Introduction
The introduction of endovascular devices during percutaneous arterial interventions disrupts the homeostatic molecular crosstalk between the bloodstream platelets, leukocytes, and vascular endothelial cells. Disruption of these essential cell-cell interactions triggers a pathogenic thrombo-inflammatory response, leading to the development of life-threatening complications over an extended period. While drug-eluting stents can mitigate the inflammatory and proliferative responses, they also impede arterial healing, necessitating a prolonged regimen of dual antiplatelet therapy to counteract the heightened risk of thrombosis [
1].
Given that thrombotic complications associated with drug-eluting stents consistently arise due to the cytotoxic effect of released drugs on endothelial cells, perpetrating exposure of the device surface to blood clotting factors, a range of strategies have been explored to enhance endothelial cell adherence to endovascular devices. These strategies include the application of surfaces coated with agents capable of capturing endothelial cell progenitors, as well as the development of biomimetic surfaces inspired by well-known endothelial cell supports such as fibrin or extracellular matrix.
However, although these strategies effectively promote endothelial cell adherence to device surfaces, their anticipated benefits have not been realized, neither for CoCr in coronary stenosis [
2] nor for flow diverters in the treatment of cerebral aneurysms [
3].
Interestingly, relying solely on endothelial adherence may prove to be inadequate. Rapid and comprehensive endothelial coverage is achieved on bare metal stents, yet their efficacy is hampered by persistent chronic thromboinflammatory local responses that lead to restenosis. Notably, the activation of leukocytes and platelets upon contact with the device can persist, despite the presence of adhered endothelial cells. This discrepancy underscores the need for a more nuanced understanding of the intricate interactions between the device and the cardiovascular tissue.
Endothelial cells play a critical role in executing essential physiological functions, including anti-inflammatory and antithrombotic processes. However, for these cells to effectively fulfill their roles, appropriate signaling cues are required.
CD31, also known as platelet endothelial cell adhesion molecule-1 (PECAM-1), is central to this context. This intricate glycoprotein is predominantly expressed by endothelial cells, and to a lesser extent, by circulating leukocytes and platelets. CD31 plays a pivotal role in circulatory homeostasis, driven by its trans-homophilic properties that regulate immune responses during cell-cell interactions, maintain vascular integrity, and support appropriate hemostasis [
4]. These critical homeostatic properties of CD31 make it a prime candidate for inspiring innovative biomimetic approaches capable of redefining the integration of endovascular devices and driving progress in cardiovascular medicine.
We aimed to harness the characteristics of CD31 membrane-distal domains 1 and 2 to "camouflage" endovascular devices, thereby augmenting biocompatibility and fostering seamless integration within the cardiovascular system.
The physiological cell-cell CD31 interaction within the circulatory system is primarily based on its membrane-distal domains 1 and 2, which, under physiological conditions, engage in trans-homophilic and reciprocal homeostatic signaling upon contact [
5,
6]. Indeed, the substantial exposure of these domains on the luminal aspect of the endothelium also provides a means for homeostatic cell-cell interaction with circulating platelets and leukocytes, significantly contributing to the endothelium's anti-inflammatory and antithrombotic characteristics.
Guided by these insights, we present an innovative approach that involves "camouflaging" stents and flow diverters through the application of peptides emulating CD31 domains 1 and 2. By adopting this strategy, the devices can be perceived as integral components of the healthy endothelium by cells that interact with their surfaces. This transformation masks the foreign identity of these devices, with the overarching goal of mitigating adverse local reactions at the implantation site and expediting more seamless and effective integration of these devices within the intricate cardiovascular milieu.
Materials and Methods
Peptides design
The biomimicking peptides inspired by human CD31 trans-homophilic interactions were designed based on the structural characterization of domains 1-2 and their strand-swapped dimer formation observed upon CD31 crystallization, as decribed by Paddock et al [
5]. The substantial IgD1/IgD1 interface and interdomain contacts between IgD1/IgD2 and IgD2/IgD2 provided critical information to inform the development of these peptides, with the aim of enhancing and replicating the cell–cell trans-homophilic interactions crucial for CD31 intercellular functions. The designed peptides were divided into four groups, referred to as Group I, Group II, Group III, and Group IVa/b.
Group I peptides were designed to mimic the region of human CD31 IgL1 spanning His71-Ser87 or Gln70-Lys89. Homocysteines Gln70hCys and Met88hCys were incorporated to form a disulfide bond and a cyclic structure. The consensus sequence QHXXLFYKDDXXFYNISSXX was derived, with certain amino acids allowing for variation. The following peptides were included in Group I: SP722, SP745, SP765, SP1374, and SP1375.
Group II peptides were designed to imitate the region of human CD31 IgL1 corresponding to Tyr107-Glu122 or 106-124 aa. The mutated 106hCys and 124hCys homocysteines enabled the cyclic formation. The consensus sequence YKSTVIXNNKEKTTXE was established with specific amino acid variations in the individual peptides. Group II peptides included SP1072 and SP1376.
Group III peptides were created to mimic the region of human CD31 IgL2 from Pro133-Lys158. Val135hCys and Cys152hCys were introduced to form disulfide bonds and cyclic structures. The consensus sequence, PXCTLDKKEXIQGGXVXVNCSVPEEK, was developed, with particular amino acids allowing for variability. The peptides in Group III included SP1071 and SP1380.
