Submitted:
09 September 2023
Posted:
12 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals and apparatus
2.2. Preparation of GO and RGO
2.3. Preparation of magnetic MgFe2O nanoparticles
2.4. Preparation of superhydrophobic magnetic PU sponges
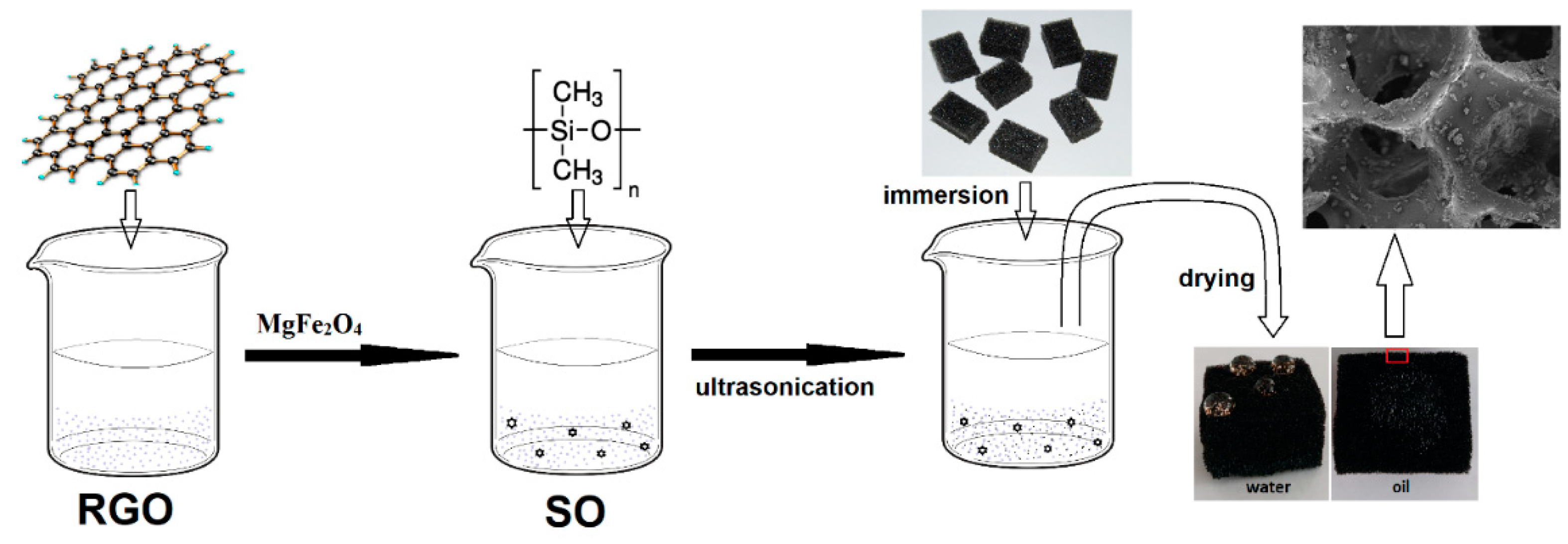
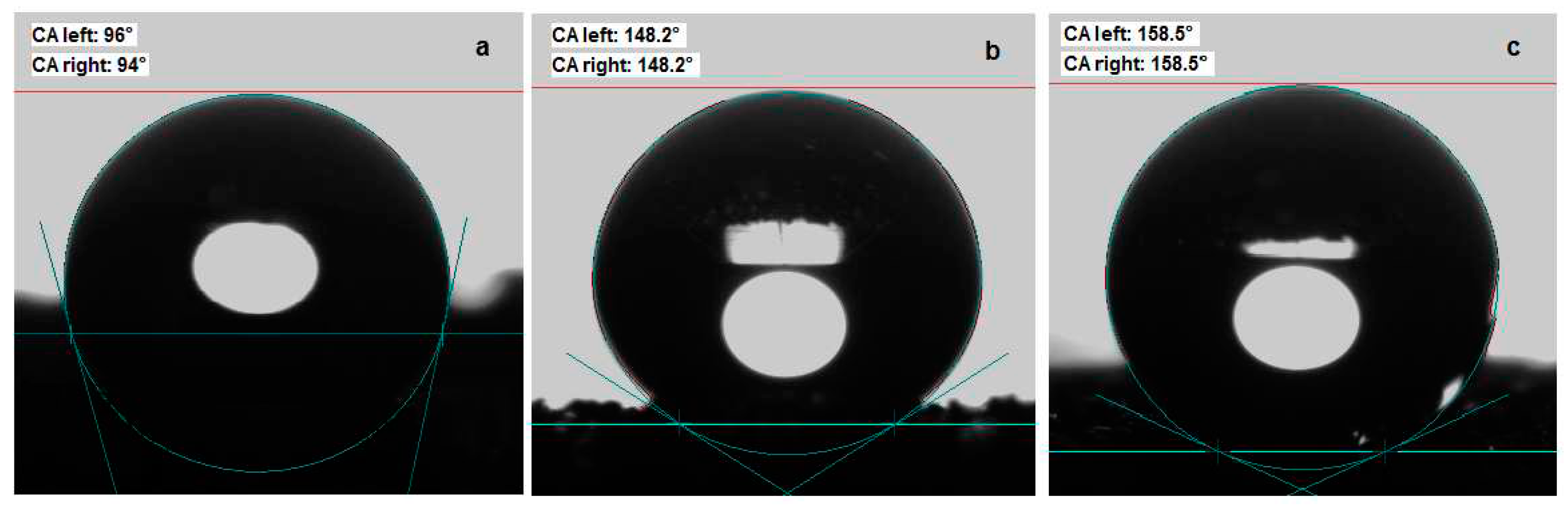
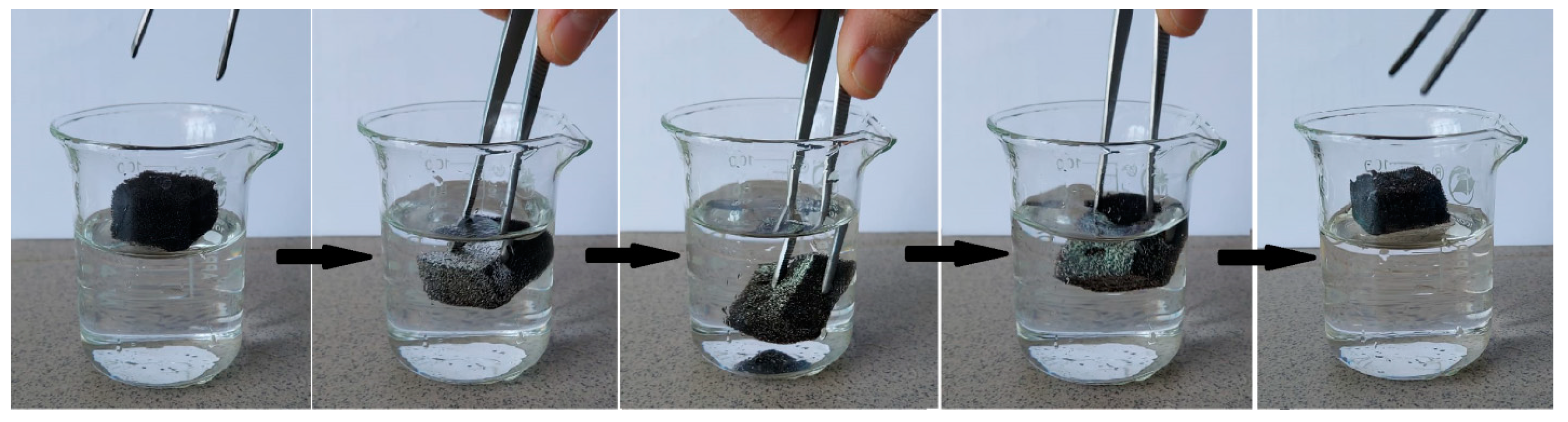
2.5. Studies of superhydrophobic/superoleophilic properties
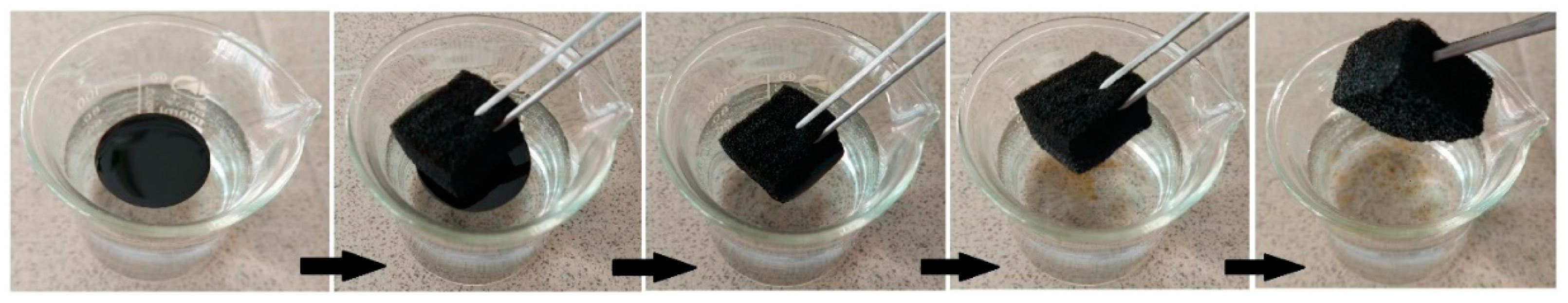
2.6. Testing oil/water separation property and oil absorption capacity
3. Results and discussion
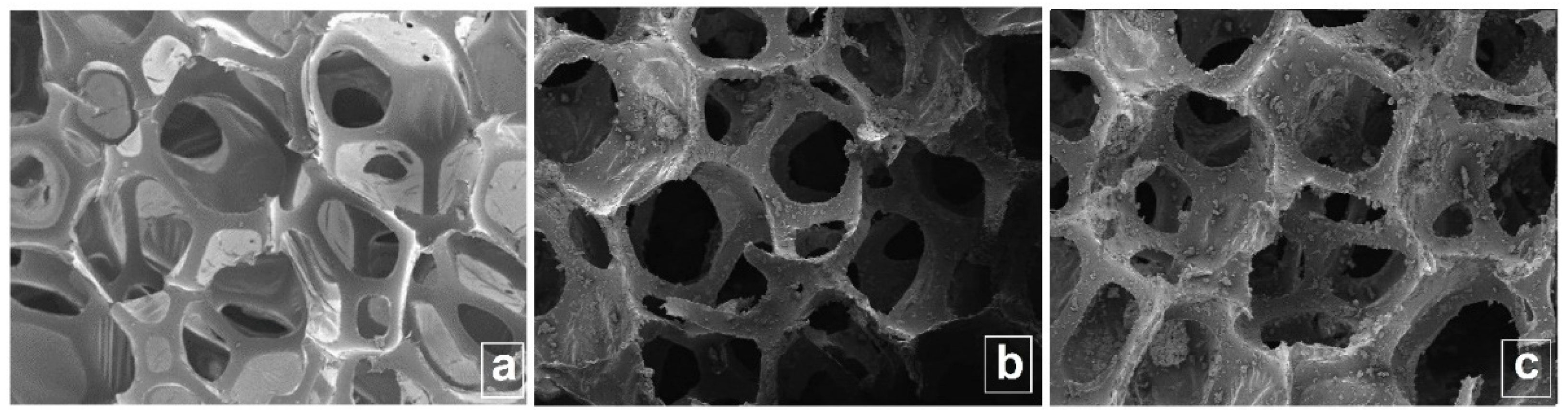
3.1. SEM morphology analysis of new superhydrophobic magnetic sponges PU/MgFe2O4/RGO/SO
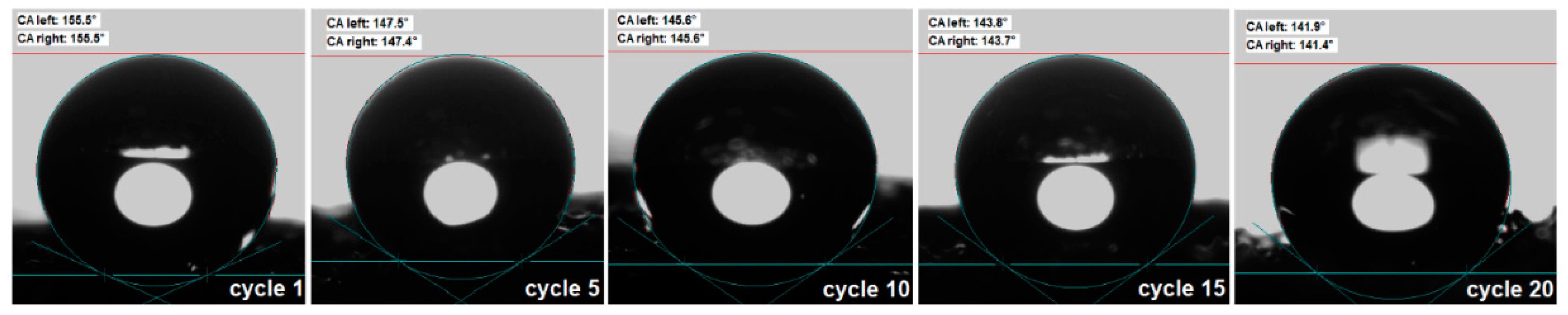
3.1. Hydrophobic and oleophilic properties of new superhydrophobic magnetic sponges PU/MgFe2O4/SO, PU/MgFe2O4/RGO/SO
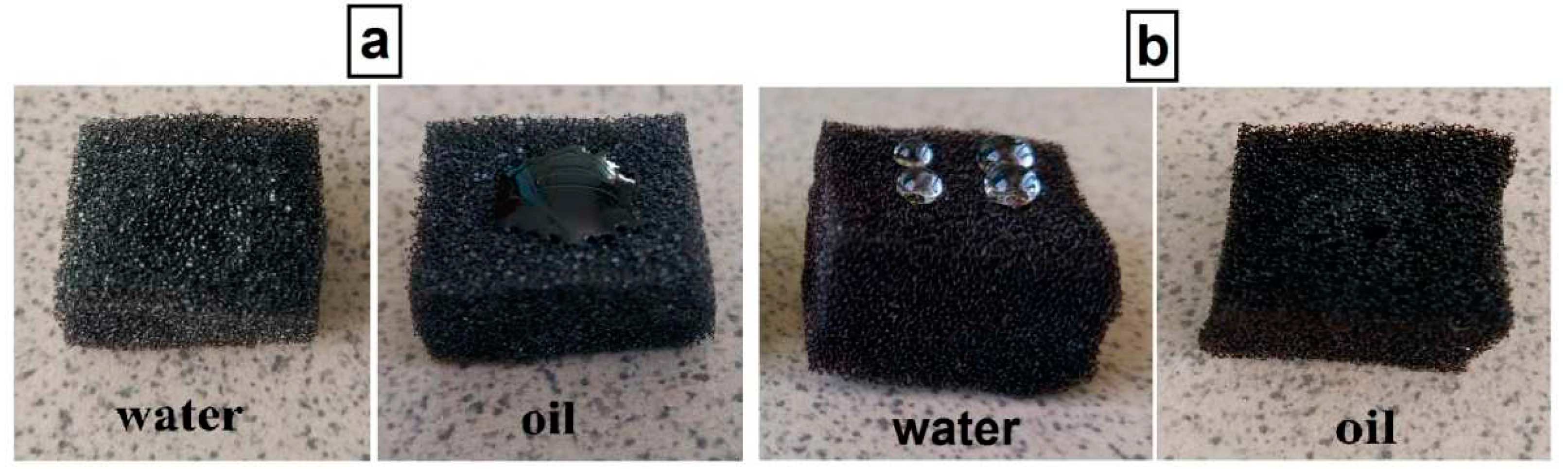
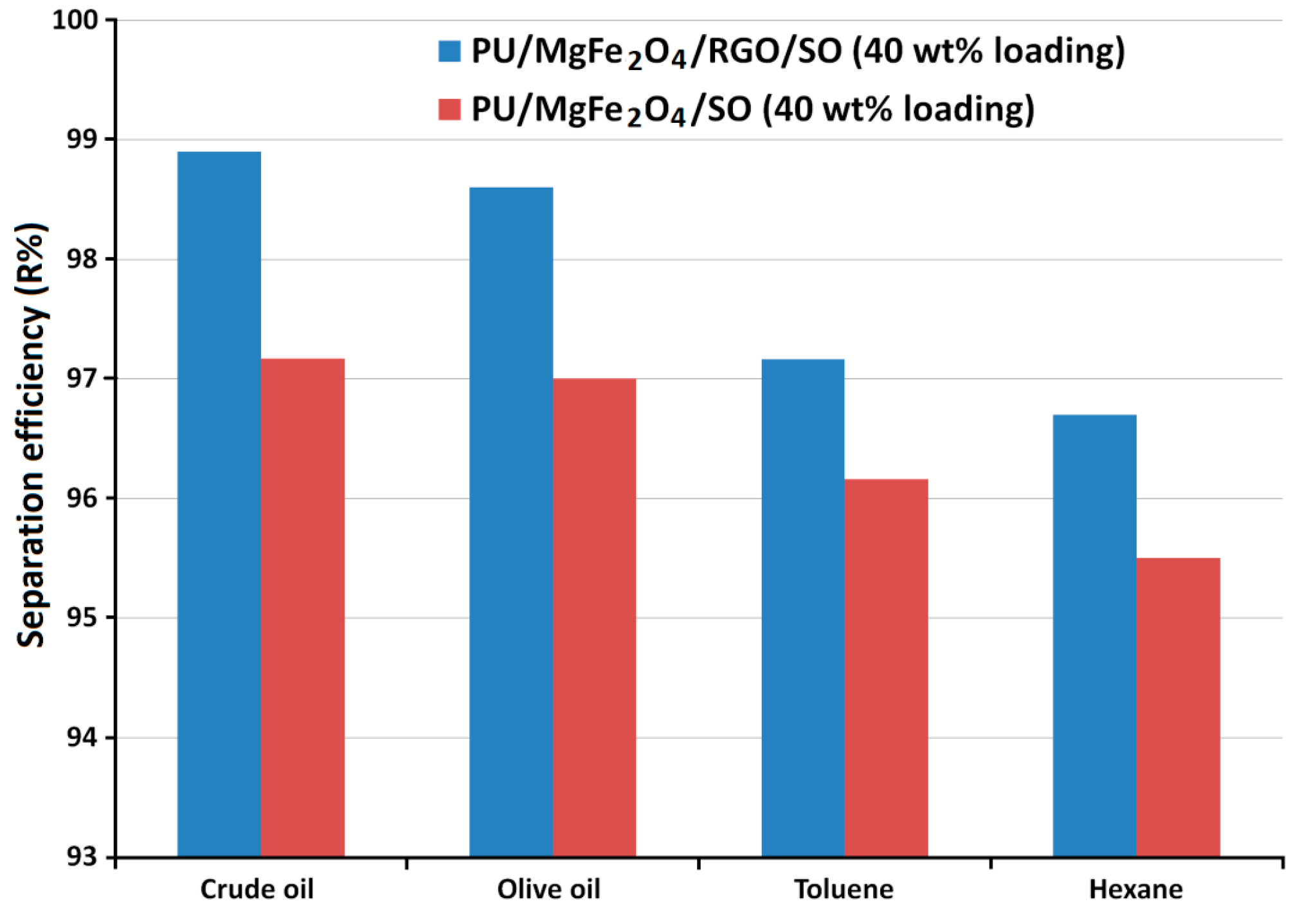
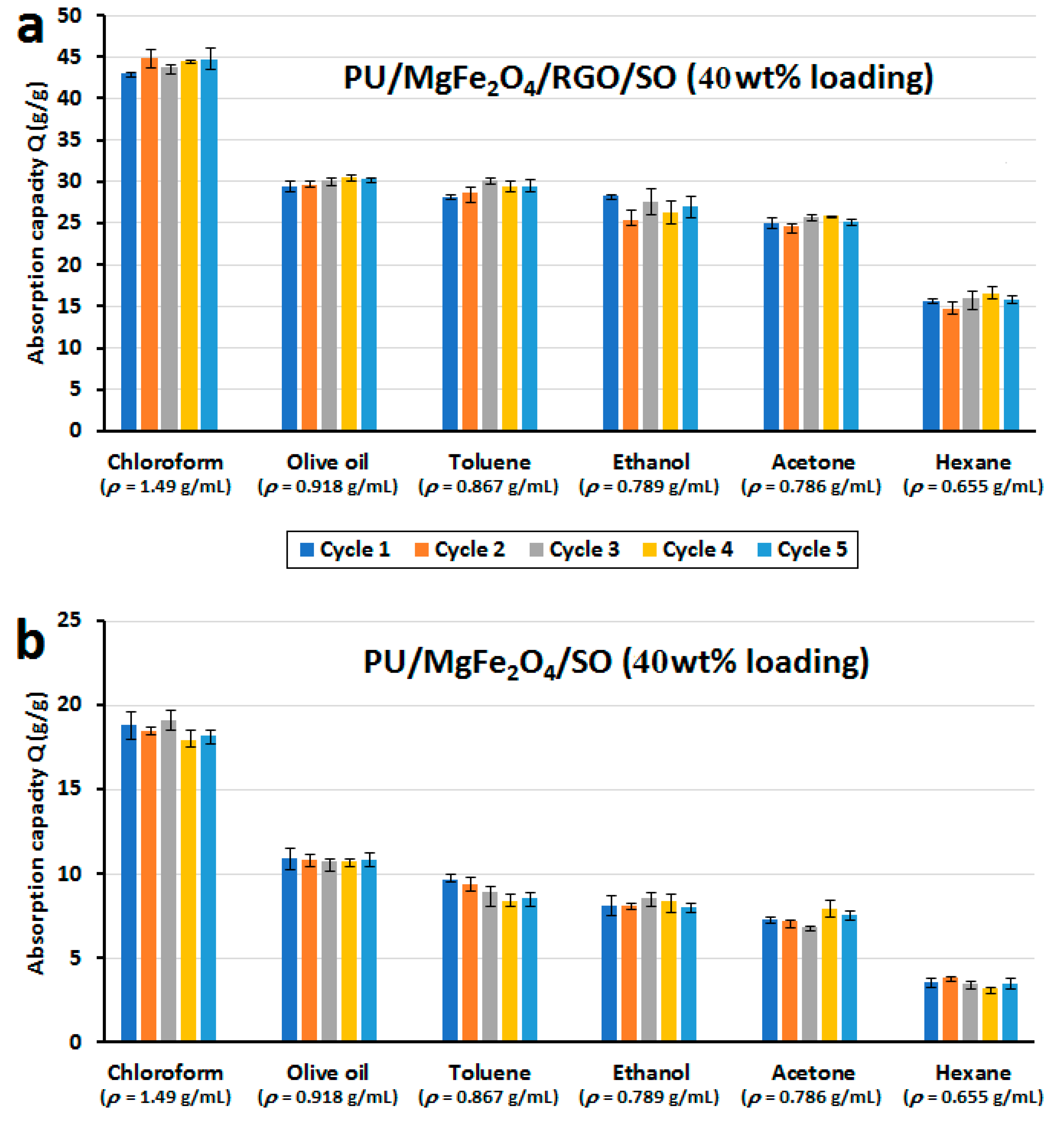
3.3. Testing oil/water separation property and oil absorption capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barbier, E.B.; Moreno-Mateos, D.; Rogers, A.D.; Aronson, J.; Pendleton, L.; Danovaro, R.; Henry, L.-A.; Morato, T.; Ardron, J.; Van Dover, C.L. Ecology: Protect the Deep Sea. Nature 2014, 505, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Liu, Q.; Zhou, J.; Ju, P.; Waterhouse, G.I.N.; Zhou, S.; Ai, S. Superhydrophobic Sponge Containing Silicone Oil-Modified Layered Double Hydroxide Sheets for Rapid Oil-Water Separations. Colloids Surf A Physicochem Eng Asp 2019, 570, 339–346. