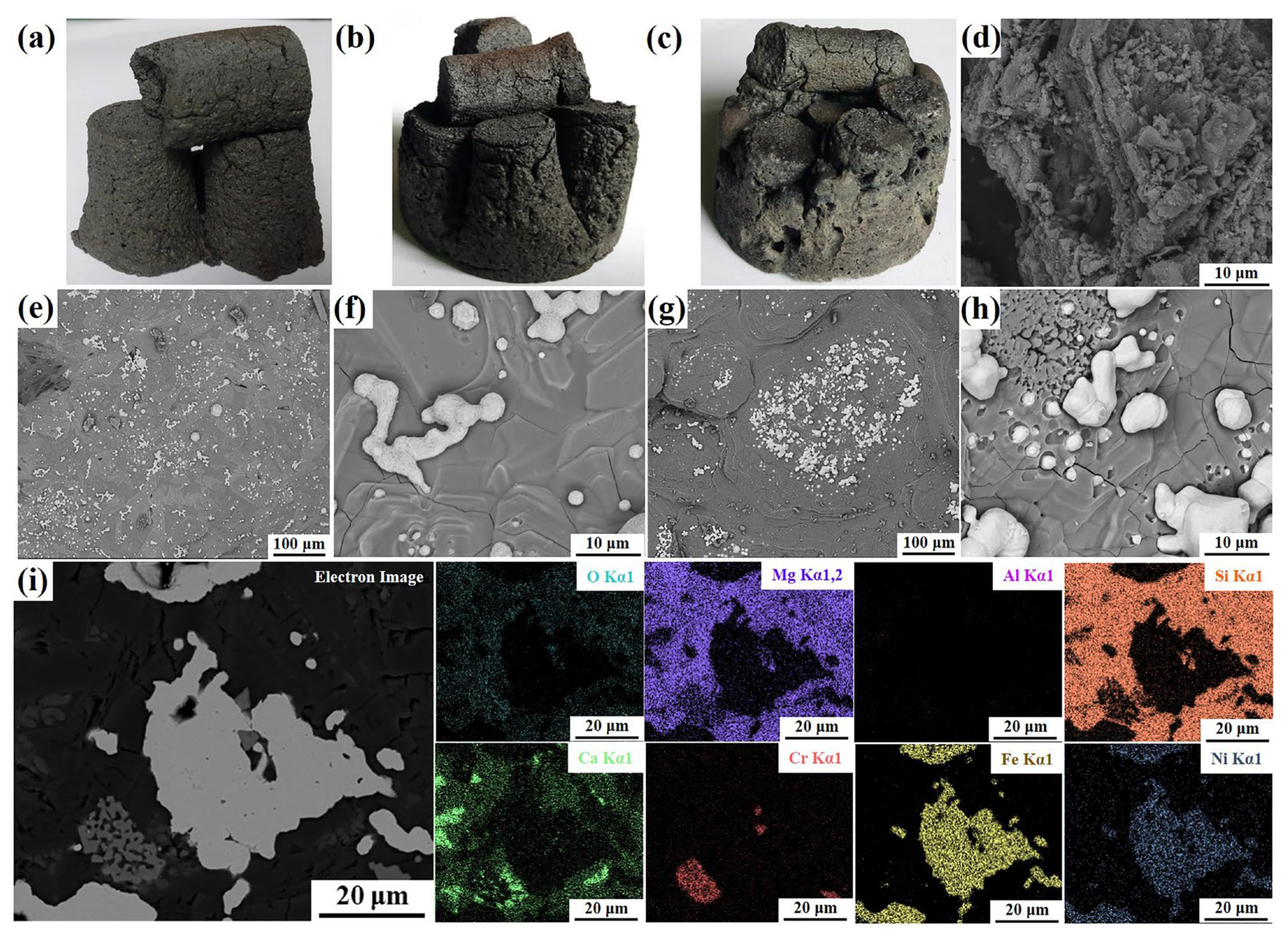
As shown in
Figure 3(a–c), after carbothermal reduction process, the melting degrees of the samples intensified as the CaF
2 additive amount increased. The samples with CaF
2 addition can not hold their original cylindrical structures (
Figure 3b,c), and their strengths were higher than those without CaF
2. The microstructures of the samples C1.4, C1.4-F6 and C1.4-F12 are shown in
Figure 3d–h, it is obvious that the melting of samples C1.4-F6 and C1.4-F12 (
Figure 3e–h) were more clearly compared to that of sample C1.4 (
Figure 3d). Moreover, a lot of particles with size of 9-25 μm were formed in sample C1.4-F12 (
Figure 3g), which was rarely in sample C1.4, indicating that the addition of CaF
2 promoted the formation of molten slag. In addition, the EDS elemental mappings of sample C1.4-F12 shown in
Figure 3i revealed the coexistence of Fe and Ni elements in the formed particles after the carbothermal reduction process. The presence of Mg, Si, and O elements within the same area suggested the formation of magnesium silicate. Furthermore, Cr
2O
3 and CaO may also exist in the reduced sample. The SEM results corroborated the formation of the ferronickel particles after the carbothermal reduction process, and the addition of CaF
2 was advantageous to the formation of ferronickel particles.
