1. Introduction
Rabies, a viral zoonotic but vaccine-preventable disease, continues to claim many lives, particularly in sub-Saharan Africa where the disease has become endemic [
1,
2]. It is a highly fatal Neglected Tropical Disease (NTD) that affects all mammals [
3] with most human cases from bites from domestic dogs [
4,
5]. The improvement in diagnosis of RABV with microscopy and growth in tissue culture were also remarkable achievements [
6]. The use of vaccines led to the elimination of rabies in most European nations [
7]. This established the disease in both humans and animals as vaccine-preventable [
8,
9].
The World Health Organization (WHO), the World Organization for Animal Health (WOAH), the Food and Agriculture Organization (FAO), and the Global Alliance for Rabies Control (GARC) have established “United Against Rabies”, as a global collaborative program that is working towards achieving the goal of “zero human rabies deaths by 2030” [
3,
10,
11]. The program to eliminate rabies in Ghana is managed by the Ghana Health Service (GHS) and the Ministry of Food and Agriculture (MOFA)- acting through the Veterinary Services Division (VSD). In Ghana, while the management of high-risk and exposed individuals with Pre-Exposure Prophylaxis and Post Exposure Prophylaxis (PEP) respectively is performed by the GHS, the major approach to the control of the disease is through the vaccination of dogs and control of dog populations by the VSD. The relatively higher cost of PEP (
$ 40 -
$ 50) for humans [
11] as compared to the cost of dog vaccines (
$ 5 -
$ 7 as of July 2023 in Ghana), makes mass vaccination of dogs an effective and relatively sustainable strategy in rabies prevention and control. The mass vaccination of dogs and cats guarantees an effective and economic approach the potential elimination/eradication of RABV in endemic regions of the world. Support from organizations such as Rabies in West Africa (RIWA), albeit impactful [
12], have not been able to sustain the vaccination rate of Ghana after the truncation of the national anti-rabies vaccination in 1997. This has resulted in gradual increase in laboratory-confirmed rabies cases both in humans and animals even though rabies cases are massively underdiagnosed and underreported [
13].
Rabies, both Africa I and Africa II lineages, is enzootic in Ghana [
2,
14,
15] with the domestic dog being the main vector [
2]. The case positivity rate of rabies in Ghana ranges from 2% to about 92 % [
16,
17,
18]. While the human deaths due to rabies are not at all striking, they are not insignificant when compared to other communicable diseases on a national level [
14,
19]. However, unlike most of these communicable diseases, the case fatality rate associated with rabies is nearly 100 % [
15]. The disease occurs throughout the year with peaks in the dry season [
19]. However, there is evidence to show that the epidemiology of the disease might be more complex than previously thought [
2]. The complexity might be heightened by the improper and under reportage of rabid cases in both humans and animals in the country. The lack of reliable data and systematic analysis of available data continues to keep rabies as a neglected disease in Ghana [
11,
20]. This might stem from socio-cultural practices where classic signs of rabies may be associated with certain superstitious beliefs or the use of herbal concoctions to treat suspected rabid humans or people bitten by suspected rabid dogs [
12,
18]. Identical observations have been made in rabies endemic regions including India [
21]. In these instances, the rabid person or animal might not be reported to a health facility for proper diagnosis and records taken. The lack of laboratory diagnosis of rabies in most suspected cases especially in humans where the diagnosis is based on symptoms, signs, and history of dog bite [
11], also contributes to the absence of data to depict the true burden of rabies disease in Ghana.
This study aims to demonstrate the occurrence of rabies in dogs and relates the results to the vaccination status of the dogs. This will help provide relevant data to inform policy and decisions on the re-adoption of yearly nationwide mass dog vaccinations in Ghana. In the least, it will contribute to the need for individuals to vaccinate their dogs as a means of preventing the transmission of rabies from dogs to humans in Ghana.