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Xu, Q.; Cai, M.; Shi, Q.; Gao, J. Highly Efficient Reusable Superhydrophobic Sponge Prepared by a Facile, Simple and Cost Effective Biomimetic Bonding Method for Oil Absorption. Sci Rep 2021, 11, 11960. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Zhao, H.-Y.; Zhu, H.-W.; Huang, J.; Shi, L.-A.; Yu, S.-H. Advanced Sorbents for Oil-Spill Cleanup: Recent Advances and Future Perspectives. Advanced Materials 2016, 28, 10459–10490. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Meng, K.; Ding, K.; Wang, Y. A Superhydrophobic Sponge with Hierarchical Structure as an Efficient and Recyclable Oil Absorbent. Chempluschem 2015, 80, 1435–1439. [Google Scholar] [CrossRef] [PubMed]
- Guselnikova, O.; Barras, A.; Addad, A.; Sviridova, E.; Szunerits, S.; Postnikov, P.; Boukherroub, R. Magnetic Polyurethane Sponge for Efficient Oil Adsorption and Separation of Oil from Oil-in-Water Emulsions. Sep Purif Technol 2020, 240, 116627. [Google Scholar] [CrossRef]
- Yu, T.; Halouane, F.; Mathias, D.; Barras, A.; Wang, Z.; Lv, A.; Lu, S.; Xu, W.; Meziane, D.; Tiercelin, N.; et al. Preparation of Magnetic, Superhydrophobic/Superoleophilic Polyurethane Sponge: Separation of Oil/Water Mixture and Demulsification. Chemical Engineering Journal 2020, 384, 123339. [Google Scholar] [CrossRef]
- Nandwana, V.; Ribet, S.M.; Reis, R.D.; Kuang, Y.; More, Y.; Dravid, V.P. OHM Sponge: A Versatile, Efficient, and Ecofriendly Environmental Remediation Platform. Ind Eng Chem Res 2020, 59, 10945–10954. [Google Scholar] [CrossRef]
- Yang, L.; Wu, S.; Chen, J.P. Modification of Activated Carbon by Polyaniline for Enhanced Adsorption of Aqueous Arsenate. Ind Eng Chem Res 2007, 46, 2133–2140. [Google Scholar] [CrossRef]
