1. Introduction
Hund’s rule of maximum multiplicity is a powerful empirical tool for determining the electron population of electronic shells. It was recently reported [
1] that the real driving force behind Hund’s rule appears to be the second law of infodynamics [
2]
where
in this time derivative is the information entropy proportional to Shannon entropy
of a discrete random variable that attains maximum
if the events are equiprobable (i.e., if the probabilities
) and vanishes for certain and impossible events
1 In other words, electron populations within an orbital minimize Shannon entropy (
2).
It is now generally accepted [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13] that information in the universe evolves, decreasing the information entropy
. Assuming that the total entropy of the universe
S is constant and is the sum of the information entropy and the physical entropy
, we obtain [
1]
This study extends the findings of [
1] to the Aufbau rule and discusses them from the perspective of emergent dimensionality [
7,
8,
9,
10,
11,
12,
13,
14]. Other applications of Shannon entropy in chemistry are known from the state of art [
15,
16,
17].
2. Hund’s rule and Shannon entropy
A simple but inventive procedure for calculating Shannon entropies of electron populations was disclosed in [
1]. Any set of
N electrons satisfies
, where
and
denote, respectively, the number of up- and down-spin electrons. Thus, the probabilities of finding ↑ and ↓ electrons within the set of
N electrons are
and
.
Definition 1 (Electron set entropy).
An electron set entropy is
This definition was disclosed in [
1] but is given here for clarity. Electrons are fermions, so electron sets populating chemical elements’ orbitals must satisfy the Pauli exclusion principle. For the s orbital, for example, which can accommodate a maximum of
electrons, the corresponding probabilities are
if the orbital accommodates one electron and
if the orbital accommodates two electrons. Thus, possible electron set entropies (
4) are
.
We do not assume any particular logarithm base
b, noting that nature uses natural logarithm in Landauer’s principle [
18] and Boltzmann/Gibbs physical entropy
. Furthermore, the author of [
1] assumes that the electron set entropies of states s
and s
are the same, but this assumption is unjustified, as we shall discuss later (cf.
Figure 8).
The situation becomes more diverse for orbitals with a larger angular momentum quantum number
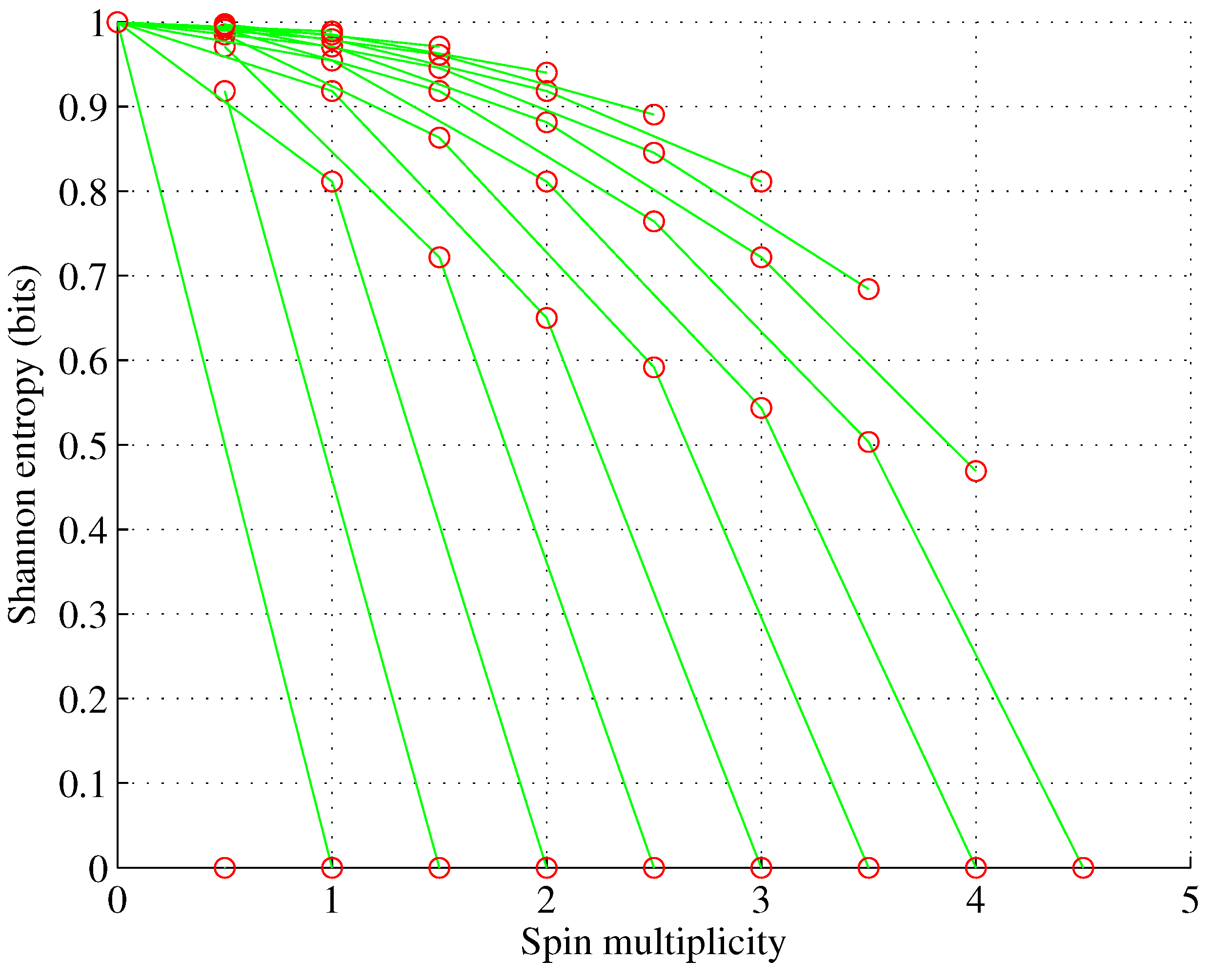
ℓ as multiple sets with different electron set entropies and spin multiplicities are possible, as shown in
Figure 1,
Figure 2,
Figure 3 and
Figure 4 for orbitals p-g.
