1. Introduction
High- strength wastewaters with high concentrations of sulfate, organic compounds and heavy metals produced during industrial processes, are usually treated via anaerobic digestion. This bioprocess effectively reduces the pollutant load in the wastewater, while simultaneously producing biomethane which is a sustainable alternative energy source. Wastewater generated from potato processing industries contains high amount of starch which has high viscosity and low solubility [
1] and for this reason is considered a highly polluted wastewater [
2]. Due to its physicochemical characteristics, starch can cause operational harms in industry’s wastewater treatment facilities, and thus, it is separated from the wastewater influent stream. After its extraction, this by-product can by hydrolysed and the produced hydrolysates can be effectively treated under anaerobic conditions for energy production in the form of methane [
3].
Upflow anaerobic sludge blanket (UASB) bioreactor is one of the most widely used processes in food processing wastewaters with high content of carbohydrates [
4] due to its low cost, high organic removal, and recovery of methane which can be used as gaseous biofuel. One of the key factors in the effective operation of upflow anaerobic treatment systems is the generation of sludge in granular form. The latter can efficiently degrade various types of wastewater with high organic loads [
5]. Each of these granules consists of many different types of anaerobic bacteria which operate symbiotically and in layers for the successful degradation of substrate. Due to the granular form, bacteria are highly tolerant to the toxic hydrogen sulfide [
6].
The last years many studies have focused on UASB systems treating starch wastewater [
5,
6,
7]. He et al. 2019 [
8] assessed the energy and economic benefits of three anaerobic bioreactors at various starchy influent COD concentrations. The authors estimated that the annual economic balance for the UASB reactor was achieved when the influent COD was 12,984 mg/L. With the utilization of biogas as source of heat and a COD influent concentration higher than 15 g/L, the system was able to achieve annual energy and economic surpluses. In addition, Lu et al. 2015 [
9] observed that when a lab-scale UASB reactor supplied with starch as the sole carbon source at a COD concentration of 1000 mg/L, a total COD removal of 98.7% was achieved with a methane yield of 0.33 L CH
4/g COD
removed applying a hydraulic retention time (HRT) of 6 hours and an organic loading rate (OLR) of 4 g COD/L-day.
Although anaerobic digestion has been widespread in recent years in the utilization of industrial wastewaters, there are several challenges that need to be addressed. The content of inhibitors in the substrate, as well as the organic loading rate applied to the anaerobic bioreactor could affect the methane production [
10]. According to Lu et al. [
9] the OLR for treating low strength starch wastewater should not exceed 8.0 g COD/L-d, with a HRT of over 3 hours. Feeding with excessive starch at a high OLR resulted in a decrease in mass transfer rate and a negative impact on the specific methanogenesis activity of granular sludge. Consequently, the performance of UASB reactor was compromised in terms of both COD removal and biogas production. In addition, Musa et al. 2019 [
11] who studied the treatment of high-strength cattle slaughterhouse wastewater in UASB reactors, noticed that the optimum OLR for organic removal efficiency >90% was 5g COD/L-day. They also pointed out that at the highest OLR of 16 g/L-d, an HRT of more than 2 days could be proved adequate to achieve a 90% degradation efficiency of the waste. The methane production is also affected by the physicochemical properties of wastewater such as carbohydrates and fats content, C/N ratio and particle size [
12]. Recently, efforts have been directed towards enhancing biogas production by adjusting nutrient content through the use of trace elements (e.g.
, Fe, Zn, Cu) and employing additives (e.g.
, biochar) that promote direct interspecies electron transfer (DIET) thereby improving digestibility through increased adsorption potential [
13,
14].
Wastewater generated from potato processing industries except for high organic load, usually has a high sulfate content which inhibits the production of methane during anaerobic treatment since the presence of sulfate affects the microbial diversity of the reactor [
15]. Sulfate-reducing bacteria (SRB) that dominate in environments with high sulfate concentration, use sulfate as electron acceptor reducing sulfate into sulfide which is toxic for anaerobic microorganisms [
16]. In addition, SRB compete with methanogens for energy sources such as acetate, starch, and H
2 and in high sulfate environments SRB outcompete methanogens since they can capture electrons more easily. Hence, the COD/SO
4-2 ratio affects the microbial community, and consequently, the performance of the anaerobic digester [
17]. According to stoichiometry, the COD/SO
4-2 ratio must be more than 0.67 for a sufficient sulfate reduction. In addition, Choi et al. [
18], observed that SRB and methanogens are very competitive when the COD/SO
4-2 ratio is below 2.7. They suggest a COD/SO
4-2 ratio of more than 2.0 for a successful anaerobic treatment.
The presence of sulfate in anaerobic digestion has gained significant interest over the last years. Using the Scopus database, we conducted a search with the keywords (
“reactor
”) AND (
“sulfur
” OR
“sulfate
”) AND (
“anaerobic digestion
”), resulting in 550 publications since 2000. Notably, there has been an upward trend in research on sulfur presence in anaerobic reactors, with 36% of the total publications emerging in the last 5 years. A graph illustrating the number of publications throughout the last 23 years is available in the SI. Since, the presence of high sulfur content in organic wastes can result in the production of hydrogen sulfide which is toxic during anaerobic digestion, significant research efforts have been dedicated to addressing the removal of H2S emissions [
19,
20,
21,
22,
23]. Although the vast majority of research has focused on the reduction of sulfate during anaerobic digestion, there are few studies examining the biomethane production from high sulfate content-wastewater. The effectiveness of the COD/SO
4-2 ratio on methane production depends on many factors such as, among others, the HRT, the OLR and the presence of metals in the influent. Many anaerobic digestion studies using different substrates have reported that a COD/SO
4-2 ratio lower than 12 can inhibit the production of methane [
24,
25,
26,
27]. Lu et al. 2016 [
28] examined the treatment of a starchy wastewater in a UASB reactor under gradually decreasing COD/SO
4-2 ratio. When the COD/SO
4-2 ratio was lower than 2, apart from COD removal which was kept constant, sulfate removal and methane production were decreased significantly at values lower than 21.2% and 0.26 ± 0.37 L/L-day respectively. However, when the ratio was more than 2, biogas production was stable near to 1.15 L/L
UASB-day with a total COD removal of 73.5–80.3%. Moreover, Hu et al. 2015 [
29] used a synthetic wastewater containing acetate, ethanol, and sulfate as substrate in a UASB reactor. The authors found out that when the COD/SO
4-2 ratio decreased from 20.0 to 0.5, the conversion of COD
influent to CH
4, dropped from 80.5% to 54.4%. Furthermore, Liu et al. 2015 [
30] detected that zero-valent iron can enhance the methane production and sulfate reduction in anaerobic granular sludge reactors only when the COD/SO
4-2 ratio is 2- 4.5.
