1. Introduction
For newborn calves to survive and resist diseases, it is crucial that they develop a strong immunity quickly. Achieving early and adequate intake of top-notch colostrum is widely recognized as the single most important management factor in determining health and survival of the neonatal calf [
1]
. Authors have demonstrated that oxidative stress (OS) impacts the immune responses of newborn calves by affecting calf lymphocyte activation, cytokine expression, and antibody production [
2,
3]. Oxidative stress can be depicted as an imbalance between oxidants and anti-oxidants in favor of the oxidants, leading to a disruption of redox signaling and control and/or molecular damage [
4]. OS results from the excessive accumulation of pro-oxidants, such as reactive oxygen species (ROS) or free radicals, leading to cell membrane disruption and damage to proteins, lipids, and DNA [
5,
6,
7].
After birth, the calf is exposed for the first time to an oxygen-rich environment once it start to breathe and this results in an increase in the production of ROS [
5], while the calf has a lower serum antioxidant capacity. Concurrently, it acquires passive immunity by consuming colostrum in the first 24 to 36 hours of life [
8]. Colostrum is a source of immunoglobulins (Ig) and immune cells, including macrophages, which use ROS-generating systems to kill bacteria [
5] Colostrum is also a source of antioxidants [
9] and ROS such as lipids or proteins. Therefore, it has been substantiated that colostrum's oxidative/antioxidative profile significantly affects the calf's oxidative status and serum IgG concentration [
5]. These observations underline the importance of improving the calf antioxidant system to prevent diseases in the neonatal and weaning periods, one of the main reasons veterinarians and producers use antimicrobials on farms.
Trace minerals are essential in the calf's health status, performance, and immune system [
10,
11,
12,
13]. Some trace elements, including selenium (Se) [
14,
15], zinc (Zn) [
16], copper (Cu) [
17], and manganese (Mn) [
18], are structural components of metalloenzymes involved in ROS neutralization (e.g., superoxide dismutase and glutathione peroxidase). Many studies have also substantiated direct links between trace minerals and enhanced cattle immune function [
3,
10,
12,
19]. It was indeed reported that Se, Zn, and Cu support the activity of various immune cells such as lymphocytes [
16], neutrophils [
14,
20,
21], macrophages [
21], monocytes [
22], B and T cells [
17,
18,
20,
23,
24]. Therefore, authors have suggested that these trace minerals might elicit increased innate and subsequent adaptive immune responses in calves [
3]. It has also been hypothesized that Mn may play a significant role in immune function in beef cattle [
11].
Deficiencies in trace elements, especially selenium and iodine, are frequently reported in Europe [
25]. In France, trace mineral supplementation in beef calves mainly consists of the administration of Se via the oral route. This method is also recommended elsewhere in the world, in other geochemical contexts such as California [
26]. However, the digestive absorption of Se and other minerals is deemed to be low [
27,
28]. Injectable trace mineral (ITM) supplements combining several trace minerals, such as Se, Cu, Zn, and Mn, have recently become available on the market, allowing rapid availability and transport of trace minerals in the blood after injection [
11]. Bates and others showed that ITM supplements reduce morbidity and mortality of dairy calves from birth to 140 days [
29]. However, to the authors' best knowledge, there are no equivalent data for newborn and pre-weaned beef calves. Therefore, this study was undertaken to investigate the effects of trace mineral supplementations, when administered either orally or subcutaneously, on morbidity, mortality, health status, and growth in beef calves and then to evaluate the potential interest of ITM supplements compared to the oral Se supplementation, which is nowadays the primary way of supplementation in French cow-calf herds.
2. Materials and Methods
Twelve cow-calf operations were selected for this study. All participated in the “Bovins Croissance” program (stands for “cattle growth”, in French), run by a French technical association promoting optimized beef herd management. All herds were located in the Auvergne area in the center of France. In the selected herds, the farm staff was capable of quickly tagging newborn calves, detecting and treating sick animals, recording health events, and maintaining farm records. Good animal welfare management was an inclusion criterion and complementary feed used in this study were approved for food-producing animals. At the end of the grazing season and before the start of the study (November), pregnant cows were fed a granulated feed supplement containing vitamins A, D3, E, C and B1, as well as Cu, Zn, Se, Co, I and polyphenol-rich plant extracts (Buffalo Tonic Axion®, Deltavit, France), according to the manufacturer’s recommendation.
According to the herd’s anticipated calving list, fifty pairs of cows and their calves to be born were enrolled in each farm from December 2020 to December 2021. The animals participating in the study remained in their environment and were intended for commercial purposes. Animals developing diseases during the study were treated according to the farm’s standard treatment protocol.
The mineral status of the herds was assessed on pooled blood samples from ten cows selected at convenience, 30 to 45 days before calving, in December. The same animals were bled again in March after calving. Blood samples were analyzed for Cu, Zn, Se, I, GSH-pxe, and erythrocyte SOD levels (all tests by IODOLAB, Grézieu-la-Varenne, France).
The 600 selected calves were allocated into two groups of 300 animals, an oral trace mineral (OTM) supplement group and an injectable trace mineral (ITM) supplement group, using a randomization scheme of permuted blocks. Within one day after birth (D0), OTM animals received 20 mg Se (as sodium selenite) as tablets (Orosel®, Octavet, France). In the ITM group, animals were injected a multi-mineral solution (Multimin® Solution for Injection for Cattle, Warburton Technology, Ireland), containing Zn (60 mg/mL), Cu (15 mg/mL), Mn (10 mg/mL), and Se (5 mg/mL), at the labelled dose of 1 mL/50 kgBW at D0, D30 and D60, so that the animals in both groups received in total 20 mg of elemental Se during the study.