However:
for , ;
for , only one electron set is possible; and
for , ;
wherein nature selects this electron set among the ones allowed by the Pauli exclusion principle that maximizes spin multiplicity. However, as shown in [
1], for
, nature also selects this electron population among the allowed ones, which minimizes the orbital Shannon entropy. This rule of populating the sublevels can be stated in the simple theorem illustrated in
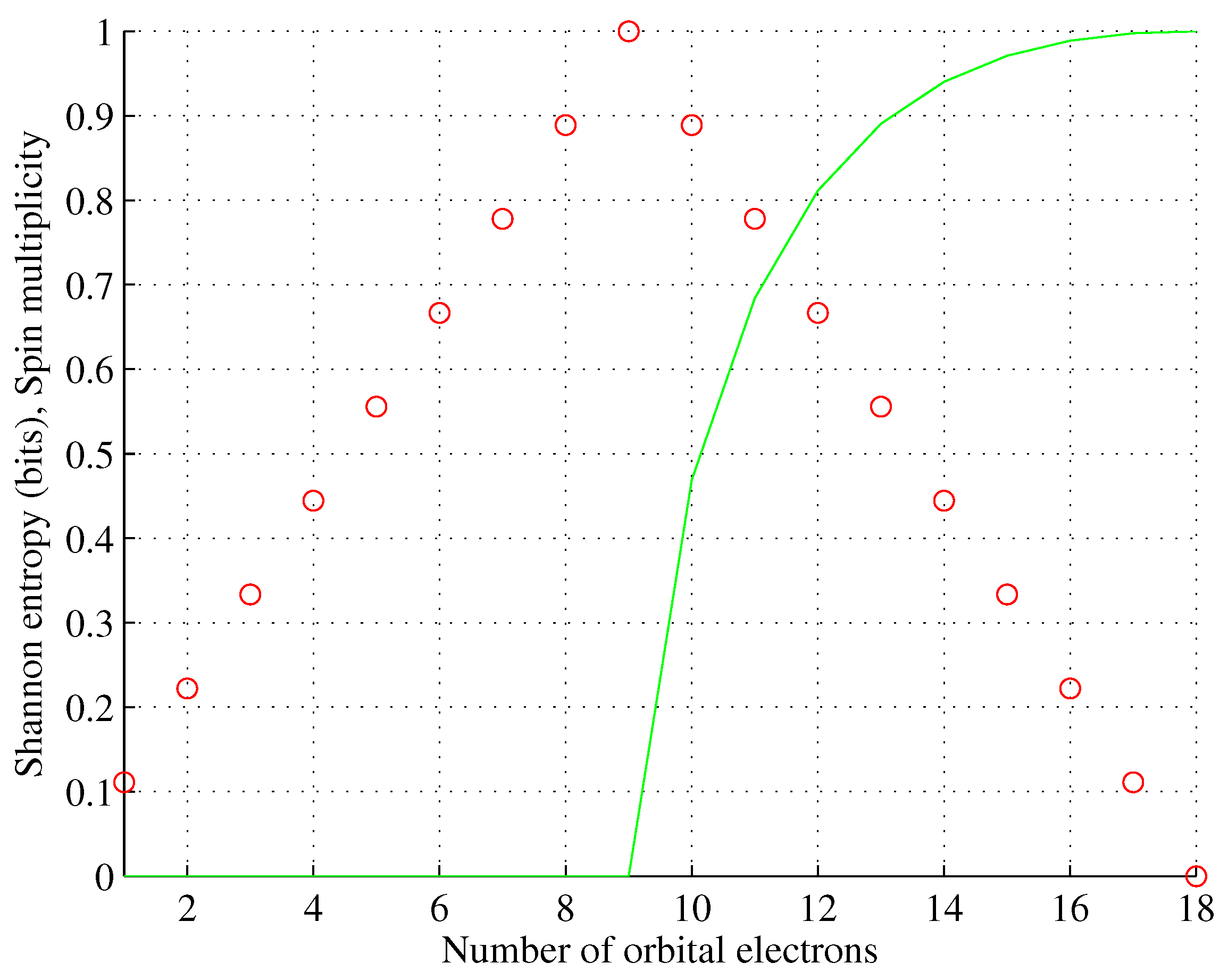
Figure 5.
Theorem 1 (Orbital entropy).
For any orbital capable of storing , electrons and storing N electrons and populated to maximize spin multiplicity (i.e. according to Hund’s rule), the orbital Shannon entropy vanishes iff and amounts
otherwise.