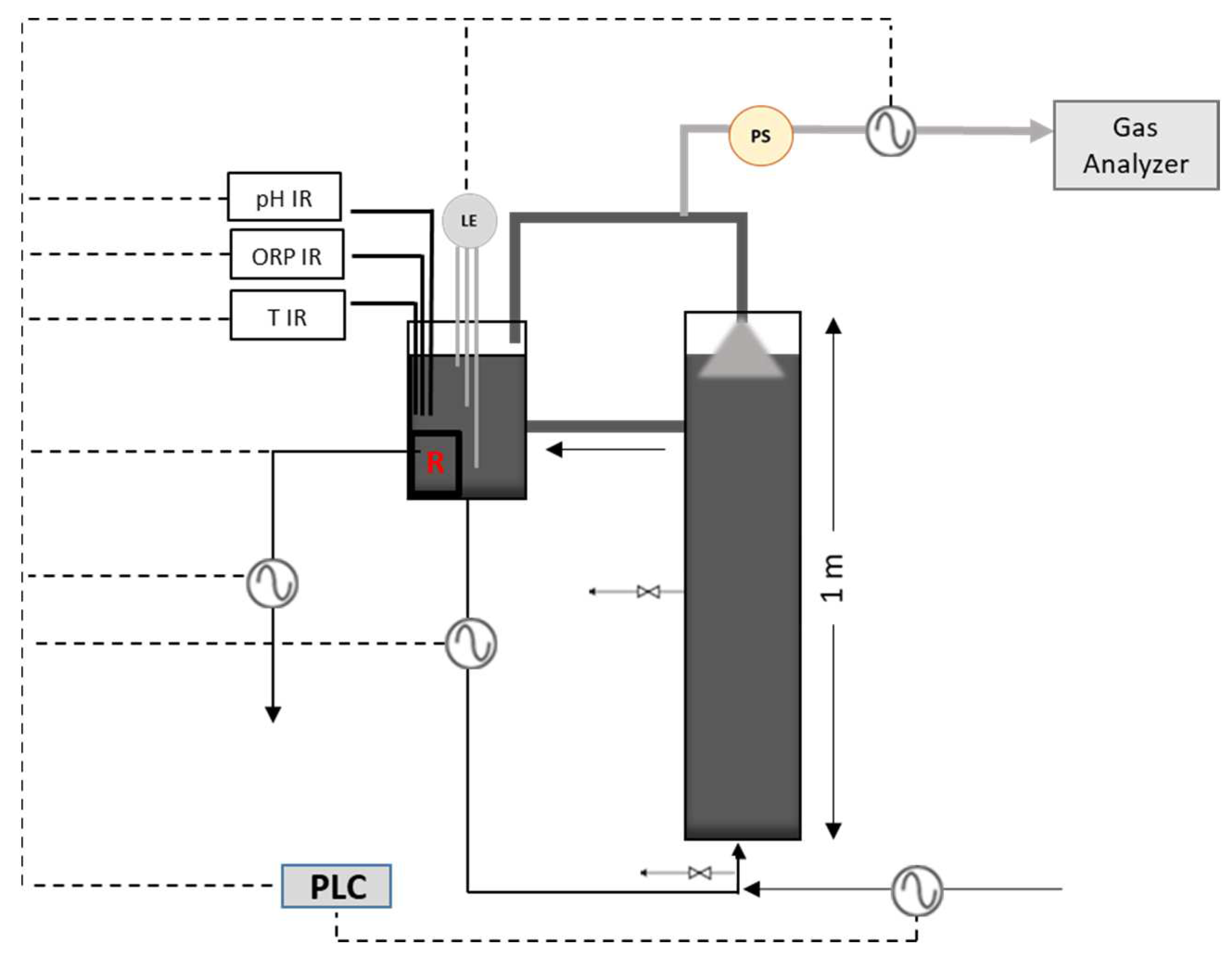
This study aims to improve the methane production and the overall performance of a UASB reactor by adding sulfate in the form of sulfuric acid to the influent. The latter consists of starch hydrolysates. Apart from sulfate, the influent was also enriched with iron ions. The UASB performance was monitored under various conditions examining different OLRs, HRTs, sulfate and iron additions. Moreover, 16S rRNA gene analysis was conducted to examine the microbial community changes before and after the sulfate addition. Our work demonstrates that appropriate sulfate concentrations in the feed, along with the presence of iron, can lead to enhanced methane yields in the UASB reactor. Monitoring the UASB reactor under various feeding conditions can lead to the identification of suitable conditions where not only the pollutant load of the waste is reduced, but also biogas rich in methane is generated. This biogas can be utilized as an alternative form of energy, aiming for economically sustainable and environmentally friendly industrial waste management.
