Submitted:
16 August 2023
Posted:
18 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurements
2.2.1. Frailty Score
2.2.2. Back Extensor Strength
2.2.3. Trunk Muscle/Fat Mass
2.3. Statistical Analyses
2.3.1. Propensity Score Matching
2.3.2. Multivariate Logistic Regression
2.3.3. Extreme Gradient Boosting
3. Results
3.1. Participant Characteristics
3.2. Linear Regression Analysis of Trunk Muscle/Fat Compositions and the Back Extensor Strength
3.3. PS Matching of the Group with the Lowest 20% Back Extensor Strength
3.4. Back Extensor Strength as a New Predictor of Frailty
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morley, J.E.; Vellas, B.; Van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.; Doehner, W.; Evans, J. Frailty consensus: a call to action. Journal of the American Medical Directors Association 2013, 14, 392–397. [Google Scholar]
- Rockwood, K.; Stadnyk, K.; MacKnight, C.; McDowell, L.; Hébert, R. A brief clinical instrument to classify frailty in elderly people. Lancet (British edition) 1999, 353, 205–206. [Google Scholar]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G. Frailty in older adults: evidence for a phenotype. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 2001, 56, M146–M157. [Google Scholar] [CrossRef] [PubMed]
- Mitnitski, A.B.; Mogilner, A.J.; Rockwood, K. Accumulation of deficits as a proxy measure of aging. TheScientificWorldJournal 2001, 1, 323–336. [Google Scholar] [PubMed]
- Xue, Q.-L. The frailty syndrome: definition and natural history. Clinics in geriatric medicine 2011, 27, 1–15. [Google Scholar] [PubMed]
- Dent, E.; Kowal, P.; Hoogendijk, E.O. Frailty measurement in research and clinical practice: a review. European journal of internal medicine 2016, 31, 3–10. [Google Scholar] [PubMed]
- Cesari, M.; Gambassi, G.; Abellan van Kan, G.; Vellas, B. The frailty phenotype and the frailty index: different instruments for different purposes. Age and ageing 2014, 43, 10–12. [Google Scholar] [CrossRef]
- Marano, L.; Carbone, L.; Poto, G.E.; Gambelli, M.; Nguefack Noudem, L.L.; Grassi, G.; Manasci, F.; Curreri, G.; Giuliani, A.; Piagnerelli, R. Handgrip strength predicts length of hospital stay in an abdominal surgical setting: the role of frailty beyond age. Aging Clinical and Experimental Research 2022, 34, 811–817. [Google Scholar]
