Submitted:
12 September 2023
Posted:
13 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Constitutive modelling
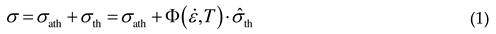
2.1. Thermal stress
2.1.1. Thermal activation effect in fixed structures


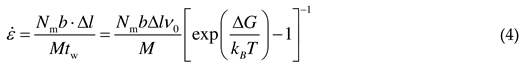
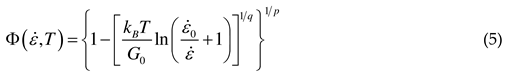

2.1.2. Microstructural evolution


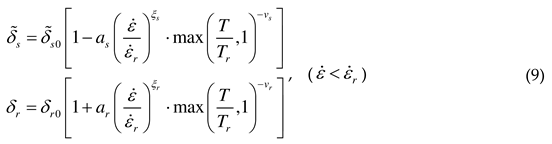


2.1.3. Threshold stress and thermal stress
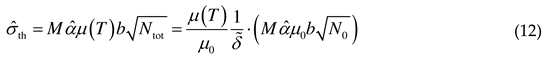
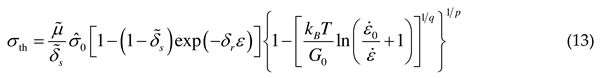
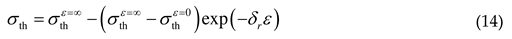
