Submitted:
23 September 2023
Posted:
25 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
- Technical or cost difficulties exist in the current utilization process for PG, and the comprehensive utilization rate remains low at less than 25%[15].
- The large stock of PG piles necessitates a corresponding market for its extensive consumption.
- Downstream products derived from PG have low added value and suffer from serious homogenization.
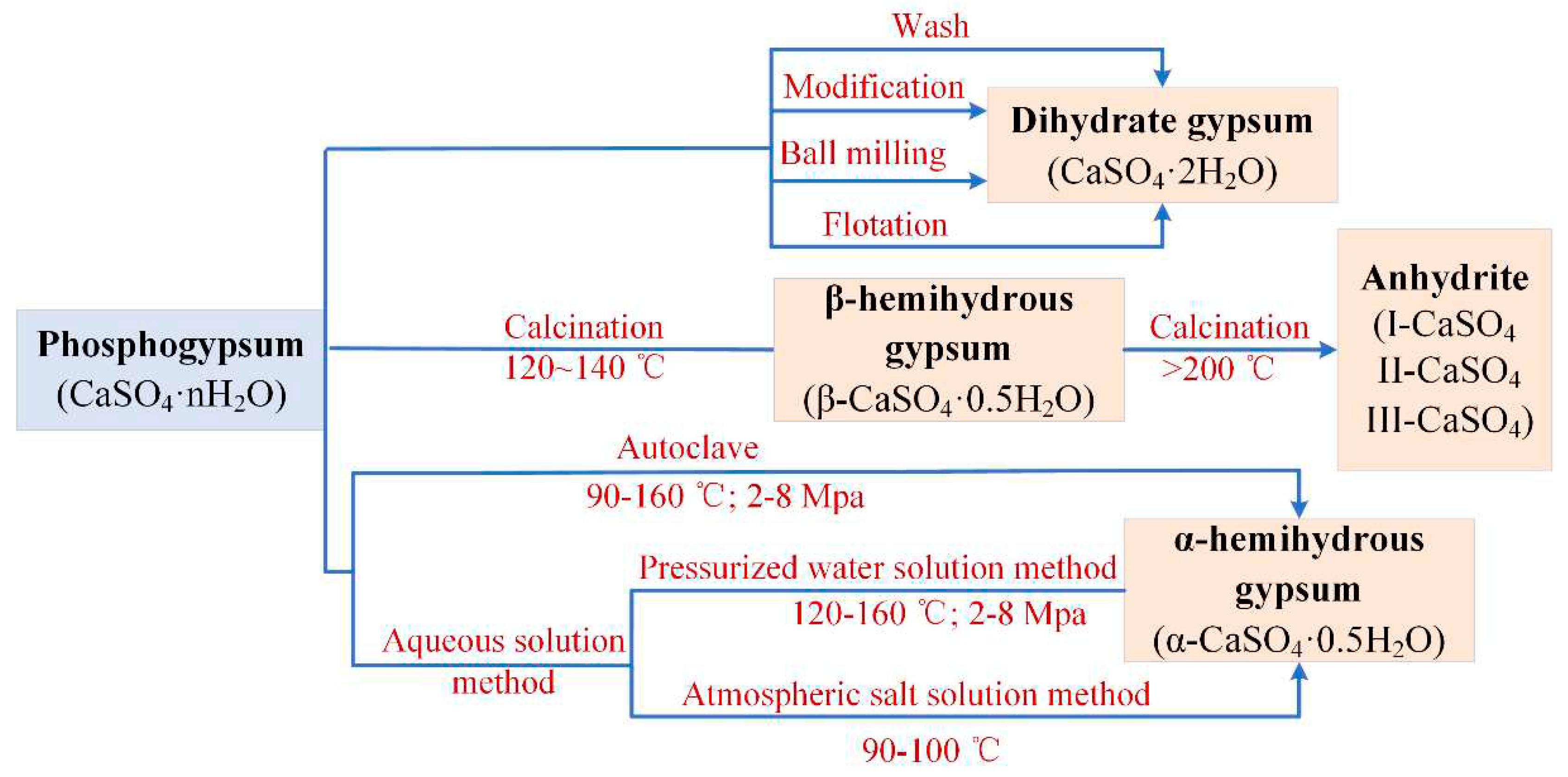
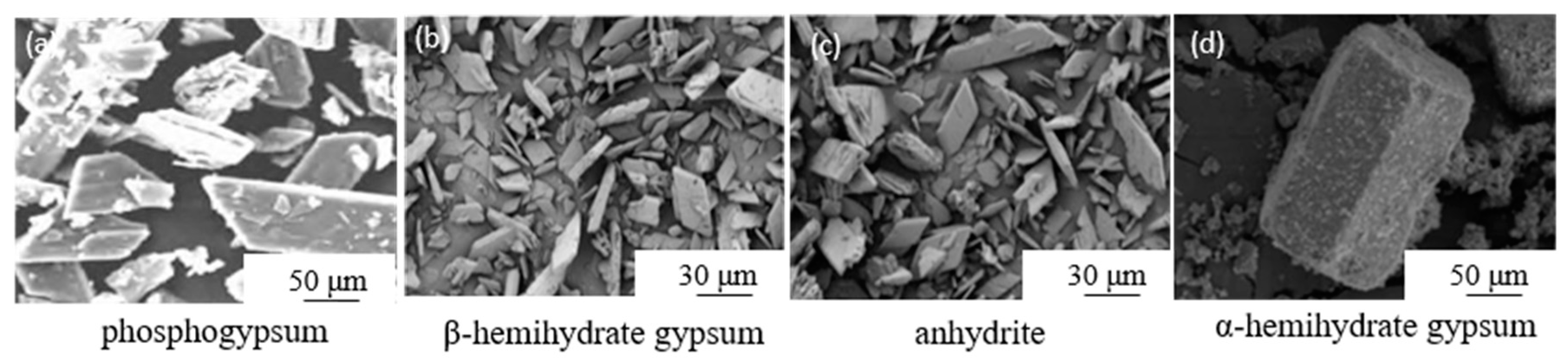
2. Dihydrate Gypsum
3. Hemihydrate Gypsum
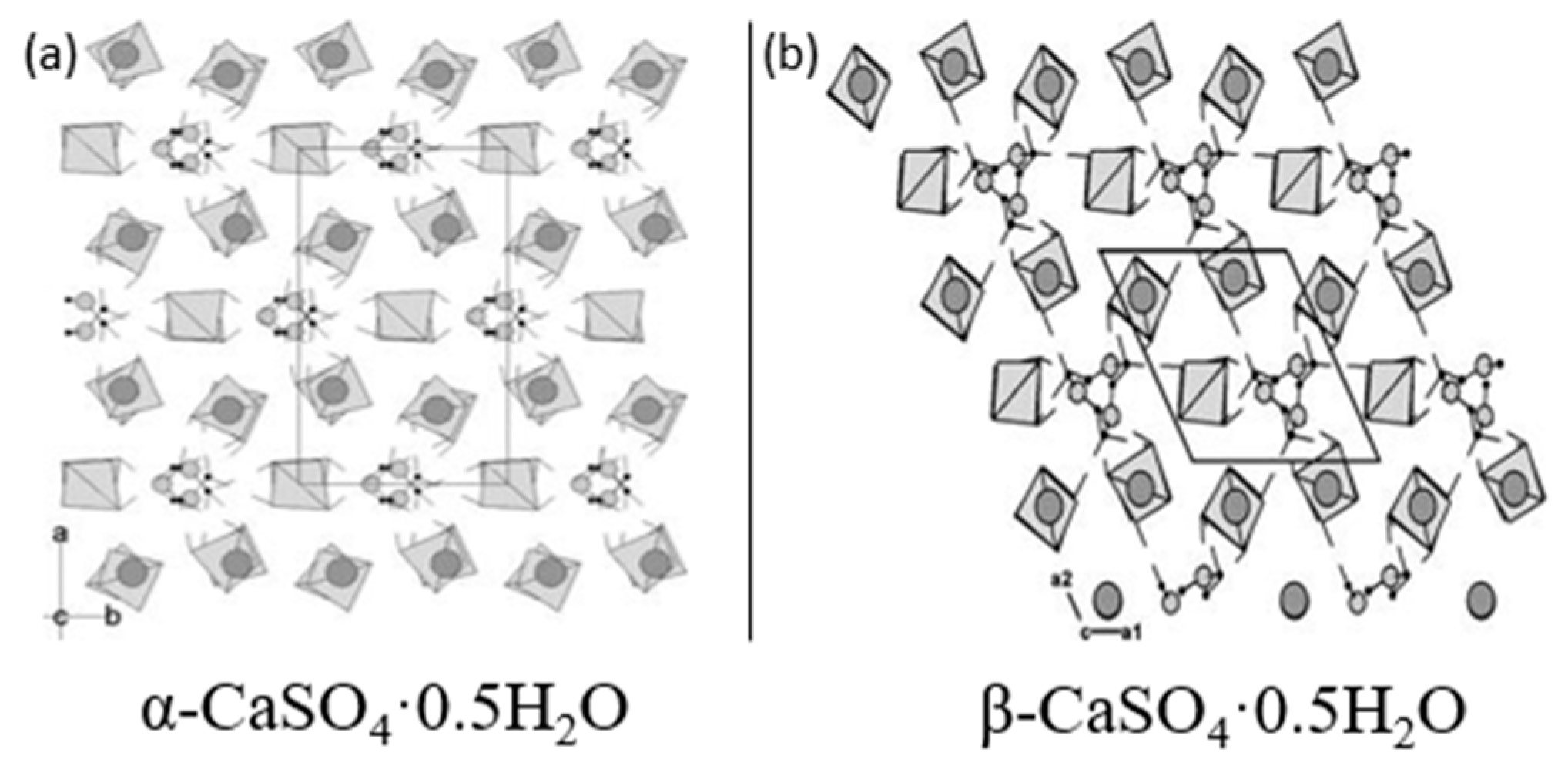
3.1. α-Hemihydrate Gypsum
3.2. β-Hemihydrate Gypsum
| Process | Experiment Condition | Crystal Morphology | Flexural Strength | Compressive Strength |
