1. Introduction
IBD, or inflammatory bowel disease, is essentially a long-term infection that affects the colon, small intestine, and rectum. Crohn's disease and ulcerative colitis are the two subtypes of IBD. There are many different symptoms associated with inflammatory bowel disease that are seen in the patients, such as acute abdominal pain, a burning feeling, diarrhoea, exhaustion, loss of appetite, malnutrition, nausea, and vomiting [
1]. According to the present IBD situation, India has a higher incidence and prevalence of these diseases than other Asian nations. IBD affects over 1.5 million people in India and almost 1.6 million people in the USA.
The study denotes that number of cases increasing in higher rate day by day. Though there are different therapeutic approach which help to treat the disease, the aim of those therapy is to inhibit and control remission. There is various conventional treatment available to diminish the symptoms like Anti-inflammatory drugs (e.g., Amino salicylates, Corticosteroids) mainly the first drug of choice in case of ulcerative colitis, Immune system suppressor (e.g., Azathioprine, Cyclosporine) use of decrease the inflammation through suppressing the immune response. Biologics are the class of drugs which also target the immune system. Other medications like Anti-diarrheal medication [
2]. Pain relivers, Antispasmodics, iron supplements also used to prevent cramps and severe intestinal bleeding. Probiotics and different herbs (Aloe vera, Butyrate, Licorice, Psyllium) are also used for the treatment. A parasite is a species that depends on another organism for sustenance diseases caused by parasites, such as those brought on by protozoa, helminths, or arthropods [
3,
4]. Studies have revealed that certain parasites, such hookworms, might influence diseases in addition to providing parasite-specific protection [
5]. Other studies have demonstrated that helminth infections can alleviate conditions like inflammatory bowel disorders or reduce their symptoms [
6]. Another study claimed that exposure to helminths could prevent colitis in animal models of IBD [
7]. Helminths and their hosts deal in a variety of ways; an effective parasite would overcome the host immune response to remain in its human host [
8,
9]. In both human and experimental settings, helminths are known to be the most potent inducers of Th2-cells, which inhibits the growth of TH1 cells [
10]. In order for a parasite to immune-modulate, a secretory route must be developed, and the inability to do so increases the risk of ulcerative colitis [
11].
2. Inflammatory Bowel Disease (IBD)
While the induction for emergent IBD is idiopathic, the recent theory on the etiology of the illness states that the long-lasting duodenal inflammation is due to a dysregulated insusceptible response to luminal microbial antigens in inherently susceptible entities [
12,
13]. The provocative process in Crohn's disease is T
H1-mediated, in case of ulcerative colitis displays both T
H1 and T
H2 cytokine profiles. Existing treatment approaches are built upon the collective use of 5-aminosalicylate and glucocorticosteroid, and immunosuppressive therapy. Newly, chimerical monoclonal antibodies to the cytokine tumor necrosis factor (TNF)-α evidenced to be more operative in the treatment of Crohn's disease [
14,
15].
2.1. Strongyloides Stercoralis
Numerous studies have shown the beneficial effects of a specific pathogenic infection with nematodes for IBD, although it is unclear what mechanism(s) underlies these effects [
8,
16,
17].
Strongyloides stercoralis, a type of intestinal nematode found in regions with high humidity such as the southern part of Asia, the Americas, Sub-Saharan Africa, and various other countries, is a commonly encountered intestinal parasite [
17]. Due to its occasional association with inducing Inflammatory Bowel Disease (IBD), particularly Ulcerative Colitis, it's crucial to take measures to prevent its presence [
5].
2.2. Blastocystis
Even though no statistically significant distinction was observed between the patients in both the non-diseased group and the control group, there was an inference that individuals suffering from IBD, particularly Ulcerative Colitis, experienced a greater likelihood of
Blastocystosis recurrence [
18].
2.3. Toxoplasma gondii
Supervising parasitic infections such as
T. gondii demands the involvement of the mediator macrophage migration inhibitory factor (MIF). Studies have established that T. gondii infection influences the damage and fatality of the small intestine among several individuals with IBD. The researchers proposed that MIF contributes to sustaining the inflammatory response initiated by oral
T. gondii infection [
18,
19,
20,
21].
2.4. Helminths
According to the results of a clinical investigation, IBD can be cured by helminth infection
6. The decreasing incidence of duodenal helminths may be associated with an increase in IBD cases in western nations.
Trichuris suis has been shown by to alleviate IBD symptoms. Patients with moderate IBD symptoms may have been exposed to helminths including
T. trichiura,
Enterobius vermicularis,
Ascaris lumbricoid, and
N. americanus as children, according to research by Weerasekara et al. showed that the development of helminths alters the production of IL-4, IL-5, IL-10, and IL-13 as well as Th2 and controlling immune responses [
22,
23].
Data suggests that certain rodent roundworms such as
Trichuris muris,
Trichinella spiralis, and
Nippostrongylus brasiliensis can influence the gut's immune state, particularly in relation to Th2 production. Previous experiments involving mice showed that early treatment with T. spiralis antigens resulted in reduced colitis severity and inferred lower death rates. This was attributed to enhanced regulation of transforming growth factor and IL-13, as well as the inhibition of interleukin production in cells. Additionally, decreased myeloperoxidase (MPO) activity and nitric oxide synthase (iNOS) expression in the colon were associated with reduced disease mortality. Notably,
T. suis was well-tolerated and demonstrated potential benefits for ulcerative colitis and Crohn's disease [
24,
25,
26].
3. Irritable Bowel Syndrome (IBS)
Although the linkage is not strong, several parasites, including
B. hominis,
Giardia spp.,
E. histolytica,
Dientamoeba fragilis, and
Trichinella spp., have been linked to the development of IBS [
27,
28,
29].
3.1. Blastocystis
It was discovered that 67% of individuals diagnosed with irritable bowel syndrome (IBS) exhibited a
Blastocystis infection, underscoring its potential importance as a health-related issue. A thorough study concentrated on 357 parasites in
Nicaraguan IBS patients [
30]. The research findings indicated that there existed no statistically notable distinction in the prevalence of colonic parasite infections caused by
B. hominis,
G. lamblia,
E. histolytica,
E. nana,
A. lumbricoides, and
H. nana when comparing individuals with IBS to the unaffected control cohort.
3.2. Giardia Spp
IBS patients who have infectious bowel dysfunction due to Giardia infection have thicker colon mucosa [
31].
3.3. Dientamoeba Fragilis
The relationship between IBS and
Dientamoeba fragilis was explored [
32]. Epidemiological studies have revealed a prevalence of 2-4% of
D. fragilis in IBS patients. However, among the 25 patients with IBS who tested positive for the parasite, no significant correlation between
D. fragilis and IBS was observed [
33,
34]. Interestingly,
D. fragilis can produce symptoms similar to IBS, leading to misdiagnosis of IBS in many patients infected with
D. fragilis [
35].
3.4. Haplorchis Taichui
H. taichui, a member of the Heterophyidae family, is prevalent in Southeast Asia. This parasite can inhabit the small intestines of animals and birds [
36]. Assimilating the metacercariae from infected cyprinoid fish causes people to become ill [
37]. Researchers have shown that
H. taichui could potentially be a causative agent for symptoms resembling those of IBS [
38].
3.5. Trichinella
First of all, it was accepted by that trichinellosis may be an additional disorder that causes IBS [
39].
3.6. Trichuris Trichiura Diniz
T. trichiura can mimic IBS symptoms and indications, hence have shown that it may lead to a false positive diagnosis [
40].
4. Gut Microbiome
Inflammatory bowel disease (IBD) occurrence is rising quickly, according to epidemiological studies, as the economy continues to increase. IBD is seen as the result of the contact between the host and microorganisms, which may include intestinal microbial factors, an aberrant immune response, and a cooperated intestinal mucosal barrier. When the balance of microbial populations is disrupted, opportunistic infections can establish themselves in the gut, increasing the chances of the host's immune system activation and promoting the onset of IBD. Identifying the specific microorganisms involved in the progression of IBD holds significant importance [
41].