4. Discussion
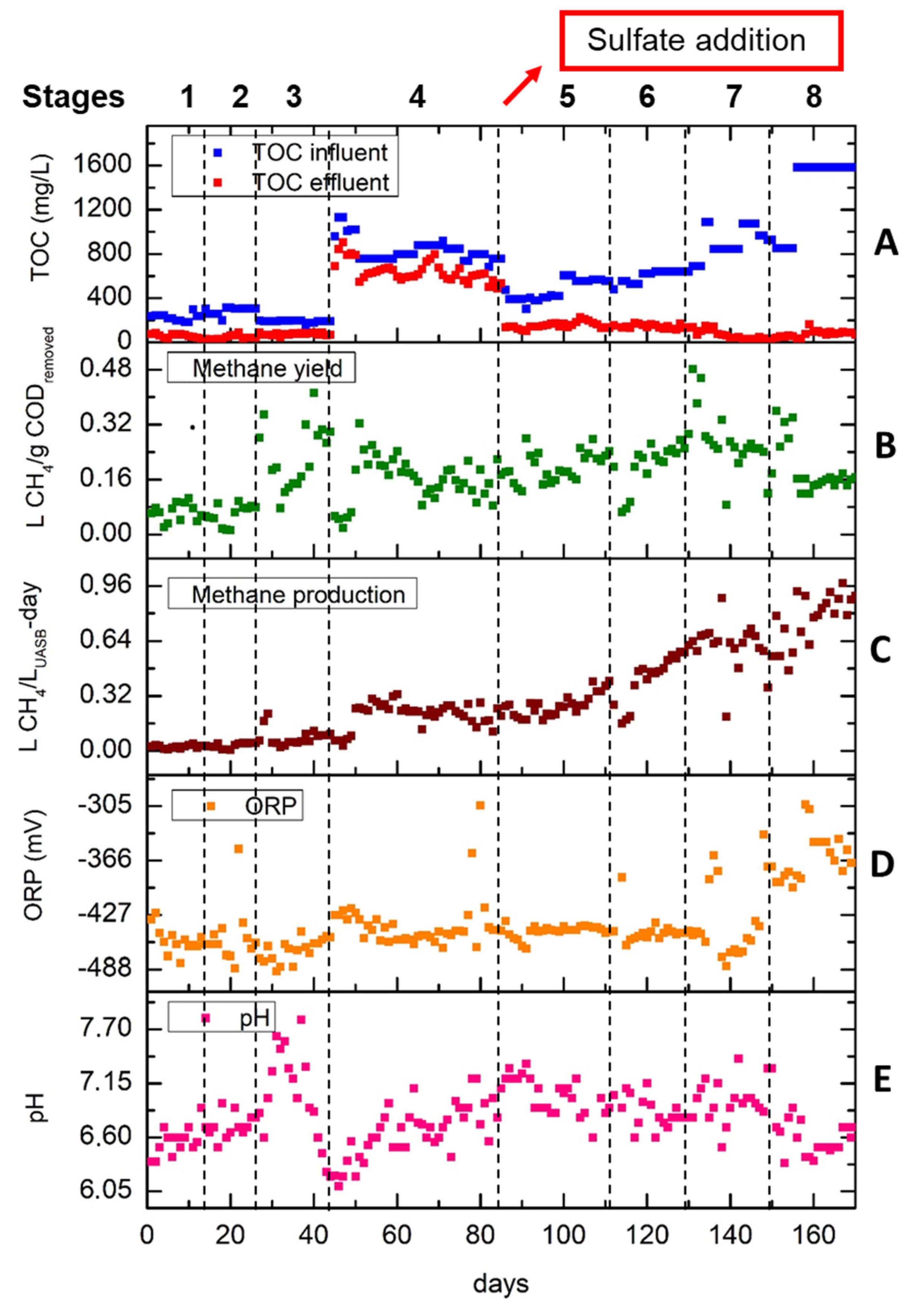
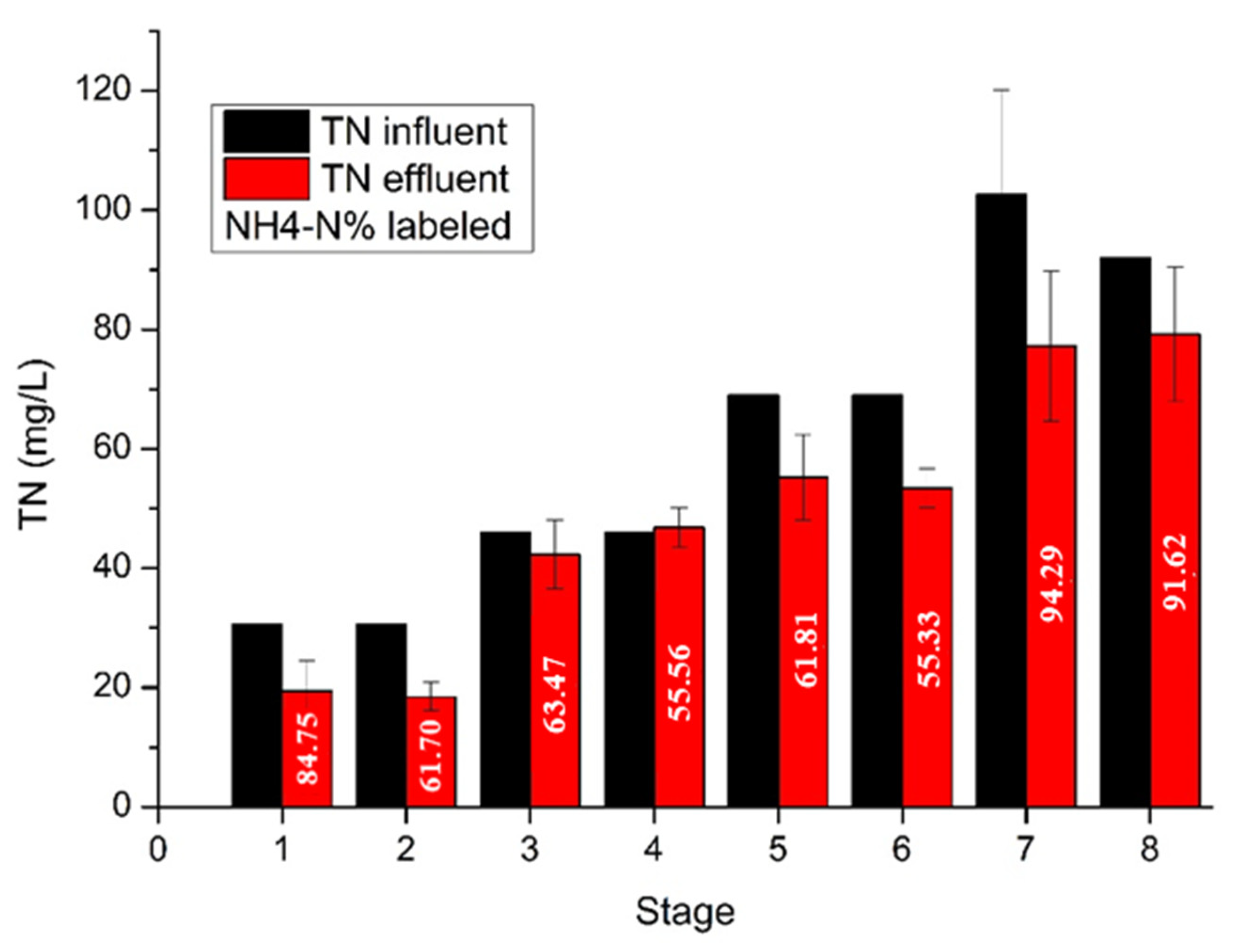
The performance of the UASB reactor showed a relative stability from the first days of its operation. The anaerobic sludge utilized as inoculum had already been activated and acclimated to potato wastewaters since it was obtained from the wastewater facilities of the same industry where the industrial starch was derived. The OLR did not exceed the value of 6 g/L
UASB-day during the whole period of experiments in order to avoid a decrease in COD removal efficiency and methane production due to the complex structure of starch [
9]. During the first 85 days of the experiment, no considerable fluctuations were observed related to HRT modifications. The increased methane production L CH
4/ L
UASB-day at stage 4 was due to the increase in OLR. After the sulfate addition, the COD/SO
4-2 ratio was maintained higher than 3, in order to avoid sulfidogenesis [
18] and keep the sludge granule integrity [
17]. Although in few other studies a low COD/SO
4-2 ratio during anaerobic processes can lead to a COD removal lower than 50%, in our study the addition of sulfate and the increase in OLR increased both the COD removal as well as the methane production [
41,
42]. The increase in COD removal can be explained by the abundance of SRB in the system that utilize organic carbon for the production of new cell metabolites [
43]. The stoichiometric ratio indicates that 1200 mg of COD is needed to reduce 1800 mg of sulfate [
44]. It has been reported that among different types of anaerobic reactors treating glucose at various COD/SO
4-2 ratios, UASB achieved the highest organic matter and sulfate removal level [
