Introduction
Cervical cancer is the fourth most prevalent cancer worldwide, and the fourth leading cause of cancer death, accounting for approximately 570,000 new cases and 311,000 deaths in 2018 (1). The global burden of cervical cancer is unevenly distributed, with > 85% of cases occurring in low- and middle-income countries (2). Over recent decades, trends in cervical cancer incidence and mortality rates have varied among countries. In the United States, there were 13,069 new cervical cancer cases and 4,175 deaths in 2015 (1), while in Japan, 10,879 new cases and 2,921 deaths were reported in 2019 (3). Among the deaths from cervical cancer in Japan, an average of 340 per year were women aged 20–44 years between 2011 and 2019 (3). Although the incidence and mortality rates of cervical cancer have decreased in many developed countries (4), there has been a significant increase in Japan in the last decade (3).
The World Health Organization recommends surgical resection of premalignant cervical lesions to reduce cervical cancer incidence and mortality in this century (4). Cold knife conization and loop-electric excisional procedure (LEEP) are considered the gold standard treatments for high-grade cervical lesions (cervical intraepithelial neoplasia grade 2 and 3; CIN2/3). However, conization, especially with a cold knife, has been associated with an increased risk of preterm delivery and premature rupture of membranes (PROM) post-treatment (5). The frequency and severity of these adverse sequelae increase with increasing cone depth and repeated treatments. Procedures such as LEEP conization and cryotherapy can also potentially induce premature delivery, although the incidence is lower compared to cold-knife conization (6). To avoid such obstetric complications in young women with CIN2/3 or AIS planning future pregnancies, less invasive yet effective procedures are desirable.
It is now widely accepted that certain types of human papillomaviruses (HPV) are the etiological factor for cervical cancer and its precursor lesions (7). While many women clear HPV infections within a few months, some have persistent infections (8). This persistent HPV infection is a prerequisite for malignant progression due to the continuous expression of HPV viral oncoproteins E6 and E7, which promote cell proliferation, genomic instability, and inhibition of host immune responses (9, 10). The most notorious HPV16 infection can be cleared by cell-mediated immunity against E2, E6, and E7 proteins of this type (10), and some therapeutic HPV vaccines have been developed to eliminate high-grade squamous intraepithelial lesions (HSILs; worse than CIN2) in some patients (11). However, HPV16 has the ability to evade host immune responses through three mechanisms; non-exposure of HPV antigens during its lifecycle, suppression of certain interferon secretions by HPV16 E6 and E7 proteins, and acquisition of immune tolerance (10). Immune tolerance is likely induced following antigen presentation by dendritic cells or macrophages without stimulation of innate immunity (local inflammation) (12).
In cosmetic dermatology, liquid phenol is utilized to peel the skin, as it penetrates deeply and kills cells (13). It has been reported that, in 82.6% of patients, HPV-related common warts cleared after 80%-phenol therapy, while in 70% they cleared after cryotherapy (14). More than 80% complete remission is observed in high-grade CINs with a single treatment with trichloroacetic acid (TCA), another skin cosmetic agent (15). To develop non-invasive treatment methods, we evaluated the efficacy of phenol and TCA therapies in treating cervical or vaginal CIN. In this non-randomized prospective study, we investigated the effectiveness and limitations of phenol therapy. Some cases required > 2 years until clearance, and others needed surgical treatment. A multivariate analysis was performed to identify factors that confer resistance to this treatment. This study could provide important information to aid the development of new therapies, including therapeutic HPV vaccines, for premalignant lesions induced by high-risk (HR) HPV infection.
Materials and Methods
Subjects and Study Design
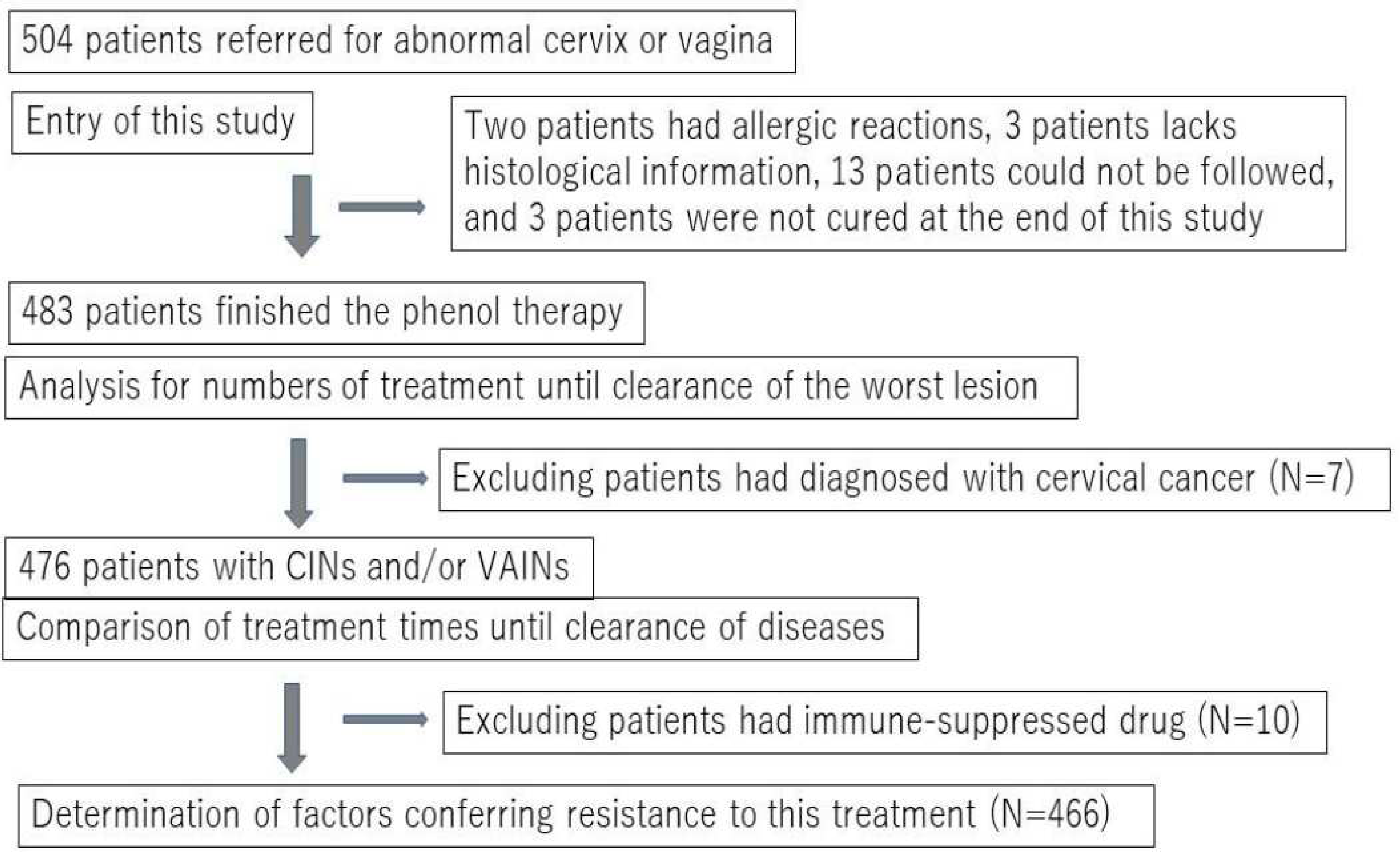
This non-randomized prospective study was conducted from September 2009 to April 2018. Initially, 504 women who were referred to two outpatient clinics in Ishikawa and Okinawa Prefectures, Japan due to abnormal Pap test results (worse than ASCUS and positive for HPV) were recruited to this study. All participants underwent a histopathological examination guided by colposcopy, and were diagnosed with low-grade and high-grade cervical or vaginal squamous intraepithelial lesions (LR or HR-SILs). For convenience, we used the terminology of intraepithelial neoplasia grades 1–3 (cervico-vaginal intraepithelial neoplasia [CIN]1–3 or vaginal intraepithelial neoplasia [VAIN]1–3) in this study. After obtaining written consent to participate in the study, which was approved by the committee of Kanazawa Medical University (#107), all subjects diagnosed with CIN, VAIN, or CIN+VaIN underwent chemical peeling therapy using liquid phenol (phenol therapy). Twenty-one subjects were excluded from the analysis, as shown in