|---|---|---|---|---|
| Pre-grinding + calcination[55] |
Temperature: 150℃ Calcination time: 2 hours |
 |
— | 6.0 |
| Calcination[50] | Temperature: 130℃ Autoclave time: 3 hours Polycarboxylate superplasticizer: 0.5 % |
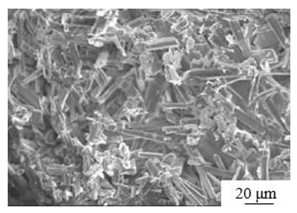 |
3.8 | 14.8 |
| Washing + calcination[51] | Temperature: 130℃ Calcination time: 1 hours |
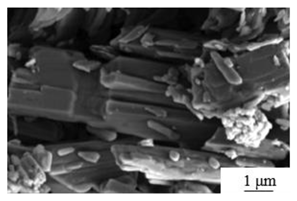 |
3.0 | 5.7 |
| Calcination[56] | Temperature: 180℃ Calcination time: 4 hours |
 |
— | 9.2 |
4. Anhydrite

5. Conclusions
- the harmless treatment of PG for producing dihydrate gypsum can serve as a cement retarder. The process is straightforward and supported by well-developed technologies. However, due to its non-competitive price, the cement industry heavily relies on natural gypsum, resulting in saturated market demand. Therefore, national and local policies are needed to promote the application's development.
- The use of hemihydrate gypsum is predominantly observed in the construction industry. In comparison to β-hemihydrate gypsum, α-hemihydrate gypsum exhibits superior physical and mechanical properties. In comparison to anhydrous gypsum, α-hemihydrate gypsum consumes less energy and facilitates scaling, thereby promoting the high-quality development of PG building materials. Consequently, the production of α-hemihydrate gypsum from PG is expected to become the dominant trend in the comprehensive utilization of PG for building materials in the future.