- Syddall, H.; Cooper, C.; Martin, F.; Briggs, R.; Aihie Sayer, A. Is grip strength a useful single marker of frailty? Age and ageing 2003, 32, 650–656. [Google Scholar] [CrossRef]
- Norman, K.; Stobäus, N.; Gonzalez, M.C.; Schulzke, J.-D.; Pirlich, M. Hand grip strength: outcome predictor and marker of nutritional status. Clinical nutrition 2011, 30, 135–142. [Google Scholar]
- Marques, A.; de Matos, M.G.; Henriques-Neto, D.; Peralta, M.; Gouveia, É.R.; Tesler, R.; Martins, J.; Gomez-Baya, D. Grip strength and depression symptoms among middle-age and older adults. In Proceedings of the Mayo Clinic Proceedings; 2020; pp. 2134–2143. [Google Scholar]
- Antonova, L.; Bucher-Koenen, T.; Mazzonna, F. Long-term health consequences of recessions during working years. Social Science & Medicine 2017, 187, 134–143. [Google Scholar]
- Farrow, M.; Biglands, J.; Tanner, S.F.; Clegg, A.; Brown, L.; Hensor, E.; O’Connor, P.; Emery, P.; Tan, A. The effect of ageing on skeletal muscle as assessed by quantitative MR imaging: an association with frailty and muscle strength. Aging clinical and experimental research 2021, 33, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Akuthota, V.; Nadler, S.F. Core strengthening. Archives of physical medicine and rehabilitation 2004, 85, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.X.; Yao, J.; Zirek, Y.; Reijnierse, E.M.; Maier, A.B. Muscle mass, strength, and physical performance predicting activities of daily living: a meta-analysis. Journal of cachexia, sarcopenia and muscle 2020, 11, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Banno, T.; Arima, H.; Hasegawa, T.; Yamato, Y.; Togawa, D.; Yoshida, G.; Yasuda, T.; Oe, S.; Mihara, Y.; Ushirozako, H. The effect of paravertebral muscle on the maintenance of upright posture in patients with adult spinal deformity. Spine deformity 2019, 7, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Granacher, U.; Gollhofer, A.; Hortobágyi, T.; Kressig, R.W.; Muehlbauer, T. The importance of trunk muscle strength for balance, functional performance, and fall prevention in seniors: a systematic review. Sports medicine 2013, 43, 627–641. [Google Scholar] [CrossRef]
