1. Introduction
Punicalagin, a polyphenol chemical, is a prevalent and significant component of pomegranates [
1]. The chemical name of Punicalagin is 2,3-(S)-hexahydroxydiphenoyl-4,6-(S, S)-Gallegly-D-glucose.(PubChem et al.,2021) [
2]. It has long been regarded as a blood tonic and is mostly used in traditional medicine in Asian nations [
2]. It is today, however, abundantly grown in areas of Southwest America, Arizona, Mexico, India, California, and Africa [
3]. The molecular formula for punicalagin is C48H28O30. Punicalagin is a transparent part solution that dissolves in methanol at a rate of 5 mg/ml(PubChem, 2012) [
5]. Punicalagin contain a polyphenol in the husk, peel, aril, fruit, juice, of
Punicalaginica granatum and
Lafoensia pacarileaves(Chaibub BA, Martins JLR et al., 2020) [
6].
The antimutagenic, antioxidant, and DNA-protective accomplishments of punicalagin, Ellagic acid (EA), and its derivatives have also been revealed [
4]. Additionally, it is used to lessen the signs and symptoms of diabetes, prostate cancer, hypertension, periodontal disease, hyperlipidemia, male infertility, vaginitis, erectile dysfunction, obesity, pediatric cerebral ischemia, Alzheimer's disease, diarrhea, bronchitis, asthma, bleeding disorders, fever, cough, inflammation, atherosclerosis, acquired immunodeficiency syndrome, mouth lesions, ulcers, skin lesions, and malaria [
3]. Numerous pharmacological effects of pomegranate polyphenols include anti-inflammatory, hepatoprotective, antigenotoxic, and anticoagulant characteristics. [
5]. Punicalagin was the primary ellagitannin found in the leaves of
Lafoensia pacaria Brazilian herb used to cure wounds and gastric ulcers [
6].
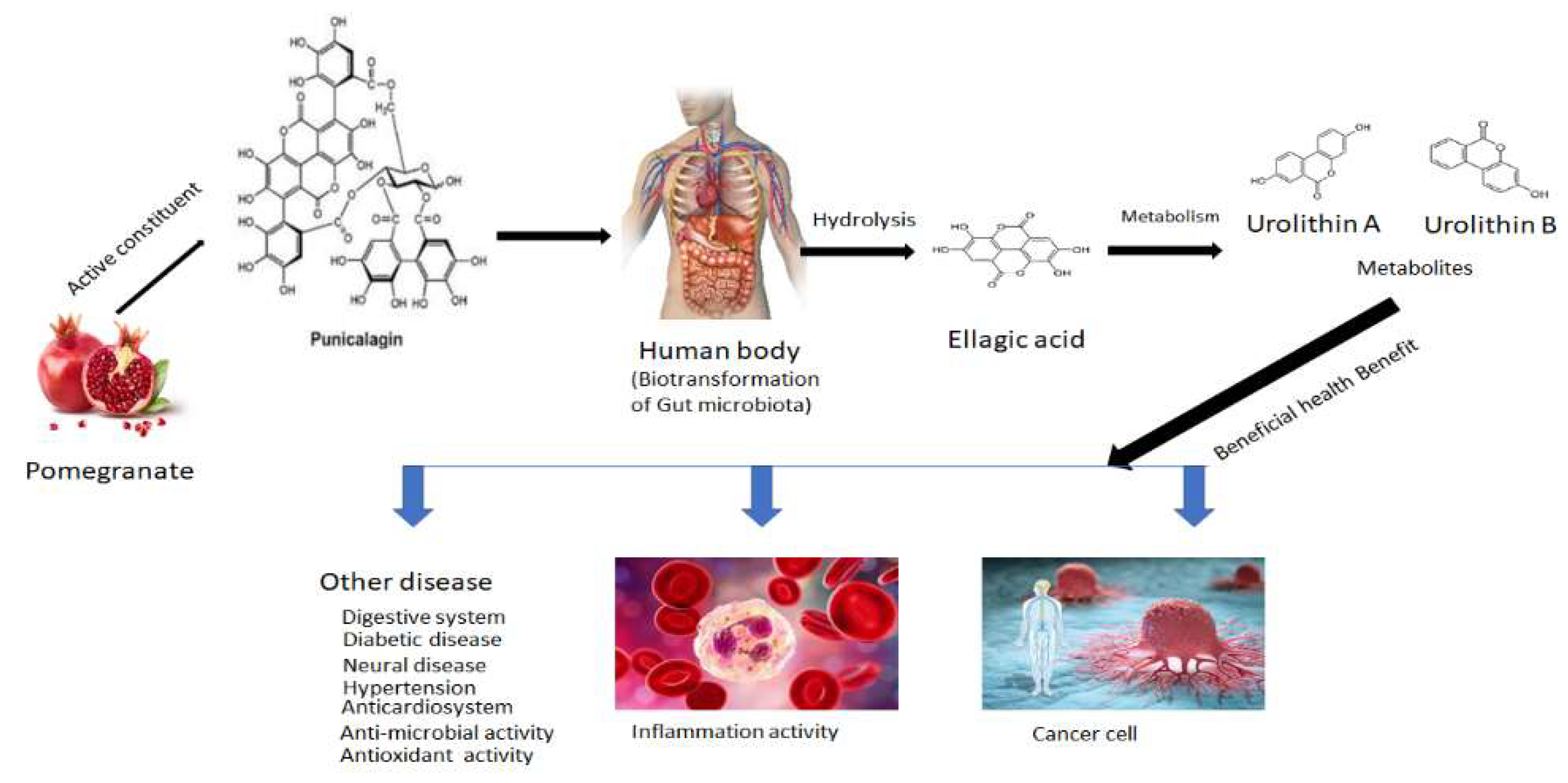
The advantages of Punicalagin are credited to polyphenols, which primarily consist of hydrolyzed tannin [
7] which shown in
Figure 1. The presence of Punicalagin and other hydrolyzed tannins which are present in pomegranate juice accounts for 87% of the antioxidant activity seen in the juice. In juice, it can accumulate to >2 g/L concentration [
8]. It is made up of glucose and is found in the middle of PUNICALAGIN. To esterify the- α-and β -anomeric forms of glucose, gallic acid dimers, ellagic acid(EA) dimers, and EA dimers are used [
7,
9]. Tannins are well known for their capacity to attach to various compounds, including DNA, metals, proteins, and polysaccharides [
10]. Kulkarni and others.it was found that punicalagin has a poor and non-specific binding to DNA but a high affinity for metal ions and bovine serum albumin [
11].
The scientific community is compensating more and more attention to natural molecules derivative from plants because they are more affordable, more bioavailable, and less hazardous than synthetic pharmaceutical drugs [
12]. In nature, polyphenols are commonly present in fruits and vegetables [
13]. Pomegranates are consumed as fruits and are also used for their antioxidant properties. Anti-inflammation therapy has the potential to treat and prevent disease [
14].High concentrations of bioactive substances, primarily phenolic acids, flavonoids, and tannins, can be found in pomegranate extract [
15]. Punicalagin has been publicized to have positive effects on both chronic [
16] and acute inflammation [
17] and to be engaged in a variety of inflammation-related processes, including immunological response [
18] macrophage and fibroblast cell activity [
19,
20] and necrosis [
18]. Punicalagin, for instance, blocks the activation of the nuclear factor of activated T cells and downregulates the mRNA and soluble protein expression of IL-2 from anti-CD3/anti-CD28stimulated murine splenic CD4+ T cell [
18].
2. Anti-cancer effect of punicalagin
Cancer is a disease in which some of the body’s cells grow uncontrollably and spread to other parts of the body. Cancer can start almost anywhere in the human body, which is made up of trillions of cells. It happens due to the DNA damage, mutations occur in DNA, etc. which occurs due to various physical, chemical, biological and environmental factors [
21].It has been shown that punicalagin and its derivative ellagic acid show anti-cancer activityby preventing DNA alterations. It was observed that the quantity of DNA damage brought on by different carcinogens was significantly reduced by these two chemicals. Punicalagin completely inhibited the production of DNA adducts by the accompanying biotransformation system with cytochrome P-450 necessary for the transformation of benzopyrene into a mutagen [
4].
