Submitted:
09 October 2023
Posted:
10 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
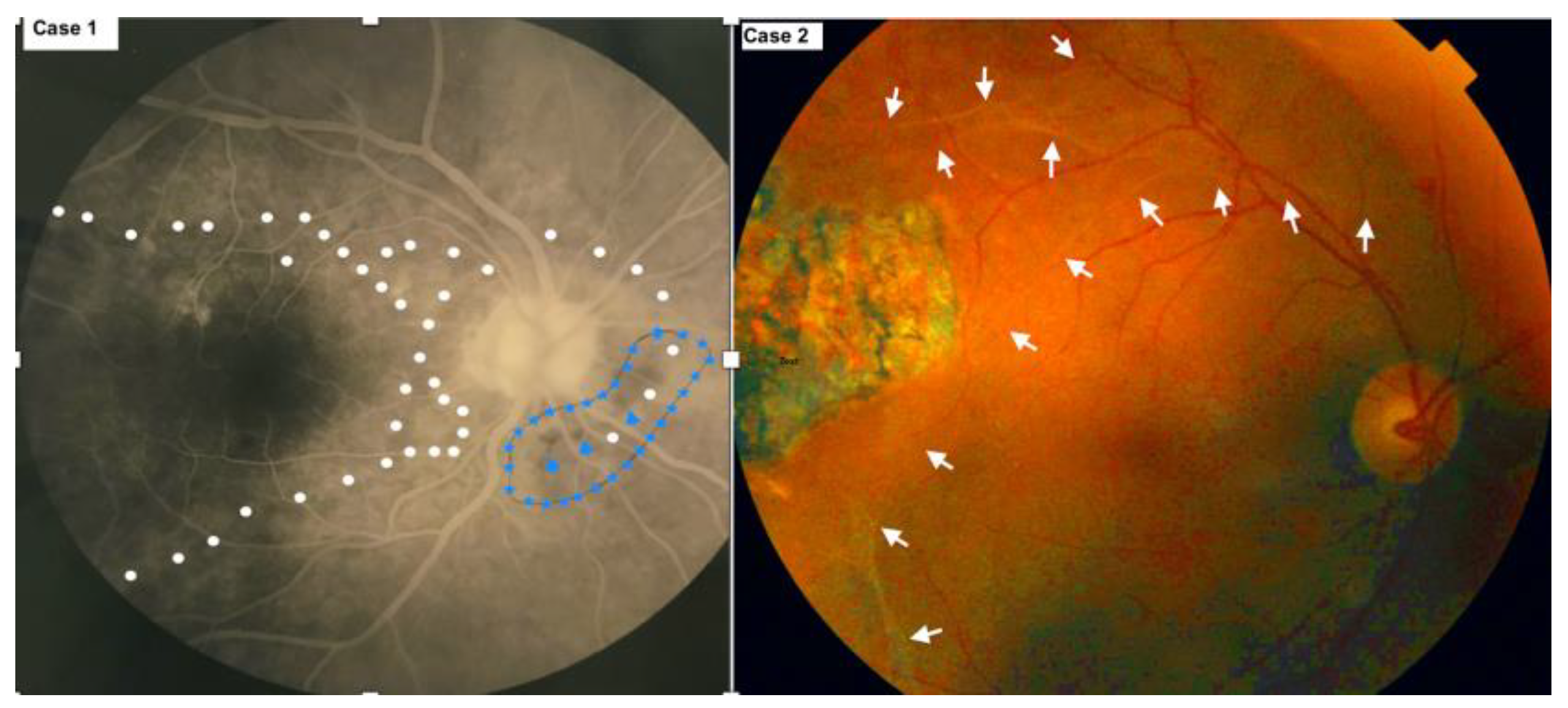
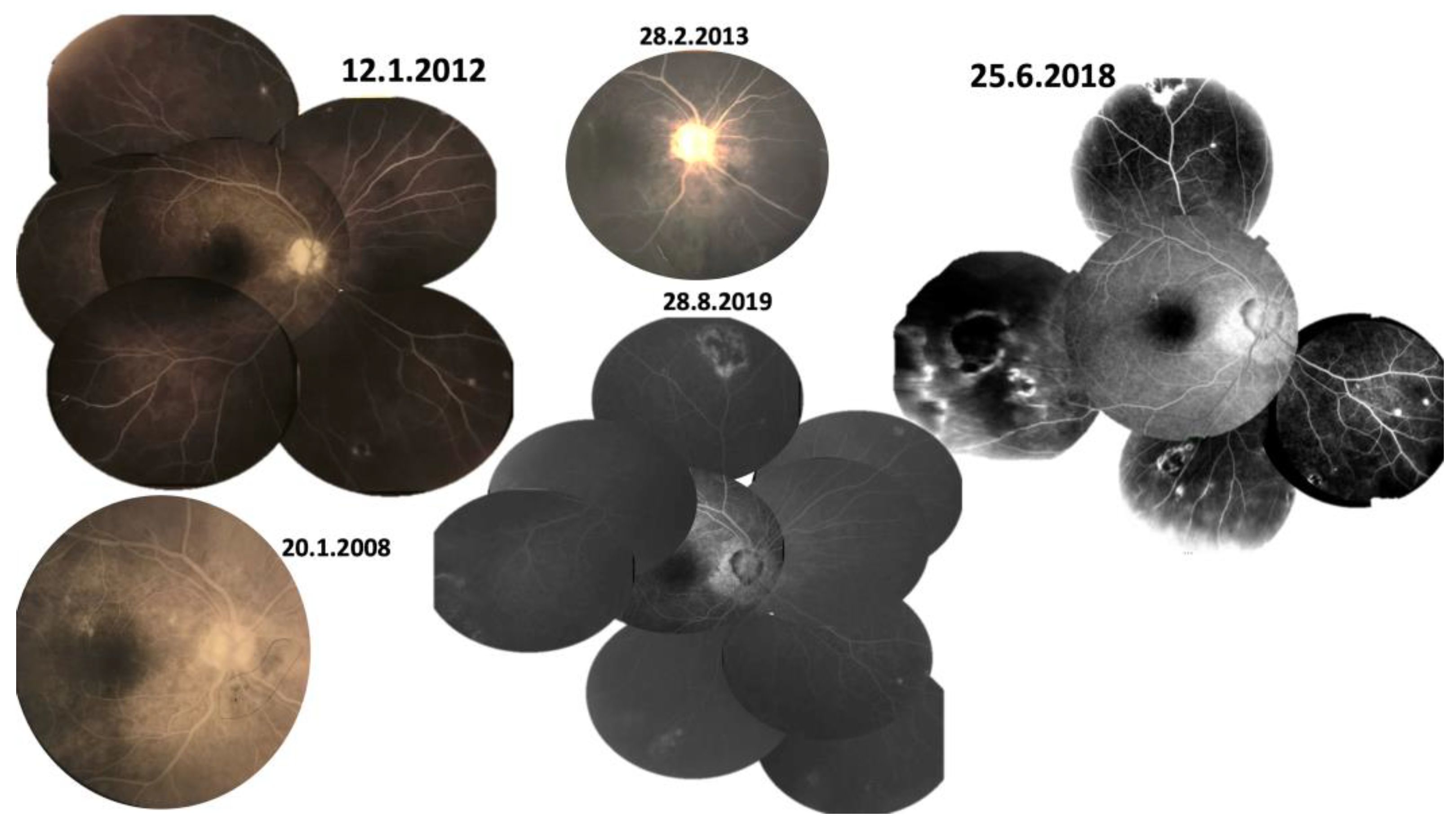
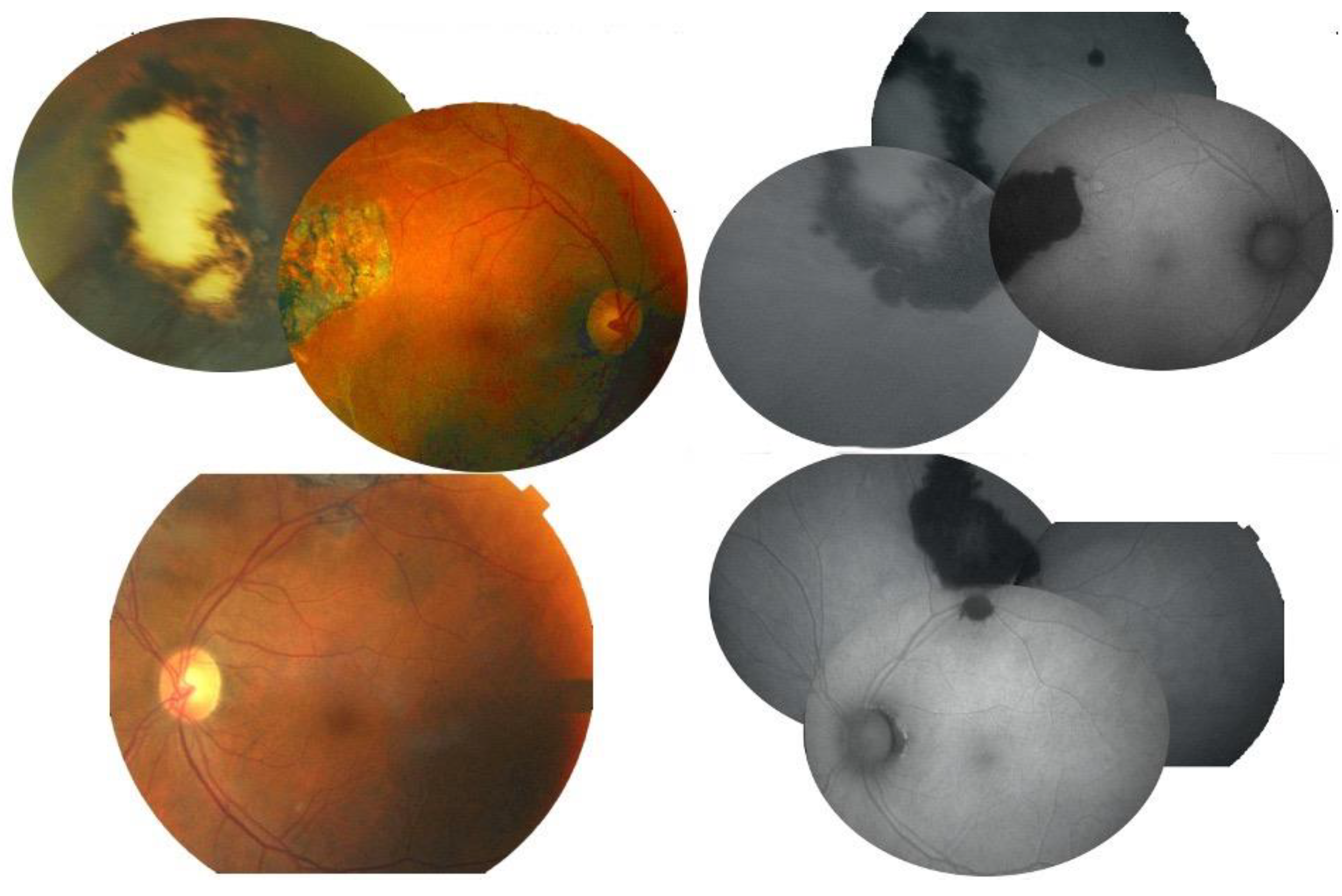
3.1. Case 1
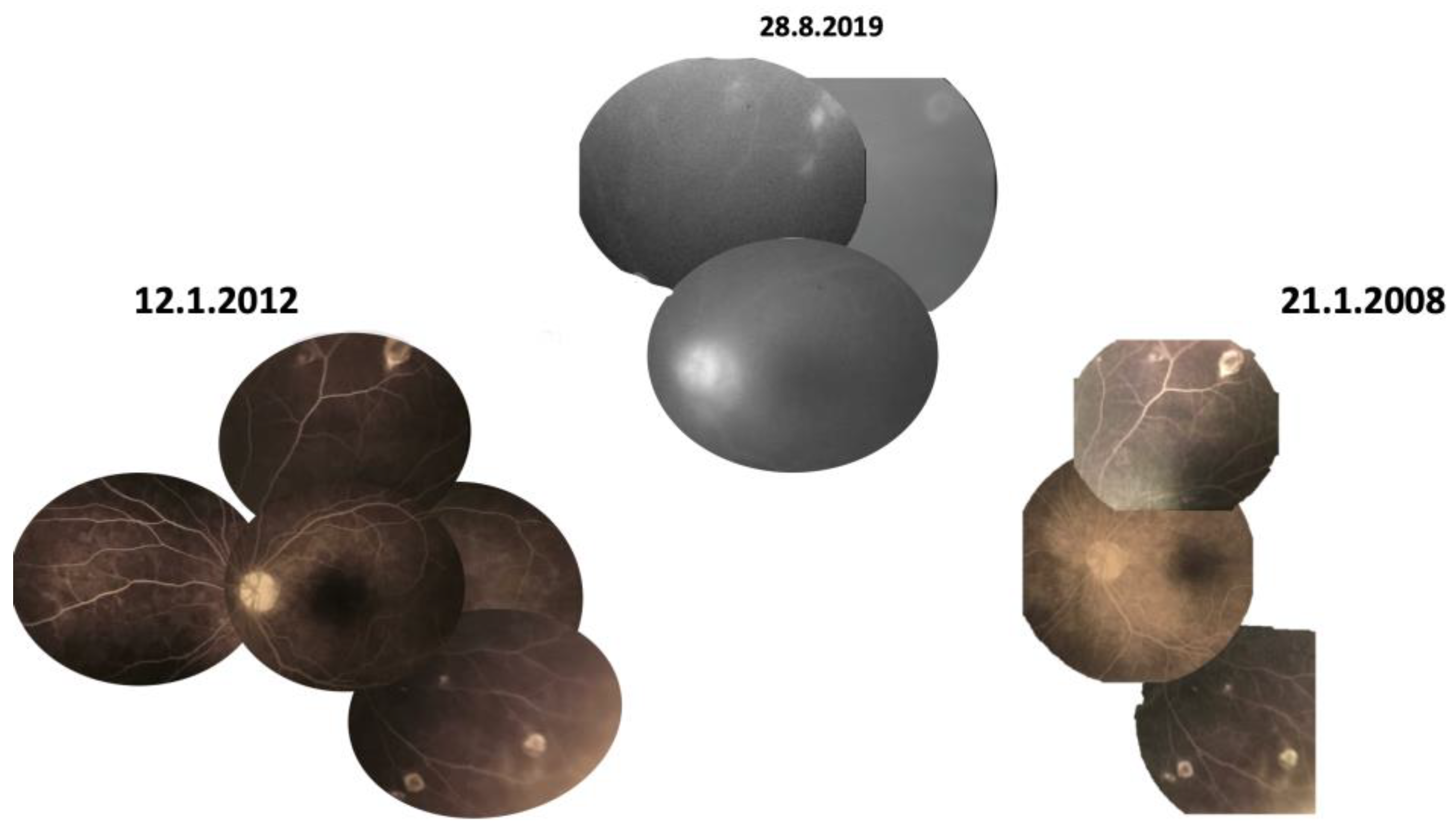