3.2. Iron and nickel contents of as-prepared ferronickel concentrates after fine grinding and magnetic separation
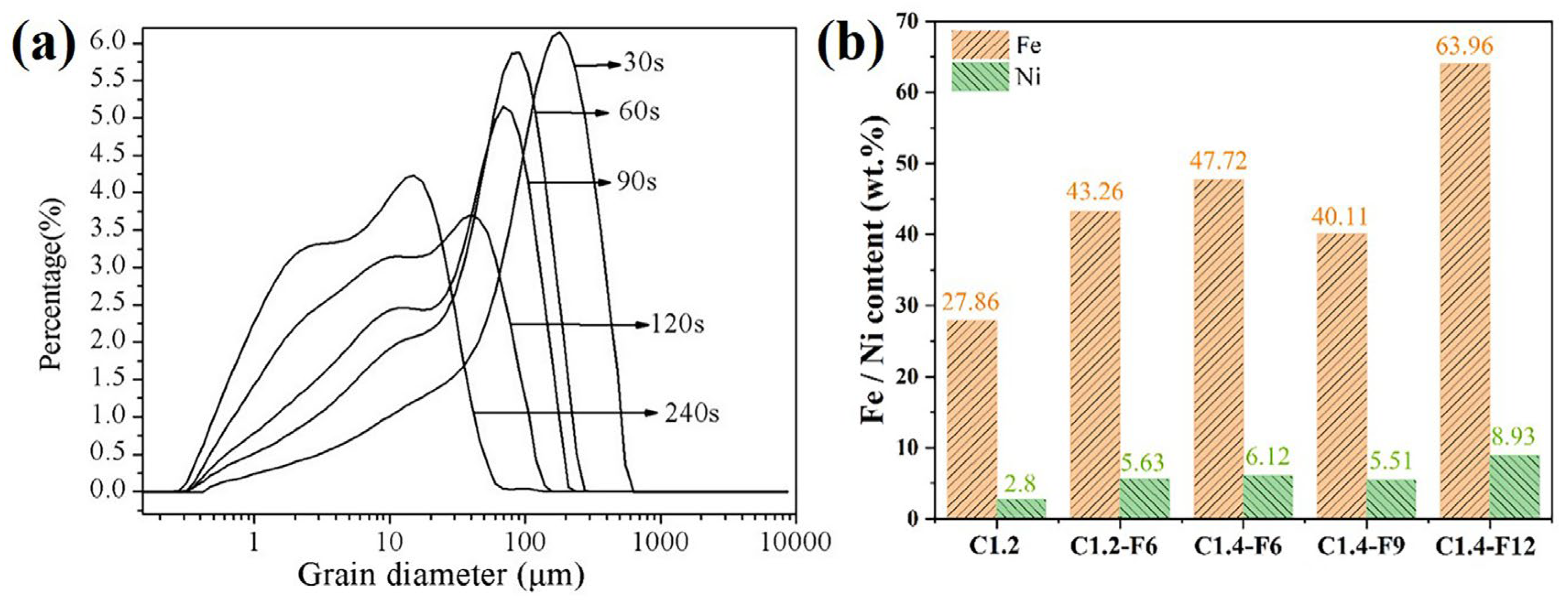
Fine grinding of the as-prepared ferronickel concentrates is beneficial to improve the iron and nickel grades by separating the ferronickel particles from the concentrates. After carbothermal reduction, the as-prepared ferronickel concentrates were fine ground, and their particle size distribution is shown in
Table 4 and
Figure 4a. Obviously, the particle size of the sample decreased with the increase of grinding time, and the D
90 of the sample was reduced to 26 μm after 240 s of fine grinding. Considering that the appropriate particle size facilitates the subsequently magnetic separation process, the optimal grinding time was selected as 240 s.