The follow-up period consisted in observing animal subjects from birth over 210 days. Farm staff had been trained prior to the start of the study to identify signs of disease and record their observations on scoring sheets. The study protocol did not require any changes in husbandry routines, and calves and dams were managed as usual. Calving difficulty was rated on a scale of 1 to 4, with 1 indicating unassisted birth and 4 indicating veterinary assistance. Diarrhea, omphalitis, and pneumonia were monitored individually and scored as present (1) when they occurred, or absent (0) at the end of the follow-up. Other conditions, if any (otitis, arthritis, interdigital phlegmon, etc.) were scored the same way but considering their very low incidence rate, all were confounded in a unique group (other diseases). The need for rehydration therapy and additional treatments represented an extra workload and was also recorded. It is difficult to determine the exact number of animals that became ill within each group since an animal could be dealing with multiple health issues, such as omphalitis, respiratory infections, and diarrhea. Each temporarily isolated incident requires an intervention on the part of the caregiver, whether the incident occurs on separate animals or on the same one, and health incidents can be counted independently of each other. We, therefore, did not consider the incidence rate (per calf-day at risk) but the cumulative incidence of health troubles for the total population at risk at the start of the observation period (2x300), and calculated a cumulated incidence of common conditions (diarrhea, pneumonia, omphalitis) on the one hand (MR1), and of all conditions on the other hand (MR2).
A technician of the local herd improvement organization “Bovins Croissance” weighed all the calves when they get 4 and 7 month-old. The body weight gain (BWG in g/day) was calculated for these two time points (BWG1, BWG2), respectively.
Passive transfer of immunity (PTI) was evaluated in the two groups between 2 and 7 days after birth by measuring the serum refractive index with an optical Brix refractometer and with an optical clinical refractometer (serum/urine), providing an estimation of IgG concentration (°Bx) and serum total protein concentration (STP, g/L). To distinguish between animals that successfully underwent PTI (≥8.4°Bx) and those for which transfer failed (<8.4°Bx), a refractive index of 8.4°Bx was been established as the appropriate threshold value [
30,
31]. Measurement lower than < 8.4°Bx was coded as 0, whereas others were coded as 1 (results treated as categorical variable). For STP, 52 g/L was considered the appropriate threshold [
32,
33].
The statistical tests used are indicated in the results section, when necessary. The Shapiro-Wilk test was used to assess the normal distribution. For all tests used in the present analysis, the significance level was set to 0.05. All calculations by Statgraphics Centurion version XVI.II software (Statgraphics Technologies, Inc., The Plains (VA), USA).
3. Results
3.1. Population baseline
The baseline of the study population is reported in
Table 1. Six hundred calves were of both sexes with sex ratio male calves:heifers of 106.9. Average body weight at birth was 42.2 (±7.8kg) and 42.1 (±6.7kg) for OTM and ITM calves, respectively. Most of the dams (>59.7%) were at least third parity beef cows. There was no significant difference between the two treatment groups regarding the parity distribution (P= 0.184). No assistance has been required for most parturitions (97,0 and 96,0% in the OTM and ITM group, respectively. P=0.871). No twin calves were included in the study.
3.2. Mineral status
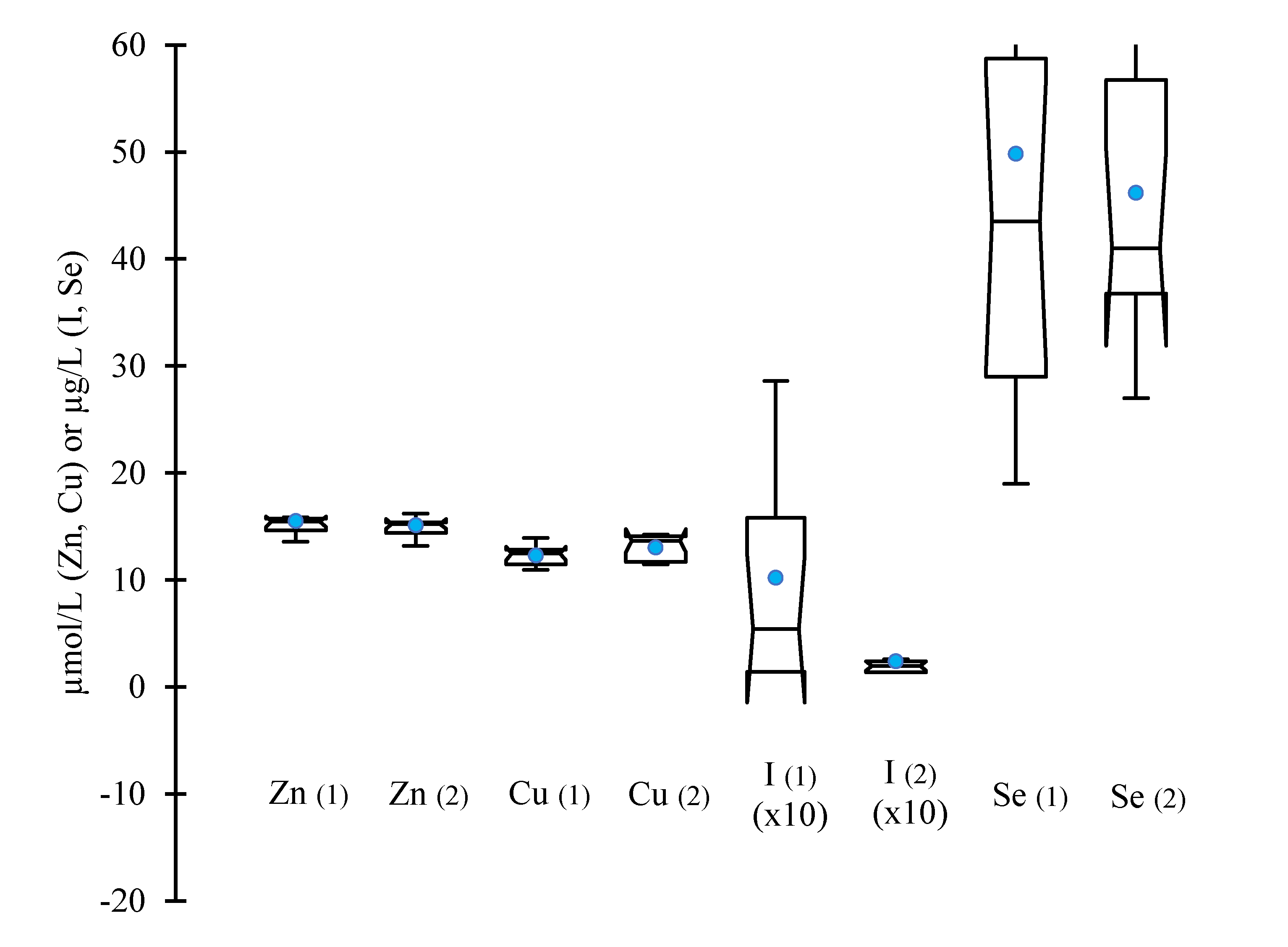
On both test days, before and after calving, the median plasma concentrations of Zn and Cu and their respective 95% confidence interval (CI
95%) (
Figure 1) fell within the typical range reported by the laboratory, with of 13.6-21.0 µmol/L for Zn and 11.8-18.0 µmol/L for Cu. Regarding inorganic I and Se, almost 33 and 41% of herds, respectively, displayed plasma concentrations lower than the typical values (>51 and 40-60 µg/L, respectively). In more than 1 herd out of 3, the Iodine plasma concentration was below the limit of quantification (15 µg/L).
