1. Introduction
A novel body-shift implant design, introduced to enable high primary stability in immediate extraction sockets, was combined with a novel alloplastic bone augmentation material placed into the circumferential jumping gaps of said sockets. This multi-centre prospective study presents 1-year plus data from a prospective single-arm cohort study. The data was collected and refined based on the following criteria:
Single-tooth immediate tooth replacement therapy (ITRT) in the maxillary incisor and canine region in both intact and labially deficient extraction sockets
Treated with circumferential jumping gap augmentation with the novel alloplastic material.
The clinical and radiographic outcomes of 31 ITRT implants were evaluated, using a novel body-shift implant design to preferentially engage the residual bony volume after immediate tooth extraction aims to optimise apical primary stability for immediate tooth replacement therapy. [
1,
2] Additionally, the relatively narrow coronal portion of this implant design increases the labial jumping gap compared with traditional tapered implants with a comparative apical volumetric profile. Combining this with an internal angle correction of 12 degrees (CoAxis), allows implant placement within the maximal residual bony ridge volume, with both the aim of optimised bone to implant contact, implant head position and creation of a circumferential jumping gap. [
3,
4]
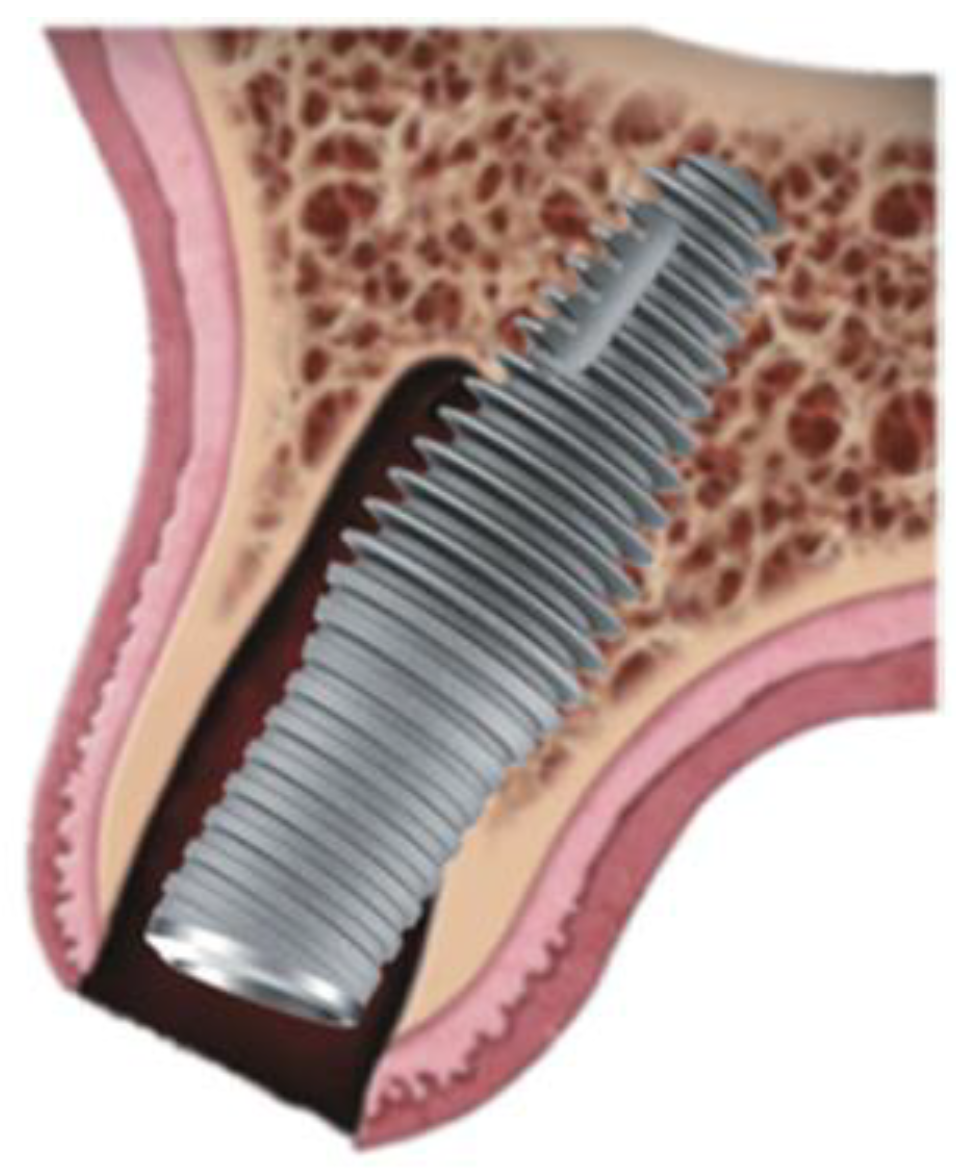
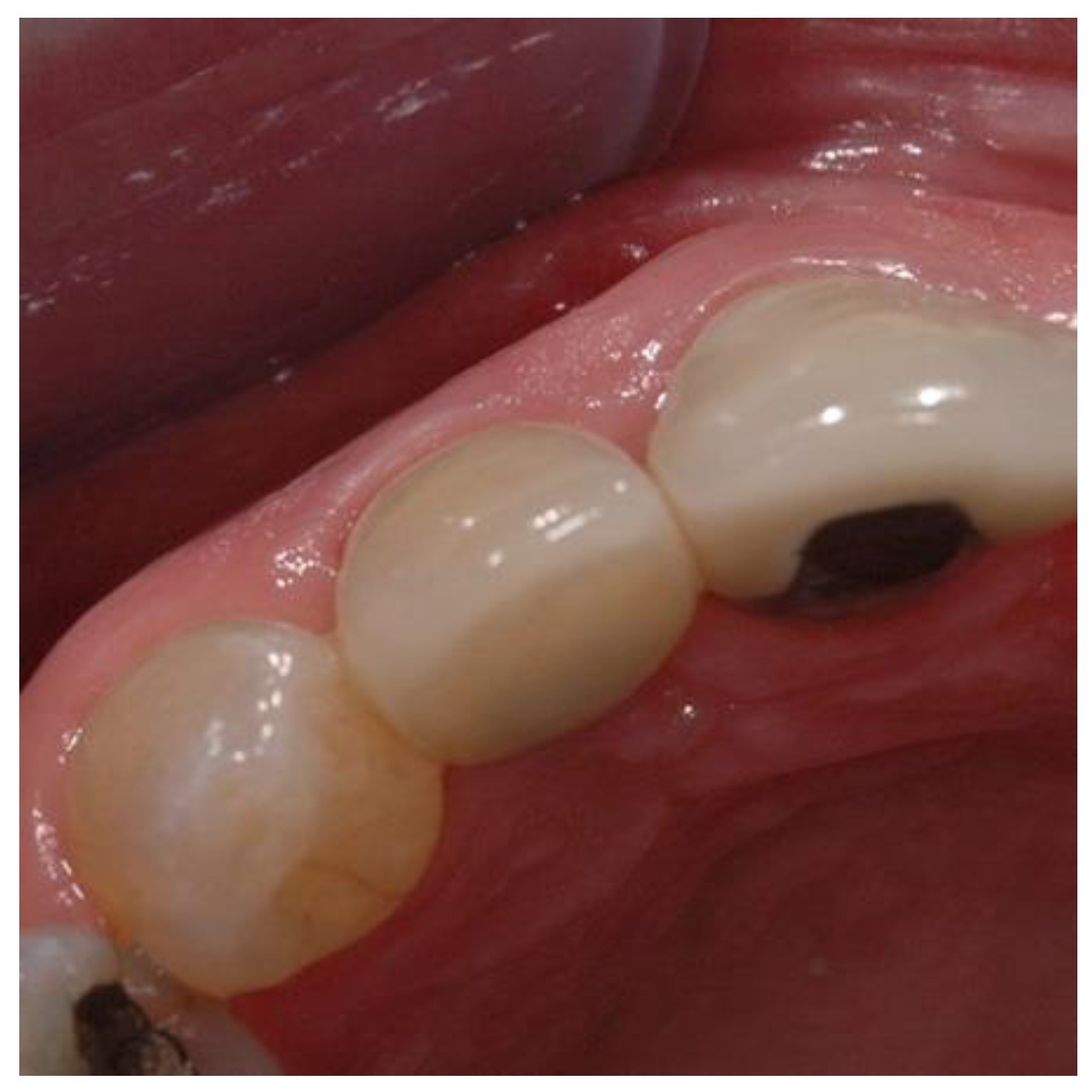
Figure 1.
Graphic of Inverta implant orientation associated with socket.
Figure 1.
Graphic of Inverta implant orientation associated with socket.
Figure 2.
Various Southern Inverta implants and drill sequence.
Figure 2.
Various Southern Inverta implants and drill sequence.
This implant was introduced in 2018 (Inverta, Southern Implants, SA) combining an internal prosthetic angle correction of 12 degrees, a body-shift feature (variations in diameter, 3D profile and thread pattern within a single implant design) focused on optimising primary apical stability, maximising coronal distance between the implant head and adjacent structures, and allowing ideal 3D implant head positioning for prosthetic emergence and larger volume for circumferential placement of grafting materials, both aiding in preservation and maintenance of ridge architecture. [
1,
3,
4,
5] The implant uses a moderately rough, sand-blasted surface micro topography. [
6]
A novel alloplastic bone augmentation material was introduced in 2015. (EthOss, EthOss Regeneration Ltd, UK). Comprising 65% Beta Tricalcium Phosphate (ß-TCP) Ca3(PO4)2 and 35% Calcium Sulphate CaSO4. This fully synthetic particulate material has been shown to result in over 50% host bone at 12 weeks with only 10-12% residual graft material. Full resorption is at 6-12 months in line with extensive published material on porous ß-TCP but may vary due to patient physiology. [
7,
8] This resorption is synchronous with new bone formation and in line with other Ca P materials [
9,
10], has an osteo-inductive potential to upregulate the host regeneration [
11]. BTCP fully resorbs [
12,
13,
14], and host bone provides an ideal foundation for long term health and stability of the hard/soft tissue complex [
15]. This is particularly critical in the aesthetic zone.
A cohort study has not yet investigated combining this novel implant and alloplastic material, for immediate tooth replacement therapy (ITRT). [
16] Therefore, the aim of this study was to present 1-year plus data from a retrospective, single-arm, multicentred study that correlated clinical and radiographic outcomes.
