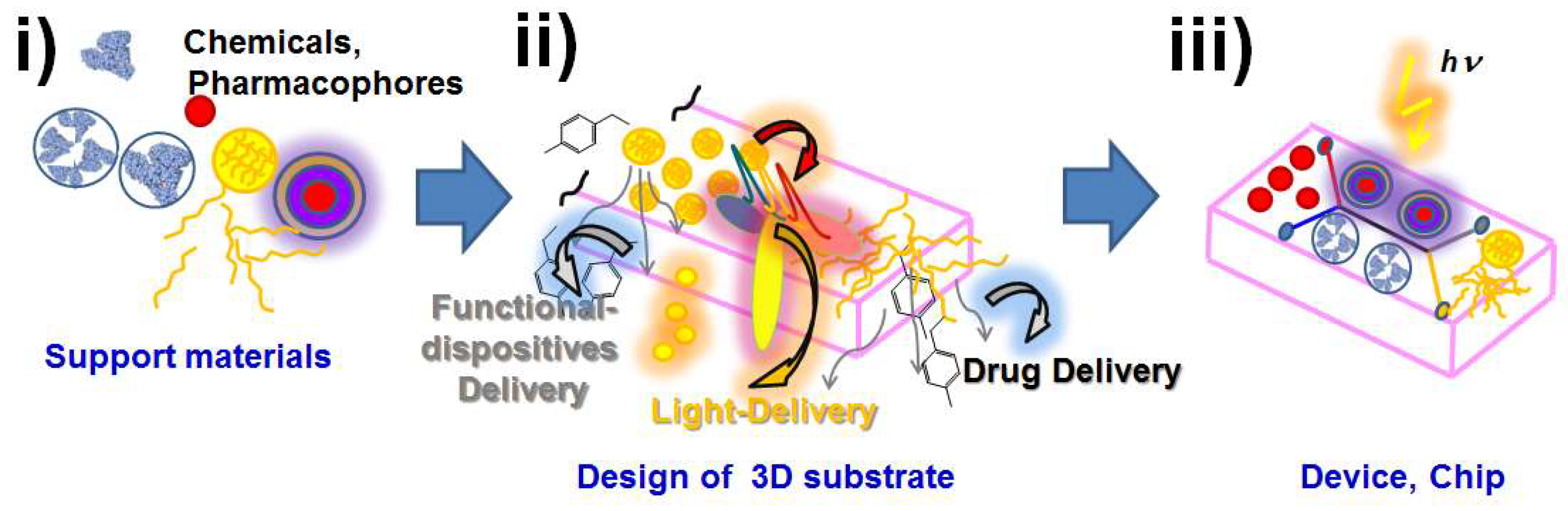
1. Introduction to functional materials by a hierarchical bottom-up design from molecules towards nanoplatforms and higher sizes of modified substrates
There is a broad spectrum of research into functional nanomaterials for varied applications.1 Research focuses on specific functions or multifunctions of nanomedicine2 such as precision medicine3 by genomic approaches4 or early diagnosis5 for a proper drug administration6. Particular interest has been paid on the control of chemistry to manipulate chemical bonds to design new pharmacophores7 and? Mimetic synthetic membrane ligands such as coronavirus variants8, and switch on/off molecular system to be activated by different strategies9 for personalized treatments in oncology10.
Here, a new proposal was developed by incorporating the control of the nanoscale11 looking for functional nanoplatforms within colloidal dispersions12. Nanochemistry allowed designing differently sized platforms in close contact and related sizes with cells and membrane components from where controlled interactions were required.13 They were assayed on nanoplatforms to provide them with an aggregated value from the perspective of the life sciences.14 Therefore new research areas were addressed. In particular we aimed at translating studies and applications in vitro and in vivo. In view of this, we developed different functions, such as drug delivery,15 biosensing16, non-classical light delivery17, bioimaging18, electronics19, 20 and quantum signaling21. However, the factor in common and challenge was focused on how interact at the right place within close distance to achieve the desired function. Hence, right delivery and specific function were also of particular interest. Therefore, we added higher sizes of substrates such as patches22 and modified substrates23 based on variable polymeric compositions24 to support the mentioned properties. A bottom-up design? was developed by a hierarchical material with different components acting in close contact with different types of real tissues such as skin.25 Moreover, surgical procedures or simple injections into deep tissues allowed being in close contact with the desired type of cells.26
Accordingly, the concept of nanodevices towards the bottom up of microdevices to be incorporated by different strategies considers: i) implantable strategies by direct deposition and contact with the targeted tissue27; ii) injections28; iii) deposition or ingestion to be adsorbed through membranes and tissues29, in addition to other types of strategies such as the use of microcapsules30. It is important to underline the role of the specific functions of devices, as well as the support material and strategy to record signalling from the mechanism developed as strategy for the targeted and desired function. In these perspectives, in this short review, we report research on nanomaterials and devices for nanomedicine applications to gain insight into implantable and related strategies to activate the desired function at the right place and time within complex biological systems.
