Submitted:
22 August 2023
Posted:
23 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
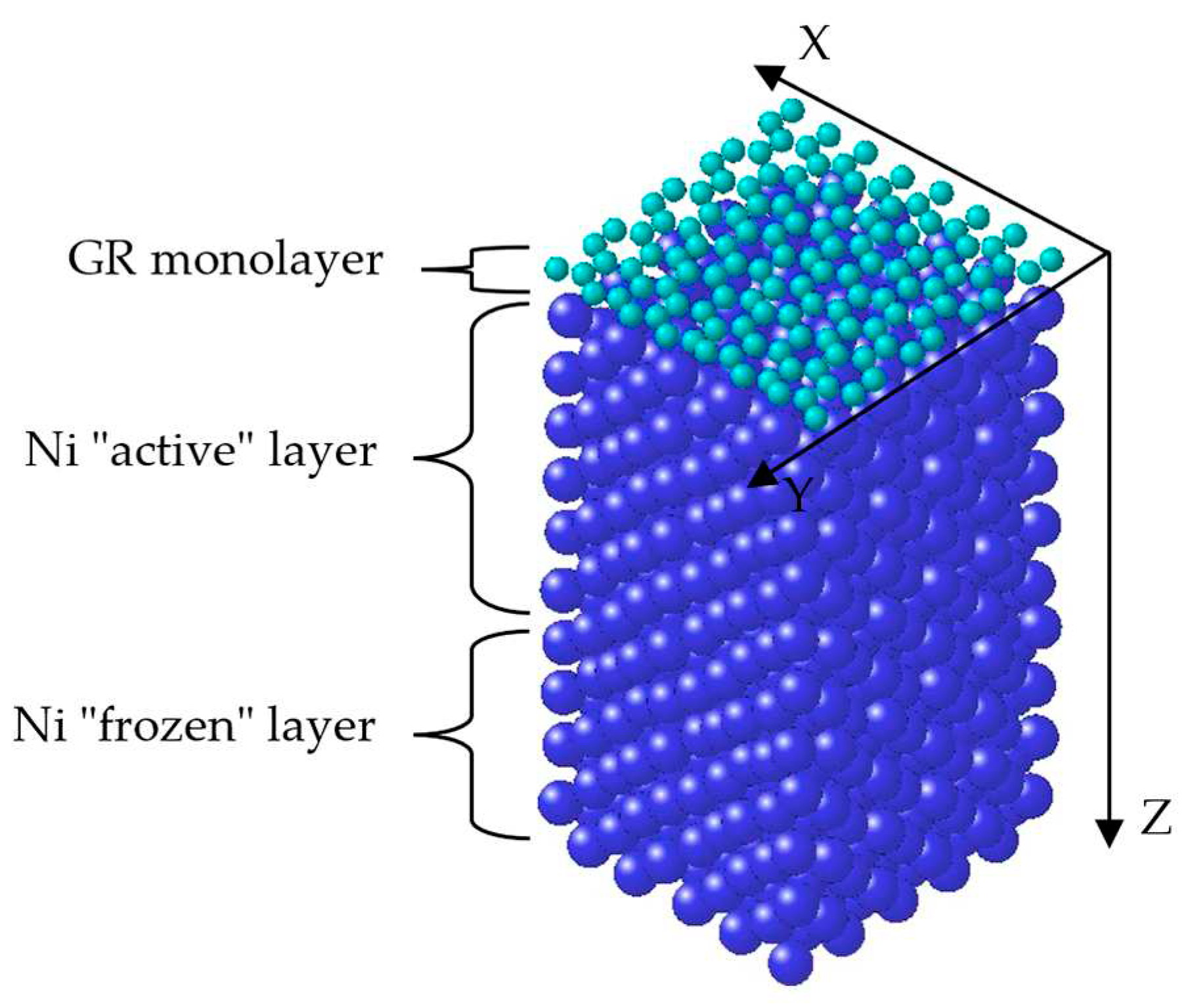
2. Simulation procedure
3. Results
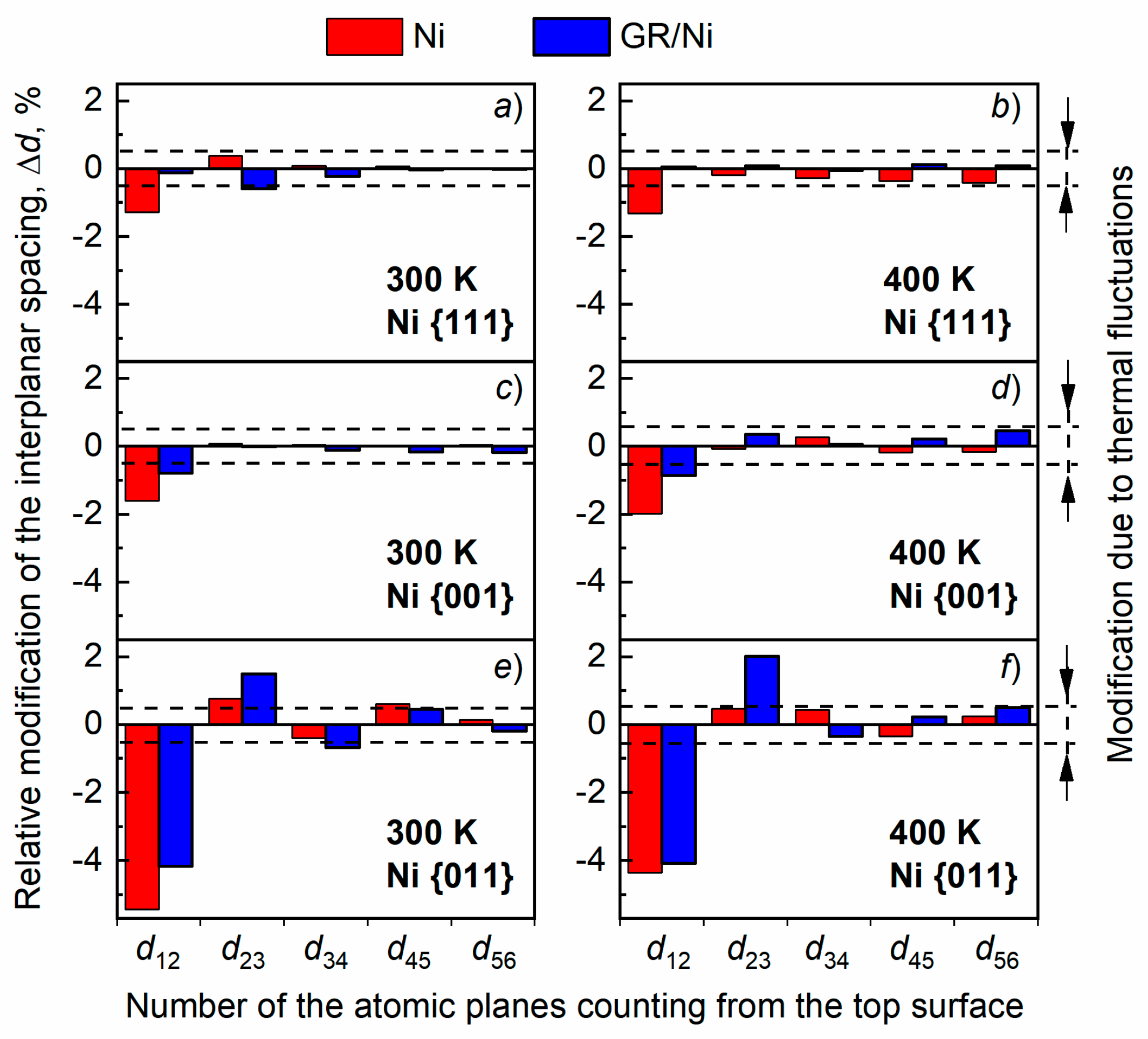
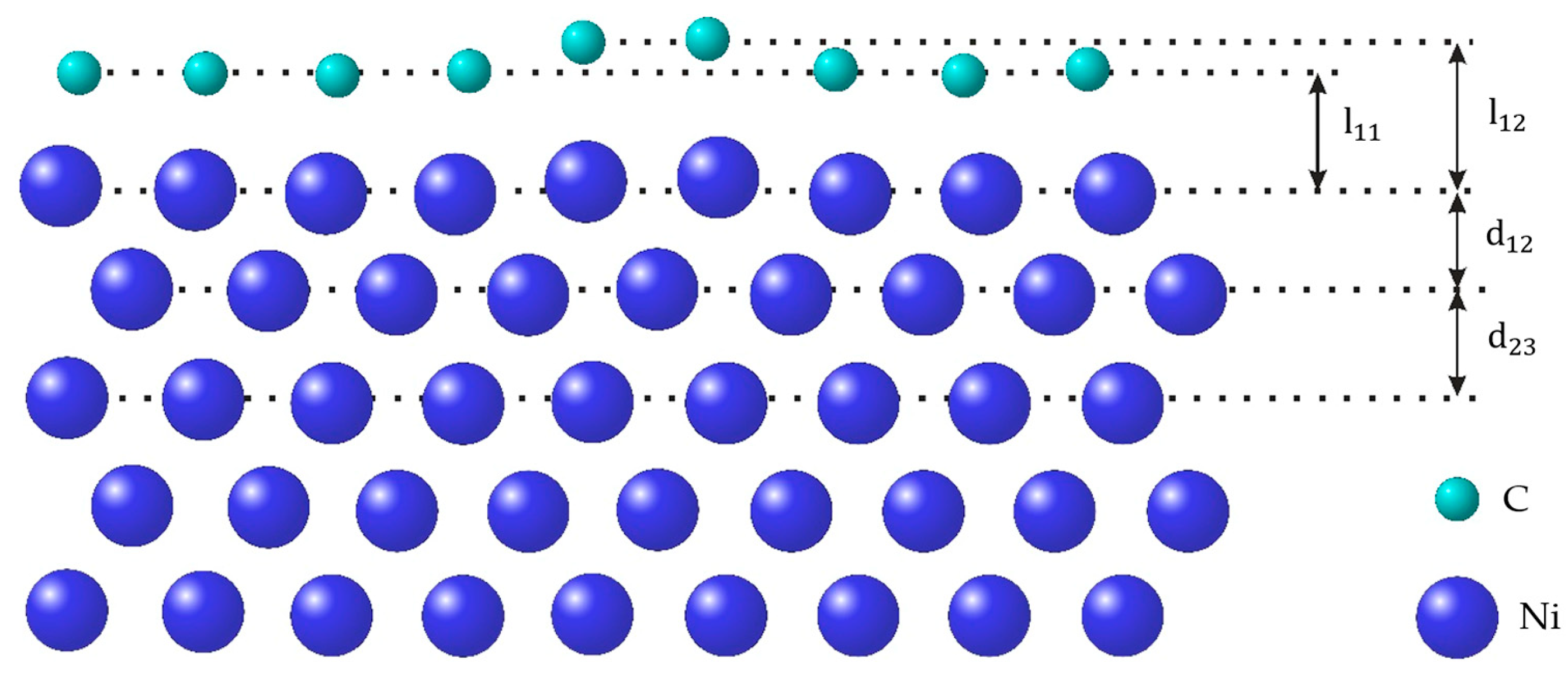
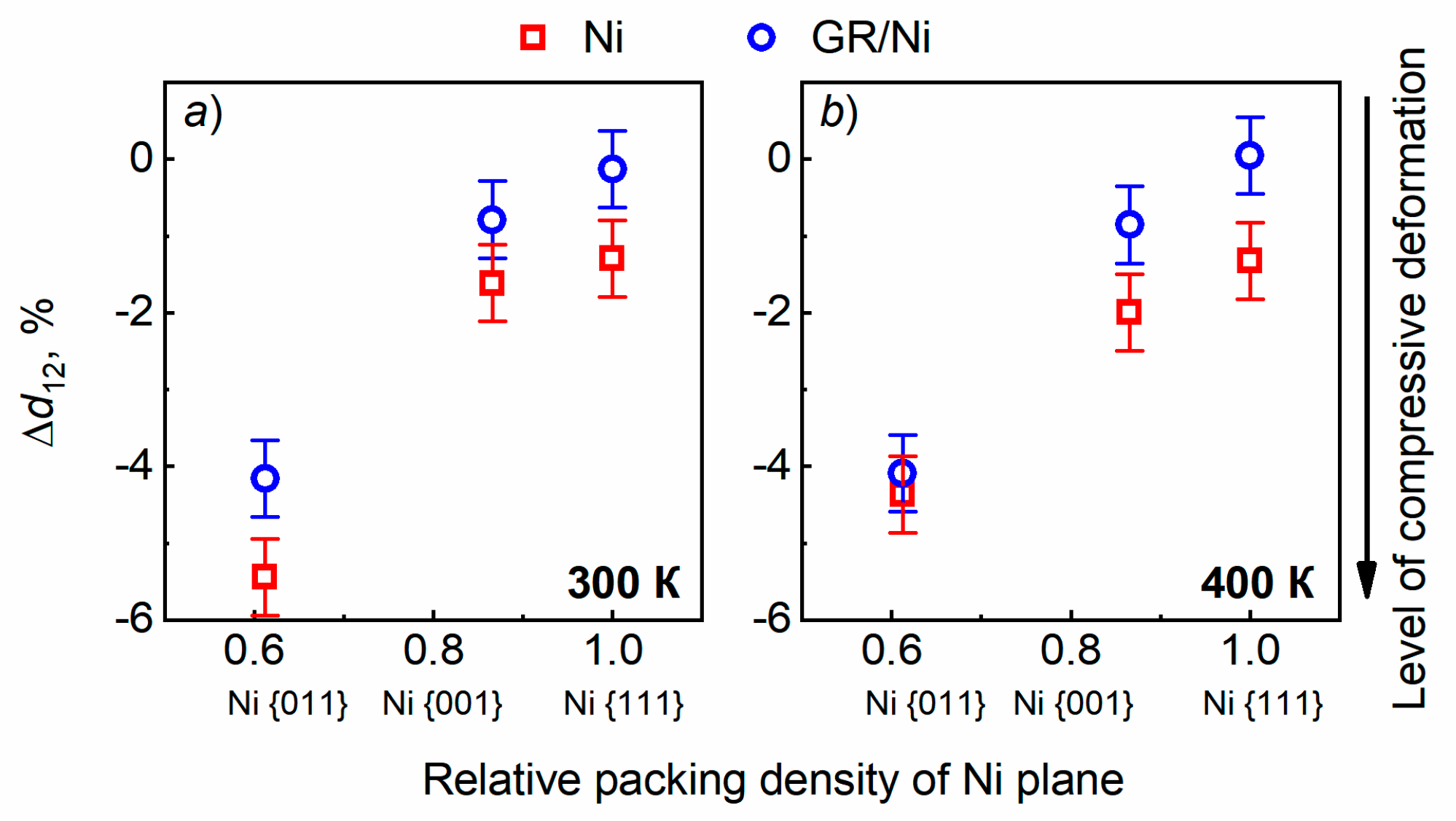
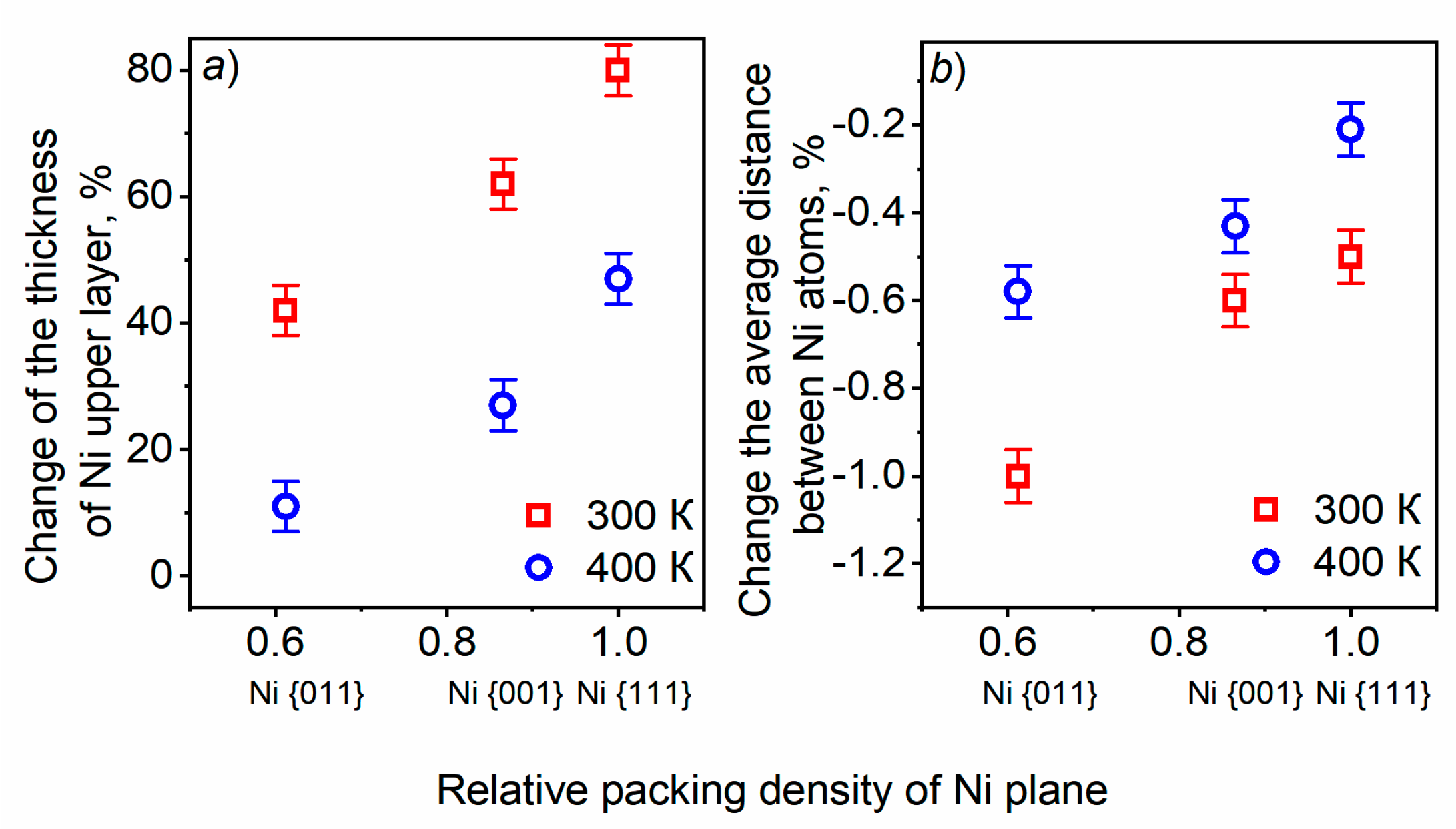
3.1. Surface relaxation of Ni of various orientations with and without GR layer Subsection
3.2. Surface reconstruction
3.2.1. GR reconstruction
3.2.2. Ni reconstruction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balandin, A.A.; Ghosh, S.; Bao, W.; et al. Superior thermal conductivity of single-layer graphene, Nano Lett. 2008, 8 (3), pp. 902-907. [CrossRef]
- Chen, S.; Moore, A. L.; Cai, W.; et al. Raman measurements of thermal transport in suspended monolayer graphene of variable sizes in vacuum and gaseous environments, ACS Nano 2011 5 (1), pp. 321-328. [CrossRef]
- Lim, S.; Park, H.; Yamamoto, G.; et al. Measurements of the electrical conductivity of monolayer graphene flakes using conductive atomic force microscopy, Nanomater. 2021, 11 (10) p. 2575. [CrossRef]