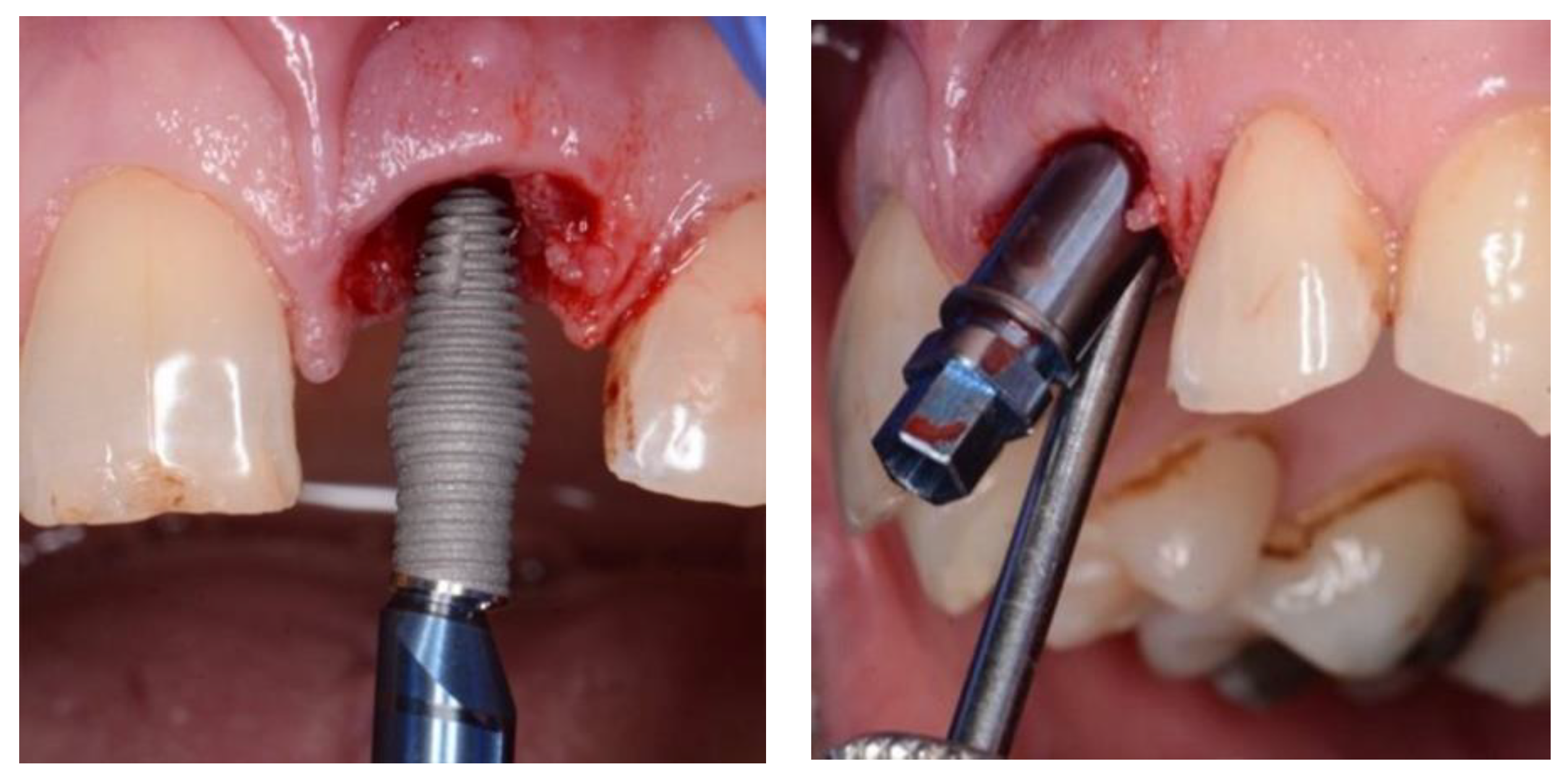
Figure 3.
and 4. Inverta placement showing labial view and angulation of placement enabled by internal angle correction (CoAxis).
Figure 3.
and 4. Inverta placement showing labial view and angulation of placement enabled by internal angle correction (CoAxis).
2. Materials and Methods
A multi-centre registry was created for this retrospective multi-centre study using the novel body-shift implant design in conjunction with the novel alloplastic bone regeneration material. Registered patients provided consent in accordance with the Declaration of Helsinki [
17]. Four study centres, three in the UK and the other in Dubai, UAE, participated in the data collection and approval. The data was extracted from the multi-centre registry based upon the following criteria:
1. single-tooth immediate tooth replacement therapy in the maxillary incisor and canine regions.
2. treatment with the novel alloplastic bone augmentation material in Types I, II, IV-A and IV-B sockets [
18].
3. CBCT evaluation using standard dental CBCT scanning units [
19,
20]
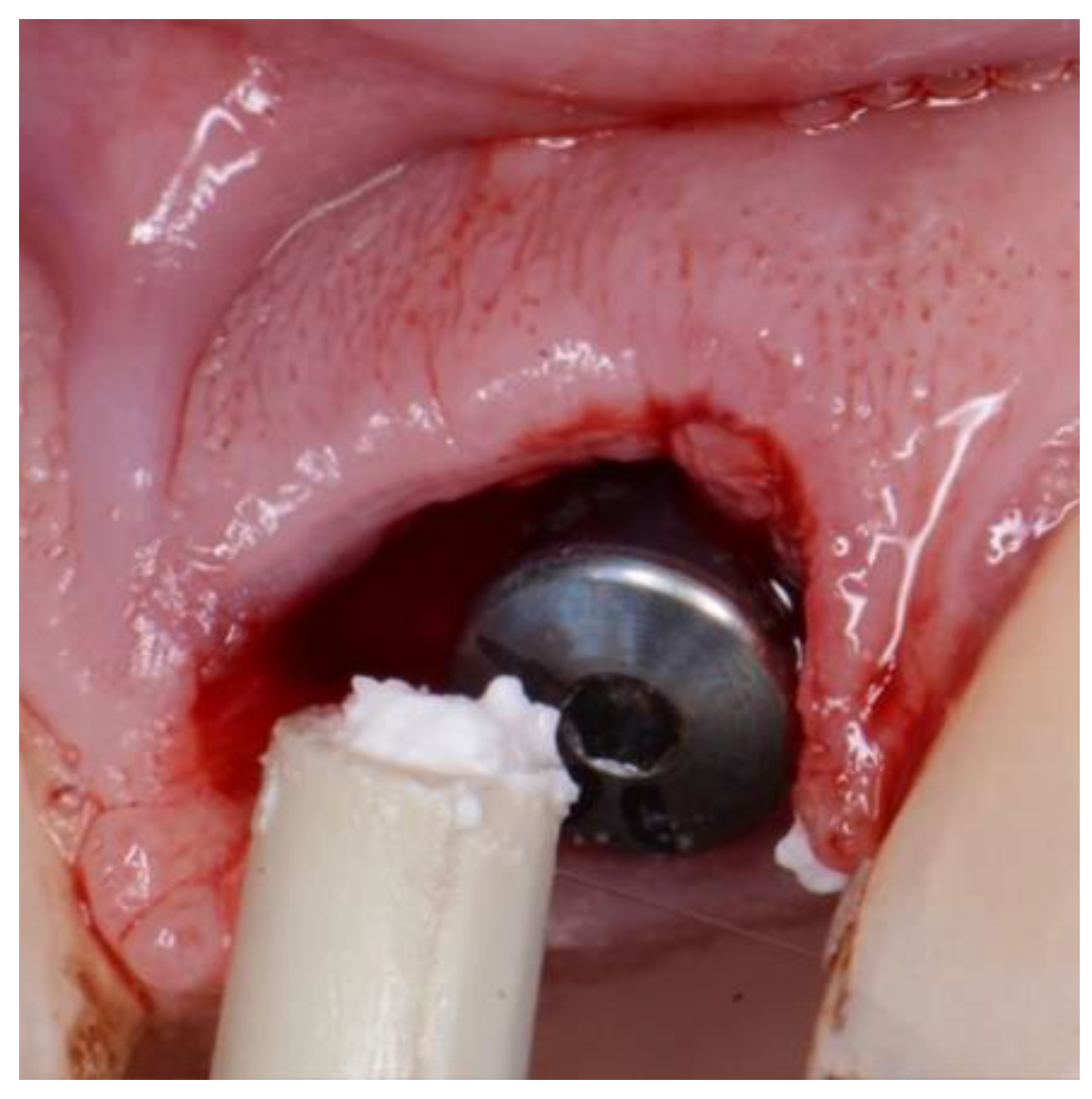
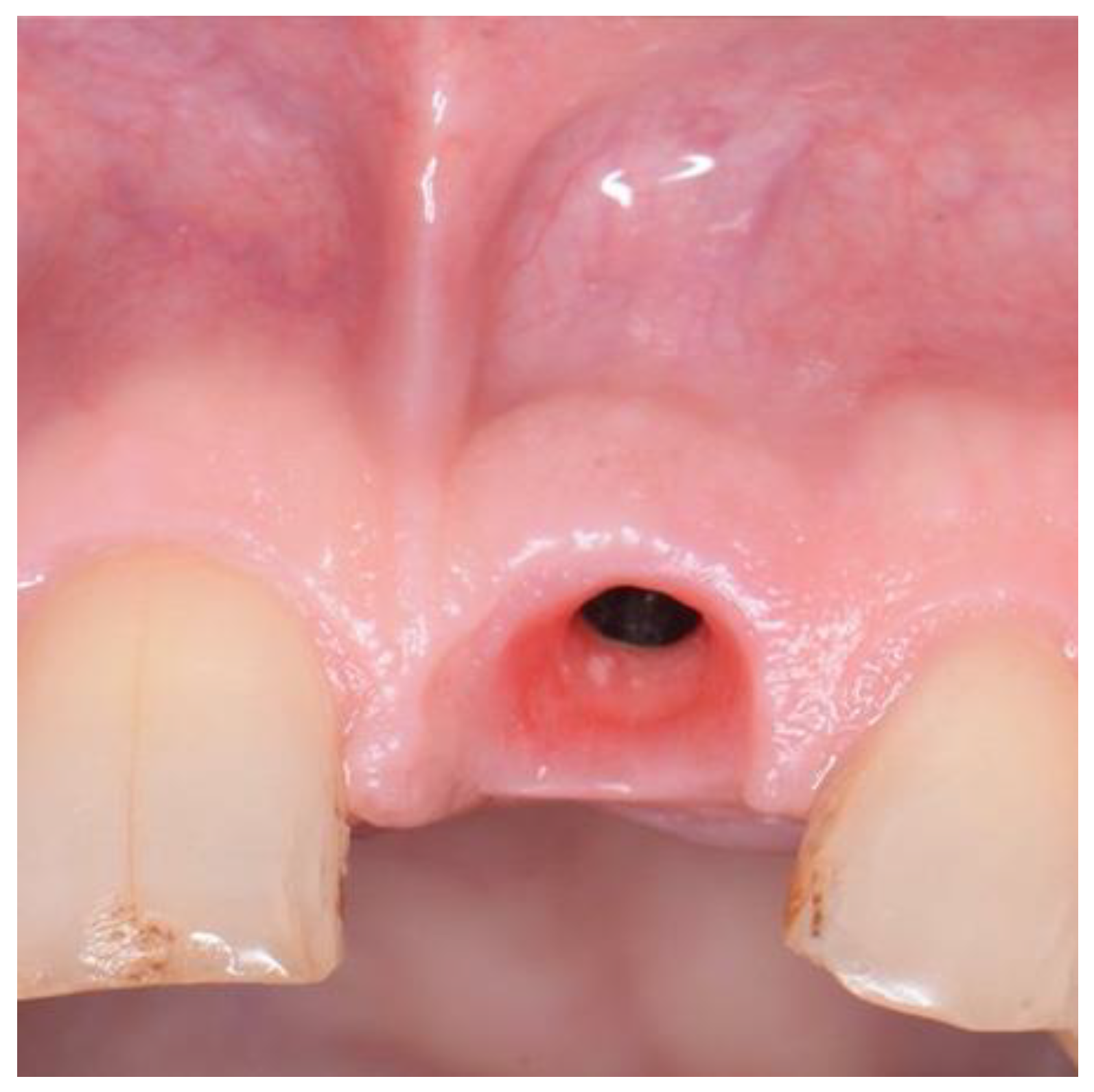
Figure 5.