Proof. According to Hund’s rule, for the spin multiplicity is equal to , while for is equal to . For electrons can freely populate available sublevels to maximize the spin multiplicity and therefore (the same for ↓). For the electrons will begin to repopulate the available sublevels following the Pauli exclusion principle up to , where , which completes the proof. □
Some researchers postulate that certain elements are exceptions to Hund’s rule. Chromium, for example, having the atomic number is between vanadium (, electron configuration [Ar]3d4s) and manganese (, [Ar]3d4s) within the periodic table of elements. Thus, it should have electron configuration [Ar]3d4s, following Hund’s rule, but instead, it has [Ar]3d4s, as one electron from 4s moves to 3d to make it more stable. But it is not Hund’s rule that is violated. It is the Aufbau rule. Hund’s rule governs the electron population of a solitary orbital only.
3. Aufbau rule and Shannon entropy
The Aufbau or Madelung energy ordering rule is another powerful empirical tool for predicting the electron configurations of chemical elements corresponding to the ground state. It correctly predicts the electron configurations of most of the elements. However, about twenty chemical elements violate the Aufbau rule, leading to intriguing exceptions and anomalies. Chromium and copper violations are attributed to a delicate balance of electron-electron repulsion and the energy gap between the 3d and 4s orbitals. Only palladium exhibits double electron promotion with all ten electrons filling the 4d orbital and therefore is often called a "double anomaly". Palladium also possesses the smallest differential entropy [
16]. This exceptional behavior is attributed to the compactness of 3d orbitals and complex electron interactions. In addition, there are no chemical elements that have orbital f
in their electron configurations, although the Aufbau rule predicts f8 for gadolinium (
) as [Xe]+6s
+4f
and for curium (
) as [Rn]+7s
+5f
. Furthermore, there are only two nondoubleton sets of consecutive elements violating the Aufbau rule. We note that for
, actual ground states are predicted. Thus, perhaps other elements, such as darmstadtium (
) and roentgenium (
) also violate the Aufbau rule.
The elements that violate the Aufbau rule are listed in
Table 1, which shows their actual electron configurations and the configurations predicted by the Aufbau rule.
==layoutwidth=297mm,layoutheight=210 mm, left=2.7cm,right=2.7cm,top=1.8cm,bottom=1.5cm, includehead,includefoot [LO,RE]0cm [RO,LE]0cm
==
As the entropies of independent systems are additive quantities, we can calculate the Shannon entropies of chemical elements by summing the orbital entropies of electron configurations using the relation (
5).
Definition 2. An element’s (ground-state) Shannon entropy is the sum of the orbital entropies in the electron configuration of this element, neglecting the principal quantum number.
For example, the electron configuration of oxygen is [He]2s
2p
→ s
s
p
, where
and
is given by the relation (
5). Thus, the Shannon entropy for oxygen is equal to
Similarly, for chromium
.
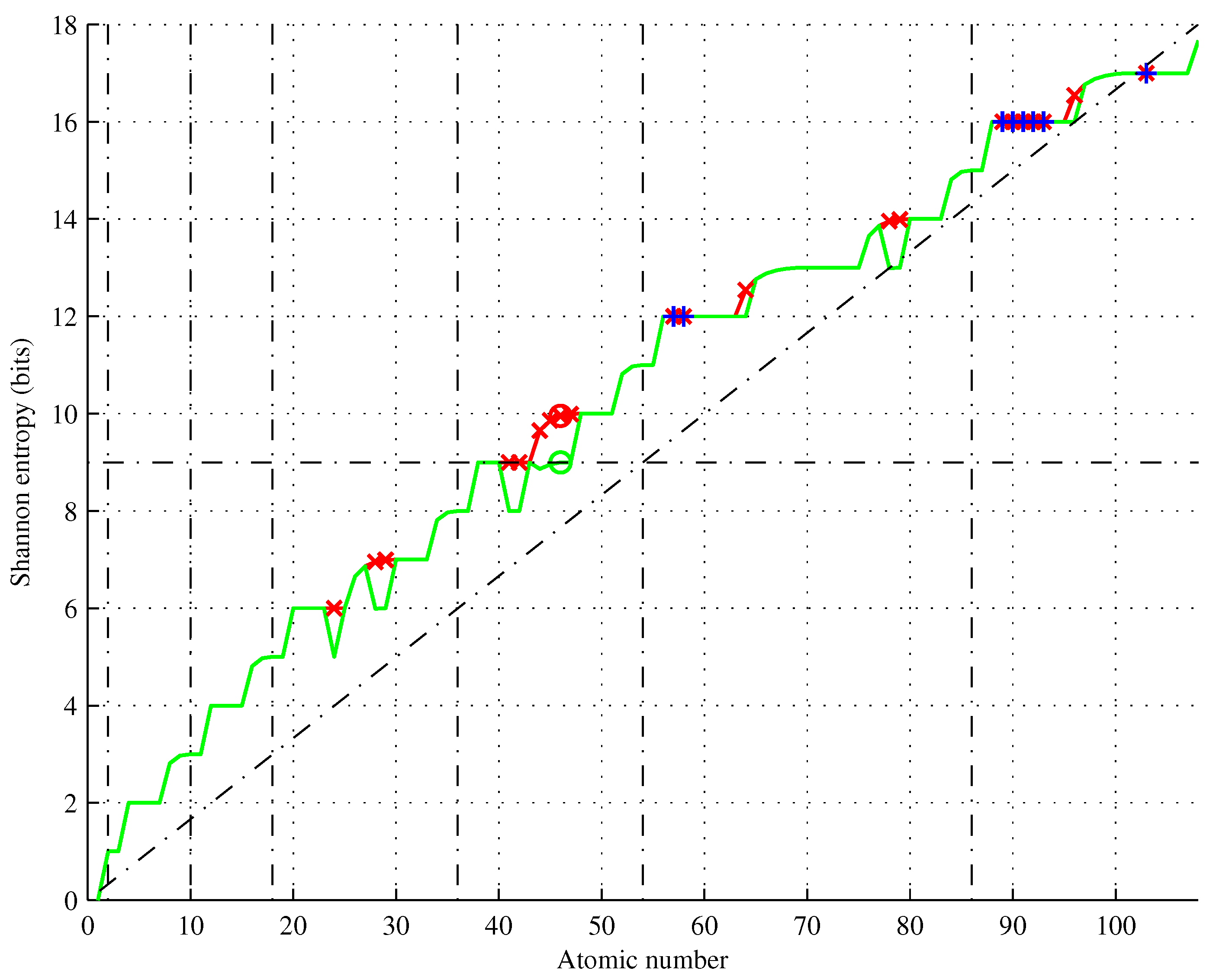
Shannon entropies
and
for
(Hassium is the heaviest element with known properties) are shown in
Figure 6 based on actual ground state configurations and configurations obtained using the Aufbau rule.