- Diaz De Tuesta, J.L.; Roman, F.F.; Marques, V.C.; Silva, A.S.; Silva, A.P.F.; Bosco, T.C.; Shinibekova, A.A.; Aknur, S.; Kalmakhanova, M.S.; Massalimova, B.K.; et al. Performance and Modeling of Ni(II) Adsorption from Low Concentrated Wastewater on Carbon Microspheres Prepared from Tangerine Peels by FeCl3-Assisted Hydrothermal Carbonization. J Environ Chem Eng 2022, 10, 108143. [Google Scholar] [CrossRef]
- Huang, S.; Shi, J. Monolithic Macroporous Carbon Materials as High-Performance and Ultralow-Cost Sorbents for Efficiently Solving Organic Pollution. Ind Eng Chem Res 2014, 53, 4888–4893. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Wang, S.; Shi, Y.; Li, J. Fabrication of Coral-like Superhydrophobic Coating on Filter Paper for Water–Oil Separation. Appl Surf Sci 2012, 261, 764–769. [Google Scholar] [CrossRef]
- Wen, Q.; Di, J.; Jiang, L.; Yu, J.; Xu, R. Zeolite-Coated Mesh Film for Efficient Oil–Water Separation. Chem. Sci. 2013, 4, 591–595. [Google Scholar] [CrossRef]
- Maria, A.; Zhuldu, K.; Serge, B. Use of Sorbents to Improve Water Quality in the Production of Reconstituted Dairy Products. Emir J Food Agric 2022. [Google Scholar] [CrossRef]
- Wang, T.; Bao, Y.; Gao, Z.; Wu, Y.; Wu, L. Synthesis of Mesoporous Silica-Shell/Oil-Core Microspheres for Common Waterborne Polymer Coatings with Robust Superhydrophobicity. Prog Org Coat 2019, 132, 275–282. [Google Scholar] [CrossRef]
