Introduction
Cancer has plagued multi-cellular organisms since their conception. Recently, I wrote a paper about how many cancer patients may have one or more mutations that are ubiquitous throughout their tumor(s) [
1,
2,
3]. The rest may at least have a small set of subclonal mutations that together cover all sequenced regions of their tumors. These mutations could be targeted by an oncolytic vector with the broadest tropism possible that only replicates and becomes hyper-virulent after detecting said mutations. I called this strategy, “Oncolytic Vector Efficient Replication Contingent on Omnipresent Mutation Engagement” (OVERCOME).
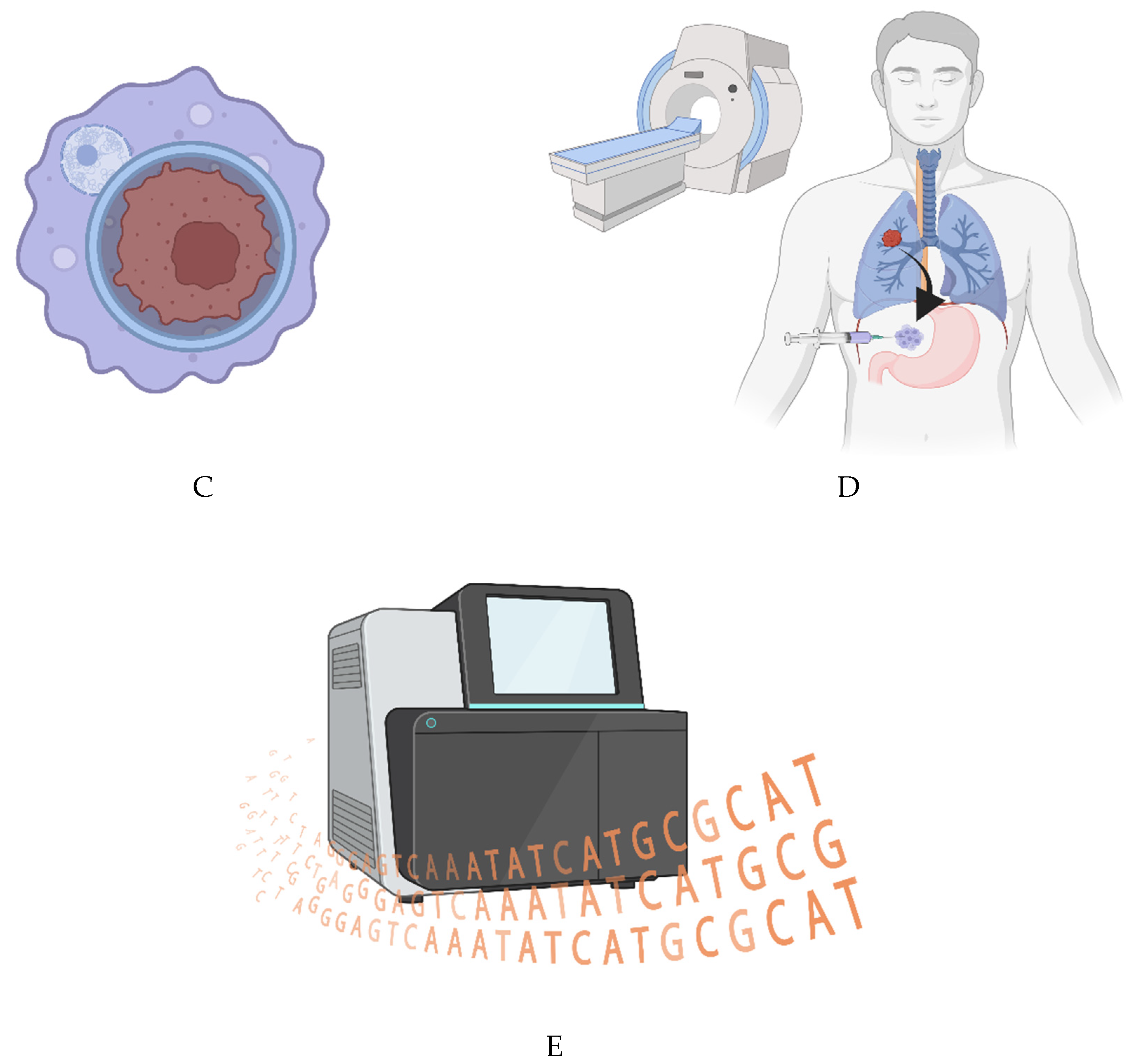
To identify these mutations, multiregion, multisample sequencing should be employed for each patient. However, tumors in certain anatomical regions are not easy to biopsy - especially in a multiregional fashion.
