Submitted:
10 January 2024
Posted:
12 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. The Historical Context
1.2. Early Developments in Therapeutic Neurofeedback
1.3. Working Models
1.3.1. The Trauma Model
1.3.2. The Dysregulation Cascade
1.3.3. The Causal Chain
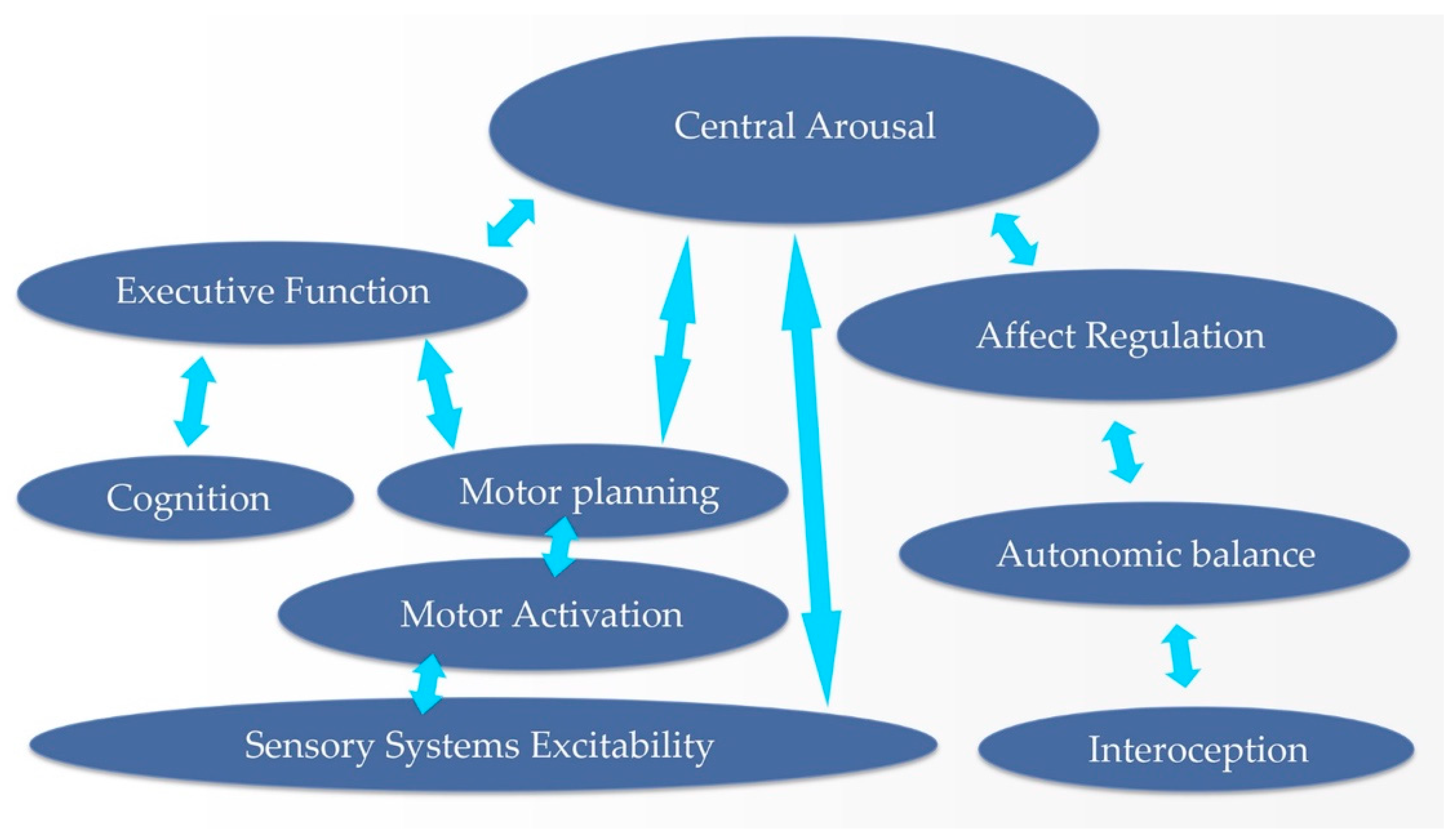
1.3.4. The Regulatory Hierarchy as Therapeutic Hierarchy
2. The Method
2.1. Cerebral Stability
2.2. Arousal Regulation
2.3. Autonomic Regulation
2.4. Affect Regulation
2.5. Executive Function
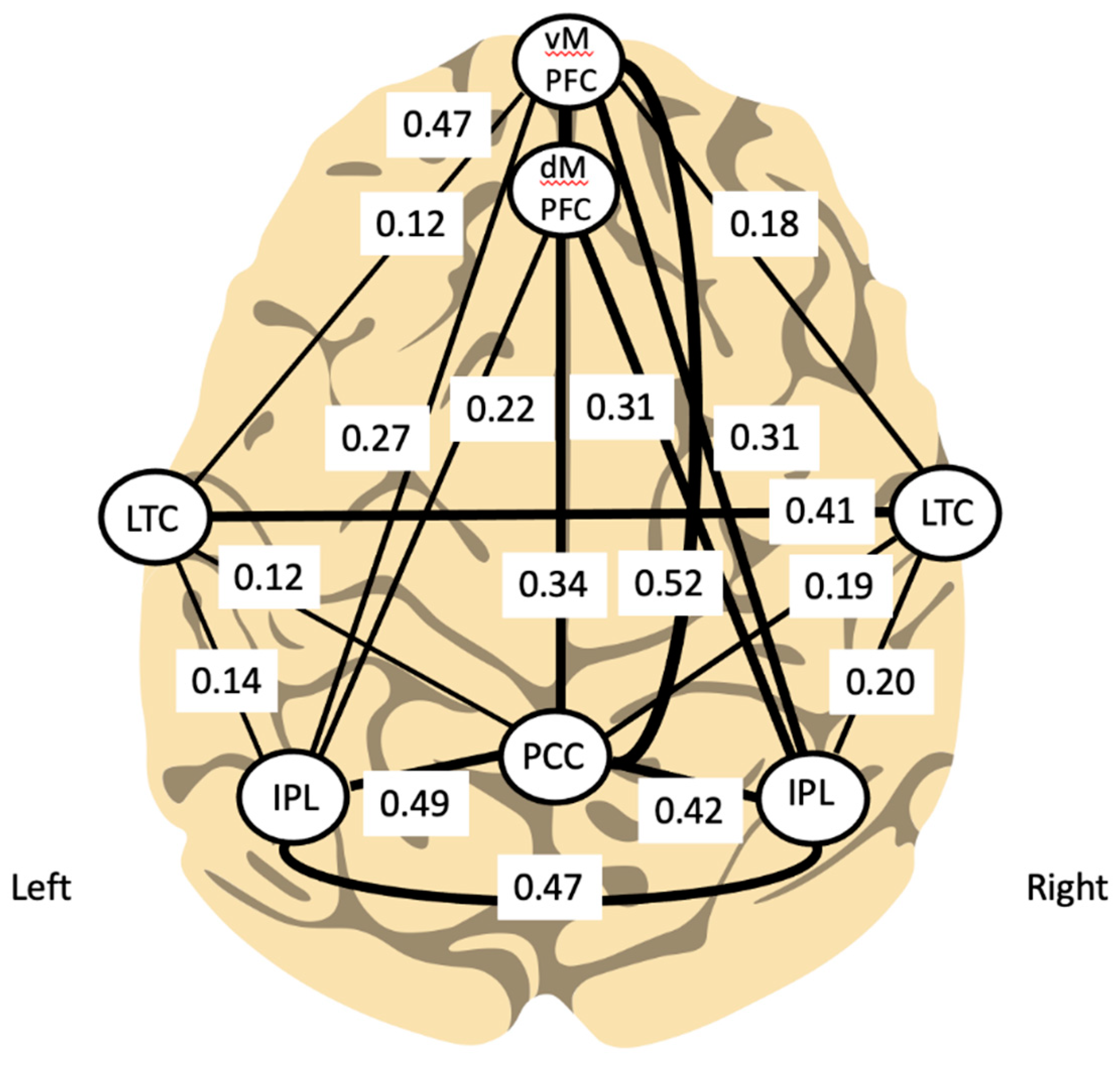
2.6. Left Parietal Heteromodal Cortex
2.7. Synchrony Training in the ILF Regime
2.8. The Frequency Domain
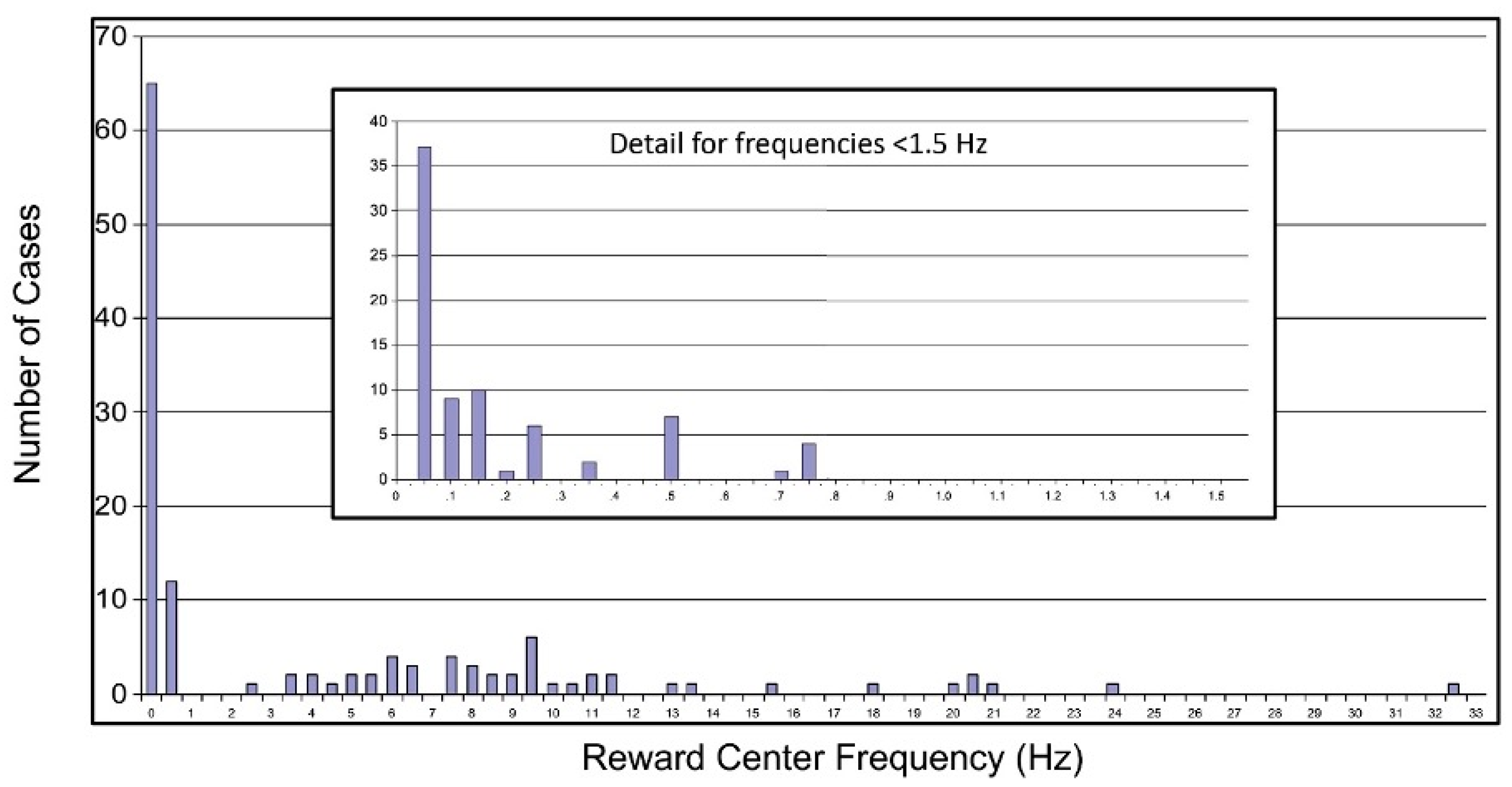
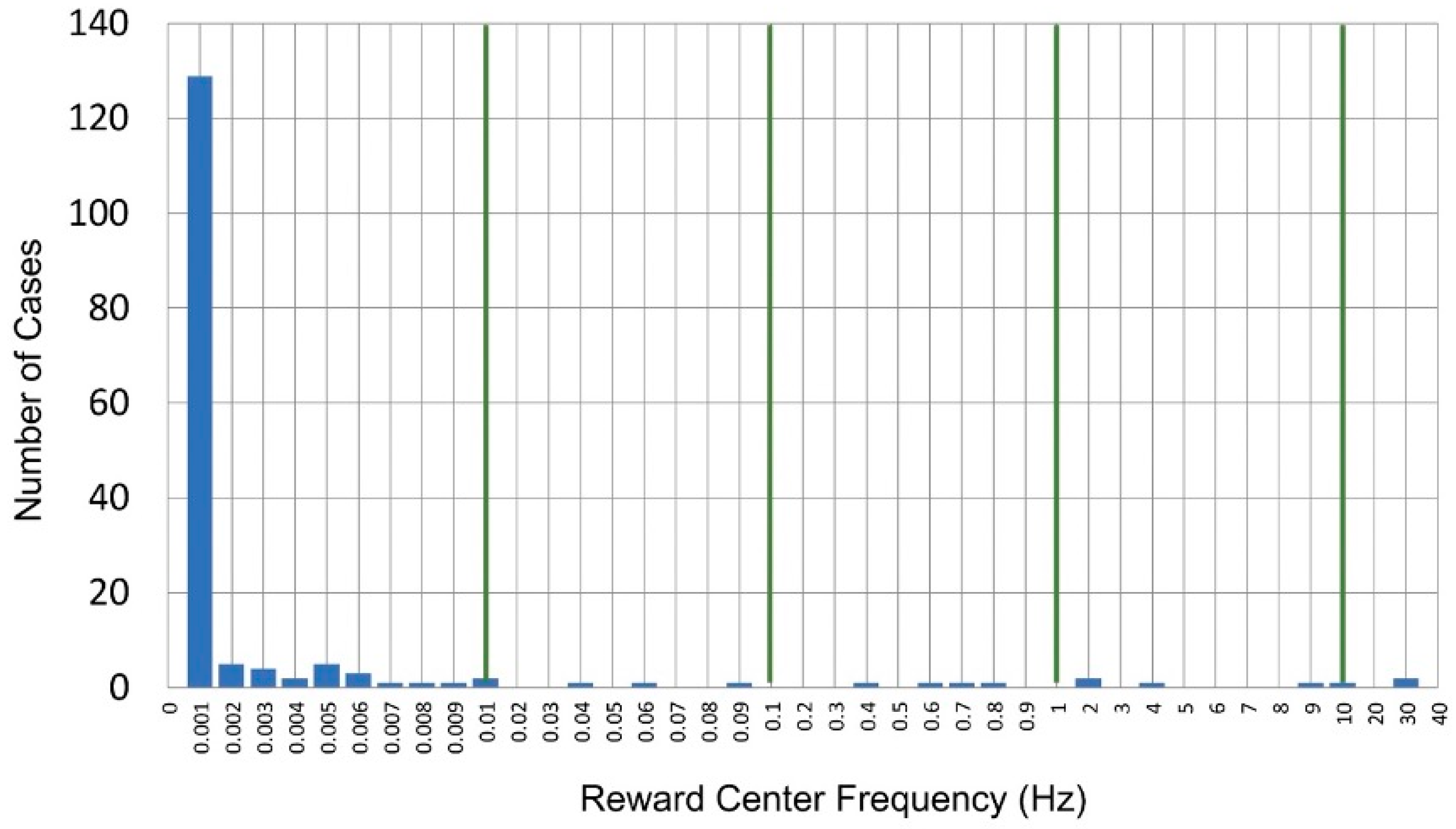
2.8.1. Extension of Training into the Infra-Low Frequency Domain
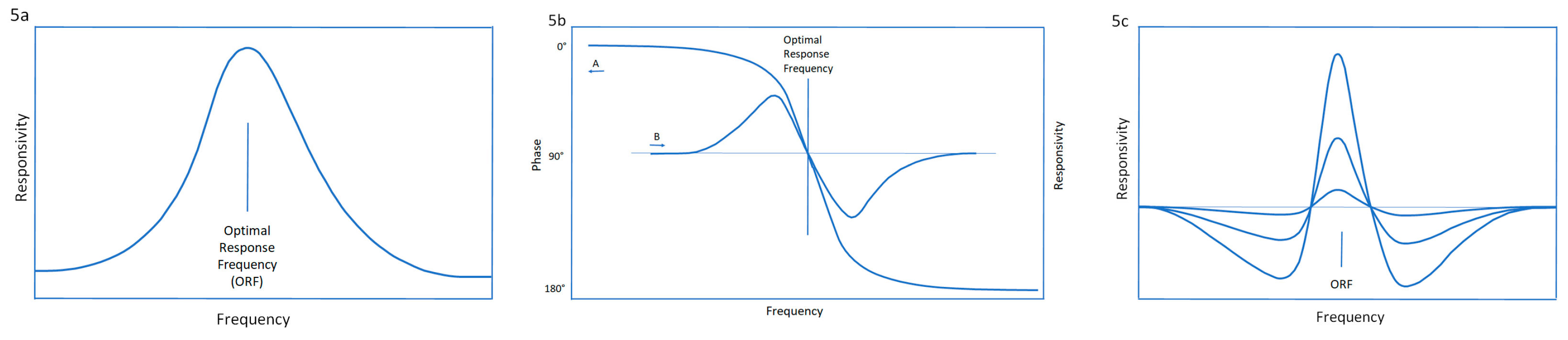
2.8.2. Optimal Response Frequency: A Resonance Phenomenon
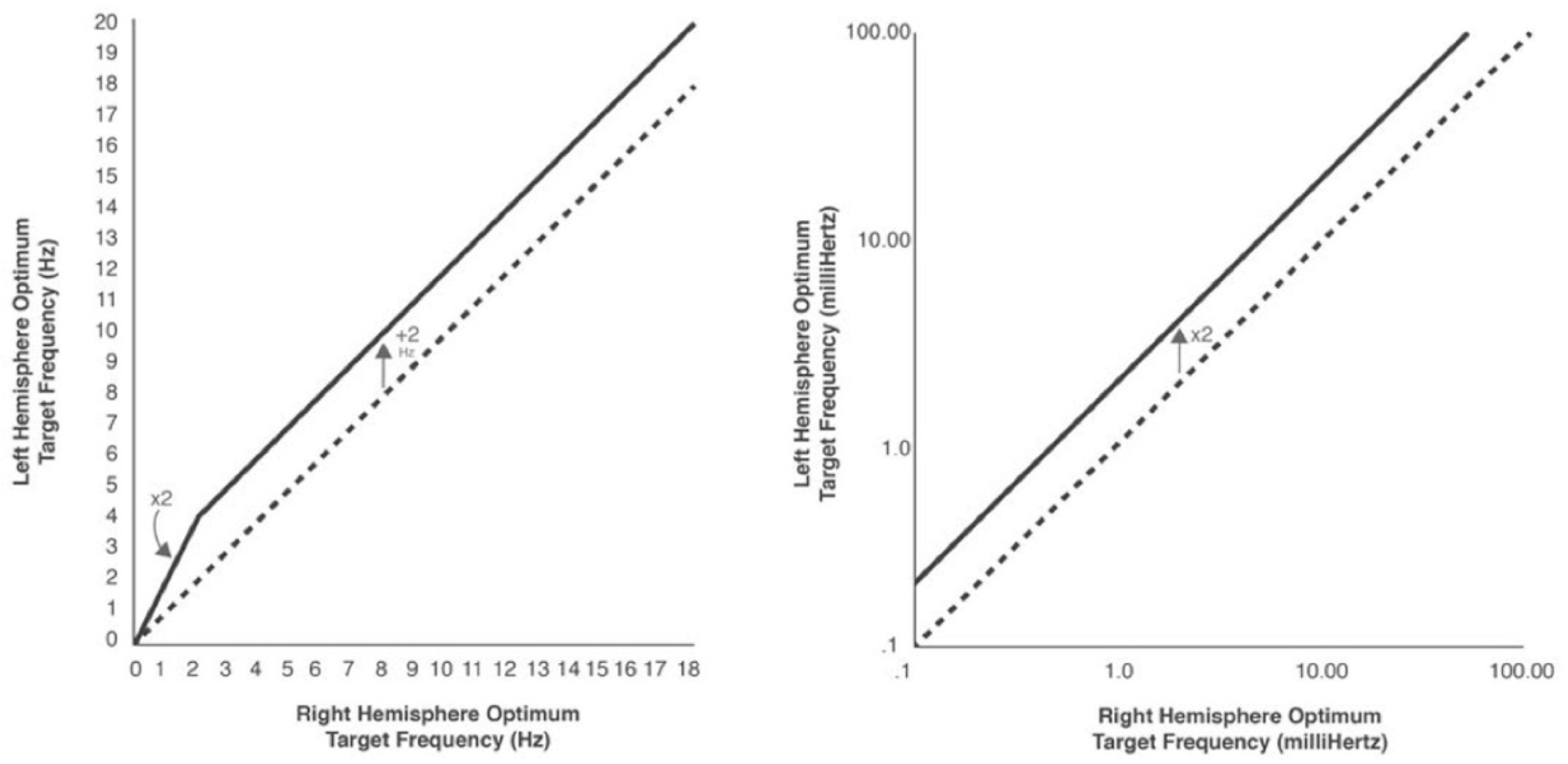
2.8.3. The Frequency Rules
2.9. Mechanisms Implications
2.9. The Neurofeedback Therapist’s ‘Systems Perspective’ and its Foundations
3. Results
3.1. Foundational Research
3.2. Representative Clinical Results
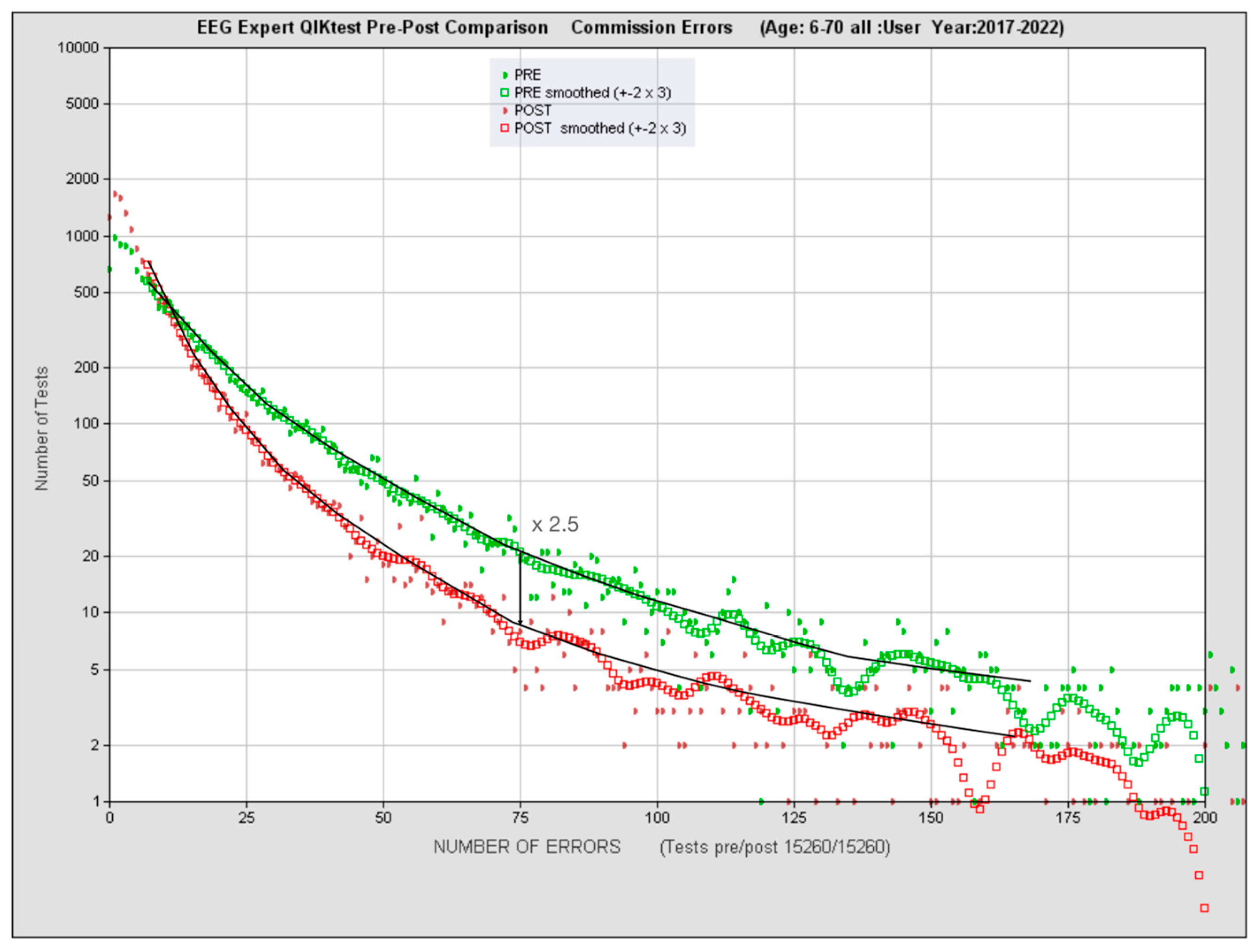
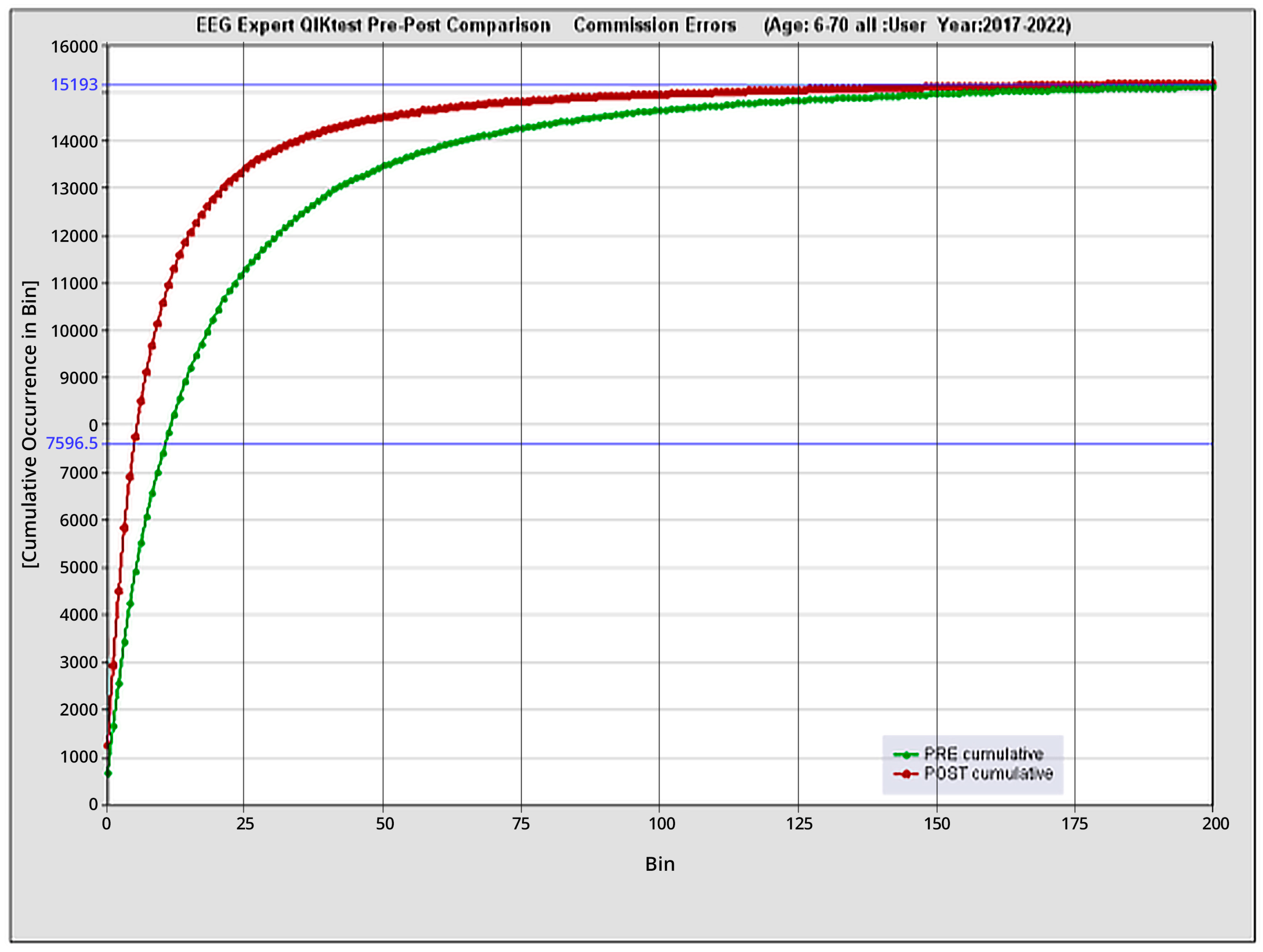
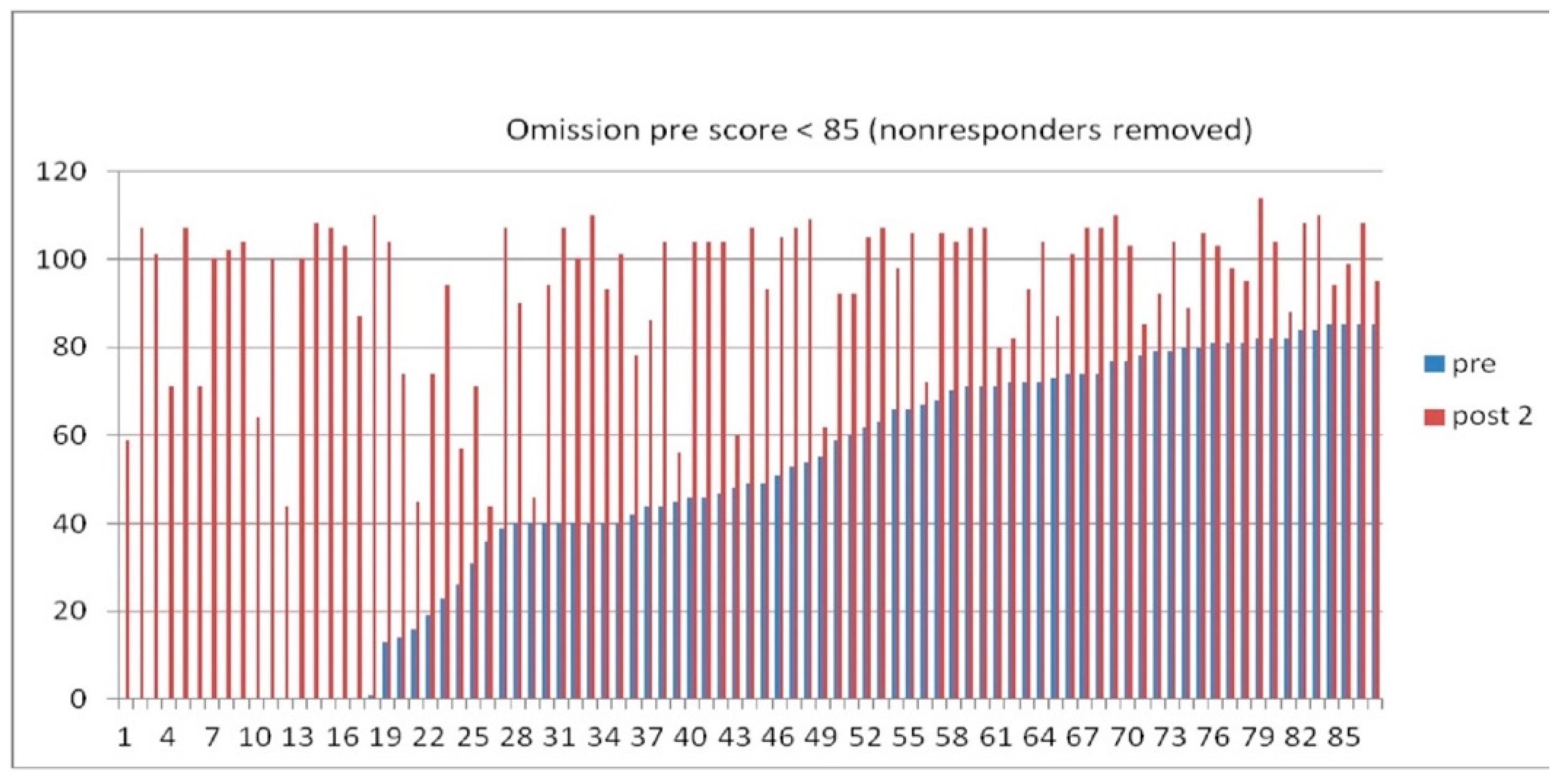
3.3. Quantitative functional appraisal for large clinical population
4. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans J.R. and Turner R., editors (2017). Rhythmic Stimulation Procedures in Neuromodulation, Elsevier, London.