2. Materials and Methods
2.1. Study Location
The study was conducted at the Korle Bu Teaching Hospital (KBTH) and the Accra Veterinary Laboratory (AVL) in the Veterinary Services Directorate (VSD) in Accra. KBTH is located at Guggisberg Avenue and has, among other facilities, an advanced laboratory unit for histopathology. AVL is located at Ring Road East, LA, and has, among other facilities, an advanced laboratory for the diagnosis of viruses, and pathogens of interest to veterinary and other zoonotic agents.
2.2. Study Population
All dogs suspected of rabies, irrespective of their breed, health, or vaccination status, were considered for this study. Other mammals, including cats and livestock, were excluded from the study. This is because nearly all rabid cases in Ghana are of and from dogs [
1,
2,
16,
22]. The minimum sample size required for this study was calculated using
[
23] at a 95 % confidence interval, a precision (m) of 0.05, and an estimated prevalence of 91.5 % [
16] and found to be 73. However, a total of 100 dogs were sampled and tested for the study. The 100 samples comprised of 50 saliva (from 50 different dogs) and 50 head / hippocampus (from 50 different dogs) samples from suspected rabid dogs reported to the AVL by veterinarians and individuals in various communities in the Greater Accra region of Ghana. While the head samples were collected between September and December 2020, the saliva samples were collected from September 2020 to June 2021.
2.3. Sample Collection and Procedures
50 brain samples were obtained from the heads of 50 different suspected rabid dogs submitted to AVL for diagnosis and confirmation of rabies. For each sample, the hippocampus was extracted, and about 90% of each hippocampus was stored together with the rest of the brain in 10 % formalin in leak-proof containers for histopathology. The remaining 10 % of each hippocampus was stored in cryovials and kept at (-80 ℃) for RNA extraction.
Saliva samples from 50 different dogs that were quarantined on suspicion of rabies were also collected. Each sample was stored in cryovials containing 500 µl of Viral Transport Medium (VTM) and kept at (-80 ℃).
All biological samples were tested for the rabies virus in a Biosafety Security Level three (BSLIII) laboratory at AVL. Histopathology of the brain tissue was conducted at the KBTH.
2.4. Data Collection
Variables measured included location, signalment, vaccination history, health status based on physical examination, number of bites and fate of the suspected rabid dog. The clinical signs recorded were limited to the signs of rabies observed e.g., hypersalivation and drastic change in behavior including increased aggression. Other information including the potential source of infection of the suspected dog was also record. This included data on the dog being attacked by another community or stray dog prior to the commencement of signs.
2.5. RNA Extraction
All samples stored at -80 ℃ were allowed to thaw and attain room temperature. 3µg of hippocampus was homogenized in a sterile 1.5 ml Eppendorf tube. A known positive brain tissue obtained from the AVL with reference number (R/24/19) serving as the positive control was also homogenized in sterile 1.5ml Eppendorf tubes. Total viral RNA of all brain and saliva samples was extracted using the Series C RNA Extraction and Purification system according to the manufacturer’s instructions and stored at -80 ℃ until required for further analysis.
2.6. Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
PCR master mix, containing Primers (61.4nmol RabPyro Forward primer: 5′ – AACACYYCTACAATGGA – 3′, 66.1nmol RabPyro reverse primer 1: 5′ - TCCAATTNGCACACATTTTGTG – 3′, 100.8nmol RabPyro reverse primer 2: 5′ – TCCARTTAGCGCACATYTTATG – 3′, and 97.7nmol RabPyro reverse primer 3: 5′ – TCCAGTTGGCRCACATCTTRTG – 3′ [
24,
25]) from Microsynth company, PCR buffer 5X, dNTPs mix 10mmol, RNase Inhibitor, One-step RT-PCR enzyme mix and RNase free water, was prepared. The master mix was then vortexed and 22.5ul were aliquoted into 0.2ml PCR tubes. 2.5ul of each of the RNA templates was transferred into each tube.