- type II anhydrous gypsum exhibits high strength, excellent water resistance, chemical corrosion resistance, and toughness. Moreover, its production capacity is significantly higher than that of β-semi-water gypsum. It can be utilized not only in road construction and mine filling materials to achieve significant consumption of PG, but also in the preparation of gypsum whiskers for applications such as paper fillers, rubber compounds, paints, and other advanced processing of novel materials. However, the production of type II anhydrous gypsum is constrained by the high calcination temperature involved in the preparation process. Moreover, type II anhydrous gypsum exhibits limited hydration activity and slow setting and hardening characteristics, necessitating careful consideration of modification treatments during its application.
Author Contributions
Funding
Conflicts of Interest
References
- Diwa R R, Tabora E U, Haneklaus N H, et al. Rare earths leaching from Philippine phosphogypsum using Taguchi method, regression, and artificial neural network analysis[J/OL]. Journal of Material Cycles and Waste Management, 2023[2023-09-12]. [CrossRef]
- Rashad A, M. Phosphogypsum as a construction material[J]. Journal of Cleaner Production, 2017, 166: 732-743. [CrossRef]
- Amrani M, Taha Y, Kchikach A, et al. Phosphogypsum recycling: New horizons for a more sustainable road material application[J]. Journal of Building Engineering, 2020, 30: 101267. [CrossRef]
- Qi J, Zhu H, Zhou P, et al. Application of phosphogypsum in soilization: a review[J/OL]. International Journal of Environmental Science and Technology, 2023[2023-08-06].
- Xiao J, Lu T, Zhuang Y, et al. A Novel Process to Recover Gypsum from Phosphogypsum[J]. Materials, 2022, 15(5): 1944. [CrossRef]
- Murali G, Azab M. Recent research in utilization of phosphogypsum as building materials: Review[J]. Journal of Materials Research and Technology, 2023, 25: 960-987. [CrossRef]
- Millán-Becerro R, Pérez-López R, Macías F, et al. Design and optimization of sustainable passive treatment systems for phosphogypsum leachates in an orphan disposal site[J]. Journal of Environmental Management, 2020, 275: 111251. [CrossRef]
- Bolívar J P, Martín J E, García-Tenorio R, et al. Behaviour and fluxes of natural radionuclides in the production process of a phosphoric acid plant[J]. Applied Radiation and Isotopes, 2009, 67(2): 345-356. [CrossRef]
- De Oliveira S M B, Da Silva P S C, Mazzilli B P, et al. Rare earth elements as tracers of sediment contamination by phosphogypsum in the Santos estuary, southern Brazil[J]. Applied Geochemistry, 2007, 22(4): 837-850. [CrossRef]
- Adeoye C, Gupta J, Demers N, et al. Variations of radon and airborne particulate matter near three large phosphogypsum stacks in Florida[J]. Environmental Monitoring and Assessment, 2021, 193(5): 284.
- El Zrelli R, Rabaoui L, Abda H, et al. Characterization of the role of phosphogypsum foam in the transport of metals and radionuclides in the Southern Mediterranean Sea[J]. Journal of Hazardous Materials, 2019, 363: 258-267. [CrossRef]
- Kulczycka J, Kowalski Z, Smol M, et al. Evaluation of the recovery of Rare Earth Elements (REE) from phosphogypsum waste – case study of the WIZÓW Chemical Plant (Poland)[J]. Journal of Cleaner Production, 2016, 113: 345-354. [CrossRef]
- Lokshin E P, Tareeva O A, Elizarova I R. On integrated processing of phosphogypsum[J]. Russian Journal of Applied Chemistry, 2013, 86(4): 463-468.