Figure 1. Therefore, a total of 483 patients were included in this study. The number of treatments required for clearance or termination (cases that needed surgery for invasive cancer) was compared among different grades.
Demographic Characteristics of Subjects
At the beginning of the study, information on age, smoking habits (direct or passive), and use of immune-suppressive drugs was obtained from all subjects. Self-report information on obstetric outcomes post-treatment was gathered from 98 subjects who became pregnant after therapy.
Procedure, Adverse Effects of the Phenol Therapy, and Follow-Up Methods
Liquid phenol has traditionally been used to treat skin warts in some Japanese clinics and in Singapore (14). We used an 89% liquid phenol solution (Maruishi Seiyaku, Japan) to treat intraepithelial lesions of the cervix or vagina. The solution was applied to wider areas (about 1 cm outside of the squamous lesion and about 2–3 cm inside the cervical canal) three to five times using a small cotton tip (3 × 15 mm). It was crucial to absorb all the liquid into the vagina with a cotton ball after this treatment to prevent phenol solution leakage to the vulva. Some patients reported dizziness, palpitations, and lower abdominal discomfort during or after this application. While these symptoms usually disappeared within 10 minutes post-treatment, in some cases they persisted for a day. Lidocaine gel was used in some cases before treatment to alleviate local pain or discomfort. Two patients chose to discontinue the treatment due to skin rash (possibly allergic reactions), while 13 patients dropped out of the study for unknown reasons (
Figure 1).
Pathological Diagnosis and HPV Test
Diagnosis was established histologically by two clinical pathologists according to the WHO classification (2014). However, for the sake of simplicity, we used previous terminology such as CIN1–3 and VaIN1–3) in the current study. During treatment, invasive cervical cancer (1A1. 1A2 and 1B1) was identified in seven patients, while the others were diagnosed with additional diseases, primarily VAIN. Therefore, the final histological diagnosis is reported in this manuscript.
Liquid-based cytology (LBC) samples for Pap tests were collected from all subjects at 4-week intervals. Diagnosis was reached through consensus between cytotechnologists and clinical pathologists (blinded to the HPV test results) in the Clinical Pathology Unit at Kanazawa Medical University Hospital or Yuai Medical Center.
A portion of each LBC sample was sent to a commercial laboratory (LSI Medience, Tokyo, Japan) for HPV genotyping. The HPV genotype was determined using a PCR-based test (Genosearch-31; MBL, Nagoya, Japan) (16). This test can detect 13 HR HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68), probable high-risk (pHR) types (26, 53, 66, 70, 73, 82), and low-risk (LR) types (6, 11, 42, 44, 54, 55, 61, 62, 71, 84, 89, and 90). To identify additional pHR-HPV types, such as HPV34, 67, and 69, the uniplex PCR method (17) was used for some CIN or VAIN cases that tested negative with the aforementioned test. In the current study, HPV was grouped as HPV16/18, higher-risk-other high-risk type (Higher risk-OHR; HPV-31, 33, 45, 52, 58), lower-risk other-high-risk (Lower risk-OHR; HPV-35, 39, 51, 56, 59, 68), probably high-risk (P-HR; HPV-26, 30, 34, 66, 67, 69, 70, 73, 82) and HPV-negative or low-risk (Negative/LR) HPV. For cases with multiple HPV types, the highest risk type was considered the primary HPV type responsible for the disease. HPV testing was performed at study entry and at the visit after disease clearance. HPV genotyping was conducted in 477 subjects at entry, and 327 cases after disease clearance.
Statistical Analysis
Statistical analysis was performed using JMP14 software. Treatment times were compared between two groups using the Mann-Whitney test. P-values <0.05 were considered to indicate statistically significant differences. Logistic regression analysis was also conducted to identify independent risk factors for treatment resistance. The cut-off number of treatments indicating treatment resistance was calculated as 13 treatments, as this exceeds the 75th percentile of number of treatments until disease clearance among all cases. In this analysis, lesion size and HPV infection pattern were omitted due to the lack of lesion size information in many cases, and the close association between HPV infection pattern and HPV group, which could confound the analysis.