The samples were placed in the thermal cycler with the following cycling conditions: Pre-PCR (50 ℃, 30 min; 95 ℃, 15 min), 45 Cycles (94 ℃, 30 s; 52 ℃, 30 s; 72 ℃, 40 s) and Holding temperatures (72 ℃, 5 min; 4 ℃, infinity) for the cDNA synthesis. The samples were then stored at - 80 ℃ until they were ready for the gel electrophoresis.
2.7. Gel electrophoresis
Gel electrophoresis was performed [
26] using a 1.5 % TAE agarose gel (agarose BP160-500, Molecular Biology Grade; Low EEO/Multipurpose, Fisher Scientific: Janssen Pharmaceuticals, Belgium) stained with ethidium bromide (Japan and Gel Red Nucleic Acid Gel Stain (10,000x), USA). The amplicons were visualized under UV transillumination after electrophoresis at 100 volts for 30 minutes with a 100 bp DNA ladder as the molecular weight marker (BioLabs, New England). The software Logger Pro 3.16.1 Demo was used to determine the migration and fragment sizes of each sample.
2.8. Histopathology
The study adopted a blind evaluation [
27] in the histologic examinations where the statuses (suspected positivity/negativity) of the brain tissues were not disclosed to the pathologists. Each tissue was prepared in a duplicate with one slide stained with Hematoxylin and Eosin (H&E) and the other stained Golgi silver nitrate stain. The negative and positive cases were made based on the observation of Negri bodies on H &E stains and distortion in the axons and ganglions in the pyramidal neurons on Golgi silver nitrate stains.
2.9. Statistical Analysis
Data was collected in excel and analyzed using Stata SE version 16 (StataCorp, College Sta-tion, TX , USA). Frequencies of various variables were determined. The bivariate relationship between positivity and each risk factor (variables) using the Chi-square test of independence was determined.
3. Results
3.1. Data Collection
Only 20 % (20/100) of all dogs sampled had received at least one vaccination within the past year. Of the vaccinated dogs, only 5 % (1/20) were from the brain samples while the remaining 95 % (19/20) were from the saliva samples.
Most dogs were suspected of being rabid when they exhibited a combination of the following signs making unnecessary noise/barking, sudden behavior change aggression, tremors, vomiting, jaw paralysis, inappetence, ataxia, and/or sudden death. Twenty-four (24%) of dogs suspected of rabies (all from the brain samples) bit 22 humans, 1 goat and another dog. These individuals were not followed to find out if they received and/or completed their PEP vaccines, died, or survived.
At least 30 % (30/100) of the suspected rabid dogs were reported to have been bitten by a stray dog at least a week and at most 2 months before their sudden death or showing signs of rabies. 60 % of the suspected rabid dogs were killed by community members after attacking people, suddenly died, found dead, or euthanized by a veterinarian.
3.2. Positivity
An overall RABV positivity of 34 % was observed in all samples. Sample-specific prevalence revealed a 58 % (29/50) and 10 % (5/50) positivity for brain and saliva samples respectively (
Table 1). All dogs that tested positive for rabies after the examination of their saliva were euthanized.