- Sterling, P. Allostasis: A model of predictive regulation. Physiol. Behav. 2012, 106, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.; Friston, K. The free-energy principle: a unified brain theory? Nat. Rev. Neurosci. 2010, 11, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Freeman, W.J. Definitions of state variables and state space for brain-computer interface. Cogn. Neurodynamics 2006, 1, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Bagdasaryan, J.; Le Van Quyen, M. Experiencing your brain: neurofeedback as a new bridge between neuroscience and phenomenology. Front. Hum. Neurosci. 2013, 7, 680. [Google Scholar] [CrossRef] [PubMed]
- Wyrwicka, W.; Sterman, M.B. Instrumental conditioning of sensorimotor cortex EEG spindles in the waking cat. Physiol. Behav. 1968, 3, 703–707. [Google Scholar] [CrossRef]
- Sterman, M.B.; Howe, R.C.; Macdonald, L.R. Facilitation of Spindle-Burst Sleep by Conditioning of Electroencephalographic Activity While Awake. Science 1970, 167, 1146–1148. [Google Scholar] [CrossRef]
- Sterman, M. Effects of brain surgery and EEG operant conditioning on seizure latency following monomethylhydrazine intoxication in the cat. Exp. Neurol. 1976, 50, 757–765. [Google Scholar] [CrossRef]
- Lubar, J.F.; Shabsin, H.S.; Natelson, S.E.; Holder, G.S.; Whitsett, S.F.; Pamplin, W.E.; Krulikowski, D.I. EEG Operant Conditioning in Intractable Epileptics. Arch. Neurol. 1981, 38, 700–704. [Google Scholar] [CrossRef]