EthOss placement into circumferential jump gap.
Figure 5.
EthOss placement into circumferential jump gap.
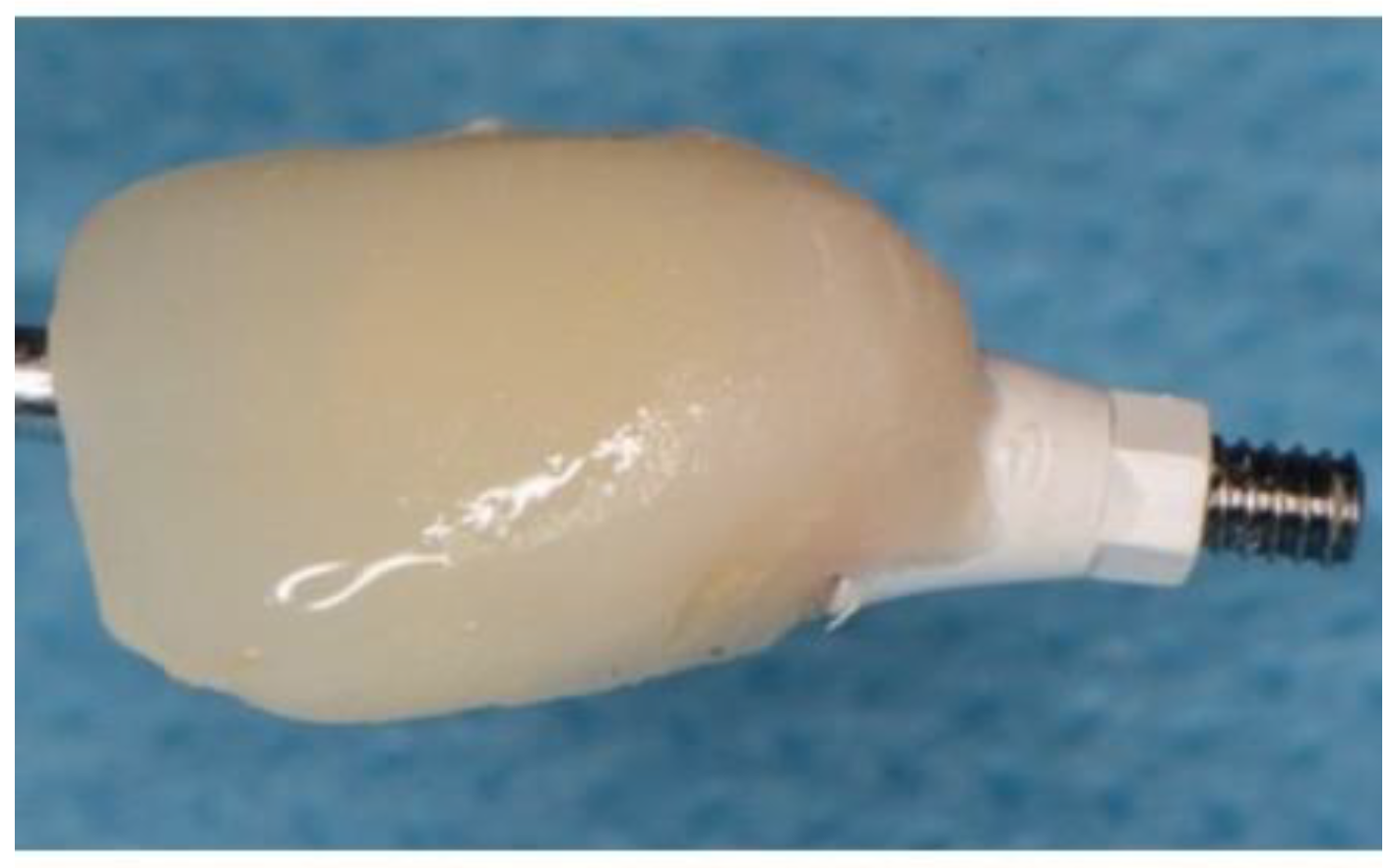
Figure 7.
Showing set Ethoss in circumferential jump gap and Cervico Temp Crown.
Figure 7.
Showing set Ethoss in circumferential jump gap and Cervico Temp Crown.
2.1. Clinical procedure
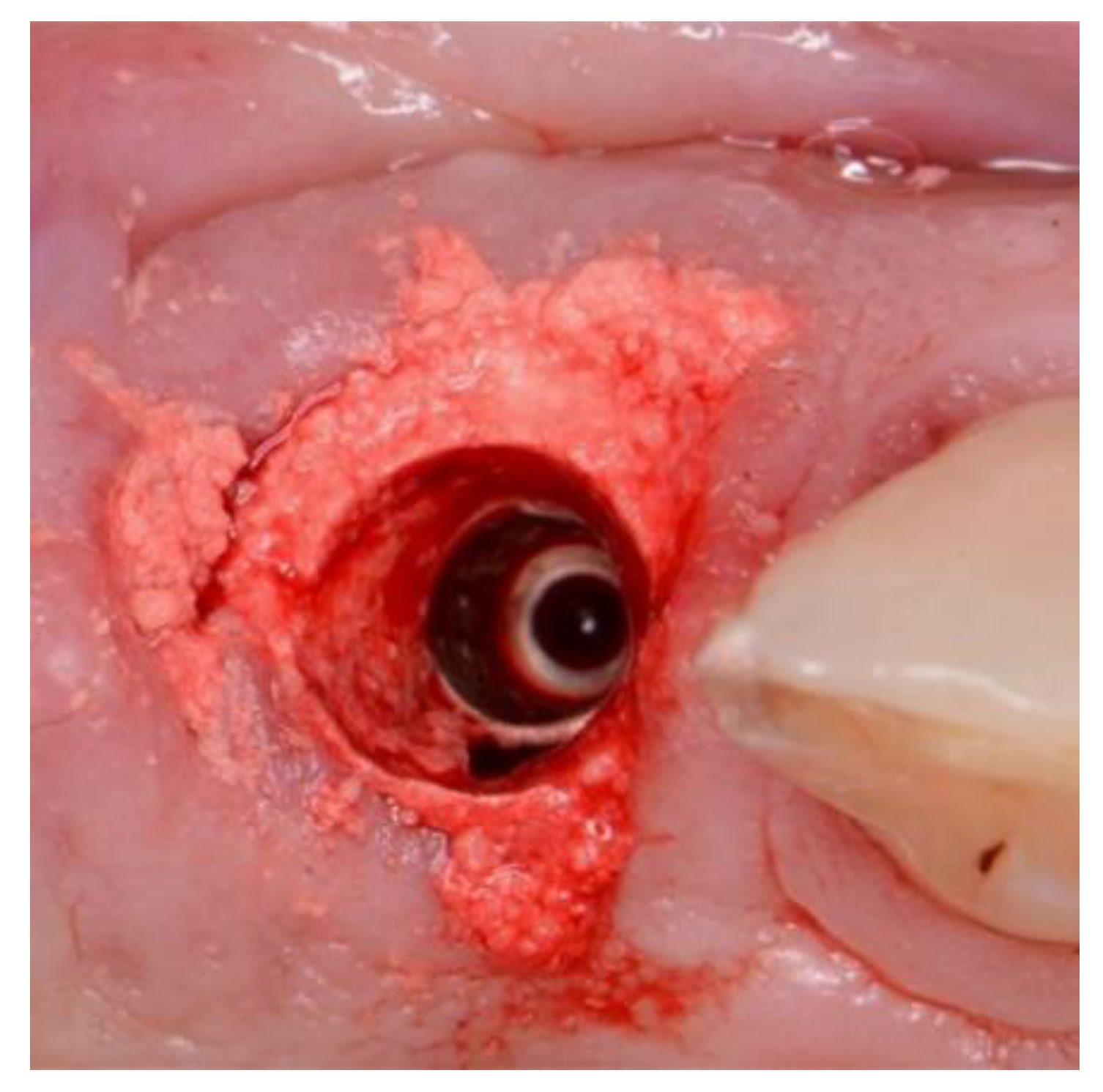
The surgical treatment protocol involved minimally traumatic tooth extraction. Residual socket debridement using both degranulation burs and sharp curettage. The osteotomy undersized by at least 0.5mm in circumference to allow placement of the novel body-shift implant of case-specific choice, 3.0 to 5.0mm from the labial free-gingival isthmus margin. A minimum immediate primary stability of 35ncm was required to facilitate immediate full contoured provisional restorations in non-occlusion [
21]. The circumferential jumping gap was filled with the novel alloplastic bone augmentation material (EthOss. EthOss Regeneration Ltd, Silsden, UK). Screw- retained provisional restorations were fabricated from direct pick up of PEEK or Titanium temporary cylinders (Southern Implants PTY, SA) These were adjusted to non-occlusion in centric and excursions. The emergence profile of the provisional screw-retained restorations is not only important for particulate graft retention but also to adapt and preform the soft tissue. [
22] Here the Cervico system (VP Innovato Holdings Ltd, Cyprus) was used by one study centre, whilst free-hand or in-house laboratory manufactured provisional shell crowns were used by the other centres. A concave subgingival emergence profile was obtained in all cases.
