1. Introduction
The study of sublethal effects of pollutants on wildlife, and specially on pollinators is essential. Honeybees face various pollutants, whatever their environment: we found pesticides especially in agricultural landscapes (Botias et al. 2017), and PAHs / heavy metals are more concentrated in urban areas (Cochard et al.2020; Gizaw et al. 2020).
Many studies have shown that pesticides greatly impact behaviorally and physiologically honeybees (feeding and activity: Azpiazu et al., 2019, Almasri et al., 2020, Lupi et al., 2020; learning: Gonalons and Farina, 2018, Williamson et al., 2013; pathogen infection: Pettis et al., 2012). Toxicity of pesticides seems to be correlated to enzymes activities used as biomarkers (Badawy et al., 2015). But only 7% of the 142 scientific experimentations published between 2005 and 2016 investigated the impact of the interaction between different insecticides and 6.3% between insecticides and fungicides (detailed in Benuszak et al., 2017). However, the effects are often synergistic and not simply additive (Almasri et al., 2020).
Other pollutants mainly found in urban areas as heavy metals have been reported to be accumulated in bees (Leita et al., 1996; Conti et al., 2001; Perugini et al., 2011; Lambert et al., 2012; Goretti et al., 2020) and act as environmental stressors on honeybees, thus increasing the expression levels of various genes involved in the detoxification metabolism in urban bees (Gizaw et al. 2020).
To our knowledge, oxidative stress response to PAHs was studied in various organisms like Arabidopsis thaliana, Mya arenaria [soft shell clam], Chlorella sp., fish (Liu et al., 2009; Pichaud et al., 2008; Santana et al., 2018; Subashchandrabose et al., 2017), but not in honeybees while we know that bees bio-accumulate PAHs (Cochard et al., 2020). However, a recent study of Nicewicz et al. (2020) has shown that the Hsp70 and defensin level were significantly higher in urban bees than in bees collected in rural apiary. High level of these two biomarkers indicate that urban insects are under greater stress factors that affect cells repair and immunity than rural insects. Moreover, the oxidative potential of urban PM are threefold-higher than rural PM according to the study of Daellenbach et al (2020).
There is therefore a real need to find a global marker of the impact of the cocktail of pollutants found in various environment on the health of pollinators. Questions remaining are: can we measure early sublethal effects of pesticides and PAH using oxidative stress? Can we use oxidative stress of honeybees to measure the impact of the actions put in place by cities to reduce pollution (PAHs/pesticides)?
Thus, the main objectives of this study were to measure the impact of the consumption of 1) a cocktail of pesticides (insecticides and fungicides) and 2) a mixture of PAHs, on the survival and oxidative stress of bees in lab experiment. We also conducted field case study where we measured oxidative stress of honeybees collected according to an increasing level of anthropization (semi-natural land, agricultural land and urban land). Based on literature, we expected a greater but slower impact of PAHs on honeybee’s oxidative stress than pesticides.
2. Material & Methods
2.1. Pesticides and PAHs diet solutions
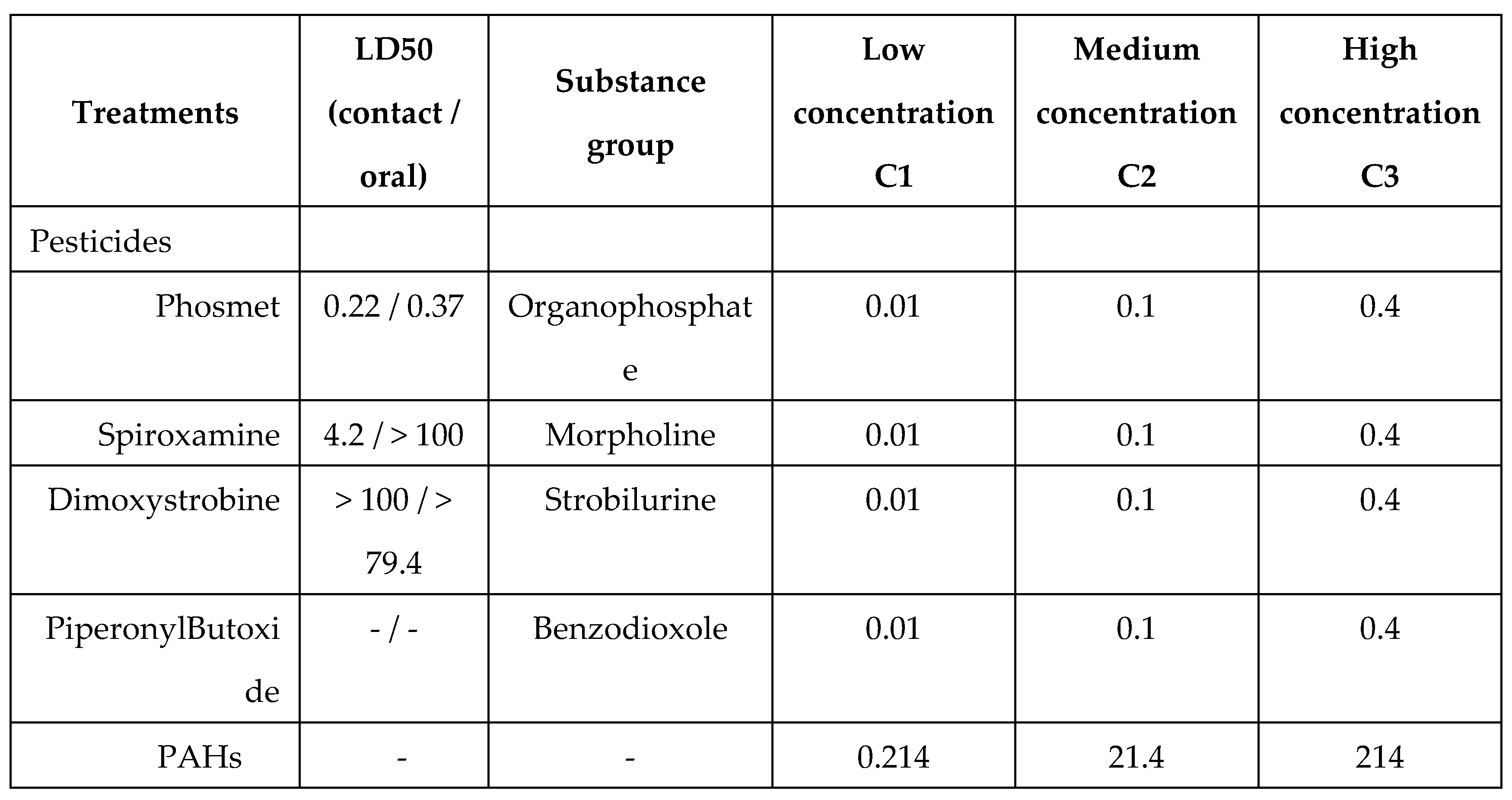
Two fungicides, one insecticide and one chemical used to enhance the insecticidal properties were chosen to compose pesticides diet: dimoxystrobine, spiroxamine, phosmet and piperonylbutoxide (≥ 98% purity, Pestanal, Sigma-Aldrich, Darmstadt, Germany). They are frequently detected in beebread samples (many years of collected samples, data not shown).
