Introduction
Hepatitis B virus (HBV) and its close relative woodchuck hepatitis virus (WHV) belong to the hepadnaviral family. Both are highly oncogenic DNA viruses which persistent infection and integration into the host’s hepatocyte genome are the main contributors to the development of hepatocellular carcinoma (HCC) [
1,
2,
3,
4]. Primary HCC is the fifth most common neoplasm in humans responsible for an estimated 830,000 deaths globally and HBV infection remains the main cause of newly diagnosed HCC at an estimated rate of 56% [
5,
6]. In eastern North American woodchucks
(Marmota monax) either naturally or experimentally infected with WHV, HCC develops in more than 90% of the animals with serologically evident chronic infection accompanied by protracted hepatitis [
7]. This provides a highly valuable, naturally occurring animal model of progression from acute to chronic hepatitis type B (CHB), self-limited resolution of acute liver inflammation, asymptomatic occult HBV infection (OBI) and its long-term pathological consequences, and the HBV-initiated oncogenic process often culminating in HCC [
7,
8]. A great advantage of this model is a stepwise progression of experimentally WHV-induced liver disease in an immunologically intact host that is inherently susceptible to a highly molecularly and pathogenically HBV-compatible virus. This infection model has been applied for basic research, and preclinical evaluations of new anti-viral and anti-cancerous agents and assessment of novel immunological approaches against CHB and HBV-associated HCC [
9,
10,
11,
12,
13].
HBV infection-associated HCC typically coincides with serum HBV surface antigen (HBsAg)-positive CHB, which could be accompanied or not by liver cirrhosis [
14]. Currently, close to 300 million people have serologically detectable chronic HBV infection and up to a million die yearly because of HBV-caused liver diseases, including CHB, liver cirrhosis, liver failure, and HCC [
15]. Nonetheless, HCC may also develop in clinically silent HBV infection (
i.e., OBI), where low levels of replicating virus persist in the absence of serum HBsAg when tested by the currently available clinical assays [
3,
16]. Pro-oncogenic properties of this form of silent HBV persistence have been identified in patients [
3,
16] and are well documented in WHV-infected woodchucks [
7,
17]. In this context, infection in woodchucks with doses lower than or equal to 1,000 virions causes persistent trace replication of infectious WHV in the absence of hepatitis, serological (immunovirological) markers of infection, and WHV-specific B cells response while WHV-specific T cell reactivity is present [
7,
19]. This form of infection was designated as primary occult infection (POI) and found to be a cause of HCC in about 20% of animals in the context of normal liver histology and apparently unaltered hepatic biochemical function [
17,
18]. A similar frequency of HCC was observed in woodchucks with secondary occult infection (SOI) continuing after resolution of acute WHV hepatitis, which is accompanied by low WHV loads (
i.e., typically below 100 copies/mL) in serum, the absence of WHsAg unless ultrasensitive detection method is applied, whereas WHV-specific antibodies and T cell responses are detectable [
7,
20]. The above findings indicated that HCC may develop long after resolution of an episode of hepatitis type B and may occur even in the absence of clinical indications of liver inflammation or cirrhosis pointing to a high carcinogenic potency of HBV.
Integration of HBV DNA into the liver genome was found to be an unwavering characteristic of HBV infection [
21,
22]. However, identification of HBV-host genomic fusions and attempts to explain their role in HCC development were essentially restricted to studies of advanced CHB with already diagnosed HCC. This led to the assumption that formation of HBV-host DNA junctions is a rather late event that occurs randomly across the hepatocyte genome. More recent studies applying high-throughput technologies tend to indicate that some of the hepatic genes could be preferred targets of HBV insertions [
23,
24]. It is of interest to note that HBV and WHV also integrate into immune cell genomes and that this may contribute to the pathogenesis of lymphoproliferative disorders [
8,
25,
26,
27]. It remained unknown until recently when the first hepadnavirus-host genomic fusions are formed in infected hepatocytes, what are the initial sites of HBV and WHV insertions and virus DNA breakpoints forming merges with host sequences, and what is their molecular format and a mechanism of creation. Since these very early events could be decisive in triggering liver oncogenesis, destabilize hepatocyte genome, and contribute to an environment supportive of pro-oncogenic perturbations which finally culminate in HCC, our laboratory investigated those issues and the results obtained are briefly summarized in this communication.
Investigational Approaches
HBV infection experiments were carried out in hepatocyte-compatible human HepaRG and HepG2-NTCP-C4 cell lines, while WHV infection study in woodchuck WCM260 hepatocyte-derived line and in woodchucks. The cell lines susceptibility to HBV or WHV infection was established in previous studies, as reported elsewhere. HepaRG cells were infected with HBV from treatment-naive patients at a multiplicity of infection (MOI) of 20 (
i.e., ~2.4 x 10
7 virus copies/~1.2 x 10
6 cells) and analyzed for virus-host DNA fusions at 15 and 30 minutes (min), 1 hour (h), 3 h and 24 h post-infection (p.i.), and at more distant time points p.i., such as 1, 2, 3, 4 and 7 weeks [
28]. In the following study, HepG2 cells overexpressing sodium taurocholate co-transporting peptide (NTCP) receptor, identified as an HBV receptor on human hepatocytes, were exposed to recombinant HBV produced by HepG2.2.15.7 cells at estimated MOI of 1000 (
i.e., ~5 x 10
8 virus copies/5 x10
5 cells) and virus integrations were examined between 15 min and 24 h p.i., as for HepaRG cells, and then at day 13 after infection [
29]. In yet another study, woodchuck WCM260 hepatocytes were infected with wild-type WHV at MOI of 20 (
i.e., ~4 x 10
9 virions/5 x10
5 cells) and investigated for WHV-host DNA fusions at 15 min and 30 min and one-hour p.i. Cells obtained at 3 h and 24 h and 13 d p.i. were analyzed for other markers of interest [
30]. The presence of WHV-host DNA integrations was also determined in liver biopsies collected at 1 h or 3 h p.i. from woodchucks intravenously infected with a wild-type WHV at 1.1 x 10
10 DNAase digestion-protected WHV virions [
28].
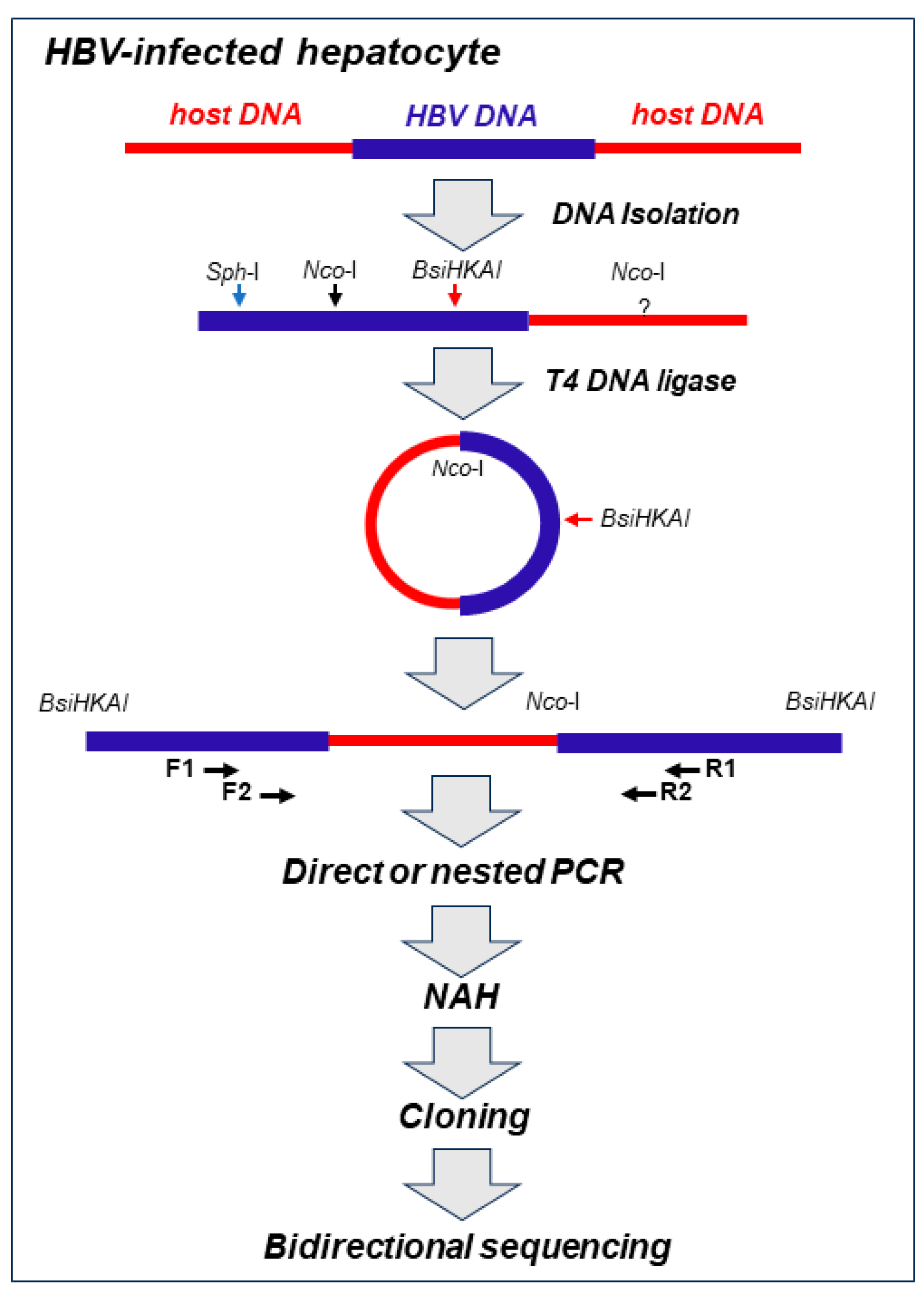
Our approach to detect virus-host genomic merges utilized HBV- or WHV genome-specific amplifications by inverse-polymerase chain reaction (inv-PCR). The specificity and the sensitivity of detection of the integrated virus sequences was enhanced by nucleic acid hybridization (NAH) of the resulting amplicons with recombinant, full-length HBV or WHV sequence as a probe (
Figure 1) [
19,
28].