- Fernández, S.; Molinero, A.; Sanz, D.; et al. Graphene-based contacts for optoelectronic devices, Micromachines 2020, 11 (10), p. 919. [CrossRef]
- Deshmukh, M.A.; Park, S.J.; Hedau, B.S.; Ha, T.J. Recent progress in solar cells based on carbon nanomaterials. J. Sol. Energy 2021, 220, 953–990. [Google Scholar] [CrossRef]
- Aepuru, R.; Aleksandrova, M.; Rao, Ch.N.; et al. Chapter 19 - Transparent conducting electrode materials for solar cell technologies. In Green Sustainable Process for Chemical and Environmental Engineering and Science. Solid State Synthetic Methods.; Eds: Boddula, I.R., Asiri, A.M., Eds.; Rahman, M.M., Eds. Publisher: Elsevier, 2021; pp. 377–407. ISBN 9780128197202. [Google Scholar] [CrossRef]
- Muchuweni, E.; Mombeshora, E.T.; Martincigh, B.S. and Nyamori V. O. Recent applications of carbon nanotubes in organic solar cells, Front. Chem. 2022, 9, p–733552. [Google Scholar] [CrossRef]
- Akhtaruzzaman, M.; Selvanathan, V.; Shahiduzzaman, M.; Hossain, M.I. Chapter 4 - Introduction to organic-inorganic hybrid solar cells. In Comprehensive guide on organic and inorganic solar cells: Fundamental concepts to fabrication methods.; Akhtaruzzaman, M., Selvanathan, V., Eds.; Publisher: Academic Press, 2022; pp. 187–193. ISBN 9780323855297. [Google Scholar] [CrossRef]