Group IVa peptides consist of heterodimers, combining peptides from Group II and Group I through covalent linkage. Group IVb peptides were also heterodimers, combining peptides from Group III and Group I. Peptides SP1379 and SP1383 were part of Groups IVa and IVb, respectively.
Peptides synthesis, structural analysis
All peptides were synthesized using standard Solid-phase Peptide Synthesis protocols with Fmoc/t-Bu chemistry and Dimethylformamide as the solvent. Due to their high sequence similarity, the individually designed peptides were screened in vitro to identify potential candidates for coating stents in future in vivo applications, based on their proven ability to induce a pro-physiological phenotype in endothelial cells.
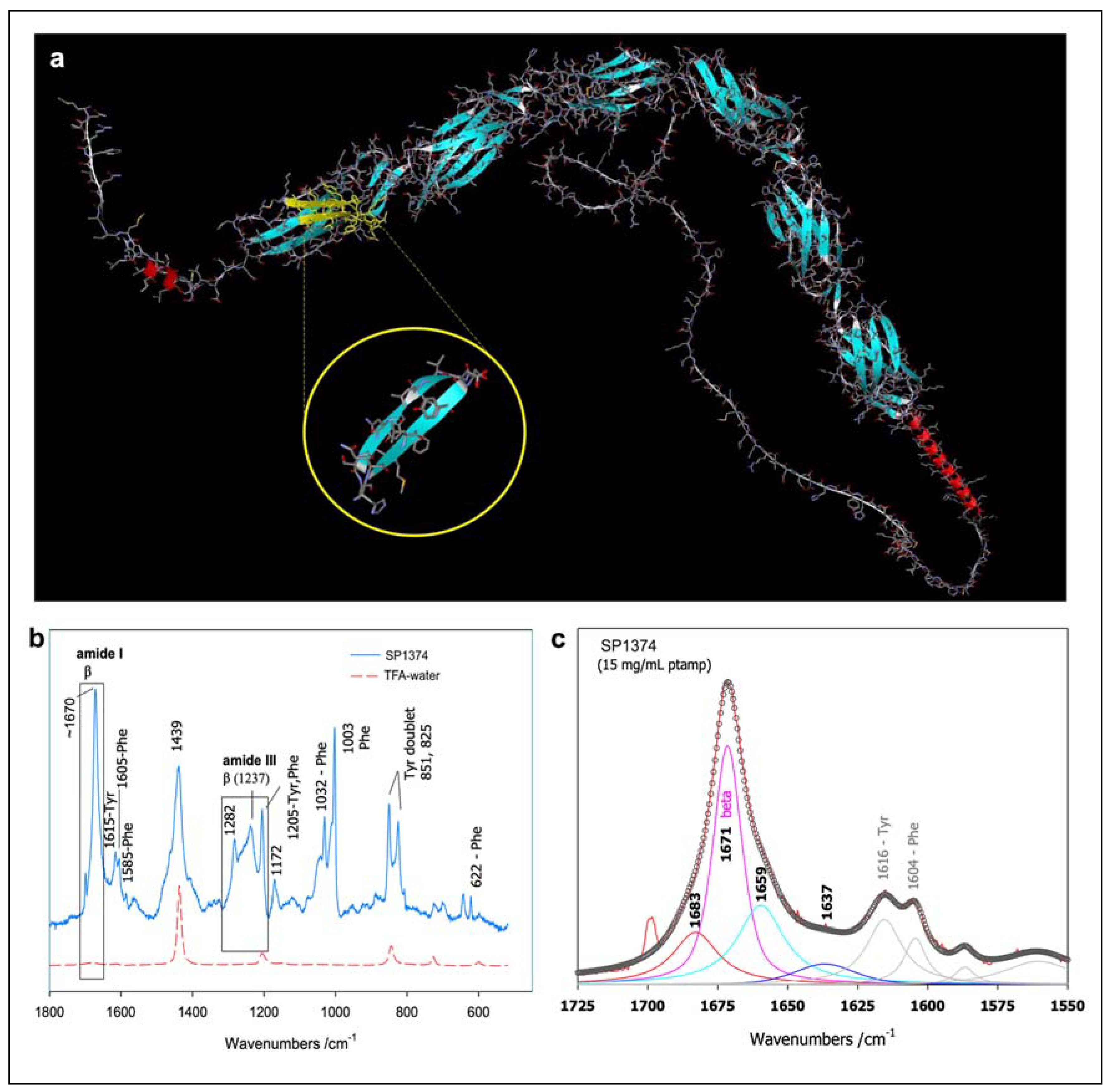
To assess their structural similarity to the endogenous protein from which the sequences were derived, the peptides were dissolved in a phosphate buffer (pH 6.8) at a concentration of 5 mM. Raman spectra were acquired by exciting ~50 μL of the sample solutions in Suprasil quartz cells (5 mm path length) using the 488 nm line emitted by an Ar+ laser (Spectra Physics). Scattered light was analyzed using a Jobin-Yvon T64000 spectrograph, equipped with a 1200 grooves/mm holographic grating and a holographic notch filter, operating in a single macro configuration. The effective spectral slit width was set at ~5 cm-1.
