Submitted:
21 August 2023
Posted:
22 August 2023
You are already at the latest version
Abstract
Keywords:
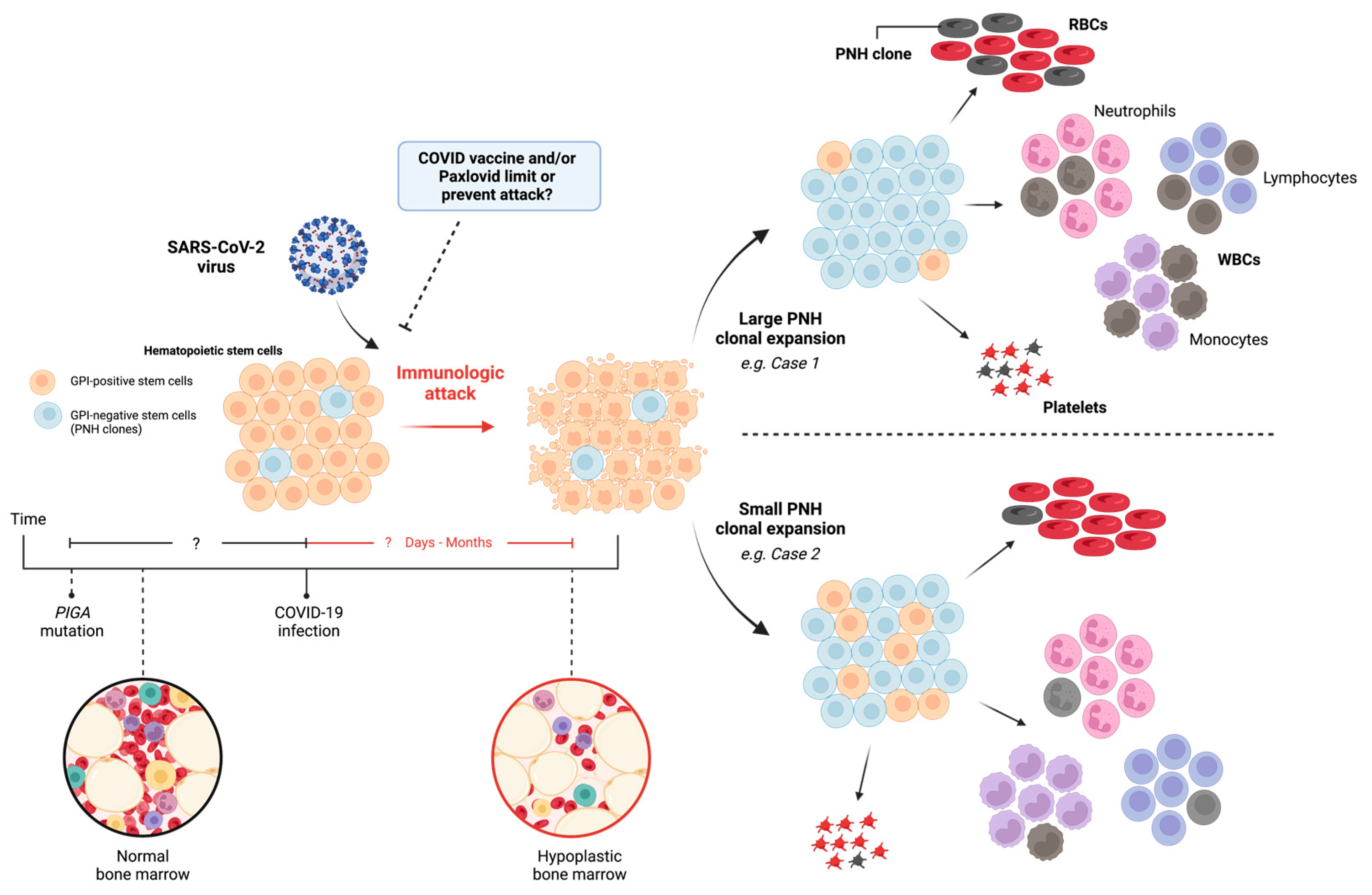
1. Introduction
2. Case Series
2.1. Case 1
2.2. Case 2
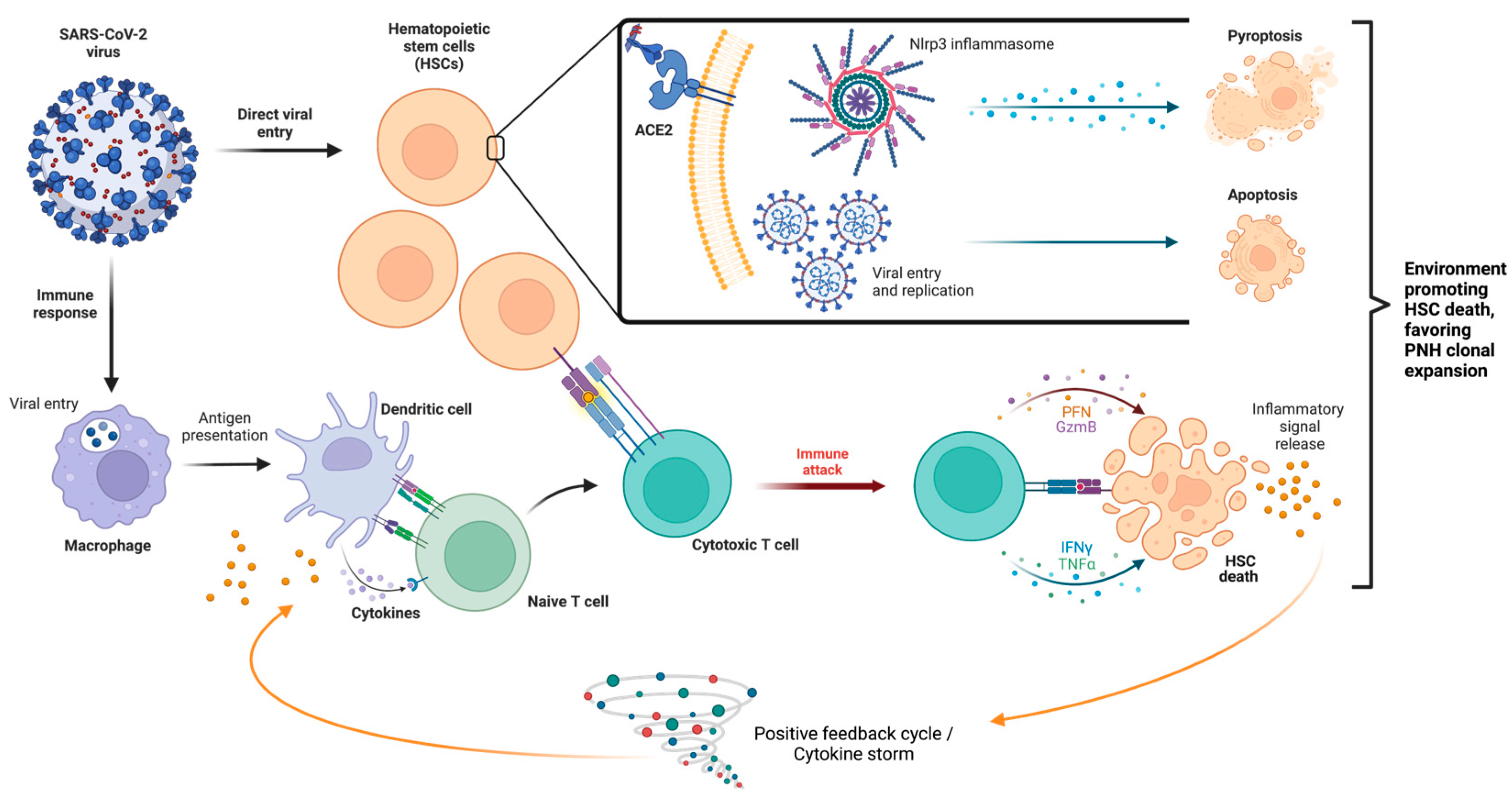
3. Discussion
4. Conclusions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jha, P.; Brown, P. E.; Ansumana, R. Counting the global COVID-19 dead. Lancet 2022, 399(10339), 1937–1938. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Niloofa, R.; Jayarajah, U.; De Mel, S.; Abeysuriya, V.; Seneviratne, S. L. Hematological Abnormalities in COVID-19: A Narrative Review. Am J Trop Med Hyg 2021, 104. [Google Scholar] [CrossRef] [PubMed]
- Hines, A.; Hakim, N.; Barrientos, J. COVID-19 infection presenting as paroxysmal nocturnal hemoglobinuria. Clin Case Rep 2021, 9 (8), e04636. [Google Scholar] [CrossRef]
- Lee, N. C. J.; Patel, B.; Etra, A.; Bat, T.; Ibrahim, I. F.; Vusirikala, M.; Chen, M.; Rosado, F.; Jaso, J. M.; Young, N. S.; et al. SARS-CoV-2 infection associated with aplastic anemia and pure red cell aplasia. Blood Adv 2022, 6, 3840–3843. [Google Scholar] [CrossRef]
- Otieno, S. B.; Altahan, A.; Kaweeta, F.; Karri, S.; Alnoor, F.; Johnson, R. Severe Hemolysis in a COVID-19 Patient with Paroxysmal Nocturnal Hemoglobinuria. Case Rep Hematol 2021, 2021, 6619177. [Google Scholar] [CrossRef]
- Sumbly, V.; Siddiqui, R.; Alshamam, M.; Kurbanova, T.; Rizzo, V. New Onset Aplastic Anemia after a COVID-19 Infection: A Case Report. American Journal of Medical Case Reports 2021, 9, 451–455. [Google Scholar]