Figure 1.
Schematic step-by-step outline of methods used for detection of HBV integration and identification of the sequences acting as the host’s HBV insertional sites and HBV DNA breakpoints in infected hepatocyte. Total DNA was extracted from infected cells and treated with restriction enzyme NcoI that cut HBV DNA at the single site and the HBV-merged host’s sequence at unknown sites. The resulting hybrid DNA fragments were circularized with T4 DNA ligase and then linearized with BsiHKAI endonuclease to facilitate PCR amplification and cloning. Nested PCR was usually performed with forward (F1 and F2) and reverse (R1 and R2) primers. The exception was a situation when a particular band was well identifiable after direct PCR by agarose gel electrophoresis and clearly displayed HBV signal by nucleic acid hybridization (NAH). NAH was routinely used to verify the presence of HBV sequences and to augment detection of the HBV-host DNA merges even when the bands carrying the merges were not apparent on the gels. This was followed by cloning of HBV-positive amplicons and bidirectional sequencing of the clones. The aim was to sequence 20-30 clones from each band analyzed. For more information see the text and references [
19,
28,
29]. For identification of WHV-host DNA merges, the approach used was identical as that above but restriction enzymes and PCR primers were specific for WHV, as reported [
19,
28].
.
Figure 1.
Schematic step-by-step outline of methods used for detection of HBV integration and identification of the sequences acting as the host’s HBV insertional sites and HBV DNA breakpoints in infected hepatocyte. Total DNA was extracted from infected cells and treated with restriction enzyme NcoI that cut HBV DNA at the single site and the HBV-merged host’s sequence at unknown sites. The resulting hybrid DNA fragments were circularized with T4 DNA ligase and then linearized with BsiHKAI endonuclease to facilitate PCR amplification and cloning. Nested PCR was usually performed with forward (F1 and F2) and reverse (R1 and R2) primers. The exception was a situation when a particular band was well identifiable after direct PCR by agarose gel electrophoresis and clearly displayed HBV signal by nucleic acid hybridization (NAH). NAH was routinely used to verify the presence of HBV sequences and to augment detection of the HBV-host DNA merges even when the bands carrying the merges were not apparent on the gels. This was followed by cloning of HBV-positive amplicons and bidirectional sequencing of the clones. The aim was to sequence 20-30 clones from each band analyzed. For more information see the text and references [
19,
28,
29]. For identification of WHV-host DNA merges, the approach used was identical as that above but restriction enzymes and PCR primers were specific for WHV, as reported [
19,
28].
.
Employment of the NAH step increased detection of the integrated viral sequences which otherwise might be omitted when the gels after electrophoresis were analyzed (see Supplementary Information to reference 28). This step also allowed elimination of rarely occurring bands which did not contain virus-specific signals which increased specificity of the virus-host DNA merges detection. Only the NAH-confirmed bands carrying viral DNA were excised from gel and DNA was extracted, cloned and bidirectionally sequenced. The host and viral DNA sequences forming junctions were identified using applicable computational software as reported [
29,
30]. A range of control experiments, including mock infections, and specificity controls ascertained validity of inv-PCR amplifications and reliability of sequencing data in each of our studies. In addition, molecular markers of HBV or WHV presence (
i.e., virus genomic DNA) and replication (
i.e., virus mRNA and covalently closed circular DNA, cccDNA) were assessed in the cells and liver biopsies examined. In this presentation, HBV- and WHV-host genomic fusions detected within the first hour after exposure to virus and referred to as the initial or the earliest integrations were of the main focus, although those identified up to 24 h p.i. or later were occasionally discussed to provide a wider picture of our findings.
Virus-Host Genomic Fusions Emerging in the First Hour after Infection
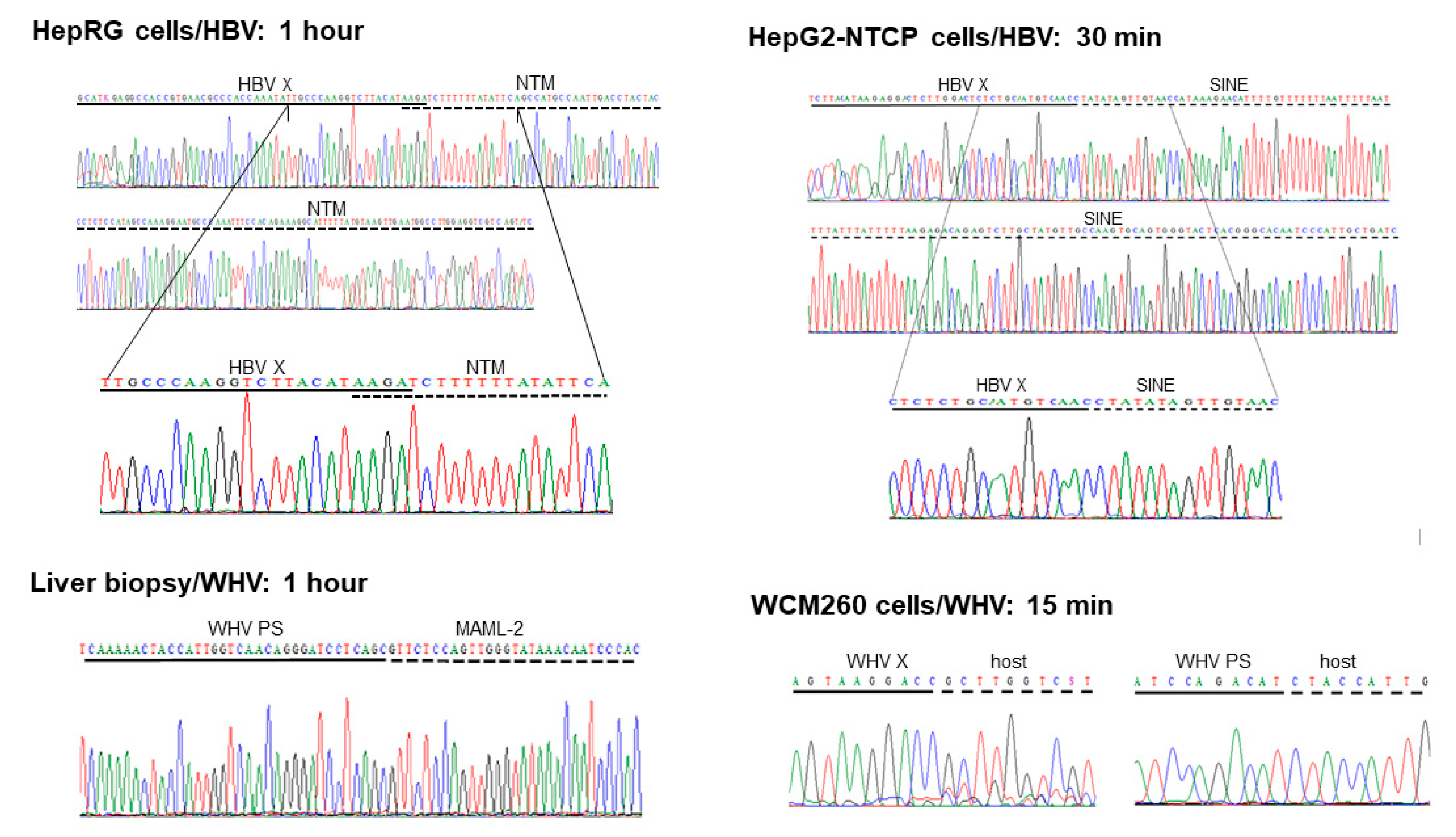
Five HBV DNA insertions into 5 distinct genes were identified at 1 h p.i. in HepaRG cells exposed to authentic, patient-derived HBV [
28]. Four of them were detected by single hits (
i.e., in a single clone), while one identified by multiple hits (
i.e., in 8 clones). This last site was in the human neurotrimin (NTM) gene located on chromosome 11q25. The gene encodes for a neural adhesion molecule and its expression was also found in stem cells and hepatic stellate cells [
31]. The fusion was created by 4-base pair overlapping homologous junction (OHJ) with the HBV sequence located in the upstream regulatory region (UPR) of the HBV X (HBx) gene that carries HBV enhancer II (Enh-II) (
Figure 2; see
Figure 3 for more information). Due to fusion with the enhancer, this HBV insertion may potentially modify hepatocyte function and influence pro-oncogenic process. Notably, the OHJ format of the HBx-NTM fusion was the only one of this type detected in our studies within one-hour after infection. All remaining HBV or WHV-host genomic merges demonstrated the format of head-to-tail junctions (HTJ) (
Figure 2). In the same study, liver biopsies acquired at 1 h or 3 h p.i. from woodchucks intravenously injected with WHV were also examined. Overall, 7 unique WHV DNA insertions into different host genes were detected at 1 h p.i. and 3 more at 3 h p.i. [
28].