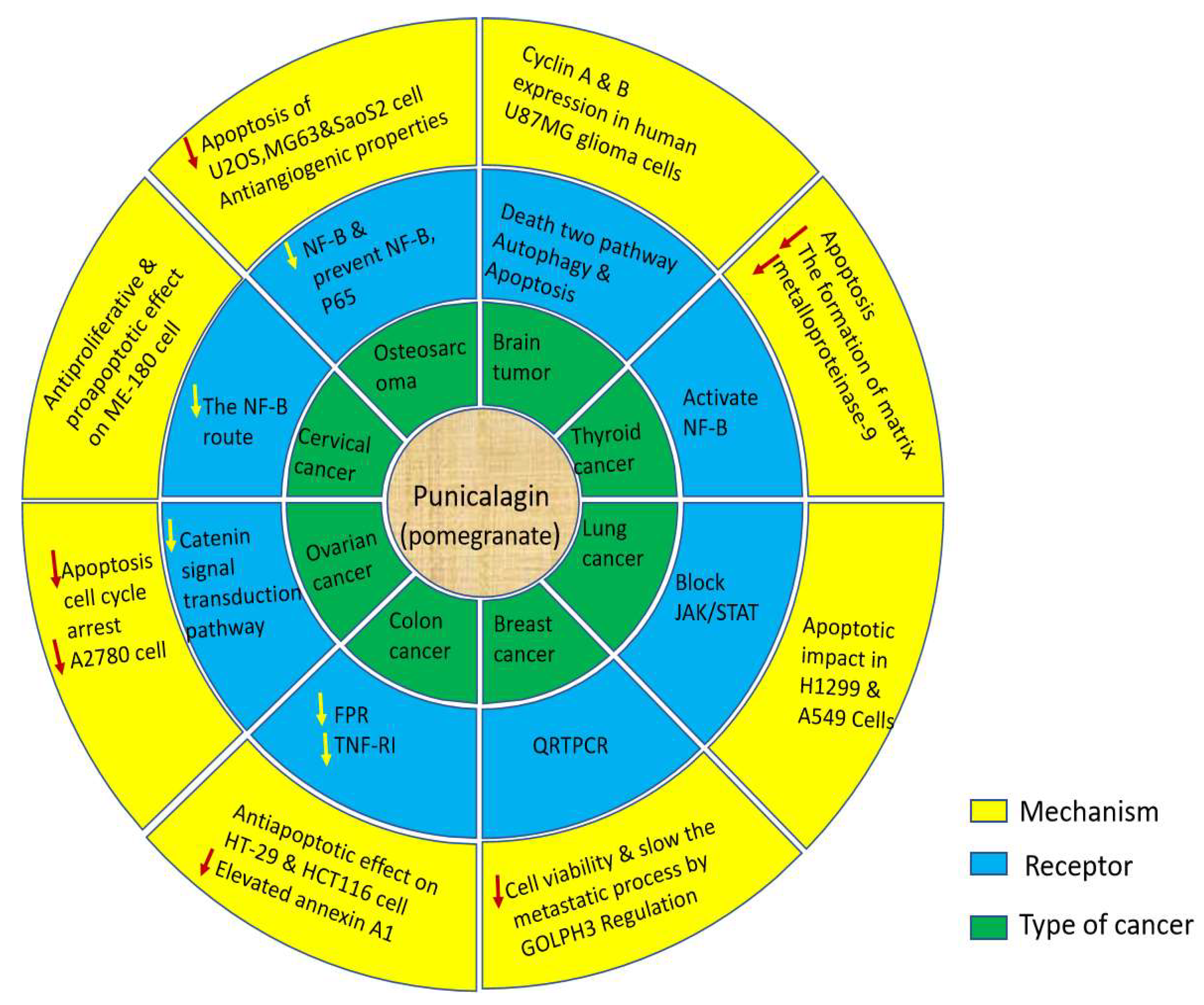
The most prevalent and destructive primary malignant tumor of the brain and central nervous system, Glioblastoma multiforme (GBM), accounts for 14.5% of all central nervous system tumors and 48.6% of malignant central nervous system tumors [
22]. Punicalagin decreases cyclins A and B expression in human U87MG glioma cells and makes them suggestively less viable by halting their cell cycle at the G2/M phase. The impact of Punicalagins leads to the promotion of cell death via two pathways, explicitly autophagy and apoptosis. There are considered a number of involvements of both pathways in punicalagin-stimulated reduction of cancer growth. Specifically, caspase 3 and 9 levels, PARP cleavage, and Bcl-2 levels were all increased by punicalagin expression, which are all typical apoptotic characteristics. Punicalagin also encouraged increase of microtubule-associated autophagy markers. AMP phosphorylation is induced by punicalagin and the proteins light chain 3 II (LC3-II), amplified kinase (AMPK), and p27Kip1. Punicalagin-induction increases autophagy via activating the LKB1-AMPK pathway [
23].
The majority of patients with thyroid cancer (TC) has accounted for more than 77.0% of all diagnoses and is the most prevalent endocrine and head-and-neck malignancy, especially in women. Punicalagin decreases thyroid cancer cells mitochondrial membrane potential, which leads to the suppression of growth and induction of apoptosis. By reducing the formation of matrix metalloproteinase-9, pomegranate peel extract reduces thyroid cancer cell migration and invasion (MMP-9) [
22]. Punicalagin therapy activates NF-B, which results in senescent growth arrest and the SASP secretory phenotype. Punicalagin causes autophagic cell death by triggering the DNA damage response in BCPAP cells from papillary thyroid carcinoma [
24].
Lung cancer remained the most common cause of cancer death worldwide in 2020, accounting for nearly 18% of all cancer deaths with 1,796,144 recorded deaths [
25]. On human lung cancer H1299 and A549 cells punicalagin was found to have a pro-apoptotic impact. Punicalagin was also revealed to block the JAK/STAT signaling pathway in lung cancer cells. Punicalagin therapy altered the profile of STAT-3 protein expression and decreased JAK-1 protein expression cytosolic fraction expression while repressing STAT-3 expression in the cytoplasm [
26]. As a result, Punicalagin appears to be able to preclude this transcription factor from moving into the nucleus. Since, STAT-3 has been demonstrated to prevent apoptosis by inducing Bcl-2 and preventing the production of Bax [
27]. It is conceivable that, punicalagin blocked the JAK/STAT pathway, which was then followed by a setback in the expression profile of Bcl-2/Bax, ultimately inducing the process and apoptosis. [
28]
Breast cancer is a major health problem for women due to its high mortality and morbidity rates. Even with adjuvant chemotherapy, the five-year survival rate for metastatic breast cancer is less than 30% [
29]. Recently IARC (International Agency for Research on Cancer) GLOBOCAN 2018 data from 185 countries revealed 2.3 million new cases of breast cancer (11.7%) and a mortality rate of 6.9% [
30]. The impact of Punicalagin concentrations greater than 50 µM could inhibit breast cancer cell growth. Vitality and minimize MCF-7 cells' invasion and migration. MDA-MB-231 cell type GOLPH3 was a golgi phosphoprotein(GOLPH) that was spoken in cells that had been transfected with or without punicalagin administration, as well as GOLPH3 expression levels. Using quantitative real-time polymerase chain reaction punicalagin prevented GOLPH3, MMP-2, and the expression of E-Cadherin was enhanced by MMP-9, N-Cadherin, and other factors. This mechanism enables punicalagin to suppress cell viability and slow the metastatic process by GOLPH3 regulation in breast cancer [
31].
In 2018, it is predicted that 1,096,000 new instances of colon cancer will be diagnosed, compared to 704,000 new cases of rectal cancer. These represent 1.8 million new cases of colon rectal cancer (CRC) collectively. In ten out of the 191 nations on the planet, men are diagnosed with CRC the most often. The most common type of cancer among women diagnosed worldwide is CRC [
30].The proapoptotic effect of punicalagin on human colorectal cancer has been observed by the increase in early apoptotic cells (in the cell line HCT116 V/PI flow cytometry) and mitochondrial release of cytochrome C [
8]. The HT-29 (human colon cancer) and HCT116 cell lines demonstrate an antiapoptotic effect of punicalagin [
32]. Also, the caspases 3/7, 8, and 9 were down regulated in HCT116 cells after treatment with punicalagin [
33].It is advantageous that punicalagin suppresses elevated annexin A1 expression protein because it appears to promote neoplastic progression in colorectal cancer [
32]. In HCT116 cells treated with punicalagin and formyl peptide receptor (FPR) inhibitors, they exposed four significantly changed proteins (applied to block annexin A1 signaling). Three heat shock proteins, 27 and 60 (HSP27 and HSP60), and tumor necrosis factor (TNF) are two of them. Catalase activity was increased, whereas receptor 1 (TNF RI) expression was down regulated refers to the Caco-2 human colon cancer cell lines. Punicalagin-induced halt of the cell cycle at the S phase and its proapoptotic action through the stimulation of the intrinsic-mitochondrial pathway [
32].
In 2020, ovarian cancer ranked third among gynecological cancers worldwide [
25]. Over 90% of all occurrences of ovarian cancer are ovarian carcinoma, making it the most prevalent kind [
34]. Punicalagin induce apoptosis, cause cell cycle arrest, and the viability of ovarian cancer cell A2780 was abridged in a dose- and time-dependent manner [
35]. Punicalagin inhibits the -catenin signal-transduction pathway and cell migration by down changeable matrix metalloproteinases and up regulating their inhibitors, and eventually suppresses the progression of ovarian cancer [
36].