2.2. Data Collection
The following data points were evaluated for this study. Mean values and Standard Deviations (SD) were calculated for each category.
2.3. Clinical Evaluation
Implant primary stability
At the time of implant placement, the insertion torque values were recorded in Newton centimetres (Ncm) using either an electric handpiece or manual surgical torque wrench.
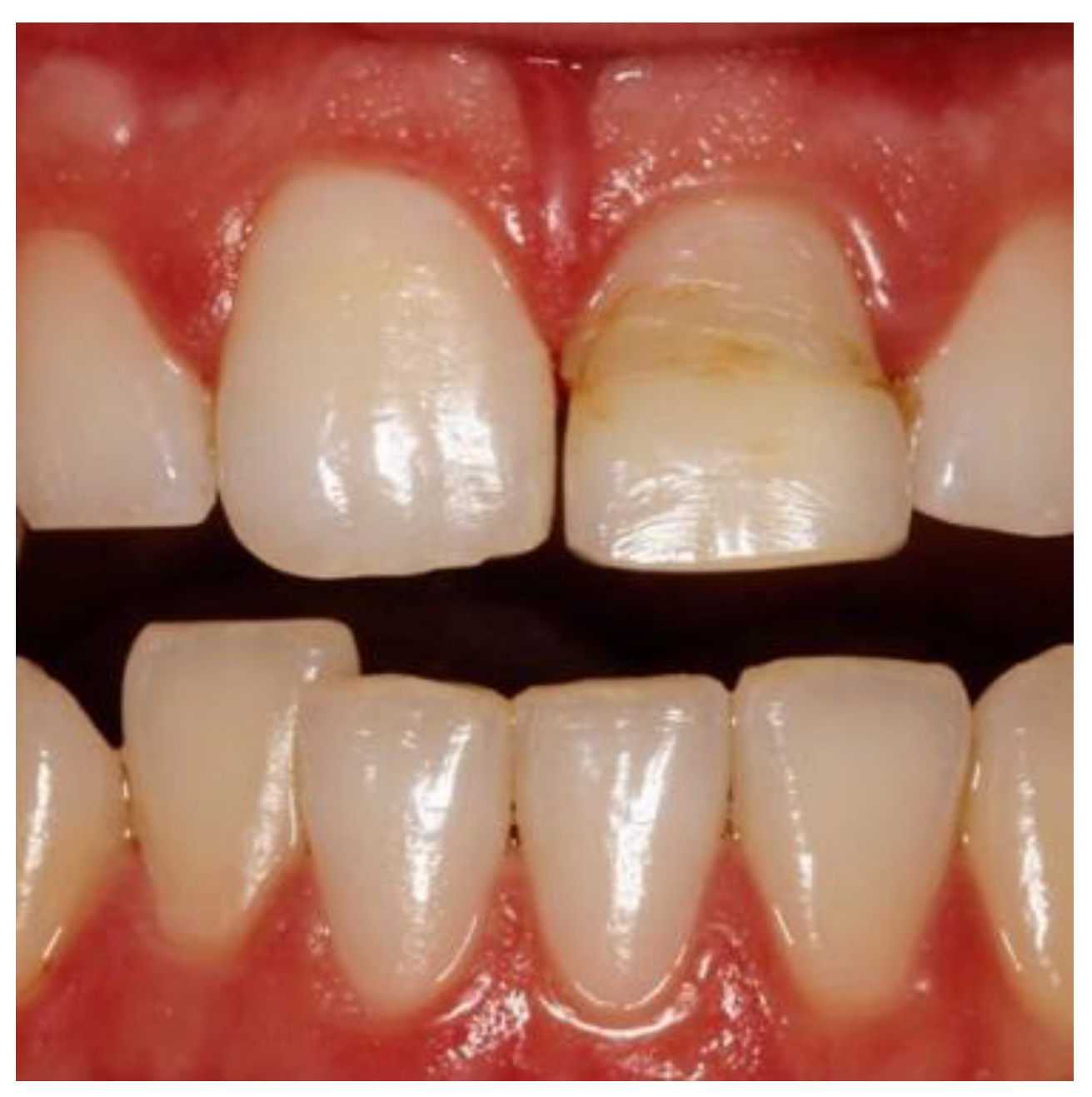
Pink Esthetic Score (PES)
High-resolution images were captured using digital single-lens reflex cameras with 105-mm macro lenses and ring flash systems at 1:1 ratio. Images were rated by the four observers and all measurements were made twice, at least 24 hours apart. Both immediate preoperative and a minimum 12-month (range 12-50 months) postoperative images were taken and evaluated [
23]. .
Figure 8.
Preoperative image used for PES evaluation.
Figure 8.
Preoperative image used for PES evaluation.
Figure 9.
Postoperative image used for PES evaluation.
Figure 9.
Postoperative image used for PES evaluation.
2.4. Radiological Evaluation
Labial plate dimension
Presence and width of the labial plate was measured prior to immediate tooth replacement therapy and at least 12 months (range 12-20 months) afterwards. Measurements were taken (in millimetres) at one level: the implant-abutment interface (IAI) equivalent to the midfacial labial plate bone crest [
24,
25] At this level, two reference points were defined. (1) the outermost aspect of the labial bone plate, and (2) the first radiographic bone-to-implant contact point connected by a straight line perpendicular to the implant body. The distance between the two points was measured using proprietary CBCT digital imaging software.
Figure 10.
11 & 12. CNCT sections immediate pre op, immediate post op and 12 months loading.
Figure 10.
11 & 12. CNCT sections immediate pre op, immediate post op and 12 months loading.
3. Results
Thirty-one maxillary single-tooth implants were included, based on the previously described criteria. The mean patient age was 59.25 years (range 24 to 79 years) with 17 male and 8 female patients. Of these 31 included implants, 54.8% were central incisors, 25.8% lateral incisors and 19.4% canines. The reasons for tooth extraction included root fracture, caries, periodontitis and unretrievable fractured posts. The circumferential labially emphasised jump gaps were all grafted with ß-tricalcium phosphate/calcium sulphate alloplast (EthOss) at the time of implant placement. Three complications were reported (1 case non-draining fistula, 1 case non-seated provisional restoration, 1 case fractured zirconia abutment). The definitive restorations were delivered between 4 hours and 18 months post implant placement. All restorations were screw-retained (n = 31). The mean insertion torque value was 58Ncm with a range of 10-100 Ncm.
3.1. Radiographic Evaluation
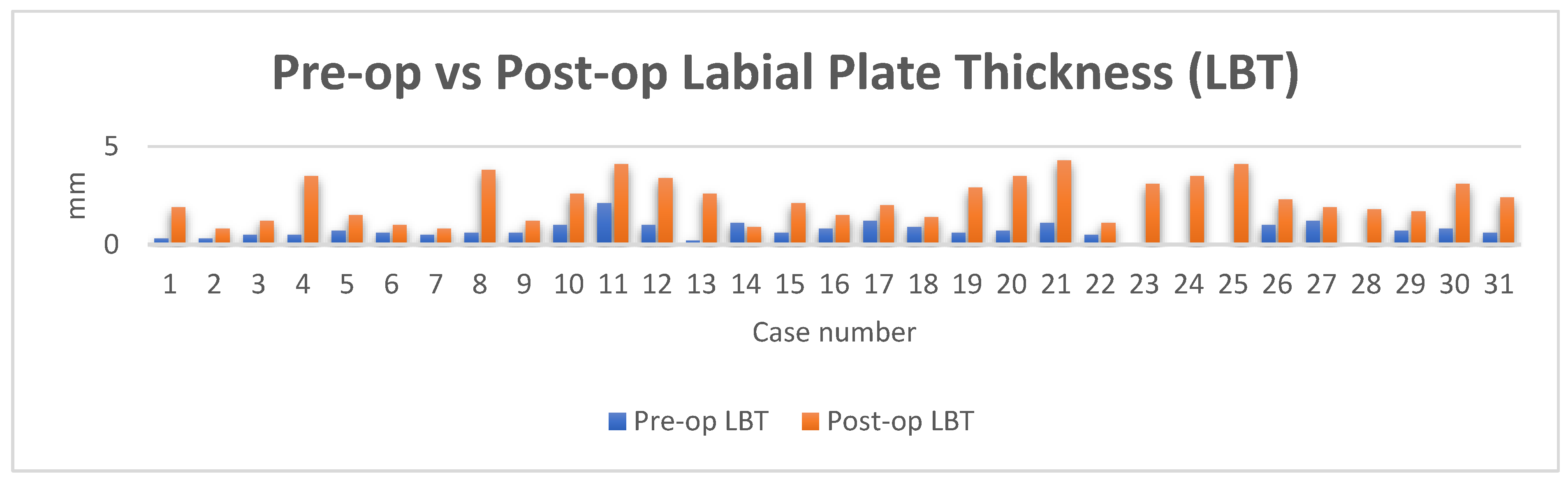
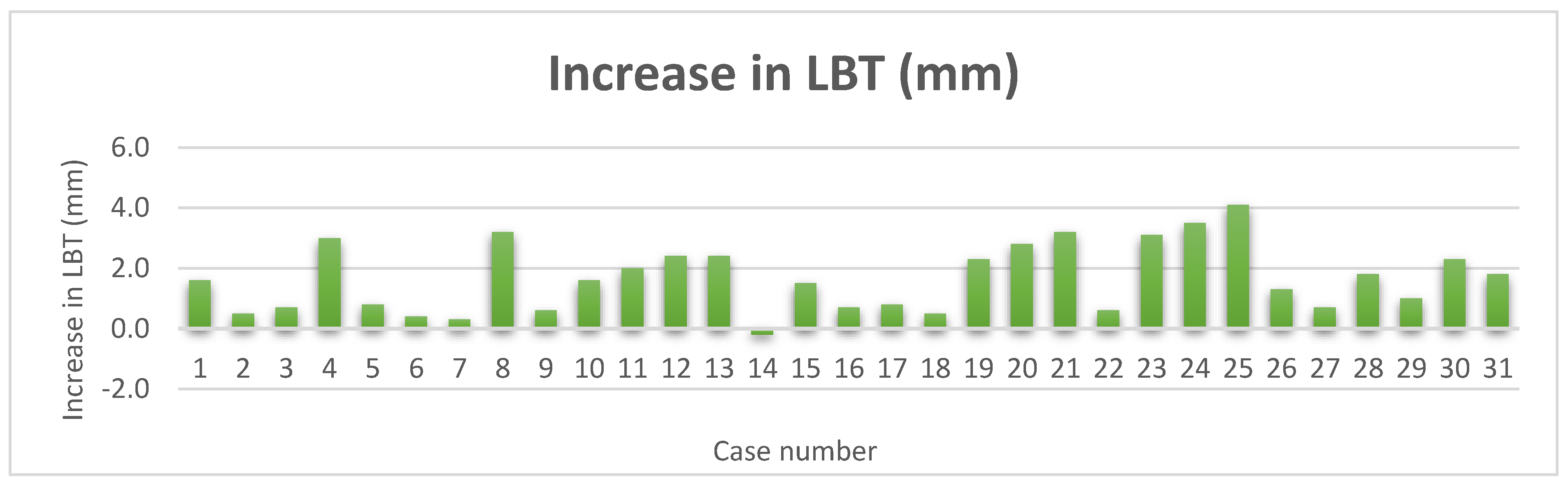
Thirty-one sets of CBCTS taken at the time of ITRT and at least twelve months after loading were available for radiographic evaluation. The mean LBT at time of ITRT was 0.7mm with a range from 0mm to 2.1mm. At follow up of at least 12 months of implant loading, the mean LBT was 2.3mm, with a range of 0.8mm to 4.3mm. This represents a mean increase in LBT of 1.7mm.