Figure 2.
Examples of the earliest HBV and WHV DNA fusions with human or woodchuck genomic sequences detected between 15 min and one hour after exposure to virus in different infection systems investigated in our studies. With exception of the overlapping homologous junction (OHJ) created by the HBV X gene (HBV X) and human neurotrimin (NTM) gene displayed on the left side of the top panel, all other virus-host genomic fusions shown have format of the head-to-tail junction (HTJ). In the bottom left panel, the virus-host DNA merge was formed by the preS sequence of WHV S (envelope) gene (WHV PS) and it was detected in liver biopsy obtained from a woodchuck at one hour after injection with WHV, as described in the text. Other abbreviations: SINE, short-interspersed nuclear element (retrotransposon); MAML-2, mastermind-like 2; WHV X, WHV X gene; host, unidentified woodchuck genomic sequence.
Figure 2.
Examples of the earliest HBV and WHV DNA fusions with human or woodchuck genomic sequences detected between 15 min and one hour after exposure to virus in different infection systems investigated in our studies. With exception of the overlapping homologous junction (OHJ) created by the HBV X gene (HBV X) and human neurotrimin (NTM) gene displayed on the left side of the top panel, all other virus-host genomic fusions shown have format of the head-to-tail junction (HTJ). In the bottom left panel, the virus-host DNA merge was formed by the preS sequence of WHV S (envelope) gene (WHV PS) and it was detected in liver biopsy obtained from a woodchuck at one hour after injection with WHV, as described in the text. Other abbreviations: SINE, short-interspersed nuclear element (retrotransposon); MAML-2, mastermind-like 2; WHV X, WHV X gene; host, unidentified woodchuck genomic sequence.
Together, the time of the first hepadnavirus DNA integration into hepatocellular genome was identical in human HepaRG cells exposed to HBV and in woodchucks infected with WHV. Regarding replication of HBV and WHV, it was detected from 1 h p.i. in both infection systems [
28].
HepG2-NTCP-C4 cells exposed to recombinant HBV at MOI of 1000 provided a more effective system for identification of virus-host integrations as judged by the twice shorter time of the appearance of the first virus-host genomic fusions [
29]. The earliest HBV insertions into two distinct genes were identified at 30 min p.i. and one more fusion with another gene at one hour after exposure to virus. One of the sites was a merger with a retrotransposon called short-interspersed nuclear element (SINE) (
Figure 2). The second was with the neuroblastoma breakpoint family member 1 (NBPF-1), a pseudogene functioning as a tumor suppressor for neuroblastomas [
32]. In addition, HBV insertion into retrotransposon termed as the mammalian apparent retrotransposon long terminal repetitive (THE-1B-LTR) element was detected at 1 h p.i. (see below). There were no HBV DNA integration signals found in HepG2-NTCP-C4 cells collected at 15 min p.i. or the cells subjected to control mock infections, but there were several more HBV DNA insertions identified in the cells harvested beyond 1 h p.i., as reported [
29]. HBV replication in HepG2-NTCP-C4 cells became evident from 1 h p.i. indicating that the detection of virus integration preceded appearance of virus nucleic acid replicative intermediates.
The earliest WHV-host integrations were also investigated in woodchuck WCM260 hepatocytes exposed to wild-type WHV [
30]. In this model, four different host genes were identified as the initial integration sites at 15 min p.i. (
Figure 2). In addition, 4 other fusions were detected at 30 min p.i. and 3 more at 1 h p.i. This showed that hepadnaviral insertions occur in fact immediately after hepatocyte first contact with infectious hepadnavirus and confirmed that this takes place before initiation of virus replication, as some of the preceding studies also indicated [
29]. This remarkably short time between the first contact with hepadnavirus and its DNA insertion into hepatocyte genome became less startling when kinetics of virus-triggered cell DNA damage and kinetic of the damage repair were recognized (see below).
Retrotransposons and Transposable Elements are Frequent Targets of the Early HBV Integration
The clonal sequencing of the early integration sites showed that, in addition to several host somatic genes creating HBV-host fusions, mobile genetic elements containing repetitive non-coding genomic sequences, such as retrotransposon and transposon elements, and genes with translocation potential were virus frequent integration targets [
28,
29]. In our study of HBV-infected HepaRG cells, HBV DNA insertions within or near the long-interspersed element-1 (LINE1 or L1) and LINE2 (or L2) sequences were identified in the first 24-h p.i. [
28]. HBV fusions with LINE1 were also detected at 24-h p.i. in Huh7-NTCP hepatocyte-like cells investigated by others [
33]. HBV also formed a fusion with the gene encoding fibronectin leucine rich transmembrane protein (FLTR2) which at its C-terminus was joined with the LINE2 sequence. These joints were found at 3 h and 24 h p.i., as well as at day 3 p.i., and all three targeted the same locus on chromosome 14. Since over twenty percent of the human genome is comprised by the LINE sequence, the finding of the HBV-LINE fusions is not surprising [
34]. In this regard, LINE1 constitutes as much as 18%, while LINE2 accounts for about 3% of the genome [
34]. LINE1 displays endonuclease and reverse transcriptase activities and transposes via target-primed retrotransposition [
34]. These properties underline LINE1 pro-oncogenic potential. It has been reported that transcripts of the HBV X gene-LINE1 chimeras have tumor fostering characteristics and may adversely affect survival of patients with HBV-associated HCC [
35]. HBV integrations into the LINE sequences have been found at large numbers in tumorous and non-tumorous liver tissues from patients with HBV-associated HCC [
36]. Notably, while fusions of the HBV X gene with LINEs occurred in significantly higher numbers in HCC tissue, the merges with HBV S gene sequences were more frequent in non-tumor tissue. It also is of interest to mention that beyond 24 h p.i., HBV DNA fusions with a retrotransposable element annotated as human satellite II (HSAT-II) DNA was identified in HepaRG cells [
28]. This merge was particularly well represented at day 14 p.i. considering the number of clones in which it was identified. The integration of HBV with the HSAT-II sequence was not reported previously, but that with similar HSAT-III were identified in human hepatoma cells and patient-derived HCC tissue [
28,
37].