The coating process for all samples, including NiTi and CoCr disks and stents, followed a three-step dip-coating protocol using copper-free click chemistry. Demineralized water and Tris buffer (10 mM, pH 8.5) served as solvents and buffers. Polydopamine (PDA) at 2 mg/mL (prepared in 10mM Tris buffer, pH 8.5, from dopamine hydrochloride) and DBCO (final concentration of 300 µM, derived from a 20 mg/mL stock solution in 10mM Tris buffer, pH 8.5) were used. CD31 peptide was introduced at 50 µg/mL.
Sample coating
The dip-coating process took place in multiwell plates on an orbital shaker for disks, while stents were placed in polypropylene round-bottom tubes on a rotary wheel, ensuring complete immersion. The PDA layer dip coating step lasted approximately 18 ± 2 hours at room temperature (protected from light). Afterward, samples underwent three rinses with demineralized water, followed by sonication to remove any residual PDA. Subsequently, samples were transferred to fresh wells/tubes containing DBCO (300 µM) and incubated overnight with continuous rotation. After another round of rinsing and sonication, samples were placed in wells/tubes containing CD31 peptide (50 µg/mL) and incubated for 2 hours with continuous rotation. After the final rinse, samples were briefly immersed in absolute ethanol for 5 seconds and air-dried. Each item underwent a thorough inspection for flawless sample coating using high-magnification 3D light microscopy (Keyence VHX-7000N) to confirm the effectiveness and completeness of the coating.
Screening in vitro
Distinct CD31 peptide coatings' impact on primary Human Aortic Endothelial Cells (HAEC) was assessed through in vitro screening. Mirror-polished nitinol disks (4.8 mm diameter, 0.15 mm thickness) underwent sequential dip-coating, either remaining bare or receiving peptide coatings. Primary Human Aortic Endothelial Cells (HAEC) were cultured on these disks for 48 hours using basal Endothelial Cell Basal Medium MV2 (Lonza), excluding growth factors.
After incubation, adherent cells on disks were rinsed, fixed, and stained. Staining included phalloidin-AlexaFluor®488 for F-actin, DAPI for DNA, and CD31 domain 1 antibody (clone 9G11)-allophycocyanin (APC) for CD31. Fluorescent elements were imaged using an Axio Observer microscope with Zeiss Zen software and analyzed using Image J® (NIH) with the "Analyze Particles" function.
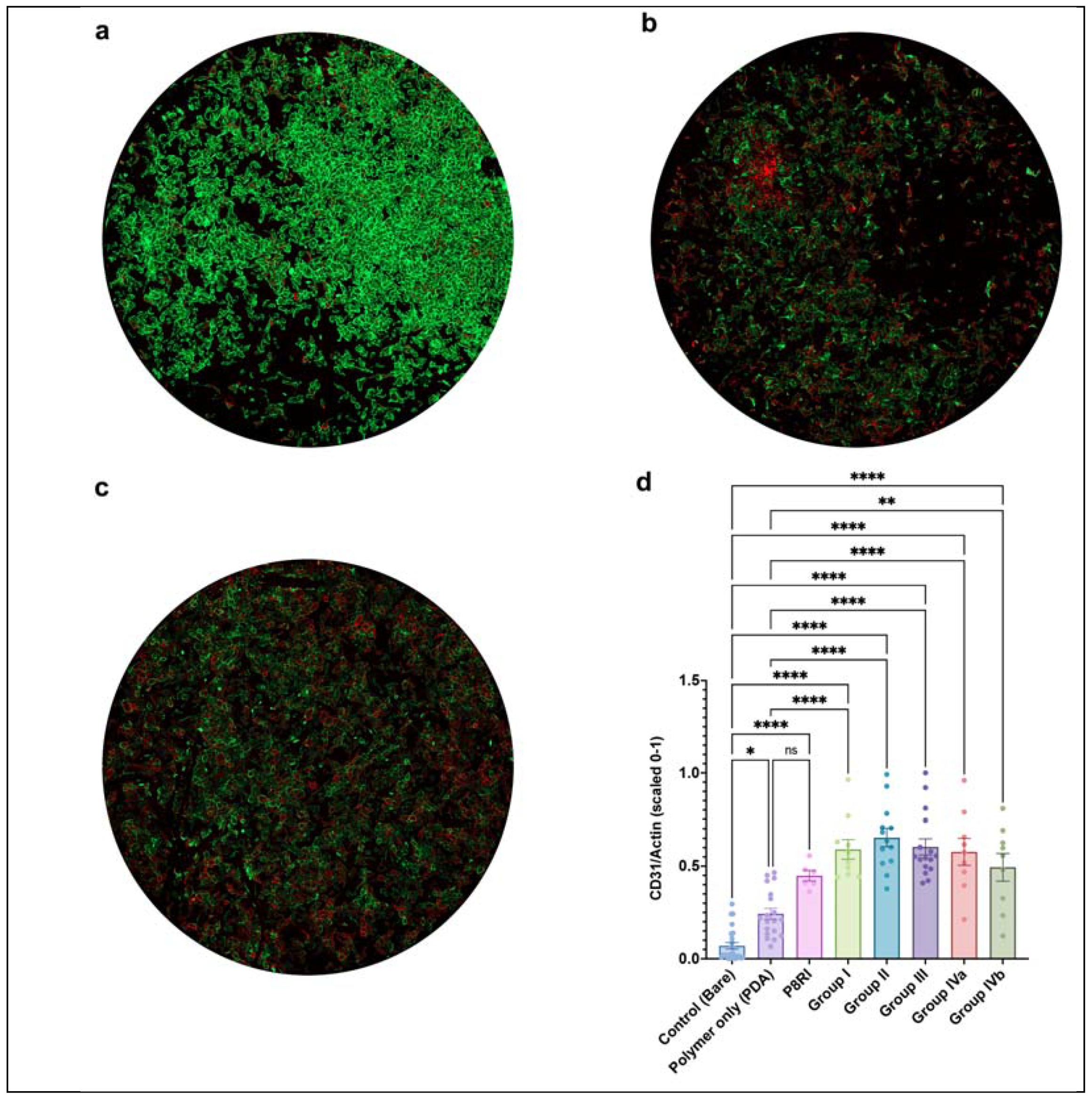
Peptides from the 5 groups underwent batch testing, and individual peptide effects were assessed across multiple experiments. Bare metal as well as discs coated with the polymer only (PDA) or a peptide targeting the cis-homophilic, juxta-membrane segment, of CD31 (P8RI [
7,
8]) were used as controls. Adherent elements were quantified as N/mm², and positive staining was assessed via integrated density (IntDens). A physiological endothelial phenotype was indicated by low intracellular F-actin and strong CD31 expression at cell borders, presented as CD31/Actin ratios on a Y-axis scaled from 0 to 1 using the formula (x-min)/(max-min).
