Submitted:
18 October 2023
Posted:
18 October 2023
You are already at the latest version
Abstract
Keywords:
1. INTRODUCTION:
2. Objectives:
- To use anthropometric data and fundamental biochemical parameters to divide T2DM patients into groups with varied degrees of obesity.
- To examine the blood levels of interleukins10,19, and 22.
- To correlate the various levels of obesity in T2DM patients with the blood IL levels.
3. METHODS:
3.1. Study design:
3.2. Study setting:
3.3. Study participants:
3.4. Institutional Review Board Statement:
3.5. Informed Consent Statement:
3.6. Inclusion criteria:
3.7. Requisites for Exclusion from patient group:
3.8. Requisites for control subjects:
3.9. Requisites for Exclusion from Controls:
3.10. Data collection:
3.10.1. T2DM patient blood specimen collection:
3.10.2. Blood samples from healthy controls:
3.10.3. Analysis of Biochemical parameters:
3.10.4. Estimation of Interleukins:
3.10.5. Interleukin-10:
3.10.6. Interleukin-19:
3.10.7. Interleukin-22:
3.10.8. Statistical assessment:
4. RESULTS:
4.1. Baseline features of the male patient group:
4.2. Baseline features of the female patient group:
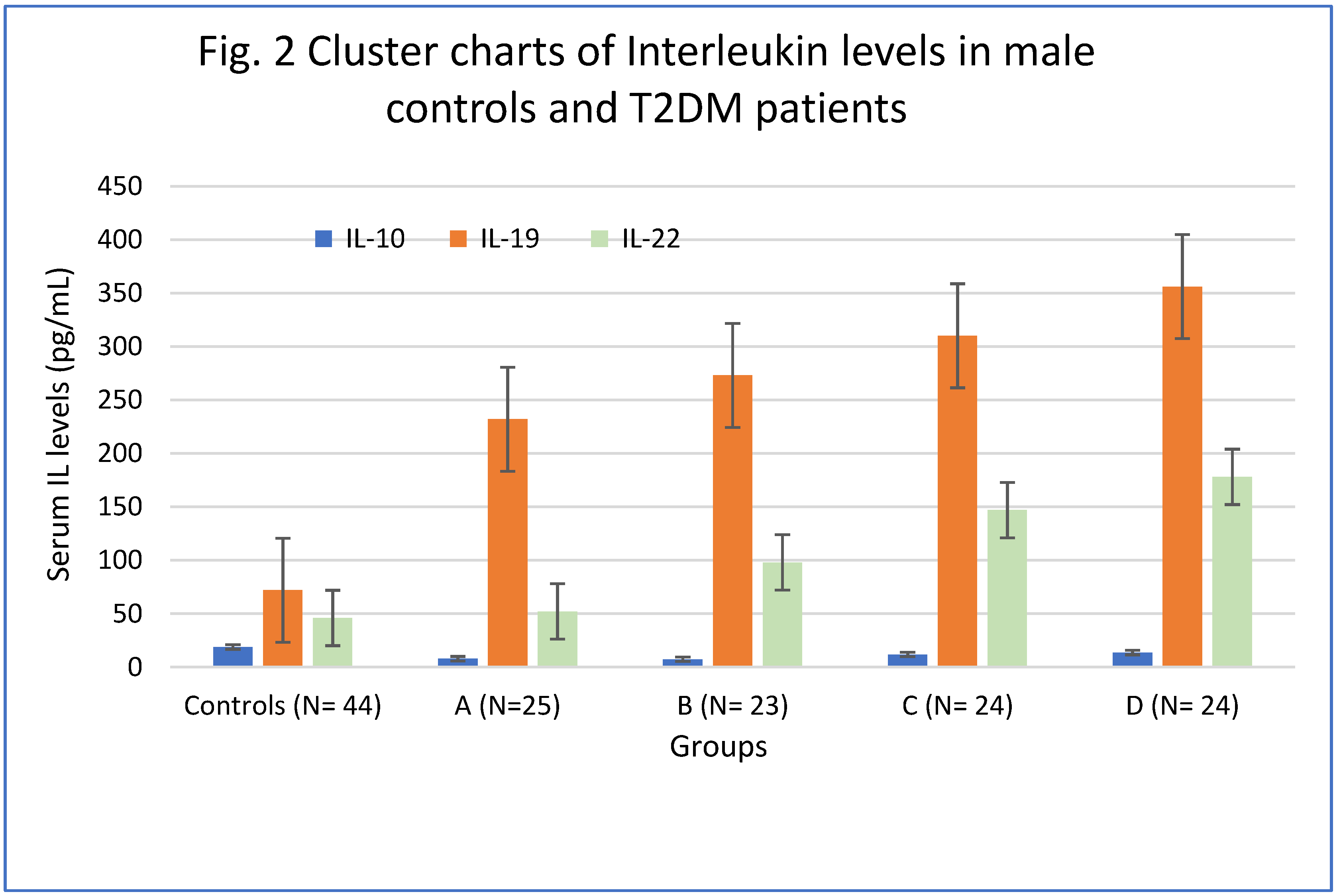
4.3. Levels of Interleukins
4.4. Interleukin-10.
4.5. Interleukin-19.
4.6. Interleukin-22.
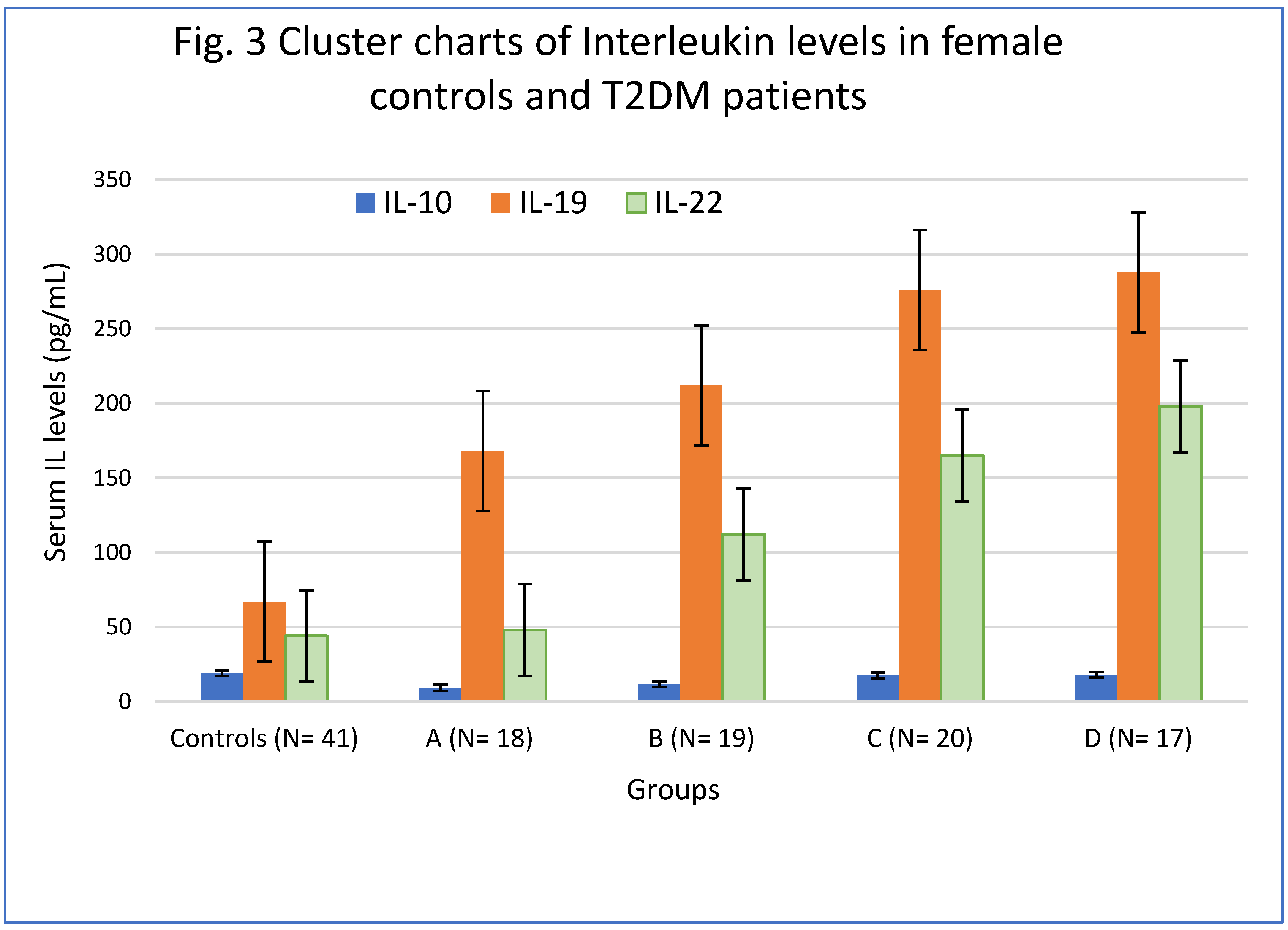
4.7. Interleukin-10 in Female groups.
4.8. Interleukin-19 in female T2DM patients.
4.9. Interleukin-22 in Female T2DM patients.
5. DISCUSSION:
5.1. Interleukin-10.
5.2. Interleukin-19.
5.3. Interleukin-22.
5.4. Limitations
6. Conclusions
Acknowledgments
References
- 1. Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. [CrossRef]