- Silva L F O, Oliveira M L S, Crissien T J, et al. A review on the environmental impact of phosphogypsum and potential health impacts through the release of nanoparticles[J]. Chemosphere, 2022, 286: 131513. [CrossRef]
- Chernysh Y, Yakhnenko O, Chubur V, et al. Phosphogypsum Recycling: A Review of Environmental Issues, Current Trends, and Prospects[J]. Applied Sciences, 2021, 11(4): 1575. [CrossRef]
- Tayibi H, Choura M, López F A, et al. Environmental impact and management of phosphogypsum[J]. Journal of Environmental Management, 2009, 90(8): 2377-2386. [CrossRef]
- Preturlan J G D, Vieille L, Quiligotti S, et al. Comprehensive Thermodynamic Study of the Calcium Sulfate–Water Vapor System. Part 1: Experimental Measurements and Phase Equilibria[J]. Industrial & Engineering Chemistry Research, 2019, 58(22): 9596-9606. [CrossRef]
- Zhou.W.; Qin.Y.; Zhao.S. et al. Preparation of α-hemihydrate gypsum by self steam curing of phosphogypsum [J]. Environmental Protection of Chemical Industry 2022, 42(6): 721-727.
- Singh M, Garg M, Verma C L, et al. An improved process for the purification of phosphogypsum[J]. Construction and Building Materials, 1996, 10(8): 597-600. [CrossRef]
- Voznesenskii A S, Ushakov E I. Temperature dependence of internal mechanical losses of gypsum stone with complex composition and structure[J]. Journal of Alloys and Compounds, 2022, 906: 164194. [CrossRef]
- López-Buendía A M, García-Baños B, Urquiola M M, et al. Evidence of a new phase in gypsum–anhydrite transformations under microwave heating by in situ dielectric analysis and Raman spectroscopy[J]. Physical Chemistry Chemical Physics, 2020, 22(47): 27713-27723.
- Zhi Z, Huang J, Guo Y, et al. Effect of chemical admixtures on setting time, fluidity and mechanical properties of phosphorus gypsum based self-leveling mortar[J]. KSCE Journal of Civil Engineering, 2017, 21(5): 1836-1843.
- Gu.Q.; Lin.X.; Zhao.S. et al. Effect of different pretreatment processes on properties of phosphogypsum. Inorganic Chemicals Industry 2022, 54(4): 17-23.
- Bumanis G, Zorica J, Bajare D, et al. Technological properties of phosphogypsum binder obtained from fertilizer production waste[J]. Energy Procedia, 2018, 147: 301-308. [CrossRef]
- Li X, Zhang Q. Dehydration behaviour and impurity change of phosphogypsum during calcination[J]. Construction and Building Materials, 2021, 311: 125328. [CrossRef]
- Guan Q, Sui Y, Zhang F, et al. Preparation of α-calcium sulfate hemihydrate from industrial by-product gypsum: A review[J]. Physicochemical Problems of Mineral Processing, 2020, 57(1): 168-181.
- Yang M, Qian J. Activation of anhydrate phosphogypsum by K2SO4 and hemihydrate gypsum[J]. Journal of Wuhan University of Technology-Mater. Sci. Ed., 2011, 26(6): 1103-1107.
- Li.H.; Zhang.H.; Zi.X. Analysis on calcination process of phosphogypsum. Inorganic Chemicals Industry: 1-14.
- Chen S, Chen J, He X, et al. Micromicelle-mechanical coupling method for high-efficiency phosphorus removal and whiteness improvement of phosphogypsum[J]. Construction and Building Materials, 2022, 354: 129220. [CrossRef]
- Li.X.; Chen.J.; Ma.B. et al. Influence of different granular distributions on physical properties of phosphogypsum-lime-fly ash system, Acta CIESC Journal 2012, 63(S1): 230-234.
- Li B, Li L, Chen X, et al. Modification of phosphogypsum using circulating fluidized bed fly ash and carbide slag for use as cement retarder[J]. Construction and Building Materials, 2022, 338: 127630. [CrossRef]
- Fang J, Ge Y, Chen Z, et al. Flotation purification of waste high-silica phosphogypsum[J]. Journal of Environmental Management, 2022, 320: 115824. [CrossRef]
- Wu.C.; Lv.W. Application of phosphogypsum in cement products. China Concrete and Cement Products 2019(1): 45-46.
- Shen.X.; Li.Z.; Hao.Y.; et al. Purification process of phosphogypsum for cement retarder production. Phosphate & Compound Fertilize 2014, 29(2): 44-46.
