Submitted:
21 September 2023
Posted:
25 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Theoretical Method
2.1. Self-Association
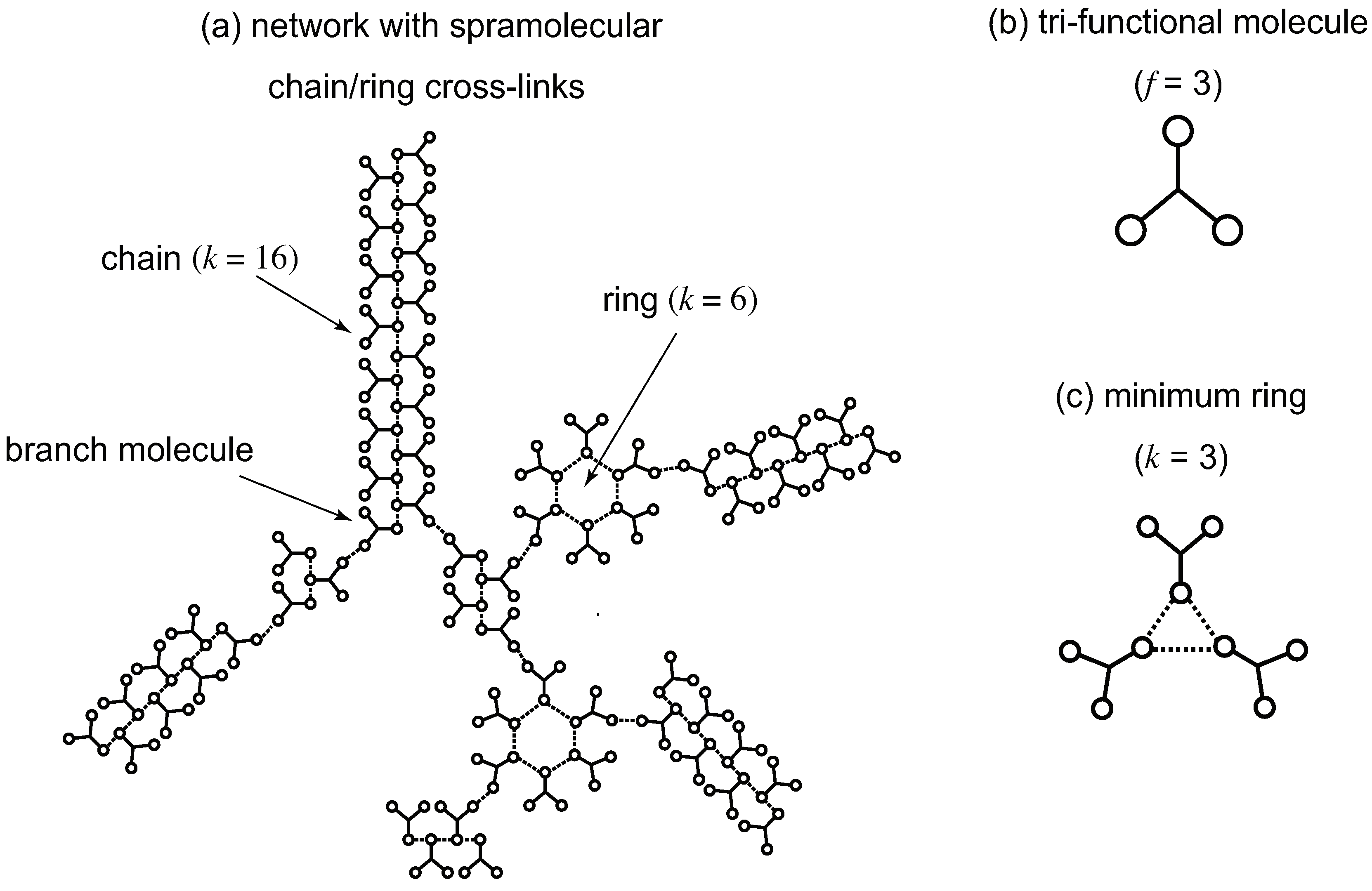
2.2. Linear growth of the Cross-Link Junctions
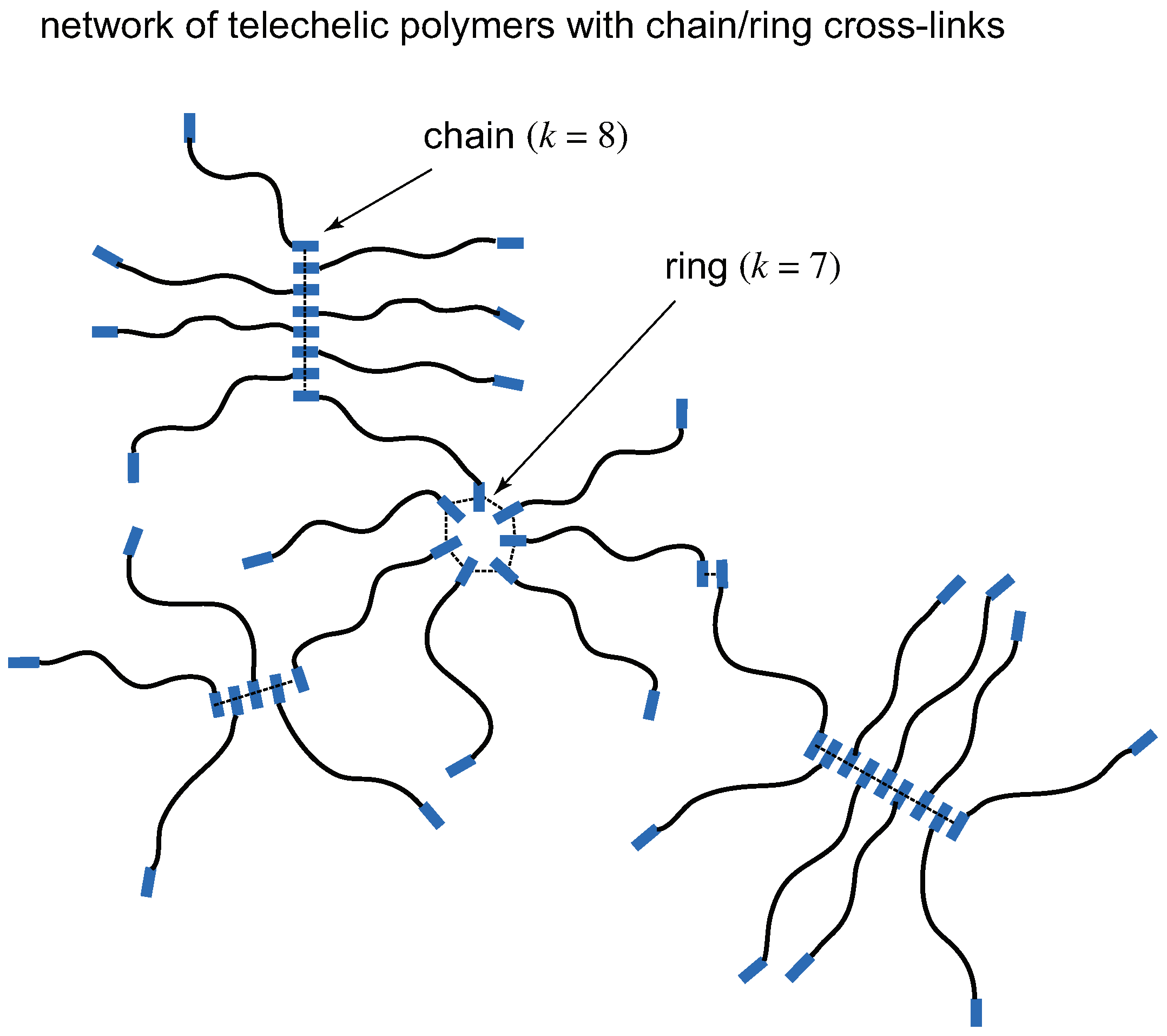
2.3. Chain/Ring Supramolecular Cross-Link Junctions
3. Metallo-Supramolecular Cross-Link Junctions
