Submitted:
04 October 2023
Posted:
08 October 2023
You are already at the latest version
Abstract
Keywords:
Introduction
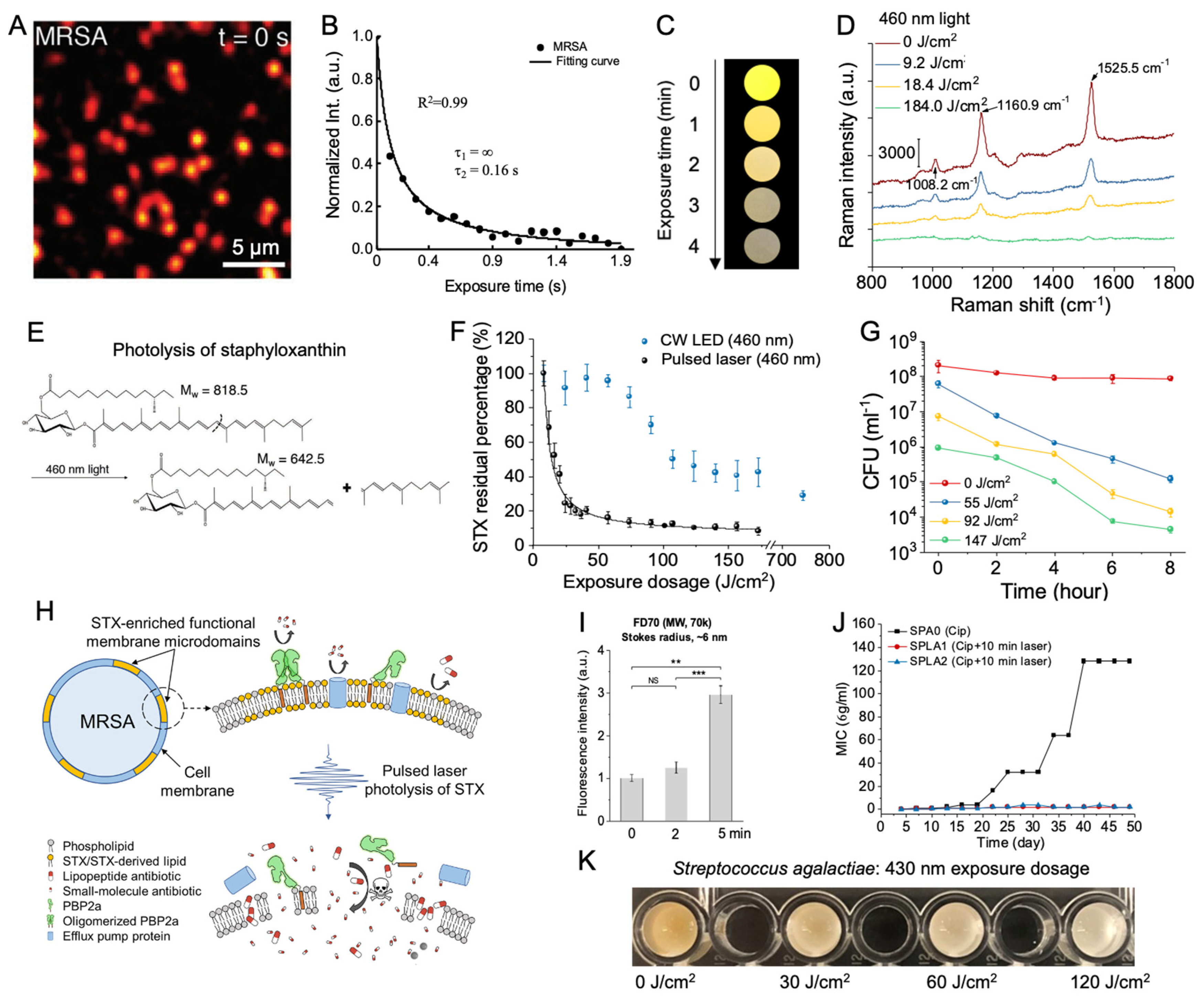
Photolysis of endogenous pigments reduces the virulence of corresponding pathogens
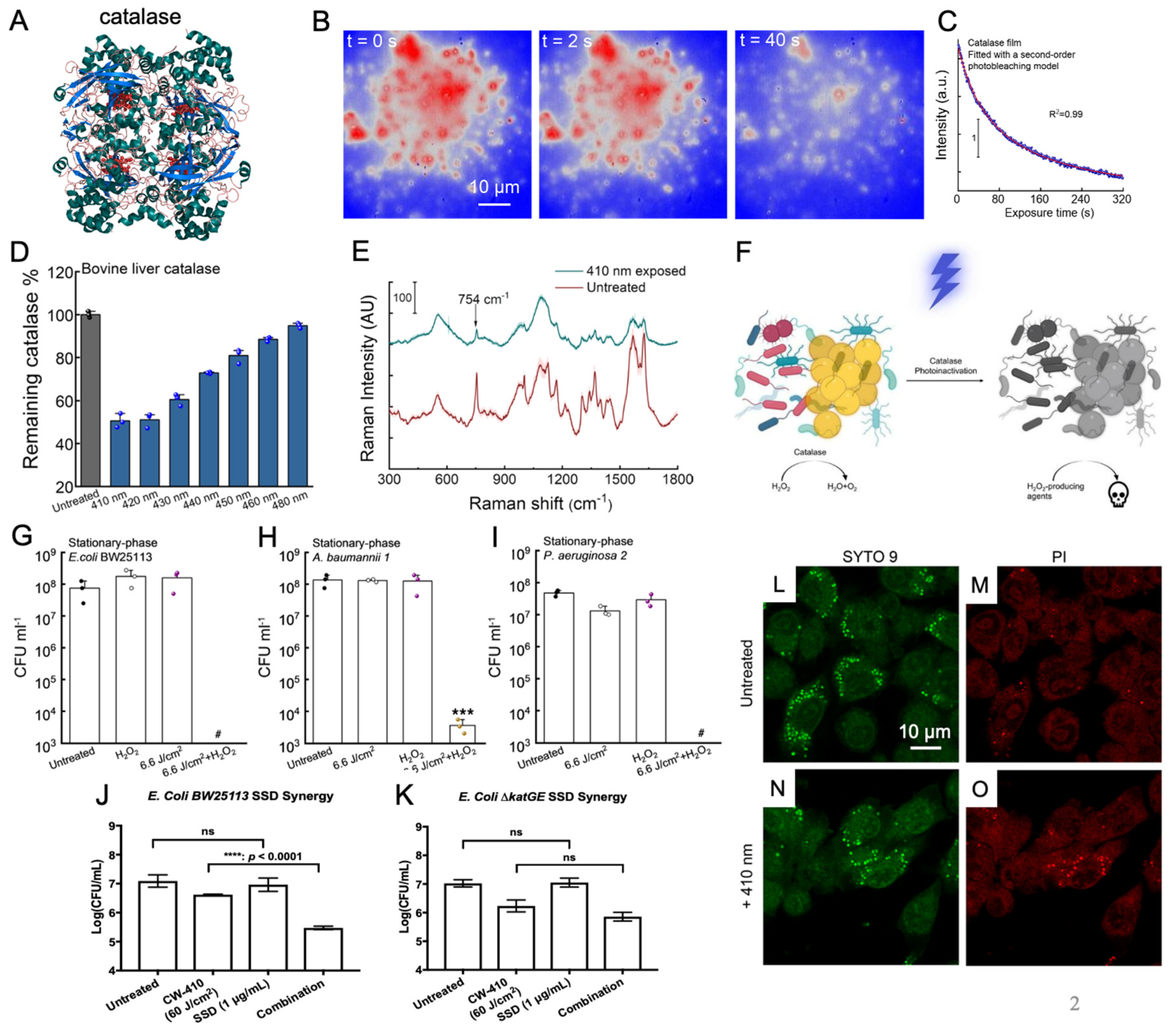
Photoinactivation of catalase sensitizes wide-ranging bacteria to reactive oxygen species
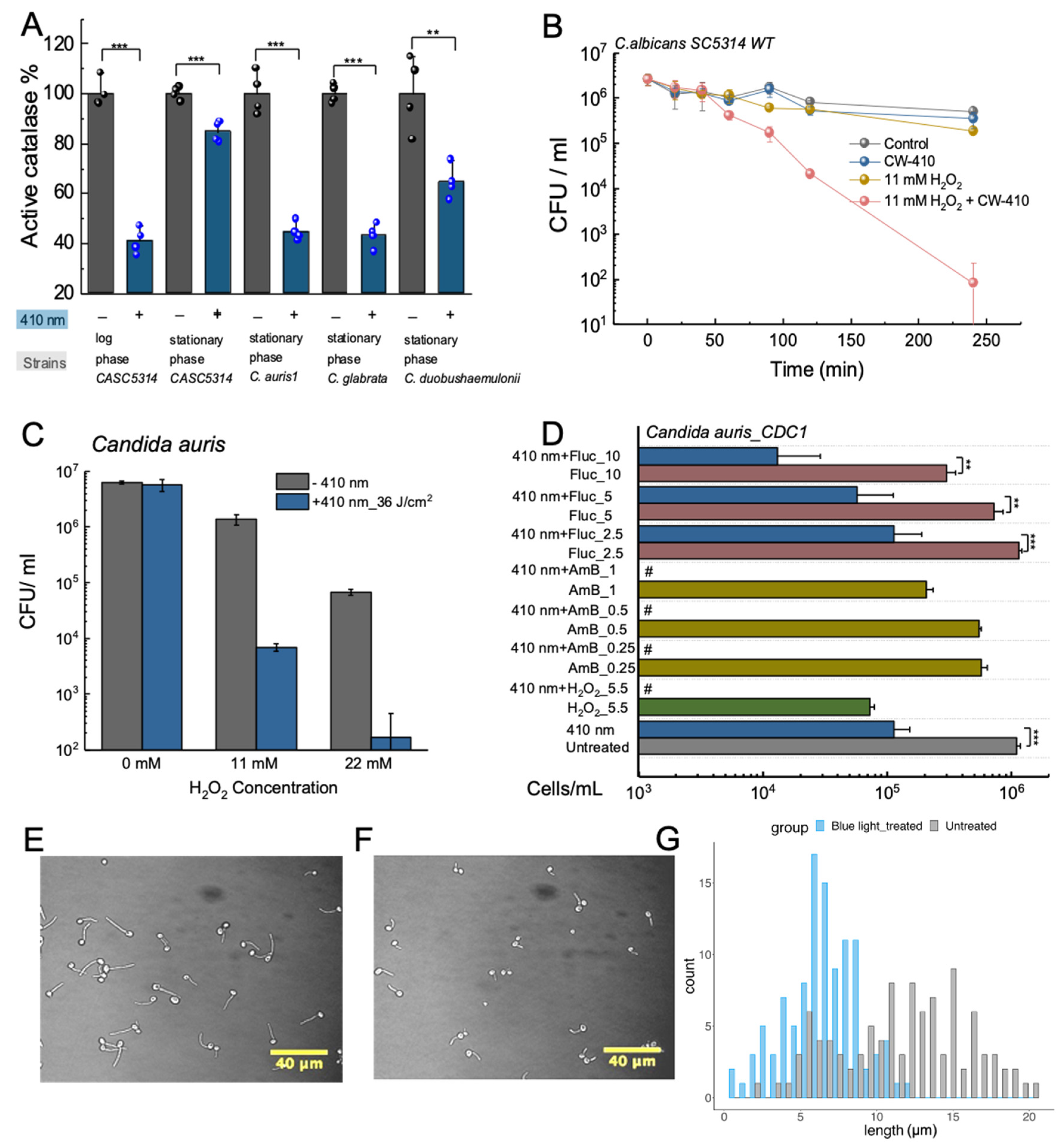
Photoinactivation of catalase sensitizes Candida spp. and Candida auris to reactive oxygen species
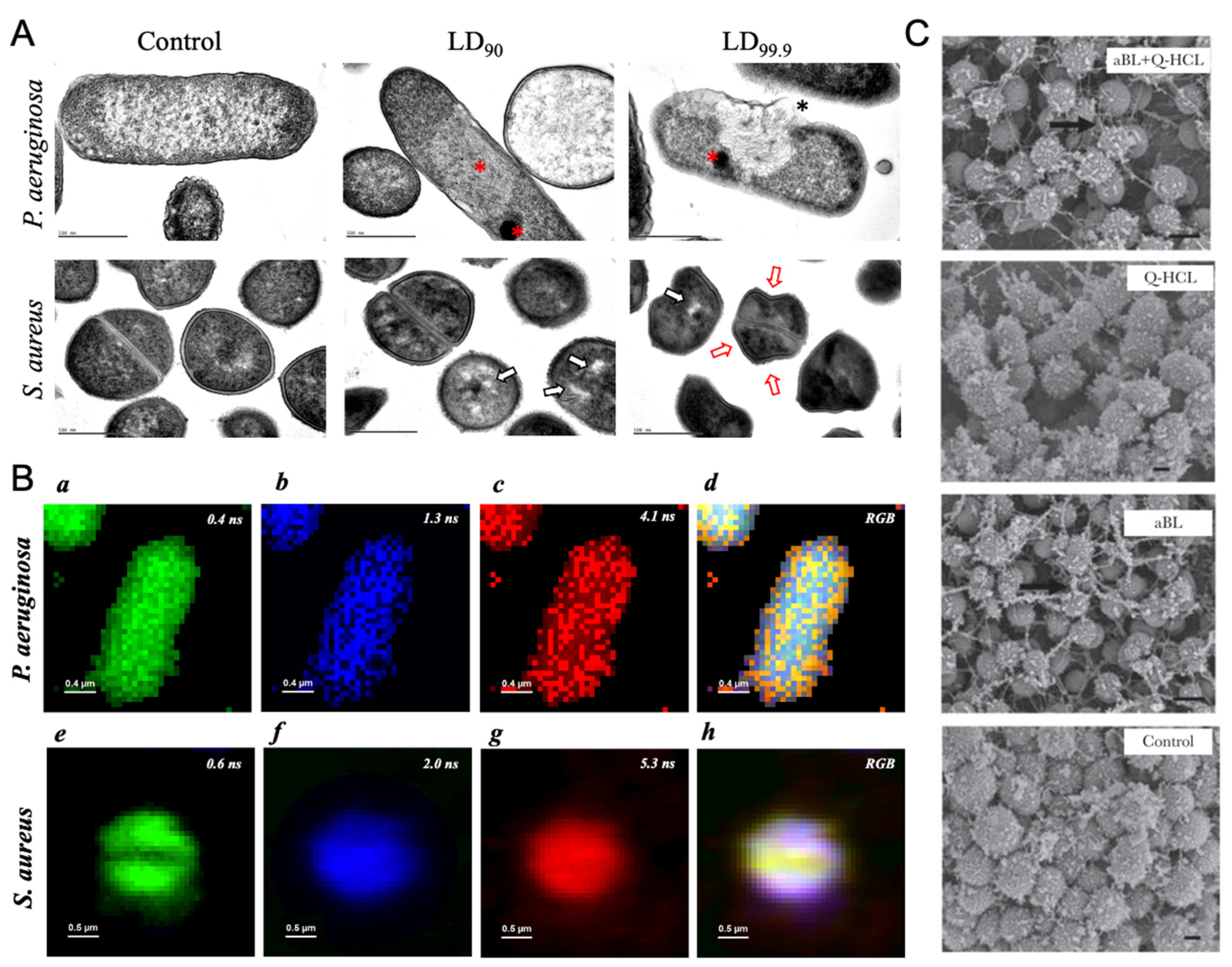
Photoexcitation of endogenous porphyrins induces intracellular ROS and triggers cellular damage
Future Directions
Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Tardivo, J.P.; Del Giglio, A.; de Oliveira, C.S.; Gabrielli, D.S.; Junqueira, H.C.; Tada, D.B.; Severino, D.; de Fátima Turchiello, R.; Baptista, M.S. Methylene blue in photodynamic therapy: From basic mechanisms to clinical applications. Photodiagnosis and Photodynamic Therapy 2005, 2, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT mechanisms. Clin Endosc 2013, 46, 24–29. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wang, Y.; Murray, C.K.; Hamblin, M.R.; Hooper, D.C.; Dai, T. Antimicrobial blue light inactivation of pathogenic microbes: State of the art. Drug Resistance Updates 2017, 33-35, 1–22. [Google Scholar] [CrossRef]
- Iwata, K.; Hagiwara, T.; Matsuzawa, H. Molecular structure and photosensitivity of polyesters with conjugated double bonds. Journal of Polymer Science: Polymer Chemistry Edition 1985, 23, 2361–2376. [Google Scholar] [CrossRef]
- Maddah, H.A.; Berry, V.; Behura, S.K. Biomolecular photosensitizers for dye-sensitized solar cells: Recent developments and critical insights. Renewable and Sustainable Energy Reviews 2020, 121, 109678. [Google Scholar] [CrossRef]
