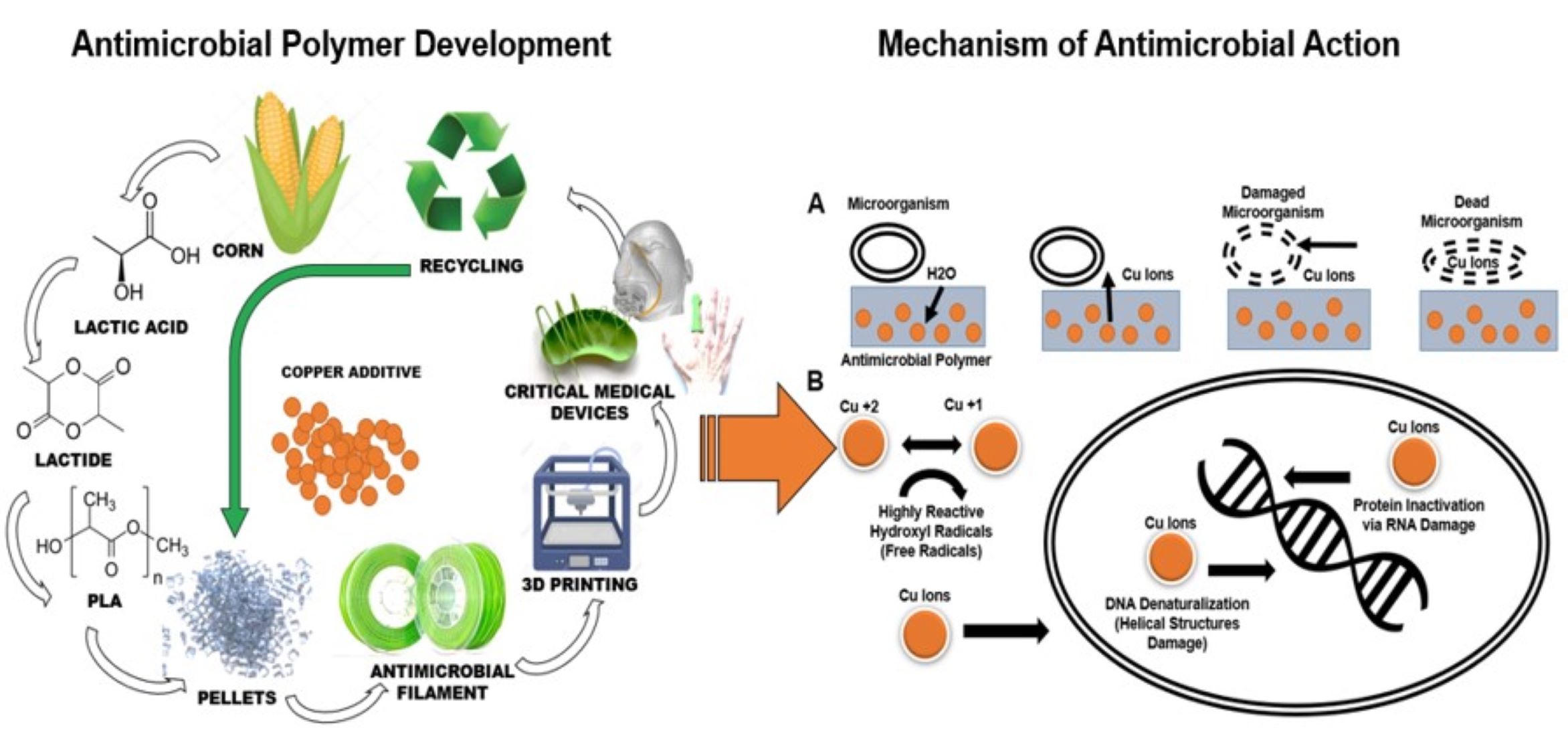
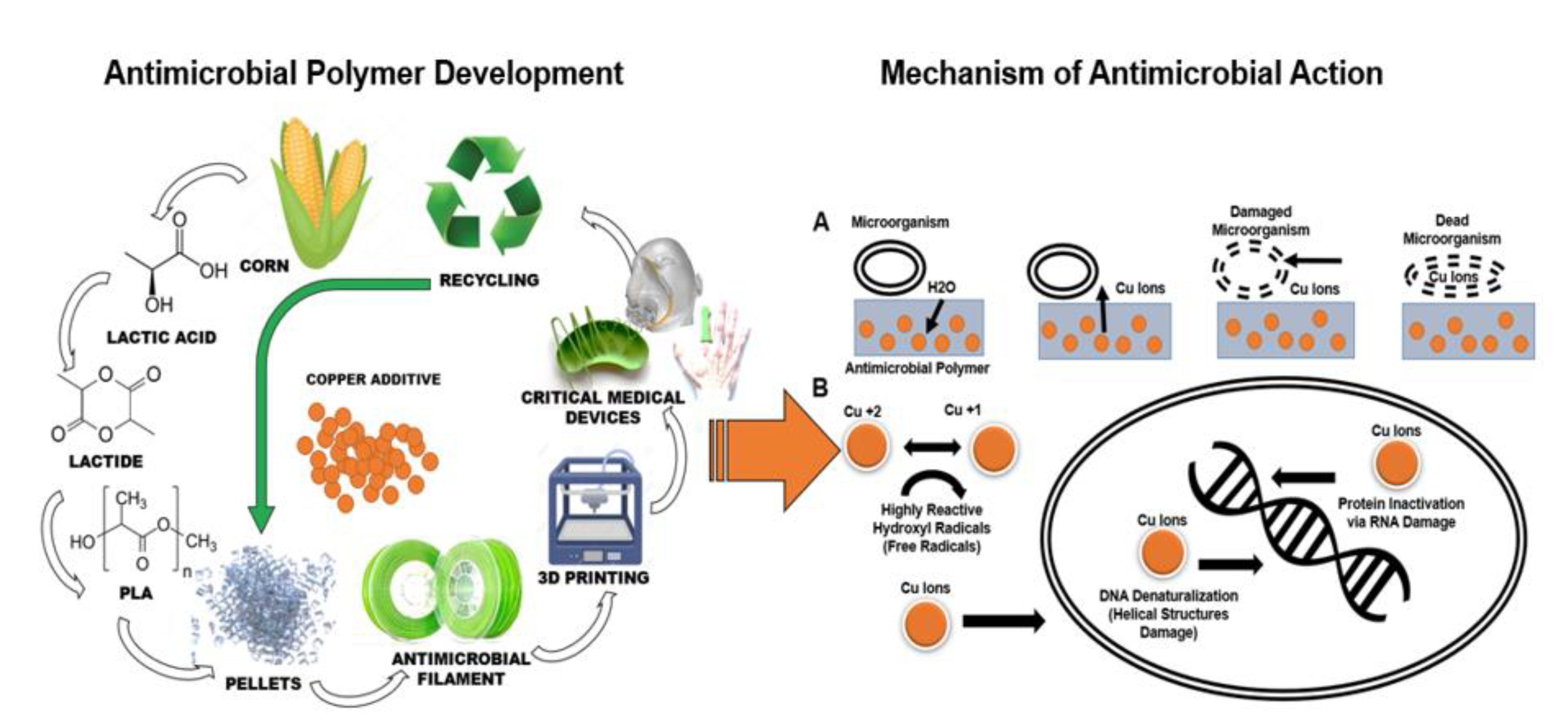
1. Introduction
The emergence and spread of SARS-CoV-2 and its variants, the causative agent responsible for the COVID-19 pandemic, resulted in a surge of cases across all continents.1,2 The Defense Protection Act was invoked by the executive governmental branch of the U.S. to increase the domestic production of medical supplies necessary for combating the current pandemic.1,2 The invocation encouraged private businesses to increase domestic production of Personal Protective Equipment (PPE) and other critical medical devices in demand during the pandemic. The Food and Drug Administration (FDA) reported that the COVID-19 outbreak significantly impacted the medical product supply chain, including supply disruptions and mass shortages of critical medical products in the U.S.3,4
Previous investigations have proposed additive manufacturing (i.e., 3D Printing) as a potential manufacturing method to assist with the shortage of critical medical devices.5 Current advancements in additive manufacturing techniques and the development of antimicrobial polymers offer the possibility of printing and customizing a wide range of critical medical devices.5 One of the main limitations for the use of polymeric materials to manufacture critical medical devices is the risk of material contamination by bacteria and viruses.2,5,6 Previous investigations have shown strong evidence to support the use of copper-based compounds as a biocidal agent7 and to enhance the antimicrobial properties of