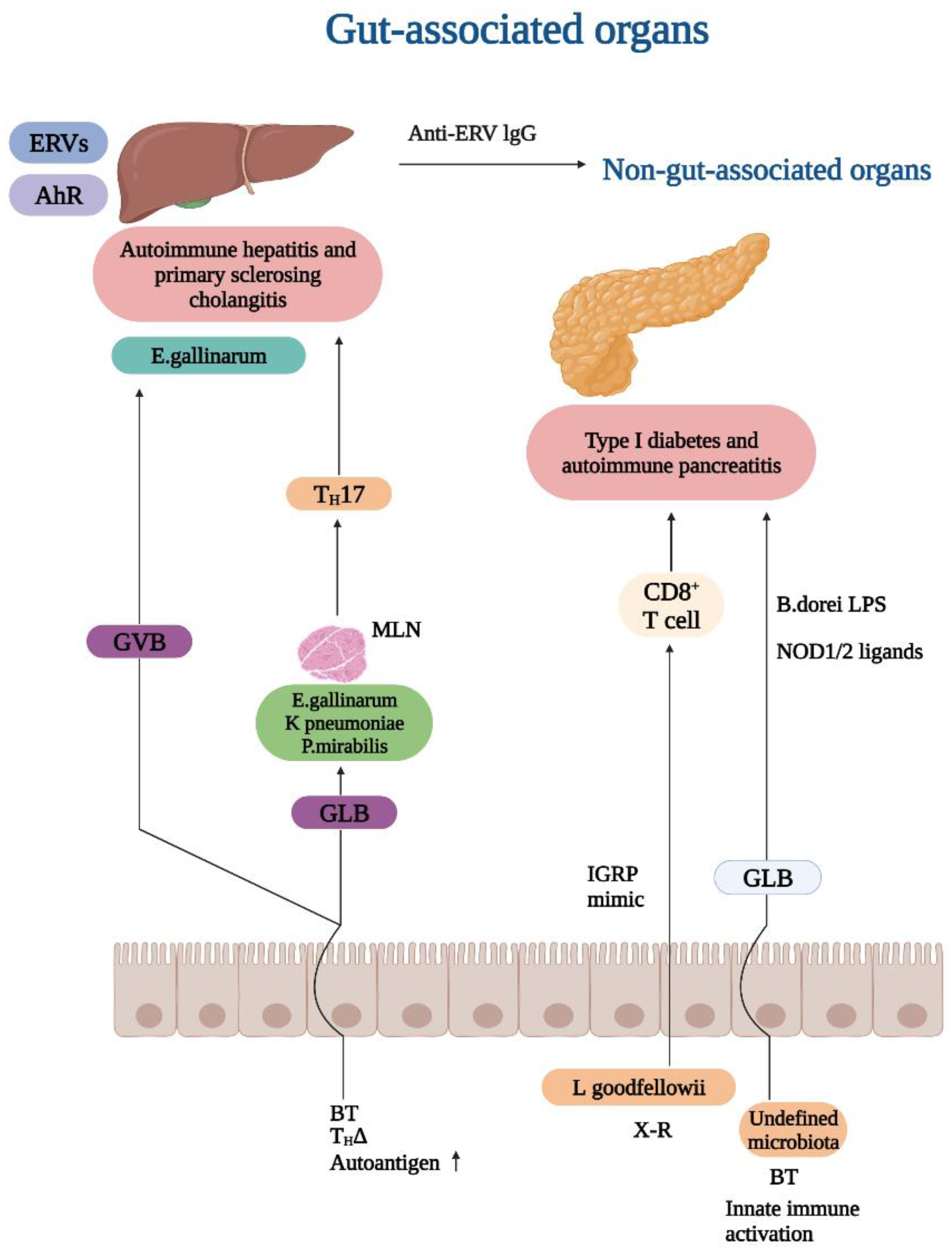
Figure 1.
Gut Microbiome of gut-associated organ.
Figure 1.
Gut Microbiome of gut-associated organ.
IBD, which affects 0.3% to 0.5% of the world's population, has two subtypes: Crohn's disease and ulcerative colitis [
42]. IBD is a kind of idiopathic inflammatory gastrointestinal illness that can arise at any time. Its onset and progression are impacted by a number of aetiologies, including immunological aspects, genetic predisposition, and the microbiota in the gut [
42]. IBD patients have a markedly different makeup of the gut microbiota than do healthy people [
43]. A 10-100 trillion-trillion-trillion-microorganism human microbiome [
44], containing bacteria, viruses, protozoa, and fungus, with bacteria having the maximum cell density (1011–1012 cells/ml). The intestinal epithelium receives a lot of energy from the gut bacteria, which may also break down carbohydrates and indigestible oligosaccharides in meals [
45]. By controlling host immune cells, good bacteria in the gut microbiota can decrease immunity [
46]. In addition to interacting with immune cells, convinced pathogenic bacteria can also produce compounds that cause intestinal injury [
47]. Genetic research has aided to recognize a number of biological archetypes that affect disease pathophysiology at a variation of separate cellular levels (e.g., T cells, B cells, and intestinal epithelial cells) [
48].
5. Role of Gut Microbiome
The human microbiome lives on the host's mucosal surfaces, including the skin, respiratory system, genitourinary tract, and other mucosal surfaces. The human gastrointestinal system is the fungal habitat that has been inspected the most. Three fungal phyla, Ascomycota, Basidiomycota, and Zygomycota, live in the human intestine [
49,
50]. In both humans and animals, the makeup of the gut microbiome looks to be far more diverse and dynamic over time than the composition of bacteria [
51]. The majority of research views fungus as intestinal commensal organisms that are acquired early in life [
52]. This has lately been contested with the assertion that healthy people' gastrointestinal tracts are not frequently colonized by fungus rather than supposing that all fungus found in human faeces samples might be accounted for by their consumption. Diet is thought to have a significant impact on the diversity and makeup of the gut microbiome [
53]. Evidence for the interconnected metabolic relationships across different kingdoms within the host was supported by the simultaneous presence of
Candida with specific bacterial genera (Prevotella and Ruminococcus) and archaeal genera (Methanobrevibacter). An indication of the involvement of fungi in altering gut balance is
Saccharomyces boulardii, a traditional herbal remedy utilized in Southeast Asia to mitigate severe diarrhea in cholera patients. This underscores the early recognition of the fungal role in shaping gut equilibrium. To counteract diarrhea and prevent intestinal colonization by Clostridium difficile following antibiotic treatment,
S. boulardii is still recommended as a probiotic [
54]. It effectively stops further C. difficile infections [
55]. Diet of the host has an impact on microbiota. It's interesting to note that interleukin (IL)-6 and IL-8 are adversely connected with prebiotic consumption, whereas circulating blood levels of GM-CSF and nondigestible fiber are positively correlated. These cytokines are decisive in the development of inflammatory bowel disease (IBD) and may be brought on by changing bacterial populations or bacterial metabolites in the intestinal lumen. As a result, the pathophysiology of IBD is dependable with a close interaction between the host bacterial microbiome and epithelial cells [
56].
Recently, there has been a great increase in patient interest in the therapeutic potential of diet-induced changes to the gut flora [
57]. In pediatric IBD patients, a number of dietary treatments have been investigated. A high-protein, low-fat diet is the Crohn's Disease Exclusion Diet (CDED), for occurrence. In mice, the particular makeup and function of the gut microbiota and metabolites are altered in auspicious ways by the ketogenic and low-carbohydrate diets [
58]. The LCD had the opposite effects from the KD when colitis was induced, protecting intestinal barrier function and increasing the expression of inflammatory cytokines [
58].