To comprehensively assess the impact of different CD31 peptide coatings on endothelialization potential, parallel scratch tests were performed. Coatings were applied to culture well bottoms, and real-time cell migration was tracked using an Incucyte S5® system, providing insights into the influence of various CD31 peptide coatings on endothelial cell migration.
Studies in vivo
The peptide SP1072 (Group II, sequence: YKSTVIVNNKEKTTAE-PEG4-K(N3)-CONH2), chosen from those exhibiting a robust CD31/Actin ratio in vitro with HAEC, was used to coat clinical grade devices (nitinol flow diverters -FD- and CoCr stents).
All in vivo procedures described in this study were conducted by PharmaLegacy Diagnostics Co. Ltd., a biotechnology research service based in Shanghai (project #PLJC22-0005). All procedures were carried out in male White New Zealand rabbits weighing 4.0 to 4.5kg, in compliance with the "Principles of Laboratory Animal Care" approved by the Institutional Animal Care and Use Committee (IACUC) of the People's Republic of China. Every effort was made to minimize the suffering of the animals and to ensure their welfare throughout the study. Rabbits were anesthetized using Acepromazine, Ketamine, and Xylazine, and post-operative analgesia was provided with Buprenorphine.
CoCr stents were successfully implanted within rabbit aortas and femoral arteries, whereas Nitinol flow diverters were positioned in the aortas and the subclavian right carotid artery, across an experimental saccular aneurysm generated using the elastase model 3 weeks before, as previously described [
8,
9]. Bare metal nitinol and CoCr devices and drug-eluting CoCr stents were used in the control groups. A minimum of 5 animals per group was included in each study.
At the time of implantation, only heparin sodium was given, to prevent acute thrombosis. Aspirin and Clopidogrel were given orally 2 days before all device implantation and pursued thereafter. A distinct experimental approach was used to evaluate the potential efficacy of the flow diverter while sparing antiplatelet therapy. In this iteration of the experiment, the experimental rabbits were not administered aspirin or any anti-P2Y12 antiplatelet medication after implantation.
Follow-up was pursued as specified in the figure legends and up to 60 days. The luminal aspect of the arterial segments within the experimental framework was meticulously analyzed using scanning electron microscopy (SEM), whereas resin cross-sections were subjected to light microscopy for detailed assessment, as previously described [
7,
8].
Results
Synthetic Peptides Characterization
We synthesized a total of 51 peptides across five groups. The sequence positions corresponding to these peptides were modeled in silico based on the CD31 domains 1 and 2 trans-dimerization interface model described by Hu et al. [
10]. Specific details for representative peptides from each group (SP1374 from Group I, SP1072 from Group II, SP1071 from Group III, SP1379 from Group IVa, SP1383 from Group IVb) are provided in
Table 1. Their positions at the trans-homophilic interface of CD31-CD31 dimers are illustrated in
Figure 1 and an example of their structural characterization using Raman spectroscopy is presented in
Figure 2.
Selection of the peptide to be used in vivo guided by the results of the screening in vitro
While all CD31 peptides consistently promoted a more physiological endothelial cell phenotype, indicated by an increased CD31/Actin ratio compared to bare metal discs, only the trans-homophilic peptides from domains 1 and 2, and not the cis-homophilic peptide from the juxtamembrane segment (P8RI) of CD31, significantly improved the physiological score compared to the polymer alone (PDA) in the in vitro screening (
Figure 3). This suggests that the specific engagement of CD31 by P8RI was limited in this assay using resting endothelial cells, possibly due to steric hindrance—the cis-homophilic position of CD31 on the cells being obscured by the large mass of the six extracellular Ig-like domains of CD31. The best results were achieved by peptides from group II. From this pool, we opted to proceed with SP1072 for coating clinical-grade nitinol flow diverters and CoCr stents due to its straightforward sequence, which implies a quicker and more straightforward scaling-up process. We also planned to conduct further in vivo testing.