- Lancaster, J.E.; Grant, J.E.; Lister, C.E.; Taylor, M.C. Skin color in apples—influence of copigmentation and plastid pigments on shade and darkness of red color in five genotypes. Journal of the American Society for Horticultural Science 1994, 119, 63–69. [Google Scholar] [CrossRef]
- Tuli, H.S.; Chaudhary, P.; Beniwal, V.; Sharma, A.K. Microbial pigments as natural color sources: current trends and future perspectives. Journal of Food Science and Technology 2015, 52, 4669–4678. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitch, E.I. The role of chlorophyll in photosynthesis. Scientific American 1965, 213, 74–83. [Google Scholar] [CrossRef]
- Herrling, T.; Jung, K.; Fuchs, J. The role of melanin as protector against free radicals in skin and its role as free radical indicator in hair. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2008, 69, 1429–1435. [Google Scholar] [CrossRef]
- Riley, P.A. Melanin. The International Journal of Biochemistry & Cell Biology 1997, 29, 1235–1239. [Google Scholar]
- Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M.S.; Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M.S. Dyes and pigments: their structure and properties. Dyes and pigments 2016, 13–29. [Google Scholar]
- Fabian, J.; Hartmann, H. Light absorption of organic colorants: theoretical treatment and empirical rules; Springer Science & Business Media: 2013; Volume 12.
- Lagashetti, A.C.; Dufossé, L.; Singh, S.K.; Singh, P.N. Fungal pigments and their prospects in different industries. Microorganisms 2019, 7, 604. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, V.T.; Martegani, E.; Giaroni, C.; Baj, A.; Bolognese, F. Bacterial pigments: A colorful palette reservoir for biotechnological applications. Biotechnology and Applied Biochemistry 2022, 69, 981–1001. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, C.; Vinithkumar, N.V.; Kirubagaran, R.; Venil, C.K.; Dufossé, L. Multifaceted applications of microbial pigments: current knowledge, challenges and future directions for public health implications. Microorganisms 2019, 7, 186. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Nizet, V. Color me bad: microbial pigments as virulence factors. Trends in Microbiology 2009, 17, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.; Strandwitz, P. Antibiotics right under our nose. Nature 2016, 535, 501–502. [Google Scholar] [CrossRef]
- Kourtis, A.P.; Hatfield, K.; Baggs, J.; Mu, Y.; See, I.; Epson, E.; Nadle, J.; Kainer, M.A.; Dumyati, G.; Petit, S. Vital signs: epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections—United States. Morbidity and Mortality Weekly Report 2019, 68, 214. [Google Scholar] [CrossRef]
- Weber, J.T. Community-associated methicillin-resistant Staphylococcus aureus. Clinical Infectious Diseases 2005, 41, S269–S272. [Google Scholar] [CrossRef]
- Pelz, A.; Wieland, K.-P.; Putzbach, K.; Hentschel, P.; Albert, K.; Gotz, F. Structure and biosynthesis of staphyloxanthin from Staphylococcus aureus. Journal of Biological Chemistry 2005, 280, 32493–32498. [Google Scholar] [CrossRef]
- Becker, K.; Skov, R.L.; von Eiff, C. Staphylococcus, Micrococcus, and other catalase-positive cocci. Manual of clinical microbiology 2015, 354–382. [Google Scholar]
- Valliammai, A.; Selvaraj, A.; Muthuramalingam, P.; Priya, A.; Ramesh, M.; Pandian, S.K. Staphyloxanthin inhibitory potential of thymol impairs antioxidant fitness, enhances neutrophil-mediated killing and alters membrane fluidity of methicillin-resistant Staphylococcus aureus. Biomedicine & Pharmacotherapy 2021, 141, 111933. [Google Scholar]
- Clauditz, A.; Resch, A.; Wieland, K.-P.; Peschel, A.; Götz, F. Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infection and immunity 2006, 74, 4950–4953. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Essex, A.; Buchanan, J.T.; Datta, V.; Hoffman, H.M.; Bastian, J.F.; Fierer, J.; Nizet, V. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. The Journal of experimental medicine 2005, 202, 209–215. [Google Scholar] [CrossRef]