- 2. Yan Y, Wu T, Zhang M, Li C, Liu Q, Li F. Prevalence, awareness and control of type 2 diabetes mellitus and risk factors in Chinese elderly population. BMC Public Health, 2022. [CrossRef]
- 3. Mir MM, Mir R, Alghamdi MAA, et al. Differential Association of Selected Adipocytokines, Adiponectin, Leptin, Resistin, Visfatin and Chemerin, with the Pathogenesis and Progression of Type 2 Diabetes Mellitus (T2DM) in the Asir Region of Saudi Arabia: A Case Control Study. J Pers Med, 1 May 2022. [CrossRef]
- 4. Mir MM, Mir R, Alghamdi MAA, et al. Potential impact of GCK, MIR-196A-2 and MIR-423 gene abnormalities on the development and progression of type 2 diabetes mellitus in Asir and Tabuk regions of Saudi Arabia. Mol Med Rep. [CrossRef]
- 5. Qadir MI, Ahmed Z. lep Expression and Its Role in Obesity and Type-2 Diabetes. Crit Rev Eukaryot Gene Expr. [CrossRef]
- 6. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. [CrossRef]
- 7. Zhou X, Guan H, Zheng L, et al. Prevalence and awareness of diabetes mellitus among a rural population in China: results from Liaoning Province. Diabet Med. [CrossRef]
- 8. Moin ASM, Butler AE. Alterations in Beta Cell Identity in Type 1 and Type 2 Diabetes. Curr Diab Rep, 2019. [CrossRef]
- 9. Yin J, Yeung R, Luk A, et al. Gender, diabetes education, and psychosocial factors are associated with persistent poor glycemic control in patients with type 2 diabetes in the Joint Asia Diabetes Evaluation (JADE) program. J Diabetes. [CrossRef]
- Liu M, Lv X, Li Y, Li J, He Y. Prevalence and Control Status of Diabetes and Related Risk Factors Among 4196 Chinese Male Older Elderly Aged ≥80 Years. Int J Gerontol. 2018;12(2):122–126. [CrossRef]