In HepG2-NTCP-C4 cells infected with HBV, one of the two earliest insertional sites detected at 30 min p.i. was a junction with retrotransposon SINE, as already mentioned. The HBV-SINE joint was found in 85% of the clones carrying the initial HBV-host DNA integrations in this infection system. This retrotransposon belongs to the non-long terminal repetitive (non-LTR) category. It is abundantly represented in human genome and implicated in oncogenic transformation [
38]. At one hour after exposure to HBV, HBV fusion with THE-1B-LTR was detected. This element is included in the mammalian apparent LTR retrotransposon (MaLR) family and is involved in the pathogenesis of non-Hodgkin’s lymphoma [
39]. THE-1B-LTR was joined with the HBV Enh-II sequence and therefore this hybrid might have a pathogenic relevance by modulating hepatocyte functions and possibly advancing development of HCC. At day 13 p.i. other HBV direct integrations or indirect merges with retrotransposons or transposable elements were detected in HepG2-NTCP-C4 cells. One of them was HBV merge with hobo activator-18
Salmo salar long terminal repeat (hAT-18-SsA) that transposes across genome via DNA-DNA fusions [
40]. The hAT-18-Ssa was joined by a non-coding sequence of chromosome-2 and that in turn was fused with the medium reiterated frequency repeat 5B (MER-5B) which also acts as transposon. Thus, this trimera was formed by the HBV sequence and two transposons. It is known that MER-5B controls expression of the gene encoding alpha fetoprotein (AFP) that occurs at high levels in fetal liver and HCC [
41]. This may suggest a connection between HBV integration and increased AFP synthesis which might be worthy of further investigation. Furthermore, HBV DNA-LINE2 fusion was identified at the same time point after infection. Overall, among HBV-host genomic junctions identified in HepG2-NTCP-C4 cells, 33% of all merges were with transposable elements and 49% of the clones carrying this type of merges belonged to category of the initial or very early HBV integrations. When the results from the HepRG and HepG2-NTCP-C4 cell lines were compared to the data from HepG2-NTCP and Huh7-NTCP investigated by others between 24 h to 7 days p.i. [
33], HBV merges with the same or comparable transposable elements were identified. These common elements included LINE1, SINE, THE-1B-related THE-Int, and MER-5B-related MER52D/41A/90A/4E1/4A [
29]. Therefore, there was relatively good compatibility between the findings obtained from different HBV infection models.
Our study documented that HBV inserts into hepatocyte repetitive non-coding sequences, such as retrotransposons and transposons, are frequent among the initial integration sites. They became detectable within 30 min after virus exposure and prior to molecular evidence of virus replication was apparent. The engagement of these mobile elements may suggest a mechanism by which HBV DNA could be dispersed across the hepatocyte genome soon after its entry into hepatocyte. Transposition of the fused HBV-mobile elements, in particular merges with virus regulatory sequences (see below), may expand the spread of virus genomic material throughout chromosomes as well as perturb organization and transcriptional activity of the hepatocellular genes. In consequence, hepatocyte function could be altered from the beginning of infection predisposing to pro-oncogenic changes during the cell lifetime which may prompt HCC development in some cases. This is consistent with the observed increase of HBV integrations within transposable elements in the livers of patients with HBV-associated HCC [
35,
36,
42,
43].
Hepadnavirus DNA Breaking Points Yielding Early Fusions with Hepatocyte Genome
Localization of HBV and WHV DNA breakpoints creating junctions with host sequences in the first hours after exposure to virus was limited to the X gene sequence owing to design of the primers applied for inv-PCR amplifications. Undeniably DNA breaks also occur in other parts of HBV and WHV genomes and they can create merges in the initial stages of infection. This was already exemplified when primers for the WHV preS genomic region were used to identify WHV DNA insertions and breakpoints in the preS and P gene sequences in liver biopsies acquired from woodchucks at one and 3 h p.i. (see
Figure 2) [
28].
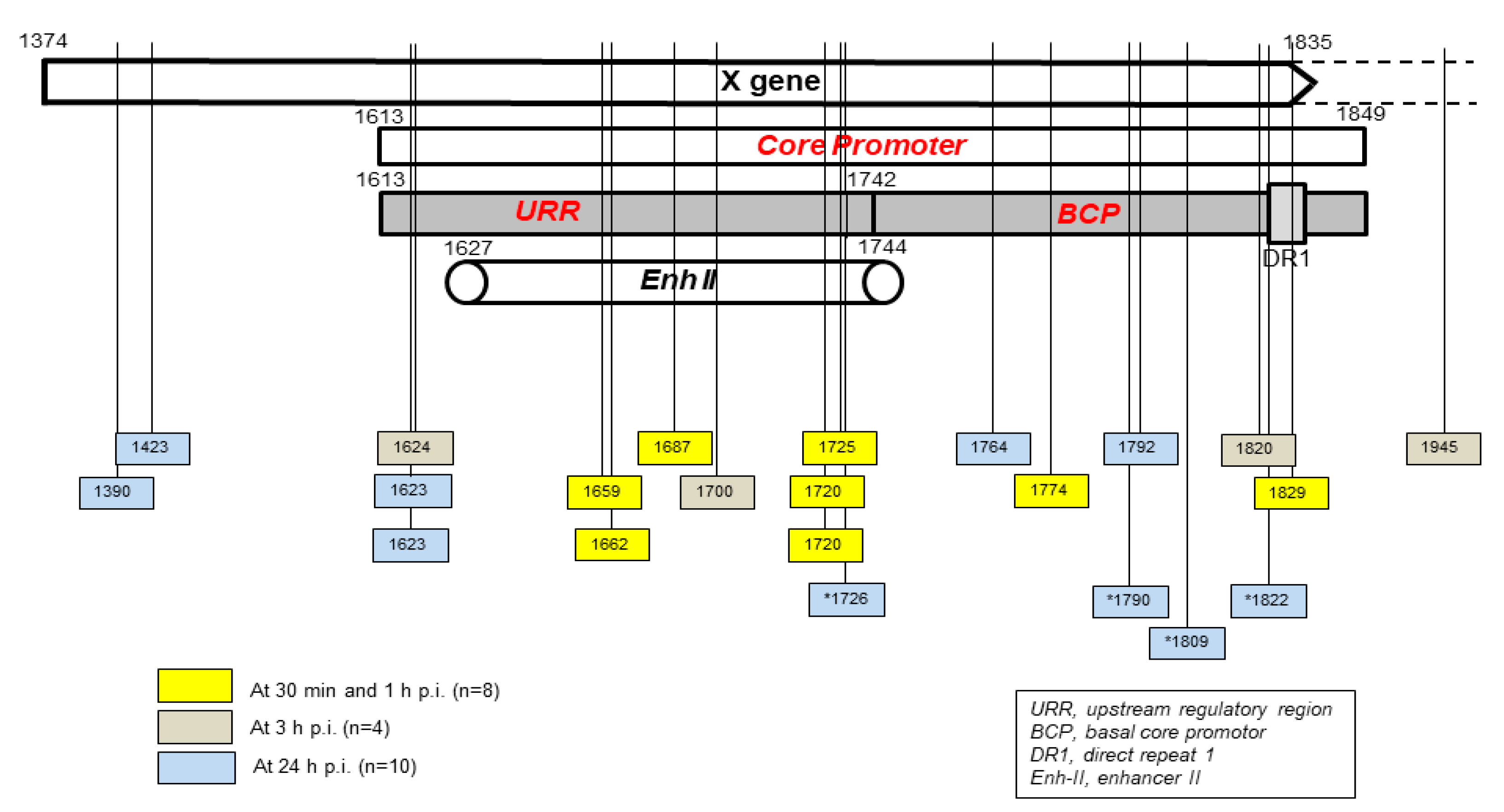
Figure 3.
Schematic presentation of the HBV X gene break points forming fusions with the human genomic sequences which were detected in hapatocyte-compatible HepRG and HepG2-NRCP-C5 cells within the first 24 hours after exposure to HBV. Yellow squares show breakpoints which formed junctions detected at 30 min and one hour after exposure to virus, red squares those identified at 3 h post-infection, and blue squares those detected at 24 h post-infection. Blue squares with stars show breakpoints reported at 24 h after infection in reference [
33]. Numbers mark nucleotide positions according to HBV DNA GenBank X79185 sequence. Abbreviations: URR, upstream regulatory region of the HBV core promoter; BCP, basal core promoter; DR1, direct repeat region; Enh II, HBV enhancer II.