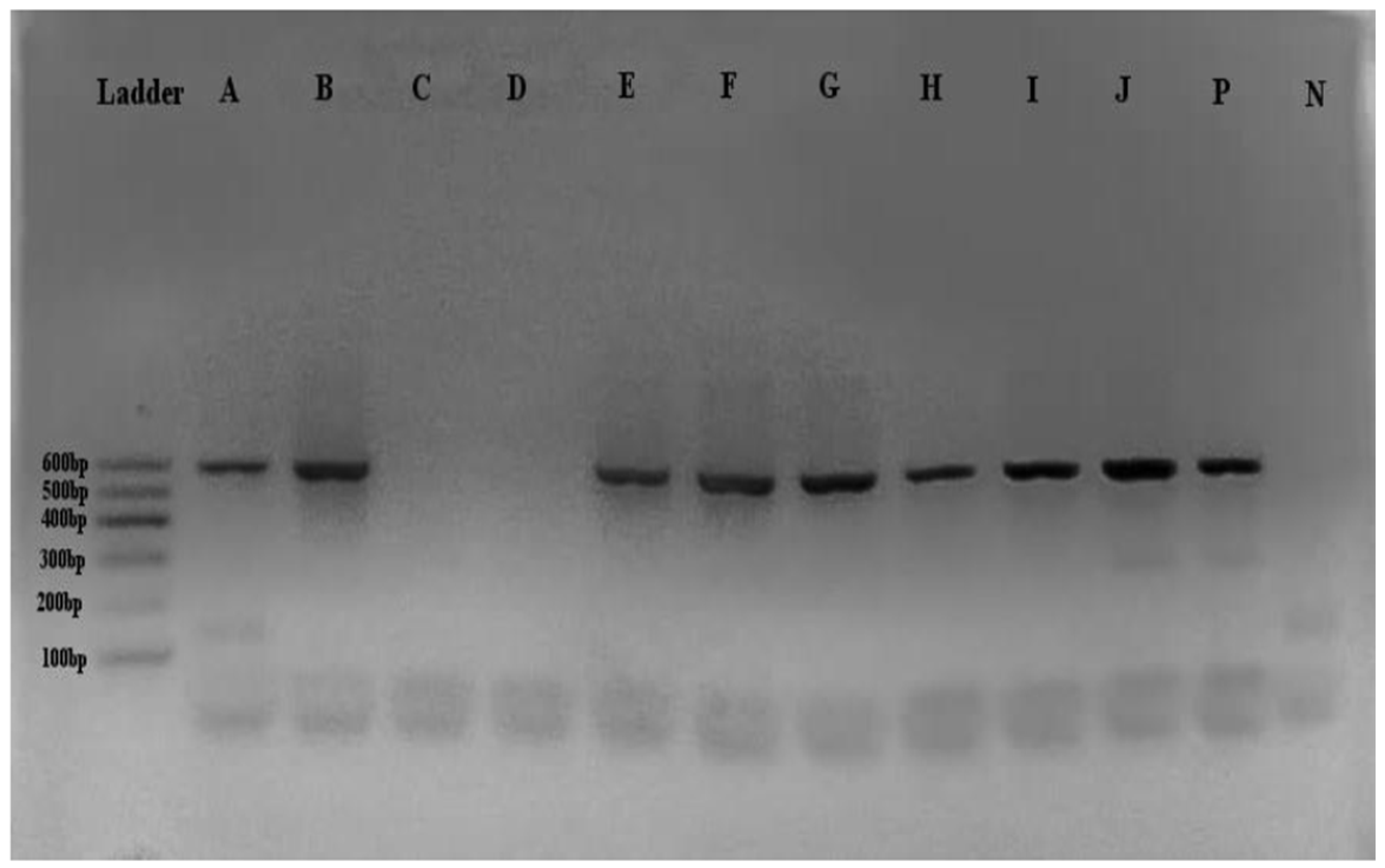
Positive samples showed a 600bp band on gel electrophoresis. There was a significant association between DNA migration to DNA fragments in the brain (p =0.0001) and saliva samples (p = 0.001).