polymers used in the development of medical devices.5,6,8,9 The use copper-based compound in polymeric matrices offers many benefits beyond those using silver compounds. Specifically, recent evidence suggesting that unlike silver, copper is naturally more effective due to their dual oxidative states (oxidative and superoxidative)10, effective across all temperatures and all levels of humidity,11 and a significantly lower bacterial resistance12 than silver. Furthermore, the cost of these metals for bulk production is very different. For example, the average price for silver is approximately $400/pound, while the average price for copper is approximately $3.50/pound, less than 1% the price of silver.13 Lastly, embedding copper particles into a polymeric matrix not only enhanced the natural properties of copper, but also enhanced those characteristics that have clinical relevance. For example, beyond the fast, strong, and long lasting antimicrobial properties of copper additives 2,5,6,14 copper ions have also been found to improve the healing process of wounds15,16 as it plays a key role in the enhancement of angiogenesis, via induction of vascular endothelial growth factor, up-regulating the activity of copper- dependent enzymes, cell proliferation and reepithelization.15,16
Biopolymers are materials that either occur naturally or are synthesized from naturally occurring biological materials, including oils, fats, sugars, or starch.
17 Unlike petroleum-based polymers, biodegradable polymers are made out of components that can be metabolized by naturally occurring microorganisms in the environment.