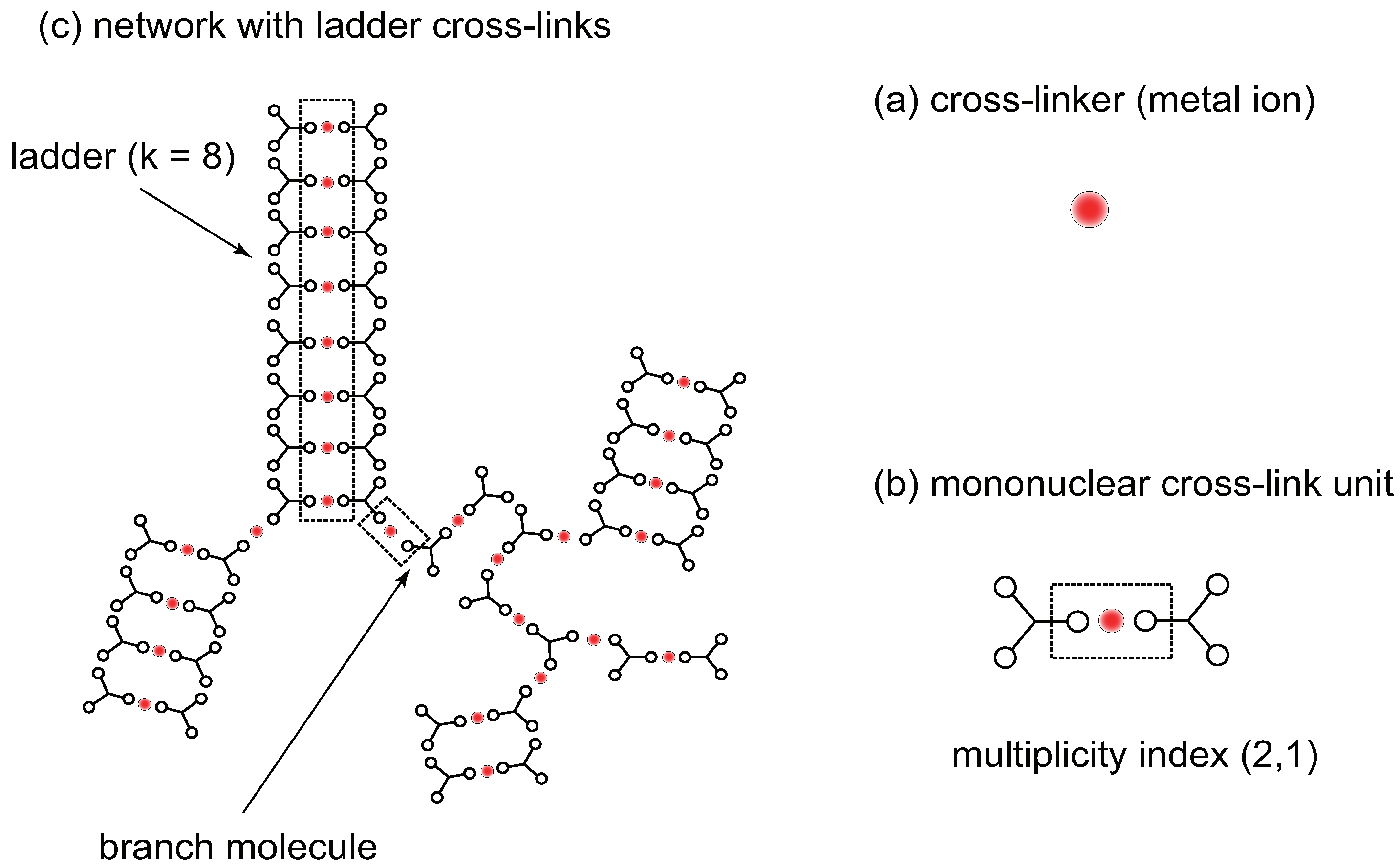
3.1. Ladder Model
3.2. Egg-Box Model
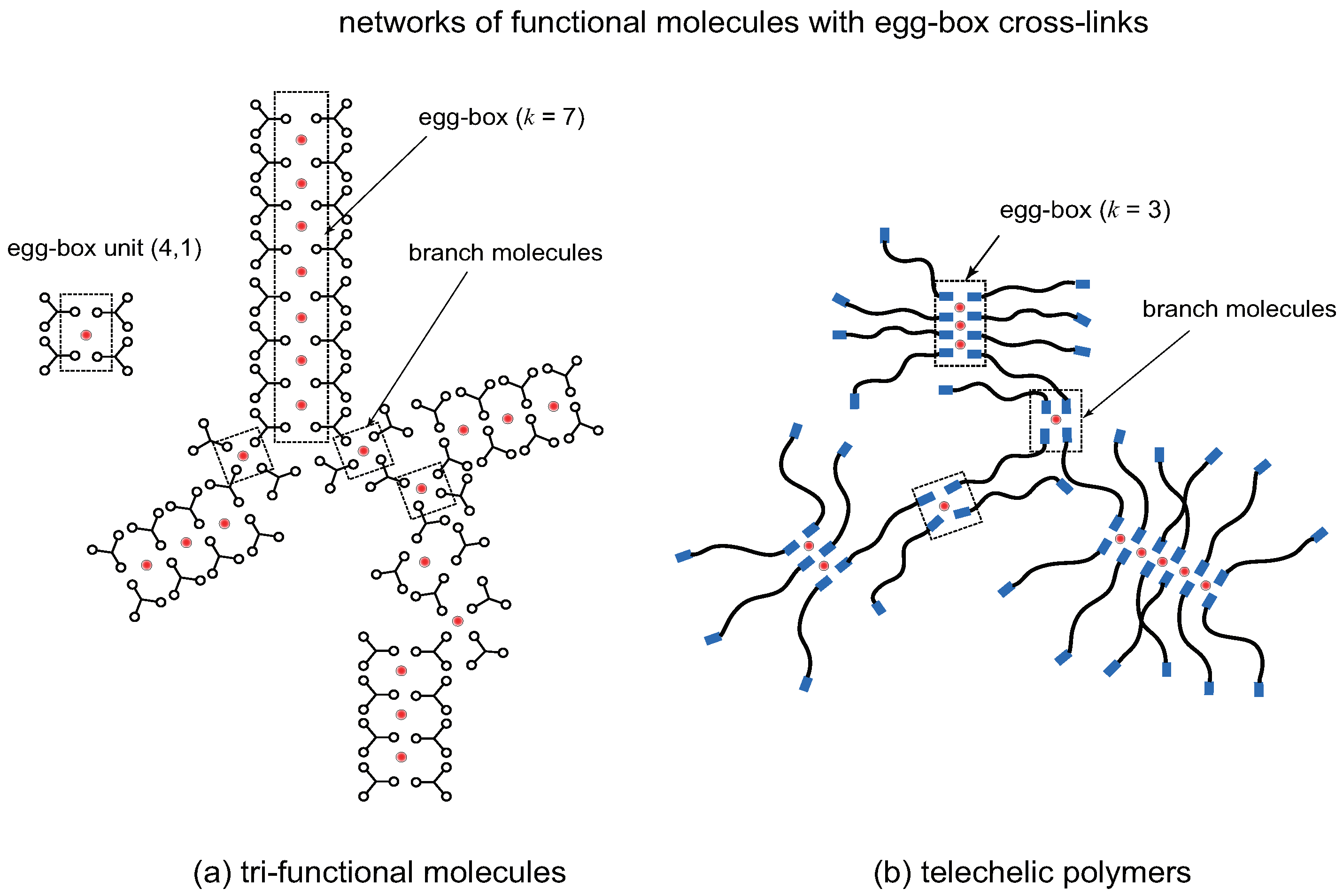
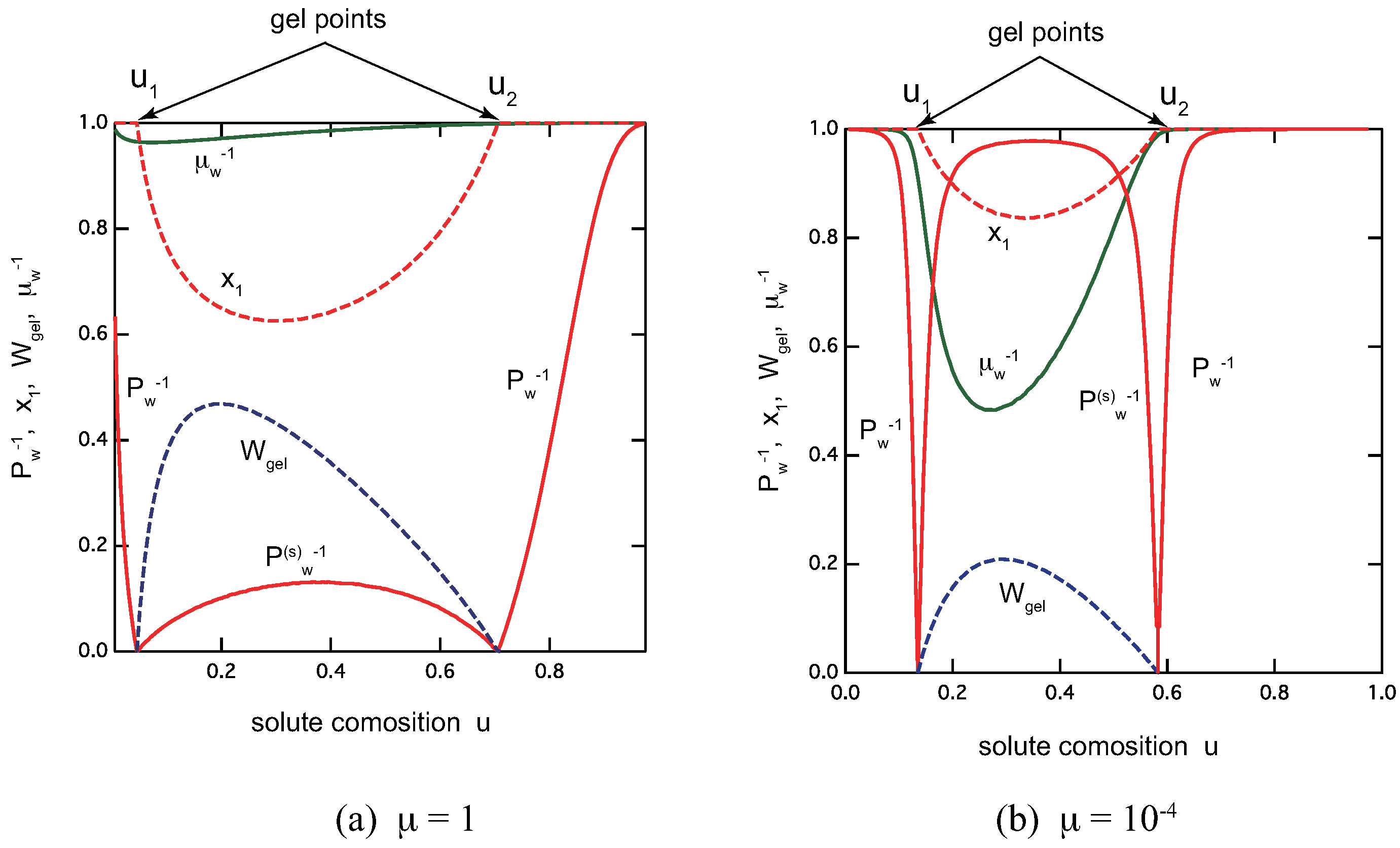
4. Discussion
5. Conclusions
- (1)
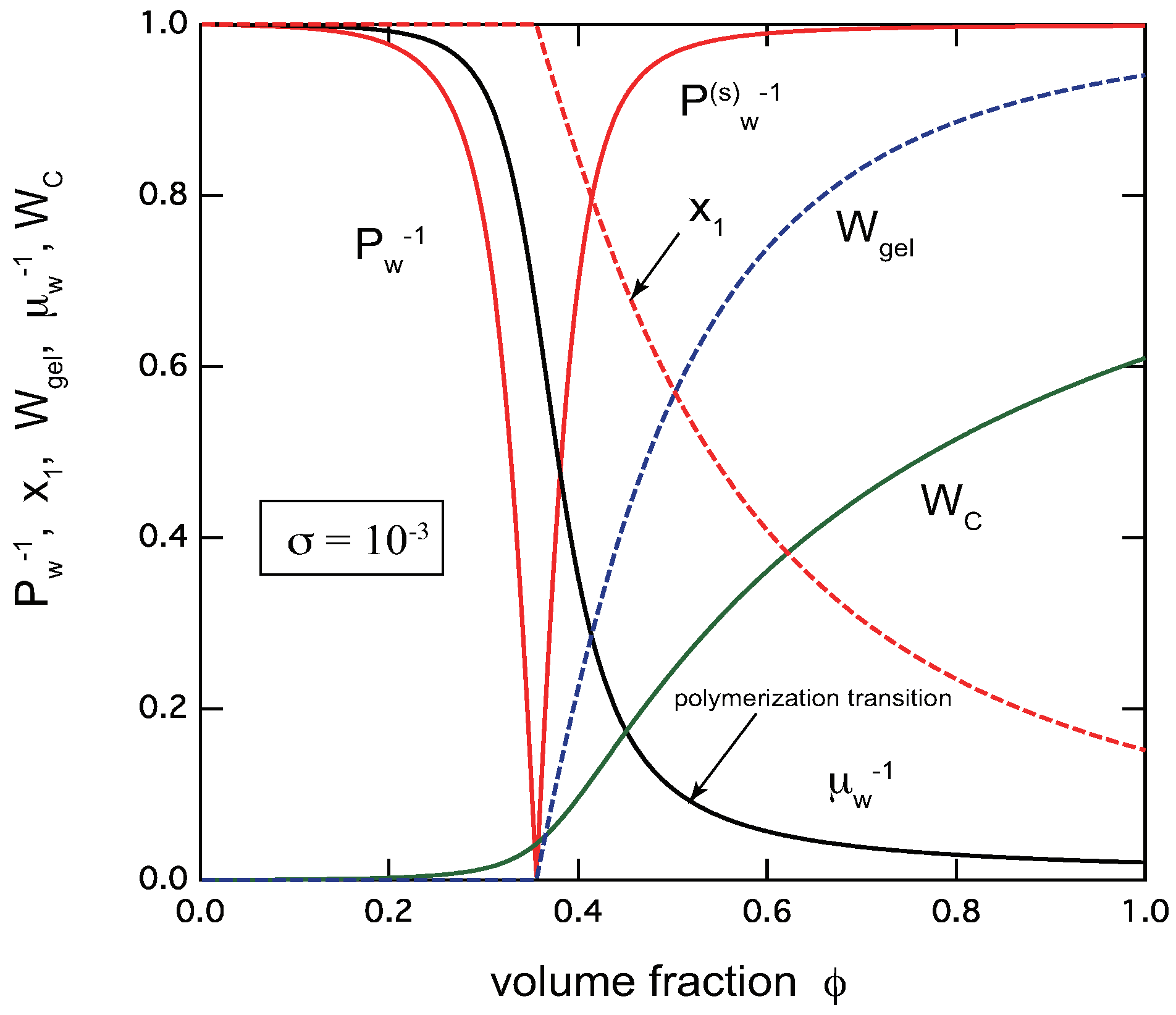
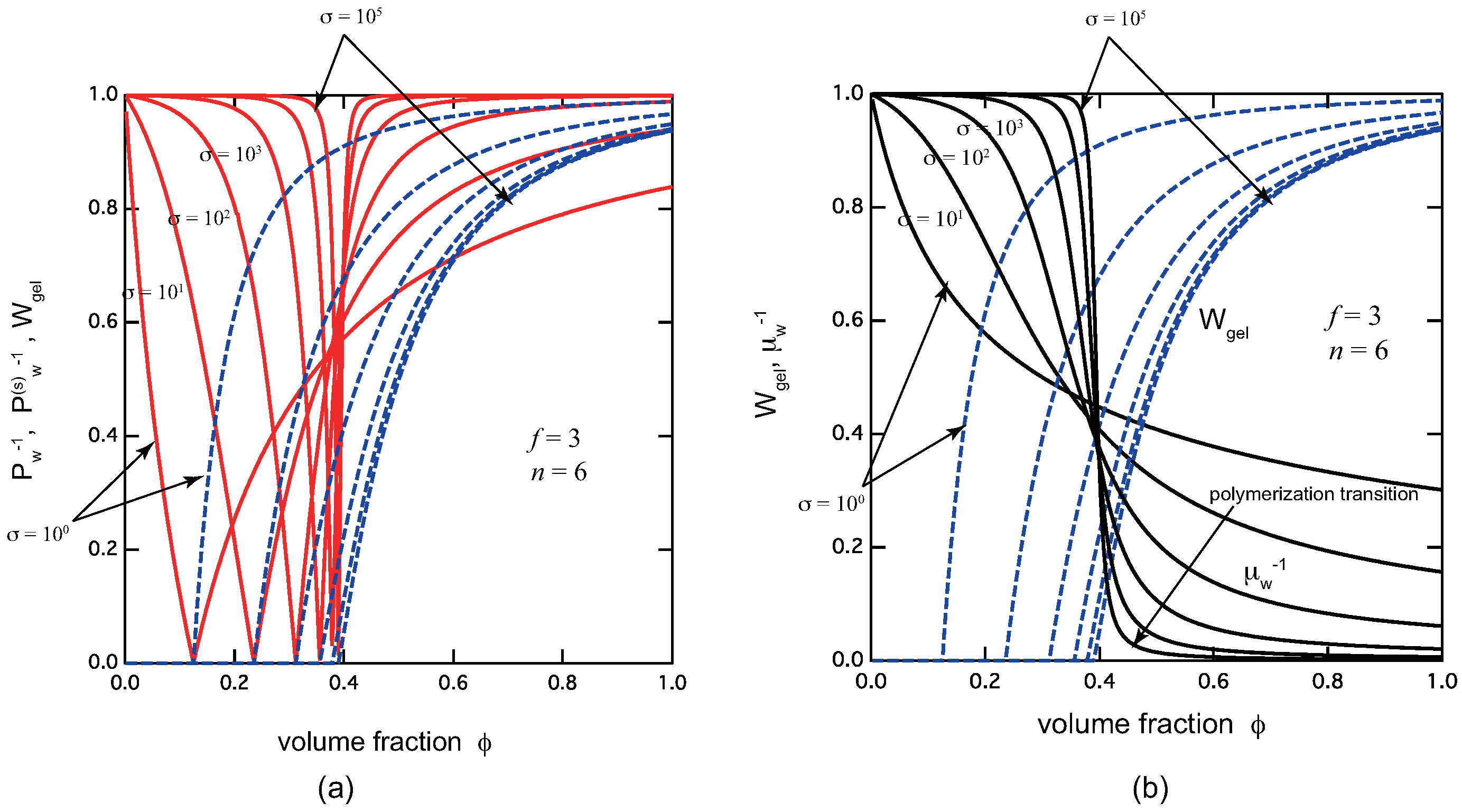
- Chain Model: In addition to the sol–gel transition, there occurs a polymerization transition at a certain concentration just after the gel point is passed under a fixed temperature. The transition is not a true phase transition in the sense that it is not accompanied by any singularity in the physical properties. In particular, the average chain length grows to infinity only in the inaccessible limit of complete reaction. However, its variation becomes sharper and sharper with the cooperativity parameter, leading eventually to a singularity at finite reactivity. The increasing sharpness of the sol–gel transition with cooperativity parameter, in particular sharp rise of the gel fraction, makes the experimental detection of the gel point easier.