Here, I explore another way of acquiring multiregion biopsies from tumors that are hard to reach via traditional means.
Addendum
One issue is how to limit the duration of expression of CRISPRa, TALE activators, or ZFP activators that are meant to increase the truncal mutation “signal” - in order to protect normal cells. One solution would be to also express switches that detect the upregulated transcript at one or more non-mutated sites. When the vector detects the target transcript at one or more non-mutated sites, transcriptional upregulation of the target(s) - and expression of the switches targeting one or more non-mutated sites - would be halted.
Two other vectors may be good options. The vaccinia virus may also be a good vector - it can accommodate 25 kb of foreign DNA and still replicate [
4]. However, I am uncertain if RNA molecules, proteins, or peptides large enough to allow for truncal mutation-dependent viral replication could escape and possibly re-enter the viral factories. The other vector would be
Salmonella Typhimurium [
5].
Finally, perhaps for neuron-based cancer, at least,
Toxoplasma gondii could eventually be helpful [
6].
The latter two options could either detect mutations from their vacuoles - or lyse their vacuoles and detect them from the cytosol.
Mechanisms
Cell free DNA
The easiest way to enact multiregion, multisample tumor sequencing may be to analyze cell-free DNA in the blood (or cerebrospinal fluid) of patients [
7,
8,
9,
10].
Bacterial competence
Bacteria also naturally colonize tumors [
11]. That in combination with the uptake of cell-free DNA via a competence mechanism could possibly help enable multiregion, multisample tumor sequencing [
12].
Macrophage phagocytosis
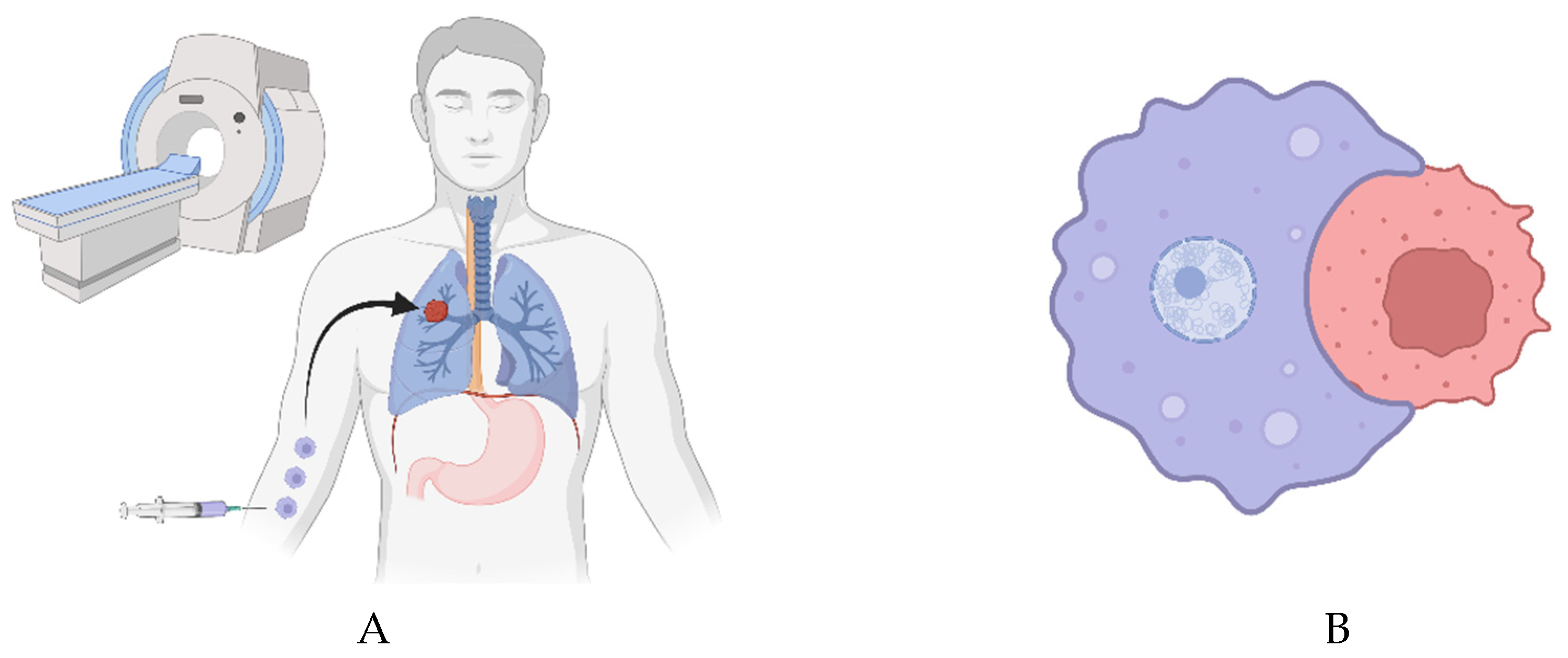
Bioengineered macrophages could be used for this purpose. They could be loaded with magnetic nanoparticles (MNPs) and steered into the heart or lung tumors using an MRI machine [
13]. Perhaps the macrophages should be induced via small molecule to chemorepel [
14] each other once they have reached the target site or sites - in order to spread out more evenly throughout the tumor(s). A gene circuit possibly involving ARHGEF37 [
15] or Cdc42 could be employed as well - to allow for vigorous, random movement within the tumor in addition to their chemorepulsion from each other.