All pesticides were dissolved in a small volume of acetone before preparation of 1mM stock solutions by adding water. Pesticides were then dissolved in Apistar© sugar syrup (Sucrose: 34%, Fructose: 33%, Glucose: 33%; ICKO Apiculture, France) following
Table 1, trying to follow field realistic concentration detected in nectar. Hayat et al. (2018) and Azpiazu et al. (2019) found pesticides residues in nectar of different flower of the same order of magnitude than our study.
20 PAHs were dissolved in sugar syrup by an external lab (Oniris, Laberca, Nantes – France, see Table S1 for compounds) using native compounds and isotopic-labelled internal standard compounds (13C-PAHs) from Promochem. PAHs were chosen as being part of the lists recommended by the EU Scientific Committer for Food (SCF), the European Union (EU) and United States Environmental Protection Agency (US EPA).
2.2. Experimental protocol
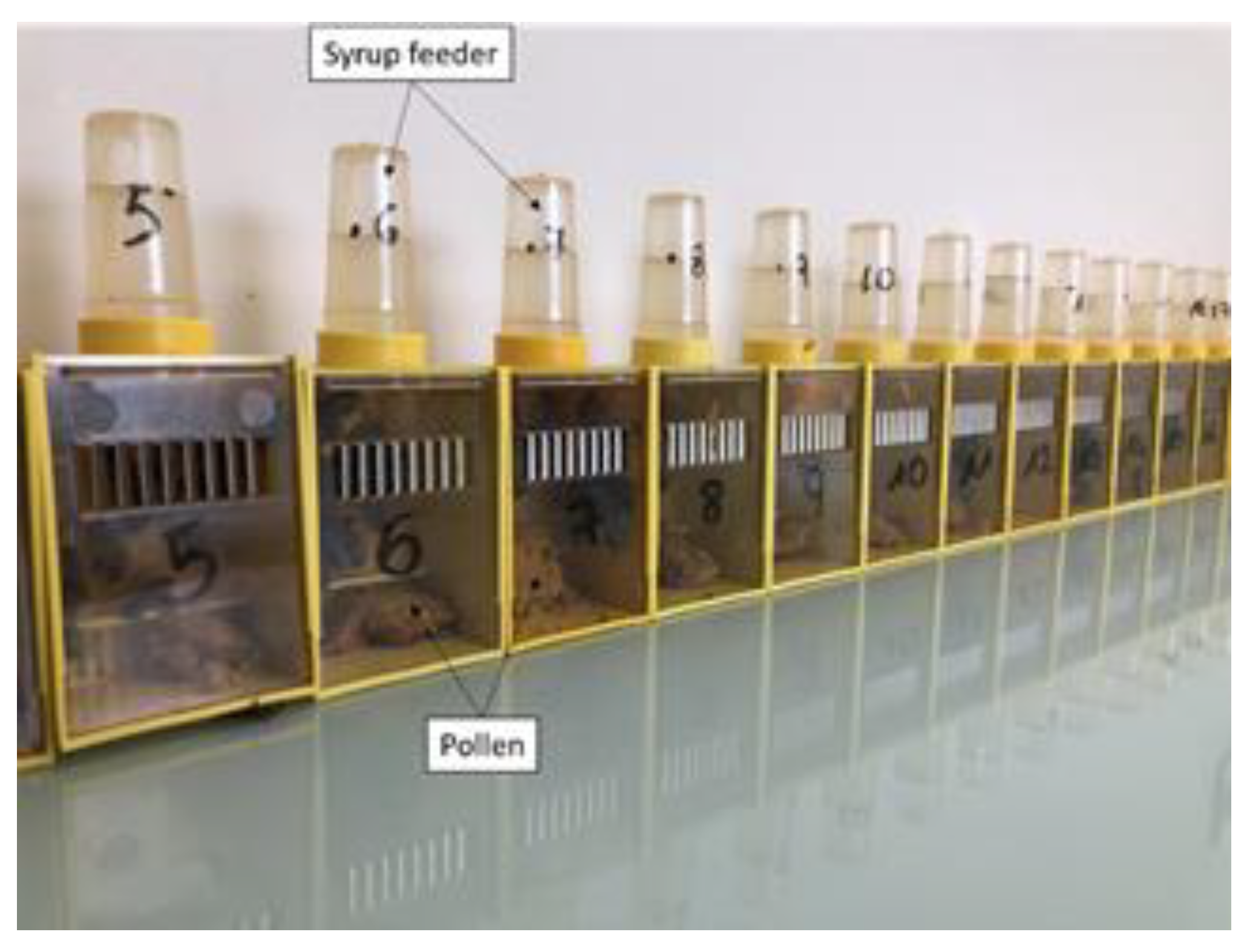
We used 9 boxes randomly assigned in one of the 3 following treatments: control, pesticides diet or PAHs diet. Twenty young honeybees (< 3 days) were placed in each box (
Figure 1). Every day, mortality was registered, and each dead bee was replaced by a marked honeybee, to exclude it from the next collected bee and maintain the same number of bees per box. The syrup container was mixed every day and filled every 7 days, noting the syrup consumption per box. Three honeybees were randomly sampled at Day 0 (control, beginning of the experiment), Day 7, Day 14, and Day 21 to measure oxidative stress. All sampled bees were immediately frozen until oxidative stress measurements.