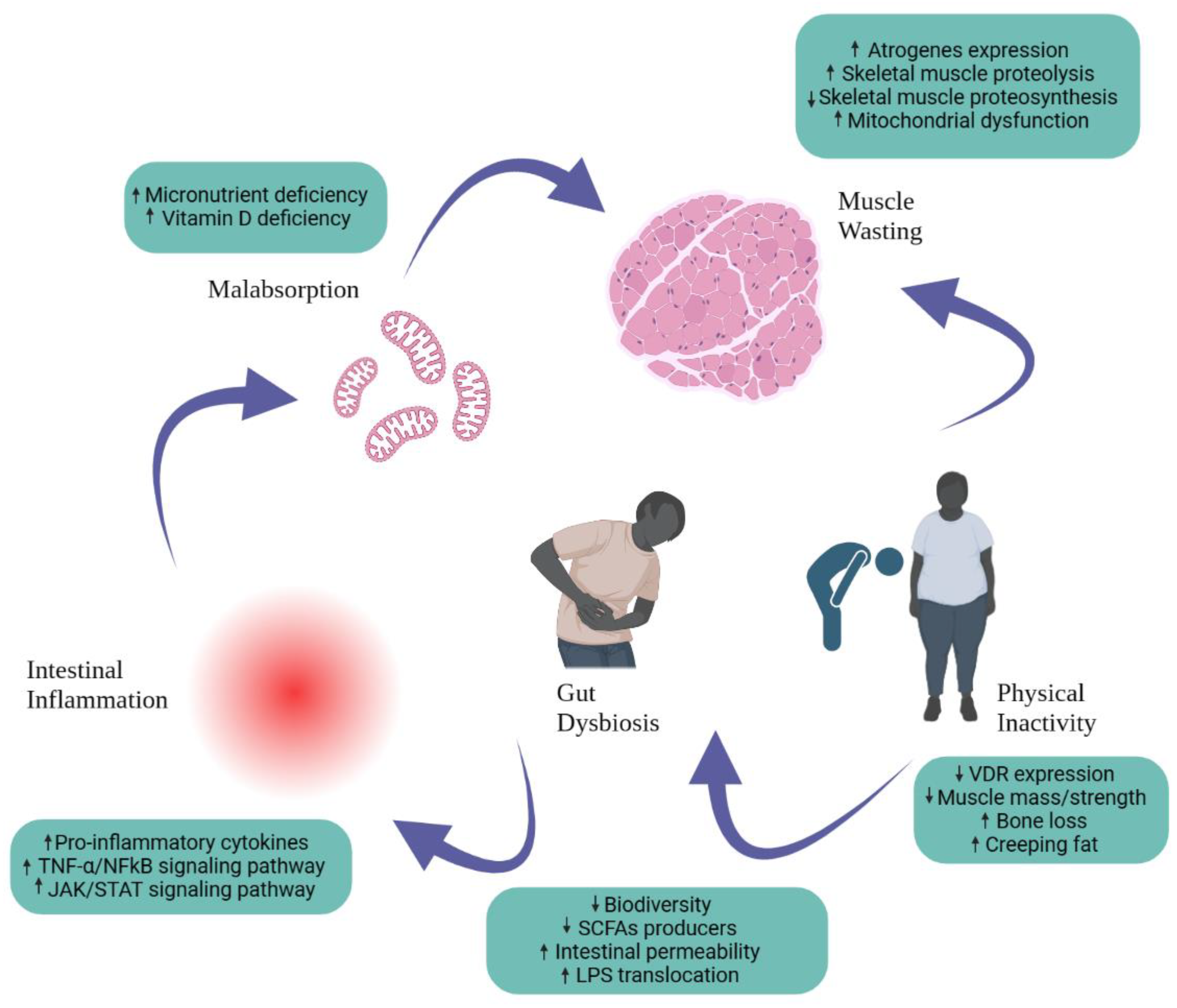
Figure 2.
Important factors contributing to the pathogenesis of the gut microbiota.
Figure 2.
Important factors contributing to the pathogenesis of the gut microbiota.
These results suggest a hopeful dietary strategy for IBD management. Importantly, this is the inaugural instance where fecal microbiota transplantation from donors who adhered to a ketogenic diet (KD) has exhibited the capacity to provide microbial advantages and alleviate colitis in recipients induced with dextran sulfate sodium (DSS) [
59]. It's vital to consider how vitamin D influences the microbiota and how this can affect cancer linked to colitis (CAC). For instance, vitamin D supplementation was found to be negatively linked with Firmicutes in a study of vitamin D-deficient pre-diabetic people (genus Rumminococcus) [
60], one of the genera where tumor numbers in colitis-related mouse studies exhibited a favorable correlation [
61]. Furthermore, vitamin D appears to play a role in fostering the formation of pattern-recognition receptors (PRRs), which could potentially enhance the protection of the colon's epithelial tissue layer against bacterial intrusion [
62].
Through the generation of protein toxins with cancerous significances, the gut microbiota plays a role in the development of CAC [
63]. Toxins can cause cancer by targeting DNA and generating genomic instability (genotoxins) or by altering cellular signaling (cytotoxins), which promotes cell growth and resistance to apoptosis. Cytolethal expanding toxin (Cdt) and colibactin are two main genotoxins that have the capacity to harm DNA [
64]. Salmonella is one of at least 30 harmful gram-negative bacteria that emit Cdts [
65]. The CdtB subunit is the sole enzymatically active component in the heterotrimer [
66]. The CdtB subunit can induce DNA single-strand breaks (SSBs) after it has entered the nucleus at low concentrations, and DNA double-strand breaks (DSBs) at large levels, triggering the DNA damage response [
61]. It is thought that, like Cdts, it results in double-strand breaks (DSBs), insufficient DNA repair, and chromosomal instability [
67]. The intestinal microbiota has the ability to create a variety of compounds that inhibit the growth of pathogenic bacteria and support intestinal homeostasis [
68]. The mucus released by Paneth cells and the bacteria in the gut are essential components of the digestive tract's chemical barrier [
69].
6. The Role of Intestinal Parasites
Intestinal parasites are often found and have a proven harmful potential. However, growing body of data shows that a sizable number of these organisms may be involved in preserving intestinal homeostasis rather than contributing to illness [
70]. Eukaryotic parasites have evolved to affect their human hosts as little as possible, the human microbiome includes some protozoan species and includes eukaryotic parasites that have evolved to affect their human hosts as little as possible. Protozoan parasites' function in the onset and course of IBD, with a focus on
Blastocystis hominis [
71,
72].
B. hominis is a prevalent protozoan parasite capable of infecting both humans and animals, with a primary affinity for the stomach. Its transmission occurs through oral and fecal routes, and it has been linked to various gastrointestinal disorders. Preliminary investigations have unveiled a noteworthy connection between protozoan infections and individuals exhibiting continuous or sporadic disease activity in ulcerative colitis (UC). Notably, B. hominis appears to be particularly widespread in such instances [
73]. Furthermore, patients who tested positive for Blastocystis had higher concentrations of the Clostridia class, Ruminococcaceae, and Prevotellaceae families, as well as the butyrate-producing bacterial genera Faecalibacterium and Roseburia, whereas patients who tested negative for Blastocystis had higher concentrations of Enterobacteriaceae, indicating that
B. hominis colonisation was not linked to the dysbiosis [
74]. However, a large number of protozoan parasites are pathogenic species that can harm the digestive system and cause illness. Entamoeba histolytica-caused colitis may seem like CD [
75]. In children with either UC or CD, cryptosporidiosis increases hospitalisation, which is one way that other parasites might aggravate the course of IBD [
76]. The lack of genetic information on intestinal protozoa and other parasites is one of the main obstacles preventing further investigation of the link between protozoan parasites and other microbiota. While genetic information on
Blastocystis and
Dientamoeba is readily accessible in public sources, there are few ribosomal DNA sequences for several other gut parasites, including widespread ones like
Entamoeba coli. Additionally, due to the high genetic variety of several common intestinal parasites, it is still unclear how dysbiosis is related to different subtypes or genotypes [
77].
The presence of intestinal protozoa in the differential diagnosis of IBS is due to the fact that they can induce symptoms similar to IBS or even substantial flare-ups of the condition. Additionally, they may cause IBS as a result of continuing low-grade inflammation brought on by chronic immune activation as a result of antigenic exposure, as occurs commonly in intestinal parasite disorders with persistent carriage/infection [
28]. The intestinal protozoa B. hominis, G. lamblia, E. histolytica, and E. coli were found in stool samples and linked to IBS-like symptoms. These parasites have been linked to an etiology of IBS in several studies [
78].
7. Fungi and Other Eukaryotic Microorganisms in the Gut
Most of the existing research on the role of the microbiota has been focused on the bacterial component. However, in recent times, microbiologists have started investigating a new taxonomic community present in the human gut – the fungal microbiota. While this group is less prevalent than the bacterial microbiota, it has not yet garnered as much attention regarding its effects on the host's health and immune system. Nevertheless, studies concerning the fungal aspect of the gut have yielded noteworthy discoveries, revealing around 300 novel species and fungal groups linked to fecal and gut contents [
79]. The primary question revolves around establishing whether the diversity of fungal taxonomy present in the gut is enduring and symbiotic, or if it is transient and merely a result of contamination. By sequencing the Internal Transcribed Spacer 1 (ITS1) regions of 98 individuals, researchers have identified a multitude of fungal species. The top three identified species are Saccharomyces, Candida, and Cladosporium. In a more recent study, a thorough investigation uncovered a significant prevalence of the Saccharomyces, Malassezia, and Candida genera. Among these,
Saccharomyces cerevisiae,
Malassezia restricta, and
Candida albicans stood out as the most commonly found species [
50].