17 Polylactic acid has been described as a commodity biopolymer that is derived from an annually renewable resource, in this case, corn (
Figure 1).
18 Thus, the advantages of polylactic acid makes this biopolymer a great candidate for the development of an antimicrobial biopolymers compatible with additive manufacturing to develop on demand and customized medical devices. Furthermore, the current antimicrobial biopolymer could significantly assist the current medical product supply chain disruptions involving the manufacturing of critical medical devices in austere clinical settings.
19 Unfortunately, there is limited knowledge about the effectiveness, longevity, and cytotoxicity of copper-based compounds embedded into a Polylactic acid matrix. Specifically, it is unknown if a biopolymer with a copper additive would retain the ability to neutralize viruses or to demonstrate a broad long-lasting biocidal effect. Thus, the purpose of the current investigation was to assess the antimicrobial efficacy, safety, and longevity of a polylactic acid-based biopolymer embedded with a copper-based composite additive. It was hypothesized that the polylactic acid-based biopolymer embedded with a copper-based composite would be highly effective against several bacterial and viral strains, remaining non-toxic and safe to skin contact. Our hypothesis was based on our preliminary investigations
2,5,6,8,9,20-25 showing strong biocidal effects of a polylactic acid-based biopolymer supplemented with a copper-based composite additive.