In India, 122,844 women receive a cervical cancer diagnosis each year, and 67,477 of them pass away from the condition. There are 432.2 million women in India who are 15 years of age or older and at risk of having cancer [
37]. Punicalagin has been demonstrated to have an effect on human cervical cancer cell lines that is antiproliferative and proapoptotic. [
38] observed the dose-dependent (up to µ100 M) cytotoxicity of Punicalagin on ME-180 cells. Similarly, Punicalagin has been shown to have a dose- (up to µ200 M) and time-dependent antiproliferative effect on HeLa cells [
36]. Punicalagin therapy causes proapoptotic Bex proteins to be upregulated and antiapoptotic Bcl-2 factors to be downregulated. In addition, Punicalagin promoted the expression of the p53 gene; a tumor suppressor 3 and 9 proapoptotic caspases [
38]. Punicalagin's effect on cervical cancer cells was obviously proapoptotic and antiproliferative by suppressing the traditional NF-B route [
38].
Every year, the United States of America diagnoses about 900 new instances of osteosarcoma. [
39] Previously, revealed that three human osteosarcoma cell lines (U2OS, MG63, and SaOS2) may be used to test the ability of Punicalagin to induce apoptosis. Treatment with Punicalagin resulted in a significant increase in both early and late apoptotic cells in all three cell lines. Additionally, a study has shown that these three cell lines were given a 24-hour Punicalagin treatment, which decreased the invasiveness of the cells in a Matrigel experiment. Punicalagin also affects NF-B signaling by inhibiting active (phosphorylated) I-B as well as preventing NF-B-p65 from moving into the nucleus (U2OS and SaOS2). Punicalagin had also shown antiangiogenic properties [
40].
3. Neurological effect of punicalagin
James Parkinson initially described Parkinson's disease (PD), a neurological, progressive condition in 1817 [
41]. Punicalagin has better effects by reducing GSK-3 activity, oxidative stress, inflammatory cascades, apoptosis, and inflammatory stress in Parkinson disease. It also restores basal ganglia neurotransmitters, which improves animal motor functions and lowers their catalepsy score. As a result, these substances may prevent the death of neuronal cells and may be effective in reversing Parkinson's disease caused by MnCl2.
Clinically key features of Alzehimer’s disease are complete dementia, including memory impairment, executive dysfunction, personality changes, and behavioural changes. The majority of patients also experience signs of a mental problem [
42]. It causes neurotoxicity by alteration in the levels of MDA and GSH, two compounds crucial in oxidative stress, as well as by the activation of apoptotic pathways. Punicalagin boosts the cerebral cortex has a high GSH concentration and suppresses lipid peroxidation. Punicalagin also suppresses the expression of proteins linked to the apoptotic pathway. Punicalagin's anti-oxidant and anti-apoptotic qualities [
43]. Punicalagin and ellagic acid, two POMX components, reduced the release of proinflammatory cytokines from microglia in vitro [
44].Punicalagin's ability to significantly reduce NFAT activation and cytokine production suggests that it can directly treat Alzehimer’s disease [
45]. Punicalagin reduced LPS-induced NF-kB pathway activation, as well as in cultured astrocytes and microglial BV-2 cells. This NF-kB pathway suppression was linked to decreased levels of amyloid and a neuroinflammatory response [
46](
Figure 2).
An estimated 3.8% of the world's population suffers from depression, with 5.0% of adults and 5.7% of persons over 60 years of age being affected (WHO). Up to 20% of people globally are affected by the genetic and epigenetic variables that contribute to the mental health illness known as depression [
47]. Punicalagin has an antidepressant-like effect that promotes climbing behavior while reducing immobility. Numerous studies suggest that Punicalagin has neuroprotective properties both in vivo and in vitro [
48]. Punicalagin was found to have a strong scavenger activity and the extract fraction that contained Punicalagin caused the reduction of LPX. Punicalagin treatment actually reduces the pro-inflammatory cytokines TNF and IL-6, which reverses the neuroinflammatory effect of LPS intervention in hippocampus slides [
49]
.
4. Inflamation effect of pinicalagin
The immune system's biological response, inflammation, can be carried on by a number of things, including pathogens, damaged cells, and toxic substances. The heart, pancreas, liver, kidney, lung, brain, digestive tract, and reproductive system may all experience acute or chronic inflammatory reactions, which may result in tissue damage or disease. Inflammatory cells are stimulated by both infectious and non-infectious stimuli, as well as by cell injury, which also activates inflammatory signaling pathways, most frequently the NF-kB, MAPK, and JAK-STAT pathways [
50]. A complex and essential element of an organism's defensive mechanism against biological, chemical, and physical assaults is inflammation. [
51]
5. Effect on IL-6/JAK/STAT3
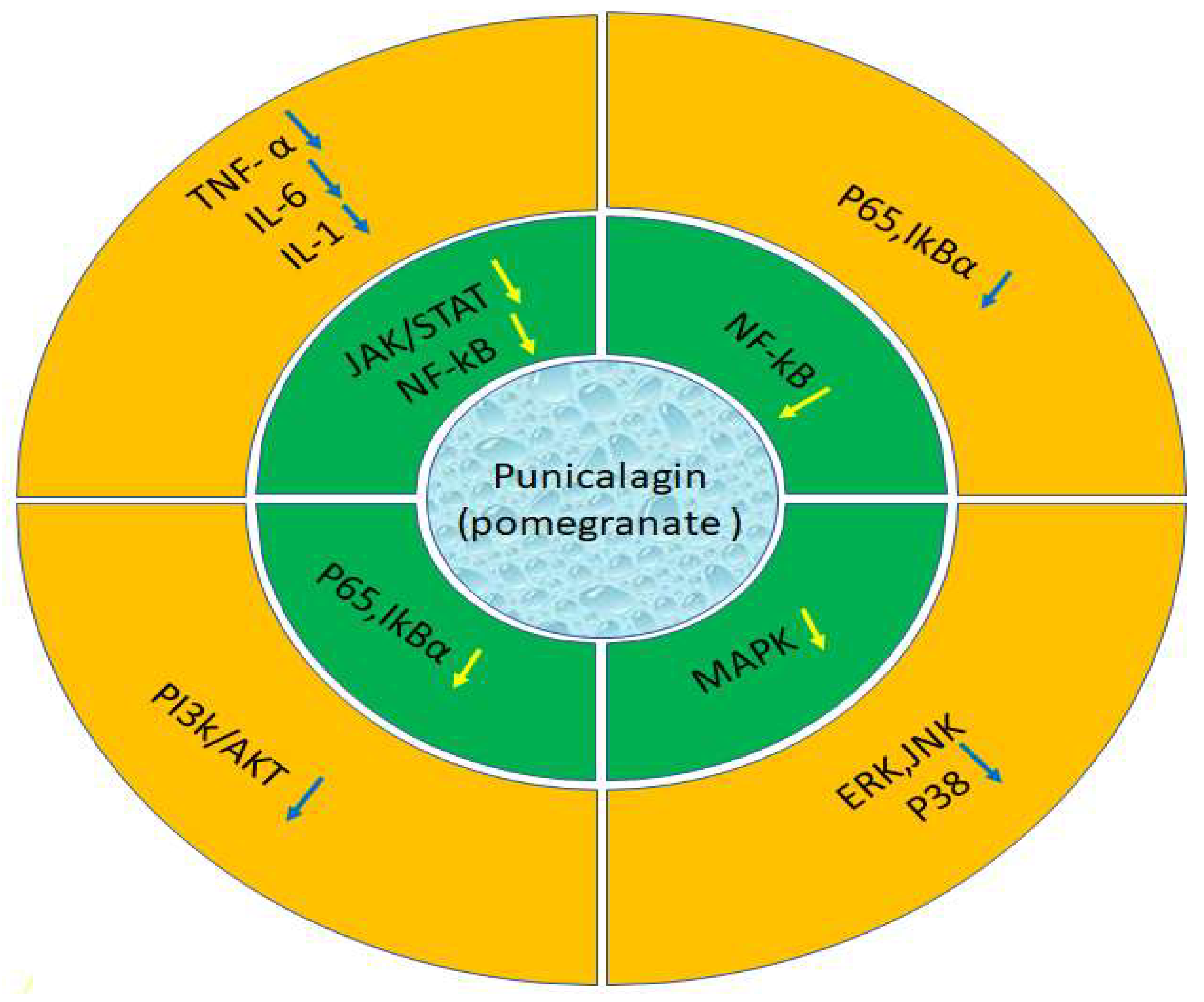
The immune system, inflammation, metabolism, and other essential life functions all involve cytokines. Inflammation is significantly influenced by the pleiotropic cytokine IL-6 [
52]. Chronic inflammatory diseases result in higher IL-6 levels. Major regulators of pro- and anti-inflammatory cytokine signaling include Janus kinases, Tyrosine Kinase 2, and STAT signaling [
53]. The JAK/STAT3 pathway is stimulated by high levels of IL-6 [
54]. Inflammatory reflection of RAW264.7 cells was triggered by LPS exposure (lipopolysaccharide). The supernatants had significantly increased levels of IL-6 and TNF-α production following 24-hour LPS stimulation. Punicalagin (50 µM) inhibited NO generation as well as the pro-inflammatory cytokines IL-6 and TNF-α in RAW264.7 cells stimulated with LPS. This resulted from pre-treatment with punicalagin (50 µM) and then treatment with LPS. It is important to note that LPS treatment alone or treatment with punicalagin alone had no impact on the basal level of IL-6 and TNF-α production in RAW 264.7 cells [
55]. There were various possible causes of ankylosing spondylitis. It was found that both increased ROS levels and the activated JAK/STAT3 signaling pathway had an impact on the pathogenic progression of ankylosing spondylitis [
56]. Punicalagin treatment considerably decreased ROS and greatly enhanced antioxidant status in ankylosing spondylitis. By controlling the primary channel of the inflammatory response, this result could be achieved by JAK/STAT3 signalling pathway [
57] (
Figure 2).