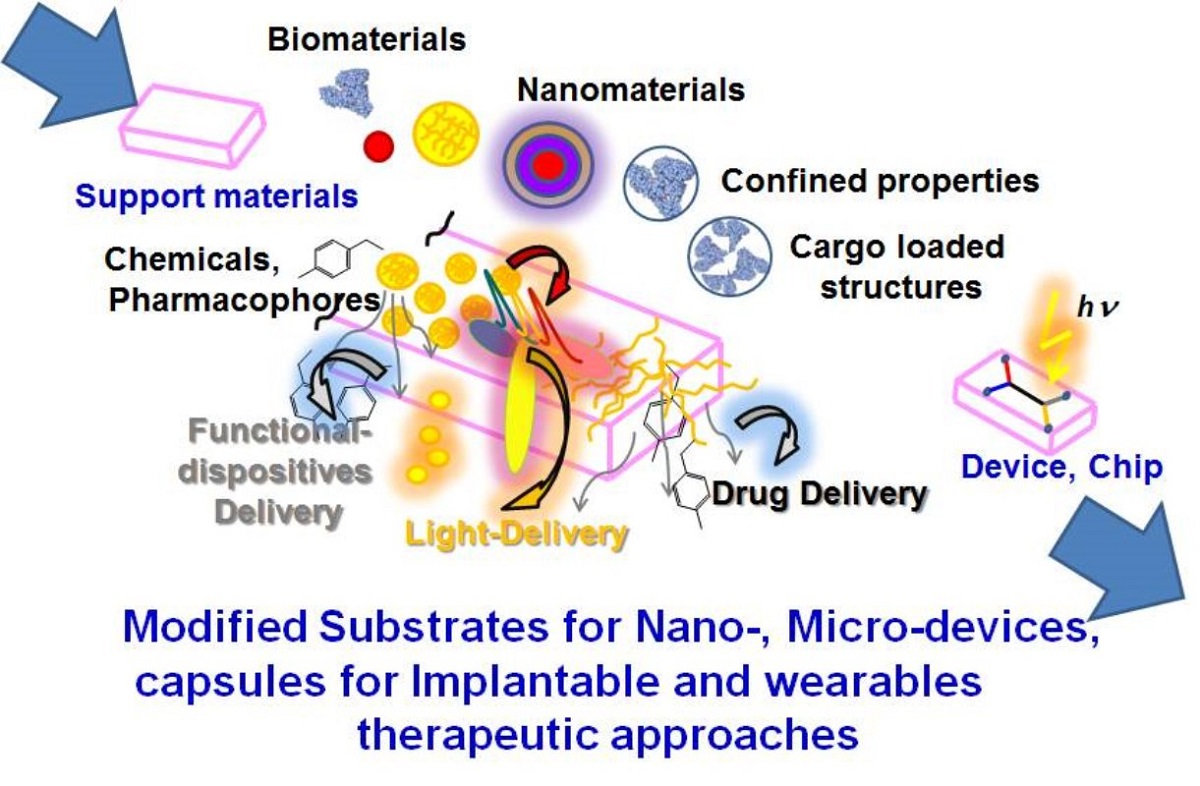
Figure 1.
Modified substrates for nano-, micro-devices, capsules for implantable and wearables therapeutic approaches. Reprinted with permissions from A. Guillermo Bracamonte et al. 2022.
Figure 1.
Modified substrates for nano-, micro-devices, capsules for implantable and wearables therapeutic approaches. Reprinted with permissions from A. Guillermo Bracamonte et al. 2022.
2. From the concept of nanomedicine to nano-, micro-, and higher sizes of modified substrates for new treatments and therapeutics
The design and control of synthesis of nanoparticles allowed opening new opportunities for fundamental research into the development of nanodevices, microdevices, and encapsulated nanomaterials with specific functionalities.31 Similarly, nanomaterials within colloidal dispersions by injectable administration32 have aroused particular interest. Likewise, new nanomaterials and new ways of administration are being developed. The incorporation of higher sizes of modified substrates such as polymeric materials modified with functional systems,33 nanoplatforms with switch on/off applications34 and other versatile and controllable approaches are in progress.
Literature has shown the design of lab-on particle35 and cargo-loaded nanoparticles36 and modified substrates such as patches. For instance, gene therapy was conducted by embryonic cell modifications with CRISP (clustered regularly interspaced short paliromic repeats)37. This technique was based on enzymatic scissors capable of modifying genetic codes by the correction of β-thalassemia mutants related to hemoglobin production disorders38. These examples show the particular need to administrate and facilitate different approaches and nanomaterials, such as bioconjugated gold nanoparticles for precision nanomedicine developments as important nanoplatforms for further modification.39 Recently, a thermo triggered release of CRISPR Cas9 System was developed by lipid encapsulated gold nanoparticles for tumor therapy.40 Despite ethical issues, this technique was shown to modify baby born new cells with interesting results.41 Thus, here we describe the design and synthesis of cargo-loaded nanoparticles based on diverse polymeric materials that act as a support and form part of the therapy strategy. Different polymeric materials showed versatile properties such as poli-lactic, malic, and galic, and related block polymers. 42
In addition, the control of the nanoscale permitted to develop studies in vivo applications in photodynamic therapy43 and related photo-stimulated phenomena in catalysis, molecular and biostructure degradation.44 Note also the design and synthesis of chemical agents such as photosensitizers and modified substrates with photo-active properties.45 Recent developments of Plasmonics materials showed high-energy electromagnetic field generation and interaction within the close surrounding.46, These effects were produced from the near field within shorter nanoscale lengths towards the far field. However, since the control of parameters and factors is still being sought, studies focuses on this and the photodegradation of molecules and membranes. The increasing need to develop high-sensitive and smart responsive materials for early diagnosis and therapeutics has opened up other research areas centered on the incorporation of nano-architectures within solid substrates and polymeric films to be then transferred to patches, devices, and wearables.47 Accordingly, control from the nanoscale to higher sizes of modified nano-composites and substrates in the microscale and higher dimensions is key in the design of new technological approaches. Further research could also lead to the miniaturization of instrumentation48 and new approaches such as implantable opto-active instruments, modified fiber optics49, fiber optic probes50, and optrodes51with transferable knowledge to propose technology on the market as in miniaturized endoscopes52 and optical probes53.
3. Micro-capsules for biosensing and drug delivery
The concept of encapsulation in the design of microdevices and nanodevices could be considered by a design of protected functional system at the molecular level or towards higher sizes within a capsule54. The functional system could be developed? from a chemical sensor, optoactive material, or related smart responsive systems applied to life sciences.55 As well it could be contemplated Nanosensors and related Nanotechnology.56 The objective of this protection by encapsulation is to prepare the functional system to respond only to the particular tissue or cell. The encapsulation protects the device against media modifications, improving administration at the right place. Moreover, the encapsulated material could be deposed by different ways of administrations. Thus, microcapsules or capsules in the macro, and micro-scale could be placed in the right tissue manually, by injection, by deposition, sticking them with adhesives films. In addition, the microcapsule after being incorporated in a specific tissue could flow through cells across membranes, and further distributions.57 Thus, independently of its administration or deposition, the capsule protects, and acts as a cargo vehicle, assisting to achieve the targeted function at the right place. Thus, the functional material then acts more efficiently than in the absence of encapsulation.