- Jo, H.; Baek, S.; Park, H.-w.; Lee, S.-A.; Moon, J.; Yang, J.E.; Kim, K.S.; Kim, J.Y.; Kang, E.K. Farmers’ cohort for agricultural work-related musculoskeletal disorders (farm) study: study design, methods, and baseline characteristics of enrolled subjects. Journal of epidemiology 2016, JE20140271. [Google Scholar] [CrossRef]
- Bassett, D.R.; Schneider, P.L.; Huntington, G.E. Physical activity in an Old Order Amish community. Medicine & Science in Sports & Exercise 2004, 36, 79–85. [Google Scholar]
- Baek, S.; Park, J.; Kyoung Kang, E.; Kim, G.; Kim, H.; Park, H.-W. Association Between Ergonomic Burden Assessed Using 20-Item Agricultural Work-Related Ergonomic Risk Questionnaire and Shoulder, Low Back, and Leg Pain in Korean Farmers. Journal of Agromedicine 2023, 28, 532–544. [Google Scholar] [CrossRef]
- Park, K.H.; Baek, S.; Kang, E.K.; Park, H.-w.; Kim, G.; Kim, S.H. The Association Between Sagittal Plane Alignment and Disc Space Narrowing of Lumbar Spine in Farmers. Annals of Rehabilitation Medicine 2021, 45, 294–303. [Google Scholar] [CrossRef]
- Pourtaheri, S.; Issa, K.; Lord, E.; Ajiboye, R.; Drysch, A.; Hwang, K.; Faloon, M.; Sinha, K.; Emami, A. Paraspinal muscle atrophy after lumbar spine surgery. Orthopedics 2016, 39, e209–e214. [Google Scholar] [CrossRef] [PubMed]
- Chun, M.Y. Validity and reliability of Korean version of international physical activity questionnaire short form in the elderly. Korean journal of family medicine 2012, 33, 144. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-w.; Baek, S.; Kim, H.Y.; Park, J.-G.; Kang, E.K. Reliability and validity of a new method for isometric back extensor strength evaluation using a hand-held dynamometer. Annals of rehabilitation medicine 2017, 41, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Park, H.-w.; Kim, G. Associations Between Trunk Muscle/Fat Composition, Narrowing Lumbar Disc Space, and Low Back Pain in Middle-Aged Farmers: A Cross-Sectional Study. Annals of Rehabilitation Medicine 2022, 46, 122. [Google Scholar] [CrossRef]
- Chen, T.; He, T.; Benesty, M.; Khotilovich, V.; Tang, Y.; Cho, H.; Chen, K. Xgboost: extreme gradient boosting. R package version 0.4-2 2015, 1, 1–4. [Google Scholar]
- Alin, A. Multicollinearity. Wiley interdisciplinary reviews: computational statistics 2010, 2, 370–374. [Google Scholar] [CrossRef]
- Johnson, J.W. A heuristic method for estimating the relative weight of predictor variables in multiple regression. Multivariate behavioral research 2000, 35, 1–19. [Google Scholar] [CrossRef]