Changes in the human mycobiota, often linked to an elevated presence of Candida spp., have been connected with distinct patterns identified in Inflammatory Bowel Diseases (IBDs). Moreover, this imbalance has been associated with a range of other conditions, such as peptic ulcers, antibiotic-associated diarrhea, hepatitis, chemotherapy-induced enteric disorders, and graft-versus-host disease [
80]. A recent comprehensive study on the human mycobiota within IBD patients has uncovered notable findings. Notably, an elevation in C. albicans was observed during episodes of inflammation. Moreover, in comparison to healthy controls, the bacterial microbiota among IBD patients displayed distinct associations with the fungal microbiome, indicating that a specific bacterial dysbiosis state might contribute to the proliferation of fungi [
81]. In their study, these researchers found a link between the levels of beneficial fungi (S. cerevisiae) and specific bacteria (
Bifidobacterium, Blautia, Roseburia, Ruminococcus) in individuals diagnosed with IBD.
Current approaches to treating IBD are centered on early administration of immune-targeted medications to reduce ongoing inflammation. Therapeutic antibodies targeting TNF-, interleukins (IL) 12 and 23, and leukocyte adhesion molecules are commonly used for IBD management. Nevertheless, with regard to the mycobiota, a potential drawback of these drugs lies in the potential for fungal overgrowth. It is worth noting that TNF-, IL-17, and IL-23 have crucial roles in immune responses against fungal infections like Candida albicans. Furthermore, certain treatment techniques are now associated with an increased risk of fungal infections, such as
histoplasmosis,
blastomycosis, and
coccidioidomycosis [
82,
83,
84]. The current state of knowledge on an etiology of IBDs clearly implies that the gut bacterial microbiota plays an important role in initiating inflammation.
8. Postdigestive Effect (Vipaka) in Ayurveda
The mechanism of drug action as per Ayurvedic ideology rests on the concept of
rasapanchak. Any therapeutic activity of either dietary item or medicine as per Ayurveda is based upon their
Rasa (taste/perception),
Vipaka (postdigestion effect),
Guna (quality),
Virya (potency) and
Prabhava (special effect) [
85]. Among these fundamentals,
Vipaka has been referred to manifest its effect as
Karma Nishthaya, i.e post-digestive irreversible process wherein biotransformation takes place. Thus,
Vipaka is a principle of pharmacokinetics among
Rasapanchak wherein the ultimate transformation decides fate of the diet/medicine and their anabolic or catabolic role in tissue or cellular nutrition [
86]. The clinicians of Ayurveda prescribe the medicine and dietary restrictions based on logical thinking and reasoning by taking Rasapanchak principles of the respective drug/diet among which
Vipaka is important. The term ‘
Vipaka’ itself denotes Vi” i.e specific, and “
Paka” refers to function assigned to
Agni (metabolic energy) [
87]. The principle of
Agni is the most unique in Ayurveda and is responsible for maintenance of health. Thus, Ayurveda has emphasized the importance of gut health which if deranged can cause various systemic pathologies. The types and seat of various
Agni in classical literature refer to the crucial metabolic transformations taking place at different levels in human body. The role of gut microbiota can be corelated to various functions of
Vipaka as they participate in metabolizing substrates into different molecules and signals which have systemic impact.
9. Role of Prakriti Phenotypes and Gut Microbiome
Ayurveda entails description of
Prakriti i.e. basic constitution which is established right from the time of conception in the embryo. This is a categorization of phenotypes based on the collective output of physical, physiological, psychological and behavioral traits of an individual as per
Dosha. The classification of
prakriti is based upon the predominance of the three
Dosha i.e.
Vata, Pitta and
Kapha. Clinically, it is divided into seven types. Among these, persons possessing
Dvandvaja prakriti i.e combined characteristics of two doshas is found at large but not considered ideal. An individual with
Sama Prakriti ie. having three doshas in an equilibrium is considered to be healthy among all [
88].
Prakriti pariksha is the basic tool to distinguish between individuals in terms of their immunity, responses to pharmacological therapies and their susceptibility to certain pathologies. Seers have further advocated diurnal regimens and seasonal regimens to the individuals for maintaining the homeostasis of Dosha in their body. There are some typical observations denoted in classics viz. individual with a predominant
kapha has a better immune status as compared to others; Cognitive functions are more pronounced in individuals of
