Submitted:
18 September 2023
Posted:
19 September 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Physiopathology associated with asynchronous LV activation
The potential role of CSP in CRT candidates
key concepts and definitions for CSP-based CRT
Clinical evidence of HBP-CRT
Clinical evidence of LBBAP-CRT
Combination of CSP with CS lead pacing-CRT
CSP-based CRT in other clinical scenarios
Current recommendations and future directions
Conclusions
Funding
Conflicts of Interest
References
- Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, Barrabés JA, Boriani G, Braunschweig F, Brignole M, Burri H, Coats AJS, Deharo JC, Delgado V, Diller GP, Israel CW, Keren A, Knops RE, Kotecha D, Leclercq C, Merkely B, Starck C, Thylén I, Tolosana JM; ESC Scientific Document Group. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021 Sep 14;42(35):3427-3520. Erratum in: Eur Heart J. 2022 May 1;43(17):1651. [CrossRef]
- Cazeau S, Ritter P, Bakdach S, Lazarus A, Limousin M, Henao L, Mundler O, Daubert JC, Mugica J. Four chamber pacing in dilated cardiomyopathy. Pacing Clin Electrophysiol. 1994 Nov;17(11 Pt 2):1974-9. [CrossRef] [PubMed]
- Cazeau S, Ritter P, Lazarus A, Gras D, Backdach H, Mundler O, Mugica J. Multisite pacing for end-stage heart failure: early experience. Pacing Clin Electrophysiol. 1996 Nov;19(11 Pt 2):1748-57. [CrossRef] [PubMed]
- Leclercq C, Cazeau S, Le Breton H, Ritter P, Mabo P, Gras D, Pavin D, Lazarus A, Daubert JC. Acute hemodynamic effects of biventricular DDD pacing in patients with end-stage heart failure. J Am Coll Cardiol. 1998 Dec;32(7):1825-31. [CrossRef] [PubMed]
- Blanc JJ, Etienne Y, Gilard M, Mansourati J, Munier S, Boschat J, Benditt DG, Lurie KG. Evaluation of different ventricular pacing sites in patients with severe heart failure: results of an acute hemodynamic study. Circulation. 1997 Nov 18;96(10):3273-7. [CrossRef] [PubMed]
- Butter C, Auricchio A, Stellbrink C, Fleck E, Ding J, Yu Y, Huvelle E, Spinelli J; Pacing Therapy for Chronic Heart Failure II Study Group. Effect of resynchronization therapy stimulation site on the systolic function of heart failure patients. Circulation. 2001 Dec 18;104(25):3026-9. [CrossRef] [PubMed]
- Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C, Garrigue S, Kappenberger L, Haywood GA, Santini M, Bailleul C, Daubert JC; Multisite Stimulation in Cardiomyopathies (MUSTIC) Study Investigators. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001 Mar 22;344(12):873-80. [CrossRef] [PubMed]
- Linde C, Leclercq C, Rex S, Garrigue S, Lavergne T, Cazeau S, McKenna W, Fitzgerald M, Deharo JC, Alonso C, Walker S, Braunschweig F, Bailleul C, Daubert JC. Long-term benefits of biventricular pacing in congestive heart failure: results from the MUltisite STimulation in cardiomyopathy (MUSTIC) study. J Am Coll Cardiol. 2002 Jul 3;40(1):111-8. [CrossRef] [PubMed]
- Auricchio A, Stellbrink C, Sack S, Block M, Vogt J, Bakker P, Huth C, Schöndube F, Wolfhard U, Böcker D, Krahnefeld O, Kirkels H; Pacing Therapies in Congestive Heart Failure (PATH-CHF) Study Group. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol. 2002 Jun 19;39(12):2026-33. [CrossRef] [PubMed]
- Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J; MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002 Jun 13;346(24):1845-53. [CrossRef] [PubMed]
- Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, Canby RC, Schroeder JS, Liem LB, Hall S, Wheelan K; Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD) Trial Investigators. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. 2003 ;289(20):2685-94. 28 May. [CrossRef] [PubMed]
- Higgins SL, Hummel JD, Niazi IK, Giudici MC, Worley SJ, Saxon LA, Boehmer JP, Higginbotham MB, De Marco T, Foster E, Yong PG. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol. 2003 Oct 15;42(8):1454-9. [CrossRef] [PubMed]
- Abraham WT, Young JB, León AR, Adler S, Bank AJ, Hall SA, Lieberman R, Liem LB, O'Connell JB, Schroeder JS, Wheelan KR; Multicenter InSync ICD II Study Group. Effects of cardiac resynchronization on disease progression in patients with left ventricular systolic dysfunction, an indication for an implantable cardioverter-defibrillator, and mildly symptomatic chronic heart failure. Circulation. 2004 Nov 2;110(18):2864-8. [CrossRef]
- Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM; Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004 ;350(21):2140-50. 20 May. [CrossRef] [PubMed]
- Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005 Apr 14;352(15):1539-49. [CrossRef]
- Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. Longer-term effects of cardiac resynchronization therapy on mortality in heart failure [the CArdiac REsynchronization-Heart Failure (CARE-HF) trial extension phase]. Eur Heart J. 2006 Aug;27(16):1928-32. [CrossRef]
- Kindermann M, Hennen B, Jung J, Geisel J, Böhm M, Fröhlig G. Biventricular versus conventional right ventricular stimulation for patients with standard pacing indication and left ventricular dysfunction: the Homburg Biventricular Pacing Evaluation (HOBIPACE). J Am Coll Cardiol. 2006 ;47(10):1927-37. 16 May. [CrossRef]
- Beshai JF, Grimm RA, Nagueh SF, Baker JH 2nd, Beau SL, Greenberg SM, Pires LA, Tchou PJ; RethinQ Study Investigators. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med. 2007 Dec 13;357(24):2461-71. [CrossRef]
- Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, Abraham WT, Ghio S, Leclercq C, Bax JJ, Yu CM, Gorcsan J 3rd, St John Sutton M, De Sutter J, Murillo J. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008 ;117(20):2608-16. 20 May. [CrossRef]
- Linde C, Gold MR, Abraham WT, St John Sutton M, Ghio S, Cerkvenik J, Daubert C; REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction Study Group. Long-term impact of cardiac resynchronization therapy in mild heart failure: 5-year results from the REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) study. Eur Heart J. 2013 Sep;34(33):2592-9. [CrossRef]
- Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA 3rd, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W; MADIT-CRT Trial Investigators. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009 Oct 1;361(14):1329-38. [CrossRef]
- Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, Hohnloser SH, Nichol G, Birnie DH, Sapp JL, Yee R, Healey JS, Rouleau JL; Resynchronization-Defibrillation for Ambulatory Heart Failure Trial Investigators. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010 Dec 16;363(25):2385-95. [CrossRef]
- Curtis AB, Worley SJ, Adamson PB, Chung ES, Niazi I, Sherfesee L, Shinn T, Sutton MS; Biventricular versus Right Ventricular Pacing in Heart Failure Patients with Atrioventricular Block (BLOCK HF) Trial Investigators. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013 Apr 25;368(17):1585-93. [CrossRef] [PubMed]
- Ruschitzka F, Abraham WT, Singh JP, Bax JJ, Borer JS, Brugada J, Dickstein K, Ford I, Gorcsan J 3rd, Gras D, Krum H, Sogaard P, Holzmeister J; EchoCRT Study Group. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med. 2013 Oct 10;369(15):1395-405. [CrossRef]
- Thibault B, Harel F, Ducharme A, White M, Ellenbogen KA, Frasure-Smith N, Roy D, Philippon F, Dorian P, Talajic M, Dubuc M, Guerra PG, Macle L, Rivard L, Andrade J, Khairy P; LESSER-EARTH Investigators. Cardiac resynchronization therapy in patients with heart failure and a QRS complex. [CrossRef]
- Gamble JHP, Herring N, Ginks M, Rajappan K, Bashir Y, Betts TR. Procedural Success of Left Ventricular Lead Placement for Cardiac Resynchronization Therapy: A Meta-Analysis. JACC Clin Electrophysiol. 2016 Feb;2(1):69-77.