- 11. Wang H, Yao J, Yin X, et al. Organisational and individual characteristics associated with glycaemic control among patients with type 2 diabetes: cross-sectional study in China. BMJ Open, 0363. [CrossRef]
- 12. Irazola V, Rubinstein A, Bazzano L, et al. Prevalence, awareness, treatment and control of diabetes and impaired fasting glucose in the Southern Cone of Latin America. PLoS One, 0183. [CrossRef]
- 13. Hu D, Fu P, Xie J, et al. Increasing prevalence and low awareness, treatment and control of diabetes mellitus among Chinese adults: the InterASIA study. Diabetes Res Clin Pract. [CrossRef]
- 14. Galicia-Garcia U, Benito-Vicente A, Jebari S, et al. Pathophysiology of Type 2 Diabetes Mellitus. Int J Mol Sci, 6275. [CrossRef]
- 15. Abdul-Ghani MA, Jayyousi A, DeFronzo RA, Asaad N, Al-Suwaidi J. Insulin Resistance the Link between T2DM and CVD: Basic Mechanisms and Clinical Implications. Curr Vasc Pharmacol. [CrossRef]
- 16. Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. [CrossRef]
- 17. Niu G, Li J, Wang H, Ren Y, Bai J. Associations of A-FABP with Anthropometric and Metabolic Indices and Inflammatory Cytokines in Obese Patients with Newly Diagnosed Type 2 Diabetes. Biomed Res Int, 8209. [CrossRef]
- 18. Urbanavičius V, Abalikšta T, Brimas G, Abraitienė A, Gogelienė L, Strupas K. Comparison of changes in blood glucose, insulin resistance indices, and adipokine levels in diabetic and nondiabetic subjects with morbid obesity after laparoscopic adjustable gastric banding. Medicina (Kaunas).
