Submitted:
09 October 2023
Posted:
09 October 2023
You are already at the latest version
Abstract
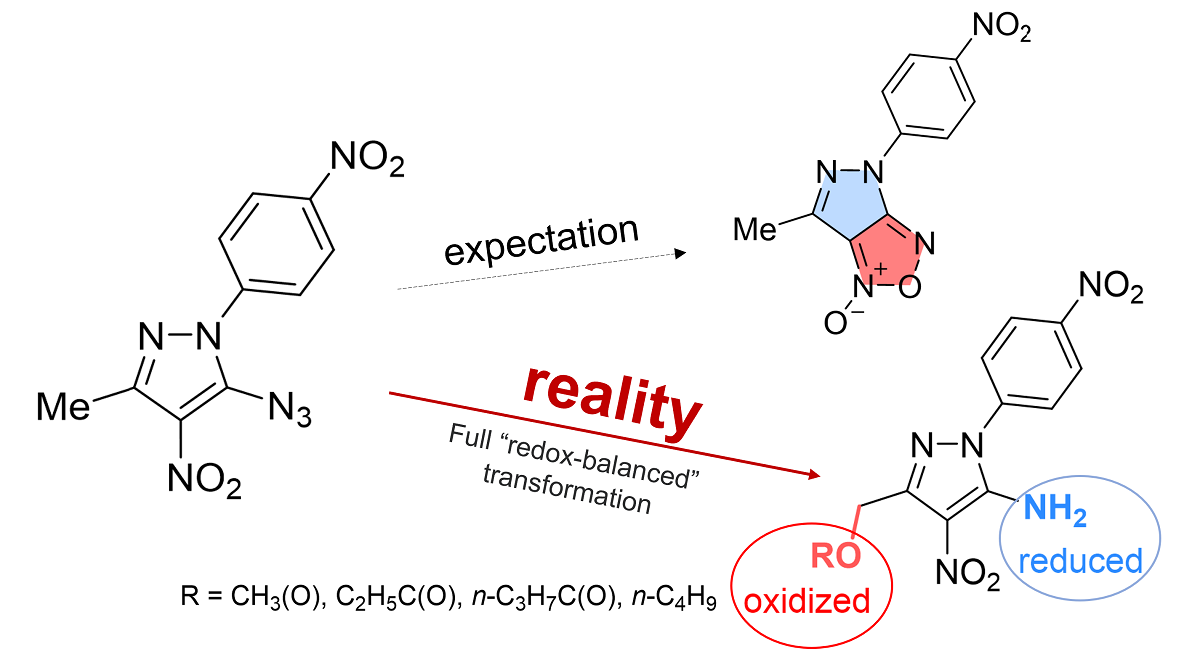
Keywords:
1. Introduction
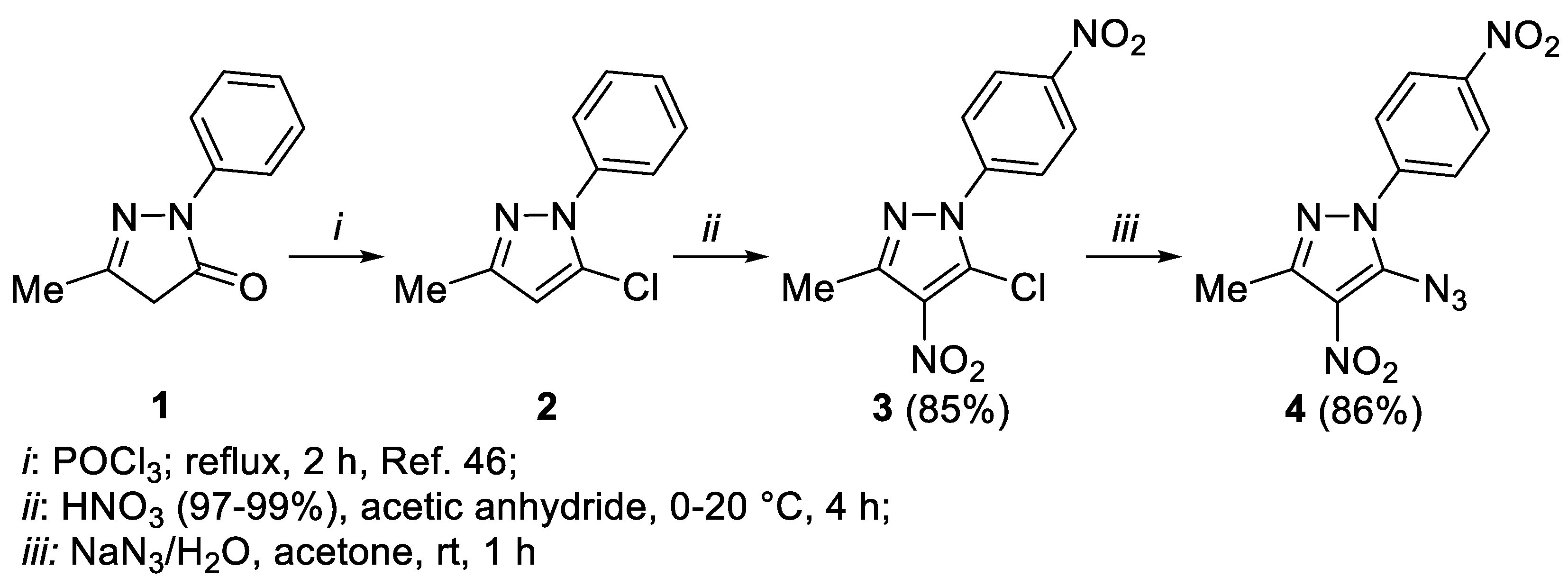
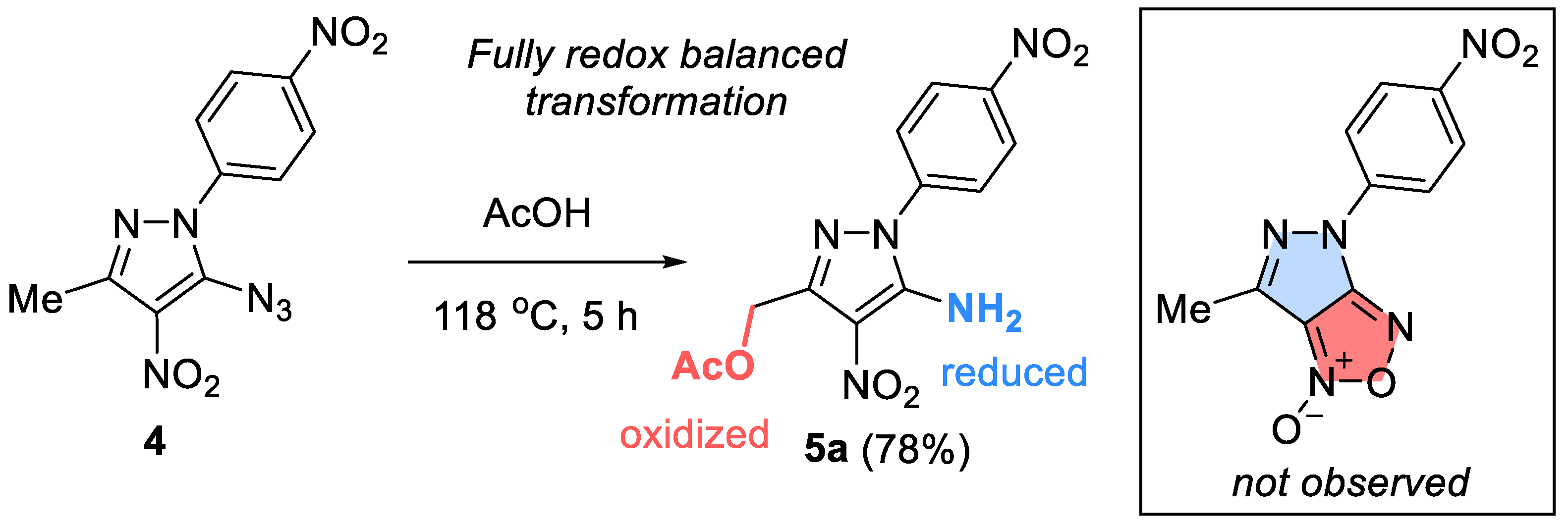
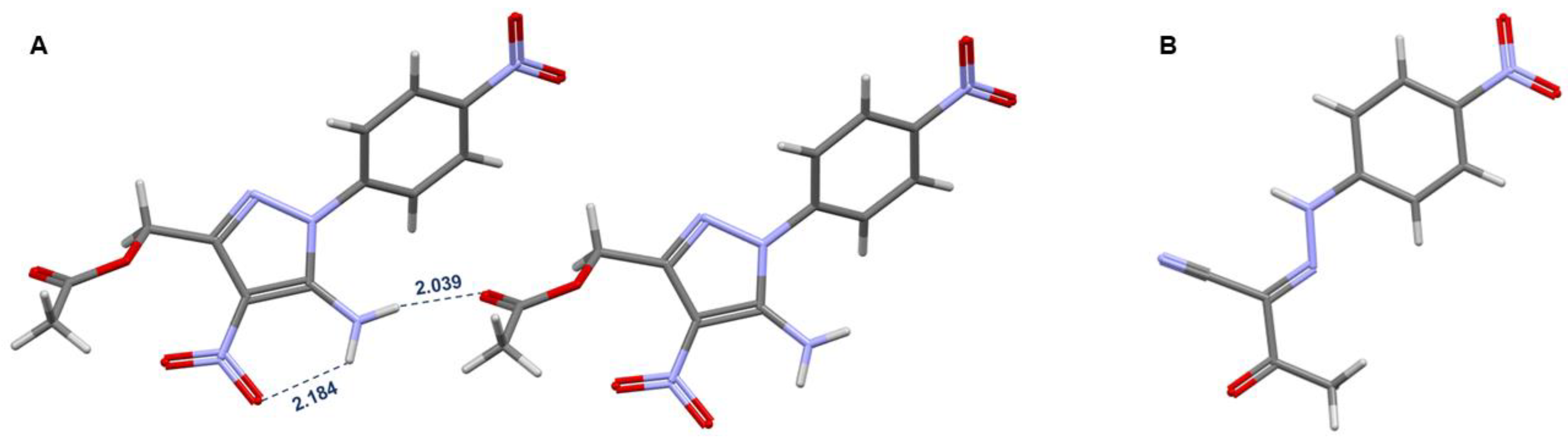
2. Results and Discussion
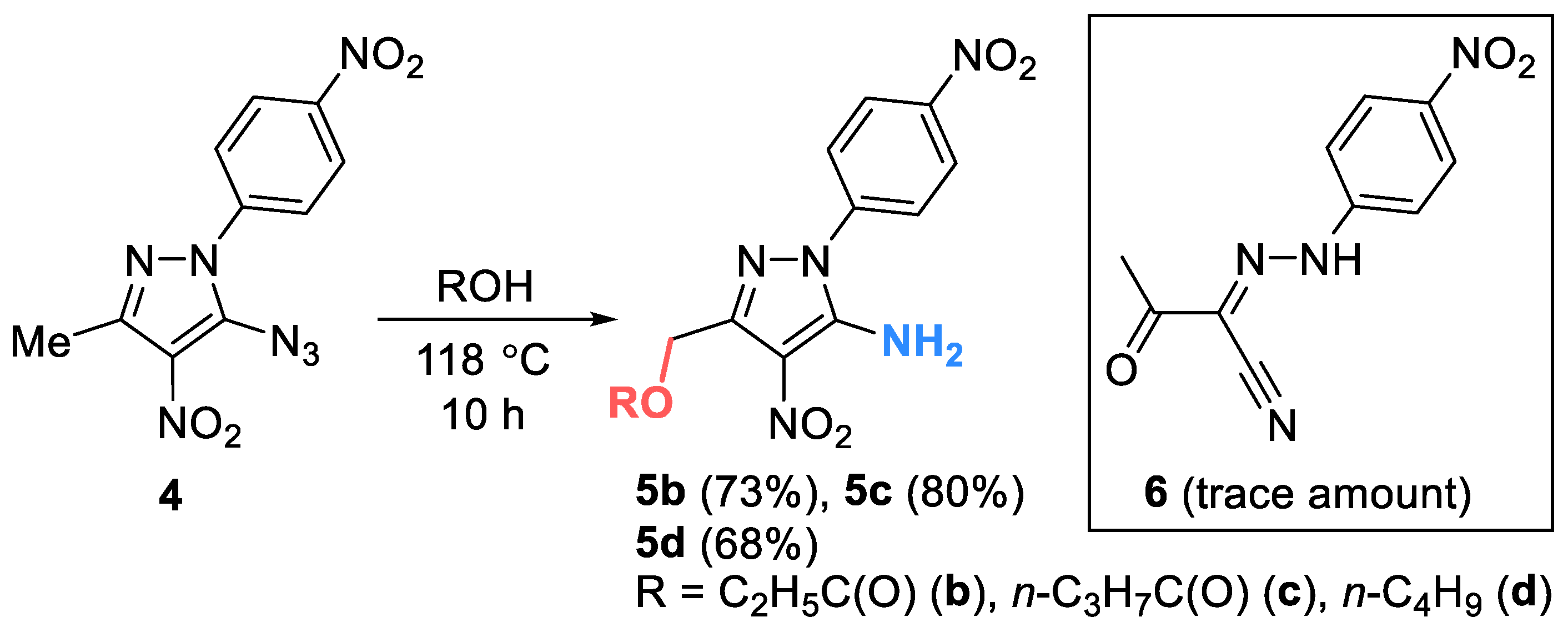
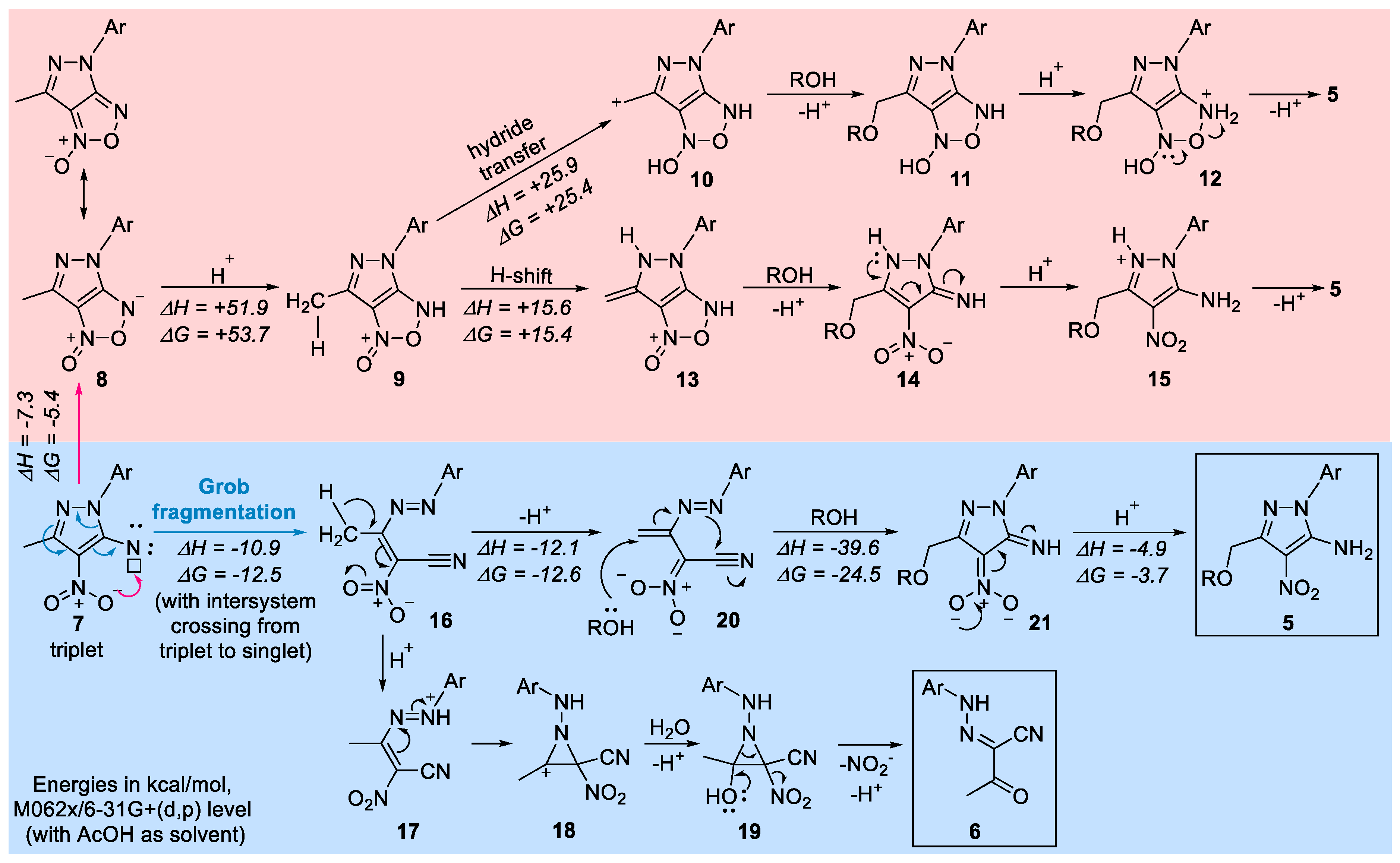
3. Materials and Methods
Chemistry
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sondhi, S.M.; Kumar, S.; Kumar, N.; Roy, P. Synthesis anti-inflammatory and anticancer activity evaluation of some pyrazole and oxadiazole derivatives. Med. Chem. Res. 2012, 21, 3043–3052. [Google Scholar] [CrossRef]
- Wei, Z.-Y.; Liu, J.-C.; Zhang, W.; Li, Y.-R.; Li, C.; Zheng, C.-J.; Piao, H.-R. Synthesis and Antimicrobial Evaluation of (Z)-5-((3-phenyl-1H-pyrazol-4-yl)methylene)-2-thioxothiazolidin-4-one Derivatives. Med. Chem. 2016, 12, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Chandrakantha, B.; Isloor, A.M.; Shetty, P.; Isloor, S.; Malladi, S.; Fun, H.K. Synthesis, characterization and antimicrobial activity of novel ethyl 1-(N-substituted)-5-phenyl-1H-pyrazole-4-carboxylate derivatives. Med. Chem. Res. 2012, 21, 2702–2708. [Google Scholar] [CrossRef]
- Ansari, A.; Ali, A.; Asif, M. ; Shamsuzzaman Review: biologically active pyrazole derivatives. New J. Chem. 2017, 41, 16–41. [Google Scholar] [CrossRef]
- Bennani, F.E.; Doudach, L.; El Rhayam, Y.; Karrouchi, K.; Cherrah, Y.; Tarib, A.; Ansar, M.; Faouzi, M.E.A. Identification of the new progress on Pyrazole Derivatives Molecules as Antimicrobial and Antifungal Agents. West Afr. J. Med. 2022, 39, 1217–1244. [Google Scholar] [PubMed]