- Moubarak G, Bouzeman A, Ollitrault J, Anselme F, Cazeau S. Phrenic nerve stimulation in cardiac resynchronization therapy. J Interv Card Electrophysiol. 2014 Oct;41(1):15-21. [CrossRef] [PubMed]
- Biffi M, Exner DV, Crossley GH, Ramza B, Coutu B, Tomassoni G, Kranig W, Li S, Kristiansen N, Voss F. Occurrence of phrenic nerve stimulation in cardiac resynchronization therapy patients: the role of left ventricular lead type and placement site. Europace. 2013 Jan;15(1):77-82. [CrossRef] [PubMed]
- Auricchio A, Heggermont WA. Technology Advances to Improve Response to Cardiac Resynchronization Therapy: What Clinicians Should Know. Rev Esp Cardiol (Engl Ed). 2018 Jun;71(6):477-484. English, Spanish. [CrossRef] [PubMed]
- Abdelrahman M, Subzposh FA, Beer D, Durr B, Naperkowski A, Sun H, Oren JW, Dandamudi G, Vijayaraman P. Clinical Outcomes of His Bundle Pacing Compared to Right Ventricular Pacing. J Am Coll Cardiol. 2018 ;71(20):2319-2330. 22 May. [CrossRef] [PubMed]
- Zanon F, Abdelrahman M, Marcantoni L, Naperkowski A, Subzposh FA, Pastore G, Baracca E, Boaretto G, Raffagnato P, Tiribello A, Dandamudi G, Vijayaraman P. Long term performance and safety of His bundle pacing: A multicenter experience. J Cardiovasc Electrophysiol. 2019 Sep;30(9):1594-1601. [CrossRef] [PubMed]
- Keene D, Arnold AD, Jastrzębski M, Burri H, Zweibel S, Crespo E, Chandrasekaran B, Bassi S, Joghetaei N, Swift M, Moskal P, Francis DP, Foley P, Shun-Shin MJ, Whinnett ZI. His bundle pacing, learning curve, procedure characteristics, safety, and feasibility: Insights from a large international observational study. J Cardiovasc Electrophysiol. 2019 Oct;30(10):1984-1993. [CrossRef] [PubMed]
- Shan P, Su L, Zhou X, Wu S, Xu L, Xiao F, Zhou X, Ellenbogen KA, Huang W. Beneficial effects of upgrading to His bundle pacing in chronically paced patients with left ventricular ejection fraction. [CrossRef] [PubMed]
- Vijayaraman P, Herweg B, Dandamudi G, Mittal S, Bhatt AG, Marcantoni L, Naperkowski A, Sharma PS, Zanon F. Outcomes of His-bundle pacing upgrade after long-term right ventricular pacing and/or pacing-induced cardiomyopathy: Insights into disease progression. Heart Rhythm. 2019 Oct;16(10):1554-1561. [CrossRef] [PubMed]
- Huang W, Chen X, Su L, Wu S, Xia X, Vijayaraman P. A beginner's guide to permanent left bundle branch pacing. Heart Rhythm. 2019 Dec;16(12):1791-1796. [CrossRef] [PubMed]
- Vijayaraman P, Subzposh FA, Naperkowski A, Panikkath R, John K, Mascarenhas V, Bauch TD, Huang W. Prospective evaluation of feasibility and electrophysiologic and echocardiographic characteristics of left bundle branch area pacing. Heart Rhythm. 2019 Dec;16(12):1774-1782. [CrossRef] [PubMed]
- Su L, Wang S, Wu S, Xu L, Huang Z, Chen X, Zheng R, Jiang L, Ellenbogen KA, Whinnett ZI, Huang W. Long-Term Safety and Feasibility of Left Bundle Branch Pacing in a Large Single-Center Study. Circ Arrhythm Electrophysiol. 2021 Feb;14(2):e009261. [CrossRef] [PubMed]
- Sharma PS, Patel NR, Ravi V, Zalavadia DV, Dommaraju S, Garg V, Larsen TR, Naperkowski AM, Wasserlauf J, Krishnan K, Young W, Pokharel P, Oren JW, Storm RH, Trohman RG, Huang HD, Subzposh FA, Vijayaraman P. Clinical outcomes of left bundle branch area pacing compared to right ventricular pacing: Results from the Geisinger-Rush Conduction System Pacing Registry. Heart Rhythm. 2022 Jan;19(1):3-11. Erratum in: Heart Rhythm. 2023 Jul;20(7):1100. [CrossRef] [PubMed]
- Vecera J, Penicka M, Eriksen M, Russell K, Bartunek J, Vanderheyden M, Smiseth OA. Wasted septal work in left ventricular dyssynchrony: a novel principle to predict response to cardiac resynchronization therapy. Eur Heart J Cardiovasc Imaging. 2016 Jun;17(6):624-32. [CrossRef] [PubMed]
- Spragg DD, Leclercq C, Loghmani M, Faris OP, Tunin RS, DiSilvestre D, McVeigh ER, Tomaselli GF, Kass DA. Regional alterations in protein expression in the dyssynchronous failing heart. Circulation. 2003 Aug 26;108(8):929-32. [CrossRef] [PubMed]
- Tan NY, Witt CM, Oh JK, Cha YM. Left Bundle Branch Block: Current and Future Perspectives. Circ Arrhythm Electrophysiol. 2020 Apr;13(4):e008239. [CrossRef] [PubMed]
- Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, Lamas GA; MOde Selection Trial Investigators. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003 Jun 17;107(23):2932-7. [CrossRef] [PubMed]
- Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, Kutalek SP, Sharma A; Dual Chamber and VVI Implantable Defibrillator Trial Investigators. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002 Dec 25;288(24):3115-23. [CrossRef] [PubMed]