Figure 3.
Schematic presentation of the HBV X gene break points forming fusions with the human genomic sequences which were detected in hapatocyte-compatible HepRG and HepG2-NRCP-C5 cells within the first 24 hours after exposure to HBV. Yellow squares show breakpoints which formed junctions detected at 30 min and one hour after exposure to virus, red squares those identified at 3 h post-infection, and blue squares those detected at 24 h post-infection. Blue squares with stars show breakpoints reported at 24 h after infection in reference [
33]. Numbers mark nucleotide positions according to HBV DNA GenBank X79185 sequence. Abbreviations: URR, upstream regulatory region of the HBV core promoter; BCP, basal core promoter; DR1, direct repeat region; Enh II, HBV enhancer II.
Taking into consideration the data from HepaRG and HepG2-NTCP-C4 cells obtained up to one-hour after exposure to virus, there were 8 HBV breakpoints found in total [
28,
29]. Six of them occurred within the X gene fragment overlapping the Enh-II sequence and the remaining two in the base core region (BCR) of the core promoter (
Figure 3). There were also 10 other breakpoints detected at 3 h and 24 h after infection. Only one of them detected at 3 h p.i. was located within Enh-II, 3 in upstream regulatory region (URR) of the core promoter just before the Enh-II sequence, three more within BCR, two at the beginning of the X gene sequence, and one outside the X gene at the beginning of the core gene (
Figure 3). By analyzing the data reported by another group which were collected at 24 h after infection of Huh7-NTCP cells with HBV [
33], we found that one of the four HBV breakpoints was within the Enh-II sequence and the remaining three within BCR. Overall, the results gave the impression that the earliest integration sites identified at 30 min and one-hour p.i. were created mainly by the breakpoints emerging in the HBV Enh-II sequence (
Figure 3). In contrast, the BCP appeared to be most prone to DNA breakages after one hour from exposure to virus,
i.e., at 3 h and 24 h p.i. This might be an interesting observation since both Enh-II and BCR are important regulatory elements relevant to HBV replication and they may also modulate expression of the merged host sequences. This would require analysis of more breakpoints within those elements and recognition of transcriptional activity and functional consequences of the resulting chimeras before this possibility becomes less elusive. WHV DNA breaks forming junctions with woodchuck genomes in liver biopsies collected in the first 3 h p.i. were also predominantly located in the WHx gene fragment overlapping the WHV BCP sequence and 5 different breakpoints were found at this location [
28]. In WCM260 hepatocytes exposed to WHV, virus integrations were discovered as early as 15 min p.i. and all WHV breakpoints found at this time point were located within the virus BCP sequence (Chauhan and Michalak, data unpublished).
Molecular Format of the Earliest Hepadnavirus-Host Genomic Junctions
The great majority of the HBV- and WHV-host genomic fusions detected in the first hour p.i. were merges of the HTJ type. In contrast, those having the OHJ format were very rare (
Figure 2) [
28,
29,
30]. Thus, among 8 HBV and 19 WHV insertional sites identified during this time period, only one (3.7%) was of the OHJ type and was created by HBV and the NTM gene sequence, as mentioned above [
28]. The same situation was seen when the host sites of HBV or WHV insertions were enumerated between one and 24 h p.i. Thus, a further 9 HBV and 3 WHV sites were identified. Among them again only one merge displayed the OHJ format implying its creation by micro-homology overlapping joining (MHOJ). This particular fusion was formed by HBV and the runt-related transcription factor 1 (RunX1) sequence at 24 h p.i. in HepG2-NTCP-C4 cells [
29]. The HTJ format of 4 other HBV-host fusions was identified in Huh7-NTCP cells exposed to recombinant HBV and investigated at 24 h p.i. by another group [
33]. Taken together, the results showed that HBV and WHV in the earliest stages of infection are joined with hepatocyte genomic sequences almost entirely via HTJs. The creation of such junctions reflects involvement of the non-homologous end joining (NHEJ) mechanism [
44,
45]. Directed by this finding and by the fact that NHEJ is involved in repair of cell dsDNA breaks, which presence is a prerequisite for HBV DNA integration [
46], we wanted to identify a molecular thread connecting these events.
Mechanism of Formation of the Initial Hepadnaviral-Host Genomic Merges
It was shown that HBV can induce oxidative stress by triggering production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) that cause cell DNA oxidation and oxidation-induced double-stranded (ds) DNA breakages [
47]. To assess if oxidative DNA damage contributes to formation of the initial hepadnavirus-host DNA junctions, woodchuck WCM260 hepatocytes in which WHV-host HTJs were formed from 15 min p.i. were investigated from this time point onwards [
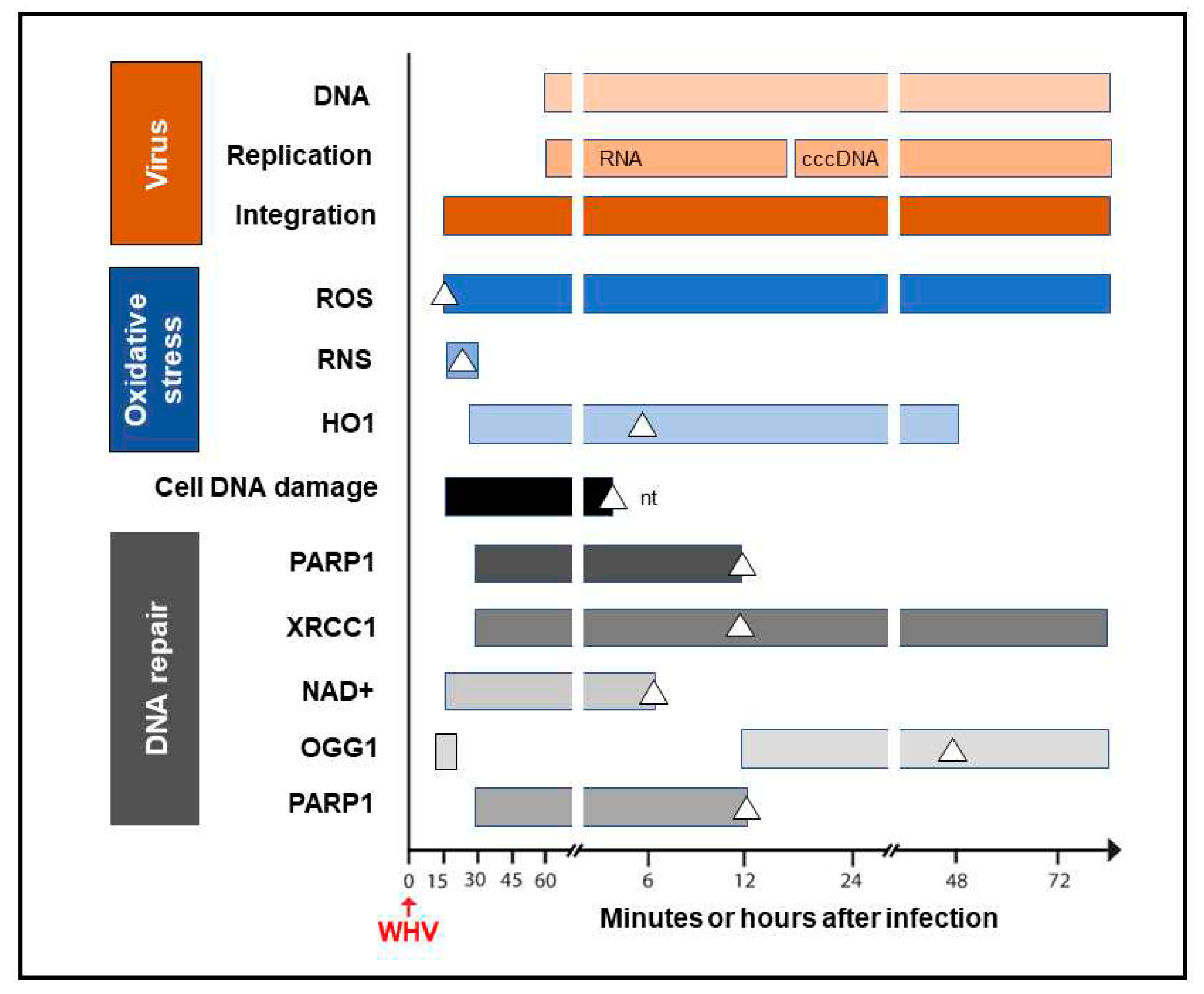
30]. We found a strong and prolonged induction of ROS but only very transient production of inducible nitric oxide (iNOS) which both became detectable from 15 min after exposure to virus (
Figure 4). Thus, while ROS reactivity remained highly elevated between 15 min and 6 h after infection, activity of iNOS increased only briefly between 15 and 30 min p.i. This coincided with microscopically evident cellular DNA damage which significantly increased between 15 min and one-hour p.i., as determined by single cell alkaline comet assay and the nuclear tail length measurements [
30]. To determine whether repair of the DNA breakages due to virus-induced oxidative stress was responsible for formation of the initial virus-host fusions, the time kinetics of transcription of poly(ADP-ribose) polymerase 1 (PARP1), which recognizes dsDNA breaks and facilitates their repair by the alternative NHEJ pathway, and X-ray repair cross-complementing protein 1 (XRCC1), which is a binding partner of PARP1 in this process, were quantified [
30]. This was supplemented with quantifications of nicotinamide adenine dinucleotide (NAD
+) activity, an indicator of PARP1 activation, heme oxygenase-1 (HO1) transcription, a marker of pro-oxidative cell stress, 8-oxyguanidine DNA glucose 1 (OGG1) transcription, an indicator of cell response to oxidative DNA damage, and by quantification of the PARP1 cleavage activity. The data revealed the synchronized induction of the PARP1 and XRCC1 transcription accompanied by augmented reactivity of NAD
+ and HO1 which all were initiated in 15 to 30 min p.i. (
Figure 4).