45]. Although pH in the influent was decreased after the addition of sulfate (ranging from 6.7 to 4.5), the robustness of the UASB system maintained the pH to adequate levels (6.3-7.4) for methane production. It has been reported that SRB can generate alkalinity that allows pH to increase [
46,
47]. The increased pH values in combination with high alkalinity promote acetogenesis and methanogenesis [
48].
The use of sulfuric acid instead of other sulfate salts in this study allows for a more controlled and precise addition of sulfates. It helps maintain an optimal acidic environment minimizing pH fluctuations. Additionally, it is highly soluble in water, ensuring that it can easily react with other substances in the experiment. During the decomposition of H
2SO
4, hydrogen ions released that are not toxic for the anaerobic bacteria -since they can be used for microbial metabolism- and do not interfere with the overall performance of the system. This allows for a more accurate assessment of the specific impact of sulfates on the microbial community without introducing any additional toxic effects that could confound the results. Moreover, sulfuric acid is commonly used in research and industrial applications due to its stability and availability. Several studies have focused on sulfuric acid pretreatment of substrate before anaerobic digestion in order to improve the methane production [
49,
50]. Lastly, the use of sulfuric acid allows for more precise dosing and easier adjustment of the sulfur content in the AD system. This flexibility is important for optimizing the process and ensuring efficient methane production. It is mentioned that sulfate released from sulfuric acid can undergo reduction by sulfate-reducing bacteria to form sulfide. This dissolved sulfide can then be oxidized to elemental sulfur through the action of ferric ions present in the system. Additionally, the released ferrous ions can react with the sulfide to form precipitates of iron sulfide [
51].