- Hill, A.; DeZern, A. E.; Kinoshita, T.; Brodsky, R. A. Paroxysmal nocturnal haemoglobinuria. Nat Rev Dis Primers 2017, 3, 17028. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X. F.; He, H. L.; Wang, S. Q.; Tang, J. Y.; Han, B.; Zhang, D. H.; Wu, L. Q.; Wu, D. P.; Li, W.; Xia, L. H.; et al. Current Treatment Patterns of Aplastic Anemia in China: A Prospective Cohort Registry Study. Acta Haematol 2019, 142, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Jalbert, J. J.; Chaudhari, U.; Zhang, H.; Weyne, J.; Shammo, J. M. Epidemiology of PNH and Real-World Treatment Patterns Following an Incident PNH Diagnosis in the US. Blood 2019, 134 (Supplement_1), 3407–3407. [Google Scholar] [CrossRef]
- Guan, W. J.; Ni, Z. Y.; Hu, Y.; Liang, W. H.; Ou, C. Q.; He, J. X.; Liu, L.; Shan, H.; Lei, C. L.; Hui, D. S. C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Taneri, P. E.; Gómez-Ochoa, S. A.; Llanaj, E.; Raguindin, P. F.; Rojas, L. Z.; Roa-Díaz, Z. M.; Salvador, D.; Groothof, D.; Minder, B.; Kopp-Heim, D.; et al. Anemia and iron metabolism in COVID-19: a systematic review and meta-analysis. Eur J Epidemiol 2020, 35, 763–773. [Google Scholar] [CrossRef]
- Hock, H.; Kelly, H. R.; Meyerowitz, E. A.; Frigault, M. J.; Massoth, L. R. Case 31-2021: A 21-Year-Old Man with Sore Throat, Epistaxis, and Oropharyngeal Petechiae. N Engl J Med 2021, 385, 1511–1520. [Google Scholar] [CrossRef]
- Chakravarthy, R.; Murphy, M. L.; Ann Thompson, M.; McDaniel, H. L.; Zarnegar-Lumley, S.; Borinstein, S. C. SARS-CoV-2 infection coincident with newly diagnosed severe aplastic anemia: A report of two cases. Pediatr Blood Cancer 2022, 69 (4), e29433. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi, A.; Parrella, A.; De Ritis, F.; Cammarota, A.; Berloco, L.; Paudice, F.; D'Angelo, G.; Aliberti, E.; Iannuzzo, G. Pancytopenia in a Case of Aplastic Anaemia/Paroxysmal Nocturnal Haemoglobinuria Unmasked by SARS-CoV-2 Infection: A Case Report. Medicina (Kaunas) 2022, 58. [Google Scholar] [CrossRef] [PubMed]