In addition, a transient 5.6-fold increase over uninfected control cells in the OGG1 gene expression (although not statistically significant) was detected between 15 and 30 min p.i. and then from 12 h p.i. onwards [
30]. PARP1 cleavage became significantly augmented between 6 and 12 h p.i., but a 2-fold increase, although statistically not significant, was identified at one-hour after infection. These quantitative measurements indicated that hepadnavirus is a strong and essentially immediate inducer of hepatocyte oxidative stress and associated DNA damage, and that the PARP1/XRRC1-initiated dsDNA NHEJ repair is involved in creation of the earliest virus-host fusions. A progressive increase in PARP1 transcription up to 6 h p.i. subsided after 12 h p.i. to the level detectable in control cells (
Figure 4). This sharp decline was accompanied by an increase in cleavage of PARP1 protein [
30]. Jointly, our data showed that the PARP1-facilitated dsDNA repair is engaged in the initial stages of infection and the NHEJ mechanism determines the HTJ format of the earliest hepadnavirus-host genomic merges. In stages beyond a few hours after infection, the virus-host junctions tend to display more often the OHJ format implying their creation by the MHOJ repair mechanism.
Figure 4.
Graphic presentation of the earliest detections and changes over time in the presence of virus genome (DNA), its replication (mRNA and cccDNA) and integration into hepatocellular genome, indicators of hepatocyte oxidative stress and oxidative DNA damage, as well as markers of the activity of components of the DNA repair pathway during the first 72-hour period after exposure to HBV or WHV. The profiles represent cumulative data from HBV and WHV infections in human and woodchuck hepatocyte-compatible cells and in woodchucks infected with WHV. White triangles mark the time at which peak expression or activity was found. White square in the OGG1 line represents a 5.6-fold increase in the gene expression over uninfected control cells which did not achieve statistically significant difference. Abbreviations: cccDNA, virus covalently closed circular DNA; ROS, reactive oxygen species; RNS, reactive nitrogen species; HO1, heme oxygenase-1; PARP1, poly(ADP-ribose) polymerase 1; XRCC1, X-ray repair cross-complementing protein 1; NAD+, nicotinamide adenine dinucleotide; OGG1, 8-oxyguanidine DNA glucose 1, and nt, not tested beyond this time point.
Figure 4.
Graphic presentation of the earliest detections and changes over time in the presence of virus genome (DNA), its replication (mRNA and cccDNA) and integration into hepatocellular genome, indicators of hepatocyte oxidative stress and oxidative DNA damage, as well as markers of the activity of components of the DNA repair pathway during the first 72-hour period after exposure to HBV or WHV. The profiles represent cumulative data from HBV and WHV infections in human and woodchuck hepatocyte-compatible cells and in woodchucks infected with WHV. White triangles mark the time at which peak expression or activity was found. White square in the OGG1 line represents a 5.6-fold increase in the gene expression over uninfected control cells which did not achieve statistically significant difference. Abbreviations: cccDNA, virus covalently closed circular DNA; ROS, reactive oxygen species; RNS, reactive nitrogen species; HO1, heme oxygenase-1; PARP1, poly(ADP-ribose) polymerase 1; XRCC1, X-ray repair cross-complementing protein 1; NAD+, nicotinamide adenine dinucleotide; OGG1, 8-oxyguanidine DNA glucose 1, and nt, not tested beyond this time point.
Conclusions and Future Directions
The propensity of HBV to integrate into human hepatocyte genome has been recognized from the commencement of studies investigating this virus, but the time when this exactly happens, the molecular format of the earliest virus-host genomic fusions, and mechanism of their formation remained unrecognized until now. Because HBV DNA integration into hepatocellular genome is considered to be the main contributor to this virus oncogenicity, this prompted our interest in elucidation of the time kinetics of creation of the earliest HBV- and WHV-host DNA merges, identification of host sequences functioning as the sites of virus initial insertions, and a mechanism of creation of these earliest virus-host junctions. The data obtained showed that the first HBV insertions became detectable in 30 min after infection with HBV in human HepG2 cells overexpressing NTCP [
29] and at one-hour after exposure to HBV when human HepaRG cells and liver biopsies from woodchucks infected with WHV were examined [
28]. Human non-coding DNA elements, including retrotransposons and transposons, constituted almost half of the early HBV insertional sites. In addition, several somatic genes including those coding factors and enzymes implicated in cell growth and cancer development were identified in the very early time points post-infection. A similar study of woodchuck hepatocytes
de novo infected with WHV showed virus DNA insertions from 15 min onwards after exposure to virus. This further revised our perception about the time required for formation of the first virus-host genomic fusions and solidified data indicating that hepadnavirus integration can precede initiation of its replication. This prompted our studies on the molecular mechanism of formation of the initial virus-host DNA merges. Since our findings showed that the initial fusions have almost exclusively the head-to-tail format, this indicated that their formation involves the NHEJ pathway. We elected to examine the alternative route for NHEJ dsDNA repair that is mediated by PARP1, which is known to operate in response to oxidative dsDNA damage in mammalian cells and is prone to joining errors potentially leading to cancer [
45,
48]. Examination of intracellular levels of ROS, cellular DNA damage, and transcription of genes involved in response to oxidative DNA damage unveiled that their statistically significant increases occurred in 15 to 30 min after infection and hence they closely coincided with formation of the first hepadnavirus-host fusions. These results collectively showed that hepadnavirus is a potent and instantaneous trigger of oxidative DNA damage in hepatocytes and swiftly activates dsDNA repair machinery. This machinery facilitates incorporation of viral DNA sequences at breakpoints into host DNA and determines the prevailing head-to-tail format of the initial DNA junctions. Interestingly, HBV and WHV regulatory elements in their X gene sequences appear to be most prone to breakage and to formation of the earliest virus-host DNA fusions. This can possibly modify expression of individual genes and compromise overall stability of hepatocyte genome, and by this create a microenvironment supportive of the oncogenic process that with time may culminate in HCC in some cases. It is feasible that a similar pro-oncogenic process can be initiated by HBV at the extrahepatic locations in which virus-host genomic merges are formed [
8,
19,
27].