Figure 13.
Pre-op vs post op LBT.
Figure 13.
Pre-op vs post op LBT.
Figure 14.
Increase in LBT.
Figure 14.
Increase in LBT.
3.2. Clinical Evaluation
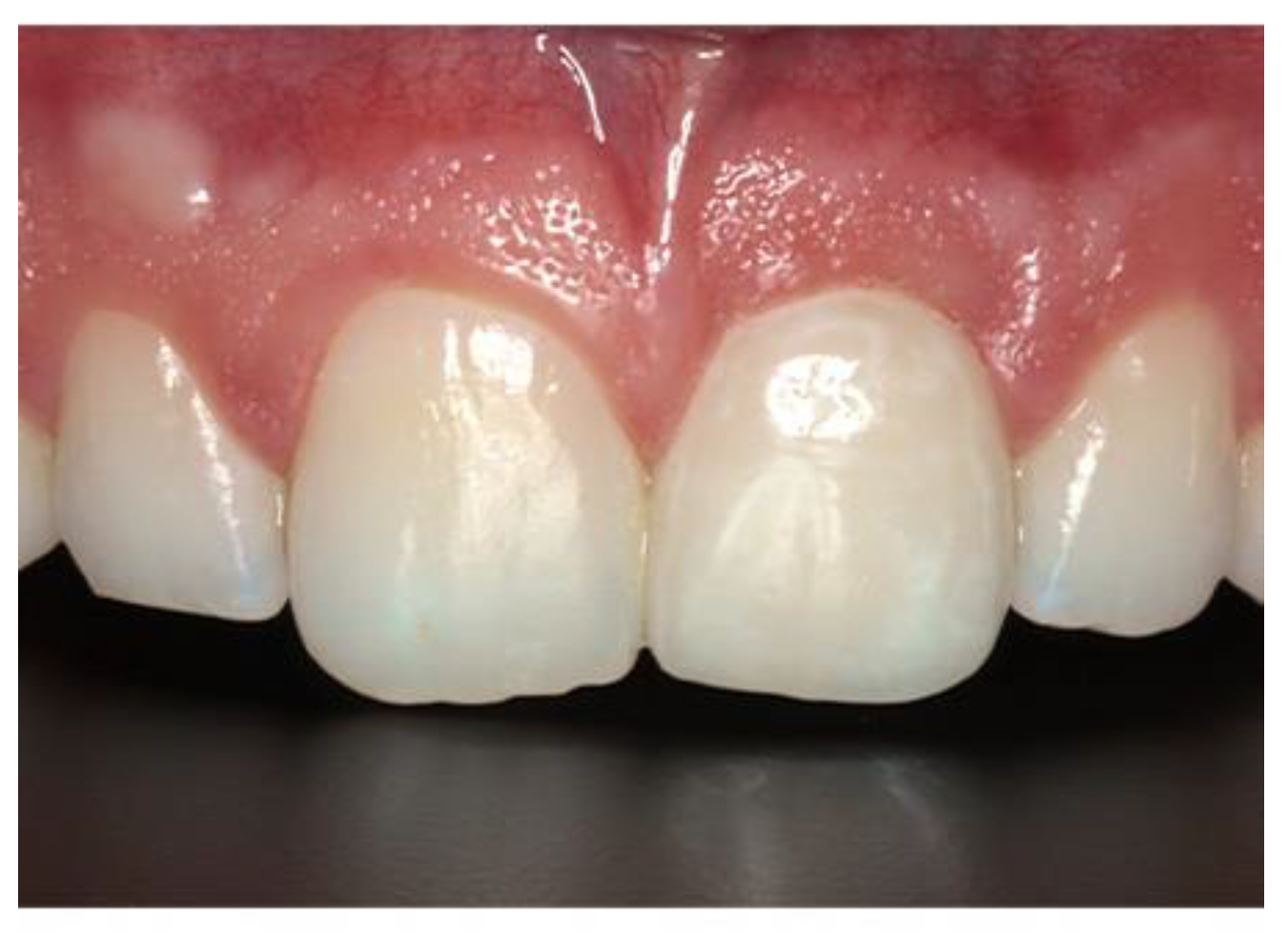
The preoperative PES mean score was 10 with a range from 5 – 13. The postoperative PES mean at least 12 months after implant loading was 12 with a range from 10 to 14
Figure 15.
Gingival architecture prior to delivery of final restoration.
Figure 15.
Gingival architecture prior to delivery of final restoration.
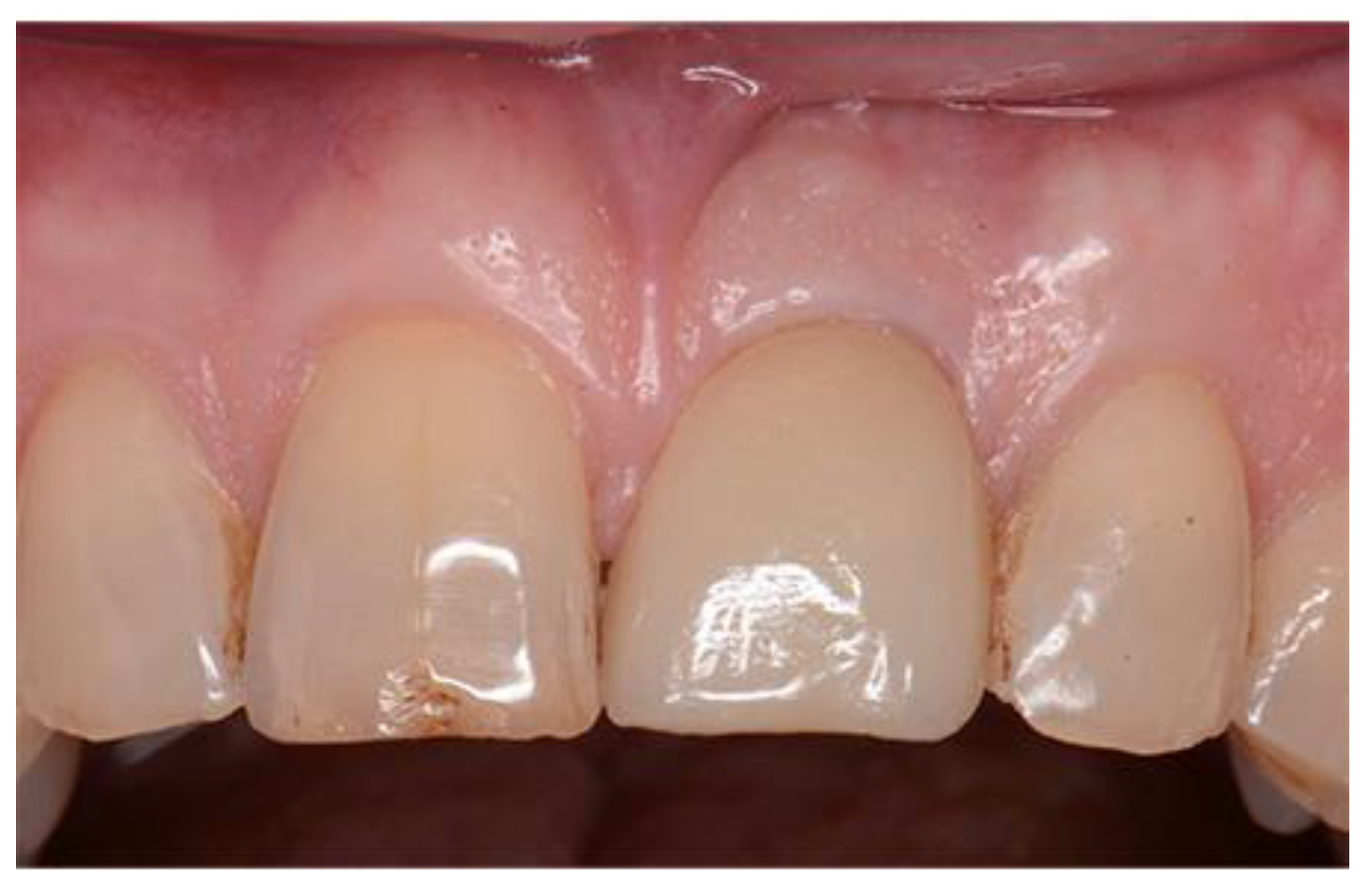
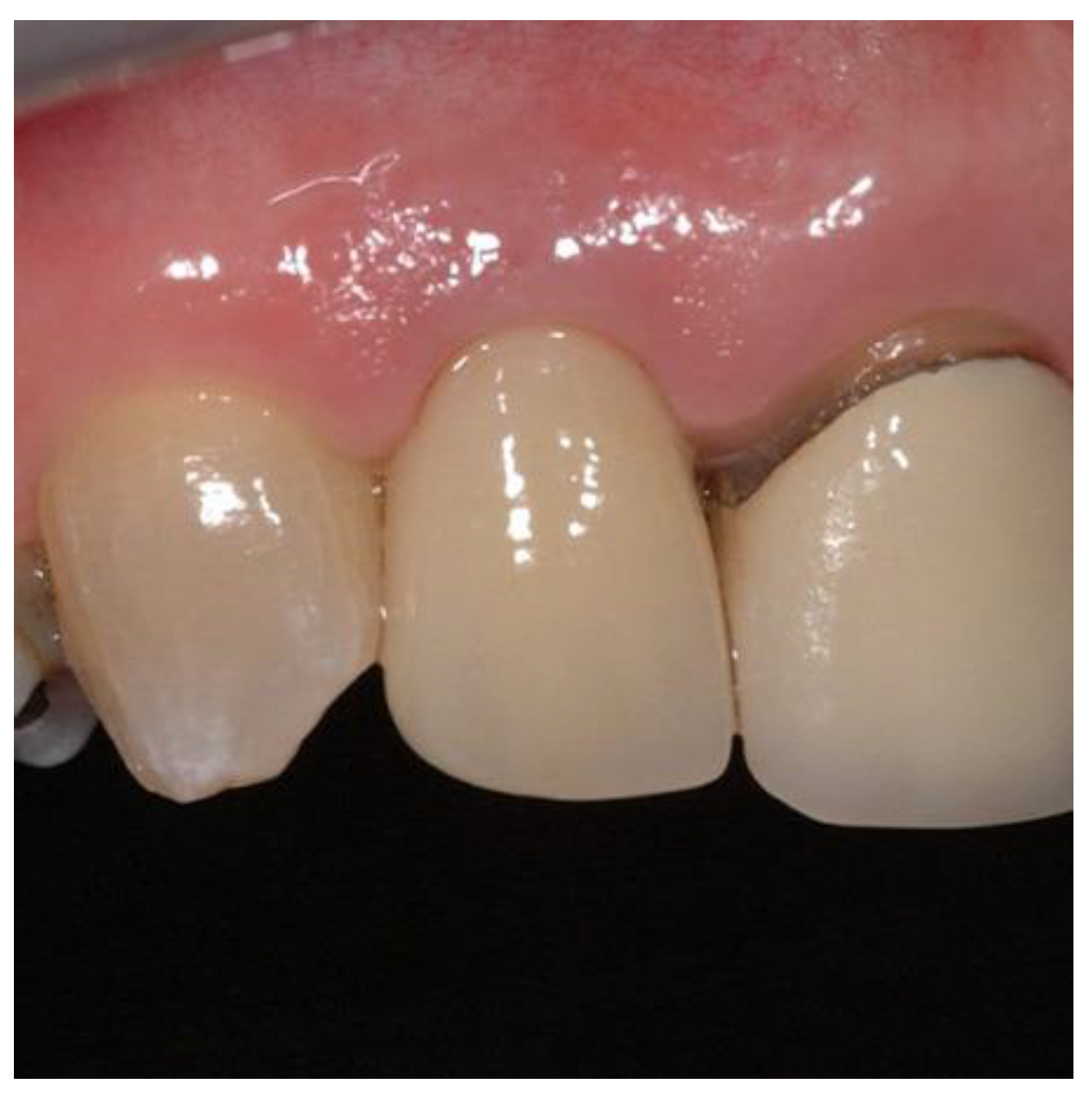
Figure 16.
Definitive restoration.
Figure 16.
Definitive restoration.
Table 1.
Key Metrics.
| Column1 |
Column2 |
Column3 |
Column4 |
| Total cases |
31.0 |
|
|
| |
|
|
|
| |
Central |
Lateral |
Canine |
| Percentage tooth type |
54.8 |
25.8 |
19.4 |
| |
|
|
|
| Mean Age (combined) |
58.8 |
|
|
| |
MALE |
FEMALE |
|
| Mean age |
53.8 |
69.2 |
|
| % sex |
67.74 |
32.26 |
|
| |
|
|
|
| Mean LBT pre-op (mm) |
0.7 |
|
|
| Mean LBT post-op (mm) |
2.3 |
|
|
| Mean increase in LBT (mm) |
1.7 |
|
|
| Range (mm) |
0 to 2.1 |
|
|
| |
Intact |
Defective |
Missing |
| Pre-op labial bone |
83.9 |
12.9 |
3.2 |
| |
|
|
|
| |
|
|
|
| ITV Ncm (mean) |
58 |
|
|
| ITV Ncm (range) |
10 to 100 |
|
|
| |
|
|
|
| PES range |
5 to 14 |
|
|
| PES Pre-op Mean |
10 |
|
|
| PES Post-op Mean |
12 |
|
|
4. Discussion
The optimal method to retain host tissues is through reduced surgery and hence the concept of ITRT was developed about 20 years ago with flapless immediate implant placement [
26]. However, there were longer term issues with the initial iteration mainly due to the selection of larger tapered implants that closely adapted to the socket after extraction [
27,
28]. Bluing of the gingiva due to the grey body of the implant being visible through the reduced or entirely absent labial gingiva is an unfortunate negative sequela. This can be followed with possible gingival recession and frank metal showing. Those are but two common issues where the implant dimensions precluded retention of endosteal bone to support the labial cortical plate [
29,
30].
It is now the consensus [
3] that we need to have an optimum implant coronal dimension to allow a buccal jump gap to graft to maintain and regenerate the buccal plate to ensure long term soft tissue stability with attached keratinized tissue [
31]. The added benefits of variable platform switching associated with sub crestal angled-correction implants has also been reported [
32].
The use of a thin buccal root section in Partial Extraction Therapy (PET), has also shown promising results in buccal plate preservation. This will be presented in a future study [
33].
Recently a novel Implant design from Southern Implants, the Inverta (Southern Implants, JHB, SA) [
3] has been released. Through its novel body-shift design, there is a narrow coronal portion to optimize the buccal jumping gap. The apical tapered portion ensures high primary stability as required for Immediate loading. The Inverta implant is offered with both a straight but also a sub crestal angle correction version, termed Co-Axis [
34] which is a 12-degree angled internal connection to assist the treating surgeon implant placement allowing optimized screw channel location.