pitta Prakriti due to their innate grasping power but same individuals are prone for adverse drug reactions. This concept is found to resonate with personalized medicine in modern era. Further, studies are being carried out for establishing the genetic basis of Prakriti, gut microbial signatures for specific Prakriti types that revealed prakriti-specific differential abundance of diverse bacterial genera etc [
89,
90].
Still, a validated Prakriti tool which is intra-operable and uniform is in process in Central Council for Research in Ayurvedic Sciences , Ministry of Ayush. The data thus acquired using a standard tool will reveal the distribution of Phenotype of Prakriti which will serve as a reference standard in future. Similarly, in case of gut microbial studies, despite the numerous research being carried out, no ready reference data about the bacterial genera of Indian population in particular is available. When both of these are accessible, only then can various association studies be planned to further validate personalized medicine approach.
10. Advancement of Shielding Immunity by Microbiota
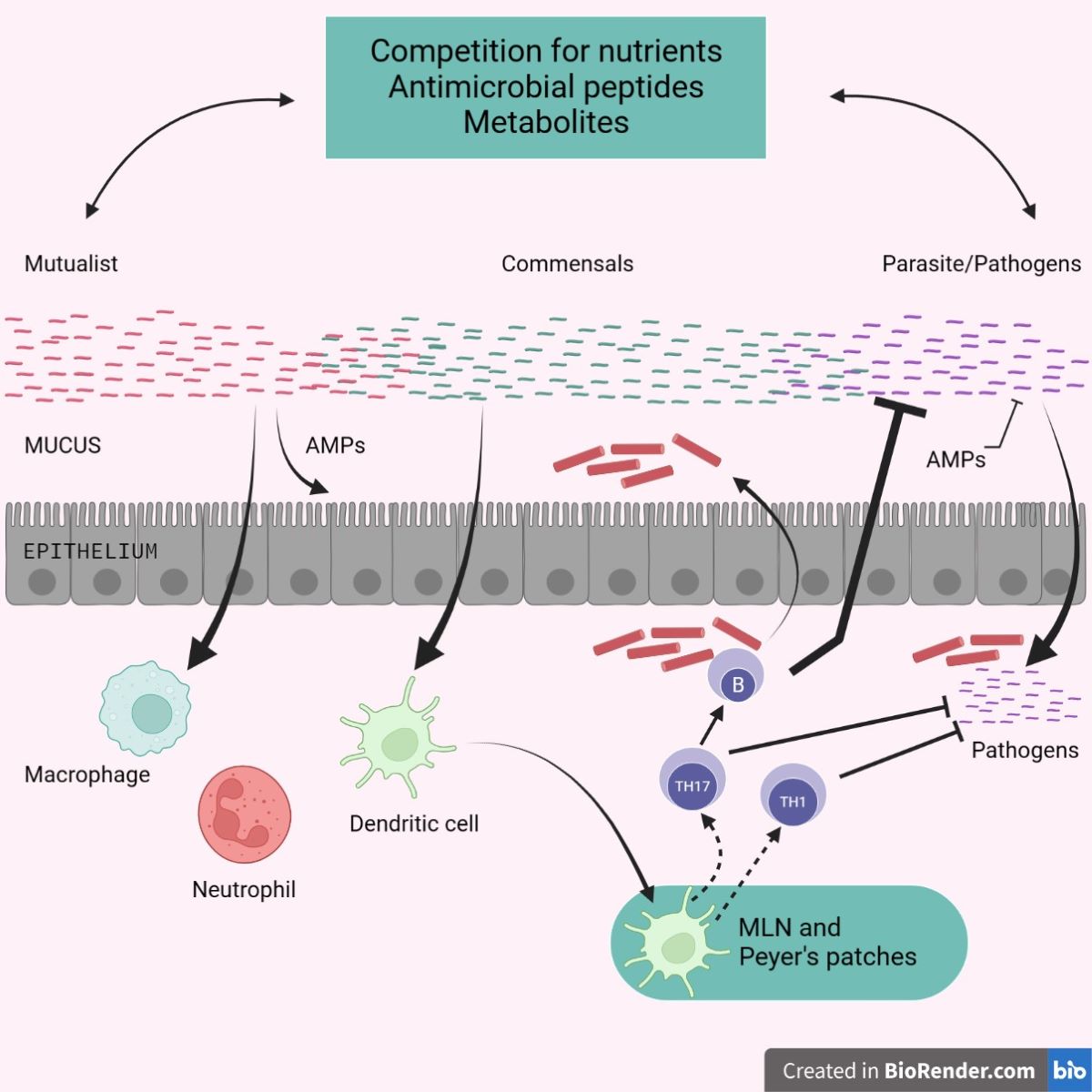
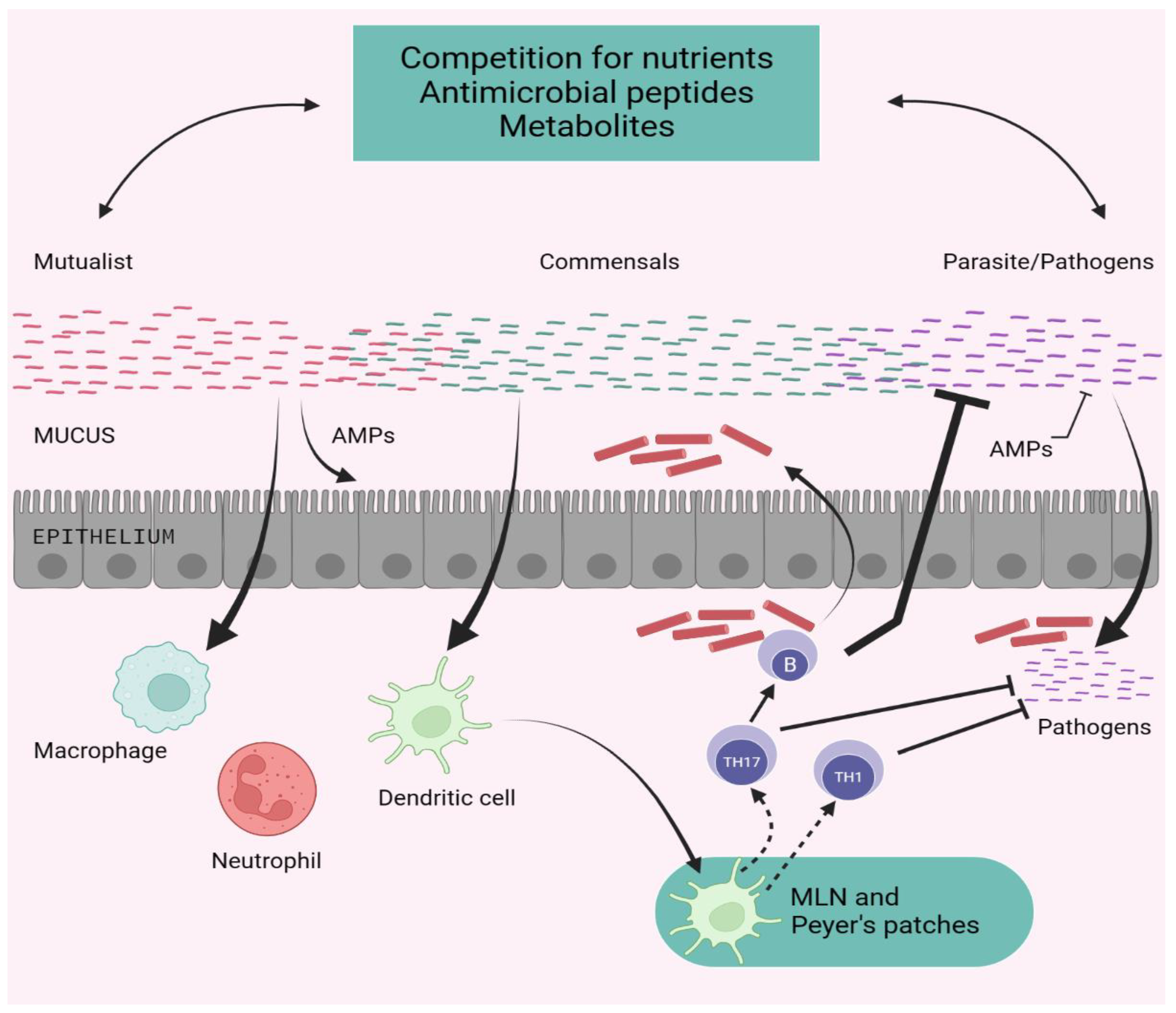
The symbiotic connection between the microbiota and its mammalian host includes mutualistic, parasitic, and commensal partnerships. The ability of a specific microbe, especially those found in the microbiota, to cause or promote disease is extremely contextual, and certain microorganisms can move from mutualist to commensal to parasite depending on the host's status of stimulation, co-infection, or localization. Commensals can inhibit pathogenic microorganisms (either naturally occurring or acquired) through several ways [
91]. Commensals can compete for nutrition while also producing antimicrobial molecules and metabolites that alter pathogen survival and virulence. Commensals can help epithelial cells produce antimicrobial peptides and strengthen tight junctions. subsequently commensals can influence the activity of dendritic cells and other innate cells both locally and systemically, promoting the production of effector T and B cell fight against pathogens. When left unchecked, the microbiota's adjuvant ability can induce inflammatory and autoimmune illnesses [
92].
Figure 3.
Promotion of Protective Immunity by the Microbiota.
Figure 3.
Promotion of Protective Immunity by the Microbiota.
The microbiota of the human gut contributes to the host's nutrient intake and synthesis, tolerates the development and variation of intestinal cells, and controls the immune system of the host. Unadorned gut illnesses, such as inflammatory bowel disease (IBD) or inflammatory bowel syndrome (IBS), are caused by differences in how they are arranged. Inflammatory bowel disease, which includes ulcerative colitis (UC) and Crohn's disease (CD), is a group of chronic inflammatory illnesses of the gut that are influenced by a combination of genetic, environmental, and internal factors. IBD has a contentious etiology, but in recent years, research on the crucial role of a tri-directional connection between the intestinal epithelium, the mucosal immune system, and the intestinal microbiota in pathogenesis has received the most attention. The cumulative frequency and early start reveal the exponential rise in the burden of inflammatory bowel disease (IBD) on healthcare systems. This elevated surge is explained by growth in the industry, allergy avoidance, existence, hygienic theory, harm to intestinal worms and gut microbial arrangement. In the past, the interferences regulating gut microbiota alignment, microfluidics-based in vitro GIT models, non-allergic effective meals, nutraceuticals, and fecal microbiota transplantation (FMT) from potent donors were some of the breakthrough techniques for illness surveillance [
93].
11. Conclusion
The alteration of immunological responses, namely the number of cytokines generated, led some nations to cautiously explore helminth larva contamination as a potential treatment for inflammatory disorders and its complications. A specific parasite infection on autoimmune illnesses has been shown in several research to have therapeutic benefits. Numerous studies including Ayurveda have suggested that treating celiac disease with hookworms helps improve gluten sensitivity. The increased prevalence of inflammatory bowel diseases, which includes as ulcerative colitis, cord disease, and IBD, is thought to be related to a decrease in the incidence of intestinal helminths. Approximately one-third of these presumptions have been highlighted in this review. But these entities nevertheless retain undetectable detailed systems. Based on the outcomes of these research findings, one can deduce that diverse parasite infections, including Toxoplasma gondii, the initiation of Crohn's disease (CD), helminth infections, and the emergence of inflammatory bowel diseases (IBD), along with the presence of Dientamoeba fragilis and B. hominis, contribute to the progression of inflammatory bowel syndrome. Acknowledging the association between autoimmune disorders and parasitic infections holds significance in assessing, detecting, and potentially averting these conditions.