Some examples of the precise deposition of functional materials with highly sensitive properties can also be found on the market, as in deposition by injection of optical lens on human eyes.58 By focusing on the design and material, different inorganic/organic materials could be used when combining inorganic salts and polymeric materials, such as thin polymeric multilayer films formed from deposition of alternated and charged inorganic/organic multilayers.59 This method has allowed producing multilayered assemblies of polyelectrolyte films on flat substrates from solution. This methodology also allows affording spherical hollowed particles60 that can be modified as cargo-loaded particles with acceptable biocompatibility. Other cases include preloading particles based on co-precipitation methods and post-loading adsorption on hollowed particles. From the pre-loading method, pH responsive dyes61 were encapsulated, acting as novel in vivo confined pH sensors in aquatic organisms62.
Nanoplatforms and microplatforms used to encapsulate smart responsive materials include: i) varied pH sensors63; ii) multicoloured silica nanoplatforms for non-classical light generation and delivery64; iii) enhanced pH sensing by plasmonic phenomena such as MEF.65, 66; and iv) cargo-loaded nanoparticles67 leading to advanced in vivo applications68. These cases show the importance of each part of the designed nanoarchitecture, addressing i) flow within biological fluids, ii) incorporating into cells across membranes,69 and iii) specific action activation of the targeted function70, 71 (Figure 2).
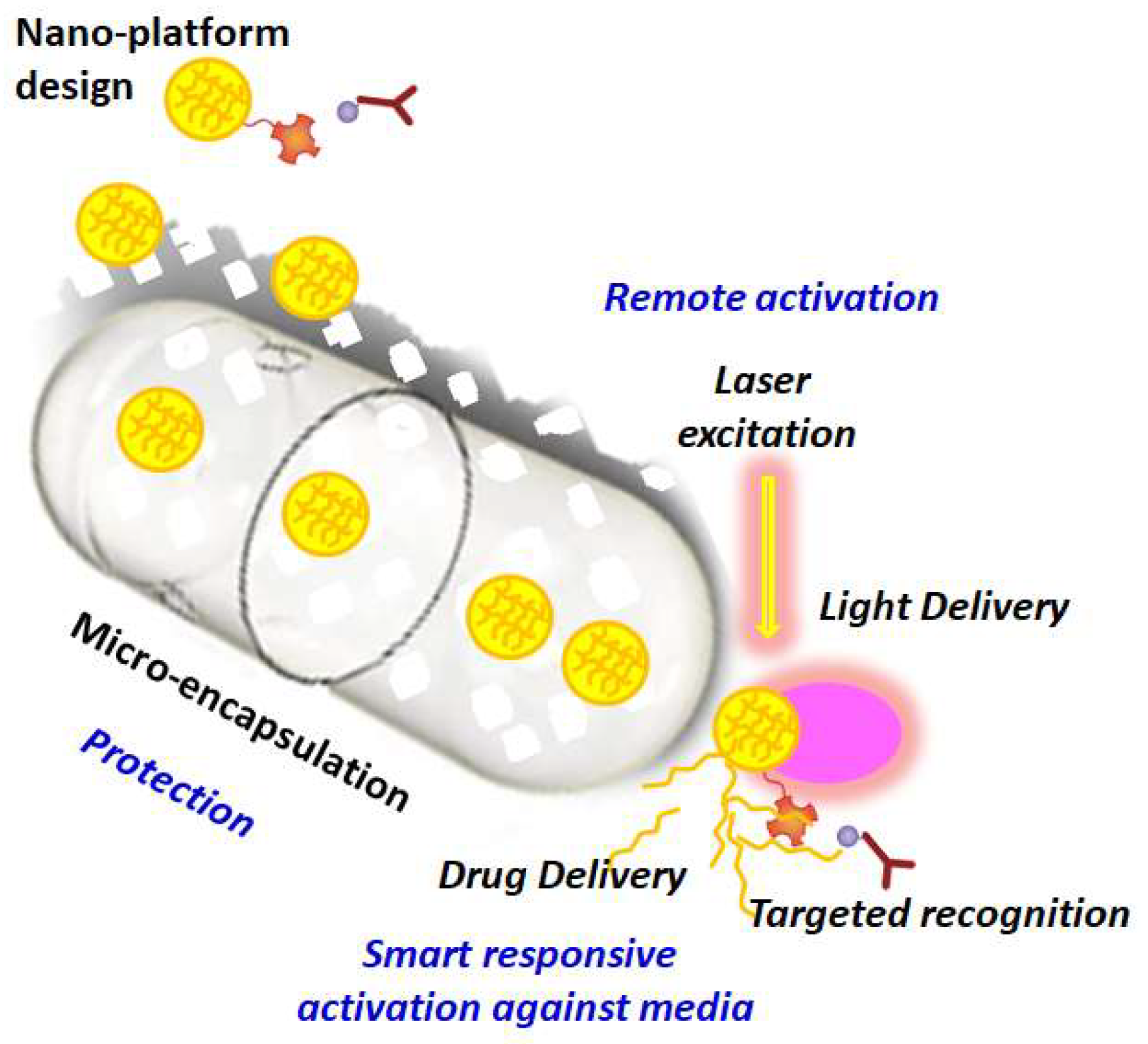
Figure 2.
Schematic representation of nano-loaded micro-capsules for biosensing and drug delivery applications using diverse functional activation strategies. Reprinted with permissions from A. Guillermo Bracamonte et al. 2022.
Figure 2.
Schematic representation of nano-loaded micro-capsules for biosensing and drug delivery applications using diverse functional activation strategies. Reprinted with permissions from A. Guillermo Bracamonte et al. 2022.
4. Implantable, portable, and wearable devices from cutting-edge knowledge to future treatments
After considering the control on the molecular scale and nanoscale towards modified substrates with higher sizes and specific functions, it could be proposed the deposition of the device on different tissues depending of needs in order to look for early diagnoses, biosensing and drug delivery. It is then possible to propose new treatments from precision medicine, where fundamental research and translational clinical applications are highly required. Material composition and biocompatibility to avoid immune reactions to the deposed material should be viewed as a device. This device could vary in size according to the application required. In these perspectives, it is known different substrate materials for implantable devices and miniaturized instrumentation.72 Similarly, wearable designs such as thin slides containing highly sensitive points of contact with the tissue provide signal tracking from biological variations.