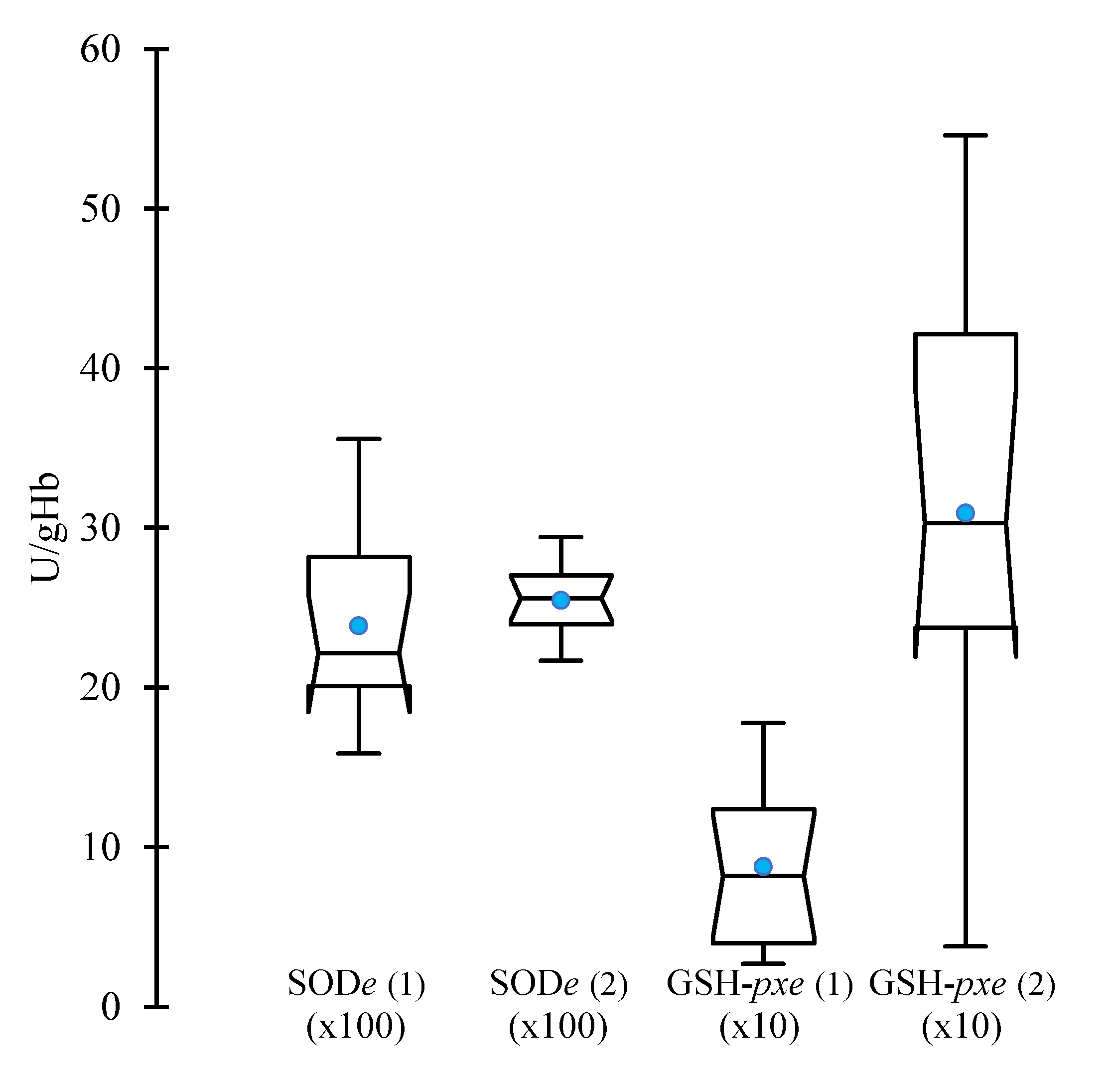
On the two test days, the median plasma activity of erythrocyte SOD and its CI
95% overpassed the typical range reported by the laboratory, generally 1400-2500 U/gHb (
Figure 2). Regarding the median GSH-
pxe plasma activity, 11 values among 12 were below the lowest reference value (155 U/gHb) for the first test. The situation sharply improved on the second test day, with 11 values (out of 12) within the typical range (P<0.05).