- Pasin, J.S.M.; Ferreira, A.P.O.; Saraiva, A.L.L.; Ratzlaff, V.; Andrighetto, R.; Machado, P.; Marchesan, S.; Zanette, R.A.; Bonacorso, H.G.; Zanatta, N.; et al. Antipyretic and antioxidant activities of 5-trifluoromethyl-4,5-dihydro-1H-pyrazoles in rats. Brazilian J. Med. Biol. Res. 2010, 43. [Google Scholar] [CrossRef]
- Faria, J.V.; Vegi, P.F.; Miguita, A.G.C.; Dos Santos, M.S.; Boechat, N.; Bernardino, A.M.R. Recently reported biological activities of pyrazole compounds. Bioorg. Med. Chem. 2017, 25, 5891–5903. [Google Scholar] [CrossRef]
- Saad, H.A.; Osman, N.A.; Moustafa, A.H. Synthesis and Analgesic Activity of Some New Pyrazoles and Triazoles Bearing a 6,8-Dibromo-2-methylquinazoline Moiety. Molecules 2011, 16, 10187–10201. [Google Scholar] [CrossRef]
- dos Santos Fernandes, G.F.; de Souza, P.C.; Marino, L.B.; Chegaev, K.; Guglielmo, S.; Lazzarato, L.; Fruttero, R.; Chung, M.C.; Pavan, F.R.; dos Santos, J.L. Synthesis and biological activity of furoxan derivatives against Mycobacterium tuberculosis. Eur. J. Med. Chem. 2016, 123, 523–531. [Google Scholar] [CrossRef]
- Zhang, Z.; Bai, Z.-W.; Ling, Y.; He, L.-Q.; Huang, P.; Gu, H.-X.; Hu, R.-F. Design, synthesis and biological evaluation of novel furoxan-based coumarin derivatives as antitumor agents. Med. Chem. Res. 2018, 27, 1198–1205. [Google Scholar] [CrossRef]
- Abdelall, E.K.A. Synthesis and biological evaluations of novel isoxazoles and furoxan derivative as anti-inflammatory agents. Bioorg. Chem. 2020, 94, 103441. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.E.; Fershtat, L.L.; Gening, M.L.; Nifantiev, N.E.; Makhova, N.N. New insight into the antiaggregant activity of furoxans. Mendeleev Commun. 2016, 26, 513–515. [Google Scholar] [CrossRef]
- Ritter, H.; Licht, H.H. Synthesis and reactions of dinitrated amino and diaminopyridines. J. Heterocycl. Chem. 1995, 32, 585–590. [Google Scholar] [CrossRef]