3.2. Case 2
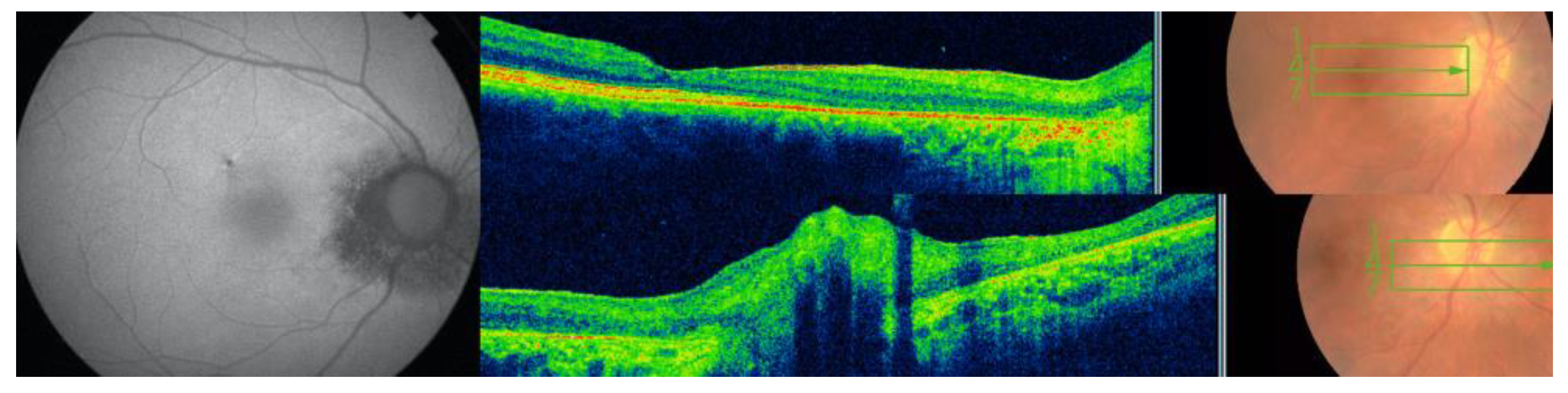
3.3. Case 3
3.4. Case 4
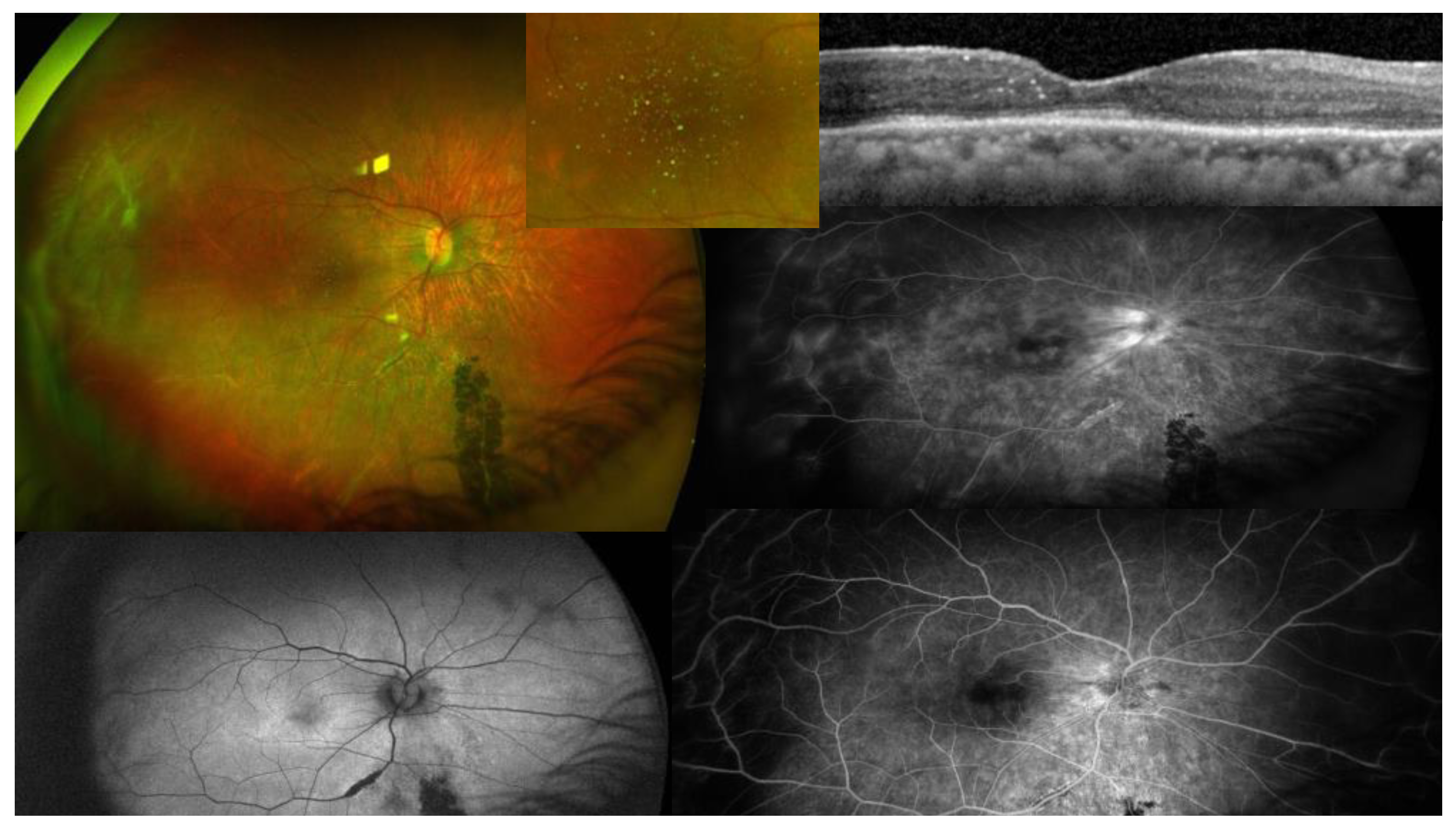
- 1.1.2009 Initial OCT scan: outer retinal thinning left macula with pigment stippling.
- 24.5.2022 Left papillophlebitis 13.5 years after initial presentation.
- 17.7.7.2022 Left papillophlebitis resolved after oral corticosteroids.
- 9.9.2022 Right papillophlebitis 100 days after left papillophlebitis.
- 19.10.2022 Disc edema right eye.
- 29.12.2022 Resolved papillophlebitis after intravitreal bevacizumab and oral corticosteroids. Peripapillary fibrosis is noted superiorly.
- 22.5.2023 last follow-up 14.5 years after presentation revealed bilateral optic atrophy and subtle subretinal tracts (arrows). Peripapillary fibrosis is noted.
Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meribo, K.; Kebede, B.; Feleke, S.M.; Mengistu, B.; Mulugeta, A.; Sileshi, M.; Samuel, A.; Deribe, K.; Tadesse, Z. Review of Ethiopian Onchocerciasis Elimination Programme. Ethiop Med J. 2017, 55, 55–63. [Google Scholar]
- Basánez, M-G. ; Pion, S.D.S.; Churcher, T.S.; Breitling LP, Little MP, Boussinesq M. River Blindness: A Success Story under Threat? PLoS Med. 2006, 3, e37. [Google Scholar]
- Hadermann A, Amaral LJ, Van Cutsem G, Siewe Fodjo JN, Colebunders R. Onchocerciasis-associated epilepsy: an update and future perspectives. Trends Parasitol. 2023, 39, 126–138. [CrossRef]
- Vinkeles Melchers, N.V.S. , Mollenkopf, S., Colebunders, R. et al. Burden of onchocerciasis-associated epilepsy: first estimates and research priorities. Infect Dis Poverty 2018, 7, 101. [Google Scholar] [CrossRef]
- Gebremedhin Gebrezgabiher G, Mekonnen Z, Yewhalaw D, Hailu A. Status of parasitological indicators and morbidity burden of onchocerciasis after years of successive implementation of mass distribution of ivermectin in selected communities of Yeki and Asosa districts, Ethiopia. BMC Public Health 2020, 20, 1233. [Google Scholar]
- McGillan P, Berry NG, Nixon GL, Leung SC, Webborn PJH, Wenlock MC, Kavanagh S, Cassidy A, Clare RH, Cook DA, Johnston KL, Ford L, Ward SA, Taylor MJ, Hong WD, O'Neill PM. Development of Pyrazolopyrimidine Anti-Wolbachia Agents for the Treatment of Filariasis. ACS Med Chem Lett. 2021, 12, 1421–1426. [CrossRef]
- Opoku NO, Bakajika DK, Kanza EM, et al. Single dose moxidectin versus ivermectin for Onchocerca volvulus infection in Ghana, Liberia, and the Democratic Republic of the Congo: A randomized, controlled, double-blind phase 3 trial. Lancet 2018. [CrossRef]
- Boussinesq, M. A new powerful drug to combat river blindness. Lancet 2018. [CrossRef]
- Ekpo UF, Eneanya OA, Nwankwo EN, Soneye IY, Weil GJ, Fischer PU, Nwaorgu OC. Persistence of onchocerciasis in villages in Enugu and Ogun states in Nigeria following many rounds of mass distribution of ivermectin. BMC Infect Dis. 2022, 22, 832. [CrossRef]
- Doğan, N. Globalisation and Ocular Parasitic Infections: A Review of Recent Studies. Turkiye Parazitol Derg 2020, 44, 239–257. [Google Scholar] [CrossRef]
- Olliaro PL, Kuesel AC, Halleux CM, Sullivan M, Reeder JC. Creative use of the priority review voucher by public and not-for-profit actors delivers the first new FDA-approved treatment for river blindness in 20 years. PLoS Negl Trop Dis 2018; 12: e0006837.
- Nicholls RS, Duque S, Olaya LA, López MC, Sánchez SB, Morales AL, et al. Elimination of onchocerciasis from Colombia: first proof of concept of river blindness elimination in the world. Parasit Vectors 2018; 11.
- Colebunders R, Basáñez MG, Siling K, Post RJ, Rotsaert A, Mmbando B, et al. From river blindness control to elimination: bridge over troubled water. Infect Dis Poverty 2018; 7: 21.
- Komlan K, Vossberg PS, Gantin RG, Solim T, Korbmacher F, Banla M, et al. Onchocerca volvulus infection and serological prevalence, ocular onchocerciasis and parasite transmission in northern and central Togo after decades of Simulium damnosum s.l. vector control and mass drug administration of ivermectin. PLoS Negl Trop Dis 2018; 12: e0006312.
- Semba RD, Murphy RP, Newland HS, et al. Longitudinal study of lesions of the posterior segment in onchocerciasis. Ophthalmology. 1990, 97, 1334–41. [CrossRef]
- Newland HS, White AT, Greene BM, Murphy RP, Taylor HR. Ocular manifestations of onchocerciasis in a rain forest area of west Africa. Brit J Ophthalmol. 1991, 75, 163–169. [Google Scholar] [CrossRef]
- Cooper PJ, Proaño R, Beltran C, Anselmi M, Guderian RH. Onchocerciasis in Ecuador: ocular findings in Onchocerca volvulus infected individuals. Brit J Ophthalmol. 1995, 79, 157–162. [Google Scholar] [CrossRef]
- Abiose A, Murdoch I, Babalola O, Cousens S, Liman I, Onyema J, Evans J, Gregory W, Jones B. Distribution and aetiology of blindness and visual impairment in mesoendemic onchocercal communities, Kaduna State, Nigeria. Kaduna Collaboration for Research on Onchocerciasis. Br J Ophthalmol. 1994, 78, 8–13. [Google Scholar] [CrossRef]
- 19. Anderson J, Fuglsang H, Marshall TF. Studies on onchocerciasis in the United Cameroon Republic. III. A four-year follow-up of 6 rain-forest and 6 Sudan-savanna villages. Trans R Soc Trop Med Hyg. 1976;70(5-6):362–373.
- Rolland A, Thylefors B, Pairault C. Evolution sur neuf ans de l'onchocercose oculaire dans une communauté villageoise d'Afrique occidentale. Bull World Health Organ. 1978, 56, 805–810. [Google Scholar]
- Dadzie KY, Remme J, Rolland A, Thylefors B. The effect of 7-8 years of vector control on the evolution of ocular onchocerciasis in West African savanna. Trop Med Parasitol. 1986, 37, 263–270. [Google Scholar]
- Anderson J, Fuglsang H, Marshall TF, Radolowicz A, Vaughan JP. Studies on onchocerciasis in the United Cameroon Republic. IV. A four-year follow-up of six rain-forest and six savanna villages. The incidence of ocular lesions. Trans R Soc Trop Med Hyg. 1978, 72, 513–515. [Google Scholar] [CrossRef]
- Budden, FH. The natural history of ocular onchocerciasis over a period of 14-15 years and the effect on this of a single course of suramin therapy. Trans R Soc Trop Med Hyg. 1976, 70, 484–491. [Google Scholar] [CrossRef]
- Banla M, Tchalim S, Karabou PK, Gantin RG, Agba AI, Kére-Banla A, Helling-Giese G, Heuschkel C, Schulz-Key H, Soboslay PT. Sustainable control of onchocerciasis: ocular pathology in onchocerciasis patients treated annually with ivermectin for 23 years: a cohort study. PLoS One. 2014, 9, e98411.