2.3. Field case study sampling
The gradient level of anthropization was chosen as in Cochard 2020 and was composed of 20 semi-natural, 37 agricultural and 23 urban apiaries, across metropolitan France (more than 10 French departments). Each apiary was composed of 3 beehives. In each beehive, 5 honeybee workers returning to the hive were randomly sampled, using a non-contaminant tool. Each honeybee was individually placed on dry ice (-80°C) and stored in standard freezer (-20°C) until laboratory analysis. The hives were not smoked during the month preceding sampling to ensure that beekeeping practices would not increase PAHs exposition of honeybees and that only the environment around the apiary had an impact on honeybee oxidative stress.
2.4. Oxidative stress measurements - Carbonylated proteins analysis
Proteins were extracted from pooled honeybee heads (4 heads per replicate; 3 replicates per experimental group) by using OxiProtemicsR extraction buffer optimised for bees, then quantified by the Bradford method (Bradford, 1976). Equal amount of whole protein extracts (20µg) for each replicate was then analyzed. Carbonylated proteins were labeled with a specific fluorescence probe (λEx/λEm = 650/665 nm) (Baraibar et al., 2013) and resolved by high-resolution electrophoresis onto 4-20% gradient SDS-PAGE. Proteins were fixed to the gel and carbonylation fluorescence signal was evidenced by fluorescence scanning. Total proteins were post-stained with SyproRubyTM protein gel stain (Molecular Probes, Invitrogen). Gel image acquisition for carbonylated and total proteins by differential fluorescence was performed using the iBright Imaging System (Thermo Fisher Scientific). Densitometric analysis of protein bands was performed using Image J analysis software (Schneider et al., 2012). The quantification was obtained from each sample, both for carbonylated and total proteins. Carbonylated proteins fluorescence signal was normalized by total protein signal for each sample in order to obtain the Carbonyl Score by using the following formula:
Carbonyl Score = Carbonylated Proteins fluorescence signal (RFU) / Total Proteins fluorescence signal (RFU).
2.5. Statistical analysis
All statistical analyses were performed using R software (version 1.2.5033). Normality was determined using Shapiro-Wilk test, before using multiple comparisons tests (Mann Whitney U test for non-parametric data, and One or Two-Ways ANOVA with Tukey’s Post Hoc comparisons tests). Survival curves were analyzed using the non-parametric Kaplan-Meier Log Rank Survival test.