3.3. Passive transfer of immunity (PTI)
In both treatment groups, the majority of calves had a successful PTI; failure of PTI (°Bx<8.4), assessed within the first week after calving, was numerically lower (χ2–test, P=0.053) in the ITM group (15.1%) than in the OTM group (21.1%). There was a strong correlation between °Bx and STP in both groups, with a Pearson’s coefficient r2=0.893 and 0.939, in the OTM and ITM group, respectively. Furthermore, the logistic regression in the whole population showed that the incidence rate of diarrhea, death, and antibiotic treatment during the course of the study was strongly correlated with °Bx (P<0.01, all endpoints). There was however no such relationship between the °Bx and the presence of pneumonia. These observations were confirmed by analyses performed by treatment group (P<0.05, all endpoints). Simple regression analysis showed no significant relationship between °Bx and parity in the whole population (P=0.262), neither in the OTM group (P=0.181), nor in the ITM group (P=0.841).
3.4. Relationship between incidence of diseases and °Bx
Based on logistic regression analysis of the entire population, a significant correlation was found between °Bx and three variables: diarrhea (P<0.001), death (P<0.001), and antibiotic treatment (P<0.001). Additionally, one-way ANOVA revealed a significant difference in mean °Bx between those without diarrhea (score 0) and those with diarrhea (score 1) (P<0.001). There was however no such relationship between the presence of pneumonia and °Bx (P=0.285).
These findings were consistent across both the OTM and ITM groups, indicating a relationship between °Bx and diarrhea, death, and antimicrobial consumption, and a lack of relationship between PTI and pneumonia.
3.5. Diarrhea
The numbers of calves that experienced diarrhea in the OTM and ITM groups were close (n=72 and 66, respectively), and did not differ significantly (
OR=0.89, CI
95% [0.69;1.31], P=0.628), (
Table 2).
3.6. Omphalitis
Only 85 calves out of 600 experienced omphalitis during the course of the study (
Table 2). Nevertheless, the odds of developing navel infection were significantly (P
=0.035) lower in the ITM group (11.0% with 33 calves) compared to the OTM group (17.3% with 52 calves).
3.7. Pneumonia
Twelve point three percent (n=37) and 13.0% (n= 39) of the calves experienced pneumonia in the OTM and ITM groups, respectively (
Table 2). The percentage of animals (0.67%, n=2) that experienced a relapse of pneumonia throughout the study period, was very low and did not differ among treatment groups.
3.8. Other conditions
Finally, 5.3% (n=16) and 2.0% (n=6) of the calves in the OTM and ITM groups (
Table 2), respectively, experienced another disease than those described above during the course of the study. ITM animals showed significantly lower odds of getting other diseases than the OTM animals (
OR=0.36, CI
95%[0.14;0.94], P
=0.037). Other diseases included arthritis, coccidiosis, undifferentiated fever, meningitis, septicemia, tracheitis, and interdigital phlegmon (
Table 3).
3.9. Cumulative incidence of health troubles
Out of the OTM and ITM groups, the former reported 177 health incidents, while the latter reported 144. This difference of 11.0% in cumulative incidence (MR2) is highly significant (n-1 χ2–test, P=0.007). However, focusing only on the three main conditions (MR1 - diarrhea, pneumonia and omphalitis), although the ITM group had a slightly lower number of health incidents, the two groups did not show a significant difference (P= 0.087).
3.10. Death
Death occurred in 3.3% (n=10) and 2.0% (n=6) of the animals of the OTM and ITM groups, respectively. Odds ratio calculation showed that the odds of dying during the study period decreased for ITM animals compared to OTM ones (
OR=0.59); however; the difference was not significant. The causes of death and their frequency are detailed in
Table 4.
3.11. Rehydration therapy
Among calves affected by diarrhea, clinical status required rehydration therapy by oral or i.v. route in 44.4% (n=32) and 36.3% (n=24), in the OTM and ITM groups, respectively. There were no significant differences between treatment groups regarding the need for rehydration, either orally or i.v. (
Table 2).
3.12. Antibiotic treatment
Forty-three point three percent (n=130) and 38.0% (n=114) of calves in the OTM and ITM groups, respectively, required medicinal treatments, most of which were antibiotics (>99%). The need for additional treatments was not statistically different between treatment groups (Kendall’s t-b, P= 0.196).
3.13. BWGs
Average BWG1 was numerically higher in the ITM group than in the OTM group [943.6 g/day (± 259.5) versus 929.1 g/day (± 254.6)], but this difference was not significant (Mann-Whitney Wilcoxon test, P=0.454). Later, mean BWG2 was 1052.7 g/day (± 180.9) in the OTM group and 1048.9 g/day (± 193.9) in the ITM group, and again the difference was not significant (P=0.826).
4. Discussion
This study was conducted in France to compare two mineral supplementation (TMS) methods for newborn calves, OTMS with a Se-rich
feed supplement (EU Directive 2002/46/EC), or ITMS with a multi-mineral solution, registered as
veterinary medicinal product (EU Regulation 2019/6). However, the study's main drawback is the absence of a control group with untreated calves. The study involved twelve herds and confirmed the subpar levels of iodine and selenium in cow-calf herds in central France. Other authors in France and Europe have already acknowledged this situation [
25,
34]. Therefore, it is ethically questionable to leave newborn calves without specific supplementation for experimental demonstration purposes, and we have refrained from doing so.
During the last decades, numerous studies have demonstrated the benefits of oral TM supplementation on beef cattle immunity, health and performance [
35]. While there is a lot of research on the impact of selenium status in pregnant cows on the health of their calves, there is limited information on supplementing newborn calves with oral selenium. Only a few papers explore this topic, and most of them lack clinical ambition [
36,
37,
38,
39,
40,
41] while muscular degeneration in calves is the most specific manifestation of selenium deficiency [
42]. Conversely, there is ample literature on injecting cocktail of trace elements into newborn calves, making it difficult to compare results. This suggests a need for more research on the oral supplementation of newborn calves in selenium.