- Avenoso, D.; Marsh, J. C. W.; Potter, V.; Pagliuca, A.; Slade, S.; Dignan, F.; Tholouli, E.; Mittal, S.; Davis, B.; Tauro, S.; et al. SARS-CoV-2 infection in aplastic anemia. Haematologica 2022, 107, 541–543. [Google Scholar] [CrossRef]
- Fara, A.; Mitrev, Z.; Rosalia, R. A.; Assas, B. M. Cytokine storm and COVID-19: a chronicle of pro-inflammatory cytokines. Open Biol 2020, 10 (4), e29433. [Google Scholar] [CrossRef]
- Ratajczak, M. Z.; Kucia, M. SARS-CoV-2 infection and overactivation of Nlrp3 inflammasome as a trigger of cytokine "storm" and risk factor for damage of hematopoietic stem cells. Leukemia 2020, 34, 1726–1729. [Google Scholar] [CrossRef] [PubMed]
- Kucia, M.; Ratajczak, J.; Bujko, K.; Adamiak, M.; Ciechanowicz, A.; Chumak, V.; Brzezniakiewicz-Janus, K.; Ratajczak, M. Z. An evidence that SARS-Cov-2/COVID-19 spike protein (SP) damages hematopoietic stem/progenitor cells in the mechanism of pyroptosis in Nlrp3 inflammasome-dependent manner. Leukemia 2021, 35, 3026–3029. [Google Scholar] [CrossRef]
- Zeng, Y.; Katsanis, E. The complex pathophysiology of acquired aplastic anaemia. Clin Exp Immunol 2015, 180, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Fattizzo, B.; Ireland, R.; Dunlop, A.; Yallop, D.; Kassam, S.; Large, J.; Gandhi, S.; Muus, P.; Manogaran, C.; Sanchez, K.; et al. Clinical and prognostic significance of small paroxysmal nocturnal hemoglobinuria clones in myelodysplastic syndrome and aplastic anemia. Leukemia 2021, 35, 3223–3231. [Google Scholar] [CrossRef] [PubMed]
| Variable | Patient Case | ||
| 1 | 2 | ||
| Age | 21 | 52 | |
| Sex | Male | Female | |
|
Interval between COVID infection and pancytopenia |
4 months | 1 month | |
| CBC | WBC ANC Platelets HGB MCV |
4.1 1.2 34 9.3 106.2 |
3.0 0.9 18 11.6 94 |
| Bone Marrow Biopsy Cellularity | 10-20% | 10-15% | |
| PNH clones | Granulocytes Monocytes RBCs |