The fine ground samples were then subjected to a magnetic separation process to extract ferronickel concentrates for chemical analysis. Their iron and nickel contents of the samples are shown in
Figure 4b. It is obvious that the contents of iron and nickel in sample C1.2-F6 exhibited significant improvement compared to the control sample C1.2 (iron: 43.26 wt.% vs 27.86 wt.% and nickel: 5.63 wt.% vs 2.8 wt.%), indicating that the introduction of CaF
2 had a substantial enhancing effect on the formation of the ferronickel concentrate. However, samples C1.2-F6 and C1.4-F6 showed closed iron and nickel contents, suggesting that the carbon ratio had little influence on the carbothermal reduction process. Furthermore, as the additive amount of CaF
2 increased from 6 wt.% to 9 wt.% and then to 12 wt.%, the initial grades of iron and nickel exhibited a decrease followed by an increase. Specifically, the iron grade decreased from 47.72 wt.% to 40.11 wt.% and then increased to 63.69 wt.%, while the nickel grade decreased from 6.12 wt.% to 5.51 wt.% and then increased to 8.93 wt.%. The improvement in the recovery of nickel and iron and concentrate grades may be attributed to the reduced surface tension of the mineral phase interface, which facilitated the growth of the iron and nickel solid solution [
12]. The decrease of iron and nickel grades for the sample with 9 wt.% CaF
2 can be caused by the entry of fine nonmagnetic material in the magnetic substance when the aggregation degree of the ferronickel was insufficient [
15]. In conclusion, the ferronickel concentrates with the highest nickel grade of 8.93 wt.% and iron grade of 63.96 wt.% were obtained with the carbon ratio of 1.4 and the CaF2 additive amount of 12 wt.%.
3.3. Effects of the additive type on the iron and nickel contents of as-prepared ferronickel concentrates
Additives were used to reduce the melting temperature and viscosity of the slag, thus enhancing the metallization, migration, and polymerization of iron and nickel elements. Moreover, slaked lime is often used to activate steel slag in metallurgy industry [
16]. In this work, after carbothermal reduction, samples C1.2-SL and C1.2-F6-SL which possessed original cylindrical shapes exhibited low melting degree and lots of cracks on the surface (as displayed in
Figure 5a,b), indicating that the addition of slaked lime reduced the cohesiveness of these samples, impeded the formation of molten slag and consequently hindered the reduction of iron and nickel.
Figure 3e–h demonstrated that the addition of CaF
2 facilitated the formation of molten slag, and then accelerated the reduction of iron and nickel from laterite nickel ore. Considering on the relatively high cost of CaF
2, Na
2SO
4 and H
3BO
3 were added to try to reduce the usage of CaF
2. Regrettably, as shown in
Figure 5c,d, both the quantity and particle size of ferronickel particles in sample C1.4-F6-S1 were obviously less than that of the sample C1.4-F6 in
Figure 3e,f. The reason can be ascribed to that the addition of Na
2SO
4 had an adverse impact on the melting degree of the samples, and subsequently hindered the formation of molten slag and the reduction of laterite nickel ore as well.