- Lantz, D.; Sterman, M.B. Neuropsychological assessment of subjects with uncontrolled epilepsy: effects of EEG biofeedback training. Epilepsia 1988, 29, 63–171. [Google Scholar] [CrossRef] [PubMed]
- Sterman, M.B. Basic Concepts and Clinical Findings in the Treatment of Seizure Disorders with EEG Operant Conditioning. Clin. Electroencephalogr. 2000, 31, 45–55. [Google Scholar] [CrossRef]
- Hook, E.B., editor (2002) Prematurity in Scientific Discovery: On Resistance and Neglect, U. California Press.
- Rockstroh B, Elbert T, Canavan AG, Lutzenberger W, Birbaumer N. 1989. Slow Cortical Potentials and Behavior, Second ed. Baltimore: Urban & Schwarzenberg.
- Birbaumer, N.; Elbert, T.; Canavan, A.G.; Rockstroh, B.; Postorino, M.; May, E.S.; Nickel, M.M.; Tiemann, L.; Ploner, M.; Ramos-Murguialday, A.; et al. Slow potentials of the cerebral cortex and behavior. Physiol. Rev. 1990, 70, 1–41. [Google Scholar] [CrossRef]
- Birbaumer, N. slow Cortical Potentials: Plasticity, Operant Control, and Behavioral Effects. Neurosci. 1999, 5, 74–78. [Google Scholar] [CrossRef]
- Strehl, U. Slow Cortical Potentials Neurofeedback. J. Neurother. 2009, 13, 117–126. [Google Scholar] [CrossRef]
- Rockstroh, B.; Elbert, T.; Birbaumer, N.; Wolf, P.; Düchting-Röth, A.; Reker, M.; Daum, I.; Lutzenberger, W.; Dichgans, J. Cortical self-regulation in patients with epilepsies. Epilepsy Res. 1993, 14, 63–72. [Google Scholar] [CrossRef]