- Wang, S.; Li, M.; Lu, Q. Filter Paper with Selective Absorption and Separation of Liquids That Differ in Surface Tension. ACS Appl Mater Interfaces 2010, 2, 677–683. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Z.; Ge, B.; Men, X.; Zhou, X.; Xue, Q. A Versatile Approach to Produce Superhydrophobic Materials Used for Oil–Water Separation. J Colloid Interface Sci 2014, 432, 105–108. [Google Scholar] [CrossRef]
- Kim, H.J.; Han, S.W.; Kim, J.H.; Seo, H.O.; Kim, Y.D. Oil Absorption Capacity of Bare and PDMS-Coated PET Non-Woven Fabric; Dependency of Fiber Strand Thickness and Oil Viscosity. Current Applied Physics 2018, 18, 369–376. [Google Scholar] [CrossRef]
- Drelich, J.; Chibowski, E.; Meng, D.D.; Terpilowski, K. Hydrophilic and Superhydrophilic Surfaces and Materials. Soft Matter 2011, 7, 9804. [Google Scholar] [CrossRef]
- Piperopoulos, E.; Calabrese, L.; Mastronardo, E.; Abdul Rahim, S.H.; Proverbio, E.; Milone, C. Assessment of Sorption Kinetics of Carbon Nanotube-based Composite Foams for Oil Recovery Application. J Appl Polym Sci 2018, 47374. [Google Scholar] [CrossRef]
- Wu, J.; Chen, J.; Qasim, K.; Xia, J.; Lei, W.; Wang, B. A Hierarchical Mesh Film with Superhydrophobic and Superoleophilic Properties for Oil and Water Separation. Journal of Chemical Technology & Biotechnology 2012, 87, 427–430. [Google Scholar] [CrossRef]
- Wang, C.; Yao, T.; Wu, J.; Ma, C.; Fan, Z.; Wang, Z.; Cheng, Y.; Lin, Q.; Yang, B. Facile Approach in Fabricating Superhydrophobic and Superoleophilic Surface for Water and Oil Mixture Separation. ACS Appl Mater Interfaces 2009, 1, 2613–2617. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Chu, Y.; Wang, Z.; Chen, N.; Lin, L.; Liu, F.; Pan, Q. Robust Superhydrophobic Polyurethane Sponge as a Highly Reusable Oil-Absorption Material. J Mater Chem A Mater 2013, 1, 5386. [Google Scholar] [CrossRef]