- Kirk JA, Kass DA. Cellular and Molecular Aspects of Dyssynchrony and Resynchronization. Card Electrophysiol Clin. 2015 Dec;7(4):585-97. [CrossRef] [PubMed]
- Nguyên UC, Verzaal NJ, van Nieuwenhoven FA, Vernooy K, Prinzen FW. Pathobiology of cardiac dyssynchrony and resynchronization therapy. Europace. 2018 Dec 1;20(12):1898-1909. [CrossRef] [PubMed]
- Merchant FM, Mittal S. Pacing induced cardiomyopathy. J Cardiovasc Electrophysiol. 2020 Jan;31(1):286-292. [CrossRef] [PubMed]
- Khurshid S, Obeng-Gyimah E, Supple GE, Schaller R, Lin D, Owens AT, Epstein AE, Dixit S, Marchlinski FE, Frankel DS. Reversal of Pacing-Induced Cardiomyopathy Following Cardiac Resynchronization Therapy. JACC Clin Electrophysiol. 2018 Feb;4(2):168-177. [CrossRef] [PubMed]
- Lu W, Lin J, Dai Y, Chen K, Zhang S. The therapeutic effects of upgrade to cardiac resynchronization therapy in pacing-induced cardiomyopathy or chronic right ventricular pacing patients: a meta-analysis. Heart Fail Rev. 2022 Mar;27(2):507-516. [CrossRef] [PubMed]
- Deshmukh P, Casavant DA, Romanyshyn M, Anderson K. Permanent, direct His-bundle pacing: a novel approach to cardiac pacing in patients with normal His-Purkinje activation. Circulation. 2000 Feb 29;101(8):869-77. [CrossRef] [PubMed]
- Huang W, Su L, Wu S, Xu L, Xiao F, Zhou X, Ellenbogen KA. A Novel Pacing Strategy With Low and Stable Output: Pacing the Left Bundle Branch Immediately Beyond the Conduction Block. Can J Cardiol. 2017 Dec;33(12):1736.e1-1736.e3. [CrossRef] [PubMed]
- Arnold AD, Shun-Shin MJ, Keene D, Howard JP, Sohaib SMA, Wright IJ, Cole GD, Qureshi NA, Lefroy DC, Koa-Wing M, Linton NWF, Lim PB, Peters NS, Davies DW, Muthumala A, Tanner M, Ellenbogen KA, Kanagaratnam P, Francis DP, Whinnett ZI. His Resynchronization Versus Biventricular Pacing in Patients With Heart Failure and Left Bundle Branch Block. J Am Coll Cardiol. 2018 Dec 18;72(24):3112-3122. [CrossRef] [PubMed]
- Sussenbek O, Rademakers L, Waldauf P, Jurak P, Smisek R, Stros P, Poviser L, Vesela J, Plesinger F, Halamek J, Leinveber P, Herman D, Osmancik P, Curila K. Left bundle branch area pacing results in more physiological ventricular activation than biventricular pacing in patients with left bundle branch block heart failure. Eur Heart J Suppl. 2023 ;25(Suppl E):E17-E24. 24 May. [CrossRef] [PubMed]
- Vijayaraman P, Dandamudi G, Zanon F, Sharma PS, Tung R, Huang W, Koneru J, Tada H, Ellenbogen KA, Lustgarten DL. Permanent His bundle pacing: Recommendations from a Multicenter His Bundle Pacing Collaborative Working Group for standardization of definitions, implant measurements, and follow-up. Heart Rhythm. 2018 Mar;15(3):460-468. [CrossRef] [PubMed]
- Burri H, Jastrzebski M, Cano Ó, Čurila K, de Pooter J, Huang W, Israel C, Joza J, Romero J, Vernooy K, Vijayaraman P, Whinnett Z, Zanon F. EHRA clinical consensus statement on conduction system pacing implantation: executive summary. Endorsed by the Asia-Pacific Heart Rhythm Society (APHRS), Canadian Heart Rhythm Society (CHRS) and Latin-American Heart Rhythm Society (LAHRS). Europace. 2023 Apr 15;25(4):1237-1248. [CrossRef] [PubMed]
- Barba-Pichardo R, Manovel Sánchez A, Fernández-Gómez JM, Moriña-Vázquez P, Venegas-Gamero J, Herrera-Carranza M. Ventricular resynchronization therapy by direct His-bundle pacing using an internal cardioverter defibrillator. Europace. 2013 Jan;15(1):83-8. [CrossRef] [PubMed]
- Lustgarten DL, Crespo EM, Arkhipova-Jenkins I, Lobel R, Winget J, Koehler J, Liberman E, Sheldon T. His-bundle pacing versus biventricular pacing in cardiac resynchronization therapy patients: A crossover design comparison. Heart Rhythm. 2015 Jul;12(7):1548-57. [CrossRef] [PubMed]
- Sharma PS, Dandamudi G, Herweg B, Wilson D, Singh R, Naperkowski A, Koneru JN, Ellenbogen KA, Vijayaraman P. Permanent His-bundle pacing as an alternative to biventricular pacing for cardiac resynchronization therapy: A multicenter experience. Heart Rhythm. 2018 Mar;15(3):413-420. [CrossRef] [PubMed]
- Huang W, Su L, Wu S, Xu L, Xiao F, Zhou X, Mao G, Vijayaraman P, Ellenbogen KA. Long-term outcomes of His bundle pacing in patients with heart failure with left bundle branch block. Heart. 2019 Jan;105(2):137-143. [CrossRef] [PubMed]
- Moriña-Vázquez P, Moraleda-Salas MT, Manovel-Sánchez AJ, Fernández-Gómez JM, Arce-Léon Á, Venegas-Gamero J, Barba-Pichardo R. Early improvement of left ventricular ejection fraction by cardiac resynchronization through His bundle pacing in patients with heart failure. Europace. 2020 Jan 1;22(1):125-132. [CrossRef] [PubMed]