In either case, once there, they could be induced via small molecule to express a chimeric antigen receptor for phagocytosis (CAR-P) [
16]. Alternatively, they can be heated via an alternating magnetic field to induce gene expression of the CAR-P [
17]. The CAR-P would target a ubiquitously expressed cell surface protein [
18,
19]. Rapid, inducible depletion of SIRPα [
20] in the macrophages via TEVp-mediated degree exposure [
21], for example, might also be of use.
Realistically, multiple CAR-Ps should probably be induced via small molecule or heating in order to target a variety of ubiquitously expressed cell surface proteins and thereby help ensure phagocytosis of cancer cells throughout the tumor(s), as some cancer cells may have lost or suppressed the expression of one or more ubiquitous cell surface proteins.
Inhibiting maturation of and lysosomal fusion with the specific phagosome carrying the target cell could be achieved in a variety of ways [
22]. After giving the macrophages some time to collect a target - they would be drawn via magnetism or chemotaxis to an extraction point in the body. Perhaps they can be magnetically drawn to the peritoneal cavity [
23] and withdrawn via needle.
Importantly, it was shown that whole cancer cells can be engulfed via this CAR-P method, as opposed to trogocytosis. However, trogocytosis was still more common. Thus, more work may be necessary before this strategy can be employed.
Perhaps if necessary, the CAR-P macrophages employed in this setting could be programmed so that trogocytosis is not possible [
24]. They could also potentially be bioengineered so that frustrated phagocytosis [
25] both leads to the chemoattraction of other CAR-P macrophages and ensures homotypic fusion once in close proximity to create multinucleated giant cells that can engulf entire, large cancer cells [
26].
An alternative solution would be to install the T-cell lytic granule system in the CAR-P macrophages [
27,
28]. They would lack granzyme B, but be able to rapidly and directionally secrete perforin to lyse the target cell while sparing the nucleus [
29,
30]. Then, they could phagocytose debris until they bind the nuclear envelope - and selectively phagocytose that and inhibit the maturation of the phagosome and phagosome-lysosome fusion. (The nucleus would be a smaller target than the cell as a whole.)
Bacterial phagocytosis
Yet another strategy would be for carrier, Irf8(-/-) [
31] macrophages to ferry non-replicating [
32,
33] facultative intracellular, phagocytic [
34], and potentially magnetosome [
35]-bearing bacteria to cancer cells. The bacteria would lyse the carrier macrophages once in the tumor region, invade tumor cells, and phagocytose their nuclei. Of course, the bacteria would also have to be programmed not to digest their cargo. Then, they would lyse the cancer cell - and could be directed chemotactically or magnetotactically to an extraction point.
Specifically, a species of phagocytic bacteria was recently identified, known as ’
Candidatus Uab amorphum’ [
23]. It is flat, spherical, or ovoid, and has a diameter of ~4-5 microns. Notably, only two genes are required for an extracellular bacterium to be able to invade target cells - an invasin gene and listeriolysin O [
36].
However, smaller bacteria (~1-2 microns in diameter) may have a higher invasion efficiency - and all currently known facultative intracellular bacteria that have evolved to invade and survive within human cells are of the smaller variety.