- Chen, R.; Das, S.R.; Jeong, C.; et al. Co-percolating graphene-wrapped silver nanowire network for high performance, highly stable, transparent conducting electrodes, Adv. Funct. Mater. 2013, 23, 5150–5158. [Google Scholar] [CrossRef]
- Kruhlov, I.; Orlov, A.; Zakiev, V.; et al. Multi-layered thin-film metal contacts for new generation solar cells. In TMS 2022 151st Annual Meeting & Exhibition Supplemental Proceedings, The Minerals, Metals & Materials Series; Publisher: Springer, Cham. 2022; pp. 431–441. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, H.; Wang, G.; et al. Interface modulations of high-performance graphene anticorrosion coatings, Prog. Org. Coat. 2023, 178, p–107463. [Google Scholar] [CrossRef]
- Al-Kuhaili, M.F. Electrical conductivity enhancement of indium tin oxide (ITO) thin films reactively sputtered in a hydrogen plasma, J. Mater. Sci.: Mater. Electron. 2020, 31, 2729–2740. [Google Scholar] [CrossRef]
- Way, А.; Luke, J.; Evans, A.D.; et al. Fluorine doped tin oxide as an alternative of indium tin oxide for bottom electrode of semi-transparent organic photovoltaic devices, AIP Adv. 2019, 9 (8), p. 085220. [CrossRef]