- Upadhyay GA, Vijayaraman P, Nayak HM, Verma N, Dandamudi G, Sharma PS, Saleem M, Mandrola J, Genovese D, Oren JW, Subzposh FA, Aziz Z, Beaser A, Shatz D, Besser S, Lang RM, Trohman RG, Knight BP, Tung R; His-SYNC Investigators. On-treatment comparison between corrective His bundle pacing and biventricular pacing for cardiac resynchronization: A secondary analysis of the His-SYNC Pilot Trial. Heart Rhythm. 2019 Dec;16(12):1797-1807. [CrossRef] [PubMed]
- Vinther M, Risum N, Svendsen JH, Møgelvang R, Philbert BT. A Randomized Trial of His Pacing Versus Biventricular Pacing in Symptomatic HF Patients With Left Bundle Branch Block (His-Alternative). JACC Clin Electrophysiol. 2021 Nov;7(11):1422-1432. [CrossRef] [PubMed]
- Huang W, Wang S, Su L, Fu G, Su Y, Chen K, Zou J, Han H, Wu S, Sheng X, Chen X, Fan X, Xu L, Zhou X, Mao G, Ellenbogen KA, Whinnett ZI. His-bundle pacing vs biventricular pacing following atrioventricular nodal ablation in patients with atrial fibrillation and reduced ejection fraction: A multicenter, randomized, crossover study-The ALTERNATIVE-AF trial. Heart Rhythm. 2022 Dec;19(12):1948-1955. [CrossRef] [PubMed]
- Whinnett ZI, Shun-Shin MJ, Tanner M, Foley P, Chandrasekaran B, Moore P, Adhya S, Qureshi N, Muthumala A, Lane R, Rinaldi A, Agarwal S, Leyva F, Behar J, Bassi S, Ng A, Scott P, Prasad R, Swinburn J, Tomson J, Sethi A, Shah J, Lim PB, Kyriacou A, Thomas D, Chuen J, Kamdar R, Kanagaratnam P, Mariveles M, Burden L, March K, Howard JP, Arnold A, Vijayaraman P, Stegemann B, Johnson N, Falaschetti E, Francis DP, Cleland JGF, Keene D. Effects of haemodynamically atrio-ventricular optimized His bundle pacing on heart failure symptoms and exercise capacity: the His Optimized Pacing Evaluated for Heart Failure (HOPE-HF) randomized, double-blind, cross-over trial. Eur J Heart Fail. 2023 Feb;25(2):274-283. [CrossRef] [PubMed]
- Li X, Qiu C, Xie R, Ma W, Wang Z, Li H, Wang H, Hua W, Zhang S, Yao Y, Fan X. Left bundle branch area pacing delivery of cardiac resynchronization therapy and comparison with biventricular pacing. ESC Heart Fail. 2020 Aug;7(4):1711-1722. [CrossRef] [PubMed]
- Vijayaraman P, Ponnusamy S, Cano Ó, Sharma PS, Naperkowski A, Subsposh FA, Moskal P, Bednarek A, Dal Forno AR, Young W, Nanda S, Beer D, Herweg B, Jastrzebski M. Left Bundle Branch Area Pacing for Cardiac Resynchronization Therapy: Results From the International LBBAP Collaborative Study Group. JACC Clin Electrophysiol. 2021 Feb;7(2):135-147. [CrossRef] [PubMed]
- Jastrzębski M, Kiełbasa G, Cano O, Curila K, Heckman L, De Pooter J, Chovanec M, Rademakers L, Huybrechts W, Grieco D, Whinnett ZI, Timmer SAJ, Elvan A, Stros P, Moskal P, Burri H, Zanon F, Vernooy K. Left bundle branch area pacing outcomes: the multicentre European MELOS study. Eur Heart J. 2022 Oct 21;43(40):4161-4173. [CrossRef] [PubMed]
- Chen X, Ye Y, Wang Z, Jin Q, Qiu Z, Wang J, Qin S, Bai J, Wang W, Liang Y, Chen H, Sheng X, Gao F, Zhao X, Fu G, Ellenbogen KA, Su Y, Ge J. Cardiac resynchronization therapy via left bundle branch pacing vs. optimized biventricular pacing with adaptive algorithm in heart failure with left bundle branch block: a prospective, multi-centre, observational study. Europace. 2022 ;24(5):807-816. 3 May. [CrossRef] [PubMed]
- Wang Y, Zhu H, Hou X, Wang Z, Zou F, Qian Z, Wei Y, Wang X, Zhang L, Li X, Liu Z, Xue S, Qin C, Zeng J, Li H, Wu H, Ma H, Ellenbogen KA, Gold MR, Fan X, Zou J; LBBP-RESYNC Investigators. Randomized Trial of Left Bundle Branch vs Biventricular Pacing for Cardiac Resynchronization Therapy. J Am Coll Cardiol. 2022 Sep 27;80(13):1205-1216. [CrossRef] [PubMed]
- Pujol-Lopez M, Jiménez-Arjona R, Garre P, Guasch E, Borràs R, Doltra A, Ferró E, García-Ribas C, Niebla M, Carro E, Puente JL, Vázquez-Calvo S, Invers-Rubio E, Roca-Luque I, Castel MÁ, Arbelo E, Sitges M, Brugada J, Tolosana JM, Mont L. Conduction System Pacing vs Biventricular Pacing in Heart Failure and Wide QRS Patients: LEVEL-AT Trial. JACC Clin Electrophysiol. 2022 Nov;8(11):1431-1445. [CrossRef] [PubMed]
- Vijayaraman P, Zalavadia D, Haseeb A, Dye C, Madan N, Skeete JR, Vipparthy SC, Young W, Ravi V, Rajakumar C, Pokharel P, Larsen T, Huang HD, Storm RH, Oren JW, Batul SA, Trohman RG, Subzposh FA, Sharma PS. Clinical outcomes of conduction system pacing compared to biventricular pacing in patients requiring cardiac resynchronization therapy. Heart Rhythm. 2022 Aug;19(8):1263-1271. [CrossRef] [PubMed]