- Zhang.C. Review on the process of phosphogypgypsum to make cement retarder. Journal of Chemical Industry & Technology 2001(3): 18-20+1.
- Fisher R D, Mbogoro M M, Snowden M E, et al. Dissolution Kinetics of Polycrystalline Calcium Sulfate-Based Materials: Influence of Chemical Modification[J]. ACS Applied Materials & Interfaces, 2011, 3(9): 3528-3537. [CrossRef]
- Sun.X. Study on preparation of structure and morphology of calcium sulfate hemihydrate based on phosphogypsum calcium sulfate dihydrate.[D] Hefei University of Technology, 2020.
- Christensen A N, Jensen T R, Nonat A. A new calcium sulfate hemi-hydrate[J]. Dalton Transactions, 2010, 39(8): 2044.
- Chen.F.; Li.J.; Chen.M. Research on preparation of high-strength α-hemihydrate gypsum from phosphogypsum and its application. Phosphate & Compound Fertilize 2019, 34(7): 13-17.
- Chen.J.; Yi.Y.; Zhang.H.; et al. Effect of autoclave parameters and impurities on preparation of α-hemihydrate gypsum from phosphogypsum. Inorganic Chemicals Industry 2022, 54(3): 91-96.
- Wang.Q.; Tian.X.; Liu.D.; et al. Effects of Slurry Concentration and pH Value on the Preparation of α-Hemihydrate Gypsum from Phosphogypsum. Metal Mine, 2022(12): 115-121.
- Yang.R.; Chen.D.; Mi.Y.; et al. Preparation of α-Semi Hydrated Gypsum from Phosphogypsum in Atmospheric Pressure Chlorine-Free Complex Salt Solution. Non-Metallic Mines 2019, 42(4): 1-5.
- Tan H, Zheng A, Kang X, et al. Influence of different additives on the mechanical performance of a-hemihydrate gypsum from phosphogypsum[J]. Materiali in Tehnologije, 2020, 54(5): 697-703.
- Du M, Wang J, Dong F, et al. The study on the effect of flotation purification on the performance of α-hemihydrate gypsum prepared from phosphogypsum[J]. Scientific Reports, 2022, 12(1): 95.
- Lu W, Ma B, Su Y, et al. Preparation of α-hemihydrate gypsum from phosphogypsum in recycling CaCl2 solution[J]. Construction and Building Materials, 2019, 214: 399-412.
- Shen.J.; Lu.D; Xv.Z. et al. Effect of Ethylenediaminetetraacetic Acid on the Preparation of α-Hemihydrate Gypsum from Phosphogypsum with Hydrothermal Autoclave Method. Bulletin of The Chinese Ceramic Society 2015, 34(10): 2816-2821.
- Bai.Y.;Li.D.;et al.Medium Crystal Agent in High-Strength Gypsum with Flue Gas Desulphurization Gypsum[J].Bulletin of The Chinese Ceramic Society,2009,37(7): 1142-1146.
- Li.D.;Guo.R.;Lin.Z.; et al. Research Status of Preparation of α-Hemihydrate Gypsum from Phosphogypsum. Bulletin of The Chinese Ceramic Society 2022, 41(3): 860-869.
- Geraldo R H, Costa A R D, Kanai J, et al. Calcination parameters on phosphogypsum waste recycling[J]. Construction and Building Materials, 2020, 256: 119406. [CrossRef]
- Yang.G.; Bo.S.; Deng.L. et al. Effect of polycarboxylate superplasticizer on the properties of β-hemihydrate phosphogypsum. New Building Materials 2023, 50(1): 124-127+153.
- Liu.M.; Wang.Q.; Zhu.C. et al.Performance Change and Application of Calcined Phosphogypsum Before and After Washing and Grinding Pre-treatment. Materials Reports 2022, 36(S1): 267-271.
- Zhang L, Mo K H, Tan T H, et al. Influence of calcination and GGBS addition in preparing β-hemihydrate synthetic gypsum from phosphogypsum[J]. Case Studies in Construction Materials, 2023, 19: e02259.
- Wu.Z.; Zhang.Y.; Zhang.L. et al. Effect of phase composition and impurity content of β-gypsum on its properties. Inorganic Chemicals Industry 2021, 53(9): 67-71.