Several aspects recognized or suggested by the studies summarized in this communication would require further investigations. Among them, complete mapping of the integration sites and the DNA breakpoints within HBV and WHV genomes forming initial junctions with host sequences would help to determine whether other hot spots, similar to the X gene/core promoter sequence, exist. This would confirm or indicate a need to modify the scope and the focus of further investigations. In this regard, determination of the ability of different HBV genotypes to form the early virus-host fusions, particularly genotypes which more frequently than others coincide with HCC development, might be interesting. We noticed two profiles of the initial HBV integration in our earliest study [
28]. Thus, while HBV inoculum of A genotype yielded within one-hour after infection merges with several genes identifiable in singular clones, HBV of C genotype produced fusions with two sites which were robustly represented in multiple clones within the same time period. Since experimental and analytical conditions were identical, this variation might be linked to virus alone. This preliminary observation requires further study to become conclusive. The finding of numerous and instantaneous HBV and WHV insertions into host somatic genes and noncoding sequences raises important issues regarding their long-term stability and possible functional and pathogenic consequences. Essentially there are no data in this regard except some indirectly applicable information derived from analysis of HBV-host junctions in nontumorous and tumorous liver tissues in advanced CHB. One of such observations is that basically the same HBV X gene/core promotor region is prone to breakpoints both immediately after virus entry to hepatocyte (see
Figure 3) and in HBV infection persisting for years in the liver [
28,
49,
50]. As it was proposed for chronically infected liver, the initial or very early HBV insertions into hepatocyte genome may predispose to clonal expansion of the cells carrying certain chimeric sequences [
50,
51]. Considering the above, although it is difficult to imagine survival of the initially infected hepatocyte for years due to its normally limited lifespan (unless immortalized), it is more acceptable to consider that daughter cells created throughout clonal expansion carry the same HBV integration profile as the hepatocytes primarily infected. The overlapping ranges of the host sequences serving as HBV initial and very late (i.e., during CHB) insertional sites might be interpreted in support of this possibility. Regarding delineation of potential functional and pathogenic/oncogenic consequences of the initial and very early virus-host chimeras there is progress in exploring approaches allowing examination of such issues. This is exemplified by identification of transcriptional activity of the HBx-LINE1 chimeras in patients with HBV-associated HCC and by showing tumor promoting properties of the transcripts in vitro [
35].
Author Contributions
TIM designed and wrote the manuscript.
Funding
Studies summarized in this communication were supported by an operating research grant from the Cancer Research Society Inc. and the Environmental Cancer Fund – Read for the Cure, Canada (PIN 22346) and in part by an operating research grant from the Canadian Institutes of Health (PJT-153001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The author acknowledges Dr. R. Chauhan’s essential contributions to the studies summarized in this communication and Dr. P.M. Mulrooney-Cousins and Ms. N.D. Churchill for their excellent technical and administrative support.
Conflicts of Interest
There are no conflicts of interest.
References
- Tarocchi, M. , Polvani S., Marroncini G., Galli A. Molecular mechanism of hepatitis B virus-induced hepatocarcinogenesis. World J Gastroenterol. 2014;20:11630–11640. [CrossRef]
- Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. 2016;64(1 Suppl):S84-S101. [CrossRef]
- Mak LY, Wong DK, Pollicino T, Raimondo G, Hollinger FB, Yuen MF. Occult hepatitis B infection and hepatocellular carcinoma: Epidemiology, virology, hepatocarcinogenesis and clinical significance. J Hepatol. 2020;73:952-964. [CrossRef]
- Russo FP, Zanetto A, Pinto E, Battistella S, Penzo B, Burra P, Farinati F. Hepatocellular carcinoma in chronic viral hepatitis: Where do we stand? Int J Mol Sci. 2022; 23:500. [CrossRef]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. [CrossRef]
- Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, McGlynn KA, Soerjomataram I. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598-1606. [CrossRef]
- Michalak, TI. Diverse virus and host-dependent mechanisms influence the systemic and intrahepatic immune responses in the woodchuck model of hepatitis B. Front Immunol. 2020;11:853. [CrossRef]
- Coffin CS, Mulrooney-Cousins PM, Michalak TI. Hepadnaviral lymphotropism and its relevance to HBV persistence and pathogenesis. Front Microbiol. 2021;12:695384. [CrossRef]
- Williams JB, Hüppner A, Mulrooney-Cousins PM, Michalak TI. Differential expression of woodchuck Toll-like receptors 1-10 in distinct forms of infection and stages of hepatitis in experimental hepatitis B virus infection. Front Microbiol. 2018;9:3007. [CrossRef]
- Liu LY, Ma XZ, Ouyang B, Ings DP, Marwah S, Liu J, Chen AY, Gupta R, Manuel J, Chen XC, Gage BK, Cirlan I, Khuu N, Chung S, Camat D, Cheng M, Sekhon M, Zagorovsky K, Abdou Mohamed MA, Thoeni C, Atif J, Echeverri J, Kollmann D, Fischer S, Bader GD, Chan WCW, Michalak TI, McGilvray ID, MacParland SA. Nanoparticle uptake in a spontaneous and immunocompetent woodchuck liver cancer model. ACS Nano. 2020;14:4698-4715. [CrossRef]
- Daffis S, Balsitis S, Chamberlain J, Zheng J, Santos R, Rowe W, Ramakrishnan D, Pattabiraman D, Spurlock S, Chu R, Kang D, Mish M, Ramirez R, Li L, Li B, Ma S, Hung M, Voitenleitner C, Yon C, Suresh M, Menne S, Cote P, Delaney WE 4th, Mackman R, Fletcher SP. Toll-Like receptor 8 agonist GS-9688 induces sustained efficacy in the woodchuck model of chronic hepatitis B. Hepatology. 2021;73:53-67. [CrossRef]
- Suresh M, Menne S. Recent drug development in the woodchuck nodel of chronic hepatitis B. Viruses. 2022;14:1711. [CrossRef]
- Corkum CP, Wiede LL, Ruble CL, Qiu J, Mulrooney-Cousins PM, Steeves MA, Watson DE, Michalak TI. Identification of antibodies cross-reactive with woodchuck immune cells and activation of virus-specific and global cytotoxic T cell responses by anti-PD-1 and anti-PD-L1 in experimental chronic hepatitis B and persistent occult hepadnaviral infection. Front Microbiol. 2022;13:1011070. [CrossRef]
- Chayanupatkul M, Omino R, Mittal S, Kramer JR, Richardson P, Thrift AP, El-Serag HB, Kanwal F. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis B virus infection. J. Hepatol. 2017;66 355-362. [CrossRef]
- World Health Organization Fact Sheet Hepatitis B. Updated June 2022. World Health Organization, Geneva, Switzerland (accessed on 17 March 2023). (accessed on 17 March 2023).
- Saitta C, Pollicino T, Raimondo G. Occult hepatitis B virus infection: An update. Viruses. 2022;14:1504. [CrossRef]
- Michalak TI, Mulrooney PM, Coffin CS. Low doses of hepadnavirus induce infection of the lymphatic system that does not engage the liver. J Virol. 2004;78:1730-8. [CrossRef]
- Gujar SA, Michalak TI. Primary occult hepadnavirus infection induces virus-specific T-cell and aberrant cytokine responses in the absence of antiviral antibody reactivity in the Woodchuck model of hepatitis B virus infection. J Virol. 2009;83:3861-76. [CrossRef]
- Mulrooney-Cousins PM, Chauhan R, Churchill ND, Michalak TI. Primary seronegative but molecularly evident hepadnaviral infection engages liver and induces hepatocarcinoma in the woodchuck model of hepatitis B. PLoS Pathog. 2014;10:e1004332. [CrossRef]
- Michalak TI, Pardoe IU, Coffin CS, et al. Occult lifelong persistence of infectious hepadnavirus and residual liver inflammation in woodchucks convalescent from acute viral hepatitis. Hepatology. 1999;29:928-38. [CrossRef]
- Brechot C, Pourcel C, Louise A, Rain B, Tiollais P. Presence of integrated hepatitis B virus DNA sequences in cellular DNA of human hepatocellular carcinoma. Nature. 1980;286:533-535. [CrossRef]
- Shafritz DA, Shouval D, Sherman HI, Hadziyannis SJ, Kew MC. Integration of hepatitis B virus DNA into the genome of liver cells in chronic liver disease and hepatocellular carcinoma. Studies in percutaneous liver biopsies and post-mortem tissue specimens. N Engl J Med. 1981;305:1067-73. [CrossRef]
- Sung WK, Zheng H, Li S, Chen R, Liu X, Li Y, Lee NP, Lee WH, Ariyaratne PN, Tennakoon C, Mulawadi FH, Wong KF, Liu AM, Poon RT, Fan ST, Chan KL, Gong Z, Hu Y, Lin Z, Wang G, Zhang Q, Barber TD, Chou WC, Aggarwal A, Hao K, Zhou W, Zhang C, Hardwick J, Buser C, Xu J, Kan Z, Dai H, Mao M, Reinhard C, Wang J, Luk JM. Genome-wide survey of recurrent HBV integration in hepatocellular carcinoma. Nat Genet. 2012;44:765-769. [CrossRef]
- Yang M, Yang G, Li F, Ou M, Li C, Chen J, Lin H, Zhang Y, Xue W, Wu Y, Xu Y, Sui W, Dai Y. HBV integrated genomic characterization revealed hepatocyte genomic alterations in HBV-related hepatocellular carcinomas. Mol Clin Oncol. 2020;13:79. [CrossRef]
- Laskus T, Radkowski M, Wang LF, Nowicki M, Rakela J. Detection and sequence analysis of hepatitis B virus integration in peripheral blood mononuclear cells. J Virol. 1999;73:1235-8. [CrossRef]
- Sinha M, Sundar K, Premalata CS, Asati V, Murali A, Bajpai AK, Davuluri S, Acharya KK, Lakshmaiah KC, Babu K G, Jacob LA, Nandan D, Velayutham D, Datta S, Jayshree RS. Pro-oncogenic, intra host viral quasispecies in diffuse large B cell lymphoma patients with occult hepatitis B virus infection. Sci Rep. 2019;9:14516.