- Elmesseri, R.A.; Saleh, S.E.; Elsherif, H.M.; Yahia, I.S.; Aboshanab, K.M. Staphyloxanthin as a potential novel target for deciphering promising anti-Staphylococcus aureus agents. Antibiotics 2022, 11, 298. [Google Scholar] [CrossRef]
- Butnariu, M. Methods of analysis (extraction, separation, identification and quantification) of carotenoids from natural products. J. Ecosyst. Ecography 2016, 6, 1–19. [Google Scholar] [CrossRef]
- Dong, P.T.; Mohammad, H.; Hui, J.; Leanse, L.G.; Li, J.; Liang, L.; Dai, T.; Seleem, M.N.; Cheng, J.X. Photolysis of staphyloxanthin in methicillin-resistant Staphylococcus aureus potentiates killing by reactive oxygen species. Advanced Science 2019, 6, 1900030. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Schlamadinger, D.E.; Desai, V.; Tauber, M.J. Triplet excitons of carotenoids formed by singlet fission in a membrane. ChemPhysChem 2011, 12, 2891–2894. [Google Scholar] [CrossRef]
- Gupta, S.; Mandal, T. Simulation study of domain formation in a model bacterial membrane. Physical Chemistry Chemical Physics 2022, 24, 18133–18143. [Google Scholar] [CrossRef]
- García-Fernández, E.; Koch, G.; Wagner, R.M.; Fekete, A.; Stengel, S.T.; Schneider, J.; Mielich-Süss, B.; Geibel, S.; Markert, S.M.; Stigloher, C. Membrane microdomain disassembly inhibits MRSA antibiotic resistance. Cell 2017, 171, 1354–1367. [Google Scholar] [CrossRef]
- Hui, J.; Dong, P.-T.; Liang, L.; Mandal, T.; Li, J.; Ulloa, E.R.; Zhan, Y.; Jusuf, S.; Zong, C.; Seleem, M.N.; et al. Photo-disassembly of membrane microdomains revives conventional antibiotics against MRSA. Advanced Science 2020, 7, 1903117. [Google Scholar] [CrossRef]
- Jusuf, S.; Hui, J.; Dong, P.-T.; Cheng, J.-X. Staphyloxanthin photolysis potentiates low concentration silver nanoparticles in eradication of methicillin-resistant Staphylococcus aureus. The Journal of Physical Chemistry C 2020, 124, 5321–5330. [Google Scholar] [CrossRef]