- Kong, S.M.; Han, Y.; Won, N.-I.; Na, Y.H. Polyurethane Sponge with a Modified Specific Surface for Repeatable Oil–Water Separation. ACS Omega 2021, 6, 33969–33975. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Tai, N.-H.; Lee, S.-B.; Kuo, W.-S. Superhydrophobic and Superoleophilic Properties of Graphene-Based Sponges Fabricated Using a Facile Dip Coating Method. Energy Environ Sci 2012, 5, 7908. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of Graphene-Based Nanosheets via Chemical Reduction of Exfoliated Graphite Oxide. Carbon N Y 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Ali, I.; Basheer, A.A.; Mbianda, X.Y.; Burakov, A.; Galunin, E.; Burakova, I.; Mkrtchyan, E.; Tkachev, A.; Grachev, V. Graphene Based Adsorbents for Remediation of Noxious Pollutants from Wastewater. Environ Int 2019, 127, 160–180. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J Am Chem Soc 1958, 80, 1339–1339. [Google Scholar] [CrossRef]
- Kudaibergenova, R.; Ualibek, O.; Sugurbekov, E.; Demeuova, G.; Frochot, C.; Acherar, S.; Sugurbekova, G. Reduced Graphene Oxide-Based Superhydrophobic Magnetic Nanomaterial as High Selective and Recyclable Sorbent for Oil/Organic Solvent Wastewater Treatment. International Journal of Environmental Science and Technology 2022, 19, 8491–8506. [Google Scholar] [CrossRef]
- Zampiva, R.Y.S.; Kaufmann Junior, C.G.; Pinto, J.S.; Panta, P.C.; Alves, A.K.; Bergmann, C.P. 3D CNT Macrostructure Synthesis Catalyzed by MgFe2O4 Nanoparticles—A Study of Surface Area and Spinel Inversion Influence. Appl Surf Sci 2017, 422, 321–330. [Google Scholar] [CrossRef]