Results
Clinical Course of Cervico-vaginal Abnormal Lesions via Phenol Therapy
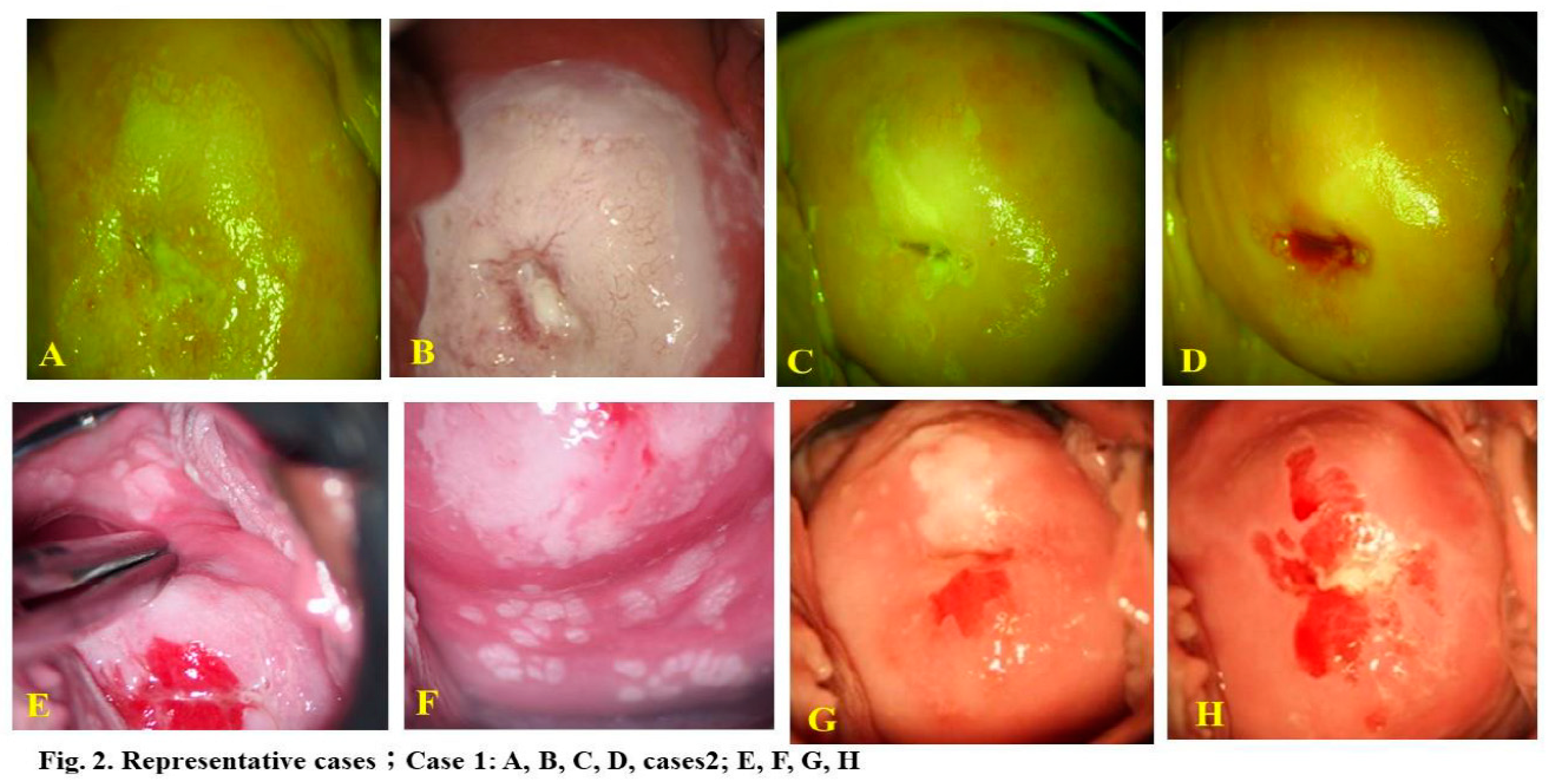
The current study analyzed 483 eligible subjects. Two representative cases are illustrated in
Figure 2. Case 1 involved a 24-year-old woman with HPV16-positive CIN3, and Case 2 involved a 20-year-old woman with multiple CIN3, VAIN2, and cervicovaginal condyloma lesions positive for HPV6, 16, 66. In these instances, 17 and 22 phenol therapy sessions were conducted, respectively, until complete lesion and HPV clearance. The former case involved a passive smoker.
The final diagnosis for 3 cases was downgraded, while it was upgraded in 16 other cases, including 7 of cervical cancer. The present study comprised subjects with CIN1 or VaIN1 (n = 129), CIN1+VaIN1 (n = 34), CIN2 or VaIN2 (n = 149), CIN2+VaIN1/2 (n = 22), CIN3 or AIS (n = 113), CIN3+AIS+VaIN (n = 19), Cancer (n = 7), and immune-suppressed CIN or VAIN (IMM-SUP; n = 10). The IMM-SUP group contained cases of CIN1 or VAIN (n = 2), CIN1+VAIN1 (n = 2), CIN2 (n = 4), and CIN3 (n = 2).