- Kotchoubey, B.; Strehl, U.; Uhlmann, C.; Holzapfel, S.; König, M.; Fröscher, W.; Blankenhorn, V.; Birbaumer, N. Modification of Slow Cortical Potentials in Patients with Refractory Epilepsy: A Controlled Outcome Study. Epilepsia 2001, 42, 406–416. [Google Scholar] [CrossRef]
- Siniatchkin, M.; Hierundar, A.; Kropp, P.; Kuhnert, R.; Gerber, W.-D.; Stephani, U. Self-regulation of slow cortical potentials in children with migraine: an exploratory study. Appl. Psychophysiol. Biofeedback 2000, 25, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Schneider, F.; Rockstroh, B.; Heimann, H.; Lutzenberger, W.; Mattes, R.; Elbert, T.; Birbaumer, N.; Bartels, M. Self-regulation of slow cortical potentials in psychiatric patients: Schizophrenia. Biofeedback Self-Regul. 1992, 17, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Gruzelier, J.; Hardman, E.; Wild, J.; Zaman, R.; Nagy, A.; Hirsch, S. Learned control of interhemispheric slow potential negativity in schizophrenia. Int. J. Psychophysiol. 1999, 34, 341–348. [Google Scholar] [CrossRef]
- Strehl, U.; Leins, U.; Goth, G.; Klinger, C.; Hinterberger, T.; Birbaumer, N. Self-regulation of slow cortical potentials—A new treatment for children with ADHD. Pediatrics 2006, 118, 1530–1540. [Google Scholar] [CrossRef]
- Plenz D, and Niebur E (2014). Criticality in Neural Systems, Wiley-VCH, Weinheim, Germany.