2. Materials and Methods
Antimicrobial Effectiveness and Longevity
Antimicrobial effectiveness was evaluated according to ISO 22196 standard protocol, designed to measure antimicrobial properties of solid plastic surfaces, by an independent laboratory (Situ Biosciences LLC, Wheeling, IL, Chicago, USA). Twelve flat test samples (5 cm x 5cm x 1 cm) were manufactured using a polylactic acid-based biopolymer supplemented with a copper-based composite additive at 1% the polymer weight (antimicrobial biopolymer). The samples were incubated for 24 hours with Staphylococcus Aureus (S. aureus), Methicillin-resistant Staphylococcus aureus (MRSA) and Escherichia Coli (E. coli) inoculums, chosen because of their relevance in causing home- and hospital-acquired infections. The first set of samples were autoclaved at 121°C for 20 minutes. The second set of samples were tested as received and following peroxyacetic/ethanol disinfection. Heat acceleration is a common method of assessing longevity using standardized and controlled environmental conditions. Antimicrobial longevity was evaluated according to ISO 22196 standard protocol. Antimicrobial longevity analysis was performed before and after a heat-based aging protocol. The aging protocol consisted in exposing the samples to a heat-based accelerating aging agents set at 55°C using standard oven for 28 days, equivalent to 1 year. Samples were incubated for 24 hours. Following inoculation, bacteria are recovered, and the concentration is determined. Performance of the treated material was measured and reported as Log10 and % reductions relative to the untreated control samples.
HCoV-229E (SARS-CoV-2 surrogate)
The antimicrobial biopolymer was assessed by an independent laboratory (Institut Pasteur de Lille, Pasture Institure Network, Lille, France) according to NF EN 14476+A2 standards used to assess chemical disinfectants and antiseptics against human coronavirus strain 229E (HCoV-229E). The composite, in powder form, was diluted onto hard water and added to a test suspension of virus mixed with an interfering substance (Bovine serum albumin at 0.3 g/L). The additive was tested at concentrations of 5%, 25%, and 50%. The mixture was maintained at 20°C for intervals of 30 seconds, 5 minutes, 1 hour and 4 hours. Following, disinfectant activity is suppressed by filtration through MicroSpin S400 HR column and dilution in ice-cold medium. The resulting solution is transferred into cell culture units and the titer of infectivity is then calculated after incubating for 6-7 days at 33°C with 5% CO2. Titer values were calculated utilizing Spearman and Kärber calculation methods. Virus infectivity reduction was calculated by the differences in log virus titers before and after treatment with the material.
HIV-1
Twenty subsamples of HIV-1, subtype B, were cultivated from an infectious clone NL4-3 with CXCR4 co-receptors (University of Chile, Clinical Hospital, Santiago, Chile) and tested against a medical device manufactured with the antimicrobial biopolymer. A split-sample-testing design with a simple blind, randomized positive and negative control groups was utilized. Subsamples were randomized into experimental and control groups, where experimental samples were further randomized into with and without noncompound groups. Samples were exposed during 15-, 60-, 120-, and 900-seconds to the device using a laboratory hood. All samples were cultured using HIV-1 Jurkat reporter cells LTR-luciferase cells (1G5) and measured at time intervals of 24-, 48-, 72-, and 96-hours post-treatment.
Listeria
A solution of Listeria monocytogenes with around 100 colony forming units per milliliter (CFU/mL) was created in an independent laboratory (Intertek Group plc: Food Services, Derby, DE21 6AS, Derbyshire, England) and tested against a single cone-shaped testing sample manufactured with the antimicrobial biopolymer. 1 mL of the Listeria monocytogenes solution was plated across 2 ALOA plates and incubated according to Listeria enumeration procedures, resulting in a concentrated solution containing 140 CFU/mL. The resultant solution of Listeria monocytogenes was subsequently tested for enumeration of Listeria following 4 hours of exposure to the cone test sample.