6. Effect on NF-κB Pathway
NF-kB has long been stared as a classic pro-inflammatory pathway. It was established in response to IL-1 and TNF-α signaling [
58]. It was without an activating stimulus, IkBs attach to sequester NF-kB dimers in the cytoplasm, concealing the signal that indicates their nuclear localization. After receiving the activating signal, the IkB proteins undergo fast polyubiquitylation and degradation, which frees NF-kB dimers to move into the nucleus and control gene expression [
59]. Punicalagin reduced tissue damage by decreasing pro-inflammatory mediators like NF-kB, TNF-α, and IL-6 while increasing antioxidant battlements by increasing Nrf2 [
60]. Punicalagin degraded IkBα and initiated the nuclear translocation of p65 in three human osteosarcoma cell lines (U2OS, MG63, and SaOS2) as well as the human osteoblast cell line (hFOB1.19), demonstrating a reduction in the activity of the NF-kB signaling pathway [
40]. Punicalagin reduced NF-kB activity in the ME-180 cervical cancer cell. Additionally, papillary thyroid carcinoma, the most dominant endocrine carcinoma, has been linked to punicalagin's anti-cancer properties [
61].
7. Effect on MAPK Pathway (Mitogen-activated protein kinases (MAPKs)
A class of serine/threonine protein kinases called mitogen-activated protein kinases (MAPKs) controls essential biological functions and cellular reactions to stress signals from the outside world. The increased activity of MAPKs, particularly p38 MAPK, and their roles in the transcriptional and translational control of the production of inflammatory mediators make them prospective targets for anti-inflammatory treatments. Preclinical data suggest that inhibitors targeting the p38 MAPK, ERKs, and JNK pathways have anti-inflammatory properties [
62]. Punicalagin markedly reduced the expression of genes that produce pro-inflammatory cytokines that are brought on by lipopolysaccharide (LPS). The suppressed levels of p38, ERK, and JNK phosphorylation in the MAPK signaling pathway were responsible for the inhibitory effects [
63].
8. Effect on PI3K/AKT/mTOR Pathway
The kinase family known as phosphoinositide-3 kinase (PI3K) regulates several biological progressions in mammalian cells, including cell development, proliferation, and survival. The primary controllers of proliferative signals, Class IA PI3Ks, are collected of a catalytic subunit (α, β, Γ ) that interacts with the p85 regulatory subunit to stimulate the AKT and mTOR pathways and control the activity of downstream effectors. Skin malignancies, psoriasis, and atopic dermatitis are a few clinical diseases well-defined by unchecked proliferation that are caused by deregulation of the PI3K/AKT/mTOR pathway in the skin. Basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC) are cutaneous malignancies that revelation hyperactivation of PI3K/AKTmTOR signaling, which is linked to tumorigenesis, hyperproliferation, and resistance to apoptosis [
64]. The expression of the pro-inflammatory enzymes cyclooxygenase-2 and nitric oxide synthase was considerably downregulated by punicalagin. Punicalagin may have an effect on the mTOR pathway because it also suppresses PI3K/AKT phosphorylation and mTOR expression [
65]. In cancer patients, the mTOR pathway is frequently deregulated. It is one of the most crucial signalling pathways in the development of cancer, which also comprises angiogenesis, apoptosis, proliferation, and treatment resistance [
66]. Punicalagin therapy reduced the survival of the thyroid cancer cell line BCPAP by activating MAPK and preventing the mTOR signaling pathway by promoting autophagy [
67] (
Figure 3).
9. Anti cardiosystem
Atherosclerosis is a chronic condition brought on by a number of variables, including heredity and dietary fat intake, which harm the artery wall and vascular function. Lipoprotein metabolism and endothelial function are both compromised in the condition. Additionally, it ranks greatly among the world's leading killers. Increased expression of adhesion molecules, including intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), which promote monocyte accumulation in the subendothelial matrix, is a hallmark of endothelial dysfunction. Macrophages and/or dendritic cells can grow from engulfing monocytes. As a result of these cells consuming native LDL and oxidised low-density lipoprotein (oxLDL), foam cells are created, which in turn cause atherosclerosis [
68,
69]. As cholesterol degradation has been revealed to be indirectly related to the occurrence of cardiovascular events, research has been undertaken that demonstrates a relationship between cholesterol degradation capacity and cardiovascular mortality. Elagitannins and their bioactive components, which the gut microbiota converts into low molecular weight molecules like urolithin B, have been shown to significantly accelerate the breakdown of cholesterol by macrophages. The results of the study showed that urolithin B was able to inhibit the formation of lipid plaques and change the expression of the ABCA1 and Scavenger Class B receptors type I (SR-BI) involved in the transport of reverse cholesterol [
70]
An important factor in the pathobiology of atherosclerotic cardiovascular disease is the dysfunction of the endothelial lining of the arterial vasculature's lesion-prone regions. In its broadest sense, endothelial cell dysfunction (ECD) refers to a collection of different non-adaptive changes in the functional phenotype that have significant effects on the control of thrombosis and hemostasis, local vascular tone and redox balance, and the orchestration of acute and chronic inflammatory responses within the arterial wall [
71](
Figure 3)
10. Anti-microbial activity
The diverse group of bacteria known as the gut microbiota performs a variety of tasks that have an impact on the host's general health. They include immune system management, nutrient metabolism, and built-in infection defense. Inflammatory chemicals that may cause inflammation in various body tissues are linked to the presence of specific bacteria [
72]. Punicalagin has been verified in studies to have antibacterial effects on both gramme-positive and gramme-negative bacteria. Punicalagin and ellagic acid have been revealed to exhibit antibacterial activity against various species of Clostridium, Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli [
9] Punicalagin has been revealed to inhibit the growth of cariogenic bacteria at high concentrations, but at subbactericidal concentrations, it inhibits the formation of biofilms and Streptococcus mutans' production of acidic and extracellular polysaccharides, indicating that punicalagin may be able to prevent tooth decay [
73]
Humans are susceptible to many viruses, yet the immune system successfully manages most of them with little harm to the host tissues. However, certain viruses do overtly damage the host, either in rare instances or as a reaction that frequently happens following infection. The characteristics of the contaminating virus, the circumstances of infection, and a number of host-controlled factors all affect the outcome [
74]. The impact of hydrolysable tannins on a panel of viruses was thoroughly examined by Lin et al. During treatment with these chemicals, the methods of virus binding, infection entry, and spread were inspected. Antiviral action was discovered against viruses that are known to enter the host cell via cell surface glycosaminoglycans (GAGs). According to the study, punicalagin was effective at suppressing several viruses at different concentrations, including the human cytomegalovirus (HCMV), the herpes simplex virus (HSV-1), the hepatitis C virus (HCV), the respiratory syncytial virus (RSV), the measles virus (MV), and the dengue virus (DENV) [
75]. Tito et al. even mentioned that punicalagin and ellagic acid reduced viral 3CL protease activity in vitro and blocked the interaction between spike protein and ACE2, implying that pomegranate extract could be used to prevent and treat SARS-CoV-2 disease [
76].