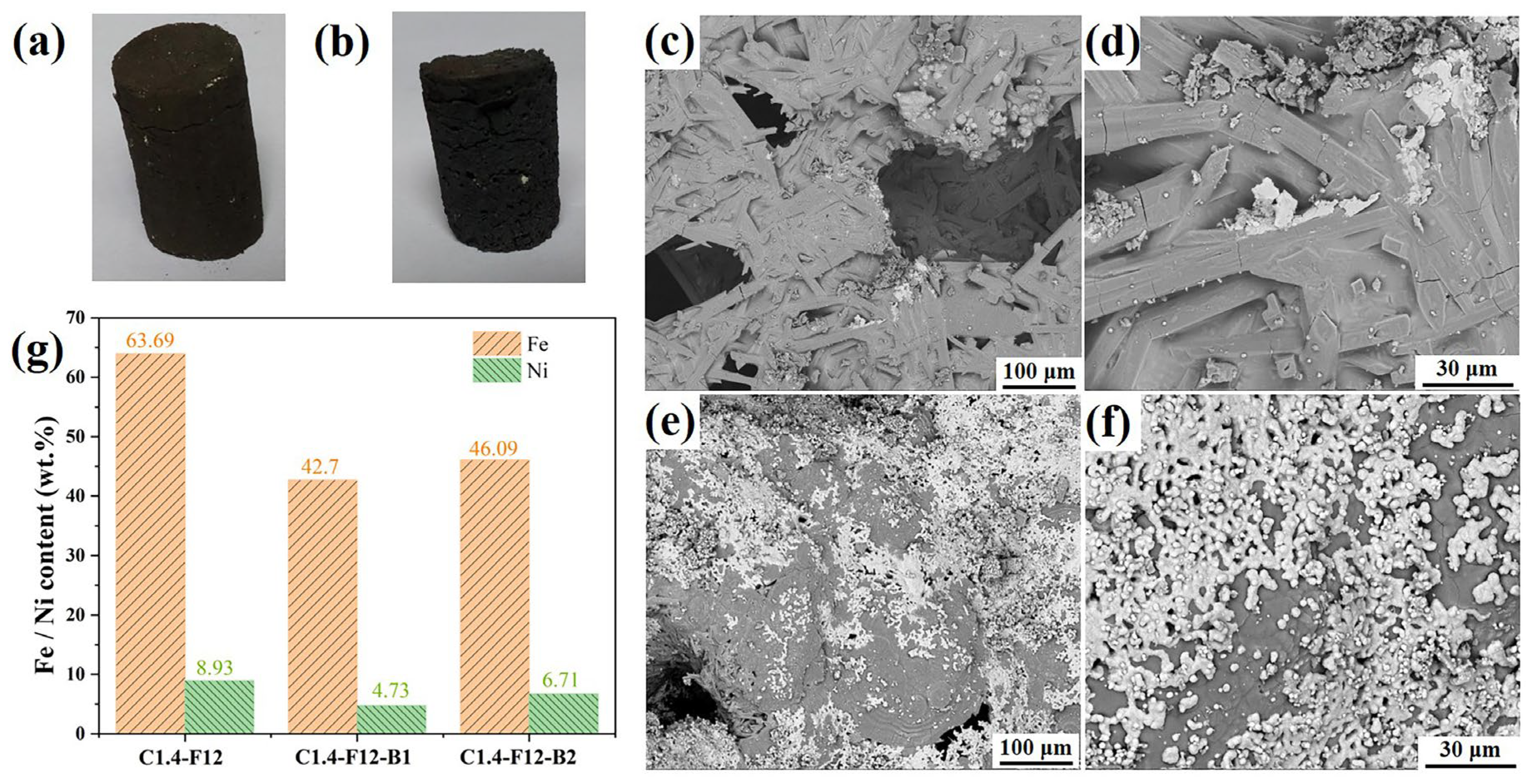
When H
3BO
3 was added into the ore, after carbothermal reduction, substantial amounts of ferronickel particles with particle sizes of 1-10 μm were observed in
Figure 5e,f, revealing that the addition of the additive significantly facilitated the formation of molten slag. Chemical analysis was employed to determine the iron and nickel contents of samples C1.4-F12-B1 and C1.4-F12-B2 and illustrated in
Figure 5g, demonstrating that the addition of H
3BO
3 led to a decrease on the iron and nickel grades compared to C1.4-F12 (iron: 46.09 wt.% vs 63.69 wt.% and nickel: 6.71 wt.% vs 8.93 wt.%). The decreased iron and nickel grades of C1.4-F12-B2 may be attributed to the difficult separation of the small size ferronickel particles since the size is far below the D
90 of sample C1.4-F12 after 240 s fine grinding (<10 μm vs 26 μm). Thus, the subsequently separation of ferronickel particles from the molten slag during the magnetic separation process became a challenging [
17].
In a word, after carbothermal reduction and magnetic separation, high-grade magnetically separable ferronickel concentrates were successfully prepared by using laterite nickel ore, anthracite and CaF2 as raw materials, the sample C1.4-F12 exhibited the highest iron grade of 63.96 wt.% and nickel grade of 8.93 wt.%. The optimal processing conditions for preparing of present high-grade ferronickel concentrates were as follows: the carbon ratio of 1.4, CaF2 addition of 12 wt.% and fine grinding time of 240 s.