- Fogel’zang, A.E.; Egorshev, V.Y.; Sinditskii, V.P.; Dutov, M.D. Organic azide structure and combustion trends. Combust. Explos. Shock Waves 1990, 26, 558–564. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Yao, Q. Synthesis and quantitative structure–activity relationship (QSAR) analysis of some novel oxadiazolo[3,4-d]pyrimidine nucleosides derivatives as antiviral agents. Bioorg. Med. Chem. Lett. 2015, 25, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Jovené, C.; Jacquet, M.; Chugunova, E.A.; Kharlamov, S.V.; Goumont, R. Synthesis and 1-oxide/3-oxide interconversion of 4-substituted benzodifuroxans: A thorough NMR and theoretical study of the structure of 4-fluoro- and 4-chloro-benzodifuroxan. Tetrahedron 2016, 72. [Google Scholar] [CrossRef]
- Chugunova, E.A.; Voloshina, A.D.; Mukhamatdinova, R.E.; Serkov, I.V.; Proshin, A.N.; Gibadullina, E.M.; Burilov, A.R.; Kulik, N.V.; Zobov, V.V.; Krivolapov, D.B.; et al. The Study of the Biological Activity of Amino-Substituted Benzofuroxans. Lett. Drug Des. Discov. 2014, 11, 502–512. [Google Scholar] [CrossRef]
- Boulton, A.J.; Middleton, D. Furazans and furazan oxides. V. Tropono[4,5-c]-, thieno[2,3-c]-, and biphenyleno[2,3-c]furazan oxides. J. Org. Chem. 1974, 39, 2956–2962. [Google Scholar] [CrossRef]
- Noto, R.; Rainieri, R.; Arnone, C. Effect of the nature of the starting aromatic ring on the cyclization of o-nitroaryl azides: kinetic and thermodynamic studies of the conversion of two azido(methoxycarbonyl)nitrothiophenes into methoxycarbonylthienofurazan oxides. J. Chem. Soc.{,} Perkin Trans. 2 1989, 127–130. [CrossRef]