- Rosenbaum, P.R.; Rubin, D.B. The central role of the propensity score in observational studies for causal effects. Biometrika 1983, 70, 41–55. [Google Scholar] [CrossRef]
- Thoemmes, F.J.; Kim, E.S. A systematic review of propensity score methods in the social sciences. Multivariate behavioral research 2011, 46, 90–118. [Google Scholar] [CrossRef] [PubMed]
- Kim, T. The impact of working hours on pregnancy intention in childbearing-age women in Korea, the country with the world’s lowest fertility rate. PLOS ONE 2023, 18, e0288697. [Google Scholar] [CrossRef]
- Austin, P.C. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biometrical Journal: Journal of Mathematical Methods in Biosciences 2009, 51, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate behavioral research 2011, 46, 399–424. [Google Scholar] [CrossRef]
- Massy-Westropp, N.M.; Gill, T.K.; Taylor, A.W.; Bohannon, R.W.; Hill, C.L. Hand Grip Strength: age and gender stratified normative data in a population-based study. BMC research notes 2011, 4, 1–5. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. Xgboost: A scalable tree boosting system. In Proceedings of the Proceedings of the 22nd acm sigkdd international conference on knowledge discovery and data mining, 2016; pp. 785–794.
- Bantis, L.E.; Nakas, C.T.; Reiser, B. Construction of confidence regions in the ROC space after the estimation of the optimal Youden index-based cut-off point. Biometrics 2014, 70, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Bradley, A.P. The use of the area under the ROC curve in the evaluation of machine learning algorithms. Pattern recognition 1997, 30, 1145–1159. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The elements of statistical learning. Springer series in statistics; New York, NY, USA, 2001. [Google Scholar]
- Jang, I.-Y.; Lee, H.Y.; Lee, E. Geriatrics fact sheet in Korea 2018 from national statistics. Annals of geriatric medicine and research 2019, 23, 50. [Google Scholar] [CrossRef]
- Batista, F.S.; Gomes, G.A.d.O.; D’Elboux, M.J.; Cintra, F.A.; Neri, A.L.; Guariento, M.E.; Souza, M.d.L.R.d. Relationship between lower-limb muscle strength and functional independence among elderly people according to frailty criteria: a cross-sectional study. Sao Paulo Medical Journal 2014, 132, 282–289. [Google Scholar] [CrossRef]