These unique aspects of the Inverta have multiple advantages to the surgeon and restorative dentist. There is an increased likelihood of correct 3-dimensional placement of an implant for high primary stability with screw retention as well as a buccally emphasized circumferential jump gap at the crestal region. This circumferential jump gap should be grafted for optimal results as seen in research [
35,
36] Here in this study, EthOss (Ethoss Regeneration, Silsden, UK) a novel synthetic particulate material comprising 65% ß-TCP and 35 % CS, which enables the material to “set” thus making it stable and proving a barrier function to soft tissue ingress in the initial healing period [
37]. Hence the requisite to use a separate Collagen type membrane can be dispensed with ensuring the optimal periosteal healing response (38).
There has been extensive Dental research on Guided Bone Regeneration [
39,
40] and the use of resorbable and non-resorbable [
41] membranes. There is also extensive research of the ability of the host periosteum in bone regeneration [
42,
43,
44]. For this reason, it is felt that use of a membrane not only impedes host blood supply to the site with up to 50% fewer blood vessels in the new bone but may also impede the host periosteal induction of Stromal Cell derived factors [
45]. These Bone Morphogenic Proteins (BMPs), attract mesenchymal stem cells to the healing site. There they can differentiate to osteoblasts, regenerating new host bone [
46,
47].
The novel ability of this alloplastic bone regeneration material to not require an exogenous membrane is of greater importance in ITRT where membrane placement poses more difficulties surgically [
48,
49] The CS element in EthOss, also shows bacteriostatic properties [
50] and improved soft tissue healing response again also beneficial in this protocol. A further benefit of the CS is when it resorbs at 3-4 weeks depending on patient physiology. The resorption creates new space between the ß-TCP particles for neo-vascular ingrowth and resultant up-regulated angiogenesis. This host up-regulation of host bone regeneration has been recently shown in a new study using Osteoprotegrin (OPG) markers [
11].
Immediate placement of the semi-conductive titanium implant has in itself shown to upregulate the host regeneration of bone and this in conjunction with earlier loading in function with the definitive restoration shows a further enhancement of upregulation via functional remodeling. [
51]
Studies have shown that we can see over 50% new host bone at 12 weeks post grafting and around only 10 % residual graft material at this time [
52,
53,
57], which results in new host bone earlier (54). This is important for long term stability of the hard and thus the soft tissue. When the Implant is in function the bone will turn over and further improve, maintaining the profile in line with Wolff [
55].
In this study we are only measuring the new buccal bone along with the PES of the soft tissue. The inter proximal bone is also of great importance in regeneration as this will ensure the long-term stability of the papillae [
56]. This will be investigated in a further study.