- Beggs, J.M.; Plenz, D. Neuronal avalanches in neocortical circuits. J. Neurosci. 2003, 23, 11167–11177. [Google Scholar] [CrossRef]
- Kitzbichler, M.G.; Smith, M.L.; Christensen, S.R.; Bullmore, E. Broadband Criticality of Human Brain Network Synchronization. PLOS Comput. Biol. 2009, 5, e1000314. [Google Scholar] [CrossRef] [PubMed]
- He, B.J.; Zempel, J.M.; Snyder, A.Z.; Raichle, M.E. The Temporal Structures and Functional Significance of Scale-free Brain Activity. Neuron 2010, 66, 353–369. [Google Scholar] [CrossRef]
- Buzsáki, G.; Watson, B.O. Brain rhythms and neural syntax: implications for efficient coding of cognitive content and neuropsychiatric disease. Dialog- Clin. Neurosci. 2012, 14, 345–367. [Google Scholar] [CrossRef] [PubMed]
- Monto, S.; Palva, S.; Voipio, J.; Palva, J.M. Very Slow EEG Fluctuations Predict the Dynamics of Stimulus Detection and Oscillation Amplitudes in Humans. J. Neurosci. 2008, 28, 8268–8272. [Google Scholar] [CrossRef]
- Thibault, R.T.; Lifshitz, M.; Raz, A. The climate of neurofeedback: scientific rigour and the perils of ideology. Brain 2017, 141, e11–e11. [Google Scholar] [CrossRef]
- Sterman, M.; Friar, L. Suppression of seizures in an epileptic following sensorimotor EEG feedback training. Electroencephalogr. Clin. Neurophysiol. 1972, 33, 89–95. [Google Scholar] [CrossRef]
- Tansey, M.A. EEG sensorimotor rhythm biofeedback training: Some effects on the neurologic precursors of learning disabilities. Int. J. Psychophysiol. 1984, 1, 163–177. [Google Scholar] [CrossRef]
- Lubar, J.F. Neocortical dynamics: implications for understanding the role of neurofeedback and related techniques for the enhancement of attention. Appl. Psychophysiol. Biofeedback 1997, 22, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, D.A.; Othmer, S. Effect of Neurofeedback on Variables of Attention in a Large Multi-Center Trial. J. Neurother. 2000, 4, 5–15. [Google Scholar] [CrossRef]
- Egner, T.; Gruzelier, J.H. Learned self-regulation of EEG frequency components affects attention and event-related brain potentials in humans. NeuroReport 2001, 12, 4155–4159. [Google Scholar] [CrossRef]
- Gruzelier, J. Critical validation studies of neurofeedback. Child Adolesc. Psychiatr. Clin. North Am. 2005, 14, 83–104. [Google Scholar] [CrossRef]
- Gruzelier, J.; Foks, M.; Steffert, T.; Chen, M.-L.; Ros, T. Beneficial outcome from EEG-neurofeedback on creative music performance, attention and well-being in school children. Biol. Psychol. 2014, 95, 86–95. [Google Scholar] [CrossRef]
- Scott, W.C.; Kaiser, D.; Othmer, S.; Sideroff, S.I. Effects of an EEG Biofeedback Protocol on a Mixed Substance Abusing Population. Am. J. Drug Alcohol Abus. 2005, 31, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.; Birbaumer, N.; Lutzenberger, W.; Gruzelier, J.H.; Kaiser, J. Neurofeedback Treatment for Attention-Deficit/Hyperactivity Disorder in Children: A Comparison with Methylphenidate. Appl. Psychophysiol. Biofeedback 2003, 28, 1–12. [Google Scholar] [CrossRef]
- Kaiser, D.A.; Othmer, S. Efficacy of SMR-beta neurofeedback for attentional processes. EEG Spectrum. 1997, 22, 299–312. [Google Scholar]
- Barnea, A.; Rassis, A.; Raz, A.; Othmer, S.; Zaidel, E. Effects of neurofeedback on hemispheric attention networks. Brain Cogn. 2004. [Google Scholar] [CrossRef]
- Othmer, S., Othmer, S.F., and Kaiser, D.A. (1999a). EEG Biofeedback: Training for AD/HD and Related Disruptive Behavior Disorders, In Understanding, Diagnosing, and Treating AD/HD in Children and Adolescents, An Integrative Approach, James A. Incorvaia, Bonnie S. Mark-Goldstein, and Donald Tessmer, editors, Aronson Press, Northvale, NJ, pp.235-296.
- Othmer, S., Othmer, S.F., and Kaiser, D.A. (1999b). EEG Biofeedback: An Emerging Model for Its Global Efficacy, In Introduction to Quantitative EEG and Neurofeedback, James R. Evans and Andrew Abarbanel, editors, Academic Press, San Diego, pp. 243-310.
- Felitti, V.J.; Anda, R.J.; Nordenberg, D.; Williamson, D.F.; Spitz, A.M.; Edwards, V.; Marks, J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. Am. J. Prevent. Med. 2: Am J Prevent Med 14, 1998; 14, 245–258. [Google Scholar]
- Brown, D.W.; Anda, R.F.; Tiemeier, H.; Felitti, V.J.; Edwards, V.J.; Croft, J.B.; Giles, W.H. Adverse Childhood Experiences and the Risk of Premature Mortality. Am. J. Prev. Med. 2009, 37, 389–396. [Google Scholar] [CrossRef]
- Rai, D.; Kosidou, K.; Lundberg, M.; Araya, R.; Lewis, G.; Magnusson, C. Psychological distress and risk of long-term disability: population-based longitudinal study. J. Epidemiology Community Heal. 2011, 66, 586–592. [Google Scholar] [CrossRef]
- Porges, S.W. (2011). The Polyvagal Theory; Neurophysiological Foundations of Emotions, Attachment, Communication, and Self-Regulation, WW Norton, New York.
- van der Kolk, B.A. The Body Keeps the Score: Memory and the Evolving Psychobiology of Posttraumatic Stress. Harv. Rev. Psychiatry 1994, 1, 253–265. [Google Scholar] [CrossRef]
- Webb, R.T.; Pedersen, C.B.; Mok, P.L. Adverse Outcomes to Early Middle Age Linked With Childhood Residential Mobility. Am. J. Prev. Med. 2016, 51, 291–300. [Google Scholar] [CrossRef]
- World Health Organization (2022). ICD 11 for Mortality and Morbidity Statistics: 6B41 Complex posttraumatic stress disorder. Available online at: http://id.who.int/icd/entity/585833559 (accessed August, 2022).