- Zhu, Q.; Pan, Q.; Liu, F. Facile Removal and Collection of Oils from Water Surfaces through Superhydrophobic and Superoleophilic Sponges. The Journal of Physical Chemistry C 2011, 115, 17464–17470. [Google Scholar] [CrossRef]
- Liang, L.; Dong, Y.; Liu, Y.; Meng, X. Modification of Polyurethane Sponge Based on the Thiol–Ene Click Reaction and Its Application for Oil/Water Separation. Polymers (Basel) 2019, 11, 2072. [Google Scholar] [CrossRef]
- Kim, H.J.; Han, S.W.; Kim, J.H.; Seo, H.O.; Kim, Y.D. Oil Absorption Capacity of Bare and PDMS-Coated PET Non-Woven Fabric; Dependency of Fiber Strand Thickness and Oil Viscosity. Current Applied Physics 2018, 18, 369–376. [Google Scholar] [CrossRef]
- Wang, N.; Deng, Z. Synthesis of Magnetic, Durable and Superhydrophobic Carbon Sponges for Oil/Water Separation. Mater Res Bull 2019, 115, 19–26. [Google Scholar] [CrossRef]
- Liu, D.; Wang, S.; Wu, T.; Li, Y. A Robust Superhydrophobic Polyurethane Sponge Loaded with Multi-Walled Carbon Nanotubes for Efficient and Selective Oil-Water Separation. Nanomaterials 2021, 11, 3344. [Google Scholar] [CrossRef]
- Liang, L.; Xue, Y.; Wu, Q.; Dong, Y.; Meng, X. Self-Assembly Modification of Polyurethane Sponge for Application in Oil/Water Separation. RSC Adv 2019, 9, 40378–40387. [Google Scholar] [CrossRef] [PubMed]
- Jamsaz, A.; Goharshadi, E.K.; Barras, A.; Ifires, M.; Szunerits, S.; Boukherroub, R. Magnetically Driven Superhydrophobic/Superoleophilic Graphene-Based Polyurethane Sponge for Highly Efficient Oil/Water Separation and Demulsification. Sep Purif Technol 2021, 274, 118931. [Google Scholar] [CrossRef]