3. Results
Antibacterial Effectiveness and Longevity
The bacterial analysis showed that the antimicrobial biopolymer was effective against
S. aureus, MRSA and E. coli inoculums (
Table 1). Specifically, prior to heat-based accelerated aging, antibacterial analysis of
S. aureus, MRSA and
E. coli inoculums exposed to the antimicrobial biopolymer for 24 hours showed a strong bacterial reduction of 99.99% for
S. aureus, 98.95% for
MRSA and 95.03% for
E. coli (
Table 1). Samples were aged for 1-year equivalent (28 days at 55°C) and exposed to the same inoculums for 24 hours. Post-aging results show the antimicrobial biopolymer to be 99.99% effective in reducing
S. aureus and MRSA, as well as 84.56% effective in reducing
E. coli.
Antiviral (HCoV-229E; SARS-CoV-2 surrogate)
The cooper composite additive used in the current antimicrobial biopolymer showed very high antiviral efficacy from the initial 30-seconds of contact, capable of reducing the viral load of Coronavirus by +99.9% in less than one hour. This process is very quick and begins in the first 30 seconds to see a rapid decrease in viral load of 60.189% (Log 0.4), a 90% (Log 1) of reduction at 5 minutes, until reaching a plateau near 60 minutes with a 99.98% of viral reduction (Log 3.6,
Figure 1).
HIV-1
The antimicrobial biopolymer showed a 54.8% average reduction on viral replication of HIV-1 following 48 hours post-treatment within 15 seconds exposure (Log 0.35 [Range: 0.3-0.38]). No differences in exposition time were observed on the device. HIV-1 viral proliferation recovers pre-treatment infectivity levels after 72 hours of exposition compared to the positive control.
Listeria
The tested cone was exposed to a Listeria monocytogenes solution with a final potency of 140 CFU/mL following plating and incubation according to Listeria enumeration procedures. The resultant Listeria colony following 4 hours of exposure to the antimicrobial polymer cone contained <10 colony forming units. Inoculated Listeria colonies were no longer viable after 4 hours of exposure to the antimicrobial biopolymer.
4. Discussion
The main findings of the present investigation showed a strong and long-lasting biocidal effect of a polylactic acid-based biopolymer embedded with a copper-based composite additive against
Staphylococcus Aureus,
MRSA, and
E. coli (
Table 1). The antimicrobial biopolymer also reduced Listeria monocytogenes from its original 140 CFU/mL down to <10 CFU/mL in 4 hours of exposure, eliminating the viability of Listeria to reproduce. Furthermore, the cytotoxicity and safety assessment of the antimicrobial biopolymer was found to be “non-toxic” and safe for human skin contact. When tested against viruses, the antimicrobial biopolymer showed strong effectiveness against the human Coronavirus (HCoV-229E a SARS-CoV-2 surrogate). Viral load reduction began after 30 seconds of exposure by approximately 60.2% (Log 0.4). Furthermore, greater concentrations of copper additive (50% additive concentration) reduced HCoV-229E viral load to 99.8% within 30 seconds of exposure (Log 2.8). Lastly, the antimicrobial biopolymer was able to disrupt HIV-1 proliferation (54.8% effective on average) within 15 seconds of exposure (Log 0.35). These findings confirmed our hypothesis that the current polylactic acid-based biopolymer embedded with a copper-based composite additive was highly effective against several bacterial and viral strains, remaining non-toxic and safe to skin contact.
Prosthetics & Orthotics
The application of antimicrobial biopolymers in prosthetics and orthotics represents a groundbreaking advancement in the field of medical device technology (
Figure 2).
6 These innovative materials are revolutionizing the way these devices are designed and manufactured, offering a multifaceted solution to some of the most pressing challenges faced by amputees and individuals with musculoskeletal conditions. By seamlessly integrating antimicrobial properties into biocompatible polymers, this technology can enhance the longevity and safety of these critical medical devices but also mitigate the risk of infections that is a common complication associated with their use.
7,26 This fusion of biopolymers with antimicrobial capabilities not only promises improved patient outcomes but also underscores the remarkable synergy between material science and medical innovation, ushering in a new era of prosthetic and orthotic care.
6,27
The most common method for 3D printed prostheses is fused deposition modeling.