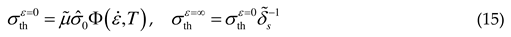
2.2. Athermal stress

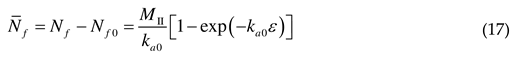
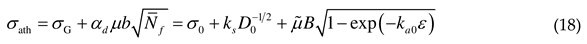
2.3. Flow stress and hardening rate
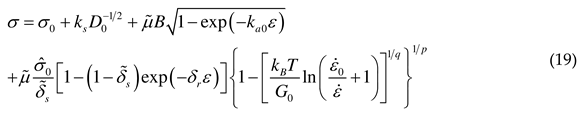
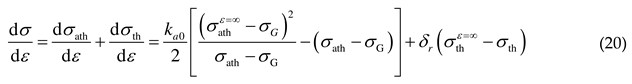
2.4. Shear modulus
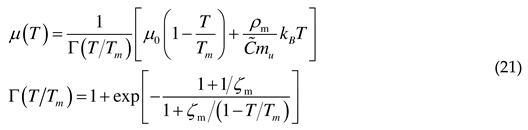
2.5. Adiabatic temperature rise
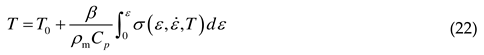
3. Application
3.1. Parameter calibration
| ρm (kg/m3) | Cp (J/kg/K) | β | μ0 (GPa) | Tm (K) | ζm | mu (amu) | |
|---|---|---|---|---|---|---|---|