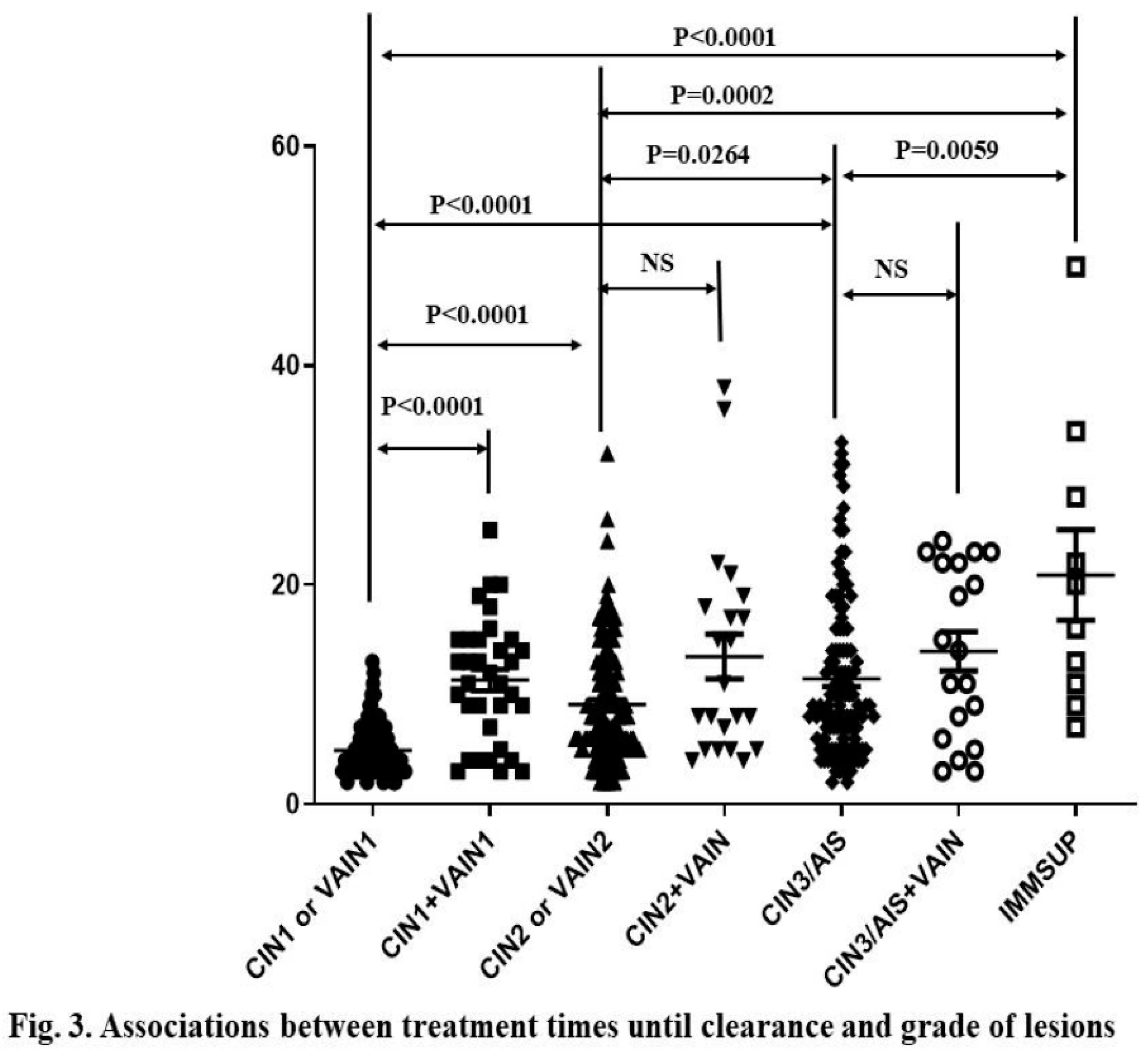
Among 476 cases excluding Cancer, the number of treatments until disease clearance ranged from 2 to 49 sessions (median = 7 sessions, 95% CI: 8.83–10.8) across all subjects. Treatment period ranged from 1 to 60 months (median = 7 months, 95% CI: 8.25–10.08) among 302 subjects with available records. The average number of sessions until disease clearance for CIN1 or VaIN1, CIN2 or VaIN2, CIN3 or AIS, and IMM-SUP was 4.9 (95% CI: 4.52-5.29), 9.1 (95% CI: 8.23–9.96), 11.4 (95% CI: 10.4–12.8), and 20.9 (95% CI: 11.6–30.2), respectively. Higher-grade CINs required more treatments than lower-grade ones (Fig.3A). IMM-SUP required more treatments than any grade of CIN or VaIN (Mann-Whitney test; P<0.05,
Figure 3A).
Conversely, the average numbers of treatment sessions until disease clearance for CIN1+VaIN1, CIN2+VaIN1/2, and CIN3+any VaIN were 11.3 (95% CI: 9.36–13.3), 13.5 (95% CI: 9.18–17.7), and 13.9 (95% CI: 10.2–17.7), respectively. There was no difference in number of treatment sessions among CIN1+VAIN1, CIN2+VAIN1/2, and CIN3/AIS+VAIN. However, co-existing CIN1 and VaIN1 cases required more treatments than those of only CIN1 or VaIN1 (Fig.3A).
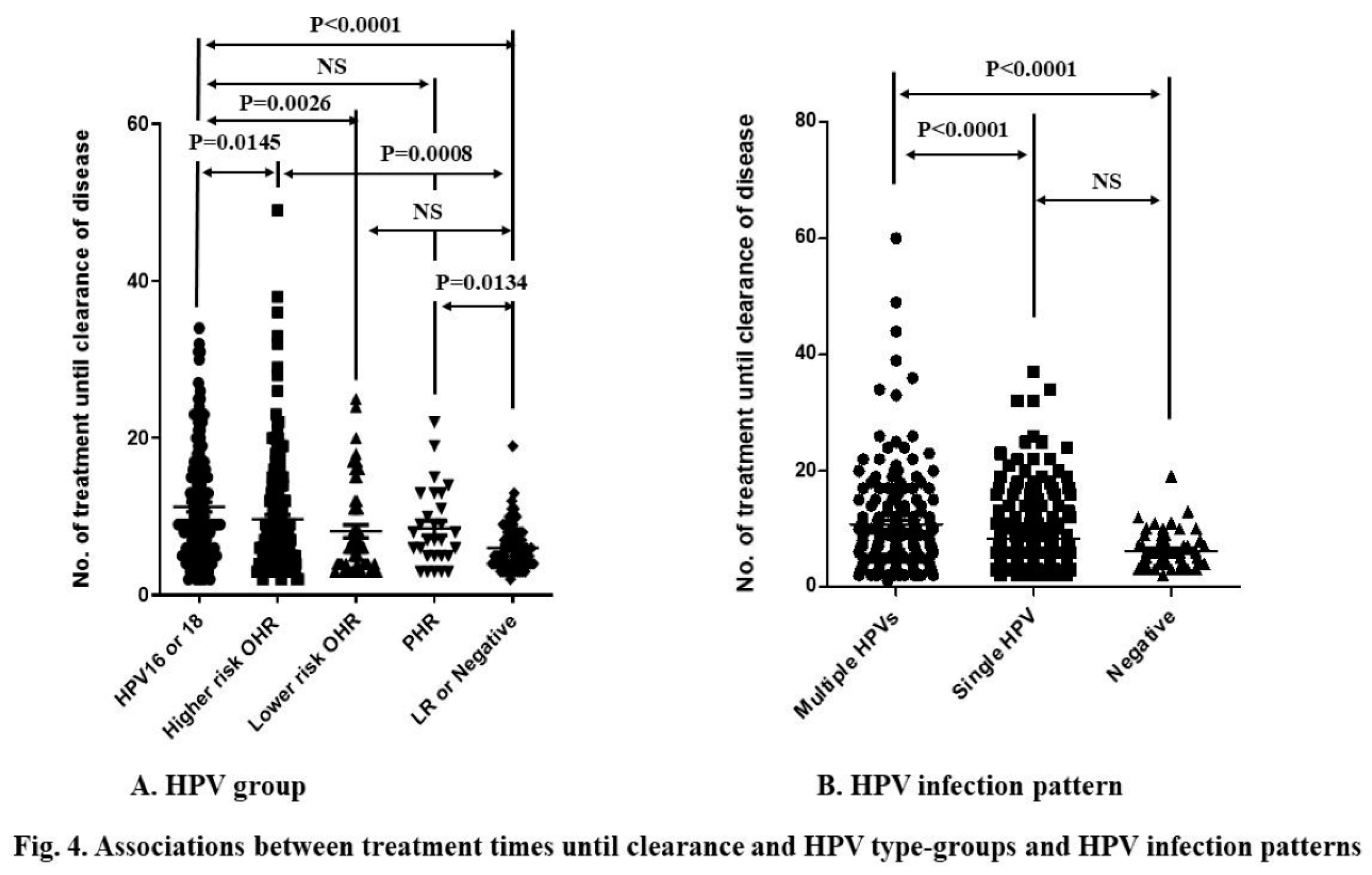
The prevalence rates of HPV16/18, Higher risk-OHR, Lower risk-OHR, P-HR, and Negative/LR were 29.6% (n = 139), 38.4% (n = 180), 10.7% (n = 50), 6.2% (n = 29), and 15.1% (n = 71), respectively. No HPV test was done in 7 cases. HPV16/18, Higher risk-OHR and P-HR HPV types required more treatments than LR/Negative. HPV16/18 needed more treatment than Higher risk OHR and Lower risk OHR tyles. However, there was no difference in treatment time between HPV16/18 and PHR HPV-types, and between Lower-risk OHR and LR/Negative (Fig.4A). Multiple HPV type infection needed more treatment than single HPV type infection and negative/LR-HPV type (
Figure 4B).