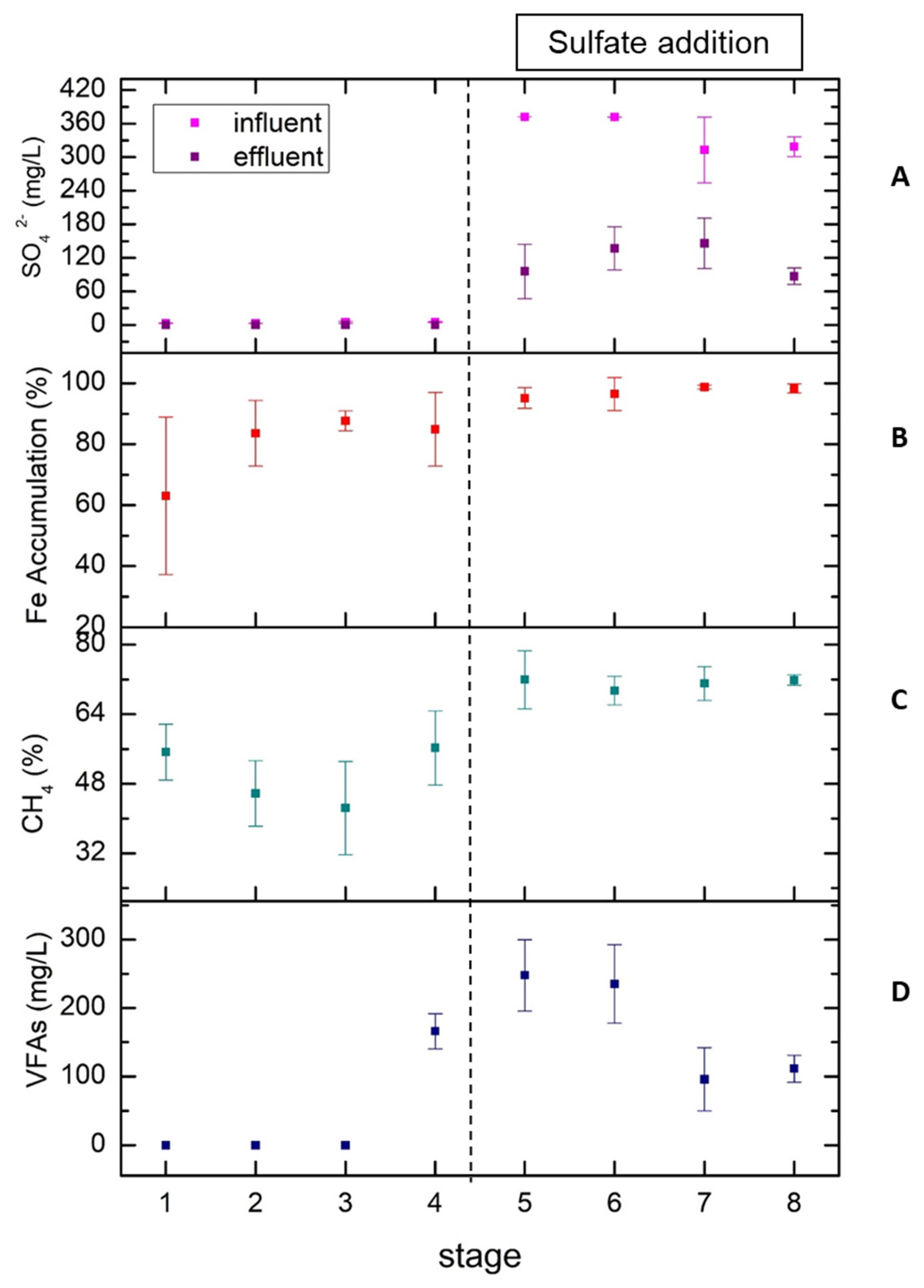
The improved performance of the UASB reactor after the sulfate addition that was observed in this study is in agreement with Lu et al. 2016 [
11] who stated that at COD/ SO
4-2 ratios of 3, 5 and 10, the respective average of methane content was 67.2%, 64.0% and 61.6% in a UASB reactor fed with starchy wastewater at an OLR of 4 g COD/L
UASB-day. According to the authors, the improvement is due to the increase in diversity of microbial community that stimulates the hydrolysis-acidification of starch and enhances the degradation of propionate, ultimately resulting in improved acetoclastic methanogenesis. In addition, at COD/SO
4-2 ratios between 2 to 10, they found a sulfate reduction of 52.7–77.1% and the sulfide produced, was almost entirely aqueous. Our study demonstrates similar results regarding sulfate reduction (59.61 ± 10.70 to 74.21 ± 13.09%), indicating no significant difference in sulfate removal among the various COD/ SO
4-2 ratios. However, when iron addition was significantly increased (stage 8), the sulfate removal was high and more stable due to the precipitation of iron sulfides. Yang et al. 2015 [
52] who studied the impact of sulfate addition (0.5-0.8 g/L) on methane production in a mesophilic anaerobic reactor feeding with acetate at an OLR of 4 g TOC/L-day, pointed out that methane production did not affected by sulfate addition, while the sulfate reduction became unstable (23-87%). In contrast with the present study, they noticed that with the increase of sulfate concentration in the substrate, the hydrogenotrophic methanogenesis was promoted and the population of
Methanosaeta sp decreased. This fact could confirm the importance and necessity of iron in the anaerobic system.
The improved and stabilized performance of the UASB reactor with low amount of VFAs at stages 7 and 8 is due to the long adaptation of microbial community to the starchy substrate, the COD/ SO
4-2 ratio values (>7) and the increased iron influent concentration. The absence of high iron concentration in the influent in combination with sulfate addition can play an important role in the increase of methane yield. The high iron accumulation in the UASB system after the sulfate addition might be explained by the fact that in the presence of sulfate, iron precipitates as ferrous sulfide. As it is mentioned above, ferrous iron in the substrate can react with sulfide produced by SRB, resulting in the precipitation of ferrous iron as FeS or FeS
2 and reducing the negative impact of sulfide on methanogenesis [
51],[
53]. In addition, the iron precipitates have a positive impact on anaerobic granular sludge enriching the microbial community [
54,
55] and granule stability [
56].
The substrate was supplemented with nitrogen to maintain a low but adequate C/N ratio during the whole period of the experiment. Zhang et al. 2022 [
57], reported that sulfate in concentrations between 200 mg/L and 1200 mg/L can promote the organic removal and methane production during anaerobic digestion of nitrogenous wastewater. After sulfate addition, nitrogen removal was increased due to the use of ammonium for microbial growth. Several studies have focused on the sulfate-reducing ammonium oxidation process explaining that ammonium is used as electron donor and sulfate as electron acceptor producing nitrogen and elemental sulfur [
58,
59]. Other recent studies explain the anoxic ammonia removal via Feammox processes where ammonium is converted to N
2 using ferrous iron as electron donor [
60,
61]. This fact can explain the high nitrogen removal in the last 4 stages, as there was an accumulated amount of iron within the reactor and the amount of iron in the substrate was high. Additional experimental data are required in the current study in order to comprehensively elucidate the significant phenomenon of nitrogen removal. This open field of research is extremely important to our research group and has great potential for further exploration.