- Zhu.P.; Peng.C.; Zhang.Y. et al. Experimental study on preparation of high quality type II anhydrous gypsum from phosphogypsum. Industrial Minerals & Processing 2018, 47(7): 19-22.
- Peng.Z.; Meng.X.; Liu.C. Study on technological parameters of preparation of β-type hemihydrous gypsum powder by phosphogypsum. S P & BMH Related Engineering 2019(3): 13-18+5.
- Ma.J.; Xie.G.; Yv.Q. et al. Experimental Study of Phosphogysum to Produce β-hemihydrate Block. Journal of Wuhan University of Technology 2015, 37(7): 20-24.
- Deng.H. Study on Phase Change Behavior and Physical and Chemical Properties of Phosphogypsum During Heat Treatment[D]. Guizhou University. 2020.
- Finot E, Lesniewska E, Goudonnet J-P, et al. Correlation between surface forces and surface reactivity in the setting of plaster by atomic force microscopy[J]. Applied Surface Science, 2000, 161(3-4): 316-322. [CrossRef]
- Freyer D, Voigt W. Crystallization and Phase Stability of CaSO 4 and CaSO 4 - Based Salts[J]. Monatshefte f�r Chemie / Chemical Monthly, 2003, 134(5): 693-719.
- Farnsworth, M. The Hydration of Anhydrite.[J]. Industrial & Engineering Chemistry, 1925, 17(9): 967-970. [CrossRef]
- Bi.Q.;Mei.Y.;Xia.J. Basic research on preparation and activity combined excitation of anhydrite-II phosphogypsum(AII). Chemical Industry and Engineering Progress 2022.1-12.
- Zhang.Y.; Yang.J.; Liu.Y. et al. Regulating technology of setting and hardening process of anhydrite-II phosphogypsum. Chemical Industry and Engineering Progress 2022, 41(10): 5637-5644.
- Cao W, Yi W, Peng J, et al. Preparation of anhydrite from phosphogypsum: Influence of phosphorus and fluorine impurities on the performances[J]. Construction and Building Materials, 2022, 318: 126021. [CrossRef]
- Liu S, Ouyang J, Ren J. Mechanism of calcination modification of phosphogypsum and its effect on the hydration properties of phosphogypsum-based supersulfated cement[J]. Construction and Building Materials, 2020, 243: 118226. [CrossRef]
- Chen.J.; Zzhang.R. Report on Ecological Environment Technology Development of Industrial By-product Gypsum Resource Utilization. Guangzhou Chemical Industry 2020, 48(24): 1-3.
- Wang.L.; Tan.H.; Li.Y. et al. Preparation of II-Anhydrite Gypsum and Effect of Cement on Its Properties. Non-Metallic Mines 2020, 43(5): 52-54+63.
| Process | Experiment Condition | Crystal Morphology |
|---|---|---|
| Modified water washing[29] | Mechanical activation + Water phase grinding Micellar agent:CH3COOH,Al2O3 |
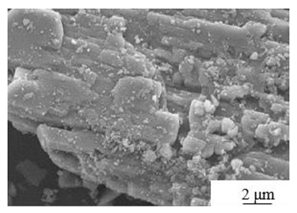 |
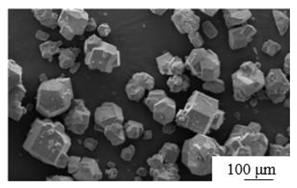