Consistent in vivo efficacy of the camouflaging coating on two endovascular devices across animal models
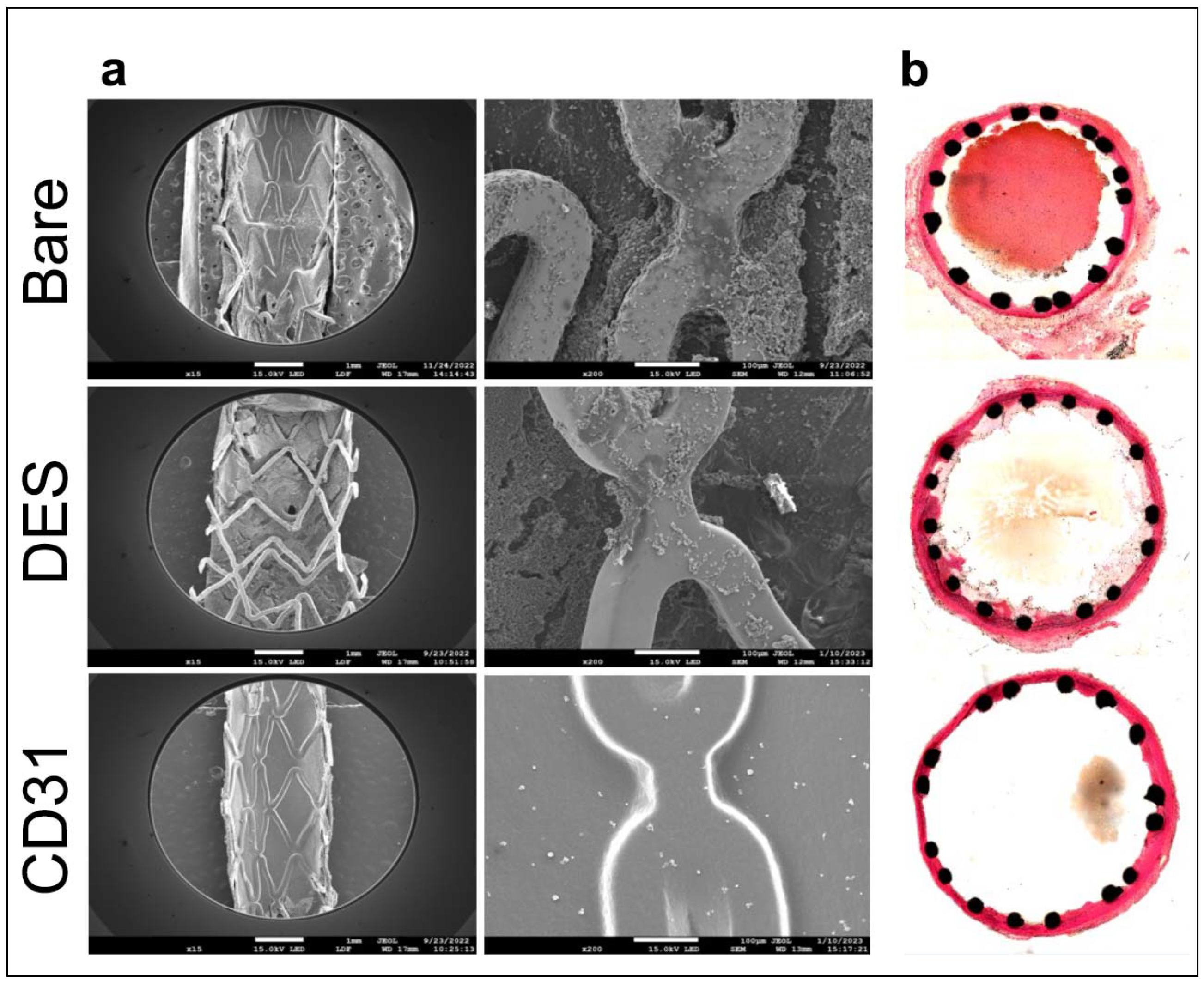
CoCr stents coated with the selected CD31 peptide demonstrated superiority over both bare-metal and drug-eluting stents. By day 7, CD31-coated CoCr stents were entirely covered by a smooth endothelium, in contrast to drug-eluting and bare-metal stents which remained largely exposed to the flowing blood. Moreover, the endothelium covering the CD31-coated stents remained entirely "clean," unlike bare-metal stents which often displayed signs of local thrombo-inflammation over the endothelialised struts (refer to
Figure 4a). The morphometric analysis of resin cross-sections confirmed the complete absence of thrombo-inflammation on the luminal side of coated stents, as well as the absence of adventitial inflammatory reactions commonly observed with bare metal devices (
Figure 4b).