- Ezzeddine FM, Pistiolis SM, Pujol-Lopez M, Lavelle M, Wan EY, Patton KK, Robinson M, Lador A, Tamirisa K, Karim S, Linde C, Parkash R, Birgersdotter-Green U, Russo AM, Chung M, Cha YM. Outcomes of conduction system pacing for cardiac resynchronization therapy in patients with heart failure: A multicenter experience. Heart Rhythm. 2023 Jun;20(6):863-871. [CrossRef] [PubMed]
- Diaz JC, Sauer WH, Duque M, Koplan BA, Braunstein ED, Marín JE, Aristizabal J, Niño CD, Bastidas O, Martinez JM, Hoyos C, Matos CD, Lopez-Cabanillas N, Steiger NA, Kapur S, Tadros TM, Martin DT, Zei PC, Tedrow UB, Romero JE. Left Bundle Branch Area Pacing Versus Biventricular Pacing as Initial Strategy for Cardiac Resynchronization. JACC Clin Electrophysiol. 2023 Aug;9(8 Pt 2):1568-1581. [CrossRef] [PubMed]
- Vijayaraman P, Sharma PS, Cano Ó, Ponnusamy SS, Herweg B, Zanon F, Jastrzebski M, Zou J, Chelu MG, Vernooy K, Whinnett ZI, Nair GM, Molina-Lerma M, Curila K, Zalavadia D, Haseeb A, Dye C, Vipparthy SC, Brunetti R, Moskal P, Ross A, van Stipdonk A, George J, Qadeer YK, Mumtaz M, Kolominsky J, Zahra SA, Golian M, Marcantoni L, Subzposh FA, Ellenbogen KA. Comparison of Left Bundle Branch Area Pacing and Biventricular Pacing in Candidates for Resynchronization Therapy. J Am Coll Cardiol. 2023 Jul 18;82(3):228-241. [CrossRef] [PubMed]
- Mariani MV, Piro A, Forleo GB, Della Rocca DG, Natale A, Miraldi F, Vizza CD, Lavalle C. Clinical, procedural and lead outcomes associated with different pacing techniques: a network meta-analysis. Int J Cardiol. 2023;377:52–9. [CrossRef] [PubMed]
- Ali N, Arnold AD, Miyazawa AA, Keene D, Chow JJ, Little I, Peters NS, Kanagaratnam P, Qureshi N, Ng FS, Linton NWF, Lefroy DC, Francis DP, Phang Boon L, Tanner MA, Muthumala A, Shun-Shin MJ, Cole GD, Whinnett ZI. Comparison of methods for delivering cardiac resynchronization therapy: an acute electrical and haemodynamic within- patient comparison of left bundle branch area, His bundle, and biventricular pacing. Europace. 2023;25(3):1060–7. [CrossRef] [PubMed]
- Hua J, Wang C, Kong Q, Zhang Y, Wang Q, Xiong Z, Hu J, Li J, Chen Q, Hong K. Comparative effects of left bundle branch area pacing, His bundle pacing, biventricular pacing in patients requiring cardiac resynchronization therapy: a network meta-analysis. Clin Cardiol. 2022;45(2):214–223. [CrossRef] [PubMed]
- Upadhyay GA, Cherian T, Shatz DY, Beaser AD, Aziz Z, Ozcan C, Broman MT, Nayak HM, Tung R. Intracardiac Delineation of Septal Conduction in Left Bundle-Branch Block Patterns. Circulation. 2019 Apr 16;139(16):1876-1888. [CrossRef] [PubMed]
- Surawicz B, Childers R, Deal BJ, Gettes LS. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram, part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119:e235–e240. [CrossRef]
- Vijayaraman P, Herweg B, Ellenbogen KA, Gajek J. His-Optimized Cardiac Resynchronization Therapy to Maximize Electrical Resynchronization: A Feasibility Study. Circ Arrhythm Electrophysiol. 2019 Feb;12(2):e006934. [CrossRef] [PubMed]
- Zweerink A, Zubarev S, Bakelants E, Potyagaylo D, Stettler C, Chmelevsky M, Lozeron ED, Hachulla AL, Vallée JP, Burri H. His-Optimized Cardiac Resynchronization Therapy With Ventricular Fusion Pacing for Electrical Resynchronization in Heart Failure. JACC Clin Electrophysiol. 2021 Jul;7(7):881-892. [CrossRef] [PubMed]
- Deshmukh A, Sattur S, Bechtol T, Heckman LIB, Prinzen FW, Deshmukh P. Sequential His bundle and left ventricular pacing for cardiac resynchronization. J Cardiovasc Electrophysiol. 2020 Sep;31(9):2448-2454. [CrossRef] [PubMed]
- Jastrzębski M, Moskal P, Huybrechts W, Curila K, Sreekumar P, Rademakers LM, Ponnusamy SS, Herweg B, Sharma PS, Bednarek A, Rajzer M, Vijayaraman P. Left bundle branch-optimized cardiac resynchronization therapy (LOT-CRT): Results from an international LBBAP collaborative study group. Heart Rhythm. 2022 Jan;19(1):13-21. [CrossRef] [PubMed]
- Feng XF, Yang LC, Zhao Y, Yu YC, Liu B, Li YG. Effects of adaptive left bundle branch-optimized cardiac resynchronization therapy: a single centre experience. BMC Cardiovasc Disord. 2022 Aug 6;22(1):360. Erratum in: BMC Cardiovasc Disord. 2022 Dec 22;22(1):558. [CrossRef] [PubMed]
- Zareba W, Klein H, Cygankiewicz I, Hall WJ, McNitt S, Brown M, Cannom D, Daubert JP, Eldar M, Gold MR, Goldberger JJ, Goldenberg I, Lichstein E, Pitschner H, Rashtian M, Solomon S, Viskin S, Wang P, Moss AJ; MADIT-CRT Investigators. Effectiveness of Cardiac Resynchronization Therapy by QRS Morphology in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT). Circulation. 2011 Mar 15;123(10):1061-72. [CrossRef] [PubMed]