Management of Cases Resistant to Therapy and Recurring Cases
In total, 52 of 483 (10.8%) underwent surgical treatment based on clinicians’ or patients’ decisions. These cases were diagnosed as CIN1 or VaIN (n = 3), CIN1+VaIN1 (n = 2), CIN2 or VAIN2 (n = 10), CIN2+VAIN1 (n = 2), CIN3 or AIS (n = 26), CIN3+VAIN (n = 2), and cancer (n = 7). One CIN3 case and two CIN1+VaIN1 cases were IMM-SUP. One CIN1+VAIN1 case required three LEEP procedures due to multiple extensive cervicovaginal lesions.
Cervical cancer IA1 was identified in one case after hysterectomy, while the remaining six cancer cases were diagnosed prior to surgery. Most of these cases were resistant to phenol therapy. The cancer diagnoses included stage-1A1(n = 3), 1A2 (n = 2) and 1B1 (n = 2), with four cases of squamous cell carcinoma (SCC) and three of adenocarcinoma (ADC). One 1A1 case underwent conization, another underwent simple hysterectomy, and the rest received radical hysterectomy with pelvic lymph node resection. All of these patients have remained disease-free as of July 2023.
Excluding cancer cases, 18 cases (3.8%) experienced recurrence of the same or lower-grade lesions after disease clearance. Recurrence of CIN2 or worse was observed in 1.5% (7/476) of subjects. Ten recurrent cases were completely cured by additional phenol therapy, three underwent LEEP surgery, and five remained classified as LSIL or ASCUS at the study’s conclusion. The latter cases were treated with TCA therapy (a chemical peeling therapy that we implemented in May 2018).
Obstetric Outcomes in Subsequent Pregnancies
Ninety-eight subjects reported their obstetric experiences post-therapy to clinicians. Of these, 97 subjects (99%) had full-term deliveries. One case resulted in premature delivery at 35 weeks without any obstetrical complications. Four subjects underwent LEEP after phenol therapy but still had normal deliveries.