An increase of the immunoglobulin concentration in calves has been demonstrated to be caused by a mineral selenium supplementation of cows before calving [
39,
43]. Many reports have already substantiated the importance of optimal calf mineral status in improving immune function during the first months of life [
29,
44,
45,
46]. In addition, other researchers have demonstrated that supplemental injections of Zn, Mn, Cu, and Se, increased humoral and cellular immune response and glutathione peroxidase activity in dairy calves [
12,
13]. Effects of selenium supplementation on the immune system of calves have been documented [
40]. High concentrations of Se in serum result an increase of the phagocytic activity of macrophages in 30 day-old calves.
The results of the present study showed that most of the supplemented calves at birth had a good PTI the first week after calving, whatever the type of supplementation they received, even if previous reports stated that Se supplementation in colostrum enhances PTI [
41,
47]. Moreover, there was no significant difference between the two populations considering the PTI. There was a strong relationship between the incidence of diarrhea, death and antibiotic treatment and failure of PTI, as already recorded in other studies [
12,
29].
Otherwise, our findings regarding the strong correlation between °Bx and STP confirmed previous results in neonatal dairy calves showing that both endpoints nicely correlate over the first ten days of age [
48]. On the other hand, we found no significant relationship between °Bx and parity in this study, while others reported significantly higher IgG concentrations in high-parity cows [
49]. Unfortunately, however, there are few studies on beef calves.
Previous studies in dairy calves showed diverse incidence rates of diarrhea but were all in line with our results showing a positive effect of ITM supplement on diarrhea. Feldmann and others demonstrated a beneficial effect of oral zinc supplementation on diarrhea [
50]. The frequency of diarrhea found by Bates et al. (2019) in dairy calves over the first 35 days was 4.9% in the multi-mineral ITM animals receiving supplementation within 24 hours after birth and 10.6% in non-treated ones (
OR=0.44, CI
95% [0.24;0.82]). Teixeira et al.(2014) reported that ITM-treated dairy calves had decreased odds of diarrhea during the first 50 days of life compared to untreated ones (41.7% vs. 49.7%, respectively, OR=0.72, CI
95%[0.54;0.98]). However, by contrast with other studies, the incidence of diarrhea was not significantly different between groups. Differences observed among studies may result from different methods.
The chance of developing omphalitis was significantly lower in the ITM group, compared to the OTM group. This observation was similar to what observed previously in dairy calves [
29]. Eleven percent and 17.3% of the ITM and OTM beef calves, respectively, developed omphalitis in our study, while Bates et al. (2019) documented an incidence rate of omphalitis of 2.6% in the ITM group and 5.0% in the untreated group with an
OR=0.55 (CI
95%[0.39;0.77] within the first 35 days of life. Neither the sex ratio, calving difficulty, nor body weight at birth can satisfactorily explain differences between groups.
The incidence rate of pneumonia was low, without any significant difference between groups. In a paper published by Teixeira et al. (2014), the incidence of pneumonia was much higher (35.2% in the ITM group and 40.0% in the non-treated group) with an OR of 0.81 (CI95%[0.61;1.10]).
During the study, we have chosen to record occurrences of any other disorder than the most frequently experienced by calves (i.e., diarrhea, omphalitis, and pneumonia) as «other diseases». Compared to data reported in dairy calves where only diarrhea and omphalitis [
29] or diarrhea, pneumonia, and otitis [
12] were observed, beef calves faced other conditions such as coccidiosis, interdigital phlegmon, arthritis, fever, meningitis, septicemia, and tracheitis besides diarrhea, omphalitis, and pneumonia in our study. Surprisingly, the overall cumulative incidence of these minor diseases differed significantly (P
=0.037) between OTM and ITM animals, whereas frequent perinatal diseases such as diarrhea and pneumonia did not show significant differences between groups.
The odds of dying for animals treated with ITM decreased compared to those treated with oral Se supplementation; however, this difference was not significant. The size of our study population and the low incidence rate of mortality could explain the lack of difference. Nevertheless, our observations are close to those reported by Bates et al. with an
OR of 0.41 (CI
95%[0.23;0.73]) for mortality in ITM-treated dairy calves compared to untreated ones within the first 35 days of follow-up, and 0.50 (CI
95%[0.32;0.80]) from birth to 140 days of life [
29]. However, Teixeira et al. (2014) reported disappointing results, with a death incidence of 3.8% of the ITM-treated dairy calves and 2.7% of the untreated calves with an
OR=1.43 (CI
95%[0.63;3.33]).
One may explain the discrepancy between studies by i) the fact that in other studies, the ITM group was compared to untreated animals, whereas in our study, the other group received oral Se supplementation, ii) the composition of the ITM used in the studies was different (Zn (60 mg/mL), Cu (15 mg/mL), Mn (10 mg/mL), and Se (5 mg/mL) in the present study and Teixeira et al. study vs. Zn (40 mg/mL), Cu (15 mg/mL), Mn (10 mg/mL), Se (5 mg/mL), and chromium (Cr) (5 mg/mL) in Bates et al. study), iii) the follow-up period was not similar between studies (210 days in our study vs. 140 days in the Bates et al. study and 50 days in the Teixeira et al. study, and iv) the dosage of ITM was different from one study to another (1 mL at D0 and D30 and then 2 mL at D60 in the present study whatever the calf’s bodyweight vs. 1 mL at D3 and D30 in the Teixeira et al. study and 1 mL/50 kg at D0, D35 and D70 in Bates et al.).
The comparison of BWG1 and BWG2 between treatment groups did not reveal any significant differences in the present experiment. Se supplementation in calves generally has no influence on calf growth, although positive effects were reported when Se supplementation is performed in deficient calves [
28]. Other reports have also described no effect of ITM supplementation on the rate of weight gain in dairy calves from birth to 140 days [
29] or in beef calves from birth to 78 days [
51], but in those two experiments, ITM group was compared to no oral specific supplementation group. Those observations are in contrast to other experiments where there was a tendency for ITM to increase overall average daily gain in beef calves [
52] or in growing heifers [
45], nonspecifically supplemented. The effect of ITM on the growth of stressed calves is also controversial. Genther and Hansen (2015) reported no effect of ITM in stressed calves during the growing period. In contrast, Richeson and Kegley (2011) described an increased gain-to-feed ratio in ITM-treated calves compared to their untreated counterparts [
53].