- Rostovtsev, V. V; Green, L.G.; Fokin, V. V; Sharpless, K.B. A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. Engl. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Pearson, T.J.; Shimazumi, R.; Driscoll, J.L.; Dherange, B.D.; Park, D.-I.; Levin, M.D. Aromatic nitrogen scanning by ipso-selective nitrene internalization. Science (80-. ). 2023, 381, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.H. Reduction of Organic Azides to Primary Amines with Lithium Aluminum Hydride. J. Am. Chem. Soc. 1951, 73, 5865–5866. [Google Scholar] [CrossRef]
- Rolla, F. Sodium borohydride reactions under phase-transfer conditions: reduction of azides to amines. J. Org. Chem. 1982, 47, 4327–4329. [Google Scholar] [CrossRef]
- Ranu, B.C.; Sarkar, A.; Chakraborty, R. Reduction of Azides with Zinc Borohydride. J. Org. Chem. 1994, 59, 4114–4116. [Google Scholar] [CrossRef]
- Becher Krystian; Krake, Niels; Brøndum, Klaus; Christensen, Nils Just; Vinader, Maria Victoria, J.P. Syntheses of o-Aminohetarenecarbaldehydes via Azides. Synthesis (Stuttg). 1989, 1989, 530–533. [Google Scholar] [CrossRef]
- Kale, A.; Medishetti, N.; Kanugala, S.; C, G.K.; Atmakur, K. Na2S-promoted reduction of azides in water: synthesis of pyrazolopyridines in one pot and evaluation of antimicrobial activity. Org. Biomol. Chem. 2019, 17, 3186–3194. [Google Scholar] [CrossRef] [PubMed]
- Zelenay, B.; Besora, M.; Monasterio, Z.; Ventura-Espinosa, D.; White, A.J.P.; Maseras, F.; Díez-González, S. Copper-mediated reduction of azides under seemingly oxidising conditions: catalytic and computational studies. Catal. Sci. Technol. 2018, 8, 5763–5773. [Google Scholar] [CrossRef]
- Staudinger, H.; Meyer, J. Über neue organische Phosphorverbindungen III. Phosphinmethylenderivate und Phosphinimine. Helv. Chim. Acta 2, 635–646.
- van Kalkeren, H.A.; Bruins, J.J.; Rutjes, F.P.J.T.; van Delft, F.L. Organophosphorus-Catalysed Staudinger Reduction. Adv. Synth. Catal. 2012, 354, 1417–1421. [Google Scholar] [CrossRef]