- Baldasseroni S, Opasich C, Gorini M, Lucci D, Marchionni N, Marini M, Campana C, Perini G, Deorsola A, Masotti G, Tavazzi L, Maggioni AP; Italian Network on Congestive Heart Failure Investigators. Left bundle-branch block is associated with increased 1-year sudden and total mortality rate in 5517 outpatients with congestive heart failure: a report from the Italian network on congestive heart failure. Am Heart J. 2002 Mar;143(3):398-405. [CrossRef] [PubMed]
- Sharma PS, Naperkowski A, Bauch TD, Chan JYS, Arnold AD, Whinnett ZI, Ellenbogen KA, Vijayaraman P. Permanent His Bundle Pacing for Cardiac Resynchronization Therapy in Patients With Heart Failure and Right Bundle Branch Block. Circ Arrhythm Electrophysiol. 2018 Sep;11(9):e006613. [CrossRef] [PubMed]
- Vijayaraman P, Cano O, Ponnusamy SS, Molina-Lerma M, Chan JYS, Padala SK, Sharma PS, Whinnett ZI, Herweg B, Upadhyay GA, Subzposh FA, Patel NR, Beer DA, Bednarek A, Kielbasa G, Tung R, Ellenbogen KA, Jastrzebski M. Left bundle branch area pacing in patients with heart failure and right bundle branch block: Results from International LBBAP Collaborative-Study Group. Heart Rhythm O2. 2022 ;3(4):358-367. 14 May. [CrossRef] [PubMed]
- Koniari I, Gerakaris A, Kounis N, Velissaris D, Rao A, Ainslie M, Adlan A, Plotas P, Ikonomidis I, Mplani V, Hung MY, de Gregorio C, Kolettis T, Gupta D. Outcomes of Atrioventricular Node Ablation and Pacing in Patients with Heart Failure and Atrial Fibrillation: From Cardiac Resynchronization Therapy to His Bundle Pacing. J Cardiovasc Dev Dis. 2023 Jun 26;10(7):272. [CrossRef] [PubMed]
- Huang W, Su L, Wu S, Xu L, Xiao F, Zhou X, Ellenbogen KA. Benefits of Permanent His Bundle Pacing Combined With Atrioventricular Node Ablation in Atrial Fibrillation Patients With Heart Failure With Both Preserved and Reduced Left Ventricular Ejection Fraction. J Am Heart Assoc. 2017 Apr 1;6(4):e005309. [CrossRef] [PubMed]
- Su L, Cai M, Wu S, Wang S, Xu T, Vijayaraman P, Huang W. Long-term performance and risk factors analysis after permanent His-bundle pacing and atrioventricular node ablation in patients with atrial fibrillation and heart failure. Europace. 2020 Dec 26;22(Suppl_2):ii19-ii26. [CrossRef] [PubMed]
- Ivanovski M, Mrak M, Mežnar AZ, Žižek D. Biventricular versus Conduction System Pacing after Atrioventricular Node Ablation in Heart Failure Patients with Atrial Fibrillation. J Cardiovasc Dev Dis. 2022 Jul 1;9(7):209. [CrossRef] [PubMed]
- Vijayaraman P, Herweg B, Verma A, Sharma PS, Batul SA, Ponnusamy SS, Schaller RD, Cano O, Molina-Lerma M, Curila K, Huybrechts W, Wilson DR, Rademakers LM, Sreekumar P, Upadhyay G, Vernooy K, Subzposh FA, Huang W, Jastrzebski M, Ellenbogen KA. Rescue left bundle branch area pacing in coronary venous lead failure or nonresponse to biventricular pacing: Results from International LBBAP Collaborative Study Group. Heart Rhythm. 2022 Aug;19(8):1272-1280. [CrossRef] [PubMed]
- Chung MK, Patton KK, Lau CP, Dal Forno ARJ, Al-Khatib SM, Arora V, Birgersdotter-Green UM, Cha YM, Chung EH, Cronin EM, Curtis AB, Cygankiewicz I, Dandamudi G, Dubin AM, Ensch DP, Glotzer TV, Gold MR, Goldberger ZD, Gopinathannair R, Gorodeski EZ, Gutierrez A, Guzman JC, Huang W, Imrey PB, Indik JH, Karim S, Karpawich PP, Khaykin Y, Kiehl EL, Kron J, Kutyifa V, Link MS, Marine JE, Mullens W, Park SJ, Parkash R, Patete MF, Pathak RK, Perona CA, Rickard J, Schoenfeld MH, Seow SC, Shen WK, Shoda M, Singh JP, Slotwiner DJ, Sridhar ARM, Srivatsa UN, Stecker EC, Tanawuttiwat T, Tang WHW, Tapias CA, Tracy CM, Upadhyay GA, Varma N, Vernooy K, Vijayaraman P, Worsnick SA, Zareba W, Zeitler EP. 2023 HRS/APHRS/LAHRS guideline on cardiac physiologic pacing for the avoidance and mitigation of heart failure. Heart Rhythm. 2023 Sep;20(9):e17-e91. [CrossRef] [PubMed]
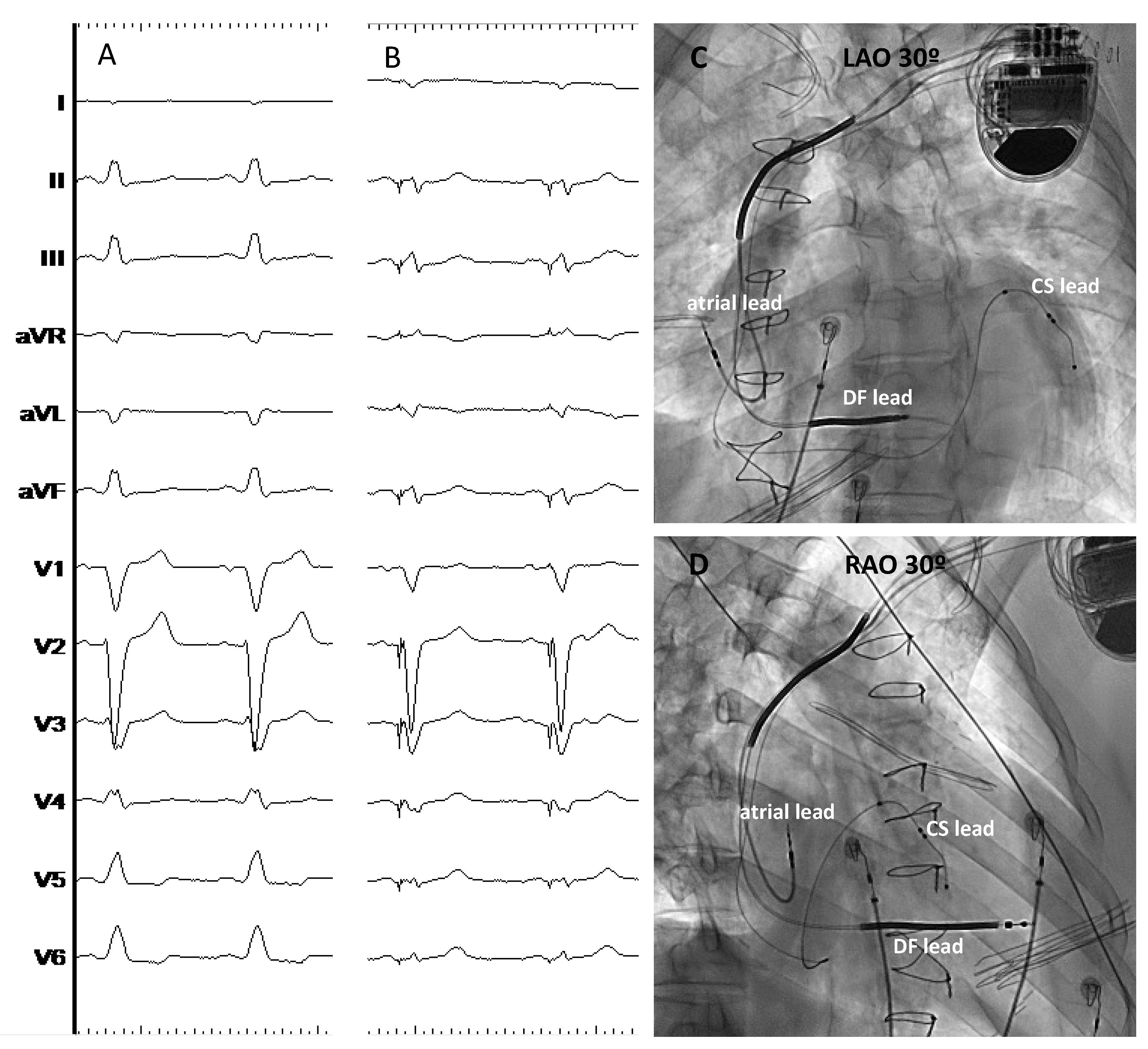
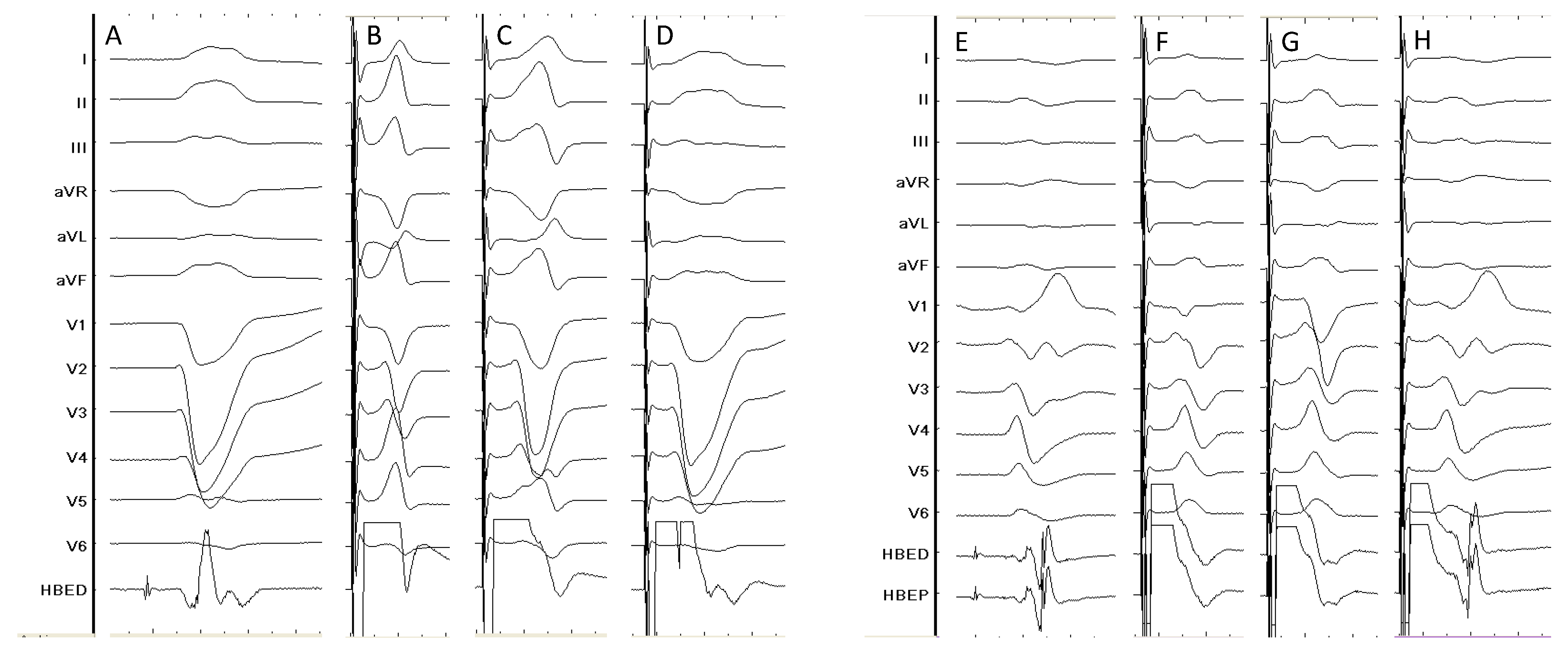

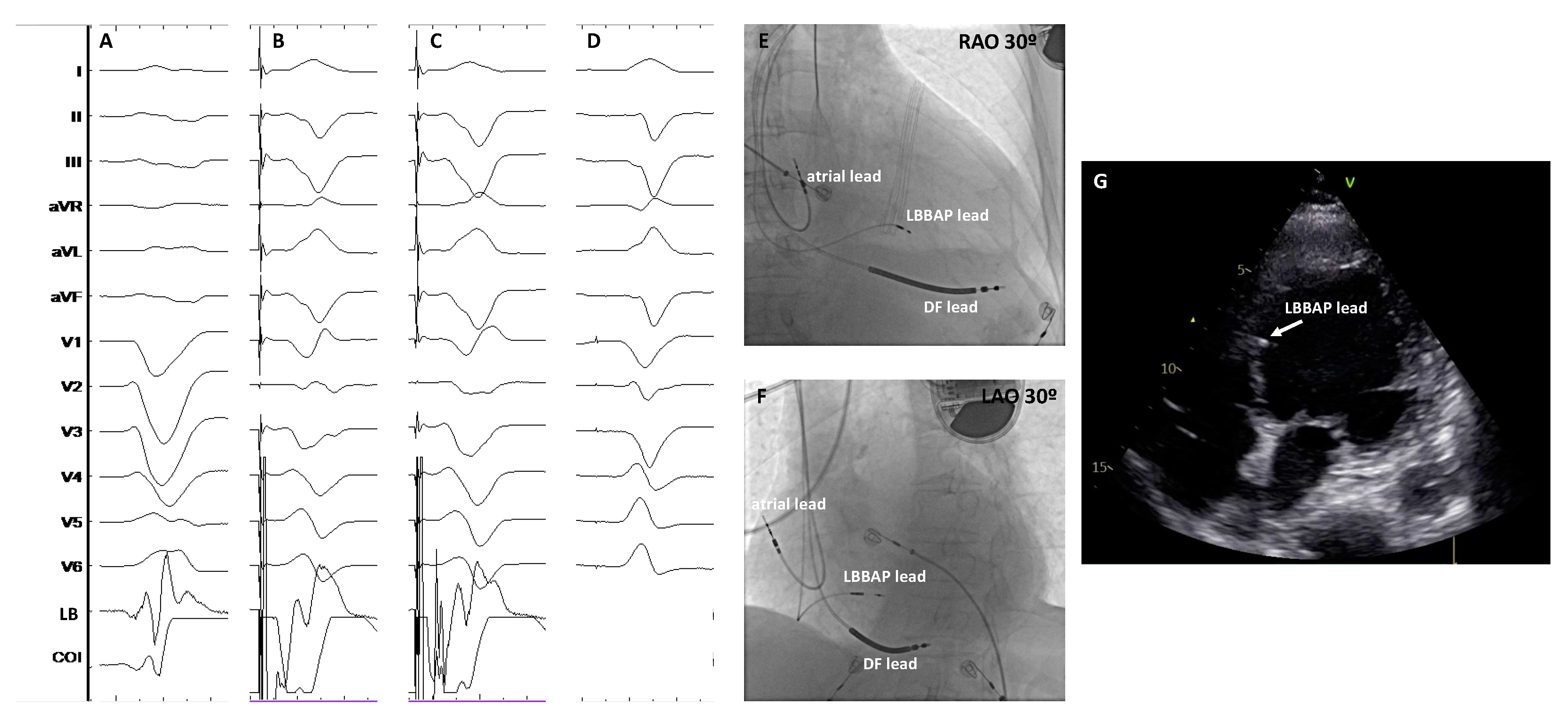
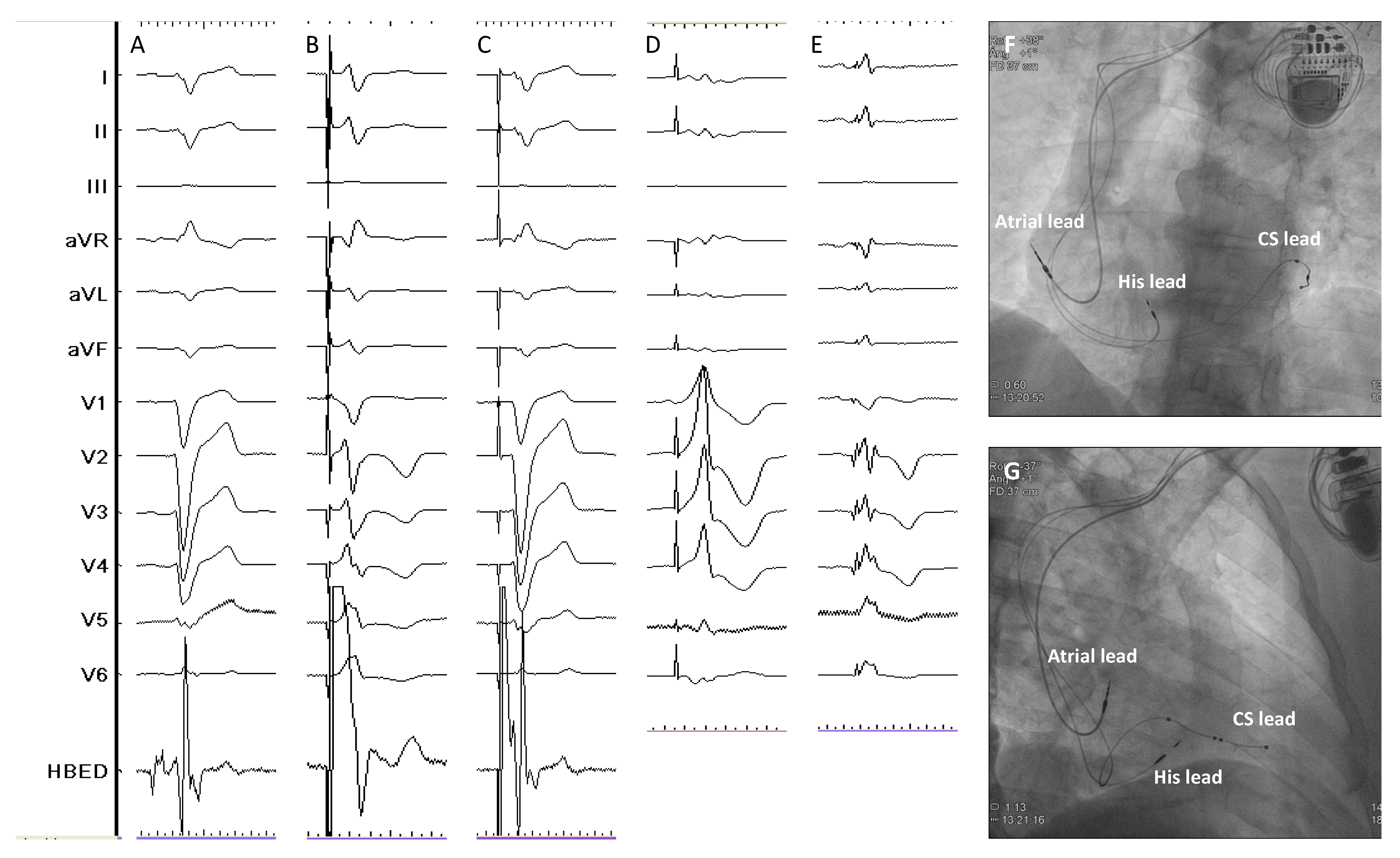
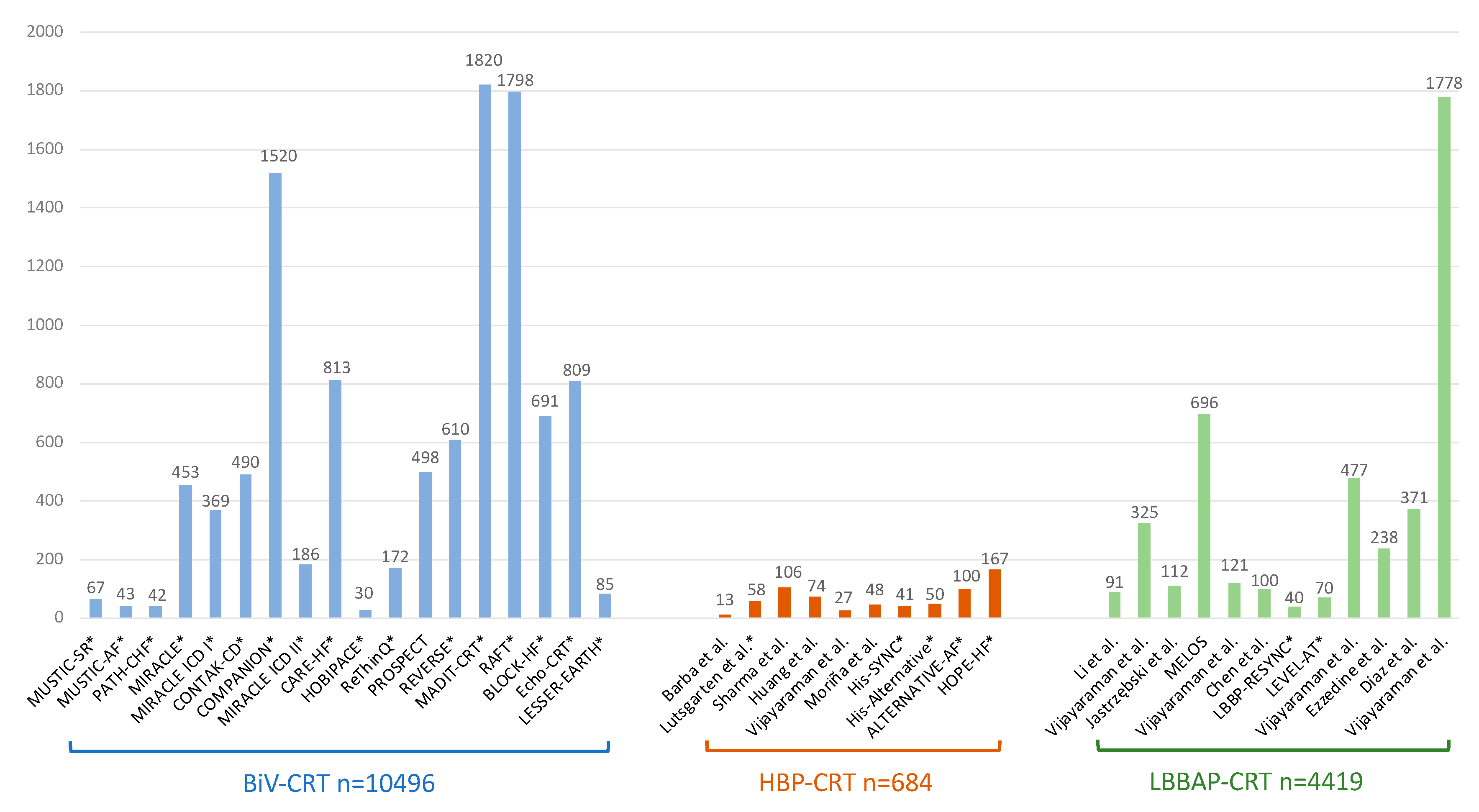
| Study | Design | Patients’ allocation | BBB correction rate | HBP threshold at implant (V)* | HBP threshold at follow-up (V)* | Mean follow-up (months) | Outcomes# | HBP lead related complications (%)# |
|---|---|---|---|---|---|---|---|---|
| Barba et al.55 Europace, 2013 | observational, retrospective, single-centre |