The need for antibiotics was 5.3 points lower in the ITM group (38.0% vs. 43.3%), which is notable considering the relatively low incidence of diseases. However, the absence of statistical differences regarding the need for antibiotic therapy between groups may rely on the fact that in our study, we compared ITM- to oral-supplemented animals. Although this result emphasizes the critical role of Se in the maintenance of health status - every animal finally got 20mg Se -, results may have been different if supplemented animals compared to untreated ones; this hypothesis deserves further investigation. In their study, Bittar et al. (2018) suggested that using trace mineral supplementation to enhance natural immunity, and thus to reduce antibiotic usage, should be part of management strategies and preventive-medicine programs [
3].
5. Conclusions
This study is the first to compare clinical benefits of an injectable multi-trace element (selenium, zinc, copper, manganese) supplementation with an oral Se supplementation in beef calves, two supplementation strategies commonly used in France.
This study again confirm the importance of the passive transfer on the incidence rate of diarrhea and mortality. However, the type of supplementation does not affect the passive transfer of immunity.
ITM supplementation effectively decreased the incidence of calf navel infection compared to Se administered orally. Furthermore, calves receiving a mixture of selenium, zinc, copper, and manganese by injection were less likely to develop diseases other than diarrhea or omphalitis than those receiving Se supplementation in tablets. Animals supplemented by injection also needed fewer antibiotics than those orally supplemented.
Injectable multi-element supplementation shows critical advantages over oral Se administration. Therefore, animal caretakers should consider it part of procedures for managing health-threatening stressful periods such as the neonatal period and weaning in beef calves.
Funding
This research was funded by VIRBAC FRANCE, Espace Azur Mercantour, 3ème Rue LID, BP 447, 06515 Carros, France.
CRediT author statement
Nicolas Herman: Conceptualization, Methodology, Investigation, Resources. Agnès Batard.: Project administration, Funding acquisition. Sébastien Geollot: Conceptualization, Writing- Original Draft. Thibault Devambez: Project administration, Supervision. Luc Durel: Conceptualization, Writing- Reviewing and Editing, Visualization.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by Animal Ethic Committee of VIRBAC S.A. (13ème rue L.I.D., Carros, France) that issued certificate #EU-ERC202012-09 for it. This ensures that ethical standards were met and that the animals' welfare was prioritized.
Conflicts of Interest
The first author declares no conflict of interest. Others are employees of VIRBAC S.A., a company that markets veterinary medicines, including MULTIMIN®.
References
- Godden, S. Colostrum Management for Dairy Calves. Vet. Clin. North Am. - Food Anim. Pract. 2008, 24, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, W.; Sordillo, L.M.; Abuelo, A. Oxidative Stress Compromises Lymphocyte Function in Neonatal Dairy Calves. Antioxidants 2021, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bittar, J.H.J.; Hurley, D.J.; Woolums, A.R.; Norton, N.A.; Barber, C.E.; Moliere, F.; Havenga, L.J.; Palomares, R.A. Effects of Injectable Trace Minerals on the Immune Response to Mannheimia Haemolytica and Pasteurella Multocida Following Vaccination of Dairy Calves with a Commercial Attenuated-Live Bacterin Vaccine. Prof. Anim. Sci. 2018, 34, 59–66. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: A Concept in Redox Biology and Medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Abuelo, Á.; Pérez-Santos, M.; Hernández, J.; Castillo, C. Effect of Colostrum Redox Balance on the Oxidative Status of Calves during the First 3 Months of Life and the Relationship with Passive Immune Acquisition. Vet. J. 2014, 199, 295–299. [Google Scholar] [CrossRef]
- Castillo, C.; Hernandez, J.; Bravo, A.; Lopez-Alonso, M.; Pereira, V.; Benedito, J.L. Oxidative Status during Late Pregnancy and Early Lactation in Dairy Cows. Vet. J. 2005, 169, 286–292. [Google Scholar] [CrossRef]
- Jacometo, C.B.; Zhou, Z.; Luchini, D.; Trevisi, E.; Corrêa, M.N.; Loor, J.J. Maternal Rumen-Protected Methionine Supplementation and Its Effect on Blood and Liver Biomarkers of Energy Metabolism, Inflammation, and Oxidative Stress in Neonatal Holstein Calves. J. Dairy Sci. 2016, 99, 6753–6763. [Google Scholar] [CrossRef]
- Weaver, D.M.; Tyler, J.W.; VanMetre, D.C.; Hostetler, D.E.; Barrington, G.M. Passive Transfer of Colostral Immunoglobulins in Calves. J. Vet. Intern. Med. 2000, 14, 569–577. [Google Scholar] [CrossRef]
- Przybylska, J.; Albera, E.; Kankofer, M. Antioxidants in Bovine Colostrum. Reprod. Domest. Anim. 2007, 42, 402–409. [Google Scholar] [CrossRef]
- Failla, M.L. Trace Elements and Host Defense: Recent Advances and Continuing Challenges. J. Nutr. 2003, 133, 1443S–1447S. [Google Scholar] [CrossRef]
- Brasche, C.; Hall, J.; Drewnoski, M.E. Effect of a Trace Mineral Injection on Performance., University of Nebraska, Lincoln, NE, USA, 2015.