Of course, on the other hand, human cell nuclei can be ~5-20 microns in diameter. Thus, while the bacteria should remain as small as possible for invasion, growth should be induced upon entering a target cancer cell’s cytoplasm. If ‘Ca. Uab amorphum’ cannot readily be engineered to be smaller and grow upon cytoplasmic entry, perhaps the genes required for said bacterium to perform phagocytosis can be transferred to a comparatively small bacterial species.
For example, a probiotic
E. Coli strain could be genetically engineered for a spherical shape, which may be a prequisite for phagocytosis [
37].
It could be given the genes required for phagocytosis, as well as multiple invasins against various ubiquitous cell surface proteins and listeriolysin O.
Growth and division could initially be suppressed via truncated RelA overexpression [
38,
39] and FtsZ inhibition [
24]. FtsZ-deficient bacteria are more susceptible to minor stressors than wild-type bacteria, but an inactive form of the protein can prevent this [
24].
Growth upon cytoplasmic entry could possibly be achieved by combining the ActA promoter [
40] with induced overexpression of a “useless” protein [
41] and inhibition of the truncated RelA protein. Some amount of RelA may be required for cellular survival, so inhibiting the non-truncated, endogenous gene copy might not be the best idea [
42].
An automated timer mechanism via a temporal promoter cascade involving the expression of early, intermediate, then late genes could be implemented for growth in the cytoplasm of target cancer cells. At the end of this time, the useless protein would be inhibited permanently and truncated RelA would be permanently re-expressed - via recombinase-mediated gene inversion.
Proteins could also potentially be secreted by the bacteria to decrease the size of the host cell nucleus or nuclei [
43].
Motility [
44] should likely be enabled during this throughout this process and after to help evade autophagy and at the end of the growth period for the bacteria to be able to search for its nuclear target. (Phagocytosis receptors should be expressed at the end of the growth period that recognize multiple ubiquitously expressed nuclear outer membrane proteins with domains present on the cytosolic leaflet of said membrane.)
Remaining strategies
While it is true that traditional needle biopsies may increase the risk of causing metastasis, the following two strategies may involve an unacceptably high risk of causing metastasis.
The macrophages perhaps could simply attach to the cancer cells and magnetotactically “tow” them to an extraction point. Matrix metalloprotease secretion by the macrophages could help to detach cancer cells from the extracellular matrix beforehand. It would be very important that they do not drop their cargo along the way.
However, the cancer cell could theoretically replicate while being towed. To prevent this, the T-cell lytic granule system mentioned before could be employed to deliver recombinases that excise genes required for division prior to towing.
Finally, cell-cell fusion [
45] could potentially be locally induced in the tumor, provided the macrophage were equipped with many chromosomally-encoded, small molecule-controlled kill switches - and could inhibit the nuclear activity of the cancer cell after fusion. Perhaps this could be achieved by rapidly surrounding the cancer cell’s nucleus or nuclei with autophagosomes that are prohibited from becoming acidic or otherwise maturing.
Brain and spinal cord tumors:
With regard to the brain and spinal cord, perhaps magnetism is all that’s required to cross the blood-brain and blood-spinal cord barrier - but perhaps not. If not, the easiest solution would probably be an intrathecal injection. If the tumor or tumors are in the brain, the MNP-loaded macrophages could be magnetically dragged up the spinal cord into the brain. This would be considered invasive, of course, but certainly not as much as drilling a burr hole in the patient’s skull.
Alternatively, magnetic resonance-guided motorized transcranial ultrasound [
46] could be used to open the blood-brain barrier at the tumor locale(s). The same technology is applicable to the spinal cord as well; MRI-guided ultrasound has already been tested in that context [
47].
Instead of magnetism, perhaps a prodrug version of deschloroclozapine [
48] can be employed that is blood-brain and blood-spinal cord barrier—permeable. It would be activated by an extracellular enzyme that is abundant in the brain and/or spinal cord [
49] and serve as a chemoattractant for the macrophages [
50]. This could be superior to magnetism because it might induce more crawling along the barrier endothelial cells until an infiltration point can be found.
The question of egress from the central nervous system is also an issue. Chemotaxis and/or magnetotaxis may again be sufficient. Or, they could also simply be directed to a site in the spinal cord for lumbar puncture-based withdrawal - again, a relatively invasive procedure. Maybe in the future, the macrophages or bacteria can somehow be directed to the lymphatic system in the brain and spinal cord and drain into a more easily accessible lymph node [
51] for needle-based extraction.