- (2)
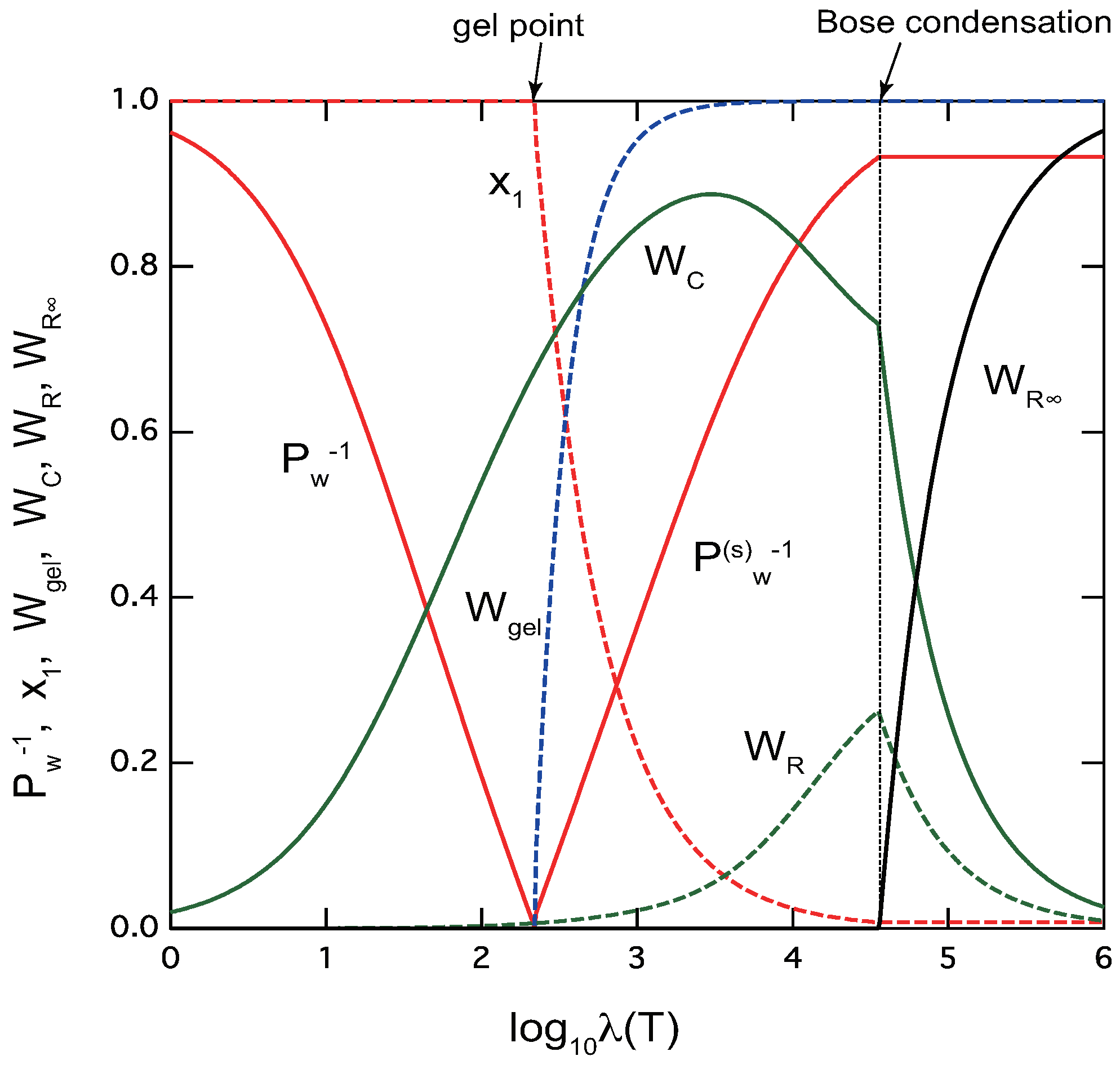
- Chain/Ring Model: Under a certain simple condition on the association constants, a new phase transition occurs at a low temperature (large ) deep in the postgel region, where the average length of rings goes to infinity. There appears a discontinuity in the physical properties at this condensation point of rings. The average molecular weight of the cross-linked polymers, the extinction probability, and the gel fraction all stay constant below this temperature. The transition is analogous to the Bose-Einstein condensation of an ideal Bose gas where finite fraction of particles falls into the condensate of zero momentum.
- (3)
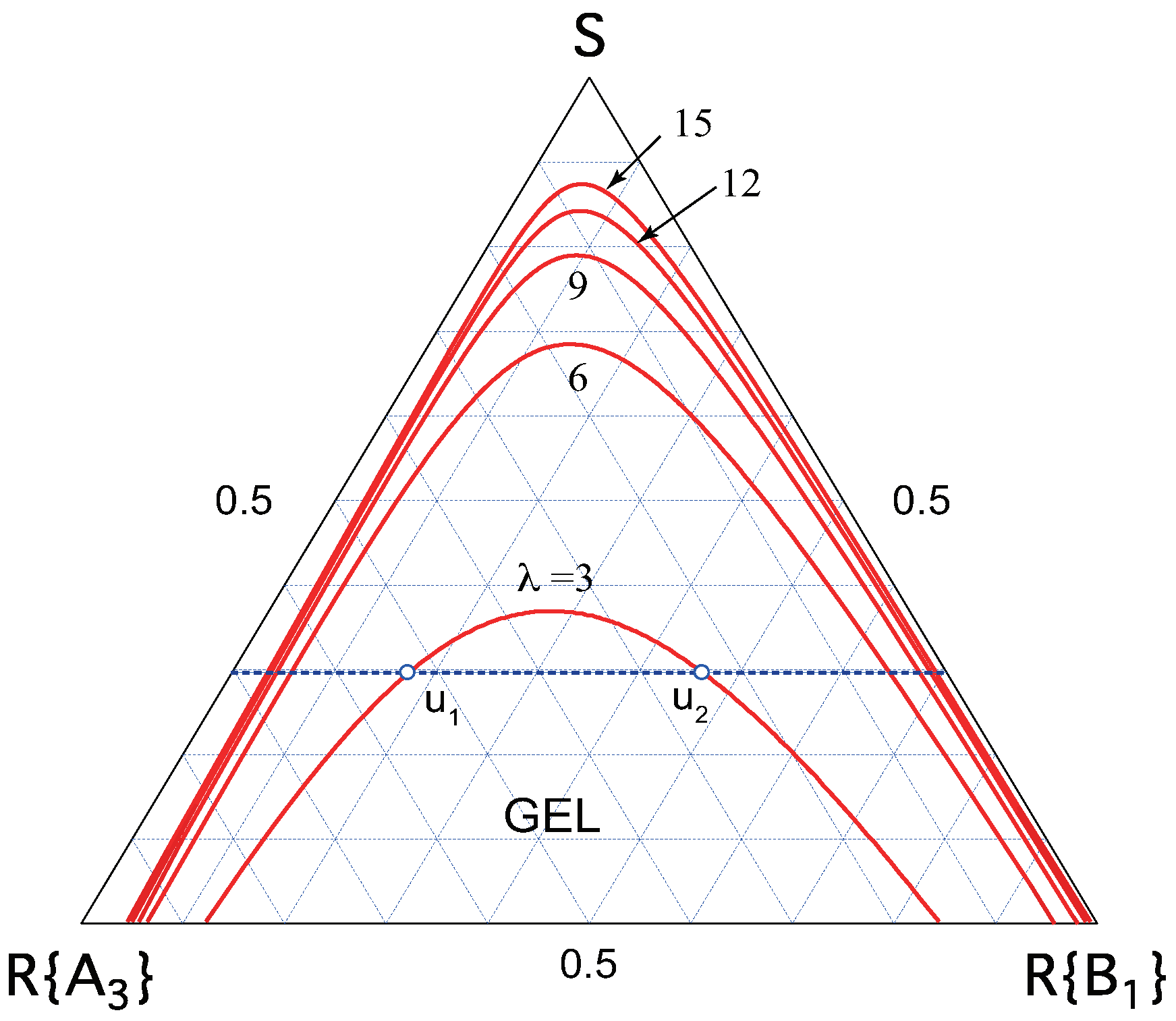
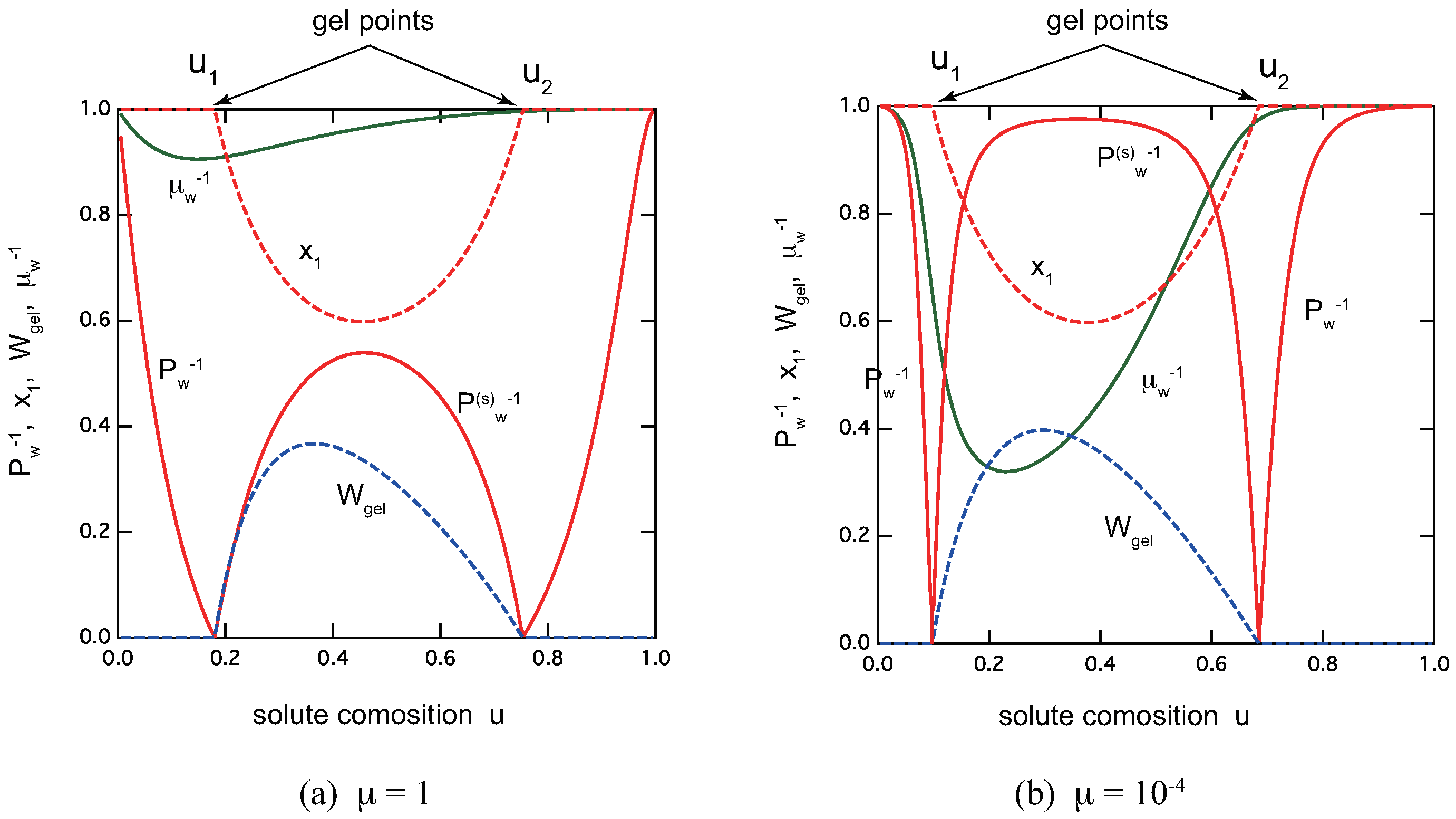
- Ladder Model: A ladder is one of the simplest structures of multi-nuclear metal-coordinated complexes. As a function of the composition u of metal ions, there occur two transitions: one from sol to gel at a low value , and the other from gel back to sol at a higher value (reentrant gel–sol transition). In the gel phase between them, there is a composition u at which the gel fraction reaches a maximum (optimal gel point). The average length of the ladder increases around this optimal gel point, but is limited within a finite value, and hence there is no polymerization transition. The ratio between the intra-layer association constant and the inter-layer one plays a role of the cooperativity parameter. The transitions become sharper with its decrease.
- (4)
- Egg-Box Model: Overall variation of physical observables is the same as the ladder model, although there are some quantitative differences. For instance, the gel fraction becomes asymmetric in the postgel region.