References
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D. T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161 (4), 1118-1132. [CrossRef]
- Seyedian, S. S.; Nokhostin, F.; Malamir, M. D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J Med Life 2019, 12 (2), 113-122. [CrossRef]
- De Silva, N. R.; Brooker, S.; Hotez, P. J.; Montresor, A.; Engels, D.; Savioli, L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol 2003, 19 (12), 547-551. [CrossRef]
- Hotez, P. J.; Bethony, J.; Bottazzi, M. E.; Brooker, S.; Diemert, D.; Loukas, A. New technologies for the control of human hookworm infection. Trends Parasitol 2006, 22 (7), 327-331. [CrossRef]
- Dave, M.; Purohit, T.; Razonable, R.; Loftus, E. V., Jr. Opportunistic infections due to inflammatory bowel disease therapy. Inflamm Bowel Dis 2014, 20 (1), 196-212. [CrossRef]
- Ruyssers, N. E.; De Winter, B. Y.; De Man, J. G.; Loukas, A.; Pearson, M. S.; Weinstock, J. V.; Van den Bossche, R. M.; Martinet, W.; Pelckmans, P. A.; Moreels, T. G. Therapeutic potential of helminth soluble proteins in TNBS-induced colitis in mice. Inflamm Bowel Dis 2009, 15 (4), 491-500. [CrossRef]
- Elliott, D. E.; Li, J.; Blum, A.; Metwali, A.; Qadir, K.; Urban Jr, J. F.; Weinstock, J. V. Exposure to schistosome eggs protects mice from TNBS-induced colitis. American Journal of Physiology-Gastrointestinal and Liver Physiology 2003, 284 (3), G385-G391.
- Elliott, D. E.; Weinstock, J. V. Helminth-host immunological interactions: prevention and control of immune-mediated diseases. Ann N Y Acad Sci 2012, 1247, 83-96. [CrossRef]
- Riffkin, M.; Seow, H. F.; Jackson, D.; Brown, L.; Wood, P. Defence against the immune barrage: helminth survival strategies. Immunol Cell Biol 1996, 74 (6), 564-574. [CrossRef]
- Maizels, R. M.; Bundy, D. A.; Selkirk, M. E.; Smith, D. F.; Anderson, R. M. Immunological modulation and evasion by helminth parasites in human populations. Nature 1993, 365 (6449), 797-805. [CrossRef]
- Raddatz, D.; Bockemühl, M.; Ramadori, G. Quantitative measurement of cytokine mRNA in inflammatory bowel disease: relation to clinical and endoscopic activity and outcome. Eur J Gastroenterol Hepatol 2005, 17 (5), 547-557. [CrossRef]
- Targan, S. R.; Murphy, L. K. Clarifying the causes of Crohn's. Nat Med 1995, 1 (12), 1241-1243.
- Zeitz, M. Pathogenesis of inflammatory bowel disease. Digestion 1997, 58 Suppl 1, 59-61.
- Hanauer, S. B.; Feagan, B. G.; Lichtenstein, G. R.; Mayer, L. F.; Schreiber, S.; Colombel, J. F.; Rachmilewitz, D.; Wolf, D. C.; Olson, A.; Bao, W.; et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet 2002, 359 (9317), 1541-1549.
- Sands, B. E.; Anderson, F. H.; Bernstein, C. N.; Chey, W. Y.; Feagan, B. G.; Fedorak, R. N.; Kamm, M. A.; Korzenik, J. R.; Lashner, B. A.; Onken, J. E.; et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med 2004, 350 (9), 876-885.
- Ince, M. N.; Elliott, D. E.; Setiawan, T.; Metwali, A.; Blum, A.; Chen, H. L.; Urban, J. F.; Flavell, R. A.; Weinstock, J. V. Role of T cell TGF-beta signaling in intestinal cytokine responses and helminthic immune modulation. Eur J Immunol 2009, 39 (7), 1870-1878.
- Schnoeller, C.; Rausch, S.; Pillai, S.; Avagyan, A.; Wittig, B. M.; Loddenkemper, C.; Hamann, A.; Hamelmann, E.; Lucius, R.; Hartmann, S. A helminth immunomodulator reduces allergic and inflammatory responses by induction of IL-10-producing macrophages. J Immunol 2008, 180 (6), 4265-4272. [CrossRef]
- Cekin, A. H.; Cekin, Y.; Adakan, Y.; Tasdemir, E.; Koclar, F. G.; Yolcular, B. O. Blastocystosis in patients with gastrointestinal symptoms: a case-control study. BMC Gastroenterol 2012, 12, 122. [CrossRef]
- Satoskar, A. R.; Bozza, M.; Rodriguez Sosa, M.; Lin, G.; David, J. R. Migration-inhibitory factor gene-deficient mice are susceptible to cutaneous Leishmania major infection. Infect Immun 2001, 69 (2), 906-911. [CrossRef]
- Terrazas, C. A.; Juarez, I.; Terrazas, L. I.; Saavedra, R.; Calleja, E. A.; Rodriguez-Sosa, M. Toxoplasma gondii: impaired maturation and pro-inflammatory response of dendritic cells in MIF-deficient mice favors susceptibility to infection. Exp Parasitol 2010, 126 (3), 348-358. [CrossRef]
- Cavalcanti, M. G.; Mesquita, J. S.; Madi, K.; Feijó, D. F.; Assunção-Miranda, I.; Souza, H. S.; Bozza, M. T. MIF participates in Toxoplasma gondii-induced pathology following oral infection. PLoS One 2011, 6 (9), e25259.
- Moreels, T. G.; Pelckmans, P. A. Gastrointestinal parasites: potential therapy for refractory inflammatory bowel diseases. Inflamm Bowel Dis 2005, 11 (2), 178-184.
- Elliott, D. E.; Summers, R. W.; Weinstock, J. V. Helminths as governors of immune-mediated inflammation. Int J Parasitol 2007, 37 (5), 457-464. [CrossRef]
- Brunet, L. R.; Dunne, D. W.; Pearce, E. J. Cytokine Interaction and Immune Responses during Schistosoma mansoni Infection. Parasitol Today 1998, 14 (10), 422-427. [CrossRef]
- Motomura, Y.; Wang, H.; Deng, Y.; El-Sharkawy, R. T.; Verdu, E. F.; Khan, W. I. Helminth antigen-based strategy to ameliorate inflammation in an experimental model of colitis. Clin Exp Immunol 2009, 155 (1), 88-95. [CrossRef]
- Summers, R. W.; Elliott, D. E.; Qadir, K.; Urban, J. F., Jr.; Thompson, R.; Weinstock, J. V. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am J Gastroenterol 2003, 98 (9), 2034-2041.
- Dogruman-Al, F.; Simsek, Z.; Boorom, K.; Ekici, E.; Sahin, M.; Tuncer, C.; Kustimur, S.; Altinbas, A. Comparison of methods for detection of Blastocystis infection in routinely submitted stool samples, and also in IBS/IBD Patients in Ankara, Turkey. PLoS One 2010, 5 (11), e15484. [CrossRef]
- Stark, D.; van Hal, S.; Marriott, D.; Ellis, J.; Harkness, J. Irritable bowel syndrome: a review on the role of intestinal protozoa and the importance of their detection and diagnosis. Int J Parasitol 2007, 37 (1), 11-20. [CrossRef]
- Spiller, R.; Garsed, K. Postinfectious irritable bowel syndrome. Gastroenterology 2009, 136 (6), 1979-1988.
- Morgan, D. R.; Benshoff, M.; Cáceres, M.; Becker-Dreps, S.; Cortes, L.; Martin, C. F.; Schmulson, M.; Peña, R. Irritable bowel syndrome and gastrointestinal parasite infection in a developing nation environment. Gastroenterol Res Pract 2012, 2012, 343812. [CrossRef]
- Dizdar, V.; Spiller, R.; Singh, G.; Hanevik, K.; Gilja, O. H.; El-Salhy, M.; Hausken, T. Relative importance of abnormalities of CCK and 5-HT (serotonin) in Giardia-induced post-infectious irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther 2010, 31 (8), 883-891. [CrossRef]
- Borody, T.; Warren, E.; Wettstein, A.; Robertson, G.; Recabarren, P.; Fontella, A.; Herdnman, K.; Surace, R. Eradication of Dientamoeba fragilis can resolve IBS-like symptoms. J Gastroenterol Hepatol 2002, 17 (Suppl), A103.