- Rosa-Fraile, M.; Rodríguez-Granger, J.; Haidour-Benamin, A.; Cuerva, J.M.; Sampedro, A. Granadaene: Proposed structure of the Group B Streptococcus polyenic pigment. Applied and Environmental Microbiology 2006, 72, 6367–6370. [Google Scholar] [CrossRef] [PubMed]
- Jusuf, S.; Dong, P.-T.; Hui, J.; Ulloa, E.R.; Liu, G.Y.; Cheng, J.-X. Granadaene photobleaching reduces the virulence and increases antimicrobial susceptibility of Streptococcus agalactiae. Photochemistry and Photobiology 2021, 97, 816–825. [Google Scholar] [CrossRef]
- Angel, D.E.; Lloyd, P.; Carville, K.; Santamaria, N. The clinical efficacy of two semi-quantitative wound-swabbing techniques in identifying the causative organism(s) in infected cutaneous wounds. International Wound Journal 2011, 8, 176–185. [Google Scholar] [CrossRef]
- Merritt, K.; Jacobs, N.J. Characterization and incidence of pigment production by human clinical group B streptococci. Journal of Clinical Microbiology 1978, 8, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, L. Understanding the regulation of Group B Streptococcal virulence factors. Future Microbiology 2009, 4, 201–221. [Google Scholar] [CrossRef]
- Liu, G.Y.; Doran, K.S.; Lawrence, T.; Turkson, N.; Puliti, M.; Tissi, L.; Nizet, V. Sword and shield: Linked group B streptococcal β-hemolysin/cytolysin and carotenoid pigment function to subvert host phagocyte defense. Proceedings of the National Academy of Sciences 2004, 101, 14491–14496. [Google Scholar] [CrossRef] [PubMed]
- Whidbey, C.; Harrell, M.I.; Burnside, K.; Ngo, L.; Becraft, A.K.; Iyer, L.M.; Aravind, L.; Hitti, J.; Adams Waldorf, K.M.; Rajagopal, L. A hemolytic pigment of Group B Streptococcus allows bacterial penetration of human placenta. Journal of Experimental Medicine 2013, 210, 1265–1281. [Google Scholar] [CrossRef]
- Bergmeyer, H.-U. Methods of enzymatic analysis; Elsevier: 2012.