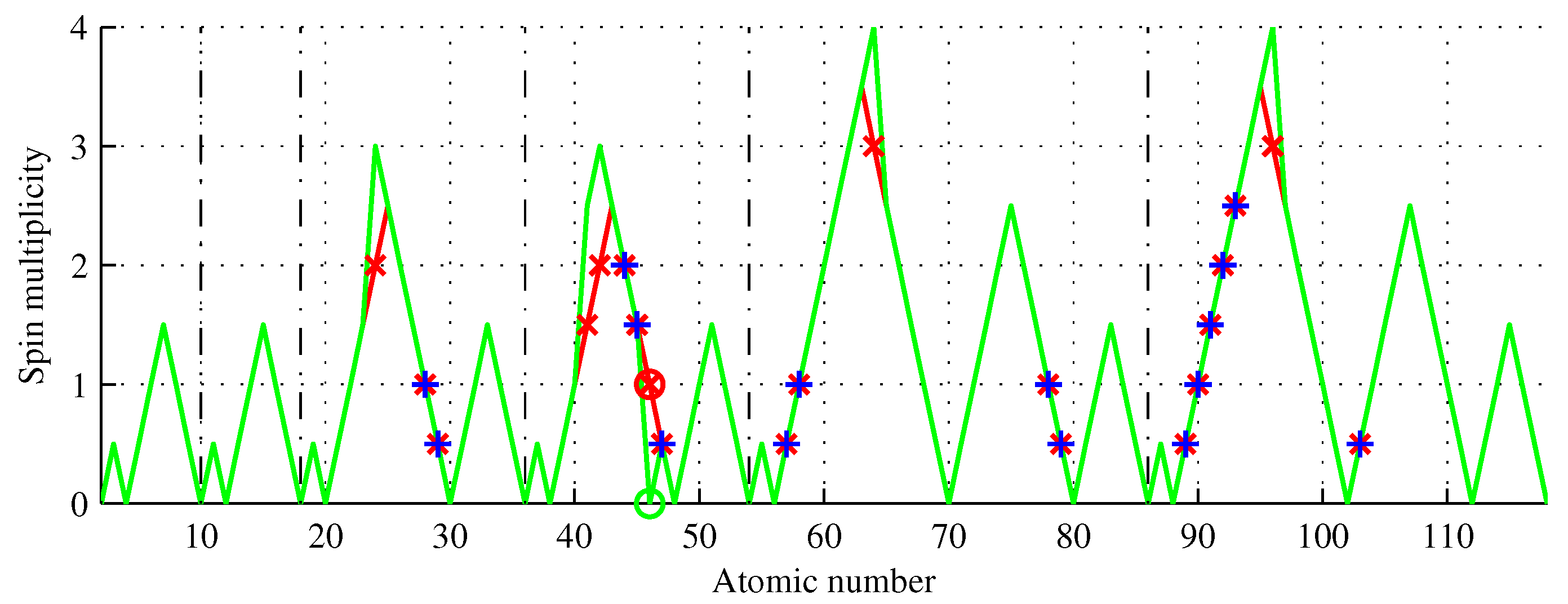
Table 1 lists the spin multiplicities of the elements that violate the Aufbau rule, i.e.,
.
Figure 7 shows the spin multiplicities of elements
.
if
Z is even and
otherwise.
The Aufbau rule reflects the periodicity of the elements within the windows defined by the noble gases’ atomic numbers, as shown in
Figure 7 and
Table 2. A simple conclusion of Theorem 1 is that the entries s
, p
-p
, d
-d
, f
-f
, etc. of the configuration of an element do not contribute to the entropy of the element, but only to the spin multiplicity. On the other hand, entries s
, p
, d
, f
, etc. contribute with
to entropy, but do not contribute to spin multiplicity. The remaining entries contribute both to the entropy and to the spin multiplicity.
Definition 3. The Aufbau core entropy of an element is , where k increases by one from for with every set of elements (cf. Table 2) to for , and so on.