- Yakoob, J.; Jafri, W.; Beg, M. A.; Abbas, Z.; Naz, S.; Islam, M.; Khan, R. Blastocystis hominis and Dientamoeba fragilis in patients fulfilling irritable bowel syndrome criteria. Parasitol Res 2010, 107 (3), 679-684. [CrossRef]
- Jimenez-Gonzalez, D. E.; Martinez-Flores, W. A.; Reyes-Gordillo, J.; Ramirez-Miranda, M. E.; Arroyo-Escalante, S.; Romero-Valdovinos, M.; Stark, D.; Souza-Saldivar, V.; Martinez-Hernandez, F.; Flisser, A.; et al. Blastocystis infection is associated with irritable bowel syndrome in a Mexican patient population. Parasitol Res 2012, 110 (3), 1269-1275. [CrossRef]
- Engsbro, A. L.; Stensvold, C. R.; Nielsen, H. V.; Bytzer, P. Treatment of Dientamoeba fragilis in patients with irritable bowel syndrome. Am J Trop Med Hyg 2012, 87 (6), 1046-1052. [CrossRef]
- Chai, J. Y.; Han, E. T.; Shin, E. H.; Sohn, W. M.; Yong, T. S.; Eom, K. S.; Min, D. Y.; Um, J. Y.; Park, M. S.; Hoang, E. H.; et al. High prevalence of Haplorchis taichui, Phaneropsolus molenkampi, and other helminth infections among people in Khammouane province, Lao PDR. Korean J Parasitol 2009, 47 (3), 243-247. [CrossRef]
- Kumchoo, K.; Wongsawad, C.; Chai, J. Y.; Vanittanakom, P.; Rojanapaibul, A. High prevalence of Haplorchis taichui metacercariae in cyprinoid fish from Chiang Mai Province, Thailand. Southeast Asian J Trop Med Public Health 2005, 36 (2), 451-455.
- Watthanakulpanich, D.; Waikagul, J.; Maipanich, W.; Nuamtanong, S.; Sanguankiat, S.; Pubampen, S.; Praevanit, R.; Mongkhonmu, S.; Nawa, Y. Haplorchis taichui as a possible etiologic agent of irritable bowel syndrome-like symptoms. Korean J Parasitol 2010, 48 (3), 225-229.
- Soyturk, M.; Akpinar, H.; Gurler, O.; Pozio, E.; Sari, I.; Akar, S.; Akarsu, M.; Birlik, M.; Onen, F.; Akkoc, N. Irritable bowel syndrome in persons who acquired trichinellosis. Am J Gastroenterol 2007, 102 (5), 1064-1069. [CrossRef]
- Diniz-Santos, D. R.; Jambeiro, J.; Mascarenhas, R. R.; Silva, L. R. Massive Trichuris trichiura infection as a cause of chronic bloody diarrhea in a child. J Trop Pediatr 2006, 52 (1), 66-68. [CrossRef]
- Qiu, P.; Ishimoto, T.; Fu, L.; Zhang, J.; Zhang, Z.; Liu, Y. The Gut Microbiota in Inflammatory Bowel Disease. Frontiers in Cellular and Infection Microbiology 2022, 12, 733992.
- Ng, S. C.; Shi, H. Y.; Hamidi, N.; Underwood, F. E.; Tang, W.; Benchimol, E. I.; Panaccione, R.; Ghosh, S.; Wu, J. C. Y.; Chan, F. K. L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2017, 390 (10114), 2769-2778. [CrossRef]
- Oligschlaeger, Y.; Yadati, T.; Houben, T.; Condello Oliván, C. M.; Shiri-Sverdlov, R. Inflammatory Bowel Disease: A Stressed "Gut/Feeling". Cells 2019, 8 (7). [CrossRef]
- Lopetuso, L. R.; Ianiro, G.; Scaldaferri, F.; Cammarota, G.; Gasbarrini, A. Gut Virome and Inflammatory Bowel Disease. Inflamm Bowel Dis 2016, 22 (7), 1708-1712. [CrossRef]
- Ramakrishna, B. S. Role of the gut microbiota in human nutrition and metabolism. J Gastroenterol Hepatol 2013, 28 Suppl 4, 9-17. [CrossRef]
- Allen-Vercoe, E.; Coburn, B. A Microbiota-Derived Metabolite Augments Cancer Immunotherapy Responses in Mice. Cancer Cell 2020, 38 (4), 452-453. [CrossRef]
- Stappenbeck, T. S.; Virgin, H. W. Accounting for reciprocal host-microbiome interactions in experimental science. Nature 2016, 534 (7606), 191-199. [CrossRef]
- Lakatos, P. L. Recent trends in the epidemiology of inflammatory bowel diseases: up or down? World J Gastroenterol 2006, 12 (38), 6102-6108.
- Hallen-Adams, H. E.; Suhr, M. J. Fungi in the healthy human gastrointestinal tract. Virulence 2017, 8 (3), 352-358. [CrossRef]
- Hoffmann, C.; Dollive, S.; Grunberg, S.; Chen, J.; Li, H.; Wu, G. D.; Lewis, J. D.; Bushman, F. D. Archaea and fungi of the human gut microbiome: correlations with diet and bacterial residents. PLoS One 2013, 8 (6), e66019. [CrossRef]
- Dollive, S.; Chen, Y. Y.; Grunberg, S.; Bittinger, K.; Hoffmann, C.; Vandivier, L.; Cuff, C.; Lewis, J. D.; Wu, G. D.; Bushman, F. D. Fungi of the murine gut: episodic variation and proliferation during antibiotic treatment. PLoS One 2013, 8 (8), e71806. [CrossRef]
- Auchtung, T. A.; Fofanova, T. Y.; Stewart, C. J.; Nash, A. K.; Wong, M. C.; Gesell, J. R.; Auchtung, J. M.; Ajami, N. J.; Petrosino, J. F. Investigating Colonization of the Healthy Adult Gastrointestinal Tract by Fungi. mSphere 2018, 3 (2). [CrossRef]
- David, L. A.; Maurice, C. F.; Carmody, R. N.; Gootenberg, D. B.; Button, J. E.; Wolfe, B. E.; Ling, A. V.; Devlin, A. S.; Varma, Y.; Fischbach, M. A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505 (7484), 559-563. [CrossRef]
- McFarland, L. V. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol 2010, 16 (18), 2202-2222.
- Madoff, S. E.; Urquiaga, M.; Alonso, C. D.; Kelly, C. P. Prevention of recurrent Clostridioides difficile infection: A systematic review of randomized controlled trials. Anaerobe 2020, 61, 102098. [CrossRef]
- Olendzki, B.; Bucci, V.; Cawley, C.; Maserati, R.; McManus, M.; Olednzki, E.; Madziar, C.; Chiang, D.; Ward, D. V.; Pellish, R.; et al. Dietary manipulation of the gut microbiome in inflammatory bowel disease patients: Pilot study. Gut Microbes 2022, 14 (1), 2046244. [CrossRef]
- Hart, L.; Verburgt, C. M.; Wine, E.; Zachos, M.; Poppen, A.; Chavannes, M.; Van Limbergen, J.; Pai, N. Nutritional Therapies and Their Influence on the Intestinal Microbiome in Pediatric Inflammatory Bowel Disease. Nutrients 2021, 14 (1). [CrossRef]
- Kong, C.; Yan, X.; Liu, Y.; Huang, L.; Zhu, Y.; He, J.; Gao, R.; Kalady, M. F.; Goel, A.; Qin, H.; et al. Ketogenic diet alleviates colitis by reduction of colonic group 3 innate lymphoid cells through altering gut microbiome. Signal Transduct Target Ther 2021, 6 (1), 154. [CrossRef]
- Levine, A.; Wine, E.; Assa, A.; Sigall Boneh, R.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn's Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157 (2), 440-450.e448. [CrossRef]
- Ciubotaru, I.; Green, S. J.; Kukreja, S.; Barengolts, E. Significant differences in fecal microbiota are associated with various stages of glucose tolerance in African American male veterans. Transl Res 2015, 166 (5), 401-411. [CrossRef]
- Healy, A. R.; Herzon, S. B. Molecular Basis of Gut Microbiome-Associated Colorectal Cancer: A Synthetic Perspective. J Am Chem Soc 2017, 139 (42), 14817-14824. [CrossRef]
- Liang, X.; Li, H.; Tian, G.; Li, S. Dynamic microbe and molecule networks in a mouse model of colitis-associated colorectal cancer. Sci Rep 2014, 4, 4985. [CrossRef]
- Gargi, A.; Reno, M.; Blanke, S. R. Bacterial toxin modulation of the eukaryotic cell cycle: are all cytolethal distending toxins created equally? Front Cell Infect Microbiol 2012, 2, 124.