- Aebi, H. [13] Catalase in vitro. In Methods in Enzymology; Academic Press: 1984; Volume 105, pp. 121–126.
- Imlay, J.A. Diagnosing oxidative stress in bacteria: not as easy as you might think. Current Opinion in Microbiology 2015, 24, 124–131. [Google Scholar] [CrossRef]
- Flannagan, R.S.; Heit, B.; Heinrichs, D.E. Antimicrobial mechanisms of macrophages and the immune evasion strategies of Staphylococcus aureus. Pathogens 2015, 4, 826–868. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nature Reviews Immunology 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Day, W.A.; Sajecki, J.L.; Pitts, T.M.; Joens, L.A. Role of catalase in Campylobacter jejuni intracellular survival. Infection and Immunity 2000, 68, 6337–6345. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.-T.; Jusuf, S.; Hui, J.; Zhan, Y.; Zhu, Y.; Liu, G.Y.; Cheng, J.-X. Photoinactivation of catalase sensitizes a wide range of bacteria to ROS-producing agents and immune cells. JCI Insight 2022, 7. [Google Scholar] [CrossRef]
- Jusuf, S.; Zhan, Y.; Zhang, M.; Alexander, N.J.; Viens, A.; Mansour, M.K.; Cheng, J.-X. Blue light deactivation of catalase suppresses Candida hyphae development through lipogenesis inhibition. Photochemistry and Photobiology 2023, 99, 936–946. [Google Scholar] [CrossRef]
- Van Acker, H.; Coenye, T. The role of reactive oxygen species in antibiotic-mediated killing of bacteria. Trends in Microbiology 2017, 25, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Goswami, M.; Mangoli, S.H.; Jawali, N. Involvement of reactive oxygen species in the action of ciprofloxacin against Escherichia coli. Antimicrobial Agents and Chemotherapy 2006, 50, 949–954. [Google Scholar] [CrossRef]
- Park, H.-J.; Kim, J.Y.; Kim, J.; Lee, J.-H.; Hahn, J.-S.; Gu, M.B.; Yoon, J. Silver-ion-mediated reactive oxygen species generation affecting bactericidal activity. Water Research 2009, 43, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Gordon, O.; Slenters, T.V.; Brunetto, P.S.; Villaruz, A.E.; Sturdevant, D.E.; Otto, M.; Landmann, R.; Fromm, K.M. Silver coordination polymers for prevention of implant infection: Thiol interaction, impact on respiratory chain enzymes, and hydroxyl radical induction. Antimicrobial Agents and Chemotherapy 2010, 54, 4208–4218. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.L.; Modak, S.M. Mechanism of silver sulfadiazine action on burn wound infections. Antimicrobial Agents and Chemotherapy 1974, 5, 582–588. [Google Scholar] [CrossRef]
- Xu, X.; Xu, L.; Yuan, G.; Wang, Y.; Qu, Y.; Zhou, M. Synergistic combination of two antimicrobial agents closing each other’s mutant selection windows to prevent antimicrobial resistance. Scientific Reports 2018, 8, 7237. [Google Scholar] [CrossRef]
- Jusuf, S.; Cheng, J.-X. Blue light improves antimicrobial efficiency of Silver sulfadiazine via catalase inactivation. Photobiomodulation, Photomedicine, and Laser Surgery 2023, 41, 80–87. [Google Scholar] [CrossRef] [PubMed]
- NOX2 complex–derived ROS as immune regulators. Antioxidants & Redox Signaling 2011, 15, 2197–2208.