- You, P.; Liu, Z.; Tai, Q.; et al. Efficient semitransparent perovskite solar cells with graphene electrodes, Adv. Mater. 2015, 27 (24), pp. 3632-3638. [CrossRef]
- Yoon, J.; Sung, H.; Lee, G.; et al. Superflexible, high-efficiency perovskite solar cells utilizing graphene electrodes: towards future foldable power sources, Energy Environ. Sci. 2017, 10, 337–345. [Google Scholar] [CrossRef]
- Luo, Q.; Ma, H.; Hou, Q.; et al. All-carbon-electrode-based endurable flexible perovskite solar cells, Adv. Funct. Mater. 2018, 28, p–1706777. [Google Scholar] [CrossRef]
- Iqbal, M.Z. ; Assad-Ur Rehman. Recent progress in graphene incorporated solar cell devices, J. Sol. Energy 2018, 169, 634–647. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.-Z.; Hupalo, M.; et al. Metals on graphene: interactions, growth morphology, and thermal stability, Crystals 2013, 3 (1), pp. 79-111. [CrossRef]
- Yang, R.T.; Goethel, P.J.; Schwartz, J.M.; Lund, C.R.F. Solubility and diffusivity of carbon in metals, J. Catal. 1990, 122 (1), pp. 206-210. [CrossRef]