11. Antioxidant activity
Oxidative stress is brought on by an imbalance between the creation and accumulation of oxygen-reactive species (ROS) in cells and tissues and the body's capacity to detoxify these reactive substances [
77]. This disparity causes damage to crucial biological macromolecules such as lipids, carbohydrates, proteins, and DNA. Additionally, the presentation of numerous chronic diseases, as well as metabolic, neurological, cardiovascular, lung, kidney, and cancer diseases, is linked to oxidative stress [
78,
79]. Antioxidants like PUN, metabolised EA, and urolithins have all been identified as having positive belongings against oxidative stress. Punicalagin and ellagic acid both have excellent antioxidant properties with some variances that may be due to variable polymerization and the quantity of unsaturated double bonds [
80]. Urolithin A, B, C, and D (also known as 8-O-methylurolithin A, 8,9-di-O-methylurolithin C, and 8,9-di-O-methylurolithin D) are used to inhibit the generation of intracellular ROS [
81].
One of the most crucial organs in the body, the liver legalizes many different bodily functions, with a focus on the metabolism, secretion, storage, and detoxification of both endogenous and foreign chemicals. Hepatic illnesses remain to be one of the biggest risks to public health because of these functions, and they are still a concern everywhere on the globe [
82]. Punicalagin inhibited the expression of cyclooxygenase-2 and the histological damage to liver tissue [
83]. Punicalagin can protect HepG2 cells against palmitate-induced lipotoxicity via activating the ERK/Nrf2 pathway; according to a study by Yan et al. Oxidative stress and apoptosis are closely connected processes. Punicalagin improves mitochondrial activity by raising mitochondrial membrane potential and reducing the production of ROS (reactive oxygen species) brought on by palmitate. Punicalagin can influence cellular antioxidant mechanisms through the ERK/Nrf2 pathway, preserving mitochondrial function [
84].
12. Antidiabetic activity
Obesity causes systemic, ongoing inflammation that can result in type 2 diabetes (T2D), insulin resistance (IR), and β-cell dysfunction. This ongoing inflammatory condition may be what causes type 2 diabetes and its long-term consequences, such as non-alcoholic fatty liver disease (NAFLD), retinopathy, cardiovascular disease, and nephropathy [
85]. Recent studies on metabolic disorders such as diabetes and obesity discovered that punicalagin, ellagic acid , and urolithin A have the ability to prevent the DPP-4, α-GLU, and lipase enzymes complicated in glucose and triglyceride metabolism. The ability of these substances to prevent adipogenesis and decrease triglyceride accumulation was validated during the differentiation of the 3T3-L1 cell line with these polyphenols. Additionally, it has been revealed that they have the capacity to alter the expression of genes that control fatty acid and glucose metabolism, including the GLUT4, FABP4, adiponectin, and PPARy genes, which are frequently employed as indicators of adipocyte differentiation [
86]. Punicalagin improves mitochondrial activity, lessens oxidative stress, and decreases inflammation, making it a potential supplement for the treatment of NAFLD [
87]. Punicalagin lowers nitrosative stress and lipid peroxidation, which benefit to minimise oxidative stress brought on by high glucose levels [
88].
13. Anti-neural disease
Reduced blood supply to the locations supplied by the clogged arteries is where the ischemic episode starts. Adenosine triphosphate (ATP), a high-energy phosphate molecule, is mostly deficient in neuronal tissues. Therefore, Ca2+ homeostasis maintenance in cerebral ischemia cells is lost because there is insufficient ATP to power extrusion pumps, and the resting membrane potential is also disturbed due to malfunction of the Na+/K+ ATPase pumps, resulting in "anoxic depolarization" [
89]. This illustration of the likely chain of events leading to ischemic neuronal death emphasises the negative consequences of oxidative and nitrosative stress in cerebral ischemia [
90]. Punicalagin decreases malondialdehyde levels, sodium-potassium adenosine triphosphatase activity, nitric oxide, protein carbonyl content, and reactive oxygen species produced by mitochondria while increasing superoxide dismutase, catalase, glutathione peroxidase, reduced glutathione, and glutathione reductase activity. Punicalagin's antioxidant capacity enables excellent repair of oxidative damage brought on by cerebral ischemia and reperfusion [
48].
14. Anti-digestive system
The digestive (GI) system is in charge of breaking down and absorbing the food and liquids that are consumed. Due to the GI tract's intricacy and the large amount of information that may be included, GI physiology could cover Diseases of the oesophagus, stomach; small intestine, colon, and rectum are referred to as GI diseases. Recurrent stomach pain and bloating, heartburn, indigestion or dyspepsia, nausea and vomiting, diarrhoea, and constipation are the main symptoms of common GI problems [
91]. The gastroprotective properties of punicalagins were studied by Chaibub et al. There are some lyophilized water fractions of
L. pacari and Punicalagin can decline gastric secretion volume, free acidity, and total acidity in mice 4 hours after pyloric ligation, as well as the rate of stomach damage in the indomethacin delivery paradigm. The lyophilic water component of L. pacari and P. punicalagin has antiulcerogenic happenings as a result of their acid antisecretory activity. After treatment, lyophilized water fraction and punicalagin significantly reduced acute gastrointestinal lesions [
91].
15. Anti-Hypertension
The antihypertensive activities of punicalagin The risk of all major cardiovascular events, such as stroke, sudden cardiac death, coronary heart disease, heart failure, abdominal aortic aneurysm, and peripheral vascular disease, is significantly increased by hypertension, which continues to be the leading cause of morbidity and mortality in the world. Clarifying the disease's complicated aetiology, which involves dysregulation of numerous homeostatic systems and impacts not just blood pressure but also the development of end-organ damage connected to hypertension, required significant improvements in our understanding of its pathogenesis [
92]. Punicalagin reduces the synthesis of luciferase that is triggered by NF-B, as well as NF-B phosphorylation and nuclear translocation of the p65 subunit. Pre-eclampsia (PE), a condition of pregnancy brought on by poor maternal nutrition and oxidative stress, is characterised by severe hypertension and a higher risk of foetal and maternal death. As a hypertension inducer, N-nitro-L-arginine methyl ester (L-NAME) was employed. Treatment with punicalagin dramatically lowered systolic and diastolic blood pressure. Punicalagin also improves angiogenic balance by upregulating tyrosine kinase-1 and VEGF receptors (vascular endothelial growth factor receptors) 1 and 2 while decreasing the expression of VEGF. Punicalagin dramatically raises the amount of nitric oxide in the placenta. Punicalagin also reduces body blood pressure and increases oxidative stress in pregnant rats that causes and induces preeclampsia, and it also balances angiogenic activity [
93].
Table 1.
Showing the different activities of extracts and their Pharmacological/Biolofical activites and receptor binding pockets.
Table 1.
Showing the different activities of extracts and their Pharmacological/Biolofical activites and receptor binding pockets.
| S.N |
Pharmacological
activities |
Punicalagin effects |
Receptor |
References |
| 1 |
Anticardio system |
Breakdoun of cholesterol by macrophages ,
It is also inhibit the farmaton of lipid plaques and change the Expression. |
It mainly affects or binds to a receptor known as scavenger class B receptor (SR-BI) type I via transport of reverse cholesterol. |
[94] |
| 2 |
Bacteria |
Punicalagin exhibit antibacterial activity against various species of Clastridium , Pseudomonas , Aeruginosa ,etc
It is also inhibited the growth of cariogenic cells (including bacteria) at high concentration but at subbactericidal concentration it inhibit the formation of biofilms and Streptococcus mutants . |
It inhibit the formation of biofilms |
[9], [73]
|
| 3 |
Viruses |
Punicalagin and ellagic acid reduced viral 3CL protease activity in interaction between spike protein and ACE2 . |
spike protein |
[76] |
| 4 |
Antioxidant activity |
Prevent the generation of intracellular ROS ,Pinicalagin inhibit the expression of cyclo-oxygenase -2 and the histological damage to liver tissue, whereas punicalagin might protected HEPG-2 cells by changing the activities of the ERK/NrF2 pathway . |
ERK/NrF2 Pathway |
[17], [95] |
| 5 |
Anti-diabetic activity |
Punicalagin and ellagic acid have the ability to inhibit the DPP-4,α-GLU and lipase enzyme involved in glucose and triglyceride metabolism |
3T3-L1 cell line, FABP4, PPARY. |
[87] |
| 6 |
Anti-neural desease |
Punicalagin – decrease malondialdehyde levels, sodium – potassium adenosine triphasphatase activity, nitric oxide, protein carbonyl content and ROS.