References
- Guenet, J. M. Thermoreversible Gelation of Polymers and Biopolymers., 2nd ed.; Academic Press, Harcourt Brace Jovanovich Publishers: London, 1992. [Google Scholar]
- te Nijenhuis, K. Thermoreversible Networks. Adv. Polym. Sci. 1997, 130, 1–252. [Google Scholar]
- Winter, H. H.; Mours, M. Rheology of Polymers near Liquid–Solid Transitions. Adv. Polym. Sci. 1997, 134, 165–234. [Google Scholar]
- Tanaka, F. Polymer Physics—Applications to Molecular Association and Thermoreversible Gelation; Cambridge University Press: Cambridge, 2011. [Google Scholar]
- Zhang, J.; Hu, Y.; Li, Y. Gel Chemistry: Interactions, Structures and Properties; Springer: Singapore, 2018. [Google Scholar]
- Thakur, V. K.; Thakur, M. K. Polymer Gels: Science and Fundamentals., 1st ed.; Springer: Singapore, 2018. [Google Scholar]
- Thakur, V. K.; Thakur, M. K. Hydrogels: Recent Advances., 1st ed.; Springer: Singapore, 2018. [Google Scholar]
- Nakano, S.; Ogiso, T.; Kita, R.; Shinyashiki, N.; Yagihara, S.; Yoneyama, M.; Katsumoto, Y. Thermoreversible gelation of isotactic-rich poly(N-isopropylacrylamide) in water. J. Chem. Phys. 2011, 135, 114903. [Google Scholar] [CrossRef]
- Taylor, M. L.; Paul Tomlins, P.; Sahota, T. S. Thermoresponsive Gels. Gels 2017, 3, 4–1. [Google Scholar] [CrossRef]
- Zhang, K.; Kun Xue, K.; Loh, X. J. Thermo-Responsive Hydrogels: From Recent Progress to Biomedical Applications. Gels 2021, 7, 77–1. [Google Scholar] [CrossRef]
- Wang, C.; Hashimoto, T.; Chuang, Y.-C.; Tanaka, T.; Chang, Y.-P.; Yang, T.-W.; Huang, M.-T. Physical Gelation of Aqueous Solutions of Atactic Poly(N-isopropylacrylamide). Macromolecules 2022, 55, 9152–9167. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Wang, Y.; Wang, C. Physical Gels of Atactic Poly(N-isopropylacrylamide) in Water: Rheological Properties and As-Derived Spinodal Temperature. Gels 2023, 9, 288–281. [Google Scholar] [CrossRef] [PubMed]
- Patrickios, C. S. Amphiphilic Polymer Co-Networks: Synthesis, Properties, Modelling and Applications; Royal Society of Chemistry: London, 2020. [Google Scholar]
- Terech, P.; Weiss, R. G. Low Molecular Mass Gelators of Organic Liquids and the Properties of Their Gels. Chem. Rev. 1997, 97, 3133–3159. [Google Scholar] [CrossRef]
- Weiss, R. G.; Terech, P. Molecular Gels: Materials with Self-Assembled Fibrillar Networks; Springer: Dordrecht, 2006; p. 978. [Google Scholar]
- Weiss, R. G. Controlling Variables in Molecular Gel Science: How Can We Improve the State of the Art? Gels 2018, 4, 25–1. [Google Scholar] [CrossRef]
- Babu, S. S.; Praveen, V. K.; Ajayaghosh, A. Functional π-Gelators and Their Applications. Chem. Rev. 2014, 114, 1973–2129. [Google Scholar] [CrossRef]
- Basu, N.; Chakraborty, A.; Ghosh, R. Carbohydrate Derived Organogelators and the Corresponding Functional Gels Developed in Recent Time. Gels 2018, 4, 52–51. [Google Scholar] [CrossRef]
- Morris, J.; Bietsch, J.; Bashaw, K.; Wang, G. Recently Developed Carbohydrate Based Gelators and Their Applications. Gels 2021, 7, 24–1. [Google Scholar] [CrossRef] [PubMed]
- Annable, T.; Buscall, R.; Ettelaie, R.; Whittlestone, D. The Rheology of Solutions of Associating Polymers: Comparison of Experimental Behavior with Transient Network Theory. J. Rheol. 1993, 37, 695–726. [Google Scholar] [CrossRef]
- Annable, T.; Buscall, R.; Ettelaie, R.; Shepherd, P.; Whittlestone, D. Influence of Surfactants on the Rheology of Associating Polymers in Solution. Langmuir 1994, 10, 1060–1070. [Google Scholar] [CrossRef]
- Yekta, A.; Xu, B.; Duhamel, J.; Adiwidjaja, H.; Winnik, M. A. Fluorescence Studies of Associating Polymers in Water: Determination of the Chain End Aggregation Number and a Model for the Association Process. Macromolecules 1995, 28, 956–966. [Google Scholar] [CrossRef]
- Kujawa, P.; Watanabe, H.; Tanaka, F.; Winnik, F. M. Amphiphhilic Telechelic Poly(N-isopropylacrylamide) in Water: From Micelles to Gels. Eur. Phys. J. E 2005, 17, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, P.; Segui, F.; Shaban, S.; Diab, C.; Okada, Y.; Tanaka, F.; Winnik, F. M. Impact of End-Group Association and Main-Chain Hydration on the Thermosensitive Properties of Hydrophobically Modified Telechelic Poly(N-isopropylacrylamide) in Water. Macromolecules 2006, 39, 341–348. [Google Scholar] [CrossRef]
- Kujawa, P.; Tanaka, F.; Winnik, F. M. Temperature-Dependent Properties of Telechelic Hydrophobically Modified Poly(N-isopropylacrylamides) in Water: Evidence from Light Scattering and Fluorescence Spectroscopy for the Formation of Stable Mesoglobules at Elevated Temperatures. Macromolecules 2006, 39, 3048–3055. [Google Scholar] [CrossRef]
- Kujawa, P.; Aseyev, V.; Tenhu, H.; Winnik, F. M. Temperature-Sensitive Properties of Poly(N-isopropylacrylamides) Mesoglobules Formed in Dilute Aqueous Solutions Heated above Their Demixing Point in Water: Evidence from Light Scattering and Fluorescence Spectroscopy for the Formation of Stable Mesoglobules at Elevated Temperatures. Macromolecules 2006, 39, 7686–7693. [Google Scholar]
- Dudowicz, J.; Freed, K. F.; Douglas, J. F. Lattice model of living polymerization. I. Basic thermodynamic properties. J. Chem. Phys. 1999, 111, 7166–7130. [Google Scholar] [CrossRef]
- Douglas, J. F.; Dudowicz, J.; Freed, K. F. Lattice model of equilibrium polymerization. VII. Understanding the role of gcooperativityh in self-assembly. J. Chem. Phys. 2008, 128, 224901–224901. [Google Scholar] [CrossRef]
- Greef, T. F. A. D.; Smulders, M. M. J.; Wolffs, M.; Schenning, A. P. H. J.; Sijbesma, R. P.; Meijer, E. W. Supramolecular Polymerization. Chem. Rev. 2009, 109, 5687–5754. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, A. D.; Salimi, S.; Hart, L. R.; Babra, T. S.; Greenland, B. W.; Hayes, W. Applications of supramolecular polymer networks. Reactive and Functional Polymers 2022, 172, 105209–1. [Google Scholar] [CrossRef]
- Yan, T.; Schröter, K.; Herbst, F.; Binder, W. H.; Thurn-Albrecht, T. Nanostructure and Rheology of Hydrogen-Bonding Telechelic Polymers in the Melt: From Micellar Liquids and Solids to Supramolecular Gels. Macromolecules 2014, 47, 2122–2130. [Google Scholar] [CrossRef]