- Fedor, Y.; Vignard, J.; Nicolau-Travers, M. L.; Boutet-Robinet, E.; Watrin, C.; Salles, B.; Mirey, G. From single-strand breaks to double-strand breaks during S-phase: a new mode of action of the Escherichia coli Cytolethal Distending Toxin. Cell Microbiol 2013, 15 (1), 1-15. [CrossRef]
- Van Elsland, D.; Neefjes, J. Bacterial infections and cancer. EMBO Rep 2018, 19 (11). [CrossRef]
- Nougayrède, J. P.; Homburg, S.; Taieb, F.; Boury, M.; Brzuszkiewicz, E.; Gottschalk, G.; Buchrieser, C.; Hacker, J.; Dobrindt, U.; Oswald, E. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 2006, 313 (5788), 848-851. [CrossRef]
- Balish, E.; Warner, T. Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. Am J Pathol 2002, 160 (6), 2253-2257. [CrossRef]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? EBioMedicine 2021, 66, 103293.
- Lee, M.; Chang, E. B. Inflammatory Bowel Diseases (IBD) and the Microbiome-Searching the Crime Scene for Clues. Gastroenterology 2021, 160 (2), 524-537. [CrossRef]
- Stensvold, C. R.; van der Giezen, M. Associations between Gut Microbiota and Common Luminal Intestinal Parasites. Trends Parasitol 2018, 34 (5), 369-377.
- Eichenberger, R. M.; Ryan, S.; Jones, L.; Buitrago, G.; Polster, R.; Montes de Oca, M.; Zuvelek, J.; Giacomin, P. R.; Dent, L. A.; Engwerda, C. R.; et al. Hookworm Secreted Extracellular Vesicles Interact With Host Cells and Prevent Inducible Colitis in Mice. Front Immunol 2018, 9, 850. [CrossRef]
- Tito, R. Y.; Chaffron, S.; Caenepeel, C.; Lima-Mendez, G.; Wang, J.; Vieira-Silva, S.; Falony, G.; Hildebrand, F.; Darzi, Y.; Rymenans, L.; et al. Population-level analysis of Blastocystis subtype prevalence and variation in the human gut microbiota. Gut 2019, 68 (7), 1180-1189. [CrossRef]
- Yamamoto-Furusho, J. K.; Torijano-Carrera, E. Intestinal protozoa infections among patients with ulcerative colitis: prevalence and impact on clinical disease course. Digestion 2010, 82 (1), 18-23. [CrossRef]
- Audebert, C.; Even, G.; Cian, A.; Loywick, A.; Merlin, S.; Viscogliosi, E.; Chabé, M. Colonization with the enteric protozoa Blastocystis is associated with increased diversity of human gut bacterial microbiota. Sci Rep 2016, 6, 25255.
- Verstockt, B.; Vermeire, S.; Van Assche, G.; Ferrante, M. When IBD is not IBD. Scand J Gastroenterol 2018, 53 (9), 1085-1088.
- Vadlamudi, N.; Maclin, J.; Dimmitt, R. A.; Thame, K. A. Cryptosporidial infection in children with inflammatory bowel disease. J Crohns Colitis 2013, 7 (9), e337-343. [CrossRef]
- Stensvold, C. R.; Lebbad, M.; Victory, E. L.; Verweij, J. J.; Tannich, E.; Alfellani, M.; Legarraga, P.; Clark, C. G. Increased sampling reveals novel lineages of Entamoeba: consequences of genetic diversity and host specificity for taxonomy and molecular detection. Protist 2011, 162 (3), 525-541. [CrossRef]
- D'Anchino, M.; Orlando, D.; De Feudis, L. Giardia lamblia infections become clinically evident by eliciting symptoms of irritable bowel syndrome. J Infect 2002, 45 (3), 169-172. [CrossRef]
- Suhr, M. J.; Hallen-Adams, H. E. The human gut mycobiome: pitfalls and potentials--a mycologist's perspective. Mycologia 2015, 107 (6), 1057-1073.
- Richard, M. L.; Sokol, H. The gut mycobiota: insights into analysis, environmental interactions and role in gastrointestinal diseases. Nat Rev Gastroenterol Hepatol 2019, 16 (6), 331-345. [CrossRef]
- Sokol, H.; Leducq, V.; Aschard, H.; Pham, H. P.; Jegou, S.; Landman, C.; Cohen, D.; Liguori, G.; Bourrier, A.; Nion-Larmurier, I.; et al. Fungal microbiota dysbiosis in IBD. Gut 2017, 66 (6), 1039-1048. [CrossRef]
- Whibley, N.; Jaycox, J. R.; Reid, D.; Garg, A. V.; Taylor, J. A.; Clancy, C. J.; Nguyen, M. H.; Biswas, P. S.; McGeachy, M. J.; Brown, G. D.; et al. Delinking CARD9 and IL-17: CARD9 Protects against Candida tropicalis Infection through a TNF-α-Dependent, IL-17-Independent Mechanism. J Immunol 2015, 195 (8), 3781-3792.
- Maher, C. O.; Dunne, K.; Comerford, R.; O'Dea, S.; Loy, A.; Woo, J.; Rogers, T. R.; Mulcahy, F.; Dunne, P. J.; Doherty, D. G. Candida albicans stimulates IL-23 release by human dendritic cells and downstream IL-17 secretion by Vδ1 T cells. J Immunol 2015, 194 (12), 5953-5960. [CrossRef]
- Ford, A. C.; Peyrin-Biroulet, L. Opportunistic infections with anti-tumor necrosis factor-α therapy in inflammatory bowel disease: meta-analysis of randomized controlled trials. Am J Gastroenterol 2013, 108 (8), 1268-1276. [CrossRef]
- Rath, S. K.; Panja, A. K.; Nagar, L.;, Shinde, A. The scientific basis of rasa (taste) of a substance as a tool to explore its pharmacological behavior. Anc Sci Life, 2014, 33, 198–202. [CrossRef]
- Ranade, A.; Gayakwad, S.; Chougule, S.; Shirolkar, A.; Gaidhani, S.; Pawar, S. D. Gut microbiota: metabolic programmers as a lead for deciphering Ayurvedic pharmacokinetics. Current Science 2020, 10, 119 (3), 451-461. [CrossRef]
- Ranade, A. V.; Shirolkar, A.; Pawar, S. D. Gut microbiota: One of the new frontiers for elucidating fundamentals of Vipaka in Ayurveda. AYU 2019, 40 (2), 75-78. [CrossRef]
- Upadhyaya, N.; Suvitha, S. V.; Yadav, S.; Yadav, C. R. A Clinical Utility of Prakriti Parikshan- An Ayurvedic Diagnostic Tool: A Brief Review. International Journal of Research in AYUSH and Pharmaceutical Sciences 2021, 5 (2), 514-520. [CrossRef]
- Govindaraj, P.; Nizamuddin, S.; Sharath, A. et al. Genome-wide analysis correlates Ayurveda Prakriti. Sci Rep 2015, 5, 15786. [CrossRef]
- Chaudhari, D.; Dhotre, D.; Agarwal, D.; Gondhali, A.; Nagarkar, A.; Lad, V.; Patil, U.; Juvekar, S.; Sinkar, V.; Shouche, Y. Understanding the association between the human gut, oral and skin microbiome and the Ayurvedic concept of prakriti. J Biosci 2019, 44 (5), 112. [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J. R.; Pfeffer, K.; Coffer, P. J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504 (7480), 451-455. [CrossRef]
- Belkaid, Y.; Hand, T. W. Role of the microbiota in immunity and inflammation. Cell 2014, 157 (1), 121-141.
- Li, S.; Jin, M.; Wu, Y.; Jung, S.; Li, D.; He, N.; Lee, M. S. An efficient enzyme-triggered controlled release system for colon-targeted oral delivery to combat dextran sodium sulfate (DSS)-induced colitis in mice. Drug Deliv 2021, 28 (1), 1120-1131.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).