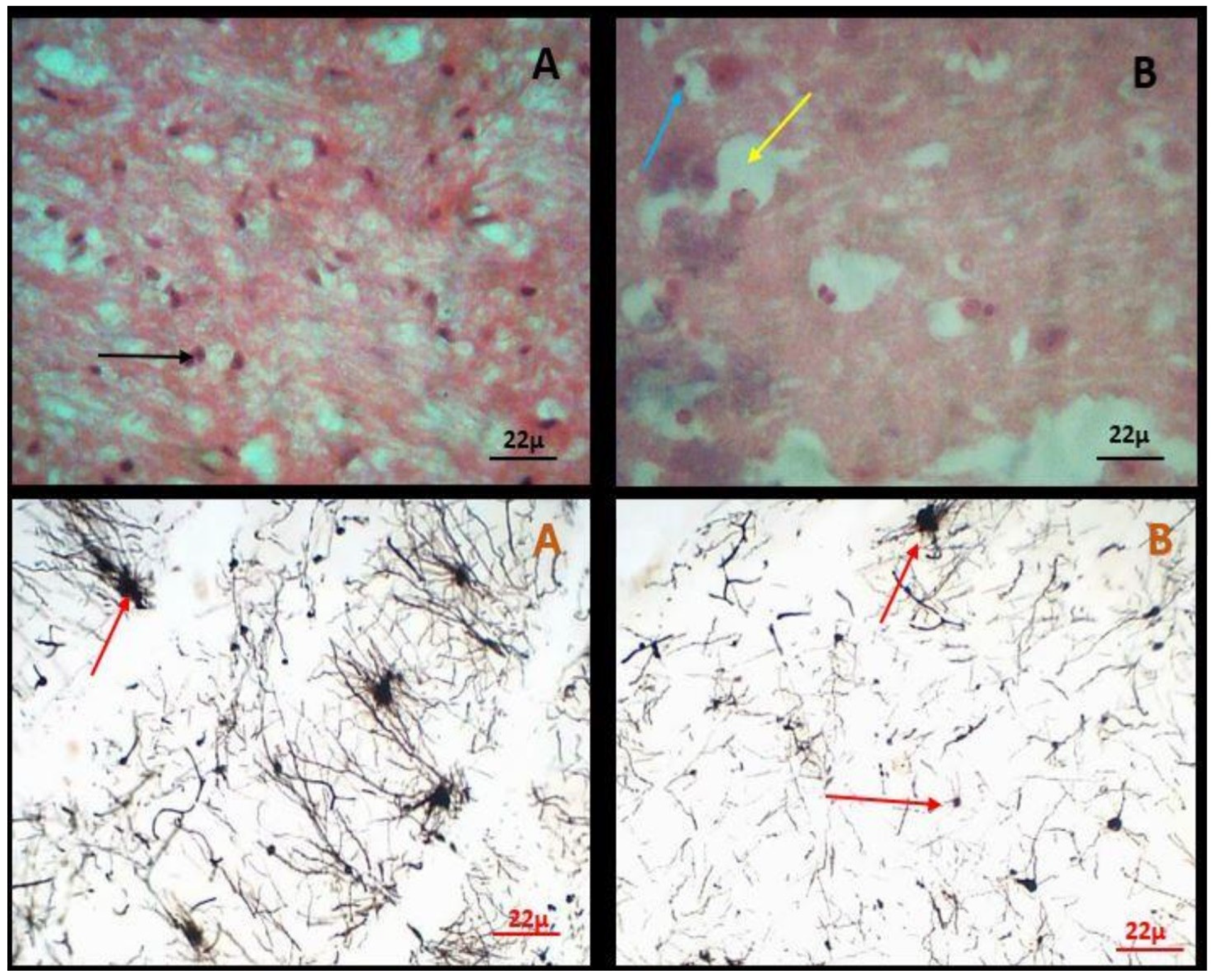
Figure 1.
Brain histomorphology in healthy and suspected rabid dogs. [A] is an H&E stain section of intact neurons (arrow) at the cerebral cortex of a healthy dog. [B] is an H&E stain section from a rabid dog showing neuronal necrosis (yellow arrow) and dense eosinophilic Negri bodies in the cerebral cortex (blue arrow). [A1] is a Golgi silver nitrate stain from a healthy dog showing aggregation of cell bodies of intact pyramidal neurons (red arrow). [B1] is a Golgi silver-nitrate stain from a rabid dog showing distortions of cell bodies in pyramidal neurons (red arrows).