| 8930 | 382 | 0.9 | 50.7 | 1356 | 0.04 | 0.057 | 63.55 |
| σG (MPa) | ks (MPa√μm) | D0 (μm) | B (MPa) | ka0 |
|---|---|---|---|---|
| 20.6 | 158 [14] | 62 | 230 [2] | 0.64 [2] |
| N0 (m-2) | M | b (nm) | kB/G0 (K-1) | p | q | (s-1) | |
|---|---|---|---|---|---|---|---|
| 1013 [39] | 3.06 [40] | 0.4 [41] | 0.256 [26] | 4.9×10-5 [15] | 2/3 [10] | 1 [10] | 107 [10] |
| as | ξs | vs | δr0 | ar | ξr | vr | (s-1) | Tr (K) | |
|---|---|---|---|---|---|---|---|---|---|
| 0.12 | 0.377 | 0.24 | 0.5 | 4.3 | 50 | 0.8 | 0 | 107 | 298 |
3.2. Simulation results
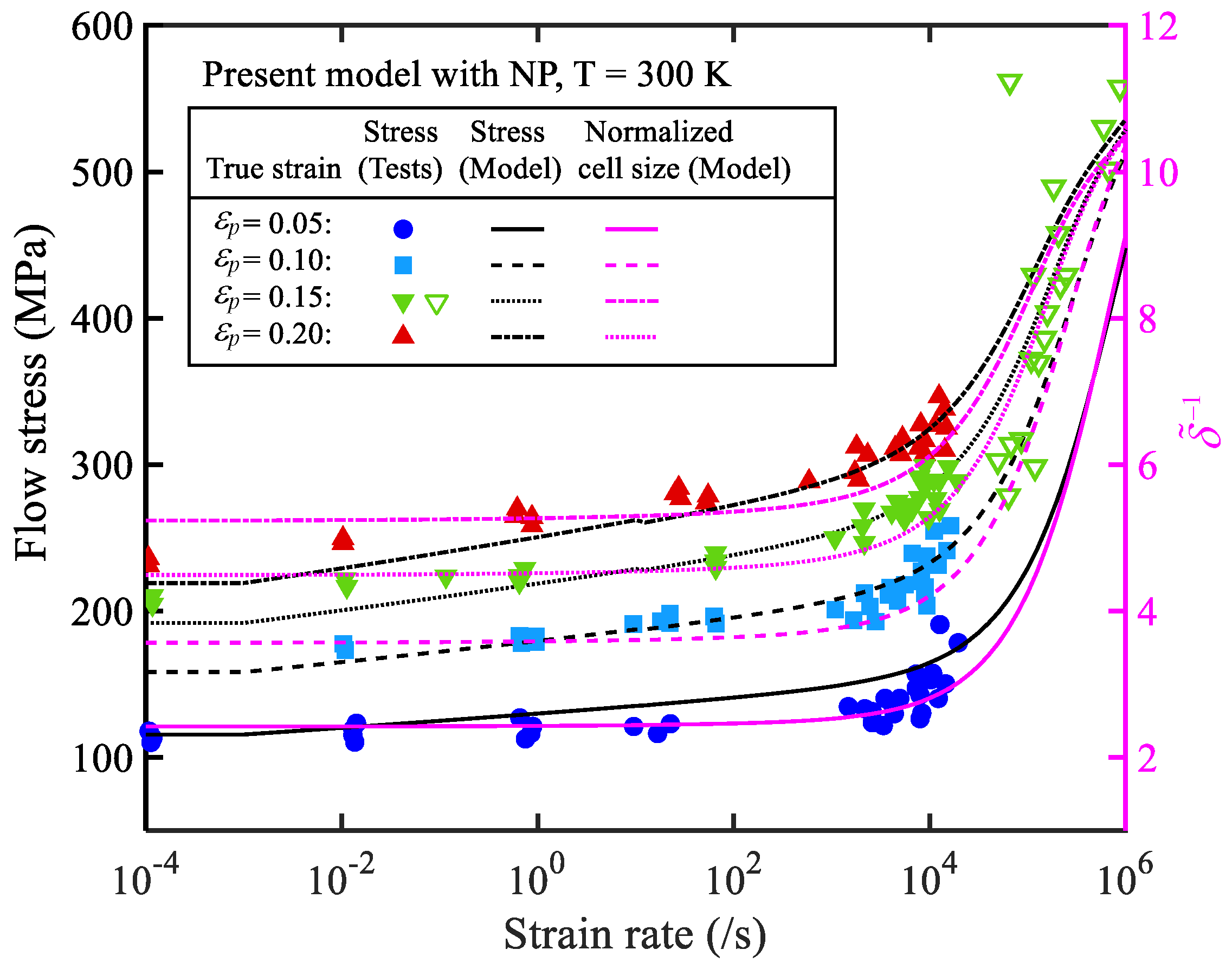
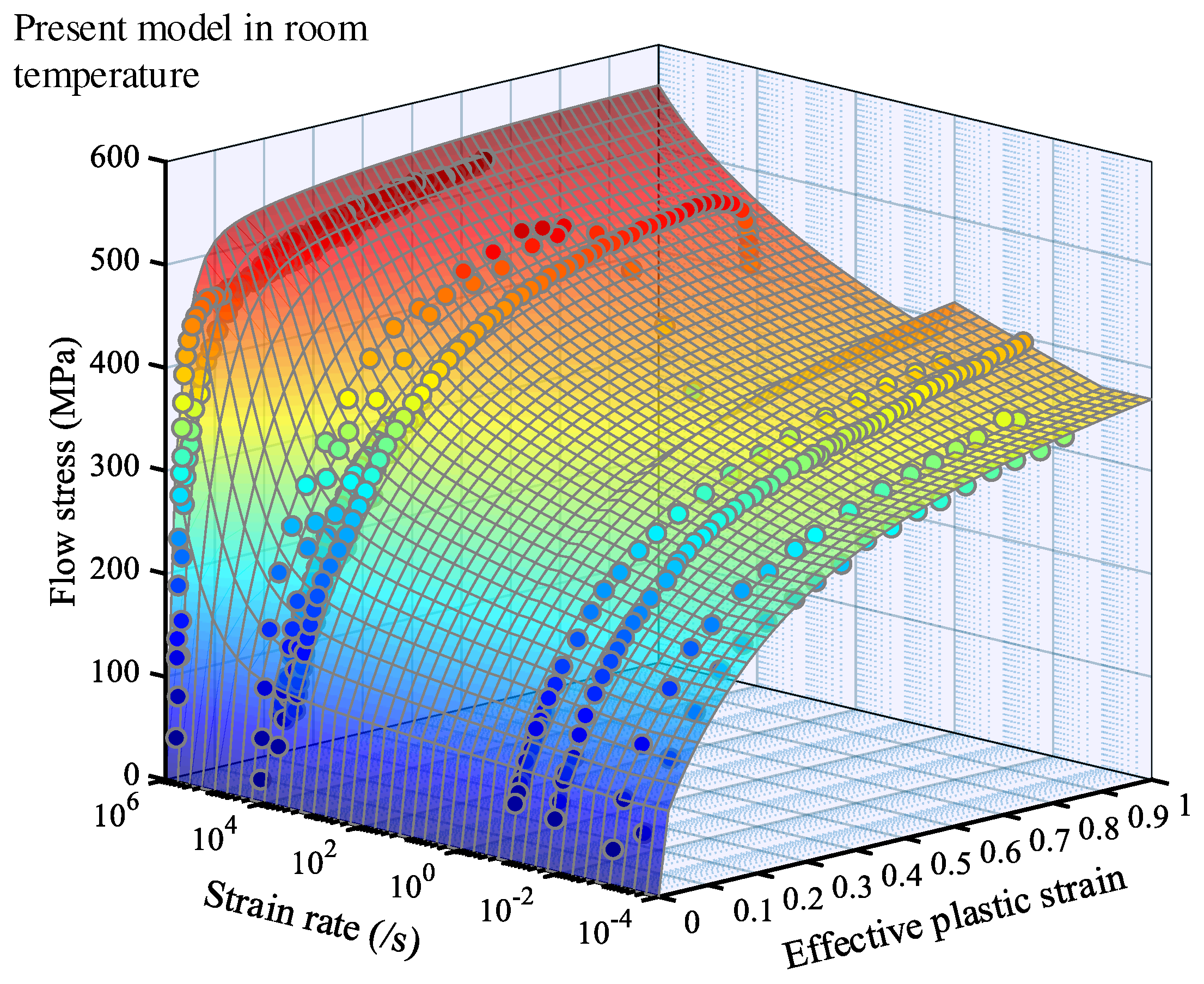
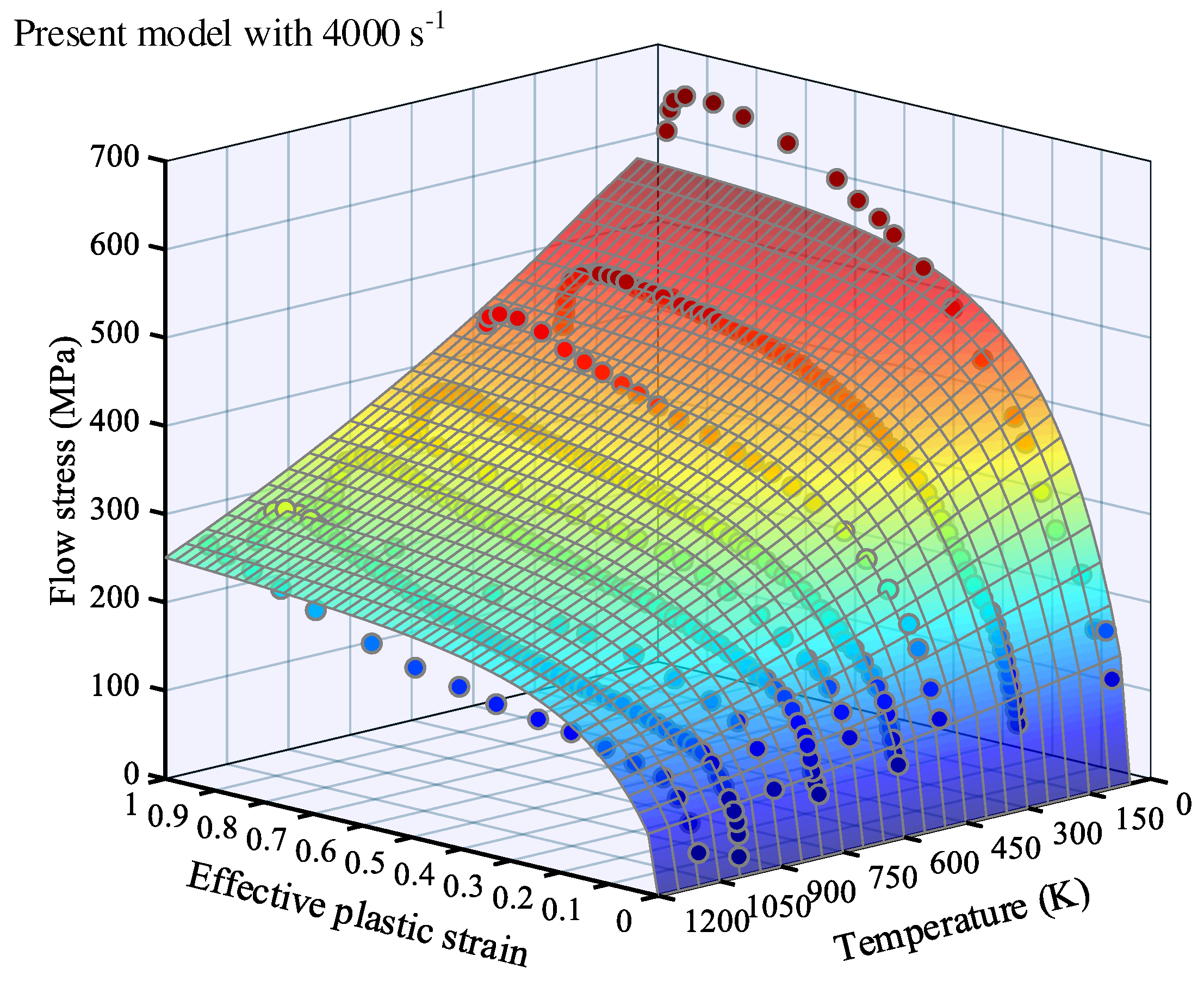
4. Result analysis and discussion
4.1. Relative error analysis

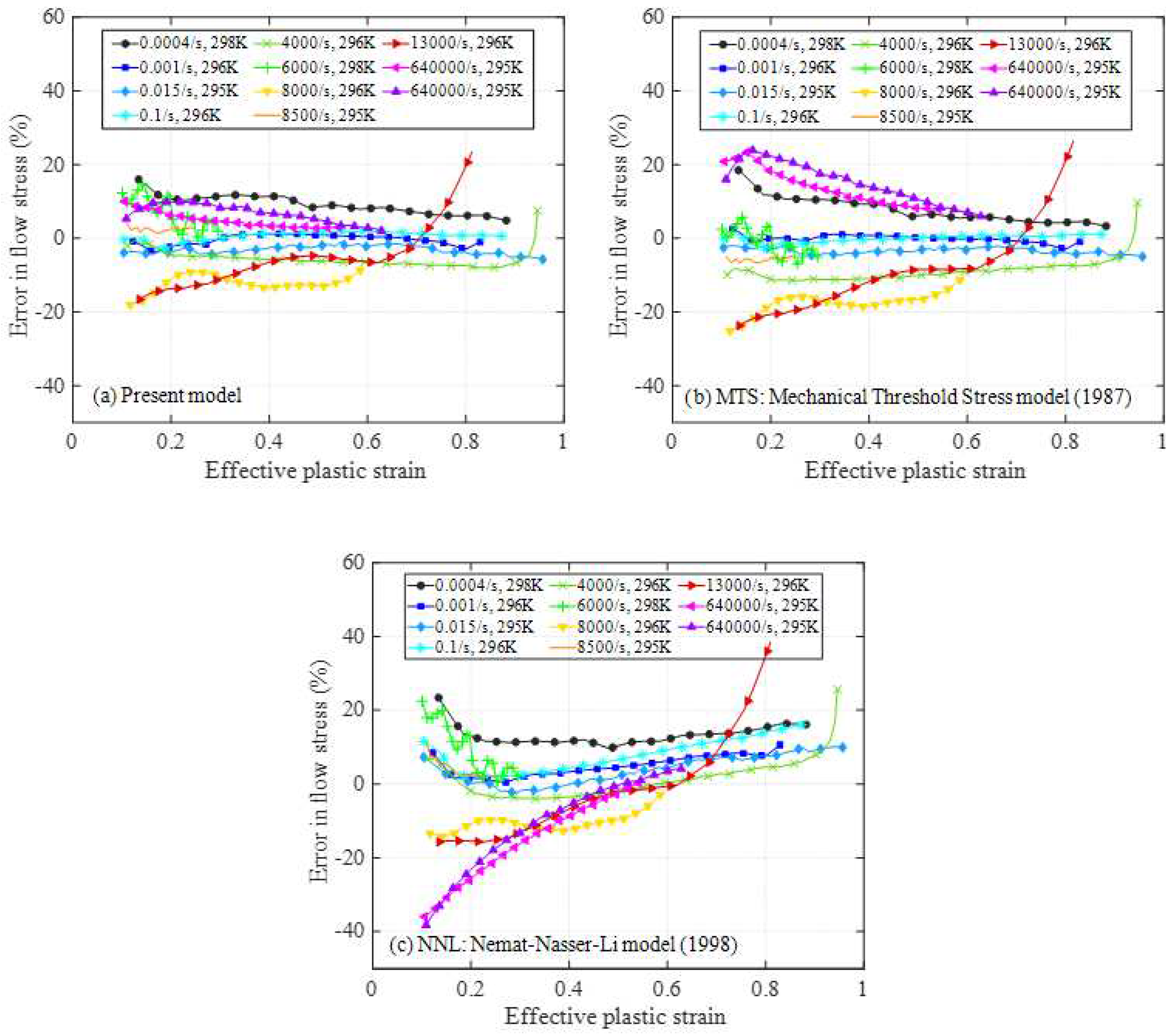
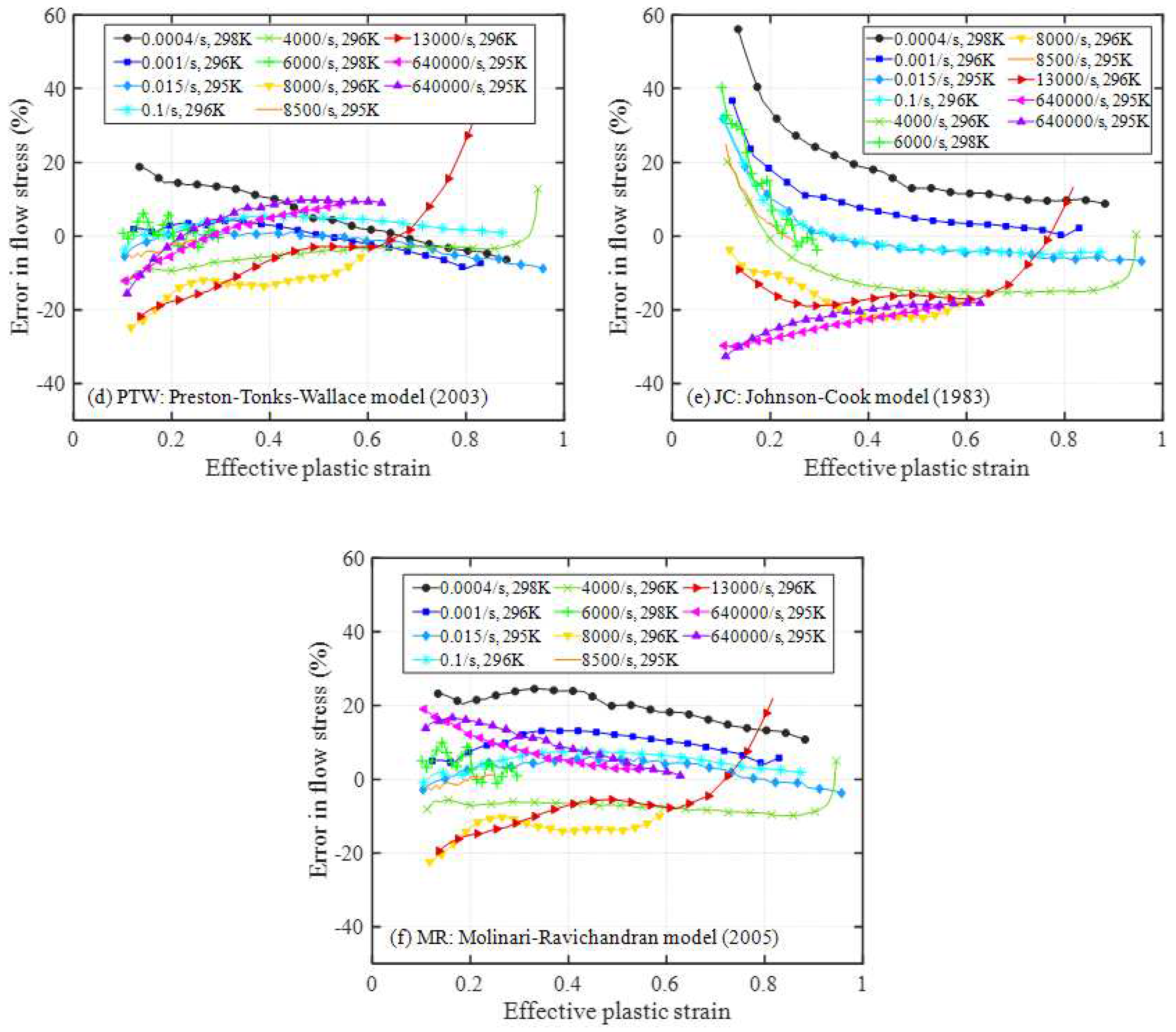
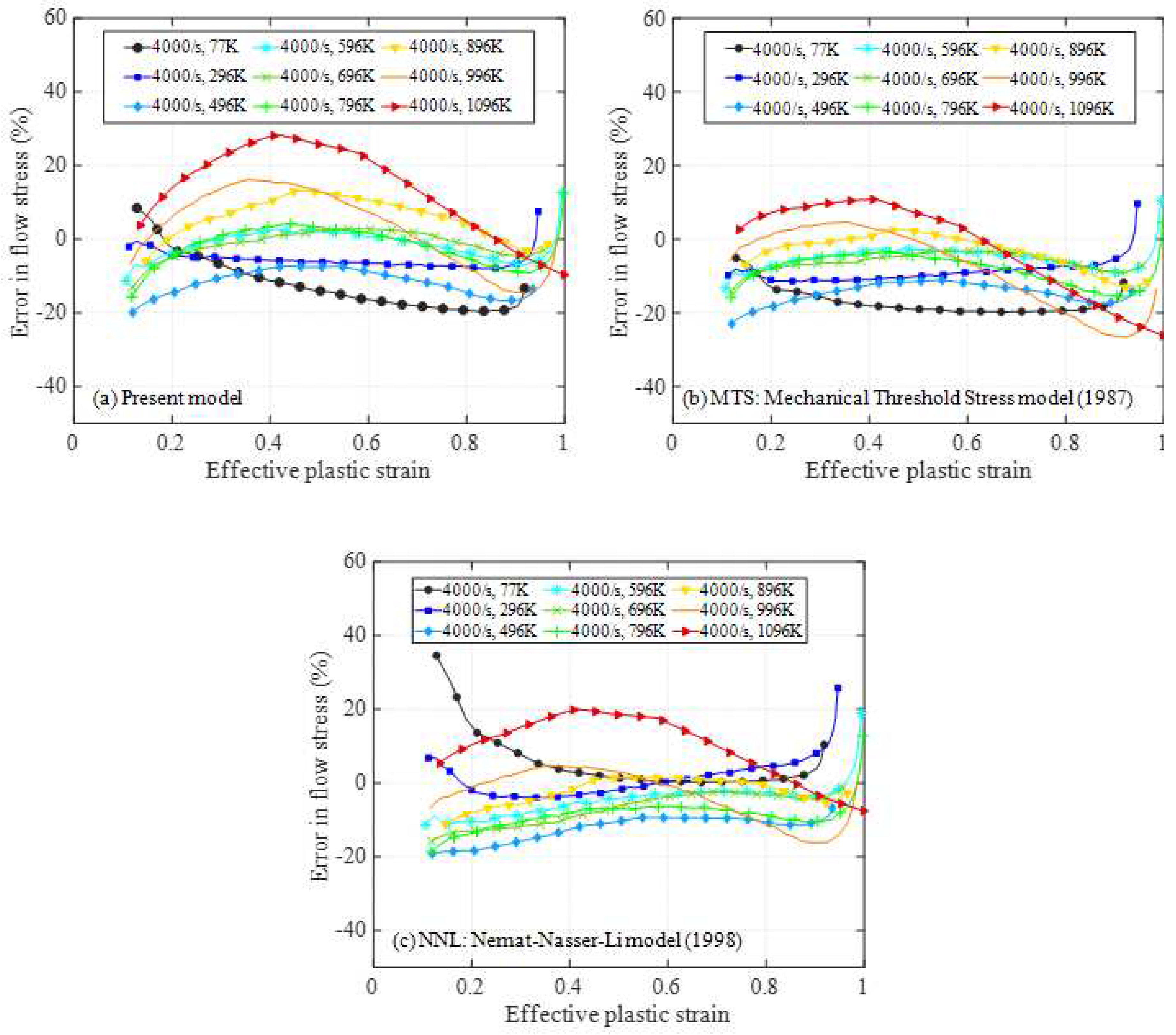
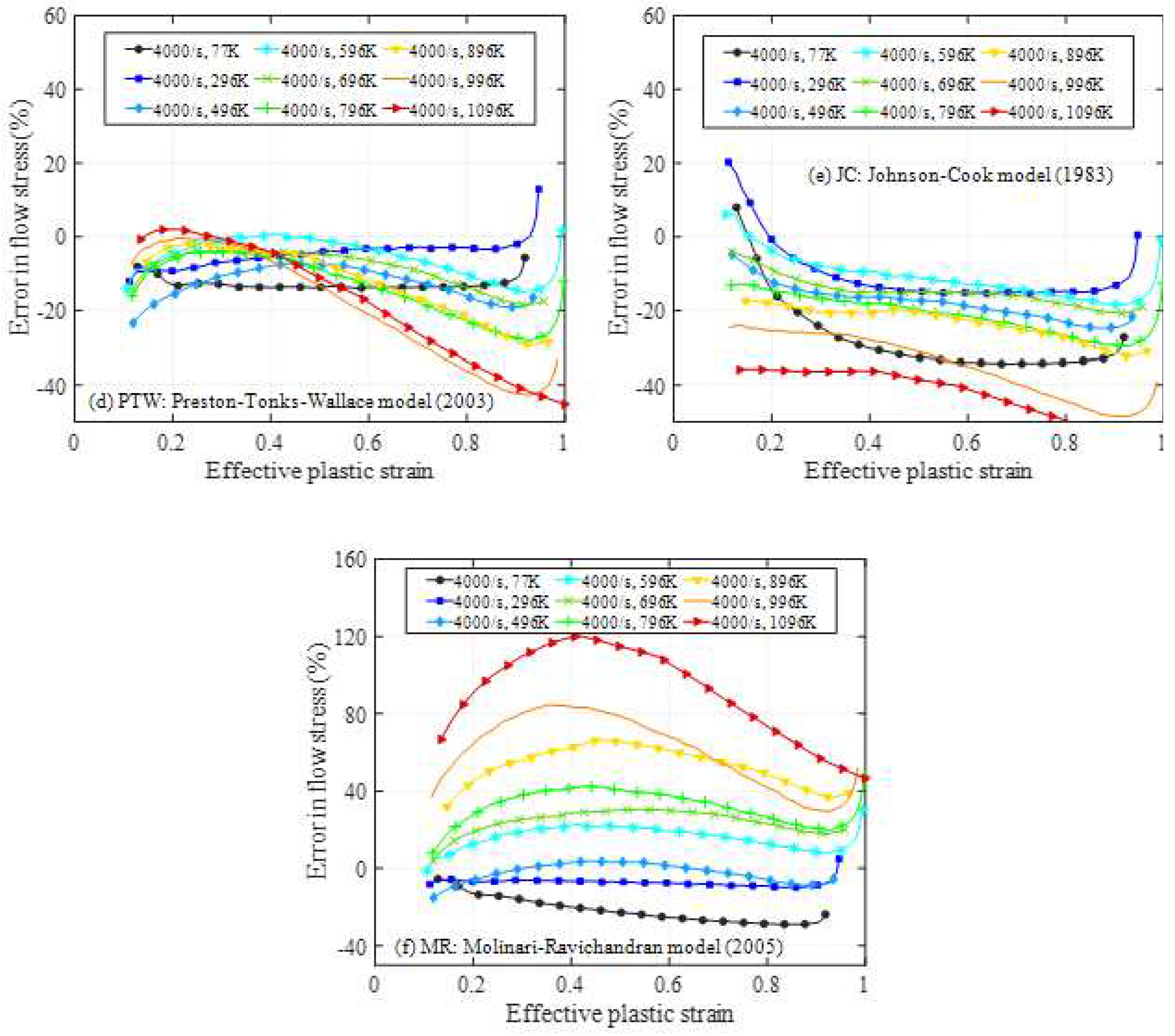
4.2. Comprehensive error analysis
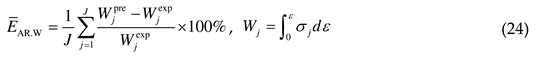

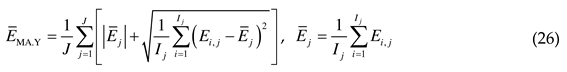
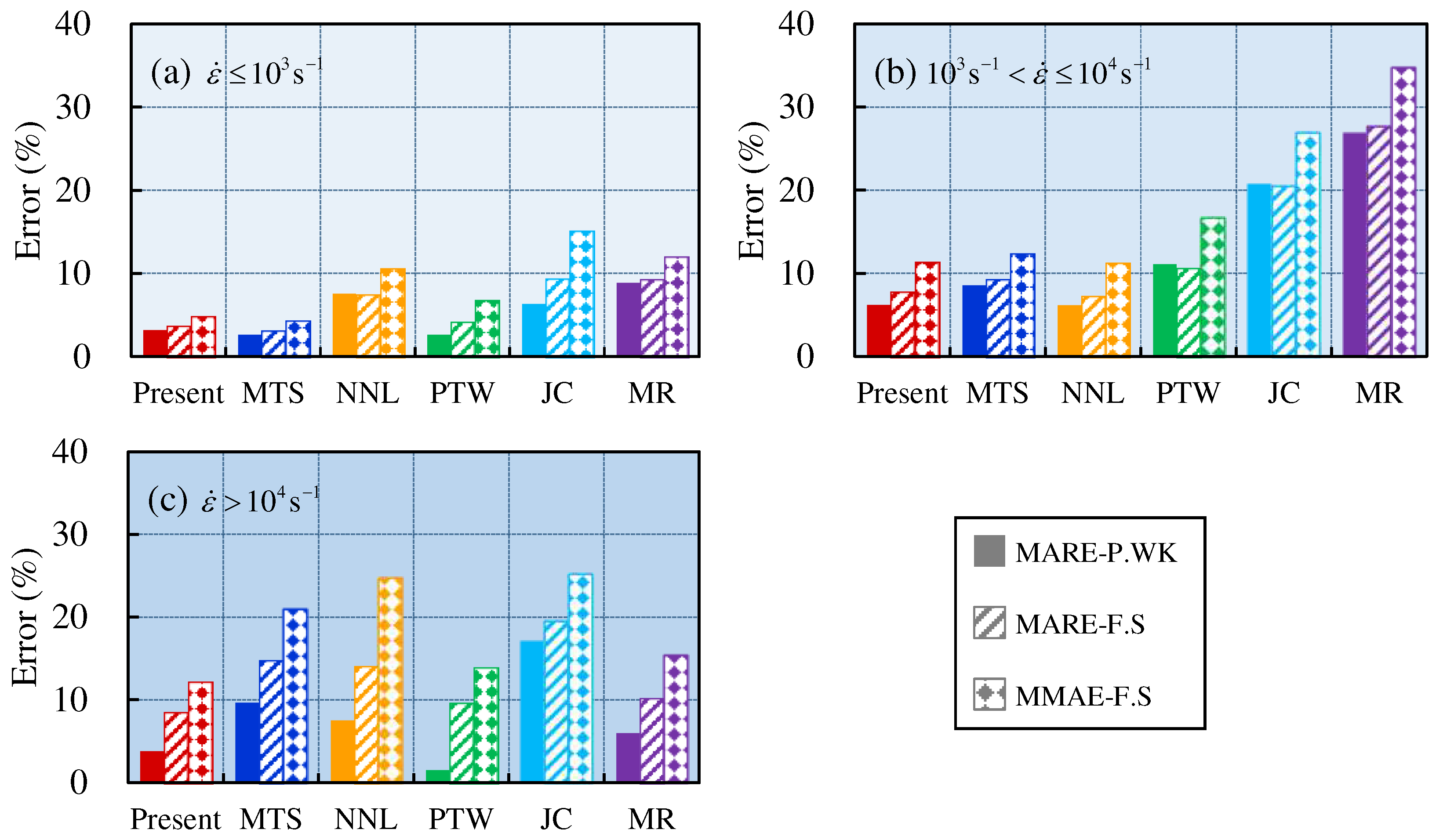
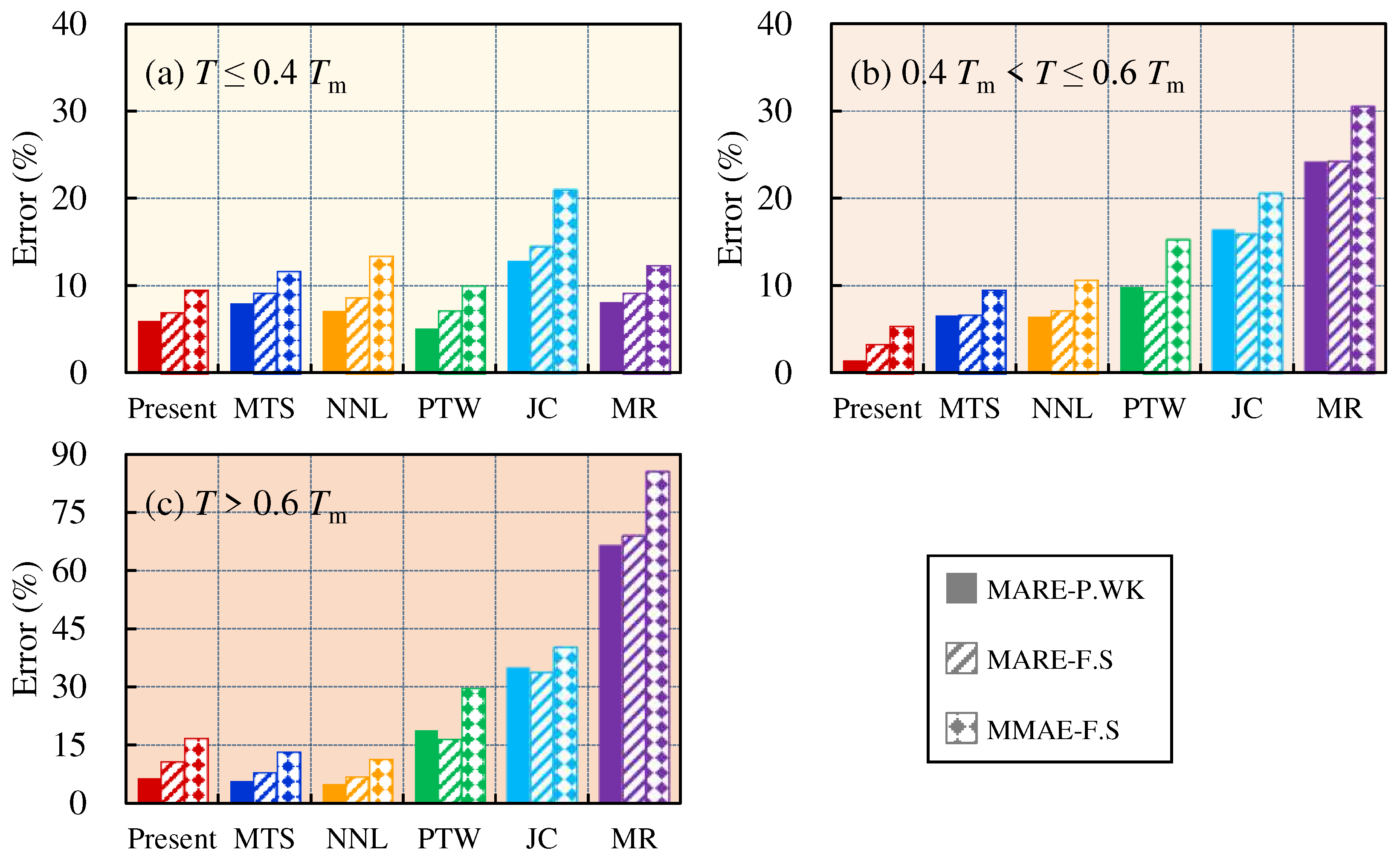