3. Results
3.1. Impact of PAH and pesticides diet on survival probability and syrup consumption
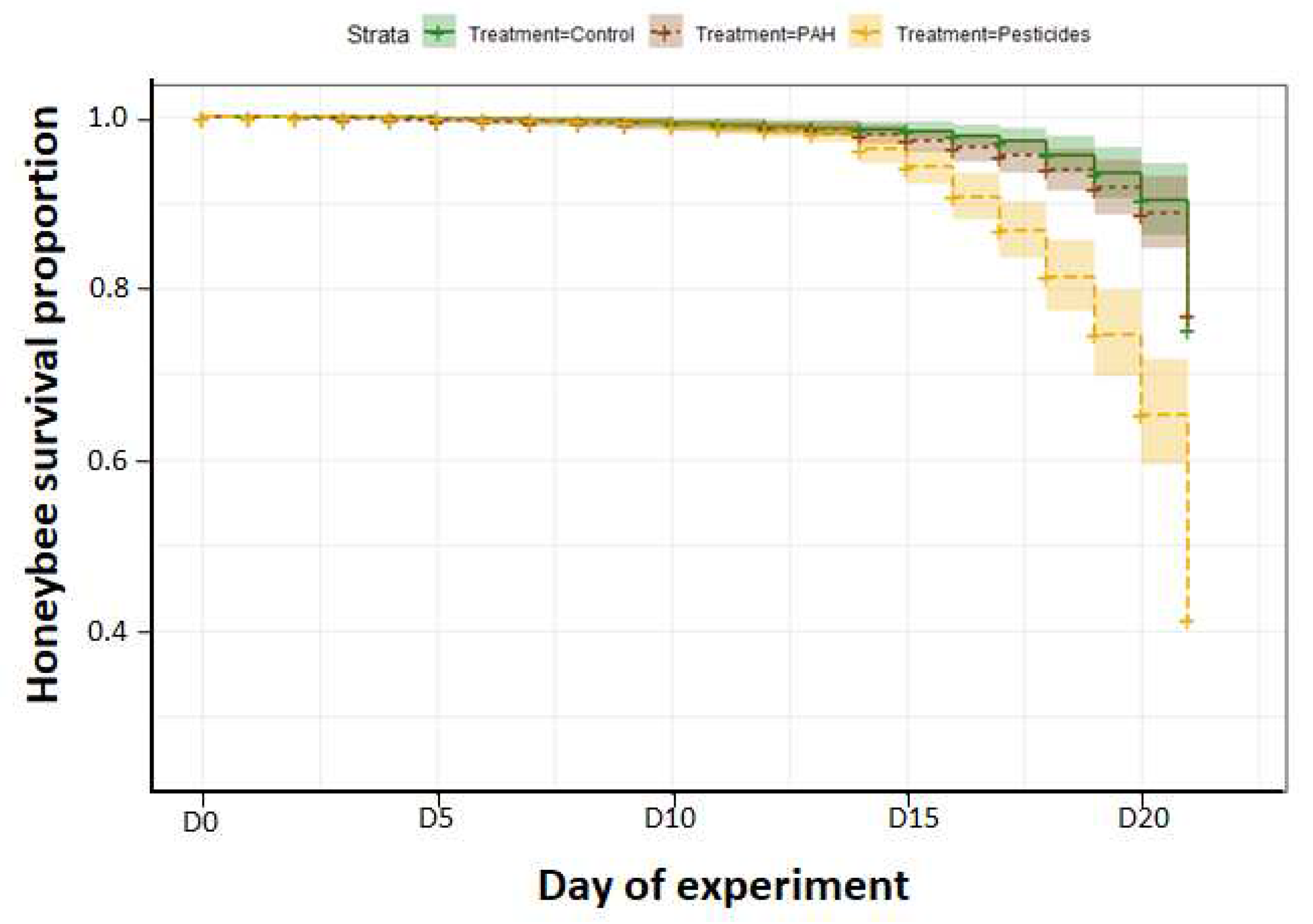
Figure 2 presents survival curves associated with each diet treatment (control, pesticides, and PAHs). Results show that the 3 treatment groups significantly differ in survival (Log-rank test, χ
2 = 74.2, df = 2, p < 0.001), with pesticides diet group having a more pronounced mortality curve from two weeks of experiment. By the end of the 21 days of experiment, we observe about 75% survival in control and PAHs groups whereas there is only 40% survival in pesticides group.
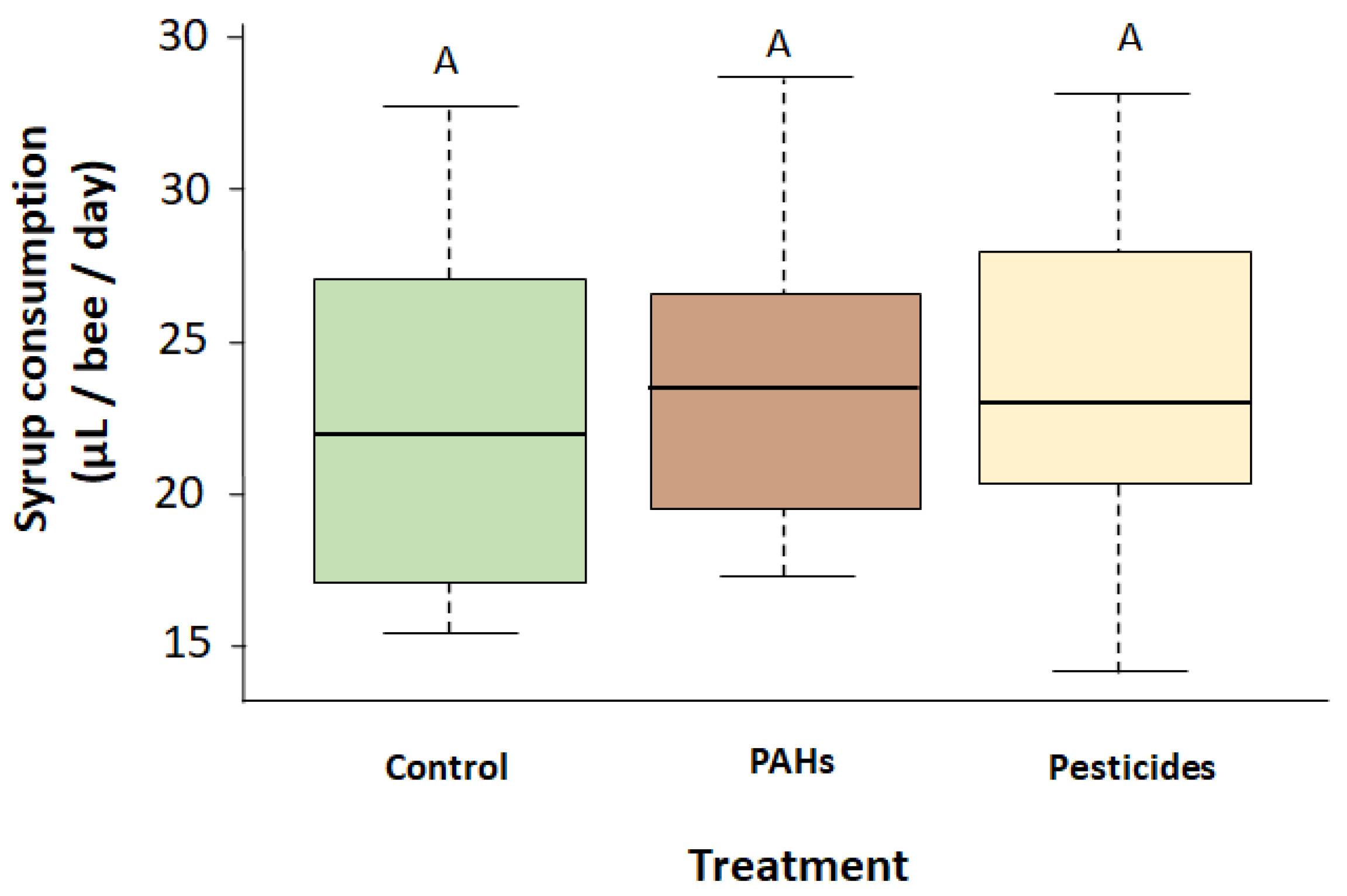
Pollutant diet had no effect on the syrup consumption (ANOVA, F
1,48 = 0.167, p = 0.685), neither concentration of the solution (ANOVA, F
2,48 = 0.160, p = 0.853), nor the interaction between pollutant diet and concentration (ANOVA, F
2,48 = 1.874, p = 0.165). We found no significant difference of consumption between control group and the two pollutant diet groups (ANOVA, F