- Teixeira, A.G. V; Lima, F.S.; Bicalho, M.L.S.; Kussler, A.; Lima, S.F.; Felippe, M.J.; Bicalho, R.C. Effect of an Injectable Trace Mineral Supplement Containing Selenium, Copper, Zinc, and Manganese on Immunity, Health, and Growth of Dairy Calves. J. Dairy Sci. 2014, 97, 1–11. [Google Scholar] [CrossRef]
- Palomares, R.A.; Hurley, D.J.; Bittar, J.H.J.; Saliki, J.T.; Woolums, A.R.; Moliere, F.; Havenga, L.J.; Norton, N.A.; Clifton, S.J.; Sigmund, A.B.; et al. Effects of Injectable Trace Minerals on Humoral and Cell-Mediated Immune Responses to Bovine Viral Diarrhea Virus, Bovine Herpes Virus 1 and Bovine Respiratory Syncytial Virus Following Administration of a Modified-Live Virus Vaccine in Dairy Calves. Vet. Immunol. Immunopathol. 2016, 178, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Maddox, J.F.; Aherne, K.M.; Reddy, C.C.; Sordillo, L.M. Increased Neutrophil Adherence and Adhesion Molecule MRNA Expression in Endothelial Cells during Selenium Deficiency. J. Leukoc. Biol. 1999, 65, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Nève, J. Physiological and Nutritional Importance of Selenium. Experientia 1991, 47, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Palomares, R.A.; Hurley, D.J.; Moliere, F.; Woolums, A.R.; Havenga, L.J.; Norton, N.A.; Sigmund, A.J.; Berger, C.E.; Berger, M.L.; Clark, M.J.; et al. Effect of Injectable Trace Minerals on the Immune Response to Bacterial Antigens after Administration of an Attenuated-Live Bacterin in Dairy Calves. In Proceedings of the Proceedings of the 29th World Buiatrics Congress; Dublin, Ireland, 2016.
- Maggini, S.; Wintergerst, E.S.; Beveridge, S.; Hornig, D.H. Selected Vitamins and Trace Elements Support Immune Function by Strengthening Epithelial Barriers and Cellular and Humoral Immune Responses. Br. J. Nutr. 2007, 98, 29S–35S. [Google Scholar] [CrossRef]
- Tomlinson, D.; Socha, M.; DeFrein, M. Role of Trace Minerals in the Immune System. In Proceedings of the Penn State Dairy Catt le Nutrition Workshop; Grantville, PA, USA; 2008; pp. 39–52. [Google Scholar]
- Genther, O.N.; Hansen, S.L. Effect of Dietary Trace Mineral Supplementation and a Multi-Element Trace Mineral Injection on Shipping Response and Growth Performance of Beef Cattle. J. Anim. Sci. 2014, 92, 2522–2530. [Google Scholar] [CrossRef]
- Cerone, S.I.; Sansinanea, A.S.; Streitenberger, S.A.; Garcia, M.C.; Auza, N.J. The Effect of Copper Deficiency on the Peripheral Blood Cells of Cattle. Vet. Res. Commun. 1998, 22, 47–57. [Google Scholar] [CrossRef]
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and Its Role in Immunity and Inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef]
- Rink, L.; Kirchner, H. Zinc-Altered Immune Function and Cytokine Production. J. Nutr 2000, 130, 1407S–1415S. [Google Scholar] [CrossRef]
- Pinna, K.; Kelley, D.S.; Taylor, P.C.; King, J.C. Research Communication: Immune Functions Are Maintained in Healthy Men with Low Zinc Intake. J. Nutr. 2002, 132, 2033–2036. [Google Scholar] [CrossRef]
- Haase, H.; Rink, L. Multiple Impacts of Zinc on Immune Function. Metallomics 2014, 6, 1175–1180. [Google Scholar] [CrossRef]
- Guyot, H.; Rollin, F. Le Diagnostic Des Carences En Iode et Sélénium Chez Les Bovins. Ann. Médecine Vétérinaire 2007, 151, 166–191. [Google Scholar]
- Davy, J.; Forero, L.; Tucker, T.; Mayo, C.; Drake, D.; Maas, J.; Oltjen, J. Efficacy of Selenium Supplementation Methods in California Yearling Beef Cattle and Resulting Effect on Weight Gain. Calif. Agric. 2016, 70, 187–193. [Google Scholar] [CrossRef]
- Meschy, F. Nutrition Minérale Des Ruminants - Nouvelle Edition; 2nd ed.; Editions QUAE, 2023.
- Mehdi, Y.; Dufrasne, I. Selenium in Cattle: A Review. Molecules 2016, 21, 545. [Google Scholar] [CrossRef]
- Bates, A.; Wells, M.; Laven, R.A.; Simpson, M. Reduction in Morbidity and Mortality of Dairy Calves from an Injectable Trace Mineral Supplement. Vet. Rec. 2019, 184, 680. [Google Scholar] [CrossRef]
- Todd, C.G.; McGee, M.; Tiernan, K.; Crosson, P.; O’Riordan, E.; McClure, J.; Lorenz, I.; Earley, B. An Observational Study on Passive Immunity in Irish Suckler Beef and Dairy Calves: Tests for Failure of Passive Transfer of Immunity and Associations with Health and Performance. Prev. Vet. Med. 2018, 159, 182–195. [Google Scholar] [CrossRef]
- Deelen, S.M.; Ollivett, T.L.; Haines, D.M.; Leslie, K.E. Evaluation of a Brix Refractometer to Estimate Serum Immunoglobulin G Concentration in Neonatal Dairy Calves. J. Dairy Sci. 2014, 97, 3838–3844. [Google Scholar] [CrossRef]
- Abuelo, Á.; Alves-Nores, V. Point-of-Care Testing in Cattle Practice: Reliability of Cow-Side Diagnostic Tests. In Pract. 2016, 38, 293–302. [Google Scholar] [CrossRef]
- Quigley, J.D. Calf Note #186. Serum Total Protein and Colostrum Replacers. Available online: https://www.calfnotes.com/pdffiles/CN186.pdf (accessed on 19 May 2023).