3.4. Thermodynamic equilibrium and melting temperature calculation
To further investigate the influence of various additives on the carbothermal reduction of the ferronickel concentrates, a thermodynamic analysis was carried out using FactSage version 6.4. The phase equilibrium was calculated by “Equilib function”, with the databases “FactPS” “FToxid” and “FShall”. The compound species of final products contained gas, liquid and solid at a pressure of 1.0 atm. By inputting the initial compositions based on the sample’s composition, and setting the temperature at 1250 °C, the thermodynamic calculations yielded phase equilibrium results, as presented in
Table 5. The result indicates that the reductions of the iron oxide and nickel oxide are thermally accessible at the current experimental condition.
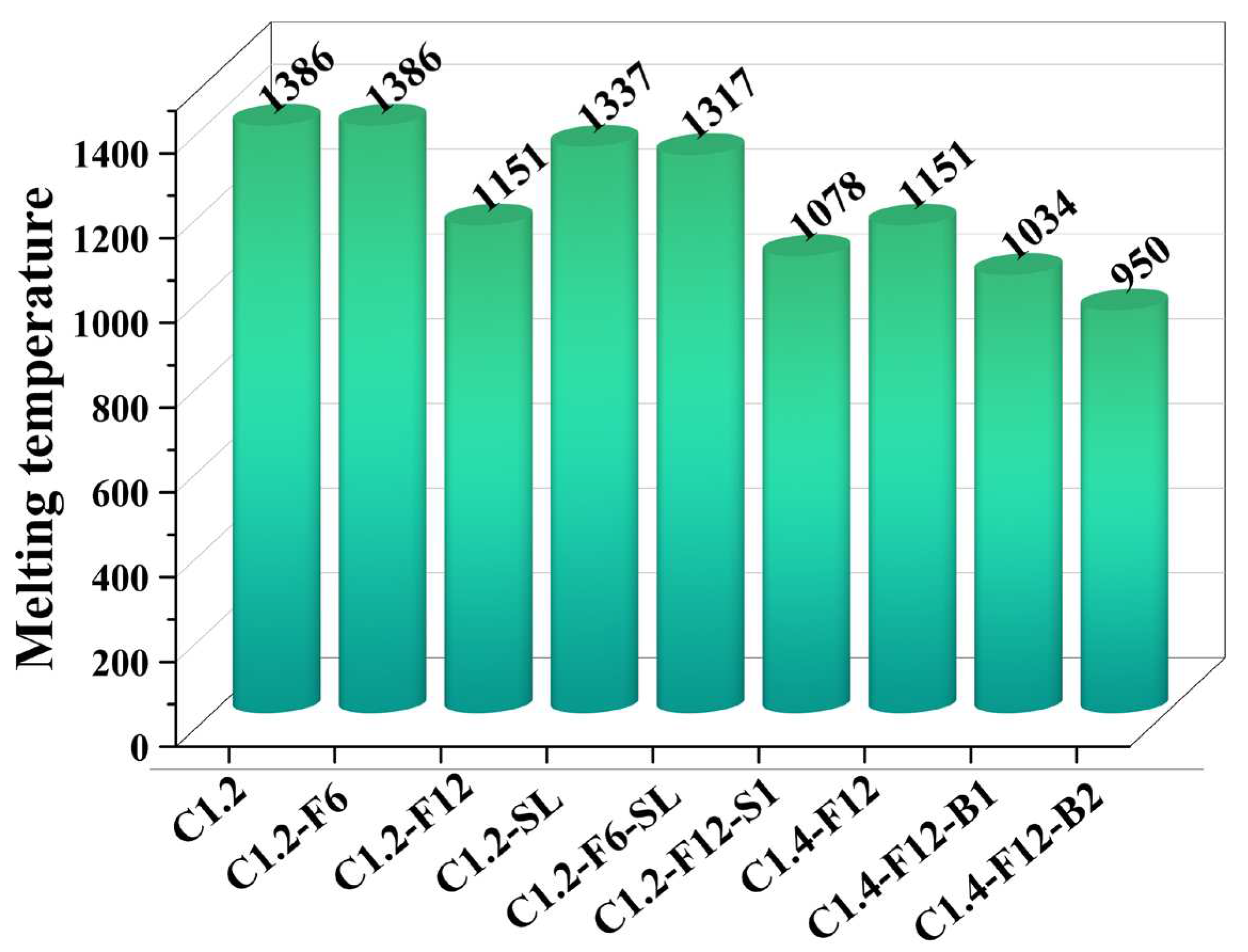
Since the iron oxide and nickel oxide reductions of these samples were all thermodynamically feasible, the main factor affecting the reduction rate of iron and nickel should be the differences in the kinetic reaction rate, which was influenced by the additives. Therefore, the equilibrium and phase transition temperature (also referred to as melting temperature) of each sample within the range of 400-1500 °C were calculated. As shown in
Figure 6, the addition of CaF
2, Na
2SO
4 and H
3BO
3 all resulted in a decrease of the melting temperature. However, the case with slaked lime only had a slight decrease compared to other additives, and the melting temperature of samples C1.2-SL and C1.2-F6-SL were both higher than the experimental temperature of 1250 °C. The most significant decrease of melting temperature (235 °C) was obtained for the case with 12 wt.% of CaF2, demonstrating the conducive role of CaF
2 on the formation of the ferronickel concentrate [
18]. Moreover, sample C1.4-F12-B2 exhibited the lowest melting temperature of 950 °C, indicating that the addition of H
3BO
3 contributed to the formation of molten slag, which was aligned with the experimental results in
Figure 5e,f.