- Herrera-Escobar, J.P.; Schneider, J.C. From Survival to Survivorship — Framing Traumatic Injury as a Chronic Condition. New Engl. J. Med. 2022, 387, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Lipton, M.L.; Kim, N.; Zimmerman, M.E.; Kim, M.; Stewart, W.F.; Branch, C.A.; Lipton, R.B.; Rubin, T.G.; Catenaccio, E.; Fleysher, R.; et al. Soccer Heading Is Associated with White Matter Microstructural and Cognitive Abnormalities. Radiology 2013, 268, 850–857. [Google Scholar] [CrossRef]
- Daneshvar, D.H.; Nair, E.S.; Baucom, Z.H.; Rasch, A.; Abdolmohammadi, B.; Uretsky, M.; Saltiel, N.; Shah, A.; Jarnagin, J.; Baugh, C.M.; et al. Leveraging football accelerometer data to quantify associations between repetitive head impacts and chronic traumatic encephalopathy in males. Nat. Commun. 2023, 14, 1–14. [Google Scholar] [CrossRef]
- Kivimäki, M.; Batty, G.D.; Pentti, J.; Shipley, M.J.; Sipilä, P.; Nyberg, S.T.; Suominen, S.B.; Oksanen, T.; Stenholm, S.; Virtanen, M.; et al. Association between socioeconomic status and the development of mental and physical health conditions in adulthood: a multi-cohort study. Lancet Public Health 2020, 5, e140–e149. [Google Scholar] [CrossRef] [PubMed]
- 54. Parian, M.; Lamberg, P.; Möckel, R.; Rosenkranz, J. Analysis of mineral grades for geometallurgy: Combined element-to-mineral conversion and quantitative X-ray diffraction. Miner. Eng. 2015, 82, 25–35. [Google Scholar] [CrossRef]
- Teicher, M.H.; Samson, J.A.; Cretton, A.; Brown, R.J.; LaFrance, W.C., Jr.; Aybek, S.; Halldorsdottir, T.; Kurtoic, D.; Müller-Myhsok, B.; Binder, E.B.; et al. Childhood Maltreatment and Psychopathology: A Case for Ecophenotypic Variants as Clinically and Neurobiologically Distinct Subtypes. Am. J. Psychiatry 2013, 170, 1114–1133. [Google Scholar] [CrossRef] [PubMed]
- Teicher MHSamson, J.A.; Anderson Carl, M.; Ohashi, K. The effects of childhood maltreatment on brain structure, function, and connectivity. Nat. Rev./Neurosci. 2016, 17, 652–666. [Google Scholar] [CrossRef]
- Teicher, M.H.; Anderson, C.M.; Ohashi, K.; Polcari, A. Childhood Maltreatment: Altered Network Centrality of Cingulate, Precuneus, Temporal Pole and Insula. Biol. Psychiatry 2013, 76, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Othmer, S.; Othmer, S.; Legarda, S.B. Clinical neurofeedback: Training brain behavior. Treat. Strateg. Pediatr. Neurol. Psychiatry 2011, 2, 67–73. [Google Scholar]
- Kirk, H.W.; Dahl, M.G. Infra Low Frequency Neurofeedback Training for Trauma Recovery: A Case Report. Front. Hum. Neurosci. 2022, 16, 905823. [Google Scholar] [CrossRef]
- Spreyermann, R. Case Report: Infra-Low-Frequency Neurofeedback for PTSD: A Therapist's Perspective. Front. Hum. Neurosci. 2022, 16, 893830. [Google Scholar] [CrossRef] [PubMed]
- Sterman, M.B.; Egner, T. Foundation and Practice of Neurofeedback for the Treatment of Epilepsy. Appl. Psychophysiol. Biofeedback 2006, 31, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Egner, T.; Sterman, M.B. Neurofeedback treatment of epilepsy: from basic rationale to practical application. Expert Rev. Neurother. 2006, 6, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.E. QEEG-Guided Neurofeedback for Recurrent Migraine Headaches. Clin. EEG Neurosci. 2011, 42, 59–61. [Google Scholar] [CrossRef]
- Legarda, S.B.; McMahon, D.; Othmer, S.; Othmer, S. Clinical Neurofeedback: Case Studies, Proposed Mechanism, and Implications for Pediatric Neurology Practice. J. Child Neurol. 2011, 26, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Quirk, D.A. Composite Biofeedback Conditioning and Dangerous Offenders: III. J. Neurother. 1995, 1, 44–54. [Google Scholar] [CrossRef]
- Behavioral Electroencephalography (2023). Ulrich, G., Editor, Cambridge Scholars Publishing ISBN (10): 1-5275-9260-X.
- Othmer, S.F., and Othmer, S. (2005) Inter-Hemispheric EEG Training: Clinical Experience and Conceptual Models, Handbook of Neurofeedback, Dynamic and Clinical Applications, Evans, J.R., editor, Haworth Press, New York, p.109-136.
- Putman, J.A.; Othmer, S.F.; Othmer, S.; Pollock, V.E. TOVA Results Following Inter-Hemispheric Bipolar EEG Training. J. Neurother. 2005, 9, 37–52. [Google Scholar] [CrossRef]
- Ross, J.A.; Van Bockstaele, E.J. The Locus Coeruleus- Norepinephrine System in Stress and Arousal: Unraveling Historical, Current, and Future Perspectives. Front. Psychiatry 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Sihn, D.; Kim, S.-P. Brain Infraslow Activity Correlates With Arousal Levels. Front. Neurosci. 2022, 16, 765585. [Google Scholar] [CrossRef]
- Othmer, S.F. (2019). Protocol Guide for Neurofeedback Clinicians, 7th Edition (2019), EEG Info, Los Angeles.
- Benioudakis, E.S.; Kountzaki, S.; Batzou, K.; Markogiannaki, K.; Seliniotaki, T.; Darakis, E.; Saridaki, M.; Vergoti, A.; Nestoros, J.N. Can Neurofeedback Decrease Anxiety and Fear in Cancer Patients? A Case Study. 2016, 25, 59–65. [Google Scholar] [CrossRef]
- Comellas, M.; Pijuan, J.; Nogués, M.; Roca, J. Efficiency analysis of a multiple axle vehicle with hydrostatic transmission overcoming obstacles. Veh. Syst. Dyn. 2018, 56, 55–77. [Google Scholar] [CrossRef]
- Othmer, S., Othmer, S. (2006). Efficacy of neurofeedback for pain management. In: Boswell M, Cole BE, eds. Weiner’s Pain Management: A Practical Guide for Clinicians. 7th ed. CRC Press; 719-739.
- Feynman, R.P. (1972). The Feynman Lectures on Physics, Vol. 1, Addison Wesley Internet-accessible: https://www.feynmanlectures.caltech.edu/I_23.html (accessed 12/27/22).
- Othmer, S. (2009). Neuromodulation Technologies: An Attempt at Classification, Chapter 1 in Introduction to QEEG and Neurofeedback: Advanced Theory and Applications (Second Edition), Thomas Budzynski, T., Evans, J.R., and Andrew Abarbanel, A., Eds, Elsevier, pp. 3-26.
- Fujimoto, K.; Kaneko, K. How fast elements can affect slow dynamics. Phys. D: Nonlinear Phenom. 2003, 180, 1–16. [Google Scholar] [CrossRef]
- Othmer, S.; Othmer, S.F.; Kaiser, D.A.; Putman, J. Endogenous Neuromodulation at Infralow Frequencies. Semin. Pediatr. Neurol. 2013, 20, 246–257. [Google Scholar] [CrossRef]
- Othmer, S.; Othmer, S. Infra-low-frequency neurofeedback for optimum performance. Biofeedback 2016, 44, 81–89. [Google Scholar] [CrossRef]
- Othmer, S. and Othmer, S.F (2017). Development of the Othmer Method of Neurofeedback (PDF). Available online: https://www.researchgate.net/publication/317868978_Development_of_the_Othmer_Method_of_Neurofeedback (accessed on 26 December 2022).
- Chiron, C.; Jambaque, I.; Nabbout, R.; Lounes, R.; Syrota, A.; Dulac, O. The right brain hemisphere is dominant in human infants. Brain 1997, 120, 1057–1065. [Google Scholar] [CrossRef]
- Aladjalova, N.A. (1964). Slow Electrical Processes in the Brain. Elsevier.
- Margulies, D.S.; Ghosh, S.S.; Goulas, A.; Falkiewicz, M.; Huntenburg, J.M.; Langs, G.; Bezgin, G.; Eickhoff, S.B.; Castellanos, F.X.; Petrides, M.; et al. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc. Natl. Acad. Sci. 2016, 113, 12574–12579. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef]
- Lehmann, D.; Ozaki, H.; Pal, I. EEG alpha map series: brain micro-states by space-oriented adaptive segmentation. Electroencephalogr. Clin. Neurophysiol. 1987, 67, 271–288. [Google Scholar] [CrossRef]
- Lehmann, D., Pascual-Marqui, R.D., Milz, P., Kochi, K., Faber, P., Yoshimura, M., Kinoshita, T. (2014). The resting microstate networks (RMN): cortical distributions, dynamics, and frequency specific information flow. Available from: http://arxiv.org/abs/1411.1949. 1949.