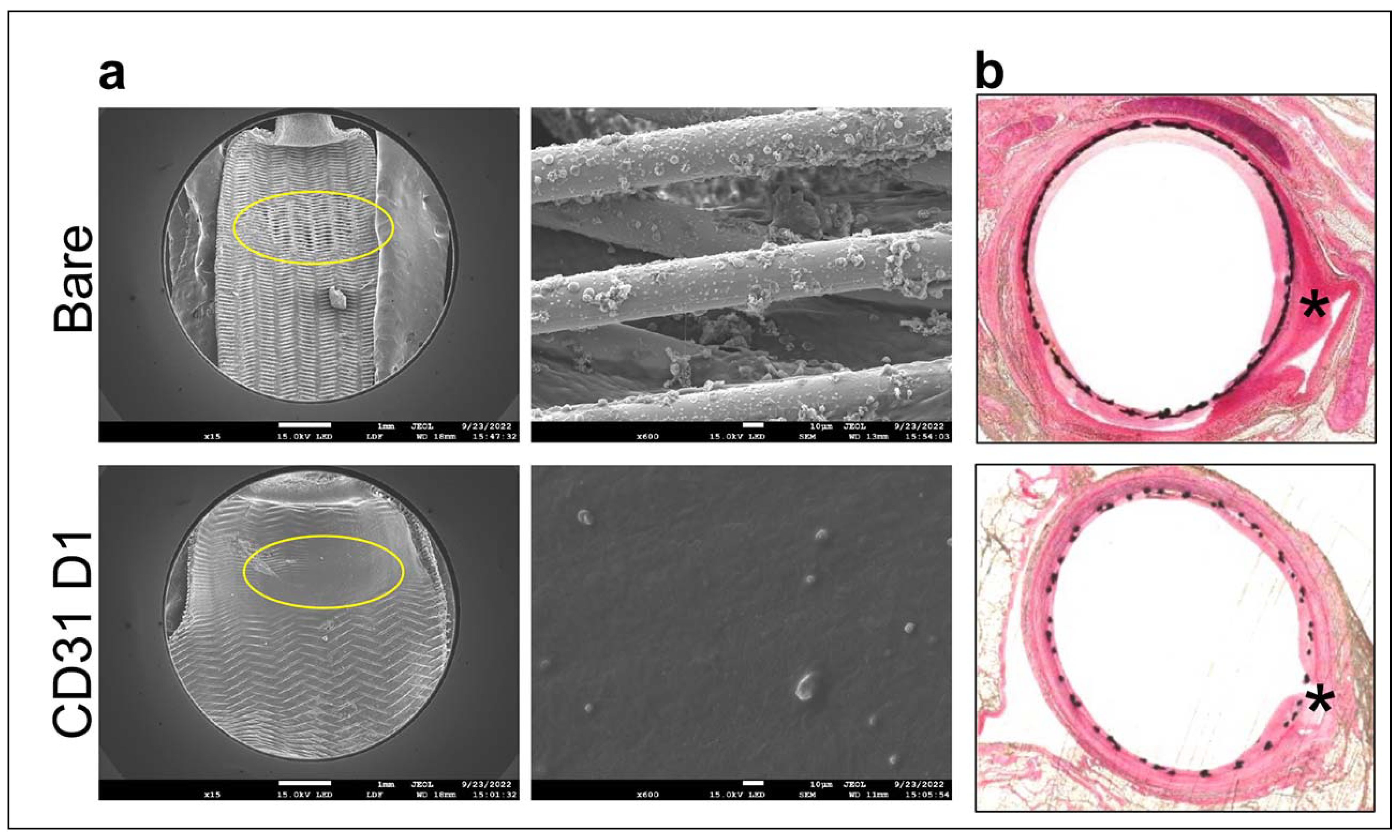
In the elastase saccular aneurysm rabbit model, the results showed that flow diverters coated with the CD31-Domain 1 mimicking peptide SP1072 promoted a smooth integration of the devices under a physiologic confluence of endothelial cells. This resulted in the formation of a "neo-arterial" wall at the entrance of the aneurysmal sac, documented by “en face” scanning electron microscopy analysis. In contrast, the group implanted with control/bare metal flow diverters exhibited an open inter-strut space at the aneurysmal entry, even two months after implantation, as depicted in
Figure 5a. Examination of resin cross-sections revealed that in the control group, fresh blood was still present in the sac (indicated by black asterisk), and flourishing periarterial inflammation was still visible even after two months (indicated by white asterisks). However, in the CD31 group, the arterial tissue appeared to have healed evenly, and the adventitia remained consistently "clean," as illustrated in
Figure 5b. In the antiplatelet treatment sparing pilot study, all animals of the CD31 peptide arm were alive at 60 days, while 2 animals (40%) died in the reference arm.
4. Discussion
The implantation of an endovascular device elicits a robust cellular response, particularly marked by thrombosis and inflammation following the activation of blood platelets and leukocytes. CD31 molecules expressed on cell surfaces play a pivotal role in this process, engaging in trans-homophilic interactions involving domains 1 and 2 [
5]. These interactions trigger a cascade of events, including the detachment of individual cells within the blood suspension and the transmission of vital physiological signals at the cell-cell interface, particularly for the endothelium. Furthermore, upon cell activation, a cis-homophilic interaction occurs between CD31 molecules on the same cells [
11], serving essential intracellular co-signaling functions [
4]. Our study was designed to explore the potential of harnessing CD31 molecule interactions among endothelial cells, platelets, and leukocytes at the blood-vessel interface and their implications for biomimetic coatings on vascular stents.