In summary, the thermodynamic calculation indicated that the addition of CaF
2 exerted a predominant influence on the reduction of iron and nickel by facilitating the formation of molten slag, which was beneficial to the reduction and formation of ferronickel concentrate in kinetics. The addition of Na
2SO
4 and H
3BO
3 can also promote the molten slag formation while the slaked lime only had a limited effect.
4. Conclusions
In this work, the influence of different additives on the carbothermal reduction of laterite nickel ore, as well as the effect of fine grinding time on the iron and nickel contents in the ferronickel concentrate were investigated. Based on experimental results, the conclusions can be drawn as follows:
The addition of Ca(OH)2 and Na2SO4 played negatively roles on the formation of iron and nickel, while CaF2 and H3BO3 were beneficial to that. The higher the amount of CaF2 and H3BO3 added, the higher the grade of the obtained ferronickel concentrate. Thermodynamic calculation also confirmed that the addition of CaF2 can reduce the melting temperature of the sample, which facilitated the reduction of iron and nickel.
Increasing the fine grinding time was beneficial for improving the grade of the ferronickel concentrate. The optimal fine grinding time of 240 s was conductive to the magnetic separation of ferronickel particles.
High-grade ferronickel concentrates with nickel of 8.93 wt.% and iron of 63.96 wt.% were obtained after reduced laterite nickel ore at 1250 °C for 15 min with a carbon ratio of 1.4 and 12 wt.% CaF2.
Author Contributions
Conceptualization, J.Z., C.C. and F.L; methodology, J.Z., F.L. and H.Z.; investigation, J.Z., C.C.; resources, H.D., Z. X. and H. Z.; data curation, J.Z.; writing—original draft preparation, J.Z.; writing—review and editing, H.D.; supervision, H. Z.; funding acquisition, S.L. and H. Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (52232002, 52072274 and 52272021) and the Open/Innovation Foundation of Hubei Three Gorges Laboratory (SK232006).
Data Availability Statement
Data will be made available on request.