- Cooper PJ, Proaño R, Beltran C, Anselmi M, Guderian RH. Onchocerciasis in Ecuador: evolution of chorioretinopathy after amocarzine treatment. Br J Ophthalmol. 1996, 80, 337–342. [CrossRef]
- Semba RD, Donnelly JJ, Young E, Green WR, Scott AL, Taylor HR. IV. Chorioretinitis elicited by Onchocerca volvulus microfilariae. Invest Ophthalmol Vis Sci. 1991, 32, 1499–507.
- Paul, E.V. , Zimmerman, LE. Some observations on the ocular pathology of onchocerciasis. Human Pathology 1970, 1, 581–594. [Google Scholar] [CrossRef]
- Rodger, FC. The pathogenesis and pathology of ocular onchocerciasis. Part IV. The pathology. Am J Ophthalmol. 1960, 49, 560–594. [Google Scholar] [CrossRef]
- Bird AC, el-Sheikh H, Anderson J, Fuglsang H. Changes in visual function and in the posterior segment of the eye during treatment of onchocerciasis with diethylcarbamazine citrate. Br J Ophthalmol. 1980, 64, 191–200. [Google Scholar] [CrossRef]
- Neumann E, Gunders AE.Pathogenesis of the posterior segment lesion of ocular onchocerciasis. Am J Ophthalmol. 1973, 75, 82–89. [CrossRef]
- Kayembe DL, Kasonga DL, Kayembe PK, Mwanza JC, Boussinesq M. Profile of eye lesions and vision loss: a cross-sectional study in Lusambo, a forest-savanna area hyperendemic for onchocerciasis in the Democratic Republic of Congo. Trop Med Int Health. 2003, 8, 83–89. [CrossRef]
- Shivalingaiah PR, Veerabhadraiah P, et al. Onchocerciasis in the Orbital Region: An Unexpected Guest From Tropics. Int J Head Neck Surg 2018, 9, 137–139. [CrossRef]
- Cooper PJ, Guderian RH, Proaño R, Taylor DW. The pathogenesis of chorioretinal disease in onchocerciasis. Parasitology Today 1997, 13, 94–98. [CrossRef]
- Thylefors B, Tønjum AM. Visual field defects in onchocerciasis. Br J Ophthalmol. 1978, 62, 462–467. [CrossRef]
- Cousens SN, Yahaya H, Murdoch I, Samaila E, Evans J, Babalola OE, Zakari M, Abiose A, Jones BR. Risk factors for optic nerve disease in communities mesoendemic for savannah onchocerciasis, Kaduna State, Nigeria. Trop Med Int Health. 1997 Jan;2(1):89-98. [CrossRef]
- Abiose A, Jones BR, Cousens SN, Murdoch I, Cassels-Brown A, Babalola OE, Alexander ND, Nuhu I, Evans J, Ibrahim UF, et al. Reduction in incidence of optic nerve disease with annual ivermectin to control onchocerciasis. Lancet. 1993 Jan 16;341(8838):130-4. [CrossRef]
- Jittamala P, Monteiro W, Smit MR, Pedrique B, Specht S, Chaccour CJ, Dard C, Del Giudice P, Khieu V, Maruani A, Failoc-Rojas VE, Sáez-de-Ocariz M, Soriano-Arandes A, Piquero-Casals J, Faisant A, Brenier-Pinchart MP, Wimmersberger D, Coulibaly JT, Keiser J, Boralevi F, Sokana O, Marks M, Engelman D, Romani L, Steer AC, von Seidlein L, White NJ, Harriss E, Stepniewska K, Humphreys GS, Kennon K, Guerin PJ, Kobylinski KC. A systematic review and an individual patient data meta-analysis of ivermectin use in children weighing less than fifteen kilograms: Is it time to reconsider the current contraindication? PLoS Negl Trop Dis. 2021 Mar 17;15(3):e0009144. [CrossRef]
- Bakajika D, Kanza EM, Opoku NO, Howard HM, Mambandu GL, Nyathirombo A, Nigo MM, Kennedy KK, Masembe SL, Mumbere M, Kataliko K, Bolay KM, Attah SK, Olipoh G, Asare S, Vaillant M, Halleux CM, Kuesel AC. Effect of a single dose of 8 mg moxidectin or 150 μg/kg ivermectin on O. volvulus skin microfilariae in a randomized trial: Differences between areas in the Democratic Republic of the Congo, Liberia and Ghana and impact of intensity of infection. PLoS Negl Trop Dis. 2022 Apr 27;16(4):e0010079. [CrossRef]
- Kura K, Milton P, Hamley JID, Walker M, Bakajika DK, Kanza EM, Opoku NO, Howard H, Nigo MM, Asare S, Olipoh G, Attah SK, Mambandu GL, Kennedy KK, Kataliko K, Mumbere M, Halleux CM, Hopkins A, Kuesel AC, Kinrade S, Basáñez MG. Can mass drug administration of moxidectin accelerate onchocerciasis elimination in Africa? Philos Trans R Soc Lond B Biol Sci. 2023 Oct 9;378(1887):20220277. [CrossRef]
- Chan CC, Nussenblatt RB, Kim MK, Palestine AG, Awadzi K, Ottesen EA. Immunopathology of ocular onchocerciasis. 2. Anti-retinal autoantibodies in serum and ocular fluids. Ophthalmology. 1987, 94, 439–43. [CrossRef]
- Van der Lelij A, Rothova A, Stilma JS, Hoekzema R, Kijlstra A. Cell-mediated immunity against human retinal extract, S-antigen, and interphotoreceptor retinoid binding protein in onchocercal chorioretinopathy. Invest Ophthalmol Vis Sci. 1990, 31, 2031–6.