7 Fused deposition modeling is a form of additive manufacturing that involves melting thin layers of plastic over each other to form a 3D structure.
28 For example, one of the most common 3D printed filament materials used to manufacture upper limb prostheses is polylactic acid.
28 Polylactic acid filament has similar properties to a thermoplastic and permits minor modifications through targeted heating once the device has been 3D printed. Polylactic acid (
Figure 2) is a low-cost material that is biodegradable and possesses thermoforming characteristics that facilitate post-processing and final adjustments of 3D printed prostheses. All these technological advances in the antibacterial properties of 3D printing filaments, along with expertise in the development of 3D printed medical devices, may encourage scientists and clinicians to work together in developing durable and affordable 3D printed prostheses for their patients.
6,29 The current investigation provides strong evidence about the antimicrobial effectiveness, longevity, and safety of the current biopolymer with potential application on prostheses and orthoses.
Food packaging
There are growing concerns about foodborne illnesses and the need to reduce food waste.26 The integration of antimicrobial properties into biodegradable polymers has paved the way for an innovative and sustainable solution.26 These advanced materials not only provide an effective shield against harmful pathogens but also extend the shelf life of perishable products, ultimately enhancing the overall quality and safety of packaged foods.26 In an era where sustainability and health-consciousness are critical, antimicrobial biopolymers in food packaging exemplify the intersection of cutting-edge technology and environmental responsibility, promising a brighter, safer future for the food industry and consumers alike. The current investigation showed that the antimicrobial biopolymer reduced Listeria monocytogenes from 140 CFU/mL down to less than 10 CFU/mL in only 4 hours of exposure, eliminating the viability of Listeria to reproduce. These findings provide new information about the antimicrobial properties of a biopolymer and the potential use in food packaging.
Austere environments
Polylactic acid is a thermoplastic polymer with relatively high-strength that can be made from annually renewable sources (
Figure 2) to manufacture artefacts for use in the biocompatible medical device market.
17 Polylactic acid is a versatile polymer compatible with additive manufacturing, as well as conventional manufacturing methods, such as injection molding.
7 The current and previous investigation suggested that the combination of copper additives with a renewable resource-based polymer can significantly improve the environmental impact when using antimicrobial biopolymers for the manufacturing of medical devices.
17 Previous literature have reported the use of additive manufacturing to develop surgical instruments designed for austere environments. Specifically, previous investigations
19,30 have used standard polylactic acid polymers to develop surgical instruments (i.e., Army/Navy retractor) strong enough to be used in the operating room and capable of supporting 13.6 kg before fracture. Thus demonstrating a potential use to manufacture on-demand surgical instruments
19,30. While high temperature sterilization methods, such as autoclaving, compromise the structural integrity of polylactic acid, lower temperatures methods, such as steam sterilization (i.e., 121°C) can be often tolerated. However, self-sanitizing material would be the appropriate choice for austere environments.
19,30
The hypoallergenic and safe nature of polylactic acid has been previously verified by the FDA and approved as a semi-permanent dermal filler and suture material.19,31 Therefore, the use of antimicrobial polylactic acid-based material has several impactful medical applications with the potential of transforming the manufacturing of medical devices, especially in remote areas.30 Currently, the sterilization of medical devices during natural disasters in remote areas, depends on portable large-chamber stream sterilizers which introduces a significant logistical burden, significantly impacting efforts to provide surgical interventions.30 Thus, the current research findings are critical for the development of a variety of antimicrobial medical devices to address potential supply chain problems involving medical care in austere medical environments.