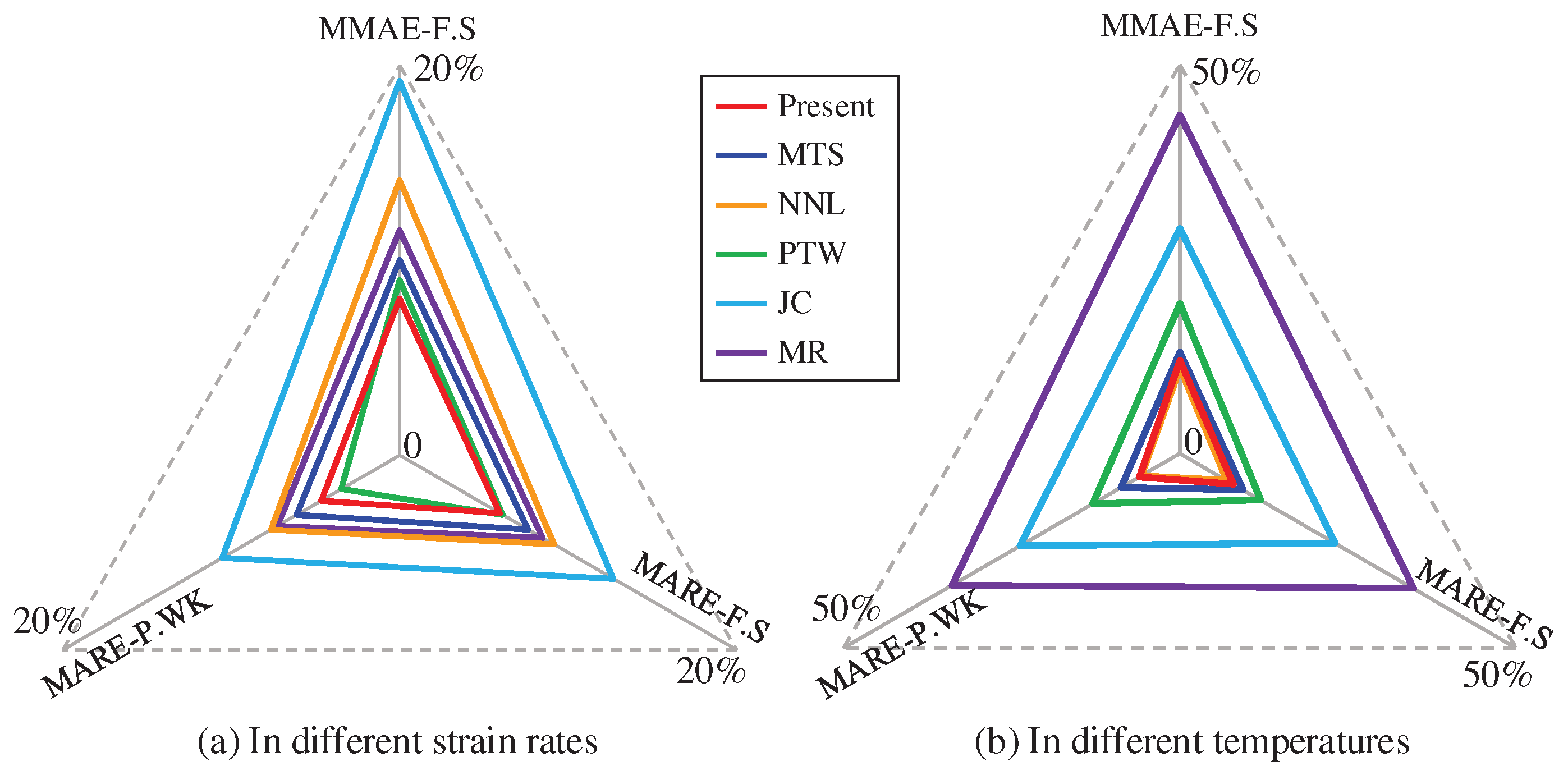
5. Conclusion
Acknowledgments
Appendix A. Source of experimental data
| Condition | ε | (s-1) | T (K) | References |
| Fixed strain. Room temperature. |
0.15 | ∈ 6×(104, 105) | 300 | [40,41] Tong and Clifton (1992, 1998) |
| 0.05, 0.10, 0.15, 0.20. |
∈ (10-4, 2×104) | Preston, Tonks, and Wallace (2003) |
| Condition | (s-1) | T (K) | References |
| Room temperature. Wide range of strain rates. |
0.015, 8500, 640000. | 295 | Follansbee and Kocks (1988) |
| 0.001, 0.1, 4000, 8000. | 296 | Nemat-Nasser and Li (1998) | |
| 0.0004, 6000. | 298 | Tanner and McGinty (1999) | |
| 13000 | 296 | Jing and Wang (2022) |
| Condition | (s-1) | T (K) | References |
| Fixed strain rate. Wide range of temperatures. |
4000 | 77, 296, 496, 596, 696, 796, 896, 996, 1096. |
Nemat-Nasser and Li (1998) |
References
- Armstrong RW, Walley SM. High strain rate properties of metals and alloys. International Materials Reviews 2008;53:105–28. [CrossRef]
- Gao CY, Zhang LC. Constitutive modelling of plasticity of fcc metals under extremely high strain rates. International Journal of Plasticity 2012;32–33:121–33. [CrossRef]
- Salvado F, Teixeira-Dias F, Walley SM, Lea L, Cardoso J. A review on the strain rate dependency of the dynamic viscoplastic response of FCC metals. Progress in Materials Science 2017;88. [CrossRef]
- Jia X, Hao K, Luo Z, Fan Z. Plastic Deformation Behavior of Metal Materials: A Review of Constitutive Models. METALS 2022;12. [CrossRef]
- Johnson GR, Cook WH. A constitutive model and data for materials subjected to large strains, Proceedings 7th International Symposium on Ballistics, The Hague: 1983, p. 541–7.