Conclusion
This magnetism-based approach is also suitable for the macrophage-mediated delivery of oncolytic vectors to tumors that would be difficult to reach via needle, of course. This has already been accomplished in mice [
4]. With regard to OVERCOME, vector replication in the carrier macrophages would be induced via small molecule, heat, or even magneto-mechanical actuation [
52] once they have reached the tumor(s). Excess iron oxide nanoparticle deposition in the body from carrier macrophage lysis may not be ideal, but they should be degraded eventually [
53].
Acknowledgments
The figure in this piece was created with BioRender.com.
References
- Renteln M. Conditional replication of oncolytic viruses based on detection of oncogenic mRNA. Gene Therapy 2018,25:1–3. [CrossRef]
- Renteln M. Correction: Conditional replication of oncolytic viruses based on detection of oncogenic mRNA. Gene Ther. 2021,28:469–469. [CrossRef]
- Renteln, MA. Promoting oncolytic vector replication with switches that detect ubiquitous mutations. CCTR 2023, 19. [Google Scholar] [CrossRef]
- Renteln, MA. Promoting oncolytic vector replication with switches that detect ubiquitous mutations. CCTR 2023, 19. [Google Scholar] [CrossRef]
- Raman V, Van Dessel N, Hall CL, Wetherby VE, Whitney SA, Kolewe EL, et al. Intracellular delivery of protein drugs with an autonomously lysing bacterial system reduces tumor growth and metastases. Nat. Commun. 2021,12:6116. [CrossRef]
- Bracha S, Hassi K, Ross PD, Cobb S, Sheiner L, Rechavi O. Engineering Brain Parasites for Intracellular Delivery of Therapeutic Proteins 2018:481192. [CrossRef]
- Murtaza M, Dawson S-J, Pogrebniak K, Rueda OM, Provenzano E, Grant J, et al. Multifocal clonal evolution characterized using circulating tumour DNA in a case of metastatic breast cancer. Nat. Commun. 2015,6:8760. [CrossRef]
- Li S, Noor ZS, Zeng W, Stackpole ML, Ni X, Zhou Y, et al. Sensitive detection of tumor mutations from blood and its application to immunotherapy prognosis. Nat. Commun. 2021, 12, 4172. [Google Scholar] [CrossRef]
- Li S, Hu R, Small C, Kang T-Y, Liu C-C, Zhou XJ, et al. cfSNV: a software tool for the sensitive detection of somatic mutations from cell-free DNA. Nat. Protoc. 2023,18:1563–83. [CrossRef]
- Escudero L, Martínez-Ricarte F, Seoane J. ctDNA-Based Liquid Biopsy of Cerebrospinal Fluid in Brain Cancer. Cancers (Basel) 2021,13:1989. [CrossRef]
- Duong MT-Q, Qin Y, You S-H, Min J-J. Bacteria-cancer interactions: bacteria-based cancer therapy. Exp. Mol. Med. 2019,51:1–15. [CrossRef]
- Cooper RM, Wright JA, Ng JQ, Goyne JM, Suzuki N, Lee YK, et al. Engineered bacteria detect tumor DNA. Science 2023,381:682–6. [CrossRef]
- Muthana M, Kennerley AJ, Hughes R, et al. Directing cell therapy to anatomic target sites in vivo with magnetic resonance targeting. Nature Communications 2015,6:8009. [CrossRef]
- Dowdell A, Paschke PI, Thomason PA, et al. Competition between chemoattractants causes unexpected complexity and can explain negative chemotaxis. Current Biology 2023,33:1704-1715.e3. [CrossRef]
- i Zhang X, Ren L, Wu J, Feng R, Chen Y, Li R, et al. ARHGEF37 overexpression promotes extravasation and metastasis of hepatocellular carcinoma via directly activating Cdc42. J. Exp. Clin. Cancer Res. 2022,41:230. [CrossRef]
- Morrissey MA, Williamson AP, Steinbach AM, et al. Chimeric antigen receptors that trigger phagocytosis. Cooper JA. ed. eLife 2018,7:e36688. [CrossRef]
- Ito A, Teranishi R, Kamei K, et al. Magnetically triggered transgene expression in mammalian cells by localized cellular heating of magnetic nanoparticles. Journal of Bioscience and Bioengineering 2019,128:355–364. [CrossRef]