- Chung, T.F.; Shen, T.; Cao, H.; et al. Synthetic graphene grown by chemical vapor deposition on copper foils, Int. J. Mod. Phys. B 2013, 27 (10), p. 1341002. [CrossRef]
- Cho, J.; Gao, L.; Tian, J.; et al. Atomic-scale investigation of graphene grown on cu foil and the effects of thermal annealing, ACS Nano 2011, 5 (5), pp. 3607-3613. [CrossRef]
- Patel, Ch.R.P.; Awasthi, K.; Yadav, T.P. Selective growth of uniform graphene films on metallic (Cu and Au) substrates, J. Int. Acad. Phys. Sci. 2022, 26 (2), pp. 175-184. https://www.iaps.org.in/journal/index.php/journaliaps/article/view/940.
- Kiraly, B.; Iski, E.V.; Mannix, A.J.; et al. Solid-source growth and atomic-scale characterization of graphene on Ag(111), Nat. Commun. 2013, 4, p–2804. [Google Scholar] [CrossRef]
- Cheianov, V.V.; Fal’ko, V.I. Selective transmission of dirac electrons and ballistic magnetoresistance of N−P junctions in graphene, Phys. Rev. B 2006, 74 (4), p. 041403. [CrossRef]
- Giovannetti, G.; Khomyakov, P. A.; Brocks, G.; et al. Doping graphene with metal contacts, Phys. Rev. Lett. 2008, 101 (2), p. 026803. [CrossRef]
- Dahal, А.; Batzill, M. Graphene–nickel Interfaces: a review, Nanoscale 2014, 6 (5), p. 2548. [CrossRef]