- Armamento-Villareal, R.; Aguirre, L.; Napoli, N.; Shah, K.; Hilton, T.; Sinacore, D.; Qualls, C.; Villareal, D. Changes in thigh muscle volume predict bone mineral density response to lifestyle therapy in frail, obese older adults. Osteoporosis international 2014, 25, 551–558. [Google Scholar] [CrossRef]
- Staiano, A.; Katzmarzyk, P. Ethnic and sex differences in body fat and visceral and subcutaneous adiposity in children and adolescents. International journal of obesity 2012, 36, 1261–1269. [Google Scholar] [CrossRef]
- Lönnqvist, F.; Thörne, A.; Large, V.; Arner, P. Sex differences in visceral fat lipolysis and metabolic complications of obesity. Arteriosclerosis, thrombosis, and vascular biology 1997, 17, 1472–1480. [Google Scholar] [CrossRef]
- Mannion, A.; Adams, M.; Cooper, R.; Dolan, P. Prediction of maximal back muscle strength from indices of body mass and fat-free body mass. Rheumatology (Oxford, England) 1999, 38, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Loosen, S.H.; Schulze-Hagen, M.; Püngel, T.; Bündgens, L.; Wirtz, T.; Kather, J.N.; Vucur, M.; Paffenholz, P.; Demir, M.; Bruners, P. Skeletal muscle composition predicts outcome in critically ill patients. Critical care explorations 2020, 2. [Google Scholar] [CrossRef] [PubMed]
- Kasukawa, Y.; Miyakoshi, N.; Hongo, M.; Ishikawa, Y.; Noguchi, H.; Kamo, K.; Sasaki, H.; Murata, K.; Shimada, Y. Relationships between falls, spinal curvature, spinal mobility and back extensor strength in elderly people. Journal of bone and mineral metabolism 2010, 28, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Granacher, U.; Lacroix, A.; Muehlbauer, T.; Roettger, K.; Gollhofer, A. Effects of core instability strength training on trunk muscle strength, spinal mobility, dynamic balance and functional mobility in older adults. Gerontology 2013, 59, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Borghuis, J.; Hof, A.L.; Lemmink, K.A. The importance of sensory-motor control in providing core stability: implications for measurement and training. Sports medicine 2008, 38, 893–916. [Google Scholar] [CrossRef] [PubMed]
- Behm, D.G.; Drinkwater, E.J.; Willardson, J.M.; Cowley, P.M. The use of instability to train the core musculature. Applied physiology, nutrition, and metabolism 2010, 35, 91–108. [Google Scholar] [CrossRef]