- Das, D.; Bishayi, B. Staphylococcal catalase protects intracellularly survived bacteria by destroying H2O2 produced by the murine peritoneal macrophages. Microbial Pathogenesis 2009, 47, 57–67. [Google Scholar] [CrossRef]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole antifungal resistance in Candida albicans and emerging non-albicans Candida Species. Frontiers in Microbiology 2017, 7. [Google Scholar] [CrossRef]
- Du, H.; Bing, J.; Hu, T.; Ennis, C.L.; Nobile, C.J.; Huang, G. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLOS Pathogens 2020, 16, e1008921. [Google Scholar] [CrossRef] [PubMed]
- Kashem, S.W.; Kaplan, D.H. Skin immunity to Candida albicans. Trends in Immunology 2016, 37, 440–450. [Google Scholar] [CrossRef]
- de Almeida, J.N.; Francisco, E.C.; Hagen, F.; Brandão, I.B.; Pereira, F.M.; Presta Dias, P.H.; de Miranda Costa, M.M.; de Souza Jordão, R.T.; de Groot, T.; Colombo, A.L. Emergence of Candida auris in Brazil in a COVID-19 intensive care unit. Journal of Fungi 2021, 7, 220. [Google Scholar] [CrossRef] [PubMed]
- Prestel, C.; Anderson, E.; Forsberg, K.; Lyman, M.; de Perio, M.A.; Kuhar, D.; Edwards, K.; Rivera, M.; Shugart, A.; Walters, M. Candida auris outbreak in a COVID-19 specialty care unit—Florida, July–August 2020. Morbidity and Mortality Weekly Report 2021, 70, 56. [Google Scholar] [CrossRef]
- Hamilton, A.J.; Holdom, M.D. Antioxidant systems in the pathogenic fungi of man and their role in virulence. Medical Mycology 1999, 37, 375–389. [Google Scholar] [CrossRef]
- Jiménez-López, C.; Lorenz, M.C. Fungal immune evasion in a model host–pathogen interaction: Candida albicans versus macrophages. PLOS Pathogens 2013, 9, e1003741. [Google Scholar] [CrossRef]
- Dong, P.-T.; Zhan, Y.; Jusuf, S.; Hui, J.; Dagher, Z.; Mansour, M.K.; Cheng, J.-X. Photoinactivation of catalase sensitizes Candida albicans and Candida auris to ROS-producing agents and immune cells. Advanced Science 2022, 9, 2104384. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Trevijano-Contador, N.; Román, E.; Sánchez-Fresneda, R.; Casas, C.; Herrero, E.; Argüelles, J.C.; Pla, J.; Cuenca-Estrella, M.; Zaragoza, O. The production of reactive oxygen species Is a universal action mechanism of amphotericin B against pathogenic yeasts and contributes to the fungicidal effect of this drug. Antimicrobial Agents and Chemotherapy 2014, 58, 6627–6638. [Google Scholar] [CrossRef]
- Linares, C.E.B.; Giacomelli, S.R.; Altenhofen, D.; Alves, S.H.; Morsch, V.M.; Schetinger, M.R.C. Fluconazole and amphotericin-B resistance are associated with increased catalase and superoxide dismutase activity in Candida albicans and Candida dubliniensis. Revista da Sociedade Brasileira de Medicina Tropical 2013, 46. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiology Reviews 2012, 36, 288–305. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami, R.; Berman, J.; Novikov, A.; Bash, E.; Shachor-Meyouhas, Y.; Zakin, S.; Maor, Y.; Tarabia, J.; Schechner, V.; Adler, A. Multidrug-resistant Candida haemulonii and C. auris, tel aviv, Israel. Emerging infectious diseases 2017, 23, 195. [Google Scholar] [CrossRef] [PubMed]
- Kohlenberg, A.; Monnet, D.L.; Plachouras, D.; group, C.a.s.c. Increasing number of cases and outbreaks caused by Candida auris in the EU/EEA, 2020 to 2021. Eurosurveillance 2022, 27, 2200846. [Google Scholar] [CrossRef]
- Geremia, N.; Brugnaro, P.; Solinas, M.; Scarparo, C.; Panese, S. Candida auris as an emergent public health problem: A current update on European outbreaks and cases. In Proceedings of the Healthcare; 2023; p. 425. [Google Scholar]
- Lyman, M.; Forsberg, K.; Sexton, D.J.; Chow, N.A.; Lockhart, S.R.; Jackson, B.R.; Chiller, T. Worsening spread of Candida auris in the United States, 2019 to 2021. Annals of Internal Medicine 2023, 176, 489–495. [Google Scholar] [CrossRef]
- Thatchanamoorthy, N.; Rukumani Devi, V.; Chandramathi, S.; Tay, S.T. Candida auris: A mini review on epidemiology in healthcare facilities in Asia. Journal of Fungi 2022, 8, 1126. [Google Scholar] [CrossRef]
- Mahl, C.D.; Behling, C.S.; Hackenhaar, F.S.; de Carvalho e Silva, M.N.; Putti, J.; Salomon, T.B.; Alves, S.H.; Fuentefria, A.; Benfato, M.S. Induction of ROS generation by fluconazole in Candida glabrata: activation of antioxidant enzymes and oxidative DNA damage. Diagnostic Microbiology and Infectious Disease 2015, 82, 203–208. [Google Scholar] [CrossRef]
- Phillips, A.J.; Sudbery, I.; Ramsdale, M. Apoptosis induced by environmental stresses and amphotericin B in Candida albicans. Proceedings of the National Academy of Sciences 2003, 100, 14327–14332. [Google Scholar] [CrossRef]