- Lenstra, D.C.; Lenting, P.E.; Mecinović, J. Sustainable organophosphorus-catalysed Staudinger reduction. Green Chem. 2018, 20, 4418–4422. [Google Scholar] [CrossRef]
- Lenstra, D.C.; Wolf, J.J.; Mecinović, J. Catalytic Staudinger Reduction at Room Temperature. J. Org. Chem. 2019, 84, 6536–6545. [Google Scholar] [CrossRef] [PubMed]
- Vil’, V.A.; Barsegyan, Y.A.; Kuhn, L.; Terent’ev, A.O.; Alabugin, I. V Creating, Preserving, and Directing Carboxylate Radicals in Ni-Catalyzed C(sp3)–H Acyloxylation of Ethers, Ketones, and Alkanes with Diacyl Peroxides. Organometallics 2023. [Google Scholar] [CrossRef]
- Kuhn, L.; Vil’, V.A.; Barsegyan, Y.A.; Terent’ev, A.O.; Alabugin, I. V Carboxylate as a Non-innocent L-Ligand: Computational and Experimental Search for Metal-Bound Carboxylate Radicals. Org. Lett. 2022, 24, 3817–3822. [Google Scholar] [CrossRef]
- Sheng Xiaolong; Tang, Mingfang; Gao, Botao; Huang, Guosheng, J.L. An Efficient Method for the α-Acetoxylation of Ketones. Synthesis (Stuttg). 2007, 2007, 1165–1168. [Google Scholar] [CrossRef]
- Ochiai, M.; Takeuchi, Y.; Katayama, T.; Sueda, T.; Miyamoto, K. Iodobenzene-Catalyzed α-Acetoxylation of Ketones. In Situ Generation of Hypervalent (Diacyloxyiodo)benzenes Using m-Chloroperbenzoic Acid. J. Am. Chem. Soc. 2005, 127, 12244–12245. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cao, F.; Yao, L.; Shi, T.; Tang, B.; Kuninobu, Y.; Wang, Z. C–N and C–O Bond Formation in Copper-Catalyzed/Mediated sp3 C–H Activation: Mechanistic Studies from Experimental and Computational Aspects. J. Org. Chem. 2020, 85, 9713–9726. [Google Scholar] [CrossRef]
- Wang, Z.; Kuninobu, Y.; Kanai, M. Copper-Mediated Direct C(sp3)–H and C(sp2)–H Acetoxylation. Org. Lett. 2014, 16, 4790–4793. [Google Scholar] [CrossRef]
- Dick, A.R.; Hull, K.L.; Sanford, M.S. A Highly Selective Catalytic Method for the Oxidative Functionalization of C−H Bonds. J. Am. Chem. Soc. 2004, 126, 2300–2301. [Google Scholar] [CrossRef]