Figure 1.
Brain histomorphology in healthy and suspected rabid dogs. [A] is an H&E stain section of intact neurons (arrow) at the cerebral cortex of a healthy dog. [B] is an H&E stain section from a rabid dog showing neuronal necrosis (yellow arrow) and dense eosinophilic Negri bodies in the cerebral cortex (blue arrow). [A1] is a Golgi silver nitrate stain from a healthy dog showing aggregation of cell bodies of intact pyramidal neurons (red arrow). [B1] is a Golgi silver-nitrate stain from a rabid dog showing distortions of cell bodies in pyramidal neurons (red arrows).
All brain samples that tested positive for RABV (visualized on the gel after RT-PCR) also tested positive after histopathological observations. More than half (58 %) of the hippocampus tissues were positive. A sample was considered positive if Negri bodies and distortion of the axons and dendrites of the pyramidal neurons were observed in the brain tissue after staining with H&E and Golgi silver nitrate stain respectively.
Figure 2.
Ethidium bromide-stained gel after cDNA synthesis of RABV using PCR. [A-J] Sample, [P] positive control, [N] Non-Template Control (NTC) of RNAse free water. The products A-J obtained after cDNA synthesis clearly showed a fragment size of 600bp.
Figure 2.
Ethidium bromide-stained gel after cDNA synthesis of RABV using PCR. [A-J] Sample, [P] positive control, [N] Non-Template Control (NTC) of RNAse free water. The products A-J obtained after cDNA synthesis clearly showed a fragment size of 600bp.
Table 2.
Distribution of positive cases in various variables measured.
Table 2.
Distribution of positive cases in various variables measured.
| Parameter |
Variable |
Total |
Positive |
%positivity |
p-value |
| Sample type |
Brain |
50 |
29 |
58.0 |
0.000 |
| Saliva |
50 |
5 |
10.0 |
| Vaccination |
Unvaccinated |
80 |
32 |
40.0 |
0.011 |
| Vaccinated |
20 |
2 |
10.0 |
| Health Status |
Healthy |
47 |
4 |
8.5 |
0.000 |
| Sick |
53 |
30 |
56.6 |
| Sex |
Male |
51 |
19 |
37.3 |
0.483 |
| Female |
49 |
15 |
30.6 |
| Age |
Puppy (<12 months) |
99 |
34 |
34.3 |
0.488 |
| Adults (>12 months) |
1 |
0 |
0.0 |
| Breed |
Local |
65 |
16 |
24.6 |
0.070 |
| Foreign/Exotic |
35 |
18 |
51.4 |
| Species bitten by rabid dogs* |
Humans |
22 |
14 |
63.6 |
0.320 |
| Dog |
1 |
1 |
100.0 |
| Goat |
1 |
0 |
0.0 |
| Attacked by a community or stray dog |
Yes |
30 |
17 |
56.7 |
0.002 |
| No |
70 |
13 |
18.6 |
Only 2 % of all dogs (2/100) were vaccinated and positive for rabies while 32 % (32/100) of the samples were unvaccinated and positive for rabies. In terms of the type of samples, all brain tissues positive for rabies were from unvaccinated dogs. 4 % (2/50) and 6 % (3/50) of the saliva samples positive for rabies were from vaccinated and unvaccinated dogs respectively.
In all, 56.7 % (17/30) of all dogs that were attacked by stray dogs tested positive for rabies. One of the dogs that tested positive for rabies had been vaccinated 5 weeks after it was bitten by a stray dog. Of the 24 dog bite cases recorded, 15 (62.5%) were from rabies positive dogs with 14 humans and a dog being the victims.