- Rule WK, Jones SE. A revised form for the Johnson–Cook strength model. International Journal of Impact Engineering 1998;21:609–24. [CrossRef]
- Wang J, Guo W-G, Li P, Zhou P. Modified Johnson-Cook description of wide temperature and strain rate measurements made on a nickel-base superalloy. Materials at High Temperatures 2017;34:157–65. [CrossRef]
- Wang Y, Zeng X, Chen H, Yang X, Wang F, Zeng L. Modified Johnson-Cook constitutive model of metallic materials under a wide range of temperatures and strain rates. Results in Physics 2021;27:104498. [CrossRef]
- Kumar Reddy Sirigiri V, Yadav Gudiga V, Shankar Gattu U, Suneesh G, Mohan Buddaraju K. A review on Johnson Cook material model. Materials Today: Proceedings 2022;62:3450–6. [CrossRef]
- Follansbee PS, Kocks UF. A constitutive description of the deformation of copper based on the use of the mechanical threshold stress as an internal state variable. Acta Metallurgica 1988;36:81–93. [CrossRef]
- Mecking H, Kocks UF. Kinetics of flow and strain-hardening. Acta Metallurgica 1981;29:1865–75. [CrossRef]
- Klepaczko, JR. Physical-state variables — the key to constitutive modeling in dynamic plasticity. Nuclear Engineering and Design 1991;127:103–15. [CrossRef]
- Mohamadnejad S, Basti A, Ansari R. Analyses of Dislocation Effects on Plastic Deformation. Multiscale Sci Eng 2020;2:69–89. [CrossRef]
- Zerilli FJ, Armstrong RW. Dislocation-mechanics-based constitutive relations for material dynamics calculations. Journal of Applied Physics 1987;61:1816–25. [CrossRef]
- Nemat-Nasser S, Li Y. Flow stress of f.c.c. polycrystals with application to OFHC Cu. Acta Materialia 1998;46:565–77. [CrossRef]
- Gao CY, Zhang LC. A constitutive model for dynamic plasticity of FCC metals. Materials Science and Engineering: A 2010;527:3138–43. [CrossRef]
- Austin RA, McDowell D. A dislocation-based constitutive model for viscoplastic deformation of fcc metals at very high strain rates. International Journal of Plasticity 2011;27:1–24. [CrossRef]
- Austin RA, McDowell DL. Parameterization of a rate-dependent model of shock-induced plasticity for copper, nickel, and aluminum. International Journal of Plasticity 2012;32–33:134–54. [CrossRef]
- Preston D, Tonks D, Wallace D. Model of plastic deformation for extreme loading conditions. Journal of Applied Physics 2003;93. [CrossRef]
- Molinari A, Ravichandran G. Constitutive modeling of high-strain-rate deformation in metals based on the evolution of an effective microstructural length. Mechanics of Materials 2005;37:737–52. [CrossRef]
- Durrenberger L, Molinari A, Rusinek A. Internal variable modeling of the high strain-rate behavior of metals with applications to multiphase steels. Materials Science and Engineering: A 2008;478:297–304. [CrossRef]
- Durrenberger L, Molinari A. Modeling of Temperature and Strain-Rate Effects in Metals Using an Internal Variable Model. Experimental Mechanics 2009;49:247–55. [CrossRef]
- Kocks UF, Argon AS, Ashby MF. Thermodynamics and kinetics of slip. Progress in Materials Science 1975;19:141–5.
- Wang ZQ, Beyerlein IJ, LeSar R. Slip band formation and mobile dislocation density generation in high rate deformation of single fcc crystals. Philosophical Magazine 2008;88:1321–43. [CrossRef]
- Gil Sevillano J, van Houtte P, Aernoudt E. Large strain work hardening and textures. Progress in Materials Science 1980;25:69–134. [CrossRef]
- Nes, E. Modelling of work hardening and stress saturation in FCC metals. Progress in Materials Science 1997;41:129–93. [CrossRef]
- Staker MR, Holt DL. The dislocation cell size and dislocation density in copper deformed at temperatures between 25 and 700°C. Acta Metallurgica 1972;20:569–79. [CrossRef]
- Estrin Y, Kubin LP. Local strain hardening and nonuniformity of plastic deformation. Acta Metallurgica 1986;34:2455–64. [CrossRef]
- Voce, E. The relationship between stress and strain for homogeneous deformations 1948.
- Hall, EO. The Deformation and Ageing of Mild Steel: III Discussion of Results. Proc Phys Soc B 1951;64:747. [CrossRef]
- Armstrong, RW. The (cleavage) strength of pre-cracked polycrystals. Engineering Fracture Mechanics 1987;28:529–38. [CrossRef]
- Meyers MA, Andrade UR, Chokshi AH. The effect of grain size on the high-strain, high-strain-rate behavior of copper. Metall Mater Trans A 1995;26:2881–93. [CrossRef]
- Yan S, Yang H, Li H, Yao X. A unified model for coupling constitutive behavior and micro-defects evolution of aluminum alloys under high-strain-rate deformation. International Journal of Plasticity 2016;85:203–29. [CrossRef]
- Kocks F, Mecking H. Physics and phenomenology of strain hardening: The FCC case. Progress in Materials Science 2003;48:171–273. [CrossRef]
- Nadal M-H, Le Poac P. Continuous model for the shear modulus as a function of pressure and temperature up to the melting point: Analysis and ultrasonic validation. Journal of Applied Physics 2003;93:2472–80. [CrossRef]
- Klepaczko J, Rusinek A, Rodríguez-Martínez JA, Pęcherski R, Arias A. Modelling of thermo-viscoplastic behaviour of DH36 and Weldox 460-E structural steels at wide ranges of strain rates and temperatures, comparison of constitutive relations for impact problems. Mechanics of Materials - MECH MATER 2009;41:599–621. [CrossRef]
- Baig M, Khan AS, Choi S-H, Jeong A. Shear and multiaxial responses of oxygen free high conductivity (OFHC) copper over wide range of strain-rates and temperatures and constitutive modeling. International Journal of Plasticity 2013;40:65–80. [CrossRef]
- Mason JJ, Rosakis AJ, Ravichandran G. On the strain and strain rate dependence of the fraction of plastic work converted to heat: an experimental study using high speed infrared detectors and the Kolsky bar. Mechanics of Materials 1994;17:135–45. [CrossRef]
- Voyiadjis GZ, Abed FH. Effect of dislocation density evolution on the thermomechanical response of metals with different crystal structures at low and high strain rates and temperatures. Archives of Mechanics 2005;57:299–343. [CrossRef]
- Humphreys FJ, Hatherly M. Recrystallization and related annealing phenomena. 2nd ed. Amsterdam ; Boston : Elsevier; 2004.
- Huang M, Rivera-Díaz-del-Castillo PEJ, Bouaziz O, van der Zwaag S. A constitutive model for high strain rate deformation in FCC metals based on irreversible thermodynamics. Mechanics of Materials 2009;41:982–8. [CrossRef]
- Banerjee, B. Taylor impact tests: Detailed report 2005.
- Banerjee, B. An evaluation of plastic flow stress models for the simulation of high-temperature and high-strain-rate deformation of metals. 2005. [CrossRef]
- Steinberg DJ, Lund CM. A constitutive model for strain rates from 10−4 to 106 s−1. Journal of Applied Physics 1989;65:1528–33. [CrossRef]
- Nix WD, Gibeling JC, Hughes DA. Time-dependent deformation of metals. Metallurgical Transactions A 1985;16:2215–26. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