For the design of a modified substrate for specific functions, different types of signaling such as light, electronics, quantum, electrical properties, electromagnetic fields, thermal and chemical signals should be considered. All controllable physical and chemical properties are of interest to transduce through space and time. However, being able to record weak signaling or amplify them through amplification strategies still proves challenging. And this is not as easy to achieve from the bulk of synthetic materials or synthetic real matrixes as well. So there are a lot of work involucred that should be developed for each design and targeted function. Moreover, it should be mentioned that this focused interest on Nanotechnology lead later with ongoing insights and new roles of devices and data recording In Vivo and In Real time for remote sensing, and diagnoses.73 Thus, it should be highlighted the Internet of Nano things in healthcare transformation by the cutting edge of Technology in progress.74
Light, managing photons and their transmissions through space and time are particularly useful to develop implantable devices. In addition, other variables should be controlled at the same time, such as biocompatibility of the materials added. Thus, photonic phenomena should be adapted to the desired application. Tuning photons and non-classical light should be compatible with tissues in order to avoid immune responses. Thus, new biomaterials are currently in progress and should be evaluated for new implantable substrates and related applications, as in signalling for biosensing and delivery treatments. In all these complex processes, interferences and background from tissues need to be considered.
It is particularly interesting to develop biomaterials in search of new substrates as in tuning light within waveguides75, transducing signalling, delivering light or even producing laser properties76. Here, plasmonics and control of high electromagnetic fields within the near field towards the far field are under study. 77 These enhanced properties could be generated from varied physical and chemical properties as well as coupling varied phenomena associated. As example it could be highlighted how it was designed a nano-bio-sensor for low concentration levels of DNA detection based on Metal Enhanced Fluorescence (MEF) coupled to Fluorescence Resonance Energy Transfer (FRET).78 In addition, recent studies provide insights into light coupling and transmission through the space, addressing different photo biological receptors in unicellular microorganism, probably developed from Bi-coloured FRET nano-emitters.79 And this is based on the effect of energy levels and consequent properties by interaction with electromagnetic fields. The electromagnetic fields could be generated from the molecular scale to the nanoscale varying in nature and intensity, which could also reach the quantum level80. Thus, it could be considered that the field is so high and versatile for tuning further Opto-active properties. Note that many of these studies were inspired from the 1946 Nobel Prize in Physics awarded to the study of spontaneous electromagnetic fields generated from metallic particles.81 It means that these phenomena are relatively new and still studied on the nanoscale and beyond.
Plasmonic devices and enhanced photodetectors, the basis of new technology, have also been developed, in addition to new strategies in remote switch on/off laser-based applications and miniaturized instrumentation such as Light-Emitting Devices (LEDs), related OLEDs, and new Plasmonics OLEDS (P-OLED) joined to mini-Optical set ups or within reduced sizes of pre-designed structures. These are new developments of biomaterials with biodegradable properties for biophotonics and implantable applications 82, such as flexible bioelectronic devices from conductive polymer based on living materials.83
In addition to control photons, the development of nano-electronics and improved electrical signalling is under study to record weak signals from tissues as in term implants with highly sensitive sensors and implants having wireless myoelectric sensors in their re-innervation sites after targeted muscle reinnervation (TMR). 84 The TMR amplifies the electrical activity of nerves at the stump of amputees by redirecting them in remnant muscles above the amputation. This signaling could be collected and transduced to prosthetics. This technology is based on highly sensitive surface electrode developments using implanted systems. This is a higher level of signal transductions from molecular transductions and electrical signals. Thus, signal was transduced from confined molecular levels towards longer lengths and scales, affording long-term implants of intramuscular sensors and nerve transfers for wireless control of robotic arms in above-elbow amputee.
Other highly sensitive implants and portable devices were reported from neuroscience such as neurophotonics, neuroimaging, and neuromedicine, in addition to portable miniaturized instrumentation at different levels; such as mini-microscopes and mini-endoscopes. Then, higher sized approaches and instruments for Bioimaging afforded to get connections between neurons in vivo85; from where, ion, molecular and neurotransmitter detections were recorded by a proper combination of miniaturized optical set-ups and molecular sensors, labellers, and nano-platforms.86 Therefore, it was even scaled up the applications to replace the upper part of the human skull with a biocompatible, re-chargeable re-fillable, and re-cleanable electrical/molecular device to safely and effectively treat and/or cure severe and currently intractable brain disorders87
Nano-chemistry and new modified substrates could lead to implantable approaches and wearable designs. Research into biosensing in cells for molecular targeting and tracking, as well as ions and neurotransmitters detections.88 Then, if this level of signal transduction is amplified, enhanced or improved by some strategy through space and time, and ideally by multimodal imaging approaches, it could lead to new technology. Examples of technology already developed and placed on the market, include portable PCR chips89, lab on-particles for early diagnosis of SARS CoV 290, and next-generation Sequencing(NGS)91 technology. The perspective of developments is particularly stimulating in this multidisciplinary research field (Figure 3).