For example, the oxygen core entropy is , while Cr, as an exception to the Aufbau rule, has core entropy .
We can, therefore, calculate Shannon entropy for different numbers of rows of
Table 2.
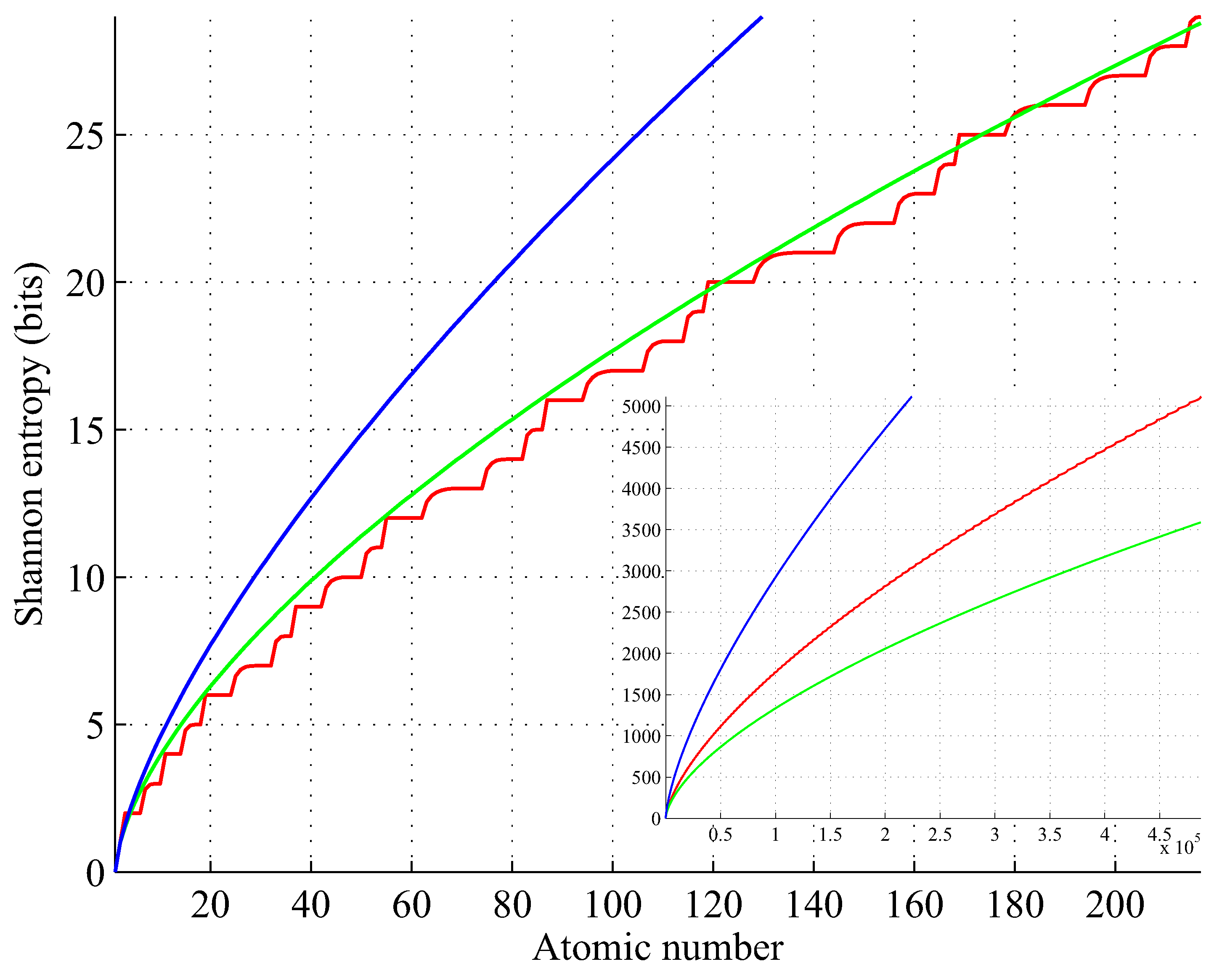
Figure 8, for example, shows the entropy for five rows, yielding
. This value is well beyond any considerable physical limit, such as Feynmanium (
), unoctquadium (
) or
, at which point the Compton wavelength of such an atom becomes smaller than the Planck length [
8,
13], which is physically implausible because the Planck area is the smallest area required to encode one bit of information [
8,
19,
20,
21]. However,
Figure 8 illustrates a trend of increasing the entropy of the elements. We conjecture that this trend continues until the element’s half-life is equal to Planck time and for
is bounded by
(cf. blue curve in the inset of
Figure 8).
Figure 8.
Shannon entropy of elements as given by the Hund and Aufbau rules (red), and its approximations (green) and (blue); , . Inset for .
Figure 8.
Shannon entropy of elements as given by the Hund and Aufbau rules (red), and its approximations (green) and (blue); , . Inset for .