2,60 = 0.218, p = 0.805,
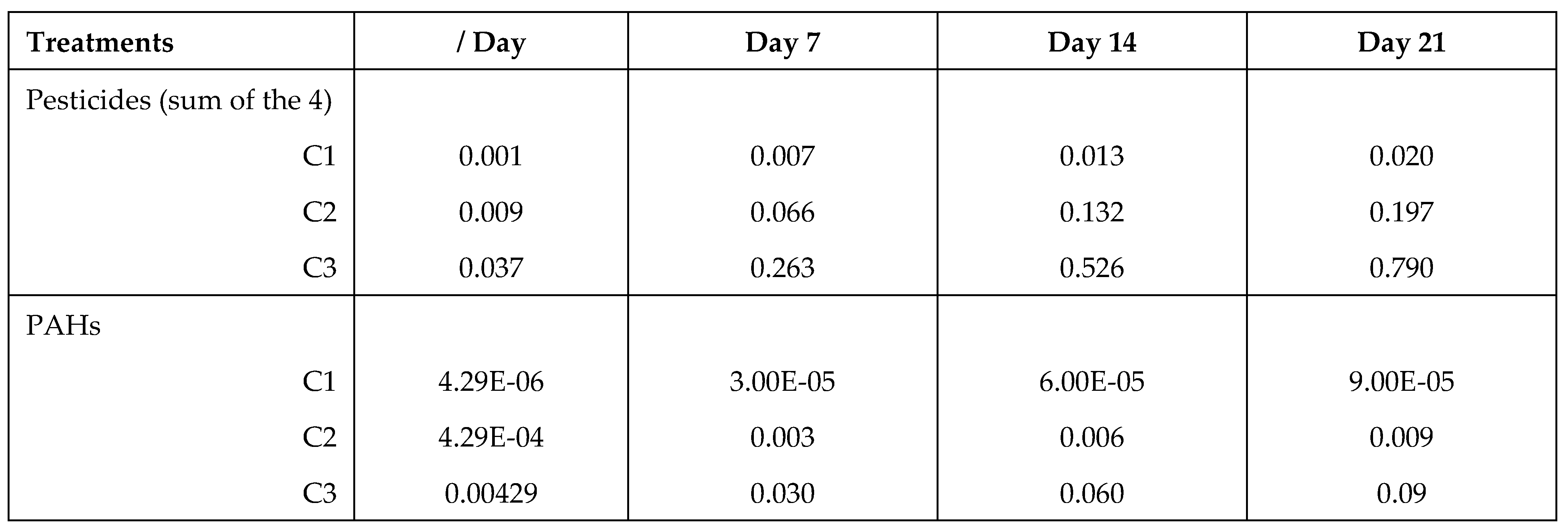
Figure 3). The mean daily consumption of syrup was 23.5 ± 1.93 µL/bee and the total consumption of pollutants per bee is presented in
Table 2.
3.2. Impact of PAH and pesticides diet on oxidative stress of honeybees
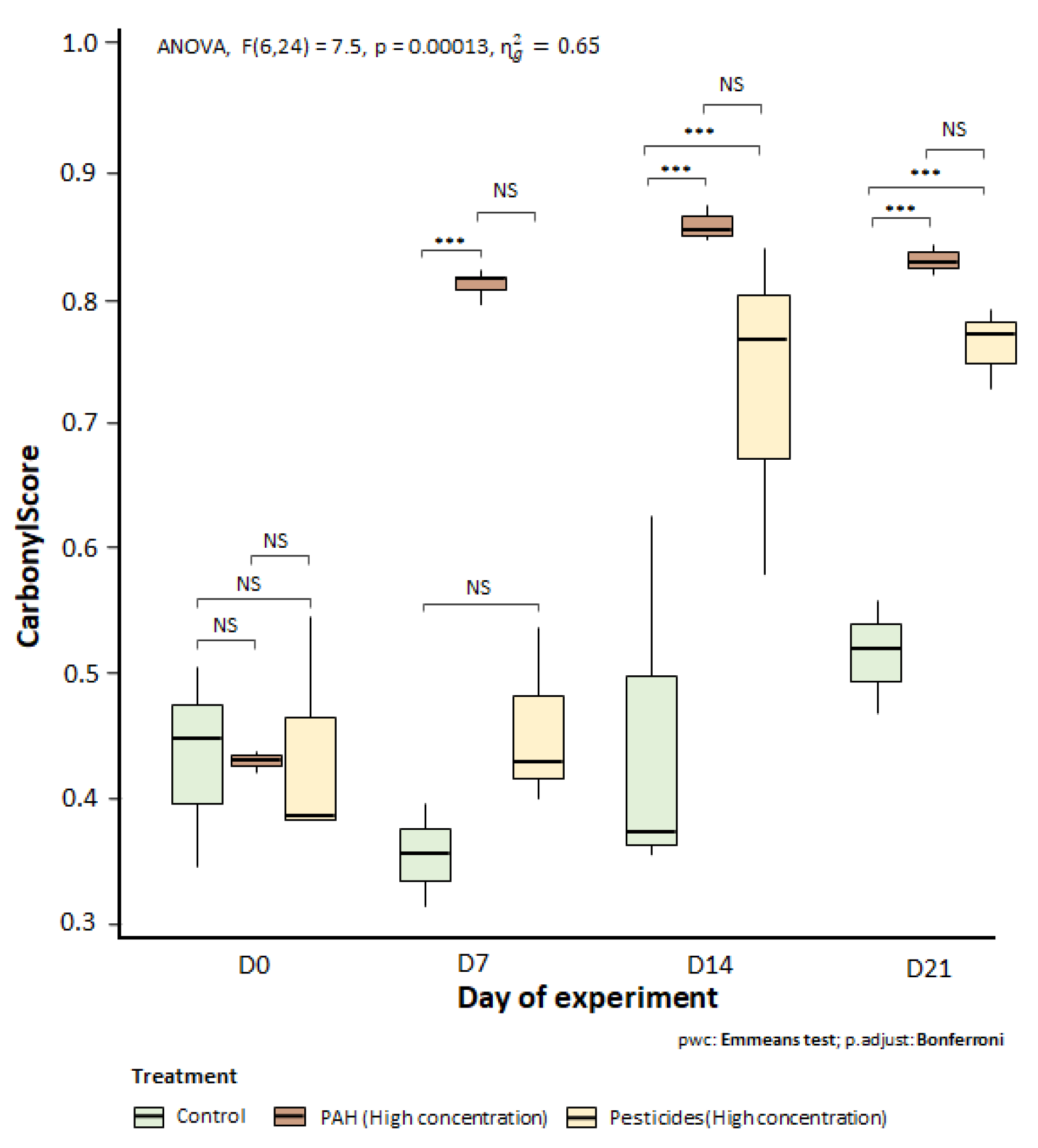
Figure 4 shows significant impact of pesticides (high concentration) and PAHs (high concentration) diet on oxidative stress compared to control group. Significant impact for PAHs diet has been detected from 7 days of experiment (ANOVA, p < 0.001) whereas significant impact of pesticides diet on oxidative stress has been detected from 14 days of experiment (ANOVA, p < 0.001). By the end of the experiment, impact of both pollutant diets was also significant (ANOVA, p < 0.001).
3.3. Field case study
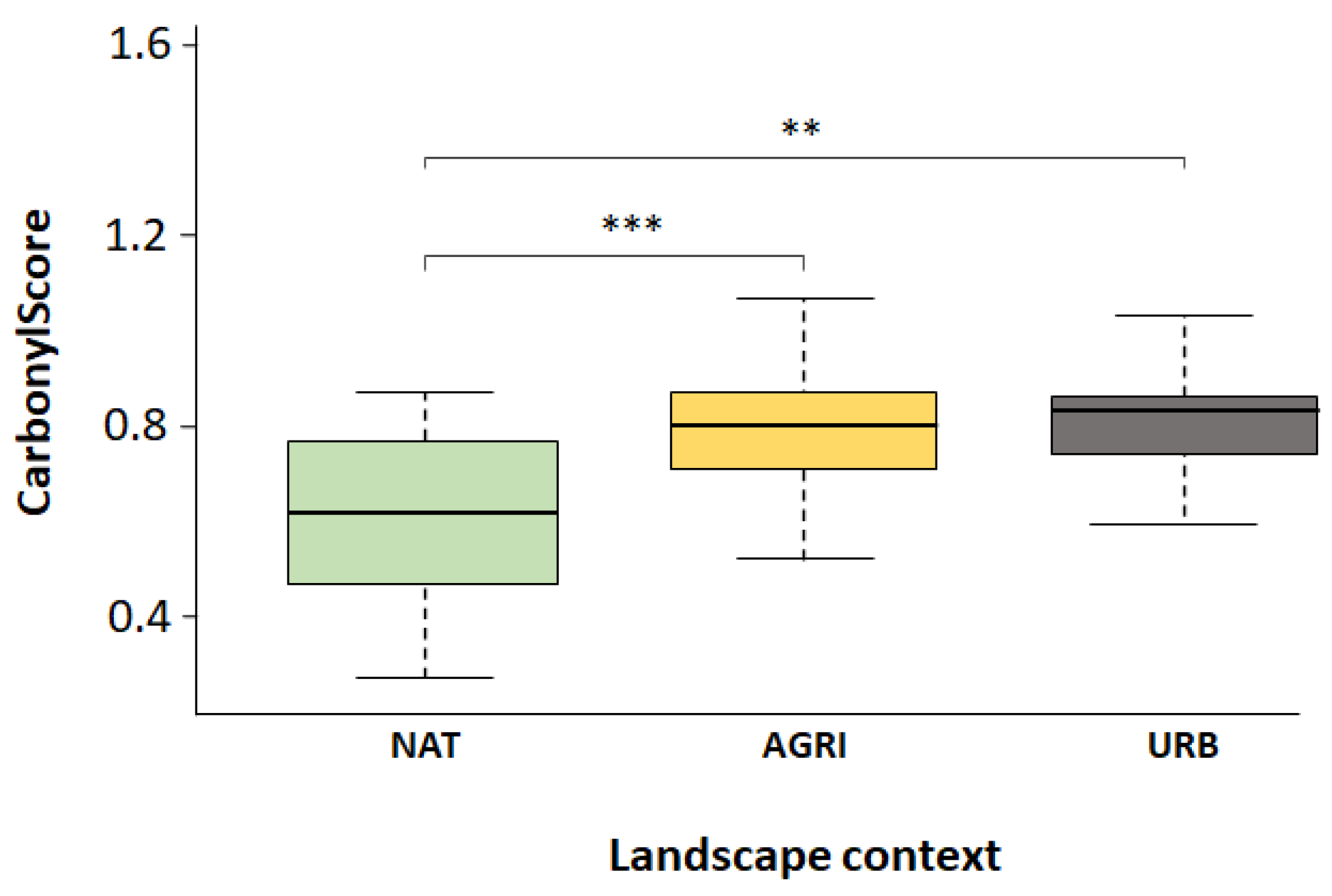
We found a significant effect of landscape context on the oxidative stress (ANOVA, 𝐹
2,77 = 9.577, p < 0.001;
Figure 5B). Honeybees sampled in semi-natural lands were found to have weaker carbonylscore than those sampled in agricultural or urban lands (natural lands: 0.62 ± 0.18, agricultural lands: 0.81 ± 0.20, urban lands: 0.80 ± 0.12).