- Brocker C, Thompson D, Matsumoto A, Nebert DW, Vasiliou V. Evolutionary divergence and functions of the human interleukin (IL) gene family. Hum Genomics. [CrossRef]
- Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol. 2012;32(1):23-63. [CrossRef]
- 21. van Exel E, Gussekloo J, de Craen AJ, et al. Low production capacity of interleukin-10 associates with the metabolic syndrome and type 2 diabetes : the Leiden 85-Plus Study. Diabetes, 1092. [CrossRef]
- 22. Snijder MB, Heine RJ, Seidell JC, et al. Associations of adiponectin levels with incident impaired glucose metabolism and type 2 diabetes in older men and women: the hoorn study. Diabetes Care, 2498. [CrossRef]
- 23. Scarpelli D, Cardellini M, Andreozzi F, et al. Variants of the interleukin-10 promoter gene are associated with obesity and insulin resistance but not type 2 diabetes in caucasian italian subjects. Diabetes, 1533. [CrossRef]
- 24. Hofmann SR, Rösen-Wolff A, Tsokos GC, Hedrich CM. Biological properties and regulation of IL-10 related cytokines and their contribution to autoimmune disease and tissue injury. Clin Immunol. [CrossRef]
- 25. Sabat R, Wolk K. Deciphering the role of interleukin-22 in metabolic alterations. Cell Biosci, 2015. [CrossRef]
- 26. Jordan WJ, Eskdale J, Boniotto M, et al. Human IL-19 regulates immunity through auto-induction of IL-19 and production of IL-10. Eur J Immunol, 1582. [CrossRef]
- 27. Ray M, Autieri MV. Regulation of pro- and anti-atherogenic cytokines. Cytokine. [CrossRef]
- 28. Jain S, Gabunia K, Kelemen SE, Panetti TS, Autieri MV. The anti-inflammatory cytokine interleukin 19 is expressed by and angiogenic for human endothelial cells. Arterioscler Thromb Vasc Biol. [CrossRef]
- 29. England RN, Autieri MV. Anti-inflammatory effects of interleukin-19 in vascular disease. Int J Inflam, 3583. [CrossRef]
- 30. Li L, ZHeng-Qing Y, Juan-Yu H, et al. Association between interleukin-19 and angiopoietin-2 with vascular complications in type 2 diabetes. J Diabetes Investig. [CrossRef]
- 31. Li L, Yu ZH, Qian L, et al. Interleukin-19 and angiopoietin-2 can enhance angiogenesis of diabetic complications. J Diabetes Complications. [CrossRef]
- 32. Chen J, Caspi RR, Chong WP. IL-20 receptor cytokines in autoimmune diseases. J Leukoc Biol. [CrossRef]
- 33. Keir M, Yi Y, Lu T, Ghilardi N. The role of IL-22 in intestinal health and disease. J Exp Med, 2019. [CrossRef]
- 34. Shen J, Fang Y, Zhu H, Ge W. Plasma interleukin-22 levels are associated with prediabetes and type 2 diabetes in the Han Chinese population. J Diabetes Investig. [CrossRef]
- 35. Sonnenberg GF, Fouser LA, Artis D. Functional biology of the IL-22-IL-22R pathway in regulating immunity and inflammation at barrier surfaces. Adv Immunol. [CrossRef]
- 36. Zenewicz LA, Flavell RA. IL-22 and inflammation: leukin' through a glass onion. Eur J Immunol, 3265. [CrossRef]
- 37. Wang X, Ota N, Manzanillo P, et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature. [CrossRef]
- 38. Yaghini N, Mahmoodi M, Asadikaram GR, Hassanshahi GH, Khoramdelazad H, Kazemi Arababadi M. Serum levels of interleukin 10 (IL-10) in patients with type 2 diabetes. Iran Red Crescent Med J.