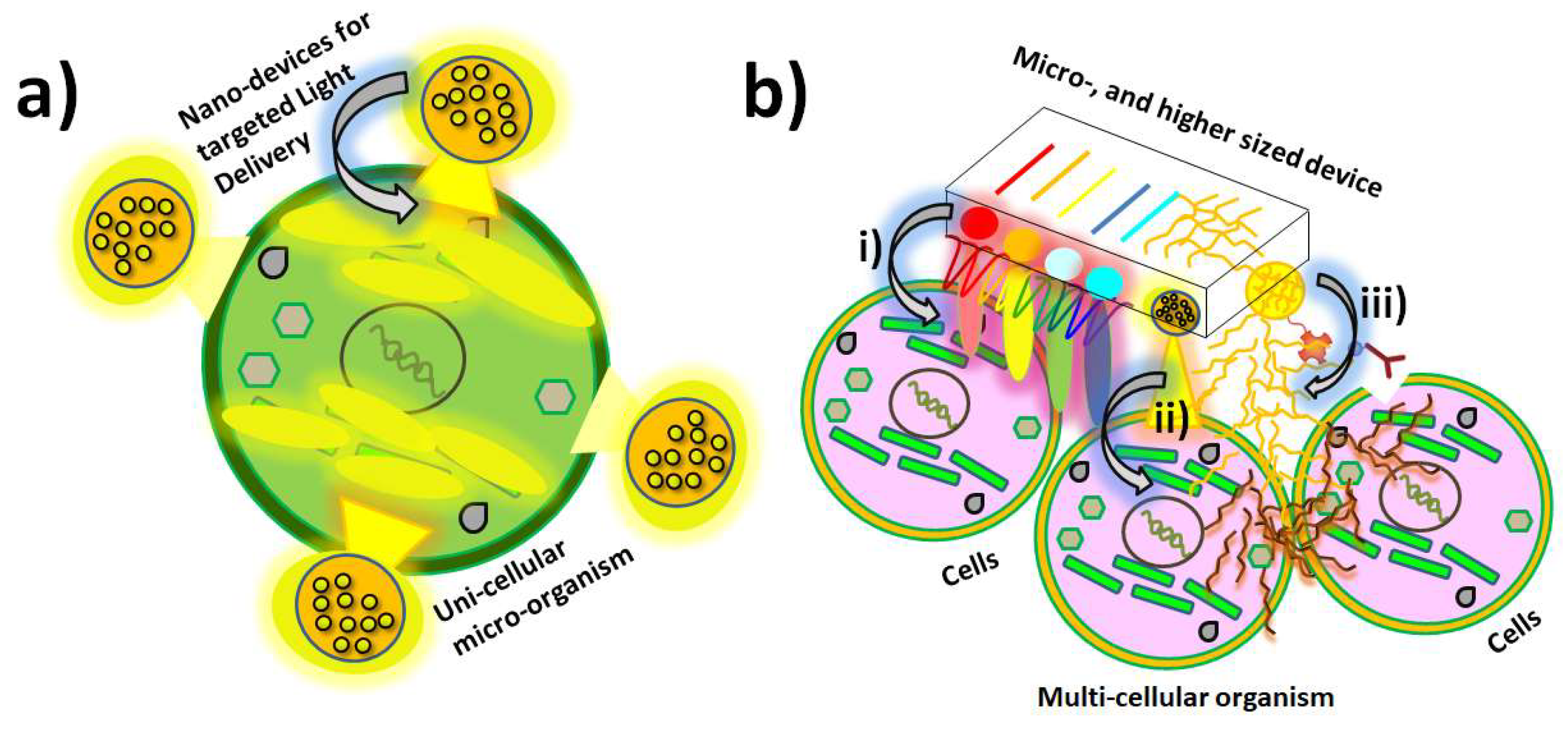
Figure 3.
Schematic representation of different device applications within variable sizes: a) Nano-device placed on uni-cellular micro-organism; and b) multifunctional micro-and higher sizes of devices interacting with a specific multi-cellular organism or tissue for i) multi-modal energy delivery modes, ii) multi-photon delivery, and iii) drug delivery based on variable design of modified substrates. Reprinted with permissions from A. Guillermo Bracamonte et al. 2022.
Figure 3.
Schematic representation of different device applications within variable sizes: a) Nano-device placed on uni-cellular micro-organism; and b) multifunctional micro-and higher sizes of devices interacting with a specific multi-cellular organism or tissue for i) multi-modal energy delivery modes, ii) multi-photon delivery, and iii) drug delivery based on variable design of modified substrates. Reprinted with permissions from A. Guillermo Bracamonte et al. 2022.
5. Conclusions and perspectives
In this communication we discussed the design of new substrates for devices focused on implantable and wearable applications. From the concept of functional materials towards the control of the nanoscale we have shown the development of applications such as nanodevices and microdevices. Many of these new nanomaterials could be incorporated in substrates improving performance. In addition, we have discussed the concept of microencapsulation to protect functional materials against interferences and biological media. This showed to be an especially interesting approach to activate functions at the targeted right place and time. Therefore, it arouses interest in biosensing such as pH in cells in vivo and in drug delivery applications. The combination of biocompatible materials and controlled physical and chemical properties could develop particular functions. Plasmonics and coupled phenomena have been shown to enhance signalling, thermal activations, and laser properties. Likewise, developments of implantable technology based on biocompatible photonic substrates.92, 93 In addition, electrical signalling detection and molecular tracking applied to tissues and organs as in biological event detections to generate bioimaging, early diagnosis, and also to optimize mechanical movements by prosthesis. Hence, there is a broad spectrum of needs and interests that should be addressed by the next generation of therapeutics and new precision medicine accessed through implantable devices and encapsulated multi-functional therapeutic nano- and micromaterials.94,
Data Availability Statement
Further information is available up on needs is provided by correspondent author.