- Guyot, H.; Saegerman, C.; Lebreton, P.; Sandersen, C.; Rollin, F. Epidemiology of Trace Elements Deficiencies in Belgian Beef and Dairy Cattle Herds. J. trace Elem. Med. Biol. 2009, 23, 116–123. [Google Scholar] [CrossRef]
- Palomares, R.A. Trace Minerals Supplementation with Great Impact on Beef Cattle Immunity and Health. Animals 2022, 12. [Google Scholar] [CrossRef]
- Kincaid, R.L.; Miller, W.J.; Neathery, M.W.; Gentry, R.P.; Hampton, D.L. Effect of Added Dietary Selenium on Metabolism and Tissue Distribution of Radioactive and Stable Selenium in Calves. J. Anim. Sci. 1977, 44, 147–151. [Google Scholar] [CrossRef]
- Żarczyńska, K.; Sobiech, P.; Tobolski, D.; Mee, J.F.; Illek, J. Effect of a Single, Oral Administration of Selenitetriglycerides, at Two Dose Rates, on Blood Selenium Status and Haematological and Biochemical Parameters in Holstein-Friesian Calves. Ir. Vet. J. 2021, 74, 9. [Google Scholar] [CrossRef]
- Szacawa, E.; Dudek, K.; Wasiak, M.; Bednarek, D.; Bederska-Łojewska, D.; Muszyńska, B.; Pieszka, M. Effect of Supplementation with the Combination of Se-Enriched Lentinula Edodes Mycelium, Exogenous Enzymes, Acidifiers, Sodium Butyrate and Silicon Dioxide Nanoparticle Feed Additives on Selected Parameters in Calves. Molecules 2022, 27, 13. [Google Scholar] [CrossRef]
- Rowntree, J.E.; Hill, G.M.; Hawkins, D.R.; Link, J.E.; Rincker, M.J.; Bednar, G.W.; Kreft, R.A. Effect of Se on Selenoprotein Activity and Thyroid Hormone Metabolism in Beef and Dairy Cows and Calves. J. Anim. Sci. 2004, 82, 2995–3005. [Google Scholar] [CrossRef]
- Salles, M.S.V.; Zanetti, M.A.; Junior, L.C.R.; Salles, F.A.; Azzolini, A.E.C.S.; Soares, E.M.; Faccioli, L.H.; Valim, Y.M.L. Performance and Immune Response of Suckling Calves Fed Organic Selenium. Anim. Feed Sci. Technol. 2014, 188, 28–35. [Google Scholar] [CrossRef]
- Hall, J.A.; Bobe, G.; Vorachek, W.R.; Estill, C.T.; Mosher, W.D.; Pirelli, G.J.; Gamroth, M. Effect of Supranutritional Maternal and Colostral Selenium Supplementation on Passive Absorption of Immunoglobulin G in Selenium-Replete Dairy Calves. J. Dairy Sci. 2014, 97, 4379–4391. [Google Scholar] [CrossRef]
- Enjalbert, F.; Lebreton, P.; Salat, O. Effects of Copper, Zinc and Selenium Status on Performance and Health in Commercial Dairy and Beef Herds: Retrospective Study. J. Anim. Physiol. Anim. Nutr. (Berl). 2006, 90, 459–466. [Google Scholar] [CrossRef]
- Guyot, H.; Spring, P.; Andrieu, S.; Rollin, F. Comparative Responses to Sodium Selenite and Organic Selenium Supplements in Belgian Blue Cows and Calves. Livest. Sci. 2007, 111, 259–267. [Google Scholar] [CrossRef]
- Hostetler, C.; Kincaid, R.; Mirando, M. The Role of Essential Trace Elements in Embryonic and Fetal Development in Livestock. Vet. J. 2003, 166, 125–139. [Google Scholar] [CrossRef]
- Arthington, J.D.; Moriel, P.; Martins, P.G.M.A.; Lamb, G.C.; Havenga, L.J. Effects of Trace Mineral Injections on Measures of Performance and Trace Mineral Status of Pre- and Postweaned Beef Calves. J. Anim. Sci. 2014, 92, 2630–2640. [Google Scholar] [CrossRef]
- Arthington, J. New Concepts in Trace Mineral Supplementation of Grazing Cattle. Hydroxy Sources, Injectable Sources and Pasture Application. In Proceedings of the 2015 Florida Ruminant Nutrition Symposium. 26th Annual Meeting.; University of Florida. Department of Animal Sciences.: Gainsville, FL, USA, 2015.
- Kamada, H.; Nonaka, I.; Ueda, Y.; Murai, M. Selenium Addition to Colostrum Increases Immunoglobulin G Absorption by Newborn Calves. J. Dairy Sci. 2007, 90, 5665–5670. [Google Scholar] [CrossRef]
- Wilm, J.; Costa, J.H.C.; Neave, H.W.; Weary, D.M.; von Keyserlingk, M.A.G. Technical Note: Serum Total Protein and Immunoglobulin G Concentrations in Neonatal Dairy Calves over the First 10 Days of Age. J. Dairy Sci. 2018, 101, 6430–6436. [Google Scholar] [CrossRef]
- Conneely, M.; Berry, D.P.; Sayers, R.; Murphy, J.P.; Lorenz, I.; Doherty, M.L.; Kennedy, E. Factors Associated with the Concentration of Immunoglobulin G in the Colostrum of Dairy Cows. Animal 2013, 7, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, H.R.; Williams, D.R.; Champagne, J.D.; Lehenbauer, T.W.; Aly, S.S. Effectiveness of Zinc Supplementation on Diarrhea and Average Daily Gain in Preweaned Dairy Calves: A Double-Blind, Blockrandomized, Placebo-Controlled Clinical Trial. PLoS One 2019, 14, e0219321. [Google Scholar] [CrossRef] [PubMed]
- Genther, O.N.; Hansen, S.L. The Effect of Trace Mineral Source and Concentration on Ruminal Digestion and Mineral Solubility. J. Dairy Sci. 2015, 98, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Rauch, J.C.; Stokes, R.S.; Shike, D.W. Evaluation of Two-Stage Weaning and Trace Mineral Injection on Receiving Cattle Growth Performance and Behavior. Transl. Anim. Sci. 2019, 3, 155–163. [Google Scholar] [CrossRef]
- Richeson, J.; Kegley, E. Effect of Supplemental Trace Minerals from Injection on Health and Performance of Highly Stressed, Newly Received Beef Heifers. Prof. Anim. Sci. 2011, 27, 461–466. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).