HBP: 16 | 81% temporarily 56% permanently |
3.1 ± 0.4 | 3.7 ± 0.5 | 31 | QRS narrowing, LVEF improvement and reduction of LVEDD and LVESD | 0 |
| Lutsgarten et al.56 Heart Rhythm, 2015 | randomized, crossover, multicentre | HBP: 29 BiVP: 29 |
72% | 1.3 ± 2.2 | 2.4 ± 4.5 | 12 | LVEF, NYHA class, 6MWT and QoL significantly improved with both HBP and BiVP | 10.3 |
| Sharma et al.57 Heart Rhythm, 2018 | observational, retrospective, multicentre |
HBP: 106 | 90% | 1.4 ± 0.9 | 2.0 ± 1.2 | 14 | QRS narrowing, LVEF and NYHA class improvement | 6.6 |
| Huang et al.58 Heart, 2019 | observational, prospective, single-centre | HBP: 74 | 97% temporarily 76% permanently |
1.9 ± 1.1 | 2.3 ± 0.9 | 37 | QRS narrowing, LVEF and NYHA class improvement | 0 |
| Moriña-Vázquez et al.59 Europace, 2020 | observational, prospective, single-centre |
HBP: 48 | 81% | 1.6 (0.9-1.9) | 0.9 (0.7-2) | 6 | QRS narrowing, LVEF and dyssynchrony parameters improvement | 0 |
| Upadhyay et al.60 Heart Rhythm, 2019 | randomized, prospective, multicentre | HBP: 21 BiVP: 20 |
52% | 2.75 (1.3-3.4) | 2 (1-3.3) | 12 | QRS narrowing, trend towards higher echo response with HBP vs BiVP | 0 |
| Vinther et al.61 JACC EP, 2021 | randomized, prospective, single-centre | HBP: 25 BiVP: 25 |
72% | 2.2 ± 1.2 | 2.4 ± 1.6 | 6 | LVEF significantly higher and LVESV significantly lower in HBP group at 6 months | 5.3 |
| Huang et al.62 Heart Rhythm, 2022 |
randomized, prospective, multicentre, crossover |
HBP: 50 BiVP: 50 |
N/A, patients with baseline narrow QRS undergoing AV node ablation | 0.9 ± 0.6 | 0.9 ± 0.6b | 9 | significant improvement in LVEF with HBP vs BiVP | 0 |
| Whinnet et al.63 Eur J Heart Fail, 2023 | randomized, crossover, multicentre | HBP: 167 | 93% | N/A | N/A | 6 | HBP did not increased peak O2 uptake but significantly improved QoL | 5.6 |
| Study | Design | Patients’ allocation | Implant success rate | Pacing threshold at implant (V) | Pacing threshold at follow-up (V) | Mean follow-up (months) | Outcomes# | LBBAP/CS lead related complications (%)# |
|---|---|---|---|---|---|---|---|---|
| Li et al.64 ESC Heart Failure, 2020 | observational, prospective, multicentre | LBBAP: 37 BiVP: 54 |
LBBAP: 81% BiVP: N/A |
LBBAP: 0.81 ± 0.30a BiVP: 1.22 ± 0.62 |
LBBAP: 0.75 ± 0.31b BiVP: 1.43 ± 0.74 |
6 | narrower QRS, greater LVEF improvement, greater echocardiographic response and higher rate of super-responders with LBBAP vs BiVP | LBBAP: 0 BiVP: N/A |
| Vijayaraman et al.65 JACC EP, 2021 | observational, retrospective, multicentre | LBBAP: 325 | 85% | 0.6 ± 0.3 | 0.7 ± 0.3 | 6 | QRS narrowing, LVEF and NYHA class improvement | 2.5 |
| Jastrzębski et al.66 Eur Heart J, 2022 | observational, retrospective, multicentre | LBBAP: 696 | 82% | N/A | N/A | 6.4 | N/A | N/A |
| Chen X et al.67 Europace, 2022 | observational, prospective, multicentre | LBBAP: 49 BiVP: 51 |
LBBAP: 98% BiVP: 91% |
LBBAP: 0.92 ± 0.20 BiVP: 1.45 ± 0.39 |
LBBAP: 0.66 ± 0.17 BiVP: 1.42 ± 0.33 |
12 | narrower QRS, greater LVEF improvement and higher rate of super-responders with LBBAP vs BiVP | LBBAP: 0 BiVP: 1.8 |
| Wang Y et al.68 JACC EP, 2022 | randomized, prospective, multicentre | LBBAP: 20 BiVP: 20 |
LBBAP: 90% BiVP: 80% |
LBBAP: 0.69 ± 0.26 BiVP: 0.92 ± 0.40 |
LBBAP: 0.82 ± 0.20 BiVP: 1.12 ± 0.67 |
6 | higher LVEF improvement and greater reduction in LVESV and NT-proBNP with LBBAP | LBBAP: 0 BiVP: 5 |
| Pujol-López et al.69 JACC EP, 2022 | randomized, prospective, single-centre | LBBAP*: 35 BiVP: 35 |
LBBAP: 77% BiVP: 94% |
LBBAP: 1.0 ± 0.4 BiVP: 1.2 ± 0.5 |
LBBAP: 0.8 ± 0.4 BiVP: 1.0 ± 0.3 |
6 | similar decrease in LVAT and LVESV; similar rates of mortality and HF hospitalization | LBBAP: 0 BiVP: 5 |
| Vijayaraman et al.70 Heart Rhythm, 2022 | observational, retrospective, multicentre | HBP: 87 LBBAP: 171 BiVP: 219 |
CSP: 86% BiVP: 75% |
HBP: 1.1 ± 0.7 LBBAP: 0.8 ± 0.4 BiVP: 1.3 ± 0.6 |
HBP: 1.1 ± 0.7 LBBAP: 0.9 ± 0.5 BiVP: 1.4 ± 0.7 |
27 | greater improvement of LVEF with CS; combined outcome of death or HF hospitalization lower with CSP vs BiVP | HBP: 2.3 LBBAP: 0.6 BiBP: 0.5 |
| Ezzedine et al.71 Heart Rhythm, 2023 | observational, retrospective, multicentre | HBP: 69 LBBAP: 50 BiVP: 119 |
N/A | HBP: 1.29 ± 1 LBBAP: 0.92 ± 0.54 BiVP: N/A |
HBP: 1.46 ± 1.14 LBBAP: 0.86 ± 0.5 BiVP: N/A |
9 | greater proportion of CRT responders in CSP groups vs BiVP. No differences in overall survival or time to first HF hospitalization | HBP: 11.1 LBBAP: 2.1 BiVP: 2.5 |