19.53% 19.77% 3.61% |
N/A 0.5% N/A |
| History of autoimmune disease | None | Anterior uveitis | |
| SARS-CoV-2 Vaccination | None | Johnson and Johnson/Janssen Seven months later, Pfizer booster |
|
| Treatment | Ravulizumab | N/A | |
| Clinical Data | Diagnosis |
Bone Marrow Biopsy Cellularity |
Onset of cytopenia after COVID infection | Therapy | Reference(s) |
| 21, M* | Hemolytic PNH / Aplastic anemia | 10-20% | 4 months | Ravulizumab | This report |
| 52, F* | Aplastic anemia (with subclinical PNH clones) | 10-15% | 1 month | Observation | This report |
| 35, M | Hemolytic PNH | Normal | 0 days ‡ |
Eculizumab, transfusion support |
[3] |
| 22, F | Severe aplastic anemia | 5% | 10 days | Sibling HSCT | [4] |
| 69, F | Severe aplastic anemia | 5-10% | 2 days | Cyclosporine, antithymocyte globulin, eltrombopag | [4] |
| 76, M | Pure red cell aplasia |
20-30% | 4 months | Cyclosporine | [4] |
| 21, M | Severe aplastic anemia (with subclinical PNH clones) |
<5% | < 1 month | Cyclosporine, antithymocyte globulin, eltrombopag; eculizumab† |
[4] |
| 69, F | Severe aplastic anemia (with subclinical PNH clones) |
5% | 5 months | Cyclosporine, antithymocyte globulin, eltrombopag | [4] |
| 19, F | Hemolytic PNH | 40-50% § |
0 days ‡ |
Eculizumab, Ravulizumab | [5] |
| 28, F | Severe aplastic anemia | 20-30% | 3 months | Cyclosporine, antithymocyte globulin, eltrombopag | [4,6] |
| 21, M | Severe aplastic anemia | <5% | 2 months | Sibling HSCT | [12] |
| 12, F | Severe aplastic anemia | 10% | 0 days ‡ |
Antithymocyte globulin, cyclosporine |
[13] |
| 18, M | Severe aplastic anemia | 10% | 0 days ‡ |
Antithymocyte globulin, cyclosporine |
[13] |
| 78, F | Hemolytic PNH / Aplastic anemia | Poor | 0 days ‡ |
-- || |
[14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