It also increases activites of some enzymes like catalase and superoxide dismutase by ATPase pump inhibition. |
Na+/K+ ATPase pumps |
[48] |
| 7 |
Anti-digestive system |
Punicalagin singnificantly reduces the acute gastrointestinal lesions,
|
This is in-vivo model |
[91] |
| 8 |
Anti-hypertension |
Punicalagin reduce the synthesis of luciferase triggered by NF-B as well as NF-B phosphorylation ,punicalagin also improve angiogenic balance by upregulating tyrosine kinase-1 and VEGF receptor 1 and 2 while decreaseing the expression of VEGF |
NF-B as well as phosphorylation and p65 |
[50] |
Conclusion
This review article contains the compilation of the all information related to different sources of Punicalagin and related derivatives (a polyphenol). It is extensively not pervasive in Punica granatum but other sources like lafoensia paccari and genus Terminalia. Punicalagin and its metabolites has shown as an inhibitory activities like angiogenesis, proliferation and induce apoptosis in osteosarcoma cancer cell, antioxidant, hepatoprotective, anti-microbial, anti-viral, neuroprotective, anti-inflammation. Other biological and pharmacological activities includes anti cancerous by inhibiting autophagy that reduces the apoptosis and suppressive proliferation of inhibiting cell viabilities. It is also reported as liver cells protective by inhibiting oxidative or nitrosative stress. Antimicrobial activities of punicalagin have been also reported by interruption of cell membrane causes damage cell viabilities. The extract of punicalagin been reported as antiviral properties by inhibiting multiplication of viral cell and their enzyme activities, therefore, reduces viral replication and transcription with very less cell toxicity. This (punicalagin and its metabolites) is also interfered cell signaling pathway against inflammation and other many pathway that proved and suggestive concludes that it improves the associated inflammatory chronic disease.
Author Contributions
For this review article has contributed many authors “Conceptualization, SY, AKY, KV, and MYA.; methodology, SY, AKY, KV, RHM, SK, SC, MK and MYA.; software, SY, AKY, KV, NH, AKS and MYA; validation, SY, AKY, KV,AT, SG AK, AKS and MYA.; formal analysis, SY, AKY, KV, and MYA; investigation, SY, AKY, KV, and MYA; resources, SY, AKY, KV, and MYA; data curation, SY, AKY, KV, NH, SK and MYA.; writing original draft preparation, SY, AKY, KV,SG, AK, AT and MYA.; writing review , SY, AKY, KV, and MYA.; visualization, SY, AKY, KV, and MYA; supervision, KV, and MYA; project administration, SY, AKY, KV, and MYA.; funding acquisition, SY.”.
Funding
This research was funded by Deanship of Scientific Research, King Khalid University, Abha, Saudi Arabia, through the Small Research Group Program grants number RGP. 1/219/44.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through a small research group program under grant number RGP. 1/219/44.
Conflicts of Interest
The authors confirm that there is no conflicting of interest.
References
- Feng, L.; Yin, Y.; Yang, X.; Tang, H.; Jiao, Q. Dynamic Variations in Punicalagin and Related Metabolic Substances in Pomegranate Fruit and Leaves During Development Periods. The Horticulture Journal advpub 2019. [Google Scholar] [CrossRef]
- Jurenka, J.S. Therapeutic applications of pomegranate (Punica granatum L.): a review. Altern Med Rev 2008, 13, 128–144. [Google Scholar]
- Abdollahzadeh, S.; Mashouf, R.; Mortazavi, H.; Moghaddam, M.; Roozbahani, N.; Vahedi, M. Antibacterial and antifungal activities of punica granatum peel extracts against oral pathogens. J Dent (Tehran) 2011, 8, 1–6. [Google Scholar]
- Zahin, M.; Ahmad, I.; Gupta, R.C.; Aqil, F. Punicalagin and ellagic acid demonstrate antimutagenic activity and inhibition of benzo [a]pyrene induced DNA adducts. Biomed Res Int 2014, 2014, 467465. [Google Scholar] [CrossRef] [PubMed]
- Giamogante, F.; Marrocco, I.; Cervoni, L.; Eufemi, M.; Chichiarelli, S.; Altieri, F. Punicalagin, an active pomegranate component, is a new inhibitor of PDIA3 reductase activity. Biochimie 2018, 147, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, C.C.; da Costa Santos, S.; de Souza Lino, R., Jr.; Bara, M.T.; Chaibub, B.A.; de Melo Reis, P.R.; Chaves, D.A.; da Silva, A.J.; Silva, L.S.; de Melo, E.S.D.; Chen-Chen, L. Chemopreventive effect and angiogenic activity of punicalagin isolated from leaves of Lafoensia pacari A. St.-Hil. Toxicol Appl Pharmacol 2016, 310, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kraszni, M.; Marosi, A.; Larive, C.K. NMR assignments and the acid-base characterization of the pomegranate ellagitannin punicalagin in the acidic pH-range. Anal Bioanal Chem 2013, 405, 5807–5816. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Adams, L.S.; Henning, S.M.; Niu, Y.; Zhang, Y.; Nair, M.G.; Heber, D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem 2005, 16, 360–367. [Google Scholar] [CrossRef]
- Venusova, E.; Kolesarova, A.; Horky, P.; Slama, P. Physiological and Immune Functions of Punicalagin. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Moilanen, J.; Karonen, M.; Tähtinen, P.; Jacquet, R.; Quideau, S.; Salminen, J.P. Biological activity of ellagitannins: Effects as anti-oxidants, pro-oxidants and metal chelators. Phytochemistry 2016, 125, 65–72. [Google Scholar] [CrossRef]
- Kulkarni, A.P.; Mahal, H.S.; Kapoor, S.; Aradhya, S.M. In vitro studies on the binding, antioxidant, and cytotoxic actions of punicalagin. J Agric Food Chem 2007, 55, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; Rollinger, J.M.; Schuster, D.; Breuss, J.M.; Bochkov, V.; Mihovilovic, M.D.; Kopp, B.; Bauer, R.; Dirsch, V.M.; Stuppner, H. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol Adv 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Sajadimajd, S.; Bahramsoltani, R.; Iranpanah, A.; Patra, J.K.; Das, G.; Gouda, S.; Rahimi, R.; Rezaeiamiri, E.; Cao, H.; Giampieri, F.; Battino, M.; Tundis, R.; Campos, M.G.; Farzaei, M.H.; Xiao, J. Advances on Natural Polyphenols as Anticancer Agents for Skin Cancer. Pharmacol Res 2020, 151, 104584. [Google Scholar] [CrossRef] [PubMed]
- Pirzadeh, M.; Caporaso, N.; Rauf, A.; Shariati, M.A.; Yessimbekov, Z.; Khan, M.U.; Imran, M.; Mubarak, M.S. Pomegranate as a source of bioactive constituents: a review on their characterization, properties and applications. Crit Rev Food Sci Nutr 2021, 61, 982–999. [Google Scholar] [CrossRef]
- Kaderides, K.; Kyriakoudi, A.; Mourtzinos, I.; Goula, A.M. Potential of pomegranate peel extract as a natural additive in foods. Trends in Food Science and Technology 2021, 115, 380–390. [Google Scholar] [CrossRef]
- Huang, M.; Wu, K.; Zeng, S.; Liu, W.; Cui, T.; Chen, Z.; Lin, L.; Chen, D.; Ouyang, H. Punicalagin Inhibited Inflammation and Migration of Fibroblast-Like Synoviocytes Through NF-κB Pathway in the Experimental Study of Rheumatoid Arthritis. J Inflamm Res 2021, 14, 1901–1913. [Google Scholar] [CrossRef]
- Fouad, A.A.; Qutub, H.O.; Al-Melhim, W.N. Nephroprotection of punicalagin in rat model of endotoxemic acute kidney injury. Toxicol Mech Methods 2016, 26, 538–543. [Google Scholar] [CrossRef]
- Lee, S.I.; Kim, B.S.; Kim, K.S.; Lee, S.; Shin, K.S.; Lim, J.S. Immune-suppressive activity of punicalagin via inhibition of NFAT activation. Biochem Biophys Res Commun 2008, 371, 799–803. [Google Scholar] [CrossRef]
- Wang, Y.; Smith, W.; Hao, D.; He, B.; Kong, L. M1 and M2 macrophage polarization and potentially therapeutic naturally occurring compounds. Int Immunopharmacol 2019, 70, 459–466. [Google Scholar] [CrossRef]
- Du, L.; Li, J.; Zhang, X.; Wang, L.; Zhang, W.; Yang, M.; Hou, C. Pomegranate peel polyphenols inhibits inflammation in LPS-induced RAW264.7 macrophages via the suppression of TLR4/NF-κB pathway activation. Food Nutr Res 2019, 63. [Google Scholar] [CrossRef]