CD31 surface molecules are constitutive, but they are susceptible to proteolytic shedding, triggered by cell membrane proteases upon cell activation. This process results in the loss of the trans-homophilic portion between domains 1 and 2, exposing a truncated CD31 primarily consisting of the juxtamembrane extracellular fragment [
12]. To interact with CD31 on platelets and leukocytes undergoing activation, we previously investigated the potential of a synthetic peptide designed to mimic the cis-homophilic juxtamembrane sequence of CD31 (P8RI). This approach demonstrated intriguing immune-regulatory effects in vitro and showed promise in vascular pathologic models in vivo. It effectively mitigated the activation of CD31-shed leukocytes and platelets in vitro, and this translated into facilitated integration of CoCr and nitinol stents within the arterial wall in vivo [
7]. However, when confronted with quiescent endothelial cells in in vitro experiments, the specific advantages provided by P8RI coating appeared relatively limited compared to the non-specific adhesive surfaces used to tie the peptide onto the devices, such as polydopamine or polyethylene glycol. This limitation likely arises from the concealed nature of the target sequence within the intact, large extracellular CD31 structure, making interaction between surface-bound P8RI and the homotypic membrane-proximal sequence on endothelial CD31 molecules improbable.
The effectiveness of P8RI coating remains evident in scenarios where the interacting cells with the device are predominantly activated, as is the case in atherosclerotic arterial segments implanted with balloon-expandable stents [
13]. However, the relative reduced ability of this peptide coating to interact with the homotypic segment of CD31 on resting cells, which predominantly come into contact with a flow diverter in a non-atherosclerotic artery, led us to consider utilizing the distal portion of CD31—specifically, the portion that interacts with the intact CD31 of resting cells.
Our data, collected at the two-month mark following the implantation of nitinol flow diverters in experimental saccular aneurysms, including the potential discontinuation of antiplatelet treatment immediately after implantation, holds great promise in the realm of endovascular devices for treating intracranial aneurysms. In this field, the rate of post-procedural complications, some of which can be fatal, remains unacceptably high, despite the introduction of several “biomimetic” surface coatings, such as a phosphorylcholine coating to mimic cell membrane composition, a fibrin-based nano-coating to render the device inert to the coagulation cascade, or a hydrophilic polymer coating featuring the endothelial glycocalix [
3]. Indeed, the mechanism underlying the presumed anti-thrombogenicity of these surface-modified flow diverters remains elusive. Additionally, there is a dearth of experimental in vitro or in vivo data substantiating a direct effect on inflammation and endothelialization. In fact, clinical studies conducted with SAPT have even indicated an increased risk of neointima formation and thromboembolic complications, highlighting the complexity of these challenges.
In contrast, the use of CD31 trans-homophilic peptides as a coating presents a unique approach that effectively addresses multiple aspects. By closely replicating the essential "leave me alone" support crucial for circulatory homeostasis, this approach demonstrates reduced activation of platelets and leukocytes both in vitro and in vivo. Furthermore, it effectively curbs neointima formation while expediting thrombus organization within the aneurysmal sac and promoting the formation of a neo-arterial wall at its entry point. This multifaceted approach holds significant promise for enhancing the safety and efficacy of endovascular devices.
Further research is warranted to gain a comprehensive understanding of the potential of CD31 trans-homophilic peptide coatings on a larger scale. This research should encompass assessments of long-term durability and exploration of clinical applications. In essence, this study marks a significant stride toward the development of more effective and biocompatible endovascular devices, with broad implications for the field of biomimetics.
5. Conclusions
Our findings suggest that the use of peptides that replicate the membrane-distal portion of CD31, which is prominently exposed on the inner side of healthy vessels, can simulate the surface of a healthy endothelium and prevent the deposition and activation of blood platelets and leukocytes that are abnormally prolonged by the presence of a foreign body at sites of endovascular stent and flow diverter implantation.
The endothelial-mimetic coating of Domain-1 used in this study can be perceived as a self-healthy surface, offering the potential to achieve rapid healing universally across all devices that come in contact with blood, such as those used for coronary, neurological, and heart valve procedures, without requiring the use of drugs to manage platelet activation or foreign-body reactions.
Further studies are necessary to investigate the efficacy and safety of CD31 domain 1 coatings on a larger scale, as well as their long-term durability and potential clinical applications. This study provides a promising step towards the development of more effective and biocompatible endovascular devices.
6. Patents
Caligiuri, G., Nicoletti, A., Skarbek, C., Bianchi, E., and Ingenito, R. (PCT/EP2022/084241) BIOMIMETIC COATING FOR ENDOVASCULAR STENT. INSERM, University Paris Cité, University Sorbonne Paris Nord., INSERM
Caligiuri, G., Nicoletti, A., Skarbek, C., Bureau, C., and Lefevre, I. (PCT/IB2022/000684) BIOMIMETIC COATING FOR ENDOVASCULAR STENT. AlchiMedics S.A.S., INSERM, University Paris Cité, University Sorbonne Paris Nord.
Author Contributions
Conceptualization, Jean Sénémaud, Charles Skarbek, Isabelle Lefevre, Buommino Elisabetta, Antonino Nicoletti, Christophe Bureau and Giuseppina Caligiuri; Data curation, Jean Sénémaud, Charles Skarbek and Giuseppina Caligiuri; Formal analysis, Jean Sénémaud, Charles Skarbek, Ran Song and Giuseppina Caligiuri; Funding acquisition, Giuseppina Caligiuri; Investigation, Jean Sénémaud, Charles Skarbek, Ran Song and Zhongcheng Pan; Methodology, Charles Skarbek, Ran Song, Zhongcheng Pan and Giuseppina Caligiuri; Project administration, Giuseppina Caligiuri; Resources, Belen Hernandez, Antonino Nicoletti, Christophe Bureau and Giuseppina Caligiuri; Supervision, Christophe Bureau; Validation, Isabelle Lefevre, Yves Castier, Christophe Bureau and Giuseppina Caligiuri; Visualization, Belen Hernandez, Isabelle Lefevre, Buommino Elisabetta and Antonino Nicoletti; Writing – original draft, Jean Sénémaud, Charles Skarbek and Giuseppina Caligiuri; Writing – review & editing, Belen Hernandez, Yves Castier, Christophe Bureau and Giuseppina Caligiuri.