Acknowledgments
We gratefully acknowledge the Département de Chimie and Centre d’Optique, Photonique et Laser (COPL), Québec, Canada, for the postdoctoral position held. We also thank the National University of Cordoba (Universidad Nacional de Cordoba, UNC), Argentina, and the National Research Council of Argentina (CONICET) for the research and teaching positions of A. G. B. Special thanks are given to the Secretary of Science and Technology of UNC (SeCyT), Argentina, for the research grant provided. We would also like to thank Prof. Denis Boudreau from COPL, Laval University, Québec, Canada, for the long-standing research Collaboration in progress, as well as to the Canadian Grants received. Thanks to Prof. Cornelia Bohne for the postdoctoral position at University of Victoria, Victoria in Vancouver Island, British Columbia, Canada. We are also grateful to Professor Burkhard König from the Institut fur Organische Chemie, Universitat Regensburg, Regensburg, Germany, for the research visit and lecture given in his laboratory to the author. We thank to Prof. Jessica Rodríguez-Fernández from Prof. J. Feldman Group at Department für Physik und CeNS Ludwig-Maximilians-Universtität, München, for encouraging the author to accept the research visit in Germany. We acknowledge Prof. Nita Sahai from University of Akron, Institute of Polymer Science and Engineering, and NASA Astrobiology Institute, Ohio, United States, for the postdoctoral position. In addition, we would like to thank our visit to Prof. Jesse Greener Laboratory, Département de Chimie, from the CQMF (Quebéc Center for Functional Materials) and CERMA (Center for Research on Advanced Materials), Université Laval, Québec, Canada. We are grateful to Dr. R. Rodríguez-Méndez from the Département de génie civil, Faculté des sciences et de génie, Université Laval Québec, Canada, with whom we shared an entrepreneurship award (9th Challenge of Ideas for Developments of Enterprises, Laval University, Canada). In addition, special thanks are also given to Prof. Valeria Amé from Centro de Investigaciones en Bioquímica Clínica e Inmunología (CIBICI), Departamento de Bioquímica Clínica, School of Chemistry, UNC, Argentina. We are also grateful to Prof. Dr. A. E. Shalan, BCMaterials, Basque Center for Materials and Nanostructures, Bilbao, Spain, and to the undergraduate student E. S. Abu Serea from BCMaterials for the design of the Artwork and related discussions. Finally, especial thanks to are given to Prof. Daniela Quinteros from Unit of Research and Development in Pharmaceutical Technology (Unidad de Investigación y Desarrollo en Tecnología Farmacéutica-UNITEFA), at Departamento de Ciencias Farmacéuticas, School of Chemistry (Dep. of Pharmaceutical Sciences), UNC, and her research group. Special mention must be made of work in collaboration by PhD student Sofia Martinez and researcher Dr. Cecilia Tettamanti in the bioconjugation of nanoparticles, nanophotonics and biophotonics.
Conflicts of Interest
The authors declare no conflict of interest. And the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Y. Sun, Z. Guo, Recent advances of bioinspired functional materials with specific wettability: from nature and beyond nature, Nanscale Horiz., 4 (2019) 52-76. [CrossRef]
- D. Gontero, M. Lessard-Viger, D. Brouard, A. G. Bracamonte*, Denis Boudreau*, Alicia V. Veglia*, Smart Multifunctional Nanoparticles design as Sensors and Drug delivery systems based on Supramolecular chemistry, Microchemical Journal, 130 (2017) 316-328. [CrossRef]
- R. Hodson, Precision Medicine, Nature Outlook, 537, 7119 (2016) 1-2.
- P. Liang C.i Ding, H. Sun, X. Xie, Y. Xu, X. Zhang, Y. Sun, Y. Xiong, W. Ma, Y. Liu, Y. Wang, J. Fang, D. Liu, Z. Songyang, C. Zho, J. Huang, Correction of β-thalassemia mutant by base editor in human embryos, Protein Cell 2017, 8(11):811–822. [CrossRef]
- J. R. Clegg, A. S. Irani, E. W. Ander, C. M. Ludolph, A. K. Venkataraman, J. X. Zhong, N. A. Peppas, Synthetic networks with tunable responsiveness, biodegradation, and molecular recognition for precision medicine applications, Sci. Adv 5:eaax7946 (2019) 1-15. [CrossRef]
- E. Zeggini , A. L. Gloyn , A. C. Barton , L. V. Wain, Translational genomics and precision medicine: Moving from the lab to the clinic, Science, 365, 6460 (2019) 1409-1413. [CrossRef]
- D. Wang , E. Heiss, K. Šmejkal, A. G. Atanasov, Bioactive Molecules and Their Mechanisms of Action, Molecules, 24 (2019) 3752 1-5. [CrossRef]
- M. Syed, A. Cilling et al., J. A. Doudna, Omicron mutations enhance infectivity and reduce antibody neutralization of SARS-CoV-2 virus-like particles, PNAS, 119, 31 (2022) 1-7. [CrossRef]
- H. Yao, Z. Yang, X. Fan, X. Song, J. He, W. Tian, A light-tunable thermoresponsive supramolecular switch with reversible and complete “off–on”/“on–off” conversion, Mater. Chem. Front., 3 (2019) 1168-1173. [CrossRef]
- D. Rosenblum, D. Peer, Omics-based nanomedicine: The future of personalized oncology, Cancer Letters, 352 (2014) 126-136. [CrossRef]
- W. In den Kirschen , W. Hutchinson , A. Guillermo Bracamonte, Conjugation Reactions of Hybrid Organosilanes for Nanoparticles and surface modifications, J. Chem. Res. Adv. (JCRA), 2, 1 (2021) 6-15.
- Huynh Mai, T. Thanh Diep, T. T. Le, V. Nguyen, Advances in colloidal dispersions : A Review, J. of Dispersion Sciences and Technology, 41, 4 (2020) 479-494. [CrossRef]
- L. R. Gomez Palacios, S. Martinez, C. Tettamanti, D. Quinteros, A. G. Bracamonte, Nano-chemistry and Bio-conjugation with perspectives on the design of Nano-Immune platforms, vaccines and new combinatorial treatments, J Vaccines Immunol, 7, 1 (2021) 049-056. http://doi.org/10.17352/jvi.000047.
- H. L. Nguyen, H. Nam Nguyen, H. H. Nguyen, M. Q. Lu, M. H. Nguyen, Nanoparticles: synthesis and applications in life science and environmental technology, Adv. Nat. Sci.: Nanosci. Nanotechnol. 6, 015008 (2015) 1-10. [CrossRef]
- .
- C. Salinas, G. Bracamonte, Design of Advanced Smart Ultraluminescent Multifunctional Nanoplatforms for Biophotonics and Nanomedicine applications, Frontiers in Drug, Chemistry and Clinical Research, 1, 1 (2018) 1-8. [CrossRef]
- D. Dalacu , P. J Poole, R. L Williams, Nanowire-based sources of non-classical light, Nanotechnology 30, 232001 (2019) 1-17. [CrossRef]
- M. Rioux, D. Gontero, A. V. Veglia, A. Guillermo Bracamonte*, D. Boudreau*, Synthesis of Ultraluminiscent gold core-shell Nanoparticles as NanoImaging Platforms for Biosensing applications based on Metal enhanced fluorescence, RSC Adv., 7 (2017) 10252-10258. [CrossRef]
- Guillermo Bracamonte, Advances in new Matter Properties and Applications of Hybrid Graphene-based Metamaterials”. Special Issue: Design and Synthesis of Graphene based Metamaterials, Current Material Science (CMS)-Recent Patents on Materials Science, Bentham Sci. Pub. E-pub Ahead of Print, Oct. BMS-CMS-2020-HT14-2468-3, 14 (2021) 1-4.