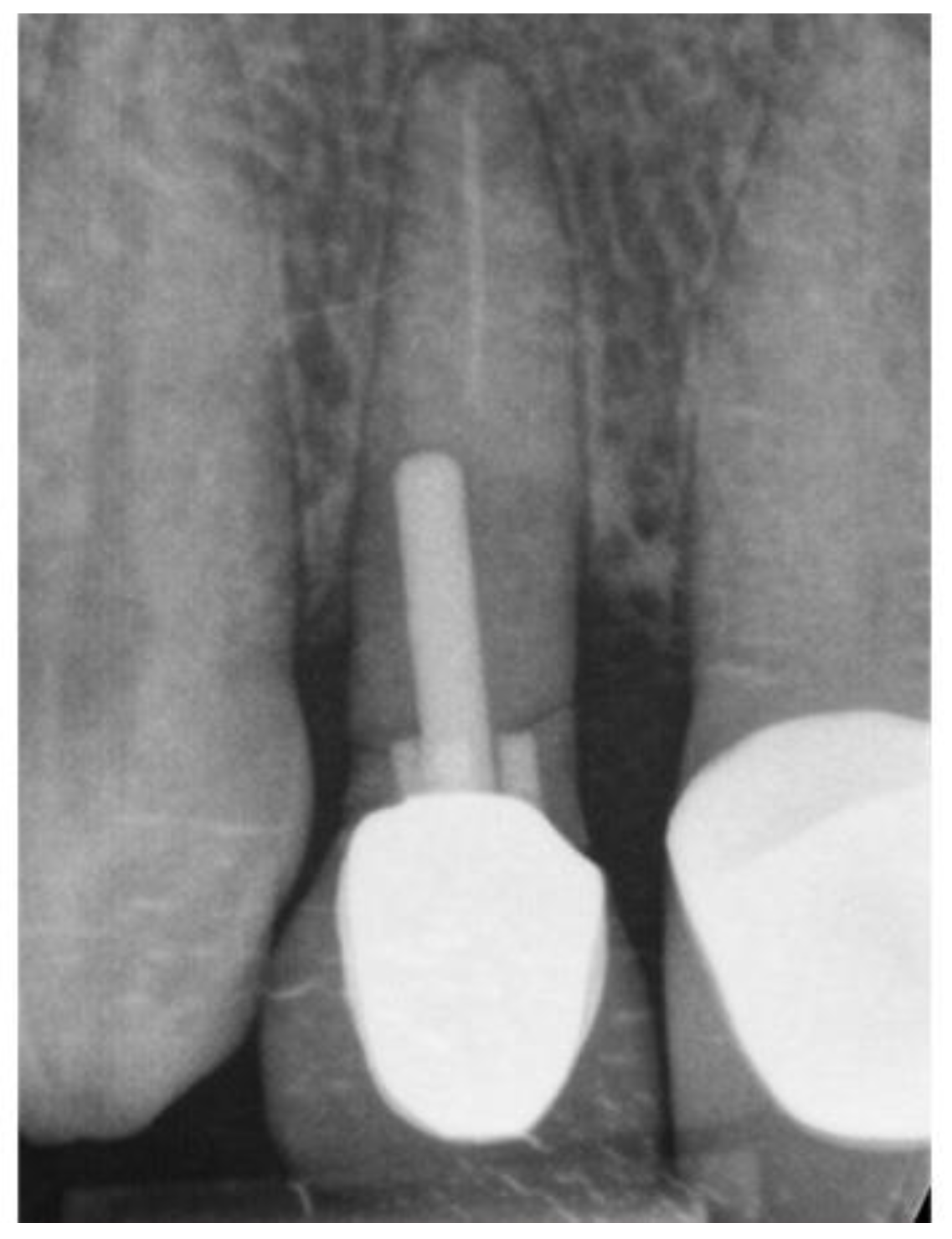
Figure 18.
Pre-op radiograph.
Figure 18.
Pre-op radiograph.
Figure 19.
Post-op ridge volume of definitive crown.
Figure 19.
Post-op ridge volume of definitive crown.
Figure 20.
Post-op labial view.
Figure 20.
Post-op labial view.
5. Conclusions
5.1. Statistical Analysis of Group Means
Table 2.
Statistical Analysis of Group Means Data.
Table 2.
Statistical Analysis of Group Means Data.
| Labial plate thickness (LBT) Pre-op (mm) |
Labial plate thickness (LBT) Post-op (mm) |
| 0.3 |
1.9 |
| 0.3 |
0.8 |
| 0.5 |
1.2 |
| 0.5 |
3.5 |
| 0.7 |
1.5 |
| 0.6 |
1.0 |
| 0.5 |
0.8 |
| 0.6 |
3.8 |
| 0.6 |
1.2 |
| 1 |
2.6 |
| 2.1 |
4.1 |
| 1 |
3.4 |
| 0.2 |
2.6 |
| 1.1 |
0.9 |
| 0.6 |
2.1 |
| 0.8 |
1.5 |
| 1.2 |
2 |
| 0.9 |
1.4 |
| 0.6 |
2.9 |
| 0.7 |
3.5 |
| 1.1 |
4.3 |
| 0.5 |
1.1 |
| 0 |
3.1 |
| 0 |
3.5 |
| 0 |
4.1 |
| 1 |
2.3 |
| 1.2 |
1.9 |
| 0.0 |
1.8 |
| 0.7 |
1.7 |
| 0.8 |
3.1 |
| 0.6 |
2.4 |
5.2. F TEST
Table 3.
F-Test Two-Sample for Variances.
Table 3.
F-Test Two-Sample for Variances.
| |
Variable 1 |
Variable 2 |
| Mean |
0.667741935 |
2.322580645 |
| Variance |
0.194258065 |
1.174473118 |
| Observations |
31 |
31 |
| df |
30 |
30 |
| F |
0.165400179 |
|
| P(F<=f) one-tail |
2.02449E-06 |
|
| F Critical one-tail |
0.543220913 |
|
5.3. T TEST (assuming unequal variances)
Table 4.
T-Test Two-Sample Assuming Unequal Variances .
Table 4.
T-Test Two-Sample Assuming Unequal Variances .
| |
Variable 1 |
Variable 2 |
| Mean |
0.667741935 |
2.322580645 |
| Variance |
0.194258065 |
1.174473118 |
| Observations |
31 |
31 |
| Hypothesized Mean Difference |
1.65483871 |
|
| Df |
40 |
|
| t Stat |
-15.75097467 |
|
| P(T<=t) one-tail |
4.76993E-19 |
|
| t Critical one-tail |
1.683851013 |
|
| P(T<=t) two-tail |
9.53987E-19 |
|
| t Critical two-tail |
2.02107539 |
|
6. Conclusions
This study has exhibited the value of the unique characteristics of these two products to enhance the success and viability of the ITRT protocol. The relatively narrow coronal portion of the Inverta implant along with the regenerative potential of Ethoss appears to have improved bone regeneration in the critical aesthetic coronal zone for enhanced tissue stability.
Although this multi-center study appears to show synergy between both this novel implant and material, further studies are required to investigate longer term stability of the treated sites, with concentration on the interdental bony septum and the effect of partial extraction therapies.
Author Contributions
All the authors contributed cases and the study was written and collated up by the lead author with the Graphs provided by the 3rd author.
Funding
No funding or free materials was given to the authors for this study. All work was conducted as daily clinical practice.
Institutional Review Board Statement
Registered patients provided consent in accordance with the Declaration of Helsinki (17).
Informed Consent Statement
All authors consent for publication of this case study.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
Peter J M Fairbairn has a competing interest in this study as a Clinical Director of EthOss Regeneration Ltd (Silsden, UK)
References
- Chu, S.J. Implant Designs in 2020: Current State of the Art Addresses Primary Stability. Compend Contin Educ Dent. 2020, 41, 432–434. [Google Scholar]
- Norton, M.R. The influence of insertion torque on the survival of immediately placed and restored single-tooth implants. J Oral Maxillofac Implants. 2011, 26, 1333–43. [Google Scholar]
- Nevins, M; Chu S.J; Jang, W; Kim D.M. Evaluation of an innovative hybrid macrogeometry dental implant in immediate extraction sockets: A histomorphometric pilot study in foxhound dogs. J Periodontics Restorative Dent 2019, 29–37.
- Östman, P.-O.; Chu, S.; Drago, C.; Saito, H.; Nevins, M. Clinical Outcomes of Maxillary Anterior Postextraction Socket Implants with Immediate Provisional Restorations Using a Novel Macro-Hybrid Implant Design: An 18- to 24-Month Single-Cohort Prospective Study. Int. J. Periodontics Restor. Dent. 2020, 40, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.J. Inverted body-shift concept in macroimplant design to enhance biologic and esthetic outcomes: A clinical report. J. Prosthet. Dent. 2020, 126, 720–726. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 1--review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 2004, 17. [Google Scholar]
- Leventis, M. D; Fairbairn P, Dontas I, et al. Biological response to ß-tricalcium phosphate/calcium sulfate synthetic graft material: an experimental study. Implant Dent. 2014:37-43.
- Leventis, M; Fairbairn, P; Kakar A; et al. Minimally invasive alveolar ridge preservation utilizing an in situ hardening ß-tricalcium phosphate bone substitute. A multicenter case series. Int J Dent. 2016.
- Habibovic, A; Malhotra P. Calcium Phosphates and Angiogenesis: Implications and Advances for Bone Regeneration. Maastricht: Treads in Biotechnology. 1: 2018;34, 2018.
- Miron, R.J.; Zhang, Q.; Sculean, A.; Buser, D.; Pippenger, B.E.; Dard, M.; Shirakata, Y.; Chandad, F.; Zhang, Y. Osteoinductive potential of 4 commonly employed bone grafts. Clin. Oral Investig. 2016, 20, 2259–2265. [Google Scholar] [CrossRef] [PubMed]
- Marzook, H.; Flifl, M.A.S.; Denewar, M.; Elsheikh, H.A.-E. Biological Impact of Alloplastic Bone Graft vs Bovine Xenograft and Allograft Materials in Bone Healing: An Experimental Study. J. Contemp. Dent. Pr. 2022, 23, 482–491. [Google Scholar] [CrossRef]
- Bohner, M; Le Gars Santoni, B; Döbelin, N. ß-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomaterialia. 2020.
- Kamitakahara, M; Ohtsuki, C; Miyazaki, T. Review Paper: Behaviour of Ceramic Biomaterials Derived from Tricalcium Phosphate in Physiological Condition. Journal of Biomaterials Applications., 2008, Vol. 23.
- Yip, I.; Ma, L.; Mattheos, N.; Dard, M.; Lang, N.P. Defect healing with various bone substitutes. Clin. Oral Implant. Res. 2015, 26, 606–614. [Google Scholar] [CrossRef]
- Liu, C. C; Solderer, A; et al. Tricalcium phosphate (containing) biomaterials in the treatment of periodontal infra-bony defects: A systemic review and meta-analysis. Journal of Dentistry. 2021.