- Menon, V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011, 15, 483–506. [Google Scholar] [CrossRef]
- Schore, A.N. Early organization of the nonlinear right brain and development of a predisposition to psychiatric disorders. Dev. Psychopathol. 1997, 9, 595–631. [Google Scholar] [CrossRef]
- Ros, T.; Ros, T.; Munneke, M.A.M.; Munneke, M.A.M.; Ruge, D.; Ruge, D.; Gruzelier, J.H.; Gruzelier, J.H.; Rothwell, J.C.; Rothwell, J.C. Endogenous control of waking brain rhythms induces neuroplasticity in humans. Eur. J. Neurosci. 2010, 31, 770–778. [Google Scholar] [CrossRef]
- Dobrushina, O.R.; Vlasova, R.M.; Rumshiskaya, A.D.; Litvinova, L.D.; Mershina, E.A.; Sinitsyn, V.E.; Pechenkova, E.V. Modulation of Intrinsic Brain Connectivity by Implicit Electroencephalographic Neurofeedback. Front. Hum. Neurosci. 2020, 14, 192. [Google Scholar] [CrossRef]
- Grin-Yatsenko, V.; Othmer, S.; Ponomarev, V.A.; Evdokimov, S.; Konoplev, Y.; Kropotov, J.D. Infra-Low Frequency Neurofeedback in Depression: Three case studies. NeuroRegulation 2018, 5, 30–42. [Google Scholar] [CrossRef]
- Grin-Yatsenko, V.A.; Kara, O.; Evdolimov, S.A.; Gregory, M.; Othmer, S.; Kropotov, J.D. Infra-Low Frequency Neurofeedback Modulates Infra-Slow Oscillations of Brain Potentials: A Controlled Study. J. Biomed. Eng. Res. 2020, 4, 1–10. [Google Scholar]
- Grin-Yatsenko, V.A.; Ponomarev, V.A.; Kropotov, J.D. The Changes of the Infra-Slow EEG Fluctuations of the Brain Potentials under Influence of Infra-Low Frequency Neurofeedback. J. Evol. Biochem. Physiol. 2023, 59, 831–840. [Google Scholar] [CrossRef]
- Sitaram, R.; Ros, T.; Stoeckel, L.; Haller, S.; Scharnowski, F.; Lewis-Peacock, J.; Weiskopf, N.; Blefari, M.L.; Rana, M.; Oblak, E.; et al. Closed-loop brain training: the science of neurofeedback. Nat. Rev. Neurosci. 2016, 18, 86–100. [Google Scholar] [CrossRef]
- Legarda, S.B.; McMahon, D.; Othmer, S.; Othmer, S. Clinical Neurofeedback: Case Studies, Proposed Mechanism, and Implications for Pediatric Neurology Practice. J. Child Neurol. 2011, 26, 1045–1051. [Google Scholar] [CrossRef]
- Bazzana, F.; Finzi, S.; Di Fini, G.; Veglia, F. Infra-Low Frequency Neurofeedback: A Systematic Mixed Studies Review. Front. Hum. Neurosci. 2022, 16, 920659. [Google Scholar] [CrossRef]
- Othmer, S.; Othmer, S.F. Post Traumatic Stress Disorder—The Neurofeedback Remedy. Biofeedback 2009, 37, 24–31. [Google Scholar] [CrossRef]