- Desai, L. V; Hull, K.L.; Sanford, M.S. Palladium-Catalyzed Oxygenation of Unactivated sp3 C−H Bonds. J. Am. Chem. Soc. 2004, 126, 9542–9543. [Google Scholar] [CrossRef]
- Wang, D.-H.; Hao, X.-S.; Wu, D.-F.; Yu, J.-Q. Palladium-Catalyzed Oxidation of Boc-Protected N-Methylamines with IOAc as the Oxidant: A Boc-Directed sp3 C−H Bond Activation. Org. Lett. 2006, 8, 3387–3390. [Google Scholar] [CrossRef]
- Desai, L. V; Malik, H.A.; Sanford, M.S. Oxone as an Inexpensive, Safe, and Environmentally Benign Oxidant for C−H Bond Oxygenation. Org. Lett. 2006, 8, 1141–1144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Khaskin, E.; Anderson, N.P.; Zavalij, P.Y.; Vedernikov, A.N. Catalytic aerobic oxidation of substituted 8-methylquinolines in PdII-2,6-pyridinedicarboxylic acid systems. Chem. Commun. 2008, 3625–3627. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Lee, M.; Dunn, A.L.; Sanford, M.S. Palladium-Catalyzed C–H Bond Acetoxylation via Electrochemical Oxidation. Org. Lett. 2018, 20, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo, M.; Ricardo Pires, J.; Viseu, I.; Magalhães, P.; Gregório, H.; Afreixo, V.; Gregório, T. Edaravone for acute ischemic stroke - Systematic review with meta-analysis. Clin. Neurol. Neurosurg. 2022, 219, 107299. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.; Kuhad, A.; Kuhad, A. Edaravone: a new hope for deadly amyotrophic lateral sclerosis. Drugs Today (Barc). 2018, 54, 349–360. [Google Scholar] [CrossRef]
- Purohit, M.K.; Chakka, S.K.; Scovell, I.; Neschadim, A.; Bello, A.M.; Salum, N.; Katsman, Y.; Bareau, M.C.; Branch, D.R.; Kotra, L.P. Structure–activity relationships of pyrazole derivatives as potential therapeutics for immune thrombocytopenias. Bioorg. Med. Chem. 2014, 22, 2739–2752. [Google Scholar] [CrossRef]