4. Discussion
The case positivity for the study was 34 %. Varied positivity rates have been reported all over the country. These include 91.4 % and 83.3 % in Cape Coast in the Central Region and Ledzokuku Krowor in the Greater Accra Region from 2005 to 2011 [
16], 91.5 % in Greater Accra Region between 2006 and 2011 [
17], about 18 % in 2014 and 3 % in 2016 in Techiman in the Bono East Region of Ghana [
22] and 2.1 % in dogs slaughtered for meat in Ghana in 2021 [
18]. The diverse range of RABV positivity across various parts of the country shows the enzootic nature of the disease in Ghana. The sample type, vaccination and health statuses and the sampled dog being attacked by a community/stray dog showed significance highlighting the importance of these parameters in the spread and maintenance of RABV.
The observed positivity rate is also an indication of the familiarity of veterinarians in Ghana with the signs and correct diagnosis of rabies. Clinical diagnosis is heavily dependent on the observed signs. Given the endemicity of rabies in Ghana and that none of the clinicians performed a rapid test before the recommendation of euthanasia, mandatory quarantine periods or laboratory tests, the value realized is encouraging. That, more than 50 % of the brain samples and 10 % of saliva samples tested positive gives credence to this position. Animals not presenting with classical rabies signs mostly fell into the saliva sample. They comprised dogs with a history of being in contact with suspected rabid dogs or displaying a drastic behavioral change. The request for further tests (for the saliva samples) while quarantining suspected dogs was to confirm the suspicion of the attending veterinarian. It shows the precautions with which dogs are recommended for euthanasia by veterinarians in Ghana. Concerning the brain samples, the recommendation of euthanasia was necessitated by both the suspicion of the veterinarian and/or the presentation of the dog to the veterinarian. In some cases, dogs were presented to the veterinarian by concerned individuals after the dogs had been attacked by community members upon suspicion of rabies. That notwithstanding, at most 42 % (21/50 of brain samples) of dogs were wrongly diagnosed as rabid, killed, or euthanized emphasizing the need for enhanced access to prompt rabies diagnosis in Ghana and public education. For instance, rapid test kits can be used as a second layer of precaution in the diagnosis of canine rabies and the decision to euthanize suspected rabid dogs. Public education on the reporting of suspected rabid dogs (or animals) to the appropriate authorities including the local veterinarian will also help reduce the killing of dogs suspected to have rabies.
About 10% of rabies vaccine dogs are incapable of mounting adequate immunity after receiving primary anti-rabies vaccination [
2]. This is a major cause of vaccine failure. Other causes of vaccine failure include improper maintenance of cold chain and administration of the vaccine at the wrong time including after exposure of the dog to the rabies virus. Therefore, the proportion of dogs that tested positive for rabies that were vaccinated (5.9 %: n = 2/34) in this study is ideal. The failure of the vaccine in one of the cases can be attributed to the administration of the vaccine at an inappropriate time. The other positive case can be safely considered as a result of the inability of the dog to mount adequate immunity before exposure to the RABV or the administration of an ineffective vaccine. An inactive or ineffective vaccine includes expired vaccines and vaccines kept above required temperatures for cold chain. Veterinarians are, thus, encouraged to ensure proper maintenance of cold chain facilities and timing in administering the anti-rabies vaccines as well as ensuring that expired vaccines are not used for vaccination. Another suggested alternative to ensure the elimination of vaccine failure is the adoption of repeated vaccination [
2]. For instance, instead of giving a single dose at 3 months and repeating yearly for puppies, as accepted in Ghana, veterinarians can consider two separate doses at 10 weeks and 12 weeks followed by yearly doses. The same approach can be considered for rabies vaccine naïve adult dogs. This method can especially be adopted in situations where the client can afford or there are ways of checking the rabies titer after administration of the first dose just to make sure enough antibodies are mounted against the challenge with RABV.