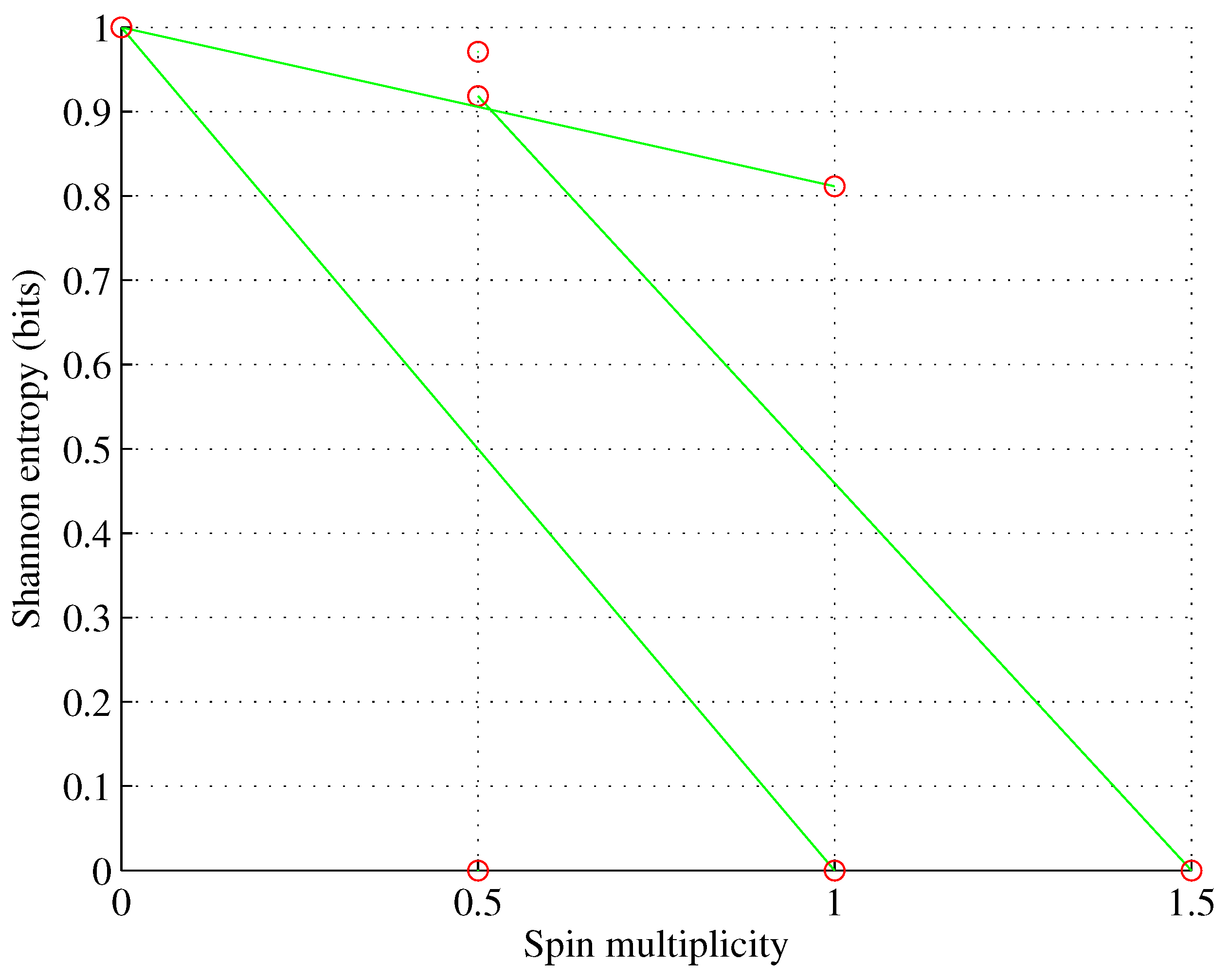
Figure 1.
Orbital p. , for , p, p, p.
Figure 1.
Orbital p. , for , p, p, p.