- Chugunova, E.A.; Timasheva, R.E.; Gibadullina, E.M.; Burilov, A.R.; Goumont, R. First Synthesis of Benzotrifuroxan at Low Temperature: Unexpected Behavior of 5,7-Dichloro-4,6-dinitrobenzo-furoxan with Sodium Azide. Propellants, Explos. Pyrotech. 2012, 37, 390–392. [Google Scholar] [CrossRef]
- dos Santos, M.S.; Oliveira, M.L. V; Bernardino, A.M.R.; de Léo, R.M.; Amaral, V.F.; de Carvalho, F.T.; Leon, L.L.; Canto-Cavalheiro, M.M. Synthesis and antileishmanial evaluation of 1-aryl-4-(4,5-dihydro-1H-imidazol-2-yl)-1H-pyrazole derivatives. Bioorg. Med. Chem. Lett. 2011, 21, 7451–7454. [Google Scholar] [CrossRef]
- Hassaneen, H.M.E.; Hassaneen, H.M.; Elnagdi, M.H. Enamines in Heterocyclic Synthesis: A Route to 4-Substituted Pyrazoles and Condensed Pyrazoles. Zeitschrift für Naturforsch. B 2004, 59, 1132–1136. [Google Scholar] [CrossRef]
- Kvaskoff, D.; Lüerssen, H.; Bednarek, P.; Wentrup, C. Phenylnitrene, phenylcarbene, and pyridylcarbenes. Rearrangements to cyanocyclopentadiene and fulvenallene. J. Am. Chem. Soc. 2014, 136, 15203–15214. [Google Scholar] [CrossRef]
- Sankaranarayanan, J.; Rajam, S.; Hadad, C.M.; Gudmundsdottir, A.D. The ability of triplet nitrenes to abstract hydrogen atoms. J. Phys. Org. Chem. 2010, 23, 370–375. [Google Scholar] [CrossRef]
- Voskresenska, V.; Wilson, R.M.; Panov, M.; Tarnovsky, A.N.; Krause, J.A.; Vyas, S.; Winter, A.H.; Hadad, C.M. Photoaffinity Labeling via Nitrenium Ion Chemistry: Protonation of the Nitrene Derived from 4-Amino-3-nitrophenyl Azide to Afford Reactive Nitrenium Ion Pairs. J. Am. Chem. Soc. 2009, 131, 11535–11547. [Google Scholar] [CrossRef] [PubMed]
- Falvey, D.E.; Gudmundsdottir, A.D. Nitrenes and Nitrenium Ions; Wiley Series of Reactive Intermediates in Chemistry and Biology; Wiley, 2013; ISBN 9780470390597.