- Carlson, J.; Ross, G.W. Neurofeedback Impact on Chronic Headache, Sleep, and Attention Disorders Experienced by Veterans with Mild Traumatic Brain Injury: A Pilot Study. Biofeedback 2021, 49, 2–9. [Google Scholar] [CrossRef]
- Gerge, A. A multifaceted case-vignette integrating neurofeedback and EMDR in the treatment of complex PTSD. Eur. J. Trauma Dissociation 2020, 4, 100157. [Google Scholar] [CrossRef]
- Winkeler, A.; Winkeler, M.; Imgart, H. Infra-Low Frequency Neurofeedback in the Treatment of Patients With Chronic Eating Disorder and Comorbid Post-Traumatic Stress Disorder. Front. Hum. Neurosci. 2022, 16, 890682. [Google Scholar] [CrossRef] [PubMed]
- Fleischman, M.J. Documenting the Impact of Infra Low Frequency Neurofeedback on Underserved Populations With Complex Clinical Presentations. Front. Hum. Neurosci. 2022, 16, 921491. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Laugesen, H. Infra-low frequency neurofeedback training in Dravet syndrome: A case study. Epilepsy Behav. Rep. 2023, 22, 100606. [Google Scholar] [CrossRef] [PubMed]
- Tschiesner, R. Infra-Low-Frequency Neurofeedback Treatment in Dysthymia: A Case Study. Behav. Sci. 2023, 13, 711. [Google Scholar] [CrossRef]
- Kirk HW (2015, 2020). Restoring the Brain, Neurofeedback as an Integrative Approach to Health. Taylor and Francis, Boca Raton FL.
- Leark, R. A., Dupuy, M.S., Greenberg, L.M., Corman, C.L. and Kindschi, C.L. (2007). TOVA: Test of Variables of Attention, Professional Guide. Los Alamitos, CA: Universal Attention Disorders Inc.
- Leth-Steensen, C.; Elbaz, Z.K.; Douglas, V.I. Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychol. 2000, 104, 167–190. [Google Scholar] [CrossRef]
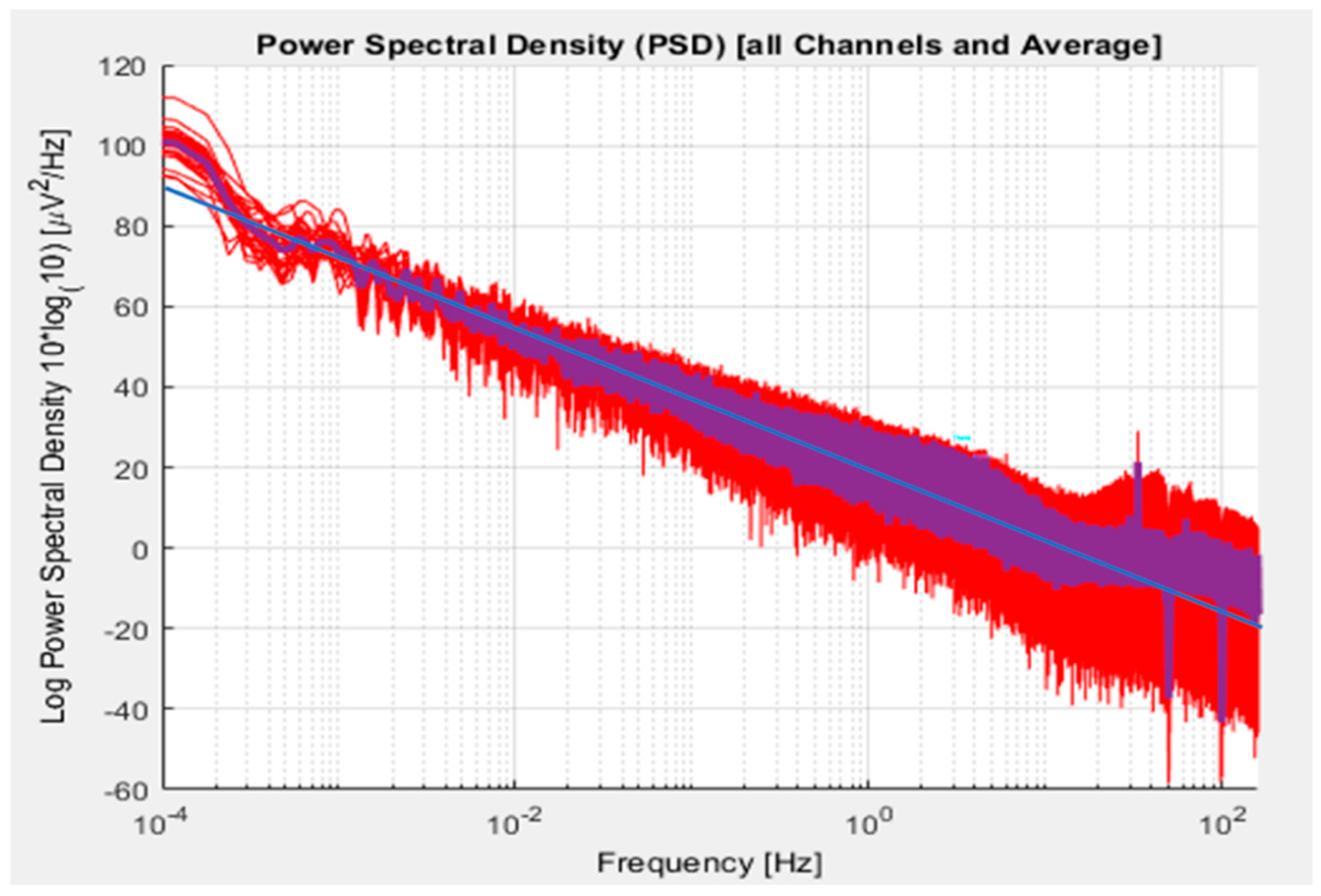
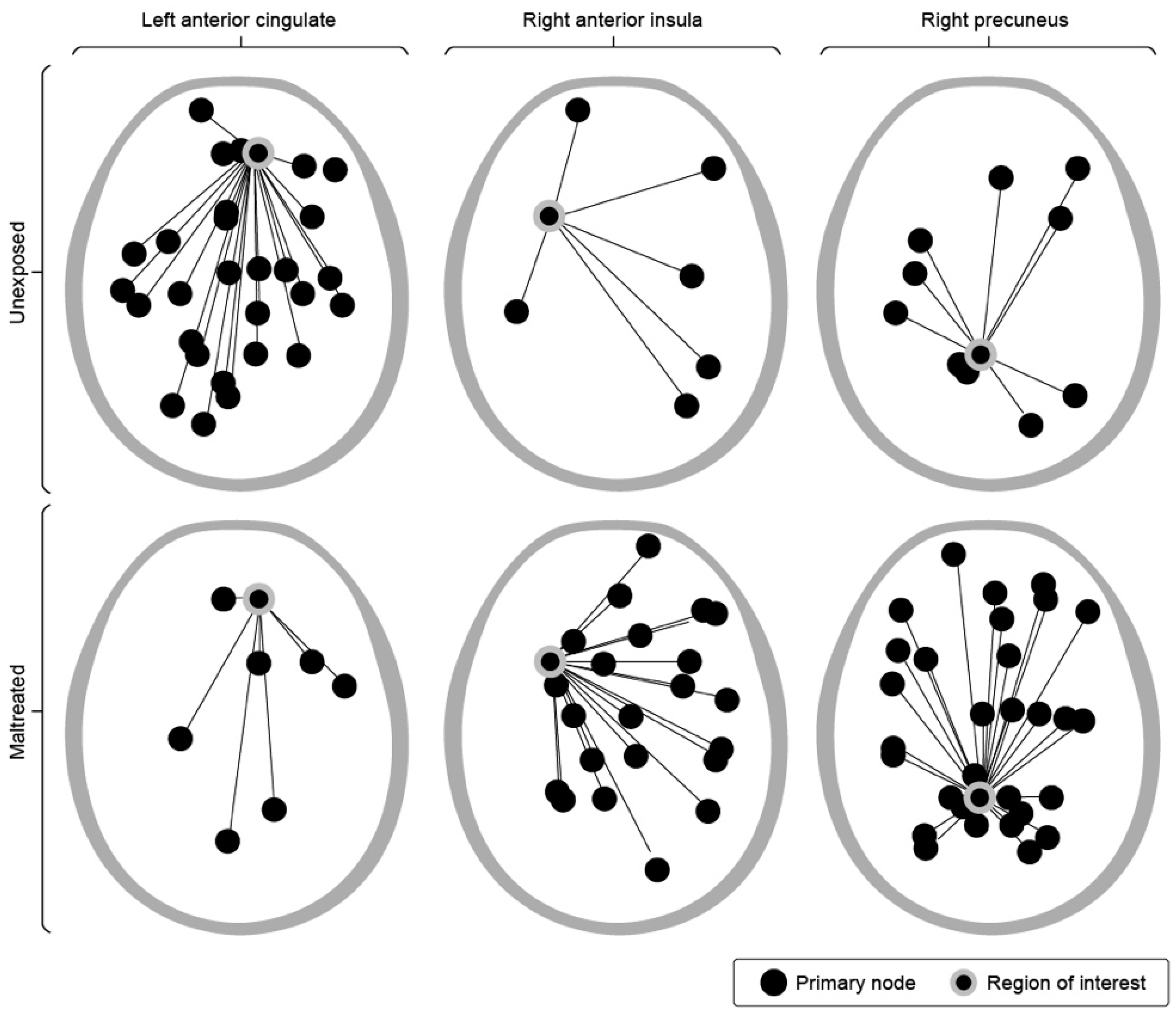
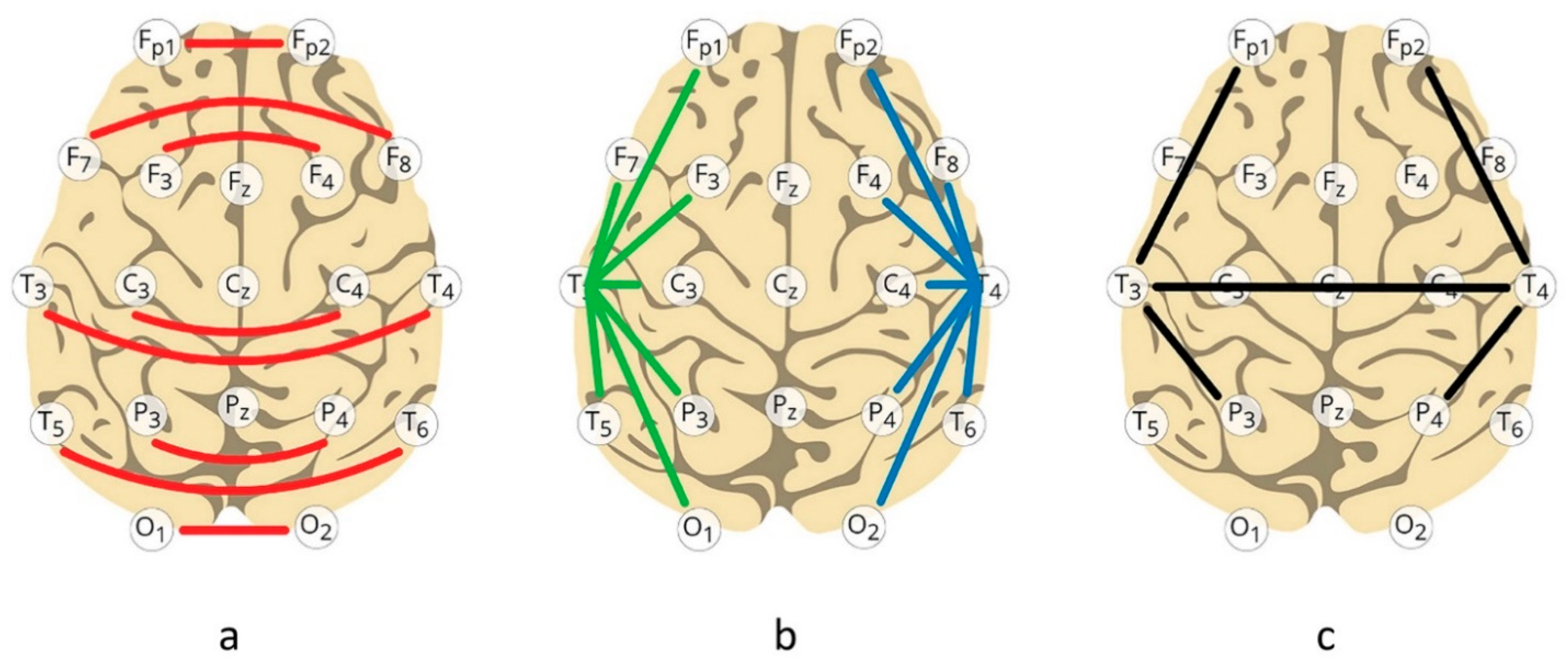
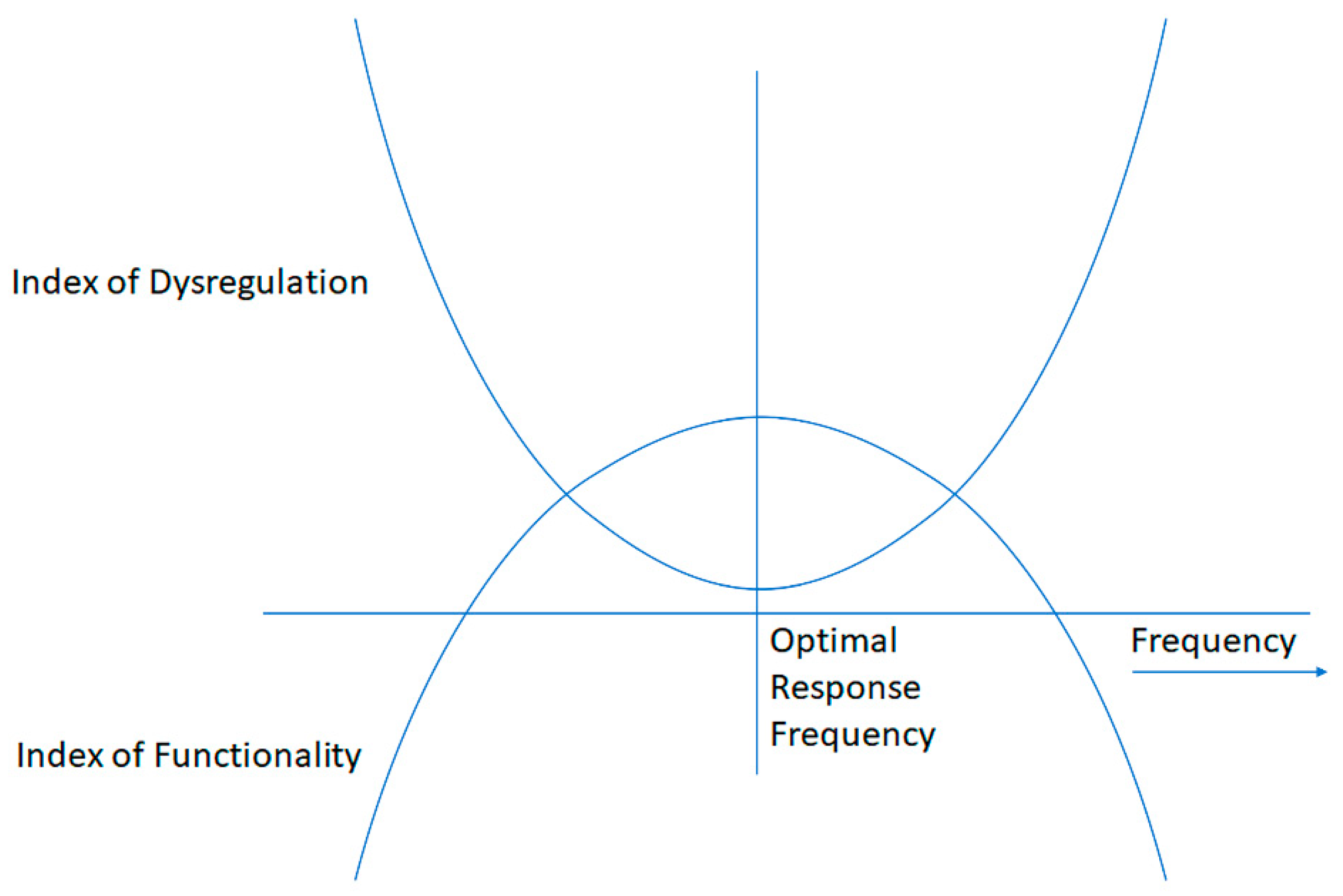
| Age Range | Category | Pre- | Post | Delta |
|---|---|---|---|---|
| 6-9 years, inclusive | Impulse control | 6 | 10 | 4.0 |
| Inattention | 6.2 | 8.5 | 2.3 | |
| Mean reaction time | 7.3 | 8.0 | 0.7 | |
| Variability in reaction time | 6.6 | 7.6 | 1.0 | |
| 10-19 years, inclusive | Impulse control | 9.0 | 17.0 | 8.0 |
| Inattention | 10.3 | 13.8 | 3.5 | |
| Mean reaction time | 12.1 | 12.6 | 0.5 | |
| Variability in reaction time | 10.4 | 11.8 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