- Sudbery, P.; Gow, N.; Berman, J. The distinct morphogenic states of Candida albicans. Trends in Microbiology 2004, 12, 317–324. [Google Scholar] [CrossRef]
- Gow, N.A.R.; van de Veerdonk, F.L.; Brown, A.J.P.; Netea, M.G. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nature Reviews Microbiology 2012, 10, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, C.A.; Vinces, M.D. Contributions of hyphae and hypha-co-regulated genes to Candida albicans virulence. Cellular Microbiology 2005, 7, 1546–1554. [Google Scholar] [CrossRef]
- Nakagawa, Y. Catalase gene disruptant of the human pathogenic yeast Candida albicans is defective in hyphal growth, and a catalase-specific inhibitor can suppress hyphal growth of wild-type cells. Microbiology and Immunology 2008, 52, 16–24. [Google Scholar] [CrossRef]
- Martin, S.W.; Konopka, J.B. Lipid raft polarization contributes to hyphal growth in Candida albicans. Eukaryotic Cell 2004, 3, 675–684. [Google Scholar] [CrossRef]
- Anjos, C.d.; Leanse, L.G.; Ribeiro, M.S.; Sellera, F.P.; Dropa, M.; Arana-Chavez, V.E.; Lincopan, N.; Baptista, M.S.; Pogliani, F.C.; Dai, T.; et al. New insights into the bacterial targets of antimicrobial blue light. Microbiology Spectrum 2023, 11, e02833–02822. [Google Scholar] [CrossRef]
- Leanse, L.G.; Dong, P.-T.; Goh, X.S.; Lu, M.; Cheng, J.-X.; Hooper, D.C.; Dai, T. Quinine enhances photo-inactivation of Gram-negative bacteria. The Journal of Infectious Diseases 2019, 221, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Gøtzsche, P. Niels Finsen's treatment for lupus vulgaris. Journal of the Royal Society of Medicine 2011, 104, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Leanse, L.G.; dos Anjos, C.; Mushtaq, S.; Dai, T. Antimicrobial blue light: A ‘Magic Bullet’ for the 21st century and beyond? Advanced Drug Delivery Reviews 2022, 180, 114057. [Google Scholar] [CrossRef]
- Garza, F.Z.C.; Born, M.; Hilbers, P.A.J.; van Riel, N.A.W.; Liebmann, J. Visible blue light therapy: Molecular mechanisms and therapeutic opportunities. Current Medicinal Chemistry 2018, 25, 5564–5577. [Google Scholar] [CrossRef]
- Wong, F.; Stokes, J.M.; Cervantes, B.; Penkov, S.; Friedrichs, J.; Renner, L.D.; Collins, J.J. Cytoplasmic condensation induced by membrane damage is associated with antibiotic lethality. Nature Communications 2021, 12, 2321. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.; Heaster, T.; Sharick, J.; Gillette, A.; Skala, M. Fluorescence lifetime imaging microscopy: fundamentals and advances in instrumentation, analysis, and applications. Journal of Biomedical Optics 2020, 25, 071203. [Google Scholar] [CrossRef] [PubMed]
- Leanse, L.G.; Dos Anjos, C.; Wang, Y.; Murray, C.K.; Hooper, D.C.; Dai, T. Effective treatment of cutaneous mold infections by antimicrobial blue light that is potentiated by quinine. The Journal of infectious diseases 2021, 224, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Sun, Y.; Zhang, Y.; Wang, W.; Dan, J.; Yao, J.; Chen, H.; Tian, F.; Sun, X.; Guo, S.; et al. Protoporphyrin IX induces a necrotic cell death in human THP-1 macrophages through activation of reactive oxygen species/c-Jun N-terminal protein kinase pathway and opening of mitochondrial permeability transition pore. Cellular Physiology and Biochemistry 2014, 34, 1835–1848. [Google Scholar] [CrossRef]
- Xue, L.; Chen, Y.Y.; Yan, Z.; Lu, W.; Wan, D.; Zhu, H. Staphyloxanthin: a potential target for antivirulence therapy. Infection and drug resistance 2019, 2151–2160. [Google Scholar] [CrossRef]
- Chen, F.; Di, H.; Wang, Y.; Cao, Q.; Xu, B.; Zhang, X.; Yang, N.; Liu, G.; Yang, C.-G.; Xu, Y.; et al. Small-molecule targeting of a diapophytoene desaturase inhibits S. aureus virulence. Nature Chemical Biology 2016, 12, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Lau, G.W.; Hassett, D.J.; Ran, H.; Kong, F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends in Molecular Medicine 2004, 10, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Konzen, M.; De Marco, D.; Cordova, C.A.S.; Vieira, T.O.; Antônio, R.V.; Creczynski-Pasa, T.B. Antioxidant properties of violacein: Possible relation on its biological function. Bioorganic & Medicinal Chemistry 2006, 14, 8307–8313. [Google Scholar]
- Björn, L.O.; Rasmusson, A.G. Photosensitivity in sponge due to cytochrome c oxidase? Photochemical & Photobiological Sciences 2009, 8, 755–757. [Google Scholar]
- Molano-Arevalo, J.C.; Jeanne Dit Fouque, K.; Pham, K.; Miksovska, J.; Ridgeway, M.E.; Park, M.A.; Fernandez-Lima, F. Characterization of intramolecular interactions of Cytochrome c using hydrogen–deuterium exchange-trapped ion mobility spectrometry–mass spectrometry and molecular dynamics. Analytical Chemistry 2017, 89, 8757–8765. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