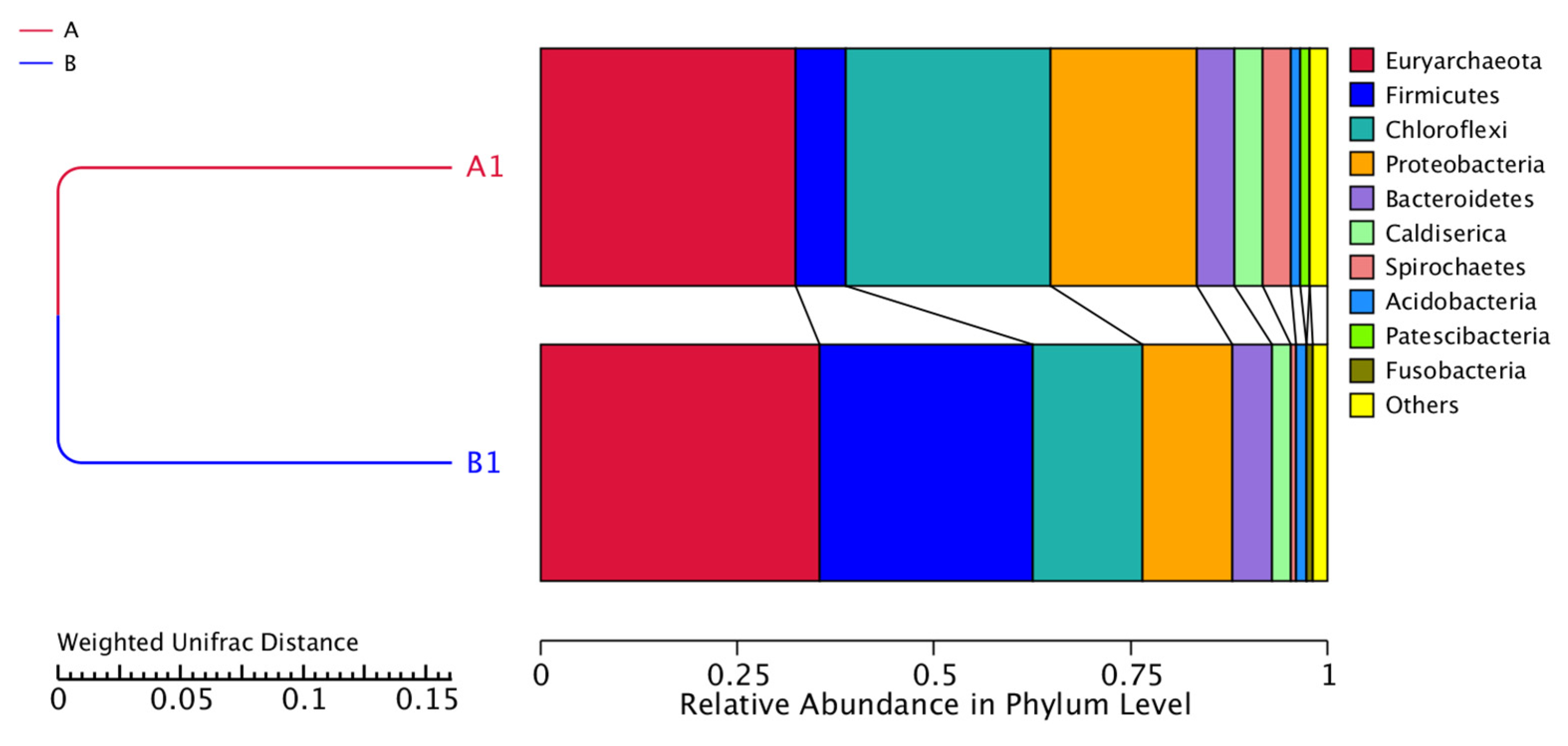
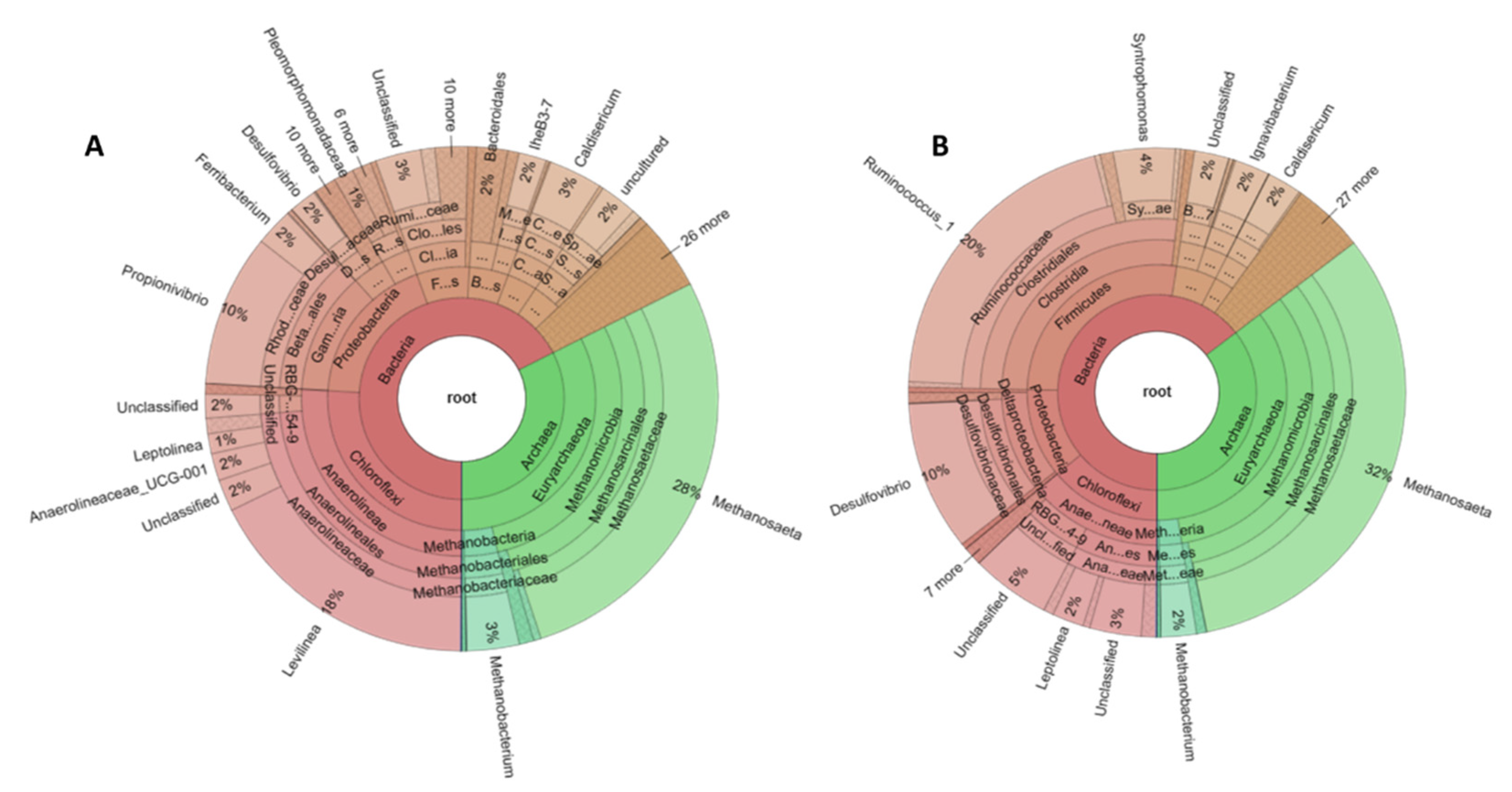
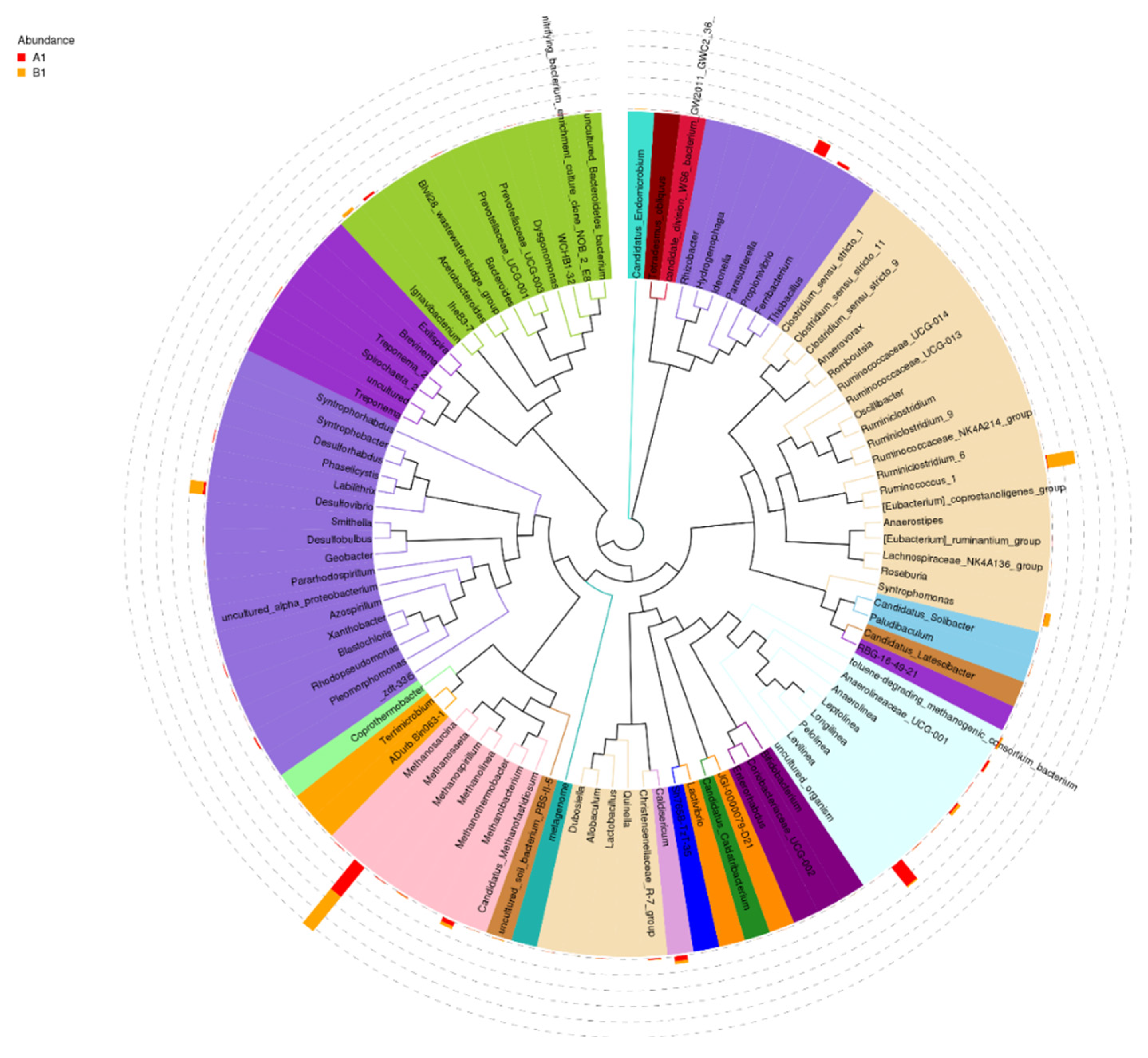
Regarding to microbial community it is worth mentioning that the archaeal Euryarchaeota were the most abundant. This phylum participates in carbon conversion during anaerobic digestion and generates methane [
62]. Microbial diversity at genus level, indicates that the most of the produced methane is resulted from acetate reduction since the relative abundance of acetoclastic
Methanosaeta sp. is higher than that of the other methanogens which use CO
2 and H
2 as source of energy [
63]. In addition, during the anaerobic fermentation of starch hydrolysates, monosaccharides are converted into acetate from Chloroflexi phylum. It is reported that the syntrophic relationship of Anaerolinae and Clostridia species improves the acidogenesis process. In
Anaerolinaceae family many species have hydrolytic fermentative properties and can easily degrade glucose molecules. In addition, some of them help in the sludge granulation [
48]. It can be noticed that
Levilinea sp. which had the highest relative abundance among the bacteria genera, as acidogens can convert carbohydrates to organic acids [
63,
64]. Furthermore,
Rhodocyclaceae family accounting for 12.23% in this study, is considered a very common microbial group in wastewater treatment processes [
66].