- 39. Giulietti A, van Etten E, Overbergh L, Stoffels K, Bouillon R, Mathieu C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-Dihydroxyvitamin D(3) works as anti-inflammatory. Diabetes Res Clin Pract. [CrossRef]
- 40. Subramanian N, Tavira B, Hofwimmer K, et al. Sex-specific regulation of IL-10 production in human adipose tissue in obesity. Front Endocrinol (Lausanne), 2022. [CrossRef]
- 41. Cartier A, Côté M, Lemieux I, et al. Sex differences in inflammatory markers: what is the contribution of visceral adiposity? Am J Clin Nutr, 1314. [CrossRef]
- 42. Camporez JP, Lyu K, Goldberg EL, et al. Anti-inflammatory effects of oestrogen mediate the sexual dimorphic response to lipid-induced insulin resistance. J Physiol, 3885. [CrossRef]
- 43. Mahr S, Menard J, Krenn V, Müller B. Sexual dimorphism in the osteoarthritis of STR/ort mice may be linked to articular cytokines. Ann Rheum Dis, 1234. [CrossRef]
- 44. Verthelyi D, Klinman DM. Sex hormone levels correlate with the activity of cytokine-secreting cells in vivo. Immunology. [CrossRef]
- 45. Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. [CrossRef]
- 46. garkava M, Pantsulaia I, Rukhadze R, Karanadze N, Chikovani T. ASSOCIATION OF IL-10 AND RESISTIN IN APPARENTLY HEALTHY ELDERLY POPULATION. Georgian Med News.
- 47. Cuneo AA, Herrick D, Autieri MV. Il-19 reduces VSMC activation by regulation of mRNA regulatory factor HuR and reduction of mRNA stability. J Mol Cell Cardiol. [CrossRef]
- Ellison S, Gabunia K, Kelemen SE, et al. Attenuation of experimental atherosclerosis by interleukin-19 [published correction appears in Arterioscler Thromb Vasc Biol. 2014 Jan; 34(1): e1. Orr, Wayne [corrected to Orr, A Wayne]]. Arterioscler Thromb Vasc Biol. 2013. [Google Scholar] [CrossRef]
- 49. England RN, Preston KJ, Scalia R, Autieri MV. Interleukin-19 decreases leukocyte-endothelial cell interactions by reduction in endothelial cell adhesion molecule mRNA stability. Am J Physiol Cell Physiol. [CrossRef]
- 50. Ouyang W, O'Garra A. IL-10 Family Cytokines IL-10 and IL-22: from Basic Science to Clinical Translation. Immunity. [CrossRef]
- 51. Herder C, Kannenberg JM, Carstensen-Kirberg M, et al. Serum levels of interleukin-22, cardiometabolic risk factors and incident type 2 diabetes: KORA F4/FF4 study. Cardiovasc Diabetol, 2017. [CrossRef]
- 52. Sabat R, Ouyang W, Wolk K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat Rev Drug Discov. [CrossRef]
- 53. Fabbrini E, Cella M, McCartney SA, et al. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology. [CrossRef]
- 54. Dalmas E, Venteclef N, Caer C, et al. T cell-derived IL-22 amplifies IL-1β-driven inflammation in human adipose tissue: relevance to obesity and type 2 diabetes. Diabetes, 1977. [CrossRef]
- 55. Hasnain SZ, Borg DJ, Harcourt BE, et al. Glycemic control in diabetes is restored by therapeutic manipulation of cytokines that regulate beta cell stress. Nat Med, 1417. [CrossRef]