Human space exploration
Another area of potential growth for the implementation of antimicrobial biopolymers compatible with additive manufacturing is human space exploration.32-34 Currently, experimental flight data has indicated altered astronaut immune function and microbial virulence during spaceflight suggesting heightened risk of contracting infectious disease during spaceflight missions.32 Additionally, several crew members expressed chronic hypersensitivity reactions in response to dysregulated immune system functioning, which could limit space mission longevity.32 Previously conducted experiments during spaceflight suggest altered microbial growth, and increased virulence and growth rates in a microgravity environment, ultimately increasing risk to a healthy population introduced to the environment. Altered microbial behavior, in conjunction with modified host susceptibility due to immune system dysregulation, threatens the health and safety of astronauts in the spaceflight environment compared to that in a normal workplace environment. The demand for multipurpose antimicrobial materials that are safe, and recyclable are of critical importance in facilitating safer, and eventually longer, spaceflight missions. For example, on earth, a cough or sneeze can spread infectious particles 3 to 6 feet, limited by gravitational forces.34 In the microgravity environment of space, infectious particles can remain airborne for prolonged periods of time leading to heightened risk of infection.34 A previous investigation suggests wearing personal protection equipment, such as respirators or face masks, can be beneficial to non-ill astronauts accompanying peers expressing signs or symptoms of a respiratory tract infection.34 Additionally, reusable facemasks still need to be sterilized between uses to curb transmission of viruses, such as COVID-19, and ensure proper protection. The previously reported immune dysfunction of astronauts in space flights, in conjunction with the microgravity-enhanced aerobiology of aerosols created from a cough or sneeze, demand the need for novel developments in preventative countermeasures that reduce the risk of viral infections in space.32-34 Thus, the results of the current investigation supported the use of antimicrobial biopolymer for development of an array of medical devices that require bacterial control, such as personal equipment, orthoses, wound dressings, and surgical equipment for human space exploration.
5. Conclusions
The present investigation showed that the antimicrobial biopolymer exhibits strong and long-lasting biocidal properties against an array of viral and bacterial inoculants. Additive manufacturing utilizing the evaluated biopolymer facilitates rapid manufacturing of critical medical devices, beneficial during global supply shortages such as that recently induced by the novel coronavirus. Furthermore, applications can extend to manufacturing medical devices that are in contact with the skin, such as prosthetic and orthotics that are susceptible to microbial and viral growth in austere environments, including natural disasters, and astronauts in space.
Author Contributions
Conceptualization, J.Z.; methodology, J.Z.; formal analysis, J.Z., A.E. and A.D.; investigation, A.E. and A.D.; resources, A.E. and A.D.; writing original draft preparation, J.Z.; writing—review and editing, A.E. and A.D.; funding acquisition, J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NASA EPSCoR grant number 21-EPSCoR2021-0023 and NASA CAN grant number 80NSSC21M0272.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. Coronavirus disease (COVID-19) Pandemic. Accessed , 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- Cortes AA, Zuñiga JM. The use of copper to help prevent transmission of SARS-coronavirus and influenza viruses. A general review. Diagnostic Microbiology and Infectious Disease. 2020/12/01/ 2020, 98, 115176. [Google Scholar] [CrossRef]
- FDA. Personal Protective Equipment for Infection Control. Accessed , 2020. https://www.fda.gov/medical-devices/general-hospital-devices-and-supplies/personal-protective-equipment-infection-control.
- FDA. Coronavirus (COVID-19) Supply Chain Update. Accessed , 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-supply-chain-update.