- Rantanen, T.; Avlund, K.; Suominen, H.; Schroll, M.; Frändin, K.; Pertti, E. Muscle strength as a predictor of onset of ADL dependence in people aged 75 years. Aging clinical and experimental research 2002, 14, 10–15. [Google Scholar]
- Bridgewater, K.J.; Sharpe, M.H. Trunk muscle training and early Parkinson’s disease. Physiotherapy Theory and Practice 1997, 13, 139–153. [Google Scholar] [CrossRef]
- Handa, N.; Yamamoto, H.; Tani, T.; Kawakami, T.; Takemasa, R. The effect of trunk muscle exercises in patients over 40 years of age with chronic low back pain. Journal of orthopaedic science 2000, 5, 210–216. [Google Scholar] [CrossRef]
- Fried, L.P.; Hadley, E.C.; Walston, J.D.; Newman, A.B.; Guralnik, J.M.; Studenski, S.; Harris, T.B.; Ershler, W.B.; Ferrucci, L. From bedside to bench: research agenda for frailty. Science of Aging Knowledge Environment 2005, 2005, pe24–pe24. [Google Scholar] [CrossRef]
- Bruunsgaard, H. Physical activity and modulation of systemic low-level inflammation. Journal of leukocyte biology 2005, 78, 819–835. [Google Scholar] [CrossRef] [PubMed]
- Taaffe, D.R.; Harris, T.B.; Ferrucci, L.; Rowe, J.; Seeman, T.E. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 2000, 55, M709–M715. [Google Scholar] [CrossRef] [PubMed]
- Batista, F.S.; Gomes, G.A.d.O.; Neri, A.L.; Guariento, M.E.; Cintra, F.A.; Sousa, M.d.L.R.d.; D’Elboux, M.J. Relationship between lower-limb muscle strength and frailty among elderly people. Sao Paulo Medical Journal 2012, 130, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Meena, M.L.; Sain, M.K.; Dangayach, G.S. Impact of posture and upper-limb muscle activity on grip strength. International Journal of Occupational Safety and Ergonomics 2019, 25, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Verna, J.L.; Mayer, J.M.; Mooney, V.; Pierra, E.A.; Robertson, V.L.; Graves, J.E. Back extension endurance and strength: the effect of variable-angle roman chair exercise training. Spine 2002, 27, 1772–1777. [Google Scholar] [CrossRef]
- Freivalds, A.; Fotouhi, D.M. Comparison of dynamic strength as measured by the cybex and mini-gym isokinetic dynamometers. International Journal of Industrial Ergonomics 1987, 1, 189–208. [Google Scholar] [CrossRef]
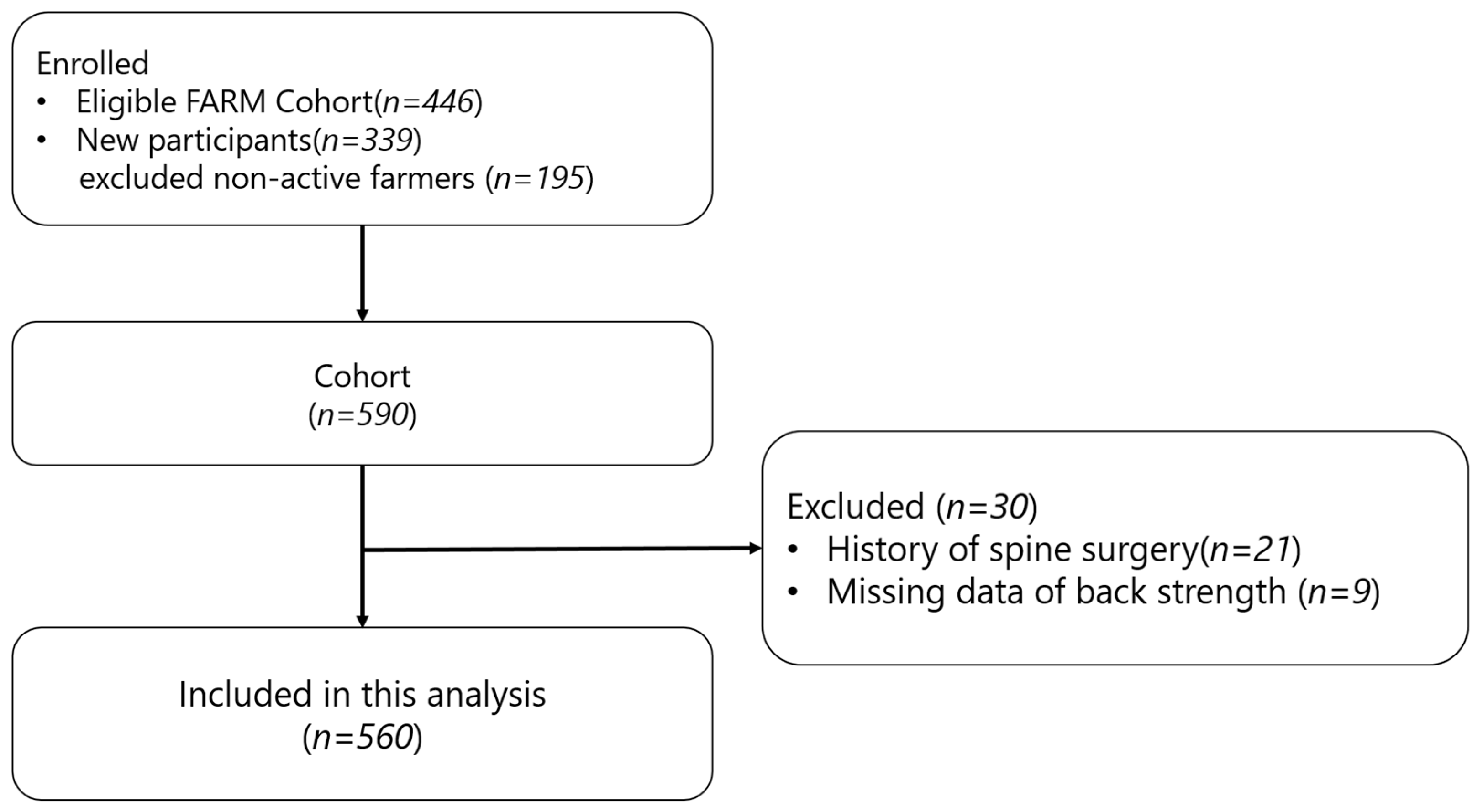