- Sultanov, F.R.; Daulbayev, Ch.; Bakbolat, B.; Mansurov, Z.A.; Urazgaliyeva, A.A.; Ebrahim, R.; Pei, S.S.; Huang, K.-P. Microwave-Enhanced Chemical Vapor Deposition Graphene Nanoplatelets-Derived 3D Porous Materials for Oil/Water Separation. Carbon Letters 2020, 30, 81–92. [Google Scholar] [CrossRef]
- Turco, A.; Malitesta, C.; Barillaro, G.; Greco, A.; Maffezzoli, A.; Mazzotta, E. A Magnetic and Highly Reusable Macroporous Superhydrophobic/Superoleophilic PDMS/MWNT Nanocomposite for Oil Sorption from Water. J Mater Chem A Mater 2015, 3, 17685–17696. [Google Scholar] [CrossRef]
- Choi, S.-J.; Kwon, T.-H.; Im, H.; Moon, D.-I.; Baek, D.J.; Seol, M.-L.; Duarte, J.P.; Choi, Y.-K. A Polydimethylsiloxane (PDMS) Sponge for the Selective Absorption of Oil from Water. ACS Appl Mater Interfaces 2011, 3, 4552–4556. [Google Scholar] [CrossRef]
- Wu, L.; Li, L.; Li, B.; Zhang, J.; Wang, A. Magnetic, Durable, and Superhydrophobic Polyurethane@Fe 3 O 4 @SiO 2 @Fluoropolymer Sponges for Selective Oil Absorption and Oil/Water Separation. ACS Appl Mater Interfaces 2015, 7, 4936–4946. [Google Scholar] [CrossRef] [PubMed]
- Parsaie, A.; Mohammadi-Khanaposhtani, M.; Riazi, M.; Tamsilian, Y. Magnesium Stearate-Coated Superhydrophobic Sponge for Oil/Water Separation: Synthesis, Properties, Application. Sep Purif Technol 2020, 251, 117105. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, R.; Ge, W.; Xie, A.; Chang, Z.; Tian, S.; Zhou, Z.; Yan, Y. 3D Macroscopic Superhydrophobic Magnetic Porous Carbon Aerogel Converted from Biorenewable Popcorn for Selective Oil-Water Separation. Mater Des 2018, 139, 122–131. [Google Scholar] [CrossRef]