The major risk factors identified in this study include the absence of vaccination, increased dog bite cases, or exposure to stray or community dogs. Vaccination of dogs has been suggested to be a major intervention that ensures the elimination of RABV [
2]. The yearly mass administration of rabid vaccines in addition to the routine and timely mop-up vaccination of unvaccinated dogs throughout the year has the potential of reducing the risk of rabies to humans to zero. However, since the truncation of the annual free anti-rabies vaccination of dogs by the government of Ghana, the vaccination coverage in the country has been progressively decreasing to less than 40 % [
16,
17,
22]. This might be the situation of vaccination of dogs in Ghana against RABV. Ironically, there is evidence to suggest an appreciably higher knowledge of rabies which does not translate into vaccination [
1]. Therefore, that a third of the samples were vaccinated was expected. This is, however, far below the estimated 70 % dog vaccination needed to achieve the elimination of rabies in endemic areas including Ghana [
10]. That the vaccination coverage bubbles around 25 % in most areas in Ghana question the ability of the country to achieve the ‘zero by 30’ goal. The alternative use of Oral Rabies Vaccines (ORV) which are considered revolutionary in eliminating rabies among wildlife [
28] can be adopted for anti-rabies campaigns as they come at a lower cost with proven effectiveness in the control of rabies in dogs [
28].
The exposure of dogs and people to stray or community animals, usually either unvaccinated or with unknown vaccination statuses, is one main route of transmission of the RABV [
17]. That more than half of the third of dogs that got exposed to stray dogs tested positive for rabies is a confirmation of this fact. The reports on rabid dogs biting people, while they were not investigated, are suggestive of the dangers of rabies in humans. It has been established that it is common for rabid dogs to attack mostly youthful males [
5,
17,
22] and their owners. Invariably, most people close to the dog or in the community are the ones likely to be bitten by a rabid dog. It is therefore imperative to ensure all domesticated and some stray/community dogs are vaccinated, and the population of stray/community dogs controlled. The effectiveness of the latter approach has been explored [
16].
The one health approach provides a palpable way to ‘achieving zero by 30’ [
10,
29]. It will involve the empowering of both the VSD and GHS in handling RABV both in animals and humans respectively. This should be backed by stakeholders at both agencies and non-governmental and governmental organizations [
5]. For instance, the reinstitution of the annual free anti-rabies campaigns should be considered a national policy. Furthermore, PEP must be made available for exposed individuals or at-risk individuals [
22] including veterinarians, hunters, pet owners, and breeders. They should also introduce systems that ensure compliance with receiving all 5 doses of PEP [
30] being used in Ghana. Additionally, advancement in surveillance [
7,
17], being it active process or passive [
31], can be useful in the control and elimination of rabies. That is, current technologies and reporting systems can be used in the reportage of suspected and confirmed cases of both human and animal rabies. An increase in education of the public [
5], veterinarians, and physicians [
29] on rabies, the importance of vaccinations, and how to approach stray or community dogs can be performed. Finally, while control of the stray dog population can be achieved by mass euthanasia, increasing awareness of animal welfare and rights suggests this method might not be appropriate; inhumane and unethical [
8]. Instead, other methods such as neutering and/or mass vaccination of stray/community dogs can better control the population of these dogs and reduce the odds of them being vectors of rabies. The use oral rabies vaccines can be used as an alternative to the parenteral vaccines [
9,
28,
32]. This will help reduce the overall cost of instituting a mass rabies vaccination scheme [
28].
Author Contributions
Conceptualization, T.O. and D.A.; methodology, T.O.; formal analysis, R.K.A.; investigation, T.O, D.A, P.T.A, D.B.; resources, T.O.; data curation, R.K.A.; writing—original draft preparation, T. O, D.A and R.K.A.; writing—review and editing, B.E, W.T, S.A.M.J, R.K.A and T.O.; visualization, R.K.A.; supervision, T.O, G.B.D; project administration, T.O, G.B.D; funding acquisition, T.O. All authors have read and agreed to the published version of the manuscript.”.