| Both (n = 560) | Male (n = 255) | Female (n = 305) | p-value | |
|---|---|---|---|---|
| Age | 58.0±7.0 | 58.5±7.0 | 57.5±6.9 | .130 |
| Waist circumference (cm) | 86.2±9.3 | 89.6±9.1 | 83.3±8.5 | < .001 |
| BMI () | 25.6±3.1 | 25.6±3.1 | 25.5±3.0 | .989 |
| TFM (cm3) | 282.3±93.6 | 272.7±100.6 | 290.3±86.7 | .059 |
| VFM (cm3) | 103.6±45.5 | 116.1±50.0 | 93.2±38.4 | < .001 |
| SFM (cm3) | 178.7±66.5 | 156.6±63.1 | 197.1±63.7 | < .001 |
| TMM (cm3) | 130.1±30.0 | 155.9±22.2 | 108.6±15.2 | < .001 |
| BMM (cm3) | 57.6±11.6 | 66.0±9.3 | 50.6±8.2 | < .001 |
| PMM (cm3) | 19.4±6.9 | 25.3±5.2 | 14.4±3.2 | < .001 |
| AMM (cm3) | 53.2±14.7 | 64.6±12.9 | 43.7±7.7 | < .001 |
| Grip strength (Kgf) | 28.7±10.2 | 37.7±7.2 | 21.3±5.1 | < .001 |
| Back extensor strength (N) | 262.7±93.8 | 321.0±96.6 | 213.9±55.9 | < .001 |
| Walking speed (m/s) | 1.0±0.2 | 1.1±0.2 | 1.0±0.2 | .005 |
| Unintentional weight loss (≥4.5kg) | 60 (10.7%) | 24 (9.4%) | 36 (11.8%) | .660 |
| Self-reported exhaustion (≥3 days/week) | 44 (7.9%) | 14 (5.5 %) | 30 (9.8%) | .163 |
| Physical activity (MET-min/week) | 5622±5657 | 5821±5453 | 5455±5827 | .673 |
| Frailty score (%) | .678 | |||
| 0 | 257 (45.9%) | 113 (44.3%) | 144 (47.2%) | |
| 1 | 189 (33.8%) | 97 (38.0%) | 92 (30.2%) | |
| 2 | 82 (14.6%) | 35 (13.7%) | 47 (15.4%) | |
| 3 | 26 (4.6%) | 8 (3.1%) | 18 (5.9%) | |
| 4 | 6 (1.1%) | 2 (0.8%) | 4 (1.3%) |
| Age < 65 (n = 470) | Age ≥ 65 (n = 90) | p-value | |
|---|---|---|---|
| Sex (male %) | 210 (44.7%) | 45 (50%) | .416 |
| Waist circumference (cm) | 85.9±9.5 | 87.5±8.2 | .099 |
| BMI () | 25.6±3.1 | 25.5±2.8 | .758 |
| TFM (cm3) | 281.8±93.4 | 284.6±95.1 | .913 |
| VFM (cm3) | 102.4±45.8 | 110.2±43.5 | .051 |
| SFM (cm3) | 179.5±66.0 | 174.4±69.2 | .302 |
| TMM (cm3) | 131.7±30.3 | 121.8±27.3 | .008 |
| BMM (cm3) | 58.4±11.4 | 53.1±11.8 | < .001 |
| PMM (cm3) | 19.7±7.0 | 17.6±5.8 | .013 |
| AMM (cm3) | 53.6±15.0 | 51.1±13.1 | .219 |
| Grip strength (Kgf) | 29.2±10.4 | 26.1±8.9 | .020 |
| Back extensor strength (N) | 266.5±93.4 | 242.4±93.7 | .015 |
| Walking speed (m/s) | 1.1±0.2 | 1.0±0.1 | < .001 |
| Unintentional weight loss (≥ 4.5kg) | 48 (10.2%) | 12 (13.3%) | .490 |
| Self-reported exhaustion (≥3 days/week) | 32 (6.8%) | 12 (13.3%) | .058 |
| Physical activity (MET-min/week) | 5646±5573 | 5498±6112 | .573 |
| Frailty score (%) | < .001 | ||