- Ahmadi, M.; Seiffert, S. Coordination Geometry Preference Regulates the Structure and Dynamics of Metallo-Supramolecular Polymer Networks. Macromolecules 2021, 54, 1388–1400. [Google Scholar] [CrossRef]
- Ahmadi, M.; Nicolella, P.; Seiffert, S. Network Percolation in Transient Polymer Networks with Temporal Hierarchy of Energy Dissipation. Macromolecules 2022, 55. [Google Scholar] [CrossRef]
- Breul, K.; Kissel, S.; Seiffert, S. Sticker Multivalency in Metallo-supramolecular Polymer Networks. Macromolecules 2021, 54, 8407–8422. [Google Scholar] [CrossRef]
- Piepenbrock, M.-O. M.; Lloyd, G. O.; Clarke, N.; Steed, J. W. Metal- and Anion-Binding Supramolecular Gels. Chem. Rev. 2010, 110, 1960–2004. [Google Scholar] [CrossRef]
- Xia, D.; Wang, P.; Ji, X.; Khashab, N. M.; Sessler, J. L.; Huang, F. Functional Supramolecular Polymeric Networks: The Marriage of Covalent Polymers and Macrocycle-Based Host–Guest Interactions. Chem. Rev. 2020, 120, 6070–6123. [Google Scholar] [CrossRef]
- Fukui, K.; Yamabe, T. A General Theory of Gel Formation with Multifunctional Interunit Junctions. Bull. Chem. Soc. Jpn 1967, 40, 2052–2063. [Google Scholar] [CrossRef]
- Tanaka, F.; Stockmayer, W. H. Thermoreversible Gelation with Junctions of Variable Multiplicity. Macromolecules 1994, 27, 3943–3954. [Google Scholar] [CrossRef]
- Tanaka, F. Thermoreversible Gelation Interfering with Phase Separation in Multicomponent Mixtures of Associating Polymers. Macromolecules 2022, 55, 5233–5248. [Google Scholar] [CrossRef]
- Flory, P. J. Thermodynamics of High Polymer Solutions. J. Chem. Phys. 1942, 10, 51–61. [Google Scholar] [CrossRef]
- Flory, P. J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, New York, 1953. [Google Scholar]
- Tanaka, F. Thermoreversible Gelation with Two-Component Mixed Cross-Link Junctions of Variable Multiplicity in Ternary Polymer Solutions. Gels 2021, 7, 89–81. [Google Scholar] [CrossRef]
- Flory, P. J. Molecular Size Distribution in Three Dimensional Polymers I. Gelation. J. Am. Chem. Soc. 1941, 63, 3083–3090. [Google Scholar] [CrossRef]
- Flory, P. J. Molecular Size Distribution in Three Dimensional Polymers II. Trifunctional Branching Units. J. Am. Chem. Soc. 1941, 63, 3091–3096. [Google Scholar] [CrossRef]
- Flory, P. J. Molecular Size Distribution in Three Dimensional Polymers III. Tetrafunctional Branching Units. J. Am. Chem. Soc. 1941, 63, 3096–3100. [Google Scholar] [CrossRef]
- Stockmayer, W. H. Theory of Molecular Size Distribution and Gel Formation in Branched-Chain Polymers. J. Chem. Phys. 1943, 11, 45–55. [Google Scholar] [CrossRef]
- Stockmayer, W. H. Theory of Molecular Size Distribution and Gel Formation in Branched Polymers II. General Cross Linking. J. Chem. Phys. 1944, 12, 125–131. [Google Scholar] [CrossRef]
- Stockmayer, W. H. Molecular Distribution in Condensation Polymers. J. Polym. Sci. 1952, 4, 69–71. [Google Scholar] [CrossRef]
- Gordon, M. Good’s Theory of Cascade Processes applied to the Statistics of Polymer Distribution. Proc. Roy. Soc. London 1962, A268, 240–257. [Google Scholar]
- Good, I. J. The Number of Individuals in the Cascade Process. Proc. Camb. Phil. Soc. 1949, 45, 360–363. [Google Scholar] [CrossRef]
- Good, I. J. Cascade theory and molecular weight averages of the sol fraction. Proc. Roy. Soc. London A 1963, 272, 54–59. [Google Scholar]
- Columbus, I.; Eren, N.; Elitsur, R.; Davidovich-Pinhas, M.; Shenhar, R. Branched Supramolecular Copolymers: Inducing Branching in Bisurea-Based Monomers Using Multi-Sulfonate Molecules. Macromolecules 2022, 55, 472–487. [Google Scholar] [CrossRef]
- Jacobson, H.; Stochmayer, W. H. Intramolecular Reaction in Polycondensations. I. The Theory of Linear Systems. J. Chem. Phys. 1950, 18, 1600–1606. [Google Scholar] [CrossRef]
- Jacobson, H.; Beckmann, C. O.; Stochmayer, W. H. Intramolecular Reaction in Polycondensations. II. Ring-Chane Equilibrium in Polydecamethylene Adipate. J. Chem. Phys. 1950, 18, 1607–1612. [Google Scholar] [CrossRef]
- Poland, D.; Scheraga, H. A. Phase Transitions in One Dimension and the Helix-Coil Transition in Polyamino Acids. J. Chem. Phys. 1966, 45, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Poland, D.; Scheraga, H. A. Theory of Helix-Coil Transitions in Biopolymers; Sec.9 E and 10 D; Academic Press: New York and London, 1970. [Google Scholar]
- Truesdell, C. On a Function which Occurs in the Theory of the Structure of Polymers. Annals of Mathematics 1945, 46, 144–157. [Google Scholar] [CrossRef]
- Fang, Y.; Al-Assaf, S.; Phillips, G. O.; Nishinari, K.; Funami, T.; Williams, P. A.; Li, L. Multiple Steps and Critical Behaviors of the Binding of Calcium to Alginate. J. Phys. Chem. B 2007, 111, 2456–2462. [Google Scholar] [CrossRef]
- Sikorski, P.; Mo, F.; Skjåk-Brœk, G.; Stokke, B. T. Evidence for Egg-Box-Compatible Interactions in Calcium-Alginate Gels from Fiber X-ray Diffraction. Biomacromolecules 2007, 8, 2098–2103. [Google Scholar] [CrossRef]
- Donati, I.; Benegas, J.; Paoletti, S. On the Molecular Mechanism of the Calcium-Induced Gelation of Pectate. Different Steps in the Binding of Calcium Ions by Pectate. Biomacromolecules 2021, 22, 5000–5019. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, J. E.; Ferry, J. D. Studies on the Cross-Linking Process in Gelatin Gels. III. Dependence of Melting Point on Concentration and Molecular Weight. J. Phys. Chem. 1954, 58, 992–995. [Google Scholar] [CrossRef]
- Tanaka, F.; Nishinari, K. Junction Multiplicity in Thermoreversible Gelation. Macromolecules 1996, 29, 3625–3628. [Google Scholar] [CrossRef]
- de Gennes, P. G. Scaling Concepts in Polymer Physics; Chap.1; Cornell University Press: Ithaca, 1979. [Google Scholar]
- Lips, A.; Clark, A. H.; Cutler, N.; Durand, D. Measurement of cooperativity of binding of calcium to neutral sodium pectate. Food Hydrocoll. 1991, 5, 87–99. [Google Scholar] [CrossRef]
- Tanaka, F.; Nakagawa, Y.; Ohta, S.; Ito, T. Thermoreversible gelation with ion-binding cross-links of varible multiplicity. J. Chem. Phys. 2019, 150, 174904–174901. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