| Díaz et al.72 JACC EP, 2023 | observational, prospective, multicentre | LBBAP: 128 BiVP: 243 |
LBBAP: 84.4% BiVP: 94.7% |
N/A | N/A | 11 | higher LVEF improvement with LBBAP; significant reduction of all-cause mortality or HF hospitalization with LBBAP | LBBAP: 7 BiVP: 6.2 |
| Vijayaraman et al.73 JACC, 2023 | observational, retrospective, multicentre | LBBAP: 797 BiVP: 981 |
N/A | LBBAP: 0.72 ± 0.4 BiVP: 1.15 ± 0.7 |
LBBAP: 0.74 ± 0.3 BiVP: 1.31 ± 0.7 |
33 | higher LVEF improvement with LBBAP and higher proportion of patients with NYHA class improvement; significant reduction of time to death or HF hospitalization with LBBAP | LBAP: 1.3 BiVP: 2.5 |
| BiVP-CRT | HBP-CRT | LBBAP-CRT | Ref. | |
|---|---|---|---|---|
| Procedural time | lower than HBP higher than LBBAP |
higher than BiVP higher than LBBAP |
lower than BiVP lower than HBP |
60,61,63, 65,68-70,72-73 |
| Fluoroscopy time | higher than HBP higher than LBBAP |
lower than BiVP comparable to LBBAP |
lower than BiVP comparable to HBP |
55,59,61,64,67-70,72,73 |
| Acute CS/CSP lead threshold | lower than HBP higher than LBBAP |
higher than BiVP higher than LBBAP | lower than BiVP lower than HBP |
55-62,64-65,67-71,73 |
| Acute haemodynamic effects | worst than HBP worst than LBBAP |
better than BiVP comparable to LBBAP |
better than BiVP comparable to HBP |
75 |
| Paced QRS duration | wider than HBP wider than LBBAP |
narrower than BiVP comparable to LBBAP |
narrower than BiVP comparable to HBP |
74-76 |
| Change in LVEF | lower than HBP lower than LBBAP |
greater than BiVP comparable to LBBAP |
greater than BiVP comparable to HBP |
74-76 |
| Follow-up CS/CSP lead threshold | lower than HBP higher than LBBAP |
higher than BiVP higher than LBBAP | lower than BiVP lower than HBP |
55-62,64-65,67-71,73 |
| CS/CSP lead-related complications | lower than HBP comparable to LBBAP |
higher than BiVP higher than LBBAP |
comparable to BiVP lower than HBP |
55-65,67-73 |
| Clinical scenarios | 2021 ESC Guideline on Cardiac Pacing and CRT1 | Clinical scenarios | 2023 HRS/APHRS/LAHRS Guideline on Cardiac Physiologic Pacing93 |
|---|---|---|---|
| HF, SR, LVEF ≤35%, LBBB, QRS ≥150 ms | BiVP-CRT (I-A) HBP if unsuccessful CS lead implantation (IIa-B) |
HF, LBBB, LVEF ≤30%, NYHA class I | BiVP-CRT (2b, B-R) |
| HF, SR, LVEF ≤35%, LBBB, QRS 130-149 ms | BiVP-CRT (IIa-B) HBP if unsuccessful CS lead implantation (IIa-B) |
HF, LBBB, QRS ≥150 ms, LVEF ≤35%, NYHA class II-IV | BiVP-CRT (1, A) HBP or LBBAP if BiVP-CRT cannot be achieved (2a, C-LD) |
| HF, SR, LVEF ≤35%, non-LBBB, QRS ≥150 ms | BiVP-CRT (IIa-B) HBP if unsuccessful CS lead implantation (IIa-B) |
HF, LBBB, QRS 120-149 ms, LVEF ≤35%, NYHA class II-IV | BiVP-CRT (1, A) if female sex BiVP-CRT (2a, B-R) for the rest |
| HF, SR, LVEF ≤35%, non-LBBB, QRS 130-149 ms | BiVP-CRT (IIb-B) HBP if unsuccessful CS lead implantation (IIa-B) |
HF, LBBB, QRS ≥150 ms, LVEF 36-50%, NYHA class II-IV | BiVP-CRT (2b, C-LD) HBP or LBBAP (2b, C-LD) |
| HF, AF, LVEF ≤35%, LBBB, QRS ≥130 ms, NYHA class III-IV | BiVP-CRT (IIa-C) HBP if unsuccessful CS lead implantation (IIa-B) |
HF, non-LBBB, LVEF ≤35%, QRS 120-149 ms, NYHA class III-IV | BiVP-CRT (2b, B-NR) HBP or LBBAP (2b, C-LD) |
| HF, LVEF ≤35%, previous PM/ICD with high VP burden | BiVP-CRT (IIa-B) HBP if unsuccessful CS lead implantation (IIa-B) |
HF, non-LBBB, LVEF ≤35%, QRS ≥150 ms, NYHA class II | BiVP-CRT (2b, B-R) HBP or LBBAP (2b, C-LD) |
| Symptomatic AF, LVEF<40% candidates for AVN ablation | BiVP-CRT (I-B) HBP if unsuccessful CS lead implantation (IIa-B) HBP (IIb-C) |
HF, non-LBBB, LVEF ≤35%, QRS ≥150 ms, NYHA class III-IV | BiVP-CRT (2a, A) HBP or LBBAP if BiVP-CRT cannot be achieved (2b, C-LD) |
| Symptomatic AF, LVEF 40-49% candidates for AVN ablation | BiVP-CRT (IIa-C) HBP if unsuccessful CS lead implantation (IIa-B) HBP (IIb-C) |
Pacemaker indication, LVEF 36-50% and anticipated high VP burden | BiVP-CRT (2a, B-R) HBP or LBBAP (2a, B-NR) |
| Symptomatic AF, LVEF ≥50% candidates for AVN ablation | BiVP-CRT (IIb-C) HBP if unsuccessful CS lead implantation (IIa-B) HBP (IIb-C) |
Pacemaker indication, LVEF 36-50%, LBBB and anticipated low VP burden | BiVP-CRT (2b, C-LD) HBP or LBBAP (2b, C-LD) |
| SR or AF, pacing indication for high degree AV block and LVEF<40% | BiVP-CRT (I-A) HBP if unsuccessful CS lead implantation (IIa-B) |
PICM with HF and high burden RVP | BiVP-CRT (1, B-NR) HBP or LBBAP (2b, C-LD) |
| AF + AVN ablation + LVEF ≤50% | BiVP-CRT (2a, B-R) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