Funding
This research was funded by the “pré-maturation” program of the University Paris Cité (StratEx, project “BEND” 2020-2022), by the RHU iVASC (ANR-16-RHUS-00010) and by a research collaborative contract between Inserm-Transfert and Alchimedics S.A.S. CS salary is supported by the French National Research Agency (ANR) as part of the future investment program integrated into France 2030, under grant agreement No. ANR-18-RHUS-0001.
Institutional Review Board Statement
All in vivo procedures described in this study were conducted by PharmaLegacy Diagnostics Co. Ltd., a biotechnology research service based in Shanghai (project #PLJC22-0005). The procedures were carried out in compliance with the "Principles of Laboratory Animal Care" approved by the Institutional Animal Care and Use Committee (IACUC) of the People's Republic of China. Every effort was made to minimize the suffering of the animals and to ensure their welfare throughout the study.
Data Availability Statement
All raw data from this study are available upon request from the corresponding author.
Conflicts of Interest
C. Skarbek, I Lefevre, C Bureau, A. Nicoletti and G. Caligiuri are the inventors of a patent related to the work presented in this manuscript (PCT/FR2022/05223 - PCT/IB2022/000684).
References
- Borovac, J.A.; D’amario, D.; Vergallo, R.; Porto, I.; Bisignani, A.; Galli, M.; Annibali, G.; A Montone, R.; Leone, A.M.; Niccoli, G.; et al. Neoatherosclerosis after drug-eluting stent implantation: a novel clinical and therapeutic challenge. Eur. Hear. J. Cardiovasc. Pharmacother. 2018, 5, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Dean, L.S. Long-term clinical observations for a biofunctionalized stent: Yet to deliver their theoretical benefits. Catheter. Cardiovasc. Interv. 2018, 91, 1219–1220. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-L.; Roalfe, A.; Chu, E.-L.; Lee, R.; Tsang, A. Outcome of Flow Diverters with Surface Modifications in Treatment of Cerebral Aneurysms: Systematic Review and Meta-analysis. Am. J. Neuroradiol. 2020, 42, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, G. Mechanotransduction, immunoregulation, and metabolic functions of CD31 in cardiovascular pathophysiology. Cardiovasc. Res. 2019, 115, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Paddock, C.; Zhou, D.; Lertkiatmongkol, P.; Newman, P.J.; Zhu, J. Structural basis for PECAM-1 homophilic binding. Blood 2016, 127, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Sundlov, J.; Zhu, J.; Mei, H.; Hu, Y.; Newman, D.K.; Newman, P.J. Atomic Level Dissection of the Platelet Endothelial Cell Adhesion Molecule 1 (PECAM-1) Homophilic Binding Interface: Implications for Endothelial Cell Barrier Function. Arter. Thromb. Vasc. Biol. 2021, 42, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Rodriguez, S.; Rasser, C.; Mesnier, J.; Chevallier, P.; Gallet, R.; Choqueux, C.; Even, G.; Sayah, N.; Chaubet, F.; Nicoletti, A.; et al. Coronary stent CD31-mimetic coating favours endothelialization and reduces local inflammation and neointimal development in vivo. Eur. Hear. J. 2021, 42, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Cortese, J.; Rasser, C.; Even, G.; Bardet, S.M.; Choqueux, C.; Mesnier, J.; Perrin, M.-L.; Janot, K.; Caroff, J.; Nicoletti, A.; et al. CD31 Mimetic Coating Enhances Flow Diverting Stent Integration into the Arterial Wall Promoting Aneurysm Healing. Stroke 2021, 52, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Altes, T.A.; Cloft, H.J.; Short, J.G.; DeGast, A.; Do, H.M.; Helm, G.A.; Kallmes, D.F. Creation of Saccular Aneurysms in the Rabbit. Am. J. Roentgenol. 2000, 174, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Zhang, H.; Liu, Q.; Hao, Q. Structural Basis for Human PECAM-1-Mediated Trans-homophilic Cell Adhesion. Sci. Rep. 2016, 6, 38655. [Google Scholar] [CrossRef] [PubMed]
- Newton, J.P.; Buckley, C.D.; Jones, E.Y.; Simmons, D.L. Residues on Both Faces of the First Immunoglobulin Fold Contribute to Homophilic Binding Sites of PECAM-1/CD31. J. Biol. Chem. 1997, 272, 20555–20563. [Google Scholar] [CrossRef] [PubMed]
- Fornasa, G.; Groyer, E.; Clement, M.; Dimitrov, J.; Compain, C.; Gaston, A.-T.; Varthaman, A.; Khallou-Laschet, J.; Newman, D.K.; Graff-Dubois, S.; et al. TCR Stimulation Drives Cleavage and Shedding of the ITIM Receptor CD31. J. Immunol. 2010, 184, 5485–5492. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, G. CD31 as a Therapeutic Target in Atherosclerosis. Circ. Res. 2020, 126, 1178–1189. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).