- Bausch-Fluck D, Hofmann A, Bock T, et al. A Mass Spectrometric-Derived Cell Surface Protein Atlas. PLOS ONE 2015,10:e0121314. [CrossRef]
- Bausch-Fluck D, Goldmann U, Müller S, et al. The in silico human surfaceome. PNAS 2018,115:E10988–E10997. [CrossRef]
- Yang H, Shao R, Huang H, Wang X, Rong Z, Lin Y. Engineering macrophages to phagocytose cancer cells by blocking the CD47/SIRPɑ axis. Cancer Med. 2019,8:4245–53. [CrossRef]
- Jungbluth M, Renicke C, Taxis C. Targeted protein depletion in Saccharomyces cerevisiae by activation of a bidirectional degron. BMC Syst. Biol. 2010,4:176. [CrossRef]
- Kinchen JM, Ravichandran KS. Phagosome maturation: going through the acid test. Nat. Rev. Mol. Cell Biol. 2008,9:781–795. [CrossRef]
- iv Wang X, Maxwell KG, Wang K, Bowers DT, Flanders JA, Liu W, et al. A nanofibrous encapsulation device for safe delivery of insulin-producing cells to treat type 1 diabetes. Sci. Transl. Med. 2021,13:eabb4601. [CrossRef]
- Gilmartin AA, Ralston KS, Petri WA. Inhibition of Amebic Cysteine Proteases Blocks Amebic Trogocytosis but Not Phagocytosis. J. Infect. Dis. 2020,221:1734–9. [CrossRef]
- Underhill DM, Goodridge HS. Information processing during phagocytosis. Nat. Rev. Immunol. 2012,12:492–502. [CrossRef]
- 26.Milde R, Ritter J, Tennent GA, Loesch A, Martinez FO, Gordon S, et al. Multinucleated Giant Cells Are Specialized for Complement-Mediated Phagocytosis and Large Target Destruction. Cell Reports 2015,13:1937–48. [CrossRef]
- Li H, Pohler U, Strehlow I, et al. Macrophage precursor cells produce perforin and perform Yac-1 lytic activity in response to stimulation with interleukin-2. Journal of Leukocyte Biology 1994,56:117–123. [CrossRef]
- Tamang DL, Alves BN, Elliott V, et al. Regulation of perforin lysis: Implications for protein disulfide isomerase proteins. Cell Immunol. 2009,255(1–2):82–92. [CrossRef]
- Heusel JW, Wesselschmidt RL, Shresta S, et al. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell 1994,76:977–987. [CrossRef]
- Voskoboinik I, Smyth MJ, Trapani JA. Perforin-mediated target-cell death and immune homeostasis. Nat. Rev. Immunol. 2006,6:940–952. [CrossRef]
- xii Gupta M, Shin D-M, Ramakrishna L, Goussetis DJ, Platanias LC, Xiong H, et al. IRF8 directs stress-induced autophagy in macrophages and promotes clearance of Listeria monocytogenes. Nat. Commun. 2015,6:6379. [CrossRef]
- Yoon YG, Koob MD. Nonreplicating Intracellular Bacterial Vector for Conjugative DNA Transfer into Mitochondria. Pharm. Res. 2012,29:1040–5. [CrossRef]
- Sánchez-Gorostiaga A, Palacios P, Martínez-Arteaga R, Sánchez M, Casanova M, Vicente M. Life without Division: Physiology of Escherichia coli FtsZ-Deprived Filaments. MBio 2016,7:e01620-16. [CrossRef]
- xv Shiratori T, Suzuki S, Kakizawa Y, Ishida K. Phagocytosis-like cell engulfment by a planctomycete bacterium. Nat. Commun. 2019,10:5529. [CrossRef]
- xvi Kolinko I, Lohße A, Borg S, Raschdorf O, Jogler C, Tu Q, et al. Biosynthesis of magnetic nanostructures in a foreign organism by transfer of bacterial magnetosome gene clusters. Nature Nanotech 2014,9:193–7. [CrossRef]
- Grillot-Courvalin C, Goussard S, Huetz F, Ojcius DM, Courvalin P. Functional gene transfer from intracellular bacteria to mammalian cells. Nat. Biotechnol. 1998,16:862–6. [CrossRef]
- Shiomi D, Sakai M, Niki H. Determination of bacterial rod shape by a novel cytoskeletal membrane protein. EMBO J. 2008,27:3081–91. [CrossRef]
- Schreiber G, Metzger S, Aizenman E, Roza S, Cashel M, Glaser G. Overexpression of the relA gene in Escherichia coli. J. Biol. Chem. 1991,266:3760–7.