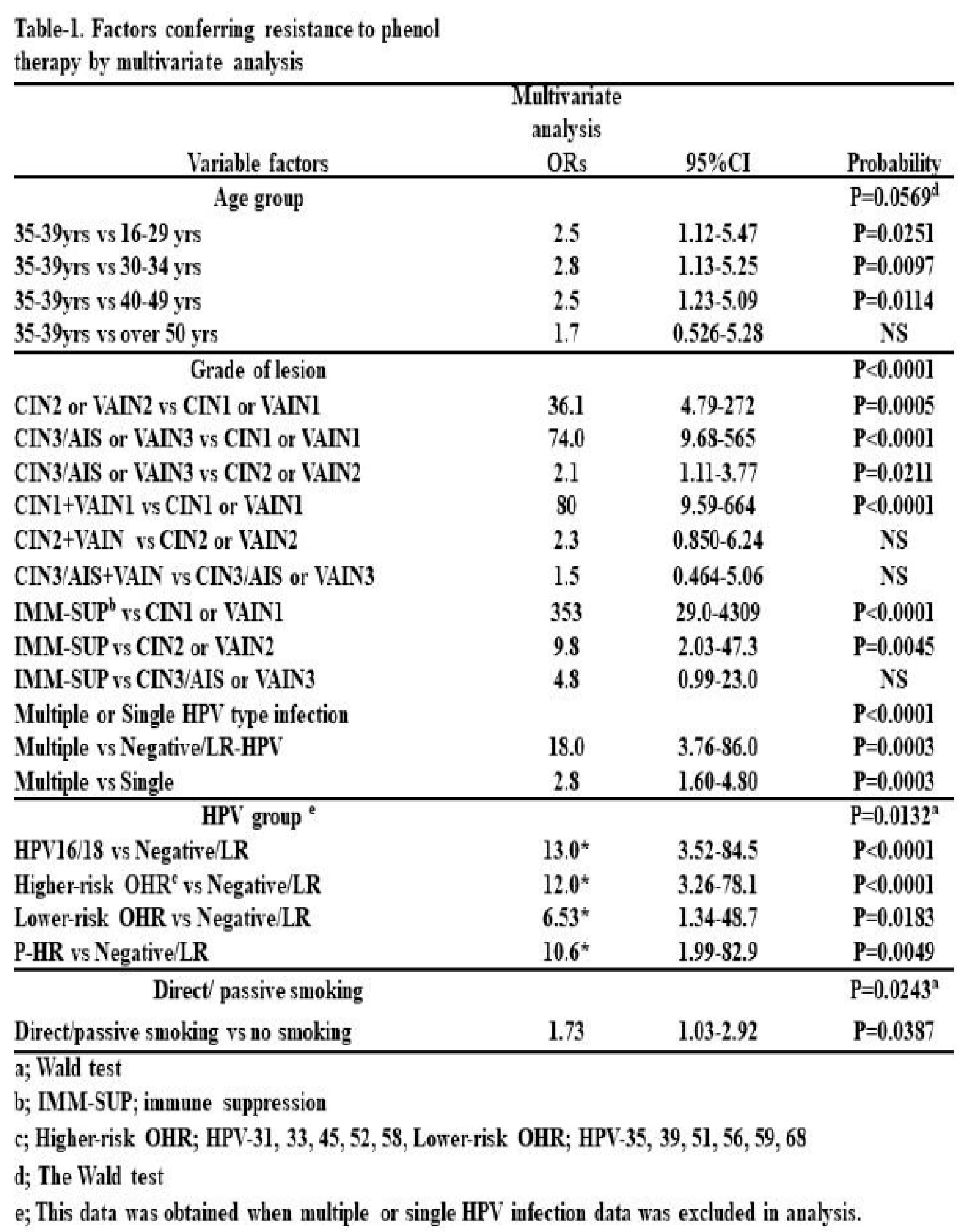
Factors Conferring Resistance to Phenol Therapy
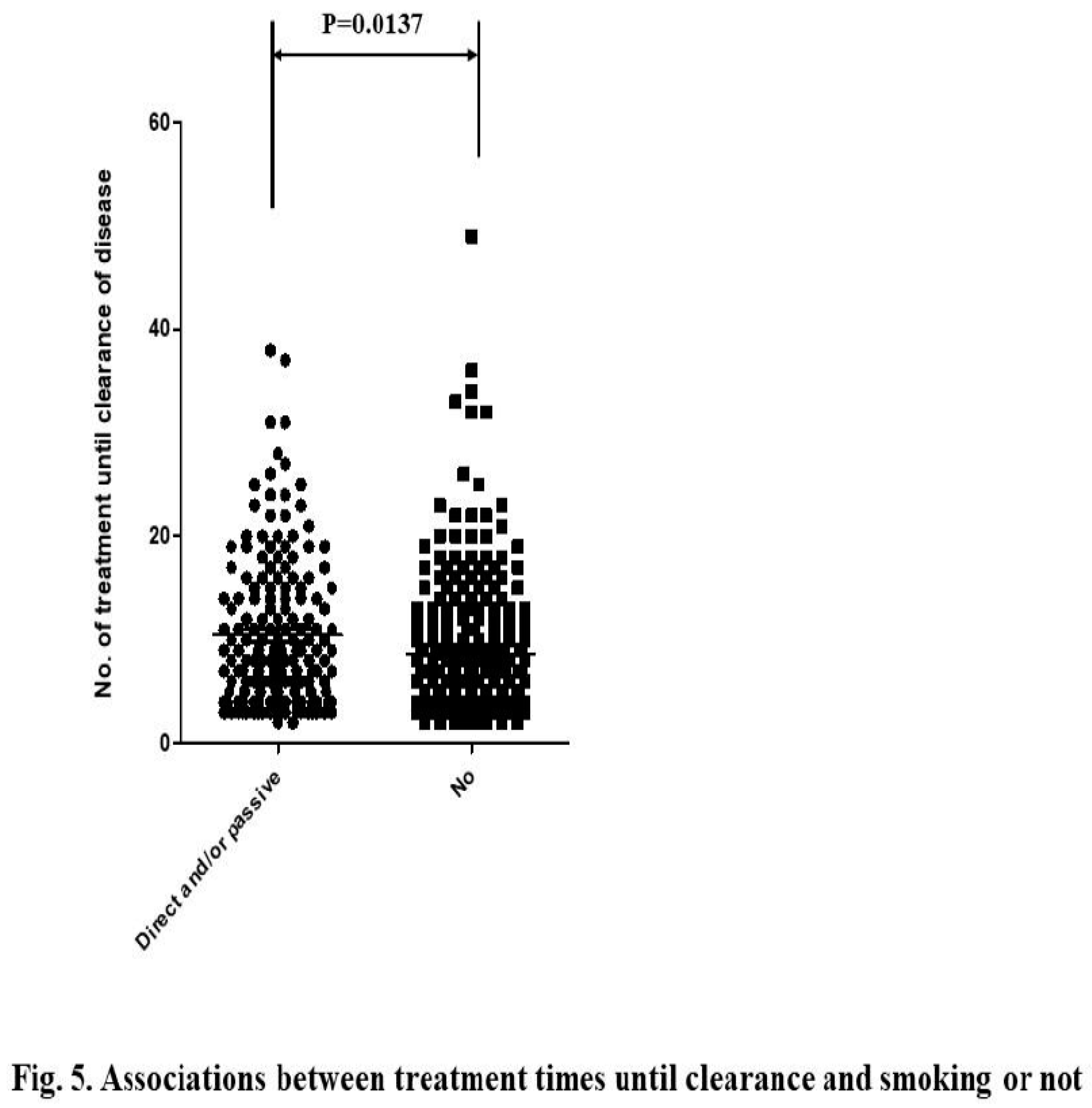
We sought to determine the factors associated with resistance to this treatment, excluding cancer cases. A univariate analysis revealed that factors such as being 35–39 years of age, having higher-grade lesions or IMM-SUP status (
Figure 3), HR HPV infection or multiple HPV type infection (
Figure 4), and active or passive smoking (
Figure 5) were significantly associated with resistance. In this analysis, there was no difference between active or passive (secondary) smokers and non-smokers.
Multivariate analysis indicated that being 35–39 years of age, having higher-grade lesions, being IMM-SUP, having multiple HPV type infections, and active or passive smoking were associated with treatment resistance (Table 1). In terms of lesion grades, higher-grade CINs and CIN1+VaIN1 were more strongly associated with resistance than lower-grade lesions and CIN1 or VaIN1, respectively. However, there was no statistical difference between CIN2+VaIN1/2 and CIN2 or VaIN1/2, or between CIN3/AIS+VaIN and CIN3/AIS. IMM-SUP was more strongly associated with resistance than CIN1 and CIN2. Multiple HPV type infections were more strongly associated with resistance than single HPV type infections and Negative/LR HPV infections. Regarding HPV risk classification, HR (HPV16/18, HR-OHR, LR-OHR) and pHR HPV infections were more strongly associated with resistance than Negative/LR HPV infections. However, no difference was observed among HPV16/18, HR-OHR, LR-OHR, and pHR-HPV types.