- He, Z.; Wu, H.; Shi, Z.; Kong, Z.; Ma, S.; Sun, Y.; Liu, X. Facile Preparation of Robust Superhydrophobic/Superoleophilic TiO 2 -Decorated Polyvinyl Alcohol Sponge for Efficient Oil/Water Separation. ACS Omega 2022, 7, 7084–7095. [Google Scholar] [CrossRef]
- Wang, C.-F.; Lin, S.-J. Robust Superhydrophobic/Superoleophilic Sponge for Effective Continuous Absorption and Expulsion of Oil Pollutants from Water. ACS Appl Mater Interfaces 2013, 5, 8861–8864. [Google Scholar] [CrossRef] [PubMed]

| Material | Adsorbed organics | Q(g/g) | References |
|---|---|---|---|
| PU/MgFe2O4/RGO/SO sponge | Crude oil, olive oil, chloroform, toluene, ethanol, acetone, hexane | Present work | |
| PU/MgFe2O4/SO sponge | Olive oil, chloroform, toluene, ethanol, acetone, hexane | 3,5-19 | Present work |
| Fe3O4-PDMS/MWNTs sponge | Dichloromethane, petroleum ether, hexane, chloroform, tetrahydrofuran, toluene, gasoline | 8,5-20 | [39] |
| PDMS sponge | Dichloromethane, toluene, transformer oil | 4,3-11 | [40] |
| PU Sponge@Fe3O @SiO4 @Fluoropolymer sponge | Petrol, Toluene, Chloroform | 17–23 | [41] |
| PU Sponge@Magnesium Stearate@Phenol formaldehyde Resin | Motor oil, Food oil, Paraffin, Gasoline,n-Hexane, Toluene | 19–38 | [42] |
| 3D macroscopic superhydrophobic magnetic porous carbon aerogel | engine oil, chloroethane and corn oil | 10,02−10,83 | [43] |
| TiO2–PVA sponge | Polyethylene glycol, CCl4, liquid paraffin, N, N-dimethylformamide, ethanol, edible oil, and n-hexane | 4,3 -13,6 | [44] |
| CNT/PDMS-coated PU sponge | Soybean oil, used motor oil, diesel oil, n-hexadecane, gasoline, n-hexane | 15-25 | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