- Katerji, M.; Duerksen-Hughes, P.J. DNA damage in cancer development: special implications in viral oncogenesis. Am J Cancer Res 2021, 11, 3956–3979. [Google Scholar] [PubMed]
- Koshy, M.; Villano, J.L.; Dolecek, T.A.; Howard, A.; Mahmood, U.; Chmura, S.J.; Weichselbaum, R.R.; McCarthy, B.J. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J Neurooncol 2012, 107, 207–212. [Google Scholar] [CrossRef]
- Wang, S.G.; Huang, M.H.; Li, J.H.; Lai, F.I.; Lee, H.M.; Hsu, Y.N. Punicalagin induces apoptotic and autophagic cell death in human U87MG glioma cells. Acta Pharmacol Sin 2013, 34, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yao, X.; Xu, S.; Pan, J.; Yu, H.; Bao, J.; Guan, H.; Lu, R.; Zhang, L. Punicalagin induces senescent growth arrest in human papillary thyroid carcinoma BCPAP cells via NF-κB signaling pathway. Biomed Pharmacother 2018, 103, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Berköz, M.; Krośniak, M. Punicalagin induces apoptosis in A549 cell line through mitochondria-mediated pathway. Gen Physiol Biophys 2020, 39, 557–567. [Google Scholar] [CrossRef]
- Fang, L.; Wang, H.; Zhang, J.; Fang, X. Punicalagin induces ROS-mediated apoptotic cell death through inhibiting STAT3 translocation in lung cancer A549 cells. J Biochem Mol Toxicol 2021, 35, 1–10. [Google Scholar] [CrossRef]
- Nielsen, M.; Kaestel, C.G.; Eriksen, K.W.; Woetmann, A.; Stokkedal, T.; Kaltoft, K.; Geisler, C.; Röpke, C.; Odum, N. Inhibition of constitutively activated Stat3 correlates with altered Bcl-2/Bax expression and induction of apoptosis in mycosis fungoides tumor cells. Leukemia 1999, 13, 735–738. [Google Scholar] [CrossRef]
- Riggio, A.I.; Varley, K.E.; Welm, A.L. The lingering mysteries of metastatic recurrence in breast cancer. British Journal of Cancer 2021, 124, 13–26. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Pan, L.; Duan, Y.; Ma, F.; Lou, L. Punicalagin inhibits the viability, migration, invasion, and EMT by regulating GOLPH3 in breast cancer cells. J Recept Signal Transduct Res 2020, 40, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, T.; Sinniah, A.; Chik, Z.; Alshawsh, M.A. Punicalagin Regulates Apoptosis-Autophagy Switch via Modulation of Annexin A1 in Colorectal Cancer. Nutrients 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Zhang, S.; Wang, B.; Huang, W.; Zheng, L.; Cheng, A. Annexin A1: A double-edged sword as novel cancer biomarker. Clin Chim Acta 2020, 504, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef]
- Tang, J.M.; Min, J.; Li, B.S.; Hong, S.S.; Liu, C.; Hu, M.; Li, Y.; Yang, J.; Hong, L. Therapeutic Effects of Punicalagin Against Ovarian Carcinoma Cells in Association With β-Catenin Signaling Inhibition. Int J Gynecol Cancer 2016, 26, 1557–1563. [Google Scholar] [CrossRef]
- Tang, J.; Li, B.; Hong, S.; Liu, C.; Min, J.; Hu, M.; Li, Y.; Liu, Y.; Hong, L. Punicalagin suppresses the proliferation and invasion of cervical cancer cells through inhibition of the β-catenin pathway. Mol Med Rep 2017, 16, 1439–1444. [Google Scholar] [CrossRef]
- Sreedevi, A.; Javed, R.; Dinesh, A. Epidemiology of cervical cancer with special focus on India. Int J Womens Health 2015, 7, 405–414. [Google Scholar]
- Zhang, L.; Chinnathambi, A.; Alharbi, S.A.; Veeraraghavan, V.P.; Mohan, S.K.; Zhang, G. Punicalagin promotes the apoptosis in human cervical cancer (ME-180) cells through mitochondrial pathway and by inhibiting the NF-kB signaling pathway. Saudi J Biol Sci 2020, 27, 1100–1106. [Google Scholar] [CrossRef]
- Morrow, J.J.; Khanna, C. Osteosarcoma Genetics and Epigenetics: Emerging Biology and Candidate Therapies. Crit Rev Oncog 2015, 20, 173–197. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, X.; Wang, H. Punicalagin inhibited proliferation, invasion and angiogenesis of osteosarcoma through suppression of NF-κB signaling. Mol Med Rep 2020, 22, 2386–2394. [Google Scholar] [CrossRef]
- Lees, A.J. Unresolved issues relating to the shaking palsy on the celebration of James Parkinson's 250th birthday. Mov Disord 2007, 22 (Suppl 17), S327–S334. [Google Scholar] [CrossRef] [PubMed]
- Lyketsos, C.G.; Carrillo, M.C.; Ryan, J.M.; Khachaturian, A.S.; Trzepacz, P.; Amatniek, J.; Cedarbaum, J.; Brashear, R.; Miller, D.S. Neuropsychiatric symptoms in Alzheimer's disease. Alzheimers Dement 2011, 7, 532–539. [Google Scholar] [CrossRef]
- Foroutanfar, A.; Mehri, S.; Kamyar, M.; Tandisehpanah, Z.; Hosseinzadeh, H. Protective effect of punicalagin, the main polyphenol compound of pomegranate, against acrylamide-induced neurotoxicity and hepatotoxicity in rats. Phytotherapy Research 2020, 34, 3262–3272. [Google Scholar] [CrossRef] [PubMed]
- Hartman, R.E.; Shah, A.; Fagan, A.M.; Schwetye, K.E.; Parsadanian, M.; Schulman, R.N.; Finn, M.B.; Holtzman, D.M. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer's disease. Neurobiol Dis 2006, 24, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Hollebeeck, S.; Winand, J.; Hérent, M.F.; During, A.; Leclercq, J.; Larondelle, Y.; Schneider, Y.J. Anti-inflammatory effects of pomegranate (Punica granatum L.) husk ellagitannins in Caco-2 cells, an in vitro model of human intestine. Food Funct 2012, 3, 875–885. [Google Scholar] [CrossRef]
- Xu, B.; Chen, X.; Lu, W.; Zhao, C.; Qiao, Y. xu et al. APL 2014, in, 2014. [Google Scholar]
- Nestler, E.J.; Barrot, M.; DiLeone, R.J.; Eisch, A.J.; Gold, S.J.; Monteggia, L.M. Neurobiology of depression. Neuron 2002, 34, 13–25. [Google Scholar] [CrossRef]
- Yaidikar, L.; Byna, B.; Thakur, S. Neuroprotective Effect of Punicalagin against Cerebral Ischemia Reperfusion-induced Oxidative Brain Injury in Rats. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association 2014, 23. [Google Scholar] [CrossRef] [PubMed]
- Olajide, O.A.; Kumar, A.; Velagapudi, R.; Okorji, U.P.; Fiebich, B.L. Punicalagin inhibits neuroinflammation in LPS-activated rat primary microglia. Mol Nutr Food Res 2014, 58, 1843–1851. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Germolec, D.R.; Frawley, R.P.; Evans, E. Markers of Inflammation, In Immunotoxicity Testing: Methods and Protocols, Dietert, R.R., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 53–73. [Google Scholar]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int Immunol 2021, 33, 127–148. [Google Scholar] [CrossRef]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int Immunopharmacol 2020, 80, 106210. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhong, L.; Zhu, J.; Xu, H.; Ma, W.; Zhang, L.; Shen, Y.; Law, B.Y.; Ding, F.; Gu, X.; Sun, H. Inhibition of IL-6/JAK/STAT3 pathway rescues denervation-induced skeletal muscle atrophy. Ann Transl Med 2020, 8, 1681. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, J.; Ren, G.; Zhang, Y.; Tan, X.; Yang, L. Punicalagin Prevents Inflammation in LPS-Induced RAW264.7 Macrophages by Inhibiting FoxO3a/Autophagy Signaling Pathway. Nutrients 2019, 11. [Google Scholar] [CrossRef]
- Zou, Y.C.; Yan, L.M.; Gao, Y.P.; Wang, Z.Y.; Liu, G. miR-21 may Act as a Potential Mediator Between Inflammation and Abnormal Bone Formation in Ankylosing Spondylitis Based on TNF-α Concentration-Dependent Manner Through the JAK2/STAT3 Pathway. Dose Response 2020, 18, 1559325819901239. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Yang, Q.; Wang, C.; Tong, W.; Xu, W. Punicalagin Exerts Protective Effects against Ankylosing Spondylitis by Regulating NF-κB-TH17/JAK2/STAT3 Signaling and Oxidative Stress. Biomed Res Int 2020, 2020, 4918239. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 2009, 1, a001651. [Google Scholar] [CrossRef]
- Prescott, J.A.; Mitchell, J.P.; Cook, S.J. Inhibitory feedback control of NF-κB signalling in health and disease. Biochem J 2021, 478, 2619–2664. [Google Scholar] [CrossRef]
- Aladaileh, S.H.; Al-Swailmi, F.K.; Abukhalil, M.H.; Ahmeda, A.F.; Mahmoud, A.M. Punicalagin prevents cisplatin-induced nephrotoxicity by attenuating oxidative stress, inflammatory response, and apoptosis in rats. Life Sci 2021, 286, 120071. [Google Scholar] [CrossRef]