- Xiao, W.; Baskes, M.I.; Cho, K. MEAM Study of Carbon Atom Interaction With Ni Nano Particle, Surf. Sci. 2009, 603 (13), pp. 1985-1998. [CrossRef]
- LAMMPS Molecular Dynamics Simulator. Available online: www.lammps.org (accessed on 17.08.23).
- Kittel, Ch.; Introduction to Solid State Physics, Publisher: Wiley India Pvt Ltd, 2012. ISBN-10 : 9788126535187.
- Gao, Y.; Lihua, L.; et al. Structure of Graphene and Its Disorders: A Review. Sci. Technol. Adv. Mater. 2018, 19 (1), pp. 613–648. [CrossRef]
- Rankin, D. CRC handbook of chemistry and physics, 89th edition, edited by David R. Lide. Crystallogr. Rev., 2009, 15 (3), pp. 223-224. [CrossRef]
- Barron, T. H. K.; White, G. Heat Capacity and Thermal Expansion at Low Temperatures. Publisher: Springer New York, 1999. ISBN 978-0-306-46198-9. [CrossRef]
- Engelmann, Y.; Bogaertsb, A.; Neyts, E.C. Thermodynamics at the Nanoscale: Phase Diagrams of Nickel–carbon Nanoclusters and Equilibrium Constants for Phase Transitions, Nanoscale 2014, 6 (20), pp. 1 1981–11987. [CrossRef]
- Frenken, J.W.M.; Smeenk, R.G. , Van der Veen, J.F. Static and dynamic displacements of nickel atoms in clean and oxygen covered Ni(001) surfaces, Surf. Sci. 1983, 135 (1-3), pp. 147-163. [CrossRef]