- Braun G, McKechnie NM, Connor V, Gilbert CE, Engelbrecht F, Whitworth JA, Taylor DW. Immunological crossreactivity between a cloned antigen of Onchocerca volvulus and a component of the retinal pigment epithelium. J Exp Med. 1991, 174, 169–177. [CrossRef]
- McGillan P, Berry NG, Nixon GL, Leung SC, Webborn PJH, Wenlock MC, Kavanagh S, Cassidy A, Clare RH, Cook DA, Johnston KL, Ford L, Ward SA, Taylor MJ, Hong WD, O'Neill PM. Development of Pyrazolopyrimidine Anti-Wolbachia Agents for the Treatment of Filariasis. ACS Med Chem Lett. 2021, 12, 1421–1426. [CrossRef]
- Saint André AV, Blackwell NM, Hall LR, Hoerauf A, Brattig NW, Volkmann L, Taylor MJ, Ford L, Hise AG, Lass JH, Diaconu E, Pearlman E. The role of endosymbiotic Wolbachia bacteria in the pathogenesis of river blindness. Science. 2002, 295, 1892–1895.
- Semba RD, Donnelly JJ, Rockey JH, Lok JB, Sakla AA, Taylor HR. Experimental ocular onchocerciasis in cynomolgus monkeys. II. Chorioretinitis elicited by intravitreal Onchocerca lienalis microfilariae. Invest Ophthalmol Vis Sci. 1988, 29, 1642–1651.
- Gyasi ME, Okonkwo ON, Tripathy K. Onchocerciasis. 2023 Feb 22. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. [PubMed]
- Cho YJ, Lee DH, Kang HM, Kim M, Koh HJ. Reversal of early central retinal vein occlusion by alleviating optic nerve edema with an intravitreal dexamethasone implant. Korean J Ophthalmol. 2014, 28, 192–193. [CrossRef]
- Murdoch I, Abiose A, Babalola O et al. Ivermectin and onchocercal optic neuritis: Short-term effects. Eye 1994, 8, 456–461. [CrossRef]
- Showler AJ, Nutman TB. Imported onchocerciasis in migrants and travelers. Curr Opin Infect Dis. 2018, 31, 393–398. [CrossRef]
- Schwartz RA, Al-Qubati Y, Zieleniewski Ł, Shah R, Kapila R. Onchocerciasis (river blindness): larva-induced eczema (onchodermatitis) from an important oculocutaneous tropical disease spilling over into North America and Europe. Int J Dermatol. 2020, 59, 1065–1070. [CrossRef]
- 51. Aziz MA, Diallo S, Diop IM, Lariviere M, Porta M. Efficacy and tolerance of ivermectin in human onchocerciasis. Lancet, 1982; 2: 171–173.
- Brattig, N.W.; Cheke, R.A.; Garms, R. Onchocerciasis (river blindness) – more than a century of research and control. Acta Tropica 2021, 218, 105677. [Google Scholar] [CrossRef]
- Korten, S.; Badusche, M.; Büttner, D.W.; et al. Natural death of adult Onchocerca volvulus and filaricidal effects of doxycycline induce local FOXP3+/CD4+ regulatory T cells and granzyme expression. Microbes Infect. 2008, 10, 313–324. [Google Scholar] [CrossRef]
- Mackenzie, C.D. A Much-Needed Advance in the Diagnosis of River Blindness. J Infect Dis. 2020, 221, 1746–1748. [Google Scholar] [CrossRef]