- W. Hutchinson, A. Guillermo Bracamonte*, Electronic properties and Pseudo-Electromagnetic Fields of Highly Conjugated Carbon Nanostructures, Special Issue: Design and Synthesis of Graphene based Metamaterials, Current Material Science (CMS)-Recent Patents on Materials Science, Bentham Sci. Pub.. E-pub Ahead of Print, Oct. Manuscript ID: BMS-CMS-2020-HT14-2468-2, 14 (2021) 1-30. [CrossRef]
- L. Nie, A. C. Nusantara, V. G. Damle, R. Sharmin, E. P. P. Evans, S. R. Hemelaar, K. J. van der Laan, R. Li, F. P. Perona Martinez, T. Vedelaar, M. Chipaux, R. Schirhag, Quantum monitoring of cellular metabolic activities in single mitochondria, Sci. Adv., 7, eabf0573 (2021) 1-8. [CrossRef]
- R. Choueiri, E. Galati, H Thérien-Aubin et al., Surface patterning of nanoparticles with polymer patches. Nature 538, (2016) 79–83. [CrossRef]
- L. Meng, T. Zeng, Y. Jin, Q. Xu, X. Wang, Surface-Modified Substrates for Quantum Dot Inks in Printed Electronics, ACS Omega, 4, 2 (2019) 4161–4168. [CrossRef]
- J. Du, Y. Li, J. Wang, C. Wang, D. Liu, G. Wang, S. Liu, Mechanically Robust, Self-Healing, Polymer Blends and Polymer/Small Molecule Blend Materials with High Antibacterial Activity, ACS Appl. Mater. Interfaces 12, 24 (2020) 26966–26972. [CrossRef]
- M Sarah. Elsayed, V. Tanda Widyaya, Y. Shafi, A. Eickenscheidt, K. Lienkamp, Bifunctional Bioactive Polymer Surfaces with Micrometer and Submicrometer-Sized Structure: The Effects of Structure Spacing and Elastic Modulus on Bioactivity, Molecules 2019, 24, 3371 1-22. [CrossRef]
- F.CellesiN.Tirelli, 12 - Injectable nanotechnology, Injectable Biomaterials, Science and Applications, Woodhead Publishing Series in Biomaterials (2011) 298-322.
- Z. Cheng, C. R. Shurer, S. Schmidt, V. K. Gupta, G. Chuang, J. Su, A. R. Watkins, A. Shetty, J. A. Spector, C.-Yuen Hui, H. L. Reesink, Matthew, J. Paszek, The surface stress of biomedical silicones is a stimulant of cellular response, Sci. Adv. 6 , eaay0076 (2020) 1-12. [CrossRef]
- Y. Li, X. Chen, R. Jin3, L. Chen, M. Dang, H. Cao, Y. Dong, B. Cai, G. Bai, J. J. Gooding S. Liu, D. Zou, Z. Zhang, C. Yang, Injectable hydrogel with MSNs/microRNA-21-5p delivery enables both immunomodification and enhanced angiogenesis for myocardial infarction therapy in pigs, Sci. Adv., 7, eabd6740 (2021) 1-19. [CrossRef]
- J.M. Rabanel, V. Aoun, I. Elkin, M. Mokhtar and P. Hildgen, Drug-Loaded Nanocarriers: Passive Targeting and Crossing of Biological Barriers, Current Medicinal Chemistry, 19 (2012) 3070-3102. [CrossRef]
- N.V.N Jyothi, P.M Prasanna, S.N Sakarkar, K.S. Prabha, P.S., Ramaiah, G.Y. Srawan, Microencapsulation techniques, factors influencing encapsulation efficiency. J. Microencapsul. 27 (2010) 187–197. [CrossRef]
- Guillermo Bracamonte, Book entitled: “Frontiers in Nano- and Micro-device Design for Applied Nanophotonics, Biophotonics and Nanomedicine”, Chapters 1-15, Bentham Science Publishers, ISBN: 978-1-68108-857-0 (Print); 978-1-68108-856-3 (Online) © 2021, Bentham Science Publishers (UAE) (2021) 1-200. https://benthambooks.com/book/9781681088563/.
- Y. Yin, W. Su, J. Zhang, W. Huang, X. Li, H. Ma, M. Tan, M. Tan, M. Tan, H. Song, G. Cao, S. Yu, D. Yu, J. Hoon Jeong, X. Zhao, H. Li, G. Nie, H. Wang, Separable Microneedle Patch to Protect and Deliver DNA Nanovaccines Against COVID-19, ACS Nano 2021, 15, 9, 14347–14359. [CrossRef]
- K. Isayama, N. Aizawa, J. Y. Kim, T. Yasuda, Modulating Photo- and Electroluminescence in a Stimuli-Responsive p-Conjugated Donor–Acceptor Molecular System, Angew.Chem.Int. Ed., 57 (2018) 11982–11986.