- Feidenhans'l, R.; Sørensen, J.E.; Stensgaard, I. Surface relaxation of Ni(110) investigated by high energy ion scattering, Surf. Sci. 1983, 134 (2), pp. 329-337. [CrossRef]
- Manasoglu, G.; Celen, R.; Akgun, M.; Kanik, M. The Effect of Graphene Coating on Surface Roughness and Friction Properties of Polyester Fabrics, Mater. Sci. 2021, 27 (4), pp. 470-476. [CrossRef]
- Kumar, H. G. P.; Xavior, M. A. Effect of graphene addition and tribological performance of Al 6061/graphene flake composite, Tribol. Mater. Surf. Interfaces 2017, 11 (2), pp. 88-97. [CrossRef]
- Kruhlov, I.O.; Vladymyrskyi, I.A.; Dubikovskyi, O.; et al. Reduction processes in Ni/Cu/Cr/Si(100) thin films under low-energy Ar+ ion bombardment. Mater. Res. Express 2020, 6 (№12), p. 126431. [CrossRef]
- Ren, S.; Cui, M.; Liu, C.; Wang, L. A comprehensive review on ultrathin, multi-functionalized, and smart graphene and graphene-based composite protective coatings. Corrosion Sci. 2023, 212, p–110939. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, H.; Wang, G.; Wang, J.; Zhu, J. Interface modulations of high-performance graphene anticorrosion coatings. Prog. Org. Coat. 2023, 178, p–107463. [Google Scholar] [CrossRef]