- Zuniga JM, Cortes A. The role of additive manufacturing and antimicrobial polymers in the COVID-19 pandemic. Expert review of medical devices. Jun 2020, 17, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, J. 3D Printed Antibacterial Prostheses. Applied Sciences. 2018, 8, 1651. [Google Scholar] [CrossRef]
- 7. Gonzalez-Henriquez CM, Sarabia-Vallejos MA, Rodriguez Hernandez J. Antimicrobial Polymers for Additive Manufacturing. International journal of molecular sciences. [CrossRef]
- Palza H, Nunez M, Bastias R, Delgado K. In situ antimicrobial behavior of materials with copper-based additives in a hospital environment. International journal of antimicrobial agents. Jun 2018, 51, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Palza H, Quijada R, Delgado K. Antimicrobial polymer composites with copper micro- and nanoparticles: Effect of particle size and polymer matrix. Journal of Bioactive and Compatible Polymers. 2015, 30, 366–380. [Google Scholar] [CrossRef]
- Lemire JA, Harrison JJ, Turner RJ. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nature Reviews Microbiology. 2013/06/01 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Michels HT, Noyce JO, Keevil CW. Effects of temperature and humidity on the efficacy of methicillin-resistant Staphylococcus aureus challenged antimicrobial materials containing silver and copper. Lett Appl Microbiol. Aug 2009, 49, 191–5. [Google Scholar] [CrossRef]
- 12. Minoshima M, Lu Y, Kimura T, et al. Comparison of the antiviral effect of solid-state copper and silver compounds. J Hazard Mater. [CrossRef]
- Statistics USBoL. Producer Price Index - Metals and metal products. Accessed September, 22, 2023. https://www.bls.gov/regions/mid-atlantic/data/producerpriceindexmetals_us_table.
- Zuniga JM, Thompson M. Applications of antimicrobial 3D printing materials in space. Journal of 3D Printing in Medicine. 2019, 3, null. [Google Scholar] [CrossRef]
- 15. Das A, Sudhahar V, Chen G-F, et al. Endothelial Antioxidant-1: a Key Mediator of Copper-dependent Wound Healing in vivo. Scientific reports, 3378. [CrossRef]
- Sen CK, Khanna S, Venojarvi M, et al. Copper-induced vascular endothelial growth factor expression and wound healing. American journal of physiology Heart and circulatory physiology. May 2002, 282, H1821–7. [Google Scholar] [CrossRef] [PubMed]
- McKeen, L. 12 - Renewable Resource and Biodegradable Polymers. In: McKeen L, ed. The Effect of Sterilization on Plastics and Elastomers (Third Edition), 3: Andrew Publishing; 2012, 2012. [Google Scholar]
- Council NR. Commodity Polymers from Renewable Resources: Polylactic Acid. National Academies Press (US). 2020. https://www.ncbi.nlm.nih.gov/books/NBK44131/.
- Rankin TM, Giovinco NA, Cucher DJ, Watts G, Hurwitz B, Armstrong DG. Three-dimensional printing surgical instruments: are we there yet? The Journal of surgical research. Jun 15 2014, 189, 193–7. [Google Scholar] [CrossRef]
- Borkow G, Covington CY, Gautam B, et al. Prevention of human immunodeficiency virus breastmilk transmission with copper oxide: proof-of-concept study. Breastfeeding medicine: the official journal of the Academy of Breastfeeding Medicine. Aug 2011, 6, 165–70. [Google Scholar] [CrossRef]
- BORKOW G, GABBAY J. Putting copper into action: copper-impregnated products with potent biocidal activities. The FASEB Journal. 2004, 18, 1728–1730. [Google Scholar] [CrossRef]
- Borkow G, Lara HH, Covington CY, Nyamathi A, Gabbay J. Deactivation of human immunodeficiency virus type 1 in medium by copper oxide-containing filters. Antimicrobial agents and chemotherapy. Feb 2008, 52, 518–25. [Google Scholar] [CrossRef]
- Borkow G, Sidwell RW, Smee DF, et al. Neutralizing viruses in suspensions by copper oxide-based filters. Antimicrobial agents and chemotherapy. Jul 2007, 51, 2605–7. [Google Scholar] [CrossRef] [PubMed]
- Borkow G, Zhou SS, Page T, Gabbay J. A novel anti-influenza copper oxide containing respiratory face mask. PloS one. Jun 25 2010, 5, e11295. [Google Scholar] [CrossRef]
- Gadi B, Jeffrey G. Copper as a Biocidal Tool. Current Medicinal Chemistry. 2005, 12, 2163–2175. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).