| Anthropometric and biochemical indices | Controls | Group A | Group B | Group C | Group D |
|---|---|---|---|---|---|
| N= 44 | N=96 | ||||
| 25 | 23 | 24 | 24 | ||
| Age | 46(28-62) | 43(27-51) | 47(29-55) | 49(30-66) | 47(32-67) |
| WHR | 0.86 (0.84-0.95) |
0.92 (0.84-0.1.05) |
0.94 (0.83-1.06) |
1.07**T (0.97-1.11) |
1.14*S (1.02-1.19) |
| BMI (kg/m2) | 21.78 ± 1.78 | 21.80 ± 2.14 | 27.44 ± 2.29 | 34.94 ± 3.18*T | 47.22± 5.70*WX |
| Fasting Glucose(mg/dL) | 92(78-116) | 115(88-130) | 114(92-138) | 116(92-144) | 124(103-152) |
| HbA1c (g/dL) | 4.9±0.88 | 7.4 ±1.02* | 8.1±1.2* | 8.7±1.48*S | 7.6±0.78* |
| Cholesterol-T | 186 (135-224) | 204**(154-230) | 214*(148-232) | 215*(142-240) | 226*(158-258) |
| HDL-C(mg/dL) | 53(38- 63) | 46(36—60) | 48(38-56) | 45(33-58) | 44(28-54) |
| LDL-C(mg/dL) | 94±32.20 | 98 (62-131) | 118** (80-136) | 120** (80-152) | 136*w (95-162) |
| TG (mg/dL) | 116(86-132) | 122(96-136) | 146(98-178) | 232*(162-256) | 242*w(162-286) |
| Anthropometric and biochemical indices | Controls | Group A | Group B | Group C | Group D |
|---|---|---|---|---|---|
| N= 41 | N=74 | ||||
| 18 | 19 | 20 | 17 | ||
| Age | 48(27-64) | 44(26-55) | 47(27-57) | 46(28-62) | 48(30-69) |
| WHR | 0.85 (0.78-0.96) |
0.91 (0.82-0.1.04) |
0.92 (0.84-1.05) |
1.06**T (0.97-1.13) |
1.10*S (1.02-1.22) |
| BMI (kg/m2) | 21.2 ± 1.66 | 21.41 ± 2.14 | 28.22 ± 2.26 | 33.88± 3.20*T | 46.48± 5.20*WX |
| Fasting Glucose(mg/dL) | 96(82-114 | 118**(90-141) | 114**(92-138) | 116**(92-144) | 124*(103-152) |
| HbA1c (g/dL) | 4.5±0.43 | 7.8±1.02* | 8.1±1.2* | 8.5±1.48*S | 8.4±0.98* |
| Cholesterol-T | 168 (132-224) | 196**(145-227) | 204**(144-238) | 215**(155-235) | 228**(162-262) |
| HDL-C(mg/dL) | 56(41- 66) | 54 (39—62) | 52(40-63) | 46(35-64) | 44(35-62) |
| LDL-C(mg/dL) | 96±28.22 | 92±28.28 | 111**±33.08 | 108**±30.30 | 132*±32.12 |
| TG (mg/dL) | 96(88-124) | 126**(94-168) | 142**(88-222) | 152**(99- 223) | 195*wx(98-245) |
| Groups | IL-10 (pg/mL) | IL-19 (pg/mL) | IL-22 (pg/mL) |
|---|---|---|---|
| Controls (N= 44) | 18.78 ± 2.21 | 72 ± 21.45 | 46 ± 12.48 |
| A (N=25) | 7.89 ± 1.45* | 232± 21.45** | 52± 17.45 |
| B (N= 23) | 7.32 ± 1.36* | 273 ± 29.66** | 98 ± 26.12***M |
| C (N= 24) | 11.78±1.87** S | 310 ± 32.24*S | 147 ± 34.65** L |
| D (N= 24) | 13.56±1.92***T | 356 ± 43.64*T | 178± 38.28*P |
| Groups | IL-10 (pg/mL | IL-19 (pg/ mL) | IL-22 (pg/ mL) |
|---|---|---|---|
| Controls (N= 41) | 19.12 ± 2.28 | 67 ± 17.42 | 44± 12.02 |
| A (N= 18) | 9.23 ± 1.52* | 168± 26.78* | 48 ± 14.08 |
| B (N= 19) | 11.68 ± 1.55** | 212± 28.22*X | 112 ± 29.56**S |
| C (N= 20) | 17.46±2.10 | 276± 35.14***Y | 165 ± 33.24**SM |
| D (N= 17) | 18.02 ± 2.14 | 288 ± 36.98***Y | 198± 37.82***TN |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