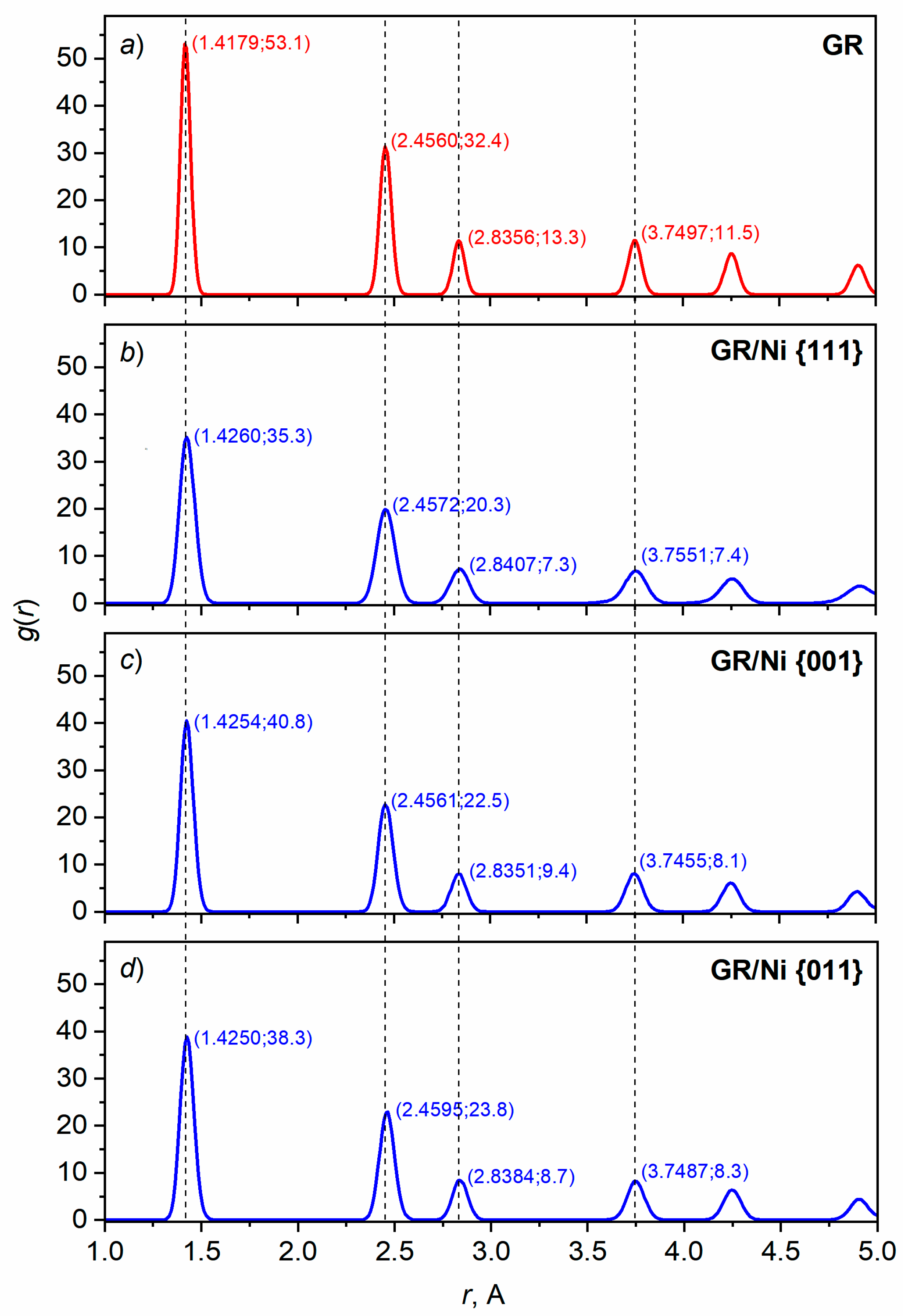
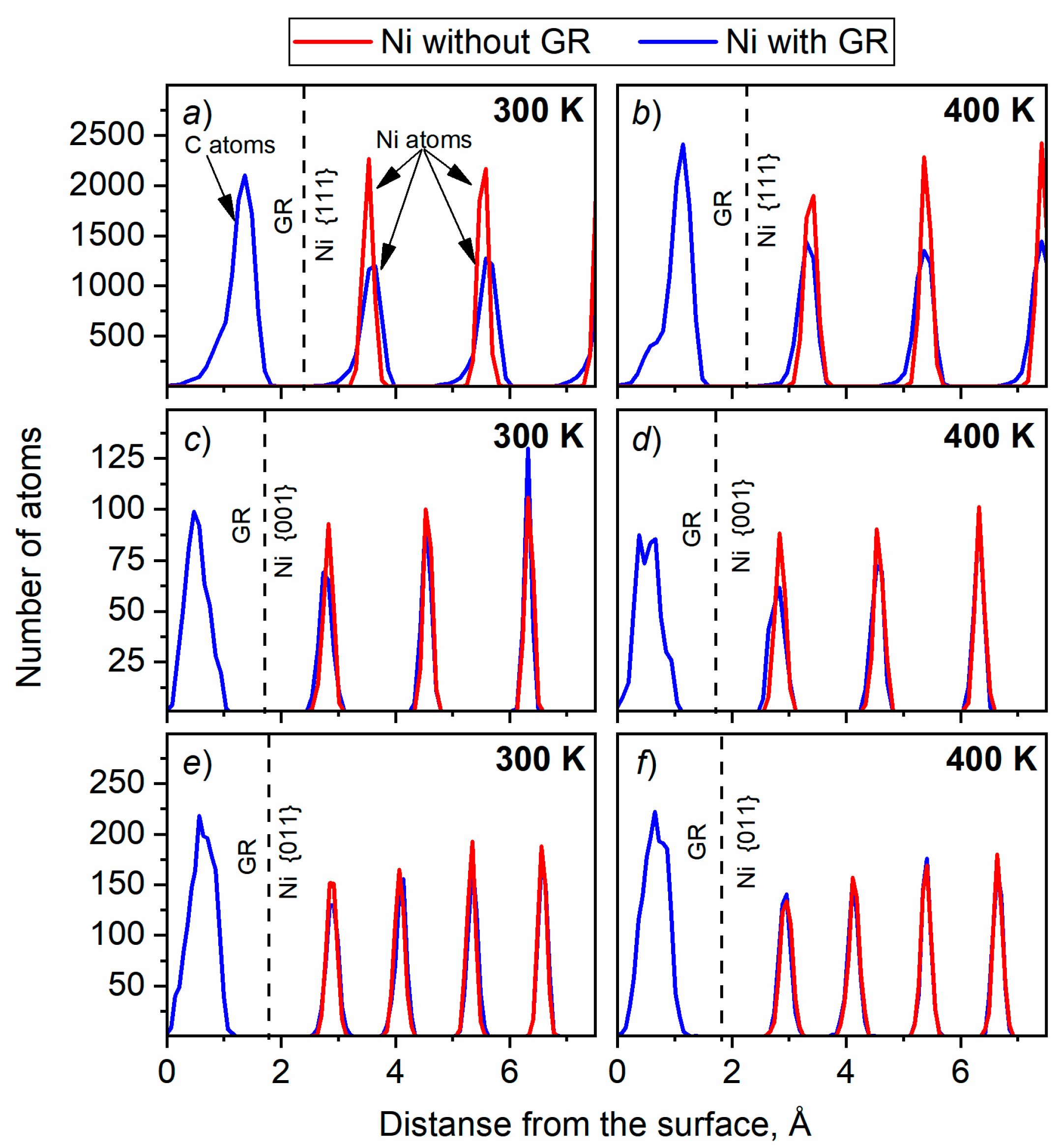

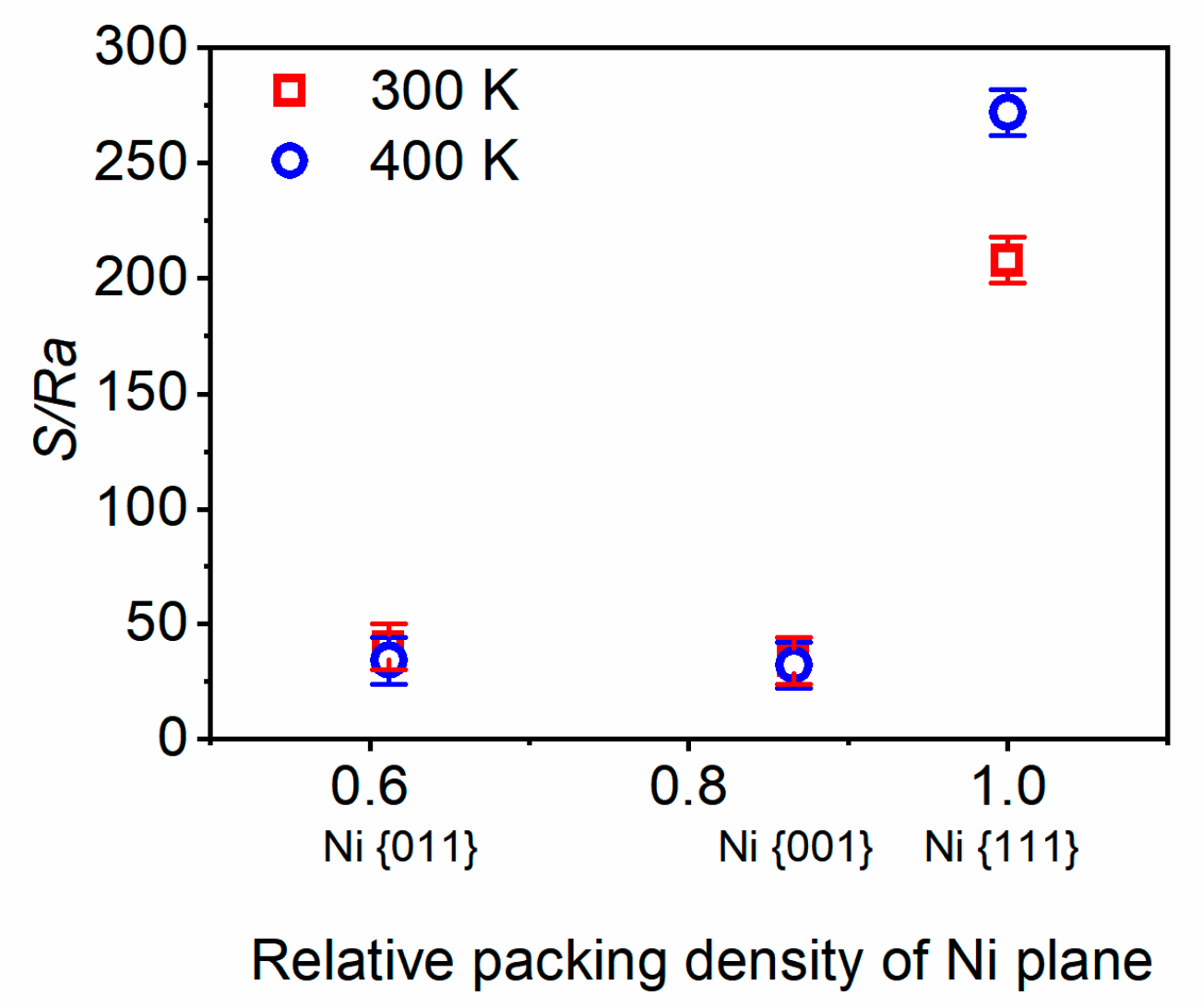
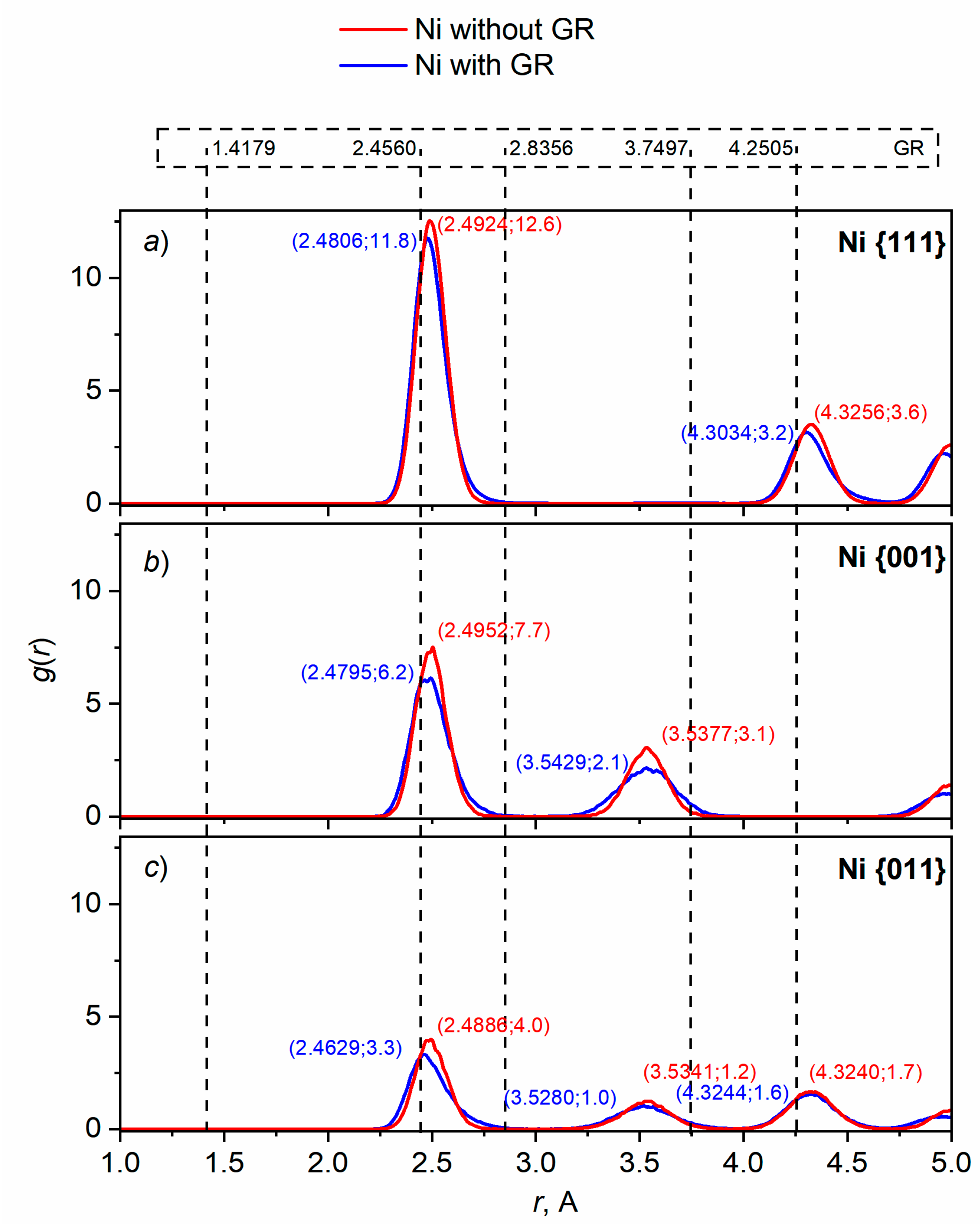
| Material | Temperature, К | Lattice constant, Å | Coefficient of linear thermal expansion, 10-6 1/К | ||
| Calculated | Reference data | Calculated | Reference data | ||
| Ni | 300 | 3.5347 | 3.523 [29] | 12.75 | 13.4 [31] |
| 400 | 3.5392 | 12.8 [32] | |||
| GR | 300 | 1.4163 | 1.42 [30] | 8.5 | |
| 400 | 1.4175 | ||||
| Ni plane | Temperature | |
| 300 К | 400 К | |
| {111} | Ra = 0.7 ± 0.05 Å | Ra = 0.55 ± 0.05 Å |
| Rmax = 1.95 ± 0.05 Å | Rmax = 1.75 ± 0.05 Å | |
| S = 146 ± 1.4 Å | S = 150 ± 1.4 Å | |
| S/Ra = 208 | S/Ra = 272 | |
| {001} | Ra = 0.35±0.05 Å | Ra = 0.4 ± 0.05 Å |
| Rmax =1.1 ± 0.05 Å | Rmax = 1.15 ± 0.05 Å | |
| S = 12 ± 1.4 Å | S = 13 ± 1.4 Å | |
| S/Ra = 34 | S/Ra = 32 | |
| {011} | Ra = 0.45 ± 0.05 Å | Ra = 0.55 ± 0.05 Å |
| Rmax = 1.2 ± 0.05 Å | Rmax =1.35 ± 0.05 Å | |
| S = 18 ± 1.4 Å | S = 19 ± 1.4 Å | |
| S/Ra = 40 | S/Ra = 34 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