- Hedtke, S.M.; Choi, Y.J.; Kode, A.; Chalasani, G.C.; Sirwani N, Jada SR, Hotterbeekx A, Mandro M, Siewe Fodjo JN, Amambo GN, Abong RA, Wanji S, Kuesel AC, Colebunders R, Mitreva M, Grant WN. Assessing Onchocerca volvulus Intensity of Infection and Genetic Diversity Using Mitochondrial Genome Sequencing of Single Microfilariae Obtained before and after Ivermectin Treatment. Pathogens. 2023, 12, 971. [Google Scholar] [CrossRef]
- El-Sayed, N.M.; Elmeya Hassan Safar, E.H. Characterization of the parasite-induced lesions in the posterior segment of the eye. Indian J Ophthalmol. 2015, 63, 881–887. [Google Scholar] [CrossRef]
- Pizem, H.; Ben-Arie-Weintrob, Y.; Naaman, E. Neuroretinitis with secondary retinal venous stasis in a patient with Schistosomiasis. Am J Ophthalmol Case Rep. 2022, 25, 101355. [Google Scholar] [CrossRef]
- Agarwal, M.; Jha, V.; Chaudhary, S.P.; Singh, A.K. Multifocal cysticercosis with optical coherence tomography findings in a child. Middle p East Afr J Ophthalmol. 2012, 19, 240–242. [Google Scholar] [CrossRef]
- Ramírez-Soto, M.C.; Tirado-Sánchez. A.; Bonifaz, A. Ocular Sporotrichosis. J Fungi (Basel). 2021, 7, 951. [Google Scholar] [CrossRef]
- Glasgow, B.J. , Brown, H.H., Foos, R.Y. Miliary retinitis in coccidioidomycosis. Am J Ophthalmol. 1987, 104, 24–27. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual authors and contributors and not of MDPI and/or the editors. MDPI and/or the editors disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |

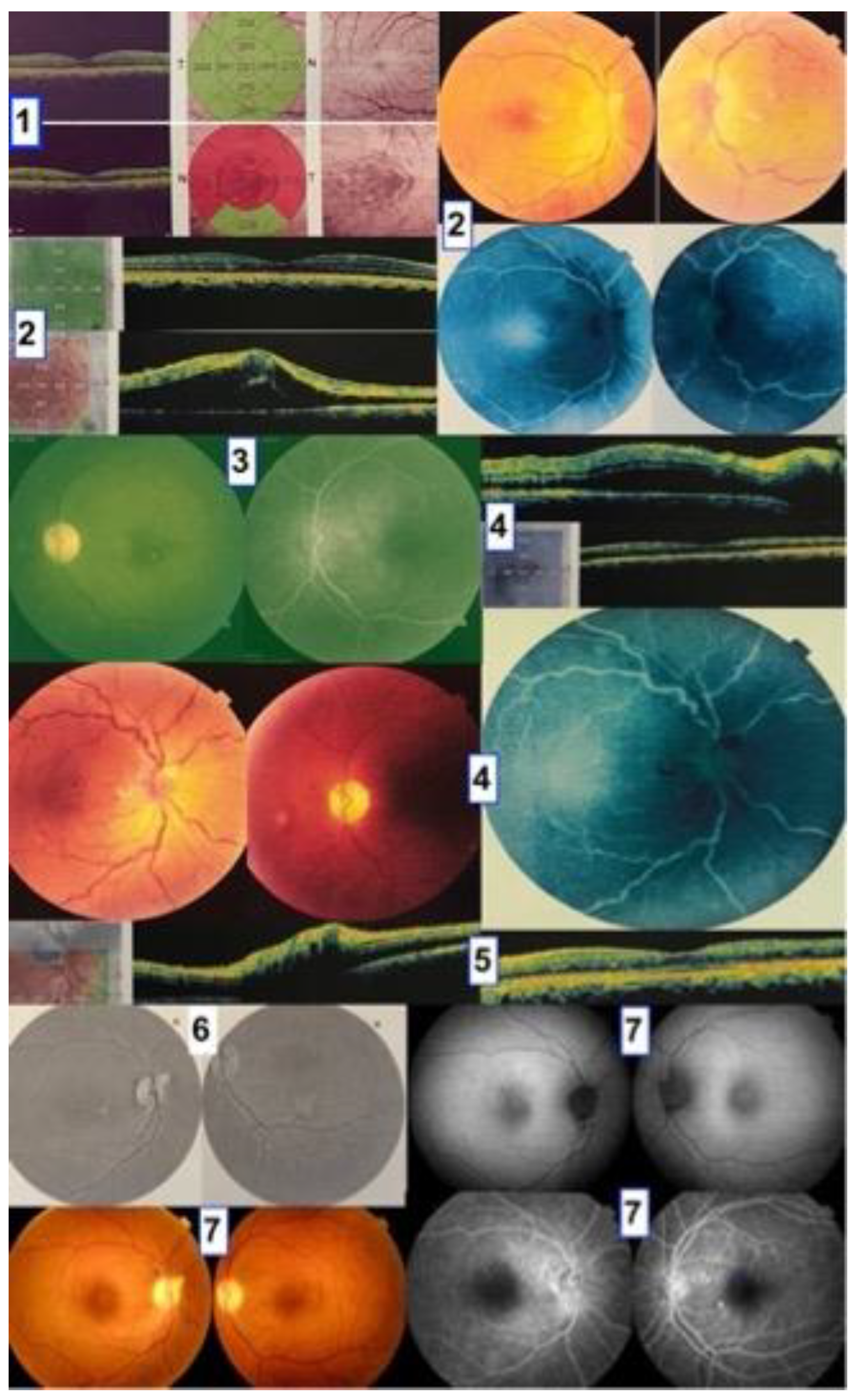
| Site | Findings | Frequency | References |
|---|---|---|---|
| Adnexa | Adnexal nodule | Rare | 32 |
| Conjunctiva | conjunctivitis | common | |
| phlyctenule | common | ||
| microfilaria | common | ||
| Cornea | motile microfilaria | 13.8%-95.7% | 7, 29, 31 |
| punctate keratitis (snowflake) | 13.80% | 16, 31 | |
| sclerosing keratitis | 5% | 16 | |
| neovascularization | rare | ||
| Sclera | microfilaria | rare | 27 |
| Anterior chamber | motile microfilaria | 13,6%-40% | 7, 16 |
| glaucoma | 1.60% | 16 | |
| iridocyclitis | 8% | 31 | |
| Lens | Cataract | infrequent | |
| Vitreous | microfilariae | infrequent | 27 |
| Optic disc | optic neuritis | 1%-5.5% | 16 |
| optic atrophy | 5.1%-57% | 16, 28, 35 | |
| microfilariae | rare | 28 | |
| epipapillary fibrosis | uncommon | 29 | |
| Retina-choroid | chorioretinitis | 38%-75% | 15, 29 |
| peripapillary atrophy | 9%-25% | 28, 29 | |
| mottled fundus | common | 29 | |
| retinal vasculitis | 1% | 16 | |
| retina tracts | 10% | 16 | |
| white intraretinal deposit | 21.40% | 16 | |
| microfilaria | rare | 27 |
| Model | Monkey | Human |
|---|---|---|
| Uveitis | Common | Common |
| RPE changes | ||
| Common | Common | |
| Disc edema/Atrophy | ||
| Common | Common | |
| Retinal vasculitis | Common | Infrequent |
| Venous engorgement | Frequent | Uncommon |
| Retinal hemorrhages | Common | Uncommon |
| Histopathology | Eosinophilic choroiditis | Eosinophilic choroiditis [27, 28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