- Lau KC, Joshi SS, Gao S, Giles E, Swidinsky K, van Marle G, Bathe OF, Urbanski SJ, Terrault NA, Burak KW, Osiowy C, Coffin CS. Oncogenic HBV variants and integration are present in hepatic and lymphoid cells derived from chronic HBV patients. Cancer Lett. 2020;480:39-47. [CrossRef]
- Chauhan R, Churchill ND, Mulrooney-Cousins PM, Michalak TI. Initial sites of hepadnavirus integration into host genome in human hepatocytes and in the woodchuck model of hepatitis B-associated hepatocellular carcinoma. Oncogenesis. 2017;6:e317. [CrossRef]
- Chauhan R, Shimizu Y, Watashi K, Wakita T, Fukasawa M, Michalak TI. Retrotransposon elements among initial sites of hepatitis B virus integration into human genome in the HepG2-NTCP cell infection model. Cancer Genet. 2019;235-236:39-56. [CrossRef]
- Chauhan R, Michalak TI. Kinetics of DNA damage repair response accompanying initial hepadnavirus-host genomic integration in woodchuck hepatitis virus infection of hepatocyte. Cancer Genet. 2020;244:1-10. [CrossRef]
- De Minicis S, Seki E, Uchinami H, Kluwe J, Zhang Y, Brenner DA, Schwabe RF. Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology. 2007;132:1937-46. [CrossRef]
- Vandepoele K, Staes K, Andries V, van Roy F. Chibby interacts with NBPF1 and clusterin, two candidate tumor suppressors linked to neuroblastoma. Exp Cell Res. 2010;316:1225-1233. [CrossRef]
- Tu T, Budzinska MA, Vondran FWR, Shackel NA, Urban S. Hepatitis B virus DNA integration occurs early in the viral life cycle in an in vitro infection model via sodium taurocholate cotransporting polypeptide-dependent uptake of enveloped virus particles. J Virol. 2018;92:e02007-17. [CrossRef]
- Ostertag EM, Kazazian HH Jr. Biology of mammalian L1 retrotransposons. Annu Rev Genet. 2001;35:501-538. [CrossRef]
- Lau CC, Sun T, Ching AK, He M, Li JW, Wong AM, Co NN, Chan AW, Li PS, Lung RW, Tong JH, Lai PB, Chan HL, To KF, Chan TF, Wong N. Viral-human chimeric transcript predisposes risk to liver cancer development and progression. Cancer Cell. 2014;25:335-349. [CrossRef]
- Giosa D, Lombardo D, Musolino C, Chines V, Raffa G, Casuscelli di Tocco F, D'Aliberti D, Caminiti G, Saitta C, Alibrandi A, Aiese Cigliano R, Romeo O, Navarra G, Raimondo G, Pollicino T. Mitochondrial DNA is a target of HBV integration. Commun Biol. 2023;6:684. [CrossRef]
- Shaul Y, Garcia PD, Schonberg S, Rutter WJ. Integration of hepatitis B virus DNA in chromosome-specific satellite sequences. J Virol. 1986;59:731-734. [CrossRef]
- Naville M, Henriet S, Warren I, Sumic S, Reeve M, Volff JN, Chourrout D. Massive changes of genome size driven by expansions of non-autonomous transposable elements. Curr Biol. 2019;29:1161-1168. [CrossRef]
- Martín-Moreno AM, Roncador G, Maestre L, Mata E, Jiménez S, Martínez-Torrecuadrada JL, Reyes-García AI, Rubio C, Tomás JF, Estévez M, Pulford K, Piris MA, García JF. CSF1R protein expression in reactive lymphoid tissues and lymphoma: Its relevance in classical Hodgkin lymphoma. PLoS One. 2015;10:e0125203. [CrossRef]
- Rubin E, Lithwick G, Levy AA. Structure and evolution of the hAT transposon superfamily. Genetics. 2001;158:949-957. [CrossRef]
- de Souza FS, Franchini LF, Rubinstein M. Exaptation of transposable elements into novel cis-regulatory elements: is the evidence always strong? Mol Biol Evol. 2013;30:1239-1251. [CrossRef]
- Zhao LH, Liu X, Yan HX, Li WY, Zeng X, Yang Y, Zhao J, Liu SP, Zhuang XH, Lin C, Qin CJ, Zhao Y, Pan ZY, Huang G, Liu H, Zhang J, Wang RY, Yang Y, Wen W, Lv GS, Zhang HL, Wu H, Huang S, Wang MD, Tang L, Cao HZ, Wang L, Lee TL, Jiang H, Tan YX, Yuan SX, Hou GJ, Tao QF, Xu QG, Zhang XQ, Wu MC, Xu X, Wang J, Yang HM, Zhou WP, Wang HY. Genomic and oncogenic preference of HBV integration in hepatocellular carcinoma. Nat Commun. 2016;7:12992. Erratum in: Nat Commun. 2016 Nov 08;7:13591. Erratum: Lee, TP [corrected to Lee, TL]. [CrossRef]
- Péneau C, Imbeaud S, La Bella T, Hirsch TZ, Caruso S, Calderaro J, Paradis V, Blanc JF, Letouzé E, Nault JC, Amaddeo G, Zucman-Rossi J. Hepatitis B virus integrations promote local and distant oncogenic driver alterations in hepatocellular carcinoma. Gut. 2022;71:616-626. [CrossRef]
- 44 Audebert M, Salles B, Calsou P. Involvement of poly(ADP-ribose) polymerase-1 and XRCC1/DNA ligase III in an alternative route for DNA double-strand breaks rejoining. J Biol Chem. 2004;279:55117-26. [CrossRef]
- Wang M, Wu W, Wu W, Rosidi B, Zhang L, Wang H, Iliakis G. PARP-1 and Ku compete for repair of DNA double strand breaks by distinct NHEJ pathways. Nucleic Acids Res. 2006;34:6170-6182. [CrossRef]
- Mason WS, Gill US, Litwin S, Zhou Y, Peri S, Pop O, Hong ML, Naik S, Quaglia A, Bertoletti A, Kennedy PT. HBV DNA integration and clonal hepatocyte expansion in chronic hepatitis B patients considered immune tolerant. Gastroenterology. 2016;151:986-998.e4. [CrossRef]
- Dandri M, Burda MR, Bürkle A, Zuckerman DM, Will H, Rogler CE, Greten H, Petersen J. Increase in de novo HBV DNA integrations in response to oxidative DNA damage or inhibition of poly(ADP-ribosyl)ation. Hepatology. 2002;35:217-2 23. [CrossRef]
- Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat Rev Cancer. 2010;10:293-301. [CrossRef]
- Chauhan R, Michalak TI. Earleist hepatitis B virus-host hepatocyte genome integration: Sites, mechanism, and significance in carcinogenesis. Hepatoma Res. 2021;7:20. [CrossRef]
- Mason WS, Liu C, Aldrich CE, Litwin S, Yeh MM. Clonal expansion of normal-appearing human hepatocytes during chronic hepatitis B virus infection. J Virol. 2010;84:8308-8315. [CrossRef]
- Mason WS, Jilbert AR, Litwin S. Hepatitis B virus DNA integration and clonal expansion of hepatocytes in the chronically infected liver. Viruses. 2021;13:210. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).