- Subkorn, P.; Norkaew, C.; Deesrisak, K.; Tanyong, D. Punicalagin, a pomegranate compound, induces apoptosis and autophagy in acute leukemia. PeerJ 2021, 9, e12303. [Google Scholar] [CrossRef]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef]
- Du, L.; Li, J.; Zhang, X.; Wang, L.; Zhang, W. Pomegranate peel polyphenols inhibits inflammation in LPS-induced RAW264.7 macrophages via the suppression of MAPKs activation. Journal of Functional Foods 2018, 43, 62–69. [Google Scholar] [CrossRef]
- Mercurio, L.; Albanesi, C.; Madonna, S. Recent Updates on the Involvement of PI3K/AKT/mTOR Molecular Cascade in the Pathogenesis of Hyperproliferative Skin Disorders. Front Med (Lausanne) 2021, 8, 665647. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, N.; Kim, H.; Talcott, S.; Mertens-Talcott, S. Pomegranate polyphenolics suppressed azoxymethane-induced colorectal aberrant crypt foci and inflammation: possible role of miR-126/VCAM-1 and miR-126/PI3K/AKT/mTOR. Carcinogenesis 2013, 34, 2814–2822. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, M.; Mihanfar, A.; Akbarzadeh, S.; Yousefi, B.; Majidinia, M. Crosstalk between miRNA and PI3K/AKT/mTOR signaling pathway in cancer. Life Sci 2021, 285, 119984. [Google Scholar] [CrossRef] [PubMed]
- Chaves, F.M.; Pavan, I.C.B.; da Silva, L.G.S.; de Freitas, L.B.; Rostagno, M.A.; Antunes, A.E.C.; Bezerra, R.M.N.; Simabuco, F.M. Pomegranate Juice and Peel Extracts are Able to Inhibit Proliferation, Migration and Colony Formation of Prostate Cancer Cell Lines and Modulate the Akt/mTOR/S6K Signaling Pathway. Plant Foods Hum Nutr 2020, 75, 54–62. [Google Scholar] [CrossRef]
- Mele, L.; Mena, P.; Piemontese, A.; Marino, V.; López-Gutiérrez, N.; Bernini, F.; Brighenti, F.; Zanotti, I.; Del Rio, D. Antiatherogenic effects of ellagic acid and urolithins in vitro. Arch Biochem Biophys 2016, 599, 42–50. [Google Scholar] [CrossRef]
- Kruth, H.S. Fluid-phase pinocytosis of LDL by macrophages: a novel target to reduce macrophage cholesterol accumulation in atherosclerotic lesions. Curr Pharm Des 2013, 19, 5865–5872. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, L.; Haller, V.; Ritsch, A. A Novel Candidate for Prevention and Treatment of Atherosclerosis: Urolithin B Decreases Lipid Plaque Deposition in apoE(-/-) Mice and Increases Early Stages of Reverse Cholesterol Transport in ox-LDL Treated Macrophages Cells. Mol Nutr Food Res 2019, 63, e1800887. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ Res 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int J Environ Res Public Health 2020, 17. [Google Scholar] [CrossRef] [PubMed]
- Gulube, Z.; Patel, M. Effect of Punica granatum on the virulence factors of cariogenic bacteria Streptococcus mutans. Microb Pathog 2016, 98, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Rouse, B.T.; Sehrawat, S. Immunity and immunopathology to viruses: what decides the outcome?
Nat Rev Immunol 2010, 10, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.T.; Chen, T.Y.; Lin, S.C.; Chung, C.Y.; Lin, T.C.; Wang, G.H.; Anderson, R.; Lin, C.C.; Richardson, C.D. Broad-spectrum antiviral activity of chebulagic acid and punicalagin against viruses that use glycosaminoglycans for entry. BMC Microbiol 2013, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Tito, A.; Colantuono, A.; Pirone, L.; Pedone, E.; Intartaglia, D.; Giamundo, G.; Conte, I.; Vitaglione, P.; Apone, F. Pomegranate Peel Extract as an Inhibitor of SARS-CoV-2 Spike Binding to Human ACE2 Receptor (in vitro): A Promising Source of Novel Antiviral Drugs. Front Chem 2021, 9, 638187. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid Med Cell Longev 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Djedjibegovic, J.; Marjanovic, A.; Panieri, E.; Saso, L. Ellagic Acid-Derived Urolithins as Modulators of Oxidative Stress. Oxid Med Cell Longev 2020, 2020, 5194508. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; Abete, P. Oxidative stress, aging, and diseases. Clin Interv Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Sun, Y.-Q.; Tao, X.; Men, X.-M.; Xu, Z.; Wang, T. In vitro and in vivo antioxidant activities of three major polyphenolic compounds in pomegranate peel: Ellagic acid, punicalin, and punicalagin. Journal of Integrative Agriculture 2017, 16, 1808–1818. [Google Scholar] [CrossRef]
- Bialonska, D.; Kasimsetty, S.G.; Khan, S.I.; Ferreira, D. Urolithins, intestinal microbial metabolites of Pomegranate ellagitannins, exhibit potent antioxidant activity in a cell-based assay. J Agric Food Chem 2009, 57, 10181–10186. [Google Scholar] [CrossRef]
- Madrigal-Santillán, E.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Sumaya-Martínez, M.T.; Gutiérrez-Salinas, J.; Bautista, M.; Morales-González, Á.; González-Rubio, M.G.-L.Y.; Aguilar-Faisal, J.L.; Morales-González, J.A. Review of natural products with hepatoprotective effects. World J Gastroenterol 2014, 20, 14787–14804. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.A.; Qutub, H.O.; Al-Melhim, W.N. Punicalagin alleviates hepatotoxicity in rats challenged with cyclophosphamide. Environ Toxicol Pharmacol 2016, 45, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Sun, W.; Wang, X.; Long, J.; Liu, X.; Feng, Z.; Liu, J. Punicalagin attenuates palmitate-induced lipotoxicity in HepG2 cells by activating the Keap1-Nrf2 antioxidant defense system. Mol Nutr Food Res 2016, 60, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Les, F.; Arbonés-Mainar, J.M.; Valero, M.S.; López, V. Pomegranate polyphenols and urolithin A inhibit α-glucosidase, dipeptidyl peptidase-4, lipase, triglyceride accumulation and adipogenesis related genes in 3T3-L1 adipocyte-like cells. J Ethnopharmacol 2018, 220, 67–74. [Google Scholar] [CrossRef]
- Landray, M.J.; Haynes, R.; Hopewell, J.C.; Parish, S.; Aung, T.; Tomson, J.; Wallendszus, K.; Craig, M.; Jiang, L.; Collins, R.; Armitage, J. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014, 371, 203–212. [Google Scholar]
- Zhong, J.; Reece, E.A.; Yang, P. Punicalagin exerts protective effect against high glucose-induced cellular stress and neural tube defects. Biochem Biophys Res Commun 2015, 467, 179–184. [Google Scholar] [CrossRef]
- Juzekaeva, E.; Gainutdinov, A.; Mukhtarov, M.; Khazipov, R. Reappraisal of anoxic spreading depolarization as a terminal event during oxygen-glucose deprivation in brain slices in vitro. Sci Rep 2020, 10, 18970. [Google Scholar] [CrossRef]
- Khoshnam, S.E.; Winlow, W.; Farzaneh, M.; Farbood, Y.; Moghaddam, H.F. Pathogenic mechanisms following ischemic stroke. Neurol Sci 2017, 38, 1167–1186. [Google Scholar] [CrossRef]
- Meerveld, B.G.-V.; Johnson, A.C.; Grundy, D. Gastrointestinal Physiology and Function. Handb Exp Pharmacol 2017, 239, 1–16. [Google Scholar]
- Taddei, S.; Bruno, R.M.; Masi, S.; Solini, A. Epidemiology and pathophysiology of hypertension, In The ESC Textbook of Cardiovascular Medicine; Williams, B. , Camm, A.J., Lüscher, T.F., Maurer, G., Serruys, P.W., Eds.; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Wang, Y.; Huang, M.; Yang, X.; Yang, Z.; Li, L.; Mei, J. Supplementing punicalagin reduces oxidative stress markers and restores angiogenic balance in a rat model of pregnancy-induced hypertension. Korean J Physiol Pharmacol 2018, 22, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Zhao, Y.; Zhang, Y.; Wang, Q.; Huang, Z.; Ding, Q.; Guo, Z.; Zhou, X.; Zhu, L.; Gu, N. The cellular uptake and cytotoxic effect of silver nanoparticles on chronic myeloid leukemia cells. Journal of biomedical nanotechnology 2014, 10, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Beeharry, M.K.; Liu, W.-T.; Yan, M.; Zhu, Z.-G. New blood markers detection technology: A leap in the diagnosis of gastric cancer. World journal of gastroenterology 2016, 22, 1202. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).