Discussion
In our study, of all subjects receiving phenol therapy, 52 required surgical intervention, demonstrating that phenol therapy alone successfully cured 89.2% of the subjects. The rate of treatment failure, defined as recurrence of CIN2 or worse, was very low at 1.9% (6/320). A meta-analysis of 96 studies on the surgical treatment of precancerous cervical lesions (CIN2 and CIN3) reported a recurrence rate of 6.6% (95% CI: 4.9–8.4) (18). The rates were found to be heterogeneous (range: 1.4–18.4) and varied by treatment procedure, being 2% for cold-knife conization and laser conization and 7% for large loop excision of the transformation zone (18). A multicenter study in Japan with 14,832 cases (median age = 37, range: 16–88 years), had a recurrence rate of 6.1% (19). Long-term follow-up studies demonstrated that the 5- and 10-year risks of developing post-treatment CIN2 and CIN3 were 16.5%, 8.6%, 18.3%, and 9.2%, respectively (20). Most subjects in the current study were followed for 5–14 years, suggesting that phenol therapy is effective and safe.
Changes in the average age at first marriage and remarriage for women from 1989 to 2018 (25.8–29.4 and 36.5–40.4 years, respectively) (21) suggest that many women who wish to have children are at high risk of developing premalignant cervical lesions. Although cervical conization with a cold knife and loop-electrical excisional procedure (LEEP) are the gold standard treatments for high-grade cervical lesions (CIN2/3) (4), such surgical treatments have an obstetric disadvantage. Relative risk (RR) for premature delivery after conization with a cold knife, laser conization, large loop excision of the transformation zone (LLETZ) is 2.7, 2.11, and 1.57, respectively (5). This risk also exists for LEEP and cryotherapy (6). Phenol therapy showed almost no adverse obstetric outcomes in 98 pregnant cases, including 4 women who received LEEP treatment after phenol therapy.
The key disadvantage of phenol therapy is the need for multiple treatments (range: 2–49 treatments; median = 7 treatments) until CIN and VaIN clearance. It may be necessary to carefully select suitable cases for this therapy and recommend alternative treatments for those who are likely to resist this therapy. Thus, we examined factors contributing to resistance to this therapy. These factors included being 35–39 years of age, having high-grade lesions (CIN2 or worse), high-risk or probable high-risk HPV type-positive, immune suppression, and smoking.
The group with the highest prevalence of cervical cancer in Japan is women aged 30–49 years, while the group with the highest prevalence of premalignant lesions (HSIL, CIN2/3) is women aged 25–34 years (3). The peak age of the incidence of cervical cancer from 2011–2015 in Japan was 40–44 years. This suggests that most women aged 35–39 years with CIN are likely to be at the highest risk for progressing to invasive cancer within 5 years. Lesion grade was another risk factor for resistance to this therapy in our analysis. However, HPV16 or 18 infection was not a stronger risk factor than infection with other high- or probable high-risk HPV types (Table 1). Lesion grade appears to be a more important predictor of treatment resistance than HPV type. However, immune suppression was a stronger predictor than any grade of CINs or VaINs, suggesting that immune suppression is the most important factor contributing to resistance to this therapy. Many studies have already suggested that the host immune response is important for persistent HPV infection and cervical cancer development (7-9). Other studies also suggest that HIV-positive individuals are at higher risk for cervical cancer (22, 23) and oropharyngeal cancer (24).
In the natural history of HPV infection leading to cancer, smoking, hormonal exposure, and HIV are additional exposures that increase the risk of progression to cancer (25). Some studies suggest that not only active smokers, but also passive (26, 27) and tertiary smokers (27), are at high risk for cervical cancer. The present study also demonstrated that active or passive smoking is associated with treatment resistance, although no difference was observed between active or passive smokers and non-smokers. An association of smoking with treatment resistance is likely to emerge in this study, as many smokers had quit smoking or avoided passive smoking due to knowledge among practitioners that smoking promotes cervical cancer development. Some studies show that smoking cessation after cancer diagnosis enhances the therapeutic response (28).
As mentioned above, immune suppression is the most important factor contributing to resistance to this therapy. It is known that smoking affects host immune response (29) against persistent HPV infection, especially in high-grade CINs. Immune tolerance to some HPV antigens or an immune-suppressive state induced by immune checkpoint inhibitors may be present in higher-grade lesions infected with high-risk HPV types (10, 12). Some therapeutic HPV vaccines have been successful in treating HPV-related pre-malignant lesions (11). However, complete clearance by such therapy has not yet been established. Treatment failure was also observed with this method, as about 11% of subjects needed surgical treatment in addition to phenol therapy. To increase the efficacy of any type of treatment for cervical pre-malignant lesions or cancer, we need to better understand the mechanisms of host immunity against persistent HPV infection and certain driver gene mutations in host cells progressing to cancer. The vaginal microbiota (30) may also be important for a comprehensive understanding of host defense systems against HPV-related cancer.
Acknowledgments
This work was granted with Research Donations from Uozu city. Method of multivariate analysis was supervised by Prof. Muneko Nishijou, Dept of Epidemiology and Public Health, Division of Innovative Research, Kanazawa Medical University.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M. Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer. Available online: https://gco.iarc.fr/today (accessed on 26 September 2020).
- Cancer Statistics. Cancer Information Service, National Cancer Center, Japan (Vital Statistics of Japan, Ministry of Health, Labour and Welfare) for incidence and mortality. 2019.
- Global strategy to accelerate the elimination of cervical cancer as a public health problem, WHO, 2000.
- Kyrgiou, M.; Athanasiou, A.; Kalliala, I.E.J.; Paraskevaidi, M.; Mitra, A.; Martin-Hirsch, P.P.; Arbyn, M.; Bennett, P.; Paraskevaidis, E. Cochrane Gynaecological, Neuro-oncology and Orphan Cancer Group. Obstetric outcomes after conservative treatment for cervical intraepithelial lesions and early invasive disease. Cochrane Database Syst. Rev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Santesso N, Mustafa RA., Wiercioch W, Kehar R, Gandhi S, Chen Y, Cheung A, Hopkins J, Khatib R, Ma B, Mustafa AA, Lloyd N, Wu D, Broutet N, Schünemann HJ, Systematic reviews and meta-analyses of benefits and harms of cryotherapy, LEEP, and cold knife conization to treat cervical intraepithelial neoplasia. International Journal of Gynecol Obstet 2016, 132, 266–271.