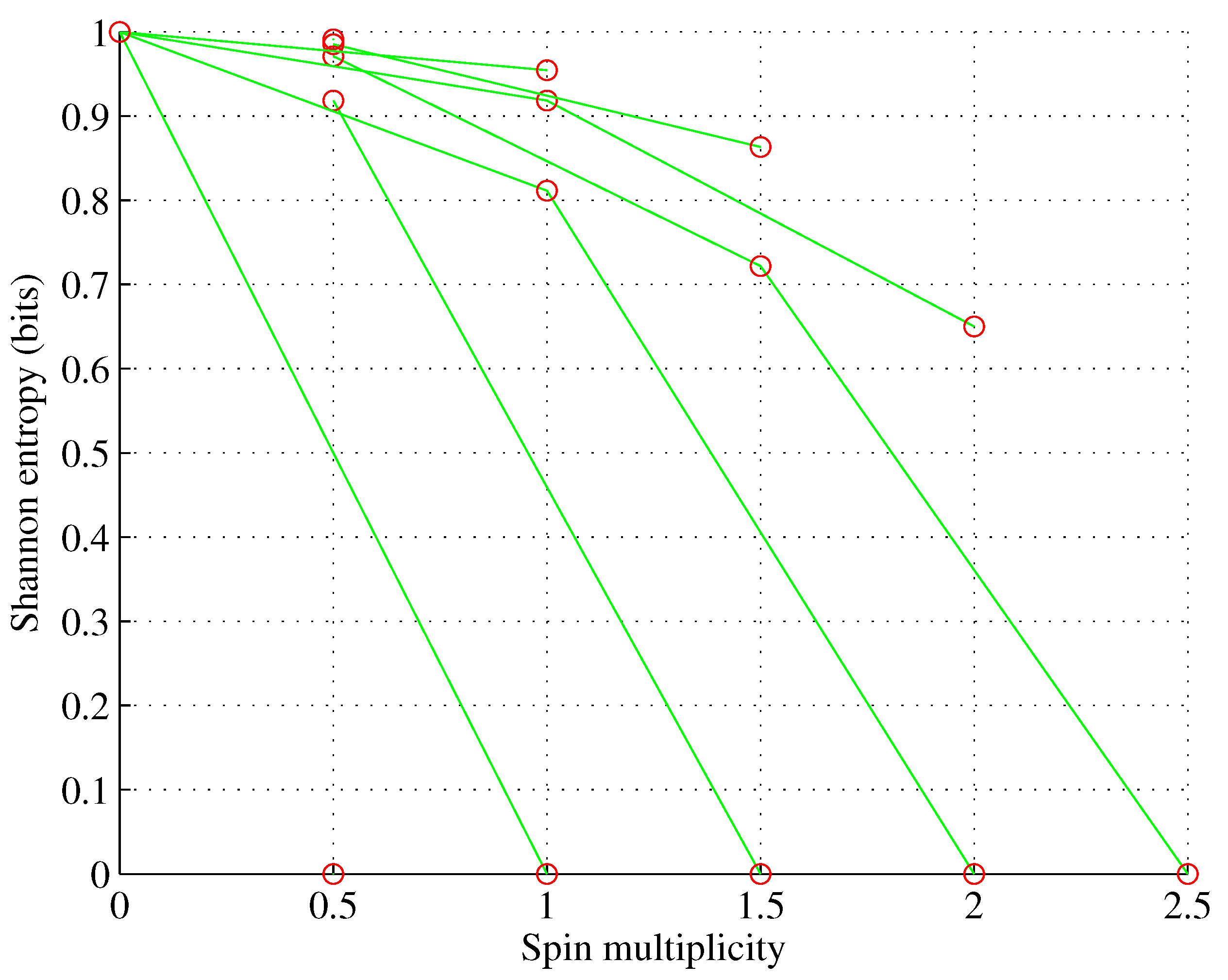
Figure 2.
Orbital d. , for , d.
Figure 2.
Orbital d. , for , d.
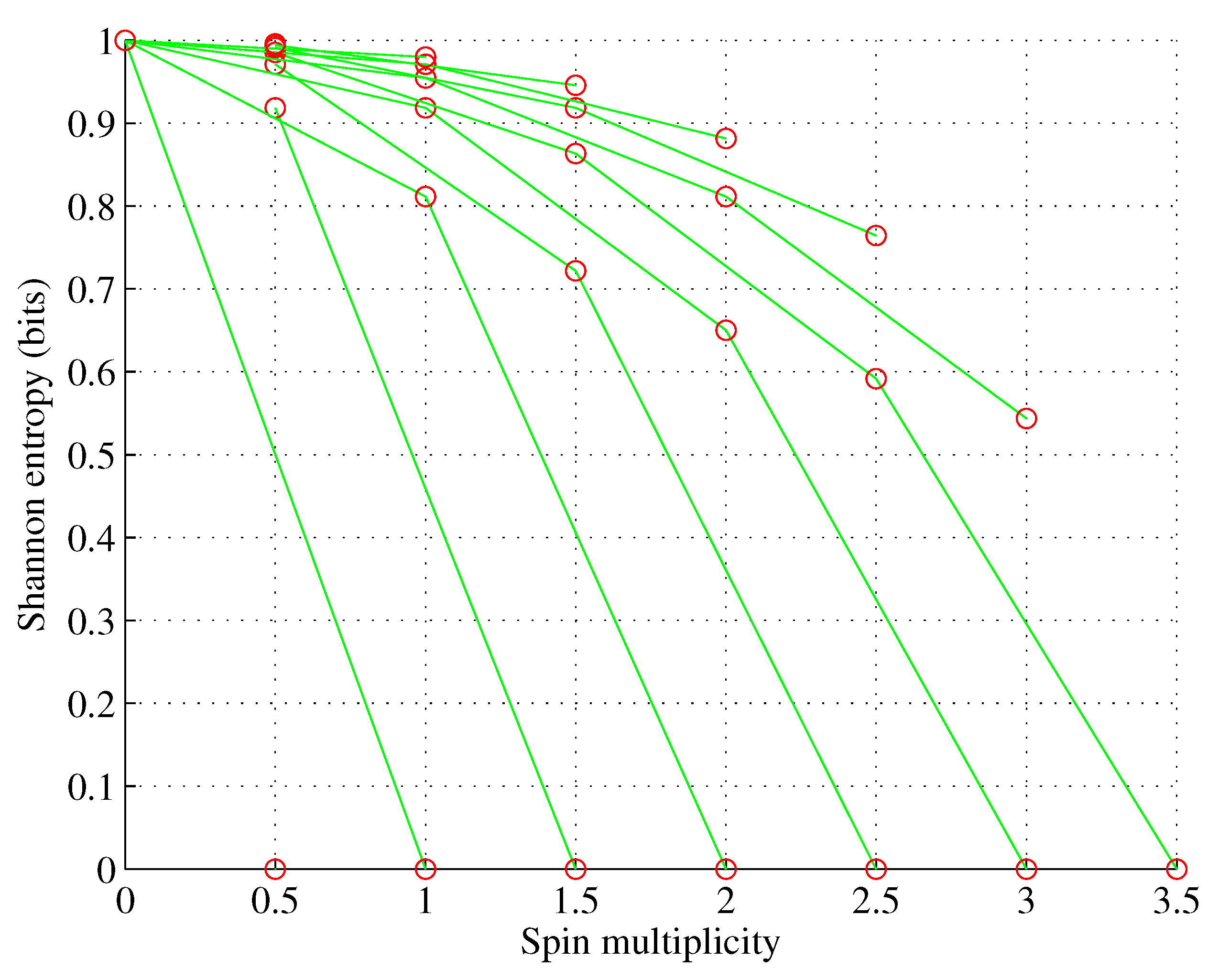
Figure 3.