- Gamborena, I; Sasaki, Y; Blatz, M.B; et al. The All-at-Once concept: Immediate implant placement into fresh extraction sockets with final crown delivery. Quintessense Dent Technol. 2019:165-177.
- National Library of Medicine. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. General Assembly of the World Medical Association. 2014.
- Elian, N.; Cho, S.-C.; Froum, S.; Smith, R.B.; Tarnow, D.P. A simplified socket classification and repair technique. Pract. Proced. aesthetic Dent. 2007, 19, 99–104. [Google Scholar] [PubMed]
- Miyamoto, Y.; Obama, T. Dental cone beam computed tomography analyses of postoperative labial bone thickness in maxillary anterior implants: comparing immediate and delayed implant placement. . 2011, 31, 215–25. [Google Scholar]
- Braut, V; Bornstein, M.M; Belser, U; Buser D. Thickness of the anterior maxillary facial bone wall - a retrospective study using cone beam computerised tomography. Journal of Periodontics and Restorative Dentistry. 2011.
- Östman, P.; Hellman, M.; Sennerby, L. Direct Implant Loading in the Edentulous Maxilla Using a Bone Density–Adapted Surgical Protocol and Primary Implant Stability Criteria for Inclusion. Clin. Implant. Dent. Relat. Res. 2005, 7, s60–s69. [Google Scholar] [CrossRef] [PubMed]
- Steigmann, M.; Monje, A.; Chan, H.-L.; Wang, H.-L. Emergence Profile Design Based on Implant Position in the Esthetic Zone. Int. J. Periodontics Restor. Dent. 2014, 34, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Fürhauser, R.; Florescu, D.; Benesch, T.; Haas, R.; Mailath, G.; Watzek, G. Evaluation of soft tissue around single-tooth implant crowns: the pink esthetic score. Clin. Oral Implant. Res. 2005, 16, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Chappuis, V; Bornstein, M.M. Influence of implant neck design on facial bone crest dimensions in the esthetic zone analysed by cone beam CT: A comparative study with a 5-to-9-year follow-up. Clinical Oral Implants Research. 2016:1055-1064.
- Chu S,J; Östman, P.O; Nicolopoulos, C; et al. Prospective muticenter clinical cohort study of a novel macro hybrid implant in maxillary anterior postextraction sockets: 1-year results. 1: Int J Periodontics Restorative Dent, 2018, 2018.
- Blanco, J.; Nuñez, V.; Aracil, L.; Muñoz, F.; Ramos, I. Ridge alterations following immediate implant placement in the dog: flap versus flapless surgery. J. Clin. Periodontol. 2008, 35, 640–648. [Google Scholar] [CrossRef]
- Covani, U.; Bortolaia, C.; Barone, A.; Sbordone, L. Bucco-Lingual Crestal Bone Changes After Immediate and Delayed Implant Placement. J. Periodontol. 2004, 75, 1605–1612. [Google Scholar] [CrossRef]
- Covani, U.; Cornelini, R.; Barone, A. Bucco-Lingual Bone Remodeling Around Implants Placed into Immediate Extraction Sockets: A Case Series. J. Periodontol. 2003, 74, 268–273. [Google Scholar] [CrossRef]
- Qahash, M; Susin, C; Polimeni, G; Hall, J; Wikesjö, UME. Bone healing dynamics at buccal peri-implantv sites., Clinical Oral Implants Research. 2008:166-172.
- Vignoletti, F; De Sanctis, M, Berglundh T, Abrahamsson I, Sanz M. Early healing of implants placed into fresh extraction sockets: an experimental study in beagle dog. II: ridge alterations. Journal of Clinical Periodontology. 2009:688-697.
- Chen, S.T; Buser, D. Esthetic outcomes following immediate and early implant placement in the anterior maxilla - A systematic review. Int J Oral Maxillofac Implants. 2014:186-215.
- Chu, S.; Saito, H.; Östman, P.-O.; Levin, B.; Reynolds, M.; Tarnow, D. Immediate Tooth Replacement Therapy in Postextraction Sockets: A Comparative Prospective Study on the Effect of Variable Platform-Switched Subcrestal Angle Correction Implants. Int. J. Periodontics Restor. Dent. 2020, 40, 509–517. [Google Scholar] [CrossRef]
- Gluckman, H.; Salama, M.; Du Toit, J. Partial Extraction Therapies (PET) Part 2: Procedures and Technical Aspects. Int. J. Periodontics Restor. Dent. 2017, 37, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, G.M; Dompkowski, D.F; Mahler, B.A; Howes, D.G. Off-Axis Implant Placement for Anatomical Considerationsc Using the Co-Axis Implant. Inside Dentistry. 2008.
- Ortega-Martinez, J.; Perez-Pascual, T.; Mareque-Bueno, S.; Hernandez-Alfaro, F.; Ferres-Padro, E. Immediate implants following tooth extraction. A systematic review. 2012, 17, e251–e261. [Google Scholar] [CrossRef]
- Tarnow, D.P; Chu, S.J; Salama, M.A; et al. Post-extraction socket implants: Part 1. The effect of bone grafting and/or provisional restoration on facial-palatal ridge dimensional change: A retrospective cohort study. Int J Periodontics Restorative Dent. 2014:323-331.
- Yang, H.L; Zhu, X. S; Chen, L; Chen, C.M; Mangham, D.C; Coulton, L.A; Aiken, S.S. Bone healing response to a synthetic calcium sulfate/ß-tricalcium phosphate graft material in the sheep vertebral body defect model. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2012:1911-1921.
- Bohner, M; Le Gars Santoni, B; Döbelin, N. ß-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomaterialia 2020:, 23–41.
- Retzepi, M.; Donos, N. Guided Bone Regeneration: biological principle and therapeutic applications. Clin. Oral Implant. Res. 2010, 21, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Benic, GI; Hämmerle, C.H.F. Horizontal bone augmentation by means of guided bone regeneration. Periodontology 2000, 2014, 13–40.
- Jung, R. E; Fenner, N; Hämmerlie, C.H.F; Zitzmann, N.U. Long-term outcome of implants placed with guided bone regeneration (GBR) using resorbable and non-resorbable membranes after 12-14 years. Clinical oral implants research. 2003:1065-1073.
- Roberts, S.J.; van Gastel, N.; Carmeliet, G.; Luyten, F.P. Uncovering the periosteum for skeletal regeneration: The stem cell that lies beneath. Bone 2015, 70, 10–18. [Google Scholar] [CrossRef]
- Weng, D; Hürzeler, M.B; Quiñones, C.R; Ohlms, A; Caffesse, R.G. Contribution of the periosteum to bone formation in guided bone regeneration: A study in monkeys. Clinical oral implants research 2000, 546–554.
- Colnot, C. Skeletal Cell Fate Decisions Within Periosteum and Bone Marrow During Bone Regeneration. J. Bone Miner. Res. 2009, 24, 274–282. [Google Scholar] [CrossRef]
- Theodoropoulou, E; Horowitz, R; Kalyvas, D. Evaluation of an In Situ Hardening ß-Tricalcium Phosphate Graft Material for Alveolar Ridge Preservation. A Histomorphometric Animal Study in Pigs. Dentistry 2018.
- Wang, Q.; Huang, C.; Xue, M.; Zhang, X. Expression of endogenous BMP-2 in periosteal progenitor cells is essential for bone healing. Bone 2011, 48, 524–532. [Google Scholar] [CrossRef]
- Samee, M.; Kasugai, S.; Kondo, H.; Ohya, K.; Shimokawa, H.; Kuroda, S. Bone Morphogenetic Protein-2 (BMP-2) and Vascular Endothelial Growth Factor (VEGF) Transfection to Human Periosteal Cells Enhances Osteoblast Differentiation and Bone Formation. J. Pharmacol. Sci. 2008, 108, 18–31. [Google Scholar] [CrossRef]
- Buser, D; Chappuis, V; Belser, U. C; Chen, S. Implant placement post extraction in esthetic tooth sites: when immediate, when early, when late? 2016, Periodontology 2000; pp. 84–102. [Google Scholar]
- Dawson, A; Martin, W. C; Polido, W.D. The SAC classification in implant dentistry. s.l.: Quintessenz Verlag, 2022. [Google Scholar]
- Al Ruhaimi, K. Effect of calcium sulphate on the rate of osteogenesis in distracted bone. Int. J. Oral Maxillofac. Surg. 2001, 30, 228–233. [Google Scholar] [CrossRef]
- Sasaki, H.; Koyama, S.; Yokoyama, M.; Yamaguchi, K.; Itoh, M.; Sasaki, K. Bone metabolic activity around dental implants under loading observed using bone scintigraphy. The International Journal of Oral and Maxillofacial Implants 2008, 23, 827–34. [Google Scholar] [PubMed]
- Leventis, M; Fairbairn, P; Dontas, I; et al. Biological response to ß-tricalcium phosphate/calcium sulfate synthetic graft material: An experimental study. Implant Dentistry 2014, 37–43.
- Leventis, M.; Fairbairn, P.; Mangham, C.; Galanos, A.; Vasiliadis, O.; Papavasileiou, D.; Horowitz, R. Bone Healing in Rabbit Calvaria Defects Using a Synthetic Bone Substitute: A Histological and Micro-CT Comparative Study. Materials 2018, 11, 2004. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.-L.; Lin, G.-H.; Fu, J.-H.; Wang, H.-L. Alterations in Bone Quality After Socket Preservation with Grafting Materials: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2013, 28, 710–720. [Google Scholar] [CrossRef]
- Wolff, J. The law of bone remodelling. Springer Berlin. 1892.
- Tarnow, D.; Elian, N.; Fletcher, P.; Froum, S.; Magner, A.; Salama, M.; Salama, H.; Garber, D.A.; Cho, S.-C. Vertical Distance from the Crest of Bone to the Height of the Interproximal Papilla Between Adjacent Implants. J. Periodontol. 2003, 74, 1785–1788. [Google Scholar] [CrossRef]
- Fairbairn, P; Leventis, M; Mangham, C; Horowitz, B. Alveolar Ridge preservation using a Novel synthetic grafting material; A case study with 2 year follow up. Case Reports in Dentistry. 2018. https://www.hindawi. 2018.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).