| 0 | 236 (50.2%) | 21 (23.3%) | |
| 1 | 157 (33.4%) | 32 (35.6%) | |
| 2 | 56 (11.9%) | 26 (28.9%) | |
| 3 | 16 (3.4%) | 10 (11.1%) | |
| 4 | 5 (1.1%) | 1 (1.1%) |
| Coefficient | Standard error | t | p-value | VIF | Relative Weight | |
|---|---|---|---|---|---|---|
| Constant | 209.661 | 42.076 | 4.983 | 8.39E-7 | ||
| AMM | 1.122 | 0.398 | 2.819 | .005 | 3.571 | 0.089 |
| PMM | 0.121 | 0.878 | 0.139 | .890 | 3.812 | 0.077 |
| BMM | 0.887 | 0.419 | 2.113 | .035 | 2.485 | 0.077 |
| VFM | 0.010 | 0.088 | 0.120 | .905 | 1.688 | 0.013 |
| SFM | 0.062 | 0.056 | 1.103 | .270 | 1.486 | -0.005 |
| Age | -1.823 | 0.508 | -3.583 | < .001 | 1.312 | -0.017 |
| Sex | 72.901 | 12.417 | 5.871 | 7.48E-9 | 3.985 | -0.118 |
| Before propensity score matching | After propensity score matching | |||||||
|---|---|---|---|---|---|---|---|---|
| Low 20% back extensor strength (n = 114) |
The higher (n = 444) |
SMD | p-value | Low 20% back extensor strength (n = 108) |
The higher (n = 279) |
SMD | p-value | |
| Age | 60.6±6.2 | 57.3±7.0 | 0.532 | < .001 | 59.9±5.7 | 59.4±5.7 | 0.002 | 0.423 |
| Female | 54.4% | 54.5% | -0.002 | 1.000 | 55.6% | 55.9% | -0.019 | 1.000 |
| Grip | 25.4±10.2 | 29.6±10.0 | < .001 | 25.2±10.3 | 28.7±9.9 | 0.001 | ||
| Wt. loss | 13.2% | 10.1% | .447 | 13.0% | 6.8% | 0.082 | ||
| Exhaust | 15.8% | 5.6% | .001 | 16.7% | 6.8% | 0.006 | ||
| Activity | 5372±5051 | 5706±5811 | .708 | 5340±4998 | 5559±5520 | 0.800 | ||
| Gait speed | 1.0±0.2 | 1.1±0.2 | < .001 | 1.0±0.2 | 1.1±0.2 | 0.002 | ||
| Risk Factor | Coefficient | Standard error | Odds ratio (95% CI) | P value |
|---|---|---|---|---|
| Back Extensor strength | -0.009 | 0.003 | 0.990 (0.983-0.997) | .008 |
| BMI | 0.026 | 0.061 | 1.027 (0.907-1.156) | .664 |
| Age | 0.084 | 0.031 | 1.088 (1.025-1.160) | .007 |
| Sex | -0.108 | 0.488 | 0.897 (0.350-2.413) | .824 |
| Constant | -6.325 |
| Characteristics | Values |
|---|---|
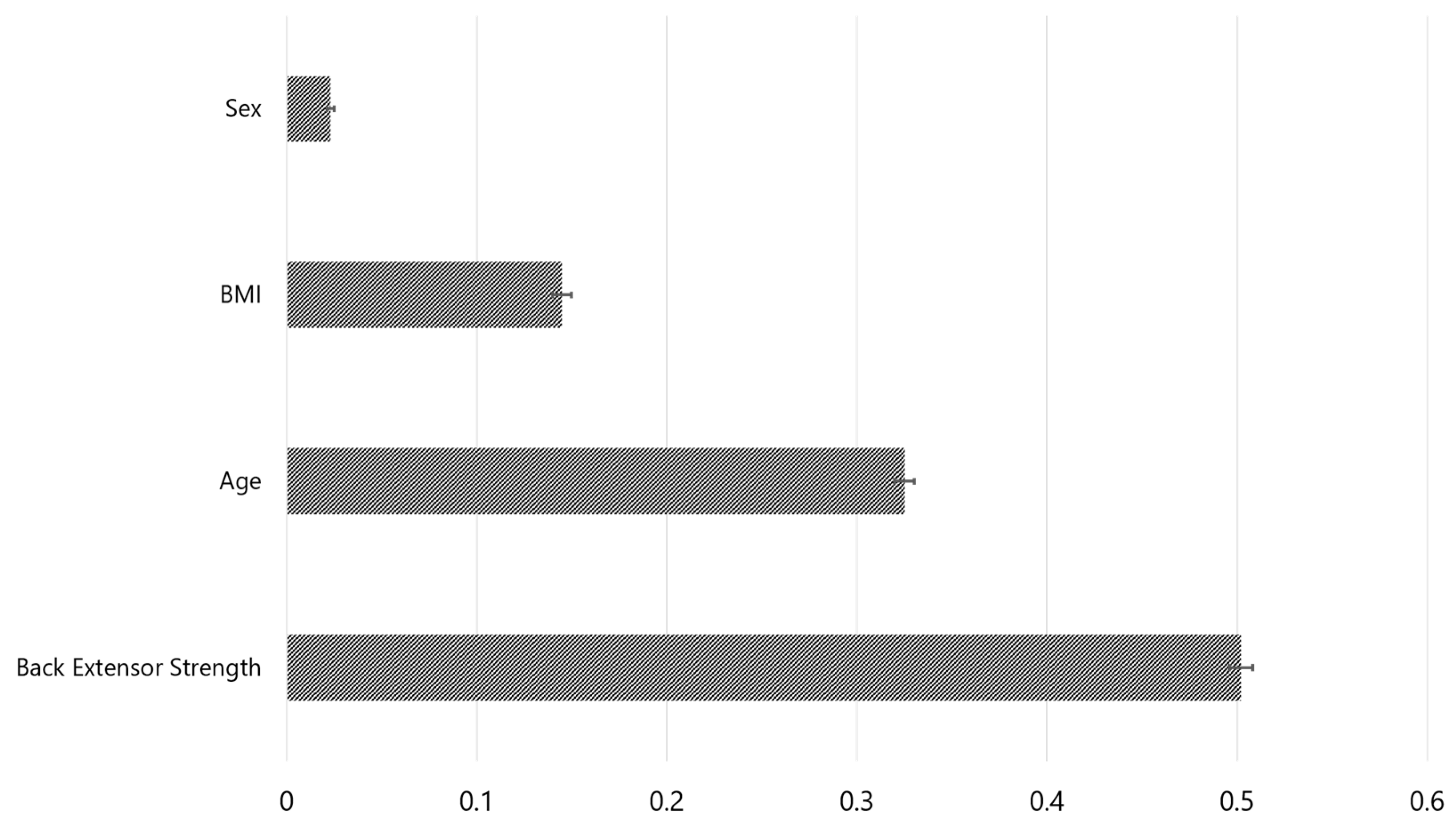
| Feature importance in Fried’s frailty prediction | |
| Back extensor strength | 0.502±0.006 |
| Age | 0.325±0.005 |
| BMI | 0.145±0.005 |
| Sex | 0.026±0.002 |
| Predictive performance of XGBoost | |
| AUC | 0.579±0.004 |
| Accuracy | 0.71±0.05 |
| Precision | 0.10±0.01 |
| Recall | 0.56±0.04 |
| F1 score | 0.15±0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