The comparison between the mean carbonylscore of honeybees from our control group (lab study) and the mean carbonylscore of honeybees from the semi-natural groups showed a significant difference (Wilcoxon test, W = 50, p = 0.0068, n = 32). Honeybees from the semi-natural group of our field study had a significantly higher oxidative stress measure than honeybees from the control group of the lab study.
4. Discussion and conclusion
The present work was composed of one lab study and one field study. The first one was designed to investigate the impact of the chronic (long-term) oral consumption of two widespread types of pollutants: pesticides (insecticides + fungicide) and PAHs on honeybee’s oxidative stress, in controlled conditions. Bee mortality was also measured in this study. The field case study was used to check whether it was possible, in real conditions, to measure significant differences in oxidative stress according to the environmental landscape context and therefore to measure the impacts of a policy to reduce pollutants in urban and rural areas.
Results of the lab study show that chronic ingestion of pesticides impact significantly more survival than chronic ingestion of PAHs. Some studies have reported an impact of pesticides on carbohydrate regulation and syrup intake (Chakrabarti et al., 2020; Cook SC 2019). In our study, honeybees were provided pollen
ad libitum and the dissolution of both pollutants in sucrose syrup did not impact syrup consumption (
Figure 3). Thus, we can suppose that difference of mortality is not due to feeding suppression (or feeding rate increase) but to the pollutants dissolved in the syrup, although we cannot rule out the impact of containment on survival (Alburaki et al., 2019). Bees have fewer genes encoding detoxifying proteins than other insects, which may explain their sensitivity, to pesticides (Claudianos et al., 2006).
Regarding PAHs groups, although their carbonylscore were high, honeybee survival was about 75% at the end of the experiment and not significantly different from the control group. Thus, oxidative stress linked to PAHs consumption does not directly induce lethal effects. To our knowledge, this is the first study considering the lifespan reduction and the sublethal impacts of PAHs on honeybees. Moreover, a recently published study has shown that the oxidative potential of urban PM are threefold-higher than rural PM (Daellenbach et al 2020). Our results are consistent with this study and show that PAHs alone are 1.5 and 1.15 more oxidant than pesticides (from 7 and 21 days of experiment respectively). Moreover and contrary to what we expected, the negative impact of PAHs on oxidative stress not only was stronger but also faster than that of pesticides.
Our lab experiment only considers the impact of chronic ingestion of pollutants and not the exposure by inhalation or contact. Direct contact exposure to pesticides experiments conducted on newly emerged honeybee are not realistic since foraging behavior do not begin before 18-28 days of life (ref Quigley et al. book chapter). Indirect contact through beebread and oral ingestion seems to be closer to reality, that’s why we chose oral ingestion exposure. In our study, we assume constant pesticide consumption, in order to investigate the worst-case scenario. However, we are aware that the concentration of pesticide residues in the nectar decreases over time (Choudhary and Sharma, 2008; Sponsler et Johnson, 2017). Thus, our field study makes it possible to consider all the routes of exposure to pollutants, and to measure their sublethal impacts thanks to the oxidation of proteins.
As stated in the literature, current studies should focus on the impacts of cocktails of pollutants (PAHs, nitrous oxide, ozone, particulate matter, and pesticides) on sublethal effects (Li et al. 2019). Our field study has attempted to provide some answers to this gap in knowledge. Significant differences in oxidative stress level were detected between honeybees sampled in semi-natural landscape and honeybees sampled in more anthropized areas (agricultural and urbanized). A previous study from Cochard et al (2020) has shown that the concentration of PAHs detected in honeybee were significantly impacted by anthropization level: the more landscape was anthropized, the higher the level of PAHs detected in honeybees. Here, oxidative stress of honeybees sampled in agricultural landscape was not significantly different from honeybees sampled in urban landscape. Considering our present study, this result means that honeybees collected from agricultural areas experience a similar oxidative stress than honeybees collected in urban areas. On the contrary, honeybees collected in semi-natural lands seems to experience less oxidative stress compared to the two other environments. However, the level of oxidative stress from semi-natural areas collected bees was still significantly higher than control groups of our lab study.
Our study brought new insights on sublethal effects of two widespread pollutants (pesticides and PAHs), and general environmental pollution on honeybees’ health. To our opinion, oxidative stress measurement could be used as a first step to detect environmental stress in honeybees before starting further research on the presence of possible pollutants in the environment of beehives. It will also be possible to determine the impact of reducing pollutant emissions into various environments.
Author Contributions
P.C. and B.P. conceived the study and conducted experiments, A.C. and M.B. carried out oxidative stress measurements, P.C. performed statistical analysis, P.C. wrote the manuscript in consultation with B.P., A.C. and M.B.