Orbital f. , for , f.
Figure 3.
Orbital f. , for , f.
Figure 4.
Orbital g. , for , g.
Figure 4.
Orbital g. , for , g.
Figure 5.
Orbital g. Spin multiplicity (red, rescaled) and associated orbital entropy (green).
Figure 5.
Orbital g. Spin multiplicity (red, rescaled) and associated orbital entropy (green).
Figure 6.
Shannon entropy of chemical elements (green) showing Aufbau rule violations (red) and elements of the same entropy (blue). Thirteen elements have entropy different than predicted by the Aufbau rule.
Figure 6.
Shannon entropy of chemical elements (green) showing Aufbau rule violations (red) and elements of the same entropy (blue). Thirteen elements have entropy different than predicted by the Aufbau rule.
Figure 7.
Spin multiplicity of chemical elements (green) showing Aufbau rule violations (red) and elements of the same multiplicity (blue). Six elements have a spin multiplicity different from that predicted by the Aufbau rule, including palladium, the only one with a lower multiplicity.
Figure 7.
Spin multiplicity of chemical elements (green) showing Aufbau rule violations (red) and elements of the same multiplicity (blue). Six elements have a spin multiplicity different from that predicted by the Aufbau rule, including palladium, the only one with a lower multiplicity.
Table 1.
Chemical elements violating Aufbau rule.
Table 1.
Chemical elements violating Aufbau rule.
| |
|
Ground state electron configuration |
Spin multiplicity |
Shannon entropy
|
| |
Z |
actual |
Aufbau |
|
|
|
|
| Cr |
24 |
[Ar]3d4s
|
[Ar]3d4s
|
3 |
2 |
|
|
| Ni |
28 |
[Ar]3d4s (or [Ar]3d4s) |
[Ar]3d4s
|
1 |
1 |
|
|
| Cu |
29 |
[Ar]3d4s
|
[Ar]3d4s
|
1/2 |
1/2 |
|
|
| Nb |
41 |
[Kr]4d5s
|
[Kr]4d5s
|
5/2 |
3/2 |
|
|
| Mo |
42 |
[Kr]4d5s
|
[Kr]4d5s
|
3 |
2 |
|
|
| Ru |
44 |
[Kr]4d5s
|
[Kr]4d5s
|
2 |
2 |
|
|
| Rh |
45 |
[Kr]4d5s
|
[Kr]4d5s
|
3/2 |
3/2 |
|
|
| Pd |
46 |
[Kr]4d
|
[Kr]4d5s
|
0 |
1 |
|
|
| Ag |
47 |
[Kr]4d5s
|
[Kr]4d5s
|
1/2 |
1/2 |
|
|
| La |
57 |
[Xe]5d6s
|
[Xe]4f6s
|
1/2 |
1/2 |
|
|
| Ce |
58 |
[Xe]4f5d6s
|
[Xe]4f6s
|
1 |
1 |
|
|
| Gd |
64 |
[Xe]4f5d6s
|
[Xe]4f6s
|
4 |
3 |
|
|
| Pt |
78 |
[Xe]4f5d6s
|
[Xe]4f5d6s
|
1 |
1 |
|
|
| Au |
79 |
[Xe]4f5d6s
|
[Xe]4f5d6s
|
1/2 |
1/2 |
|
|
| Ac |
89 |
[Rn]6d7s
|
[Rn]5f7s
|
1/2 |
1/2 |
|
|
| Th |
90 |
[Rn]6d7s
|
[Rn]5f7s
|
1 |
1 |
|
|
| Pa |
91 |
[Rn]5f6d7s
|
[Rn]5f7s
|
3/2 |
3/2 |
|
|
| U |
92 |
[Rn]5f6d7s
|
[Rn]5f7s
|
2 |
2 |
|
|
| Np |
93 |
[Rn]5f6d7s
|
[Rn]5f7s
|
5/2 |
5/2 |
|
|
| Cm |
96 |
[Rn]5f6d7s
|
[Rn]5f7s
|
4 |
3 |
|
|
| Lf |
103 |
[Rn]5f7s7p
|
[Rn]5f6d7s
|
1/2 |
1/2 |
|
|
Table 2.
Pattern in the Aufbau rule and element entropies.
Table 2.
Pattern in the Aufbau rule and element entropies.
| Z |
|
g
|
f
|
d
|
p
|
|
g
|
f
|
d
|
p
|
| 2-17 |
2 |
|
|
|
6 |
2 |
|
|
|
6 |
| 18-53 |
2 |
|
|
|
6 |
2 |
|
|
|
6 |
| 54-117 |
2 |
|
|
|
6 |
2 |
|
|
|
6 |
| 118-217 |
2 |
|
|
|
6 |
2 |
|
|
|
6 |