- Carra, C.; Bally, T.; Albini, A. Role of conformation and electronic structure in the chemistry of ground and excited state o-pyrazolylphenylnitrenes. J. Am. Chem. Soc. 2005, 127, 5552–5562. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Bettinetti, G.; Minoli, G. Photodecomposition of Some Para-Substituted 2-Pyrazolylphenyl Azides. Substituents Affect the Phenylnitrene S−T Gap More Than the Barrier to Ring Expansion. J. Am. Chem. Soc. 1999, 121, 3104–3113. [Google Scholar] [CrossRef]
- Tomioka, H.; Ichikawa, N.; Komatsu, K. Photochemistry of 2-(methoxycarbonyl)phenyl azide studied by matrix-isolation spectroscopy. A new slippery energy surface for phenylnitrene. J. Am. Chem. Soc. 1993, 115, 8621–8626. [Google Scholar] [CrossRef]
- Becher, J.; Brøndum, K.; Krake, N.; Pluta, K.; Simonsen, O.; Molina, P.; Begtrup, M. An unexpected ring opening–ring closure reaction of 5-azido-4-formylpyrazole. J. Chem. Soc.{,} Chem. Commun. 1988, 541–542. [CrossRef]
- Dehaen, W.; Becher, J. Synthesis of 5-amino-4-cyanopyrazoles via ring opening-ring closure of 5-azido-4-iminomethylpyrazoles isolation of the intermediate. Tetrahedron Lett. 1991, 32, 3565–3568. [Google Scholar] [CrossRef]
- Alabugin, I. V.; Bresch, S.; Dos Passos Gomes, G. Orbital hybridization: A key electronic factor in control of structure and reactivity. J. Phys. Org. Chem. 2015, 28, 147–162. [Google Scholar]
- Juaristi, E.; dos Passos Gomes, G.; Terent’ev, A.O.; Notario, R.; Alabugin, I. V Stereoelectronic Interactions as a Probe for the Existence of the Intramolecular α-Effect. J. Am. Chem. Soc. 2017, 139, 10799–10813. [Google Scholar] [CrossRef]
- Nigst, T.A.; Antipova, A.; Mayr, H. Nucleophilic Reactivities of Hydrazines and Amines: The Futile Search for the α-Effect in Hydrazine Reactivities. J. Org. Chem. 2012, 77, 8142–8155. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Belyakova, Y.Y.; Radulov, P.S.; Novikov, R.A.; Medvedev, M.G.; Krivoshchapov, N. V; Korlyukov, A.A.; Alabugin, I. V; Terent′ev, A.O. Inverse α-Effect as the Ariadne’s Thread on the Way to Tricyclic Aminoperoxides: Avoiding Thermodynamic Traps in the Labyrinth of Possibilities. J. Am. Chem. Soc. 2022, 144, 7264–7282. [Google Scholar] [CrossRef] [PubMed]
- Secrieru, A.; O’Neill, P.M.; Cristiano, M.L.S. Revisiting the Structure and Chemistry of 3(5)-Substituted Pyrazoles. Molecules 2019, 25. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Elguero, J.; Liebman, J.F. The annular tautomerism of imidazoles and pyrazoles: The possible existence of nonaromatic forms. Struct. Chem. 2006, 17, 439–444. [Google Scholar] [CrossRef]
- Vasilevsky, S.F.; Gold, B.; Mikhailovskaya, T.F.; Alabugin, I. V Strain control in nucleophilic cyclizations: reversal of exo-selectivity in cyclizations of hydrazides of acetylenyl carboxylic acids by annealing to a pyrazole scaffold. J. Phys. Org. Chem. 2012, 25, 998–1005. [Google Scholar] [CrossRef]
- APEX2 (Version 2.1), SAINTPlus. Data Reduction and Correction Program (Version 7.31A), Bruker Advansed X-ray Solutions, BrukerAXS Inc.,Madison, Wisconsin, USA, 2006.
- G.M. Sheldrick. SADABS, Program for empirical X-ray absorption correction, Bruker-Nonius, 1990 - 2004.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