Propionivibrio sp. that has a high relative abundance accounting for 9.75% utilizes sugars as energy source and produce propionate. In this study, the organic acids produced during acidogenesis may be oxidized by
Ferribacterium sp. (relative abundance of 2.4%) [
67] and ferric iron from the substrate may be used as their electron acceptor. The amount of iron in the substrate was already high due to the FeSO
4 7H
2O addition during the chemical oxidation of starch. In addition, some species from Caldiserica phylum are related to iron reduction in combination with sulfide oxidation [
68].
Calsidericum sp. with the relative abundance of 3.4% can reduce sulfur compounds such as elemental sulfur, thiosulfate or sulfite that were in the anaerobic system [
69].
The low COD/SO
4-2 ratio, the high OLR as well as the increased iron concentration led to changes in microbial diversity as it is observed from the genomic analysis of sample B. Regarding to archaea community, the relative abundance of
Methanosaeta sp. was increased accounting for 32.25% while all the other archaea were diminished or remained at similar levels (
Table 8). Consequently, the produced methane after the addition of sulfate derived from the acetate consumption instead of hydrogenotrophic metabolic pathway.
Ruminococcus sp. that showed the highest relative abundance in bacterial community, belong to acidogenic bacteria and produce H
2 and short chain acids from soluble carbohydrates. Theoretically, 4 mol of H
2 per mol of glucose can be produced [
70]. This genus reduces a proton to hydrogen with reduced electron carrier NADH or reduced ferredoxin [
71,
72]. Iron has a crucial role in electron transfer and hydrogen formation since it is the active site of ferredoxin [
73]. Yin et al. 2021 [
74] who studied the hydrogen production from sewage sludge, showed that ferrous iron can improve the H
2 yield as ferredoxin hydrogenase activated by Fe
2+ supplementation. The authors also pointed out that the addition of ferrous sulfate enhances the degradation of the organic compounds. In our study, the cumulative addition of iron seems to affect the acceleration of
Ruminococcus sp. population. The increase in
Desulfovibrio sp. relative abundance should be directly related to the addition of sulfate.
Desulfovibrio sp. bacteria belong to sulfate reducing bacteria (SRB) that reduce sulfate by using H
2 as electron donors [
16]. In addition, some species of this genus have iron reduction abilities too [
75]. The increase in sulfate removal efficiency, expect for the precipitation as ferrous sulfide, can be also explained from the increase in the SRB’s electron acceptors [
46]. Paulo et al. 2015 [
76] and Colleran et al. 1995 [
77] have observed that SRB exhibit a higher affinity for H
2 in comparison with methanogenic bacteria. This characteristic gives to the SRB a competitive edge in environments where sulfates are abundant. On top of that, Paulo et al. 2015 [
76], mentioned that hydrogen offers a thermodynamic advantage over acetate as an electron donor for sulfate reduction. Niu et al. 2023 [
78] investigated the sulfate removal rate under different influent sulfate concentrations in a UASB reactor treating acid mine drainage and observed that sulfate reduction by SRB is more favorable in communities with higher species diversity.
Furthermore, an increase in
Syntrophomonas sp. population from 0.3 to 4% was observed. This microbial group produces acetate from fatty acids degradation and it is proved that has a syntrophic role in anaerobic oxidation of butyrate. Zhang et al. 2016 [
79], investigated the DIET syntrophic methanogenesis in presence of Fe
3O
4 in lake sediments and observed an acceleration of methane production due to iron.
Syntrophomonas sp. associates with methanogenic bacteria and bacteria that utilize hydrogen. Direct interspecies electron transfer (DIET) between bacteria that reduce iron and methanogenic archaea can improve the methane production [
53]. Moreover, there was a noticeable increase in the relative abundance of
Ignavibacterium sp., from 0.2% to 2.3%. Ignavibacterium species have been identified as potential participants in the Feammox process [
80,
81]. These bacteria can use ferric iron as electron acceptor while oxidizing ammonium to nitrogen gas. This fact can confirm that the high nitrogen removal observed is due to N
2 production from
Ignavibacterium sp. Ιt can be concluded that after the addition of sulfate,
Ruminococcus sp. and
Syntrophomonas sp. produce hydrogen and acetate respectively from the carbohydrates, provided by the starch hydrolysates, that can be directly utilized by
Desulfovibrio sp. The latter outcompete hydrogenotrophic methanogenic bacteria. Therefore,
Methanosaeta sp. predominates in the microbial community and methane production is derived almost completely from acetate.
In summary, all the conditions applied after the sulfate addition, affect the UASB performance as follows:
The methane percentage in the total biogas and the methane production rate reached 71% and 0.84 L/LUASB-day, respectively, due to the increased amount of Methanosaeta sp. and direct interspecies electron transfer.
The organic removal efficiency reached 95% due to the high amount of Ruminococcus sp. that utilize the soluble carbohydrates from the substrate.
The amount of VFAs was kept low since some genera such as Syntorphomonas and Desulfovibrio can effectively use them as substrate.
The sulfate removal reached 72% due to the reduction of sulfate from Desulfovibrio sp.
Iron accumulation was increased to 98% probably due to precipitation as iron sulfide.
During stages 5, 6, 7, and 8, the nitrogen removal achieved respective values of 19.87%, 22.58%, 24.66%, and 13.92% probably due to the oxidation of ammonium from Ignavibacterium sp.