References
- Almasri, H. et al. Mixtures of an insecticide, a fungicide and a herbicide induce high toxicities and systemic physiological disturbances in winter Apis mellifera honey bees. Ecotoxicol. Environ. Saf. 203, (2020). [CrossRef]
- Azpiazu, C. et al. Chronic oral exposure to field-realistic pesticide combinations via pollen and nectar: effects on feeding and thermal performance in a solitary bee. Sci. Rep. 9, 1–11 (2019). [CrossRef]
- Badawy, M. E. I. , Nasr, H. M. & Rabea, E. I. Toxicity and biochemical changes in the honey bee Apis mellifera exposed to four insecticides under laboratory conditions. Apidologie 46, 177–193 (2015). [CrossRef]
- Baraibar MA, Ladouce R, Friguet B. (2013). Proteomic quantification and identification of carbonylated proteins upon oxidative stress and during cellular aging. J Proteomics 30 (92) :63-70. [CrossRef]
- Benuszak, J. , Laurent, M. & Chauzat, M. P. The exposure of honey bees (Apis mellifera; Hymenoptera: Apidae) to pesticides: Room for improvement in research. Sci. Total Environ. 587–588, 423–438 (2017). [CrossRef]
- Botías, C. , David, A., Hill, E. M. & Goulson, D. Quantifying exposure of wild bumblebees to mixtures of agrochemicals in agricultural and urban landscapes. Environ. Pollut. 222, 73–82 (2017). [CrossRef]
- Bradford Marion, M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72 (1–2), 248-254.
- Chakrabarti, P. , Carlson, E. A., Lucas, H. M., Melathopoulos, A. P. & Sagili, R. R. Field rates of SivantoTM (flupyradifurone) and Transform® (sulfoxaflor) increase oxidative stress and induce apoptosis in honey bees (Apis mellifera L.). PLoS One 15, e0233033 (2020).
- Choudhary, A. & Sharma, D. C. Dynamics of pesticide residues in nectar and pollen of mustard (Brassica juncea (L.) Czern.) grown in Himachal Pradesh (India). Environ. Monit. Assess. 144, 143–150 (2008). [CrossRef]
- Cochard, P. et al. PAH7 concentration reflects anthropization: A study using environmental biomonitoring with honeybees. Sci. Total Environ. 751, 141831 (2021). [CrossRef]
- Conti, M. E. & Botrè, F. Honeybees and their products as potential bioindicators of heavy metals contamination. Environ. Monit. Assess. 69, 267–282 (2001). [CrossRef]
- Daellenbach, K. R. et al. Sources of particulate-matter air pollution and its oxidative potential in Europe. Nature 587, 414–419 (2020). [CrossRef]
- Gizaw, G. et al. Effect of environmental heavy metals on the expression of detoxification-related genes in honey bee Apis mellifera. Apidologie 51, 664–674 (2020). [CrossRef]
- Gonalons, C. M. & Farina, W. M. Impaired associative learning after chronic exposure to pesticides in young adult honey bees. J. Exp. Biol. 221, (2018). [CrossRef]
- Goretti, E. et al. Heavy metal bioaccumulation in honey bee matrix, an indicator to assess the contamination level in terrestrial environments. Environ. Pollut. 256, 113388 (2020). [CrossRef]
- Hayat, K. , Afzal, M., Aqueel, M. A. & Khan, Q. M. Determination of Insecticide Residues in Fruits, Vegetables, Pollen, Nectar and Ground Water of Punjab (Pakistan). J. Agric. Res. 56, 95–105 (2018).
- Lambert, O. et al. Polycyclic aromatic hydrocarbons: Bees, honey and pollen as sentinels for environmental chemical contaminants. Chemosphere 86, 98–104 (2012). [CrossRef]
- Leita, L. , Muhlbachova, G., Cesco, S., Barbattini, R. & Mondini, C. Investigation of the use of honey bees and honey bee products to assess heavy metals contamination. Environ. Monit. Assess. 43, 1–9 (1996). [CrossRef]
- Lewis, K.A. , Tzilivakis, J., Warner, D. and Green, A. (2016) An international database for pesticide risk assessments and management. Human and Ecological Risk Assessment: An International Journal, 22(4), 1050-1064. [CrossRef]
- Liu, H. et al. An oxidative stress response to polycyclic aromatic hydrocarbon exposure is rapid and complex in Arabidopsis thaliana. Plant Sci. 176, 375–382 (2009). [CrossRef]
- Lupi, D. et al. Effects of Pesticides and Electromagnetic Fields on Honeybees: A Field Study Using Biomarkers. Int. J. Environ. Res. 14, 107–122 (2020). [CrossRef]
- Nicewicz, Ł. , Nicewicz, A. W., Kafel, A. & Nakonieczny, M. Set of stress biomarkers as a practical tool in the assessment of multistress effect using honeybees from urban and rural areas as a model organism: a pilot study. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef]
- Perugini, M. et al. Heavy metal (Hg, Cr, Cd, and Pb) contamination in urban areas and wildlife reserves: Honeybees as bioindicators. Biol. Trace Elem. Res. 140, 170–176 (2011). [CrossRef]
- Pettis, J. S. , Vanengelsdorp, D., Johnson, J. & Dively, G. Pesticide exposure in honey bees results in increased levels of the gut pathogen Nosema. Naturwissenschaften 99, 153–158 (2012). [CrossRef]
- Pichaud, N. et al. Oxidative stress and immunologic responses following a dietary exposure to PAHs in Mya arenaria. Chem. Cent. J. 2, (2008). [CrossRef]
- Santana, M. S. et al. Biomarker responses in fish exposed to polycyclic aromatic hydrocarbons (PAHs): Systematic review and meta-analysis. Environ. Pollut. 242, 449–461 (2018). [CrossRef]
- Schneider, C. , Rasband, W. & Eliceiri, K. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9, 671–675. [CrossRef]
- Sponsler, D. B. & Johnson, R. M. Mechanistic modeling of pesticide exposure: The missing keystone of honey bee toxicology. Environ. Toxicol. Chem. 36, 871–881 (2017). [CrossRef]
- Subashchandrabose, S. R. , Wang, L., Venkateswarlu, K., Naidu, R. & Megharaj, M. Interactive effects of PAHs and heavy metal mixtures on oxidative stress in Chlorella sp. MM3 as determined by artificial neural network and genetic algorithm. Algal Res. 21, 203–212 (2017). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).