- Bosch, F.X.; Lorincz, A.; Munoz, N.; Meijer, C.J.L.M.; Shah, K.V. The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 2002, 55, 244–265. [Google Scholar] [CrossRef] [PubMed]
- Winer, R.L.; Lee, S.-K.; Hughes, J.P.; Adam, D.E.; Kiviat, N.B.; Koutsky, L.A. Genital Human Papillomavirus Infection: Incidence and Risk Factors in a Cohort of Female University Students. Am. J. Epidemiology 2003, 157, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR, Stanley MA. The biology and life-cycle of human papillomaviruses Vaccine. 2012;30 Suppl 5:F55-70.
- Sasagawa, T.; Takagi, H.; Makinoda, S. Immune responses against human papillomavirus (HPV) infection and evasion of host defense in cervical cancer. J. Infect. Chemother. 2012, 18, 807–815. [Google Scholar] [CrossRef]
- Hancock G, Hellner K, Dorrell L. Therapeutic HPV vaccines. Best Pract Res Clin Obstet Gynaecol. 2018; 47: 59-72.
- Tindle, R.W. Immune evasion in human papillomavirus-associated cervical cancer. Nat. Rev. Cancer 2002, 2, 59–64. [Google Scholar] [CrossRef]
- Truchuelo, M.; Cerdá, P.; Fernández, L. Chemical Peeling: A Useful Tool in the Office. Actas Dermosifiliogr. 2017, 108, 315–322. [Google Scholar] [CrossRef]
- Banihashemi M, Pezeshkpoor F, Yazdanpanah M J, Family S. Efficacy of 80% phenol solution incomparison with cryotherapy in thetreatment of common warts of hands. Singapore Med 2008, 49, 1035–1037.
- Geisler, S.; Speiser, S.; Speiser, L.; Heinze, G.; Rosenthal, A.; Speiser, P. Short-Term Efficacy of Trichloroacetic Acid in the Treatment of Cervical Intraepithelial Neoplasia. Obstetrics & Gynecology 2016, 127, 353–359. [Google Scholar] [CrossRef]
- Sasagawa, T.; Maehama, T.; Ideta, K.; Irie, T. ; Fujiko Fujiko Itoh J-HERS Study Group Population-based study for human papillomavirus (HPV) infection in young women in Japan: A multicenter study by the Japanese human papillomavirus disease education research survey group (J-HERS). J. Med Virol. 2015, 88, 324–335. [Google Scholar] [CrossRef]
- Okodo M, Okayama K, Teruya K et al. Uniplex E6/E7 PCR method detecting E6 or E7 genes in 39 human papillomavirus types. J Med Virol 2018, 90, 981–988. [CrossRef] [PubMed]
- Arbyn, M.; E Redman, C.W.; Verdoodt, F.; Kyrgiou, M.; Tzafetas, M.; Ghaem-Maghami, S.; Petry, K.-U.; Leeson, S.; Bergeron, C.; Nieminen, P.; et al. Incomplete excision of cervical precancer as a predictor of treatment failure: a systematic review and meta-analysis. Lancet Oncol. 2017, 18, 1665–1679. [Google Scholar] [CrossRef] [PubMed]
- Murakami, I.; Ohno, A.; Ikeda, M.; Yamashita, H.; Mikami, M.; Kobayashi, Y.; Nagase, S.; Yokoyama, M.; Enomoto, T.; Katabuchi, H. Analysis of pathological and clinical characteristics of cervical conization according to age group in Japan. Heliyon 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Kocken, M.; Helmerhorst, T.J.; Berkhof, J.; A Louwers, J.; Nobbenhuis, M.A.; Bais, A.G.; Hogewoning, C.J.; Zaal, A.; Verheijen, R.H.; Snijders, P.J.; et al. Risk of recurrent high-grade cervical intraepithelial neoplasia after successful treatment: a long-term multi-cohort study. Lancet Oncol. 2011, 12, 441–450. [Google Scholar] [CrossRef]
- Changes in marriage age in Japan; fig/table.1-1-9 published in 2020 from Ministry of Health, Labour and Welfare, Japan.
- Castle, P.E.; Einstein, M.H.; Sahasrabuddhe, V.V.; Mbbs, V.V.S. Cervical cancer prevention and control in women living with human immunodeficiency virus. CA: A Cancer J. Clin. 2021, 71, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.M.; Tully, S.; Franceschi, S. Carcinogenicity of Human Papillomavirus (HPV) Types in HIV-Positive Women: A Meta-Analysis From HPV Infection to Cervical Cancer. Clin. Infect. Dis. 2017, 64, 1228–1235. [Google Scholar] [CrossRef]
- Gillison, M.L. Oropharyngeal cancer: a potential consequence of concomitant HPV and HIV infection. Curr. Opin. Oncol. 2009, 21, 439–444. [Google Scholar] [CrossRef]
- de Sanjosé, S.; Brotons, M.; Pavón, M.A. The natural history of human papillomavirus infection. Best Pr. Res. Clin. Obstet. Gynaecol. 2018, 47, 2–13. [Google Scholar] [CrossRef]
- Su B, Qin W, Xue F, Wei X, Guan Q, Jiang W, Wang S, Xu M, Yu S. The relation of passive smoking with cervical cancer: A systematic review and meta-analysis. Medicine (Baltimore). 2018; 97: e13061. Medicine (Baltimore) 2018, 97, e13061. [CrossRef]
- Wen, Q.; Wang, X.; Lv, J.; Guo, Y.; Pei, P.; Yang, L.; Chen, Y.; Du, H.; Burgess, S.; Hacker, A.; et al. Association between involuntary smoking and risk of cervical cancer in Chinese female never smokers: A prospective cohort study. Environ. Res. 2022, 212, 113371. [Google Scholar] [CrossRef]
- Chellappan, S. Smoking Cessation after Cancer Diagnosis and Enhanced Therapy Response: Mechanisms and Significance. Curr Oncol. 2022, 29, 9956–9969. [Google Scholar] [CrossRef] [PubMed]
- Feifei Qiu 1, Chun-Ling Liang 1, Huazhen Liu 1, Yu-Qun Zeng 2, Shaozhen Hou 3, Song Huang 3, Xiaoping Lai 3, Zhenhua Da. Impacts of cigarette smoking on immune responsiveness: Up and down or upside down? Oncotarget 2017, 8, 268–284.
- Mitra, A.; MacIntyre, D.A.; Ntritsos, G.; Smith, A.; Tsilidis, K.K.; Marchesi, J.R.; Bennett, P.R.; Moscicki, A.-B.; Kyrgiou, M. The vaginal microbiota associates with the regression of untreated cervical intraepithelial neoplasia 2 lesions. Nat. Commun. 2020, 11, 1999. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).