- Büke F, Grilli J, Cosentino Lagomarsino M, Bokinsky G, Tans SJ. ppGpp is a bacterial cell size regulator. Current Biology 2022,32:870-877.e5. [CrossRef]
- Reniere ML, Whiteley AT, Hamilton KL, John SM, Lauer P, Brennan RG, et al. Glutathione activates virulence gene expression of an intracellular pathogen. Nature 2015,517:170–3. [CrossRef]
- Basan M, Zhu M, Dai X, Warren M, Sévin D, Wang Y-P, et al. Inflating bacterial cells by increased protein synthesis. Mol. Syst. Biol. 2015,11:836. [CrossRef]
- Taylor CM, Beresford M, Epton HAS, Sigee DC, Shama G, Andrew PW, et al. Listeria monocytogenes relA and hpt mutants are impaired in surface-attached growth and virulence. J. Bacteriol. 2002,184:621–8. [CrossRef]
- Kume K, Cantwell H, Burrell A, Nurse P. Nuclear membrane protein Lem2 regulates nuclear size through membrane flow. Nat. Commun. 2019,10:1871. [CrossRef]
- Goldberg MB, Theriot JA. Shigella flexneri surface protein IcsA is sufficient to direct actin-based motility. Proc. Natl. Acad. Sci. U S A 1995,92:6572–6. [CrossRef]
- Xu C, Ren X-H, Han D, et al. Precise Detection on Cell–Cell Fusion by a Facile Molecular Beacon-Based Method. Anal. Chem. 2022,94:17334–17340. 1734; 0. [CrossRef]
- xxvii Magnin R, Rabusseau F, Salabartan F, Mériaux S, Aubry J-F, Le Bihan D,; et al. Magnetic resonance-guided motorized transcranial ultrasound system for blood-brain barrier permeabilization along arbitrary trajectories in rodents. J. Ther. Ultrasound 2015, 3, 22. [CrossRef] [PubMed]
- xxviii Weber-Adrian D, Thévenot E, O’Reilly MA, Oakden W, Akens MK, Ellens N,; et al. Gene delivery to the spinal cord using MRI-guided focused ultrasound. Gene Ther. 2015, 22, 568–77. [CrossRef] [PubMed]
- xxix Nagai Y, Miyakawa N, Takuwa H, Hori Y, Oyama K, Ji B, et al. Deschloroclozapine, a potent and selective chemogenetic actuator enables rapid neuronal and behavioral modulations in mice and monkeys. Nat. Neurosci. 2020, 23, 1157–67. [Google Scholar] [CrossRef] [PubMed]
- l Yevtodiyenko A, Bazhin A, Khodakivskyi P, Godinat A, Budin G, Maric T, et al. Portable bioluminescent platform for in vivo monitoring of biological processes in non-transgenic animals. Nat. Commun. 2021, 12, 2680. [Google Scholar] [CrossRef] [PubMed]
- li Park JS, Rhau B, Hermann A, McNally KA, Zhou C, Gong D, et al. Synthetic control of mammalian-cell motility by engineering chemotaxis to an orthogonal bioinert chemical signal. Proc. Natl. Acad. Sci. U S A 2014, 111, 5896–901. [Google Scholar] [CrossRef] [PubMed]
- lii Xu J-Q, Liu Q-Q, Huang S-Y, Duan C-Y, Lu H-B, Cao Y, et al. The lymphatic system: a therapeutic target for central nervous system disorders. Neural Regen. Res. 2022, 18, 1249–56. [Google Scholar] [CrossRef]
- liii Beltran-Huarac J, Yamaleyeva DN, Dotti G, Hingtgen S, Sokolsky-Papkov M, Kabanov AV. Magnetic Control of Protein Expression via Magneto-mechanical Actuation of ND-PEGylated Iron Oxide Nanocubes for Cell Therapy. ACS Appl. Mater. Interfaces 2023, 15, 19877–91. [Google Scholar] [CrossRef] [PubMed]
- Mazuel F, Espinosa A, Luciani N, et al. Massive Intracellular Biodegradation of Iron Oxide Nanoparticles Evidenced Magnetically at Single-Endosome and Tissue Levels. ACS Nano 2016, 10, 7627–7638. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).