Acknowledgments
The authors would like to thank the Analytical & Testing Center of Wuhan University of Science and Technology for the help on SEM analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hang, G.; Xue, Z.; Wu, Y. J.; Zhang, B. , Effect of CaF2 on the aggregation and growth of ferronickel particles in the self-reduction of nickel laterite ore. Metall. Res. Technol. 2021, 118, 407. [Google Scholar] [CrossRef]
- Wang, Z.; Li, B.; Feng, Z.; Hui, S.; Yang, Y.; Wang, H. , Extraction of ferronickel concentrate by reduction roasting-magnetic separation from low grade laterite nickel ore under the action of compound additives. Mater. Trans. 2022, 63, 1197–1204. [Google Scholar] [CrossRef]
- Tian, H.; Guo, Z.; Zhan, R.; Pan, J.; Zhu, D.; Yang, C.; Pan, L. , Effective and economical treatment of low-grade nickel laterite by a duplex process of direct reduction-magnetic separation & rotary kiln-electric furnace and its industrial application. Powder Technol. 2021, 394, 120–132. [Google Scholar]
- Yuan, S.; Zhou, W.; Li, Y.; Han, Y. , Efficient enrichment of nickel and iron in laterite nickel ore by deep reduction and magnetic separation. T. Nonferr. Metal. Soc. 2020, 30, 812–822. [Google Scholar] [CrossRef]
- Shi, R.; Li, X.; Cui, Y.; Zhao, J.; Zou, C.; Qiu, G. , Coupled preparation of ferronickel and cementitious material from laterite nickel ores. Materials 2020, 13, 4992. [Google Scholar] [CrossRef]
- Forster, J.; Pickles, C. A.; Elliott, R. , Microwave carbothermic reduction roasting of a low grade nickeliferous silicate laterite ore. Miner. Eng. 2016, 88, 18–27. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, C.; Li, B.; Wei, Y. , Migration and aggregation behavior of nickel and iron in low grade laterite ore with new additives. Metals 2021, 11, 2033. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, X.; Luo, Y.; Pan, J.; Bai, B. , Reduction smelting low ferronickel from pre-concentrated nickel-Iron ore of nickel laterite. High Temp. Mat. Pr-Isr. 2016, 35, 1031–1036. [Google Scholar] [CrossRef]
- Dong, J.; Wei, Y.; Lu, C.; Zhou, S.; Li, B.; Ding, Z.; Wang, C.; Ma, B. , Influence of calcium chloride addition on coal-based reduction roasting of low-nickel garnierite ore. Mater. Trans. 2017, 58, 1161–1168. [Google Scholar] [CrossRef]
- Ma, B.; Yang, W.; Xing, P.; Wang, C.; Chen, Y.; Lv, D. , Pilot-scale plant study on solid-state metalized reduction–magnetic separation for magnesium-rich nickel oxide ores. Int. J. Miner. Process. 2017, 169, 99–105. [Google Scholar] [CrossRef]
- Zhu, D.; Pan, L.; Guo, Z.; Pan, J.; Zhang, F. , Utilization of limonitic nickel laterite to produce ferronickel concentrate by the selective reduction-magnetic separation process. Adv. Powder Technol. 2019, 30, 451–460. [Google Scholar] [CrossRef]
- Ma, B.; Xing, P.; Yang, W.; Wang, C.; Chen, Y.; Wang, H. , Solid-state metalized reduction of magnesium-rich low-nickel oxide ores using coal as the reductant based on thermodynamic analysis. Metall. Mater. Trans. B 2017, 48, 2037–2046. [Google Scholar] [CrossRef]
- Hang, G.; Xue, Z.; Wang, J.; Wu, Y. , Mechanism of calcium sulphate on the aggregation and growth of ferronickel particles in the self-reduction of saprolitic nickel laterite ore. Metals 2020, 10, 423. [Google Scholar] [CrossRef]
- Qu, G.; Zhou, S.; Wang, H.; Li, B.; Wei, Y. , Production of ferronickel concentrate from low-grade nickel laterite ore by non-melting reduction magnetic separation process. Metals 2019, 9, 1340. [Google Scholar] [CrossRef]
- Ma, B.; Li, X.; Yang, W.; Hu, D.; Xing, P.; Liu, B.; Wang, C. , Nonmolten state metalized reduction of saprolitic laterite ores: Effective extraction and process optimization of nickel and iron. J. Clean. Prod. 2020, 256, 120415. [Google Scholar] [CrossRef]
- Cao, W.; Yang, Q. , Properties of a carbonated steel slag-slaked lime mixture. J. Mater. Civil Eng. 2015, 27, 04014115. [Google Scholar] [CrossRef]
- Farrokhpay, S.; Filippov, L. , Challenges in processing nickel laterite ores by flotation. Int. J. Miner. Process. 2016, 151, 59–67. [Google Scholar] [CrossRef]
- Cao, Z.; Ma, B.; Zhou, J.; Chen, Y.; Wang, C. , The study for reduction roasting of laterite residue in the presence of CaF2. Process. Saf. Environ. 2022, 168, 1–9. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).