| Mechanical grinding[30] | PG: fly ash: lime mass ratio of 50:30:20 mixed Grinding time: 15min Sample preparation additives: 5% cement, 3% AC reinforcer and 0.5% polycarboxylic acid water reducer |
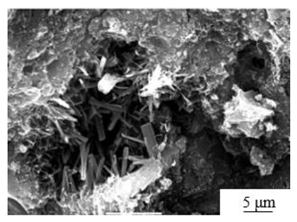 |
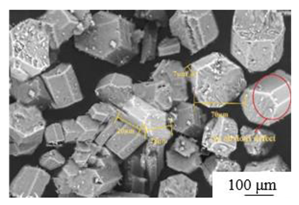
| Modification[31] | Modifier: 6wt% calcium carbide slag and 4wt%CFB fly ash | 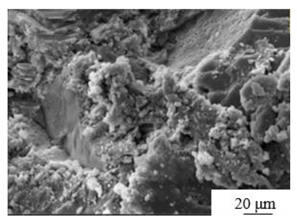 |
| Reverse direct flotation[32] | Collector: Kerosene Foaming agent: industrial grade pine oil |
 |
| Process | Principle | Advantage | Disadvantage | |
|---|---|---|---|---|
| Autoclave | Process of heating and pressurization in saturated water vapor removing of 1.5 molecules of water |
|
|
|
| Aqueous solution method |
Pressurized water solution method | Crystallization reaction of PG and water solution under pressure | High product quality |
|
| Atmospheric salt solution method | Crystallization reaction between PG and salt solution at atmospheric pressure |
|
|
|
| Process | Experiment Condition | Crystal Morphology | Flexural Strength | Compressive Strength | L/D ratio |
|---|---|---|---|---|---|
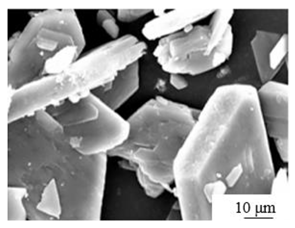
| Ball milling + Autoclave [43] | pH: 5 Temperature: 140℃ Autoclave time: 2 hours Crystal modifier: Maleic acid |
 |
8.2 | 25.4 | — |
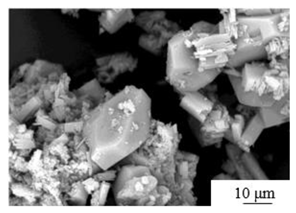
| Flotation + Autoclave [44] | Temperature: 140℃ Autoclave time: 2 hours Crystal modifier: 0.13wt% Maleic acid 2.0wt% Aluminum sulfate |
 |
6.09 | 40.96 | 0.7: 1 |
| Atmospheric salt solution [45] | Temperature: 97±1℃ Time: 2 hours Crystal modifier: maleic acid |
 |
4.7 | 37.6 | 1.0: 1 |
| Pressurized aqueous solution [46] | Temperature: 130℃ pH: 7.0 Autoclave time: 4 hours Crystal modifier: EDTA |
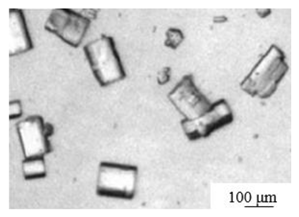 |
— | — | 1.5: 1 |
| Process | Experiment Condition | Crystal Morphology | Flexural Strength | Compressive Strength |
|---|---|---|---|---|
| Calcination + grinding[61] | Temperature: 800℃ Calcination time: 1.6 hours Potassium alum + LA-1 |
 |
>6.5 | 36.5 |
| Calcination[62] | Complexing agent (β-hemihydrate gypsum 6%、Modified steel slag 3%、K2SO4 2%、Calcium aluminate cement 0.5% ) | 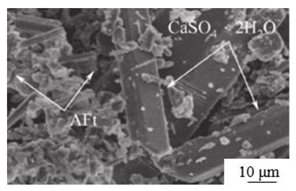 |
> 5 | 15.4 |
| Calcination [63] | Temperature: 800℃ Calcination time: 1 hours Addition: Ca (H2PO4) ·H2O and NaF |
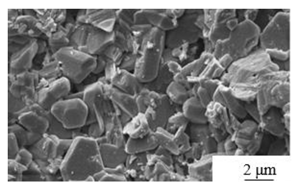 |
> 6 | > 20 |
| Calcination[64] | Temperature: 600℃ Calcination time: 1.5 hours |
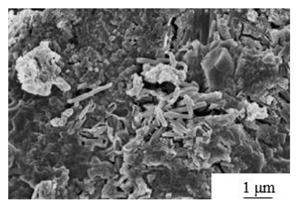 |
7-12 | 25-100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).