- Guillermo Bracamonte*, D. Brouard, M. Lessard-Viger, D. Boudreau*, A. V. Veglia Nano-supramolecular complex synthesis: switch on/off enhanced fluorescence control and molecular release using a simple chemistry reaction, Microchemical Journal, 128 (2016) 297–304. [CrossRef]
- Martin Ame, S. A. Serea, A. Shalan, A. G. Bracamonte*, Detection of Viruses and Development of New Treatments: Insights into Antibody-Antigen Interactions and Multifunctional Lab-On-Particle for SARS CoV-2”, J Nanotechnol Nanomaterials, Scientific Archives (Creative Commons Attribution License), 2, 2 (2021) 67-75. [CrossRef]
- C. I Salinas, A. G. Bracamonte, Design of advanced smart ultraluminescent multifunctional nanoplatforms for biophotonics and nanomedicine applications, Frontiers Drug Chemistry Clinical Res, 2018 - 1(1): 1-8. [CrossRef]
- P. Liang, Y. Xu, X. Zhang, C. Ding, R. Huang, Z. Zhang, J. Lv, X. Xie, Y. Chen, Y. Li, Y. Sun, Y. Bai, Z. Songyang, W. Ma, C. Zhou, J. Huang , CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes , Protein Cell, 6, 5 (2015) 363–372. [CrossRef]
- P. Liang, C. Ding, H. Sun, X. Xie, Y. Xu, X. Zhang, Y. Sun, Y. Xiong, W. Ma, Y. Liu, Y. Wang, J. Fang, D. Liu, Z. Songyang, C. Zhou, J. Huang, Correction of β-thalassemia mutant by base editor in human embryos, Protein Cell, 8, 11 (2017) 811–822. [CrossRef]
- P. Wang, L. Zhang, W. Zheng, L. Cong, Z. Guo, Y. Xie, Le Wang, R. Tang, Q. Feng, Y. Hamada, K. Gonda, Z. Hu, X. Wu, X. Jiang, Thermo triggered Release of CRISPR Cas9 System by Lipid Encapsulated Gold Nanoparticles for Tumor Therapy, Angew.Chem. Int.Ed. 57 (2018) 1491–1496. [CrossRef]
- P. Wang, L. Zhang, W. Zheng, L. Cong, Z. Guo, Y. Xie, Le Wang, R. Tang, Q. Feng, Y. Hamada, K. Gonda, Z. Hu, X. Wu, X. Jiang, Thermo triggered Release of CRISPR Cas9 System by Lipid Encapsulated Gold Nanoparticles for Tumor Therapy, Angew.Chem. Int.Ed. 57 (2018) 1491–1496. [CrossRef]
- V. Marx, The CRISPR children, Nature Biotechnology, New feature (2021) 1-5. [CrossRef]
- Y. Yi, J. H. Kim, H. W. Kang, H. S. Oh, S. W. Kim, M. H. Seo, A polymeric nanoparticle consisting of mPEG-PLA-Toco and PLMA-COONa as a drug carrier: improvements in cellular uptake and biodistribution, Pharm Res. , 22, 2 (2005) 200-8. [CrossRef]
- H. Shik, D. Y. Lee, Near Infrared Responsive cancer Photothermal and Photodynamic Therapy using Gold Nanoparticles, Polymers, MDPI, 10, 9 (2018) 961-973. [CrossRef]
- .
- L. Tsakalakos et al., Review: The role of photonics in energy, J. of Photonics for Energy, 5, 050997 (2015) 1-45. [CrossRef]
- S. Yazdi, J. R. Daniel, N. Large, G. C. Schatz, D. Boudreau, E. Ringe, Reversible Shape and Plasmon Tuning in Hollow AgAu Nanorods, Nano Lett. 16 (2016) 6939−6945. [CrossRef]
- C. Salinas, A. G. Bracamonte, From Microfluidics to Nanofluidics and signal Wave-guiding for Nanophotonics, Biophotonics resolution and Drug Delivery, Frontiers in Drug, Chemistry and Clinical Research, 2 (2019) 1-6. [CrossRef]
- M. R. Descour, A. H. O. Kärkkäinen, J. D. Rogers, C. Liang, R. S. Weinstein, J. T. Rantala, B. Kilic, E. Madenci, R. R. Richards-Kortum, E. V. Anslyn, R. D. Dupuis, R. J. Schul, C. G. Willison, C. P. Tigges, Toward the Development of Miniaturized Imaging Systems for Detection of Pre-Cancer, IEEE J. of Quantum Electronics, 38, 2 (2002) 122-130. [CrossRef]
- E. J. Smith, Z. Liu, Y. Mei, O. G. Schmidt, Combined Surface Plasmon and Classical Waveguiding through Metamaterial Fiber Desig, Nano Lett., 10 (2010) 1-5. [CrossRef]
- T. Guo, Fiber Grating-Assisted Surface Plasmon Resonance for Biochemical and Electrochemical Sensing, J. of Light Wave Technology, 35, 16 (2017) 3323-3333.
- S. Dufour, Y. De Koninck, Optrodes for combined optogenetics and electrophysiology in live animals, Neurophotonics, 2, 3, 031205 (2015) 1-14. [CrossRef]
- D. Jacob , A. I. Ramsaran, A. J. Mocle, L. M. Tran, Chen Yan, P. W. Frankland, S. A. Josselyn, A Compact Head-Mounted Endoscope for In Vivo Calcium Imaging in Freely Behaving Mice, Current Protocols in Neuroscience, 84, 1 (2018) 1-4. [CrossRef]
- Y.-Chao Li, H.-Bao Xin, H.-Xiang Lei, L.-L. Liu, Y.-Ze Li, Y. Zhang, B.-J. Li, Manipulation and detection of single nanoparticles and biomolecules by a photonic nanojet, Light: Science & Applications, 5, e16176 (2016) 1-9. [CrossRef]
- Z. Zhang, L. Shang, Smart ingestible devices: orally delivering macromolecules and beyond, Cell Press, 4, 11 (2021) P3379 3381. [CrossRef]
- R. Ramos,J. Bernard, F. Ganachaud, Ali Miserez, Protein-Based Encapsulation Strategies: Toward Micro- and Nanoscale Carriers with Increased Functionality, Small Science, 2, 3 (2022) 2100095. [CrossRef]
- Editor, Vikas Mittal, Encapsulation Nanotechnologies, Print ISBN:9781118344552; Online ISBN:9781118729175; Copyright © 2013 Scrivener Publishing LLC. All rights reserved. [CrossRef]
- Ed. By I. Meglinski, Biophotonics for Medical Applications, Woodhead Publishing series in Biomaterials: number 82, imprint of Elsevier, USA and UK Copyright©2015. ISBN: 978-0-85709-662-3 (print); ISBN: 978-0-85709-674-6 (on-line).
- https://www.zeiss.com/meditec/int/products/iols/monofocal-iols/ct-lucia-family.html.
- R.K. Iler, Multilayers of Colloidal particles, J. of Colloid Interface, Sci., 21 (1966) 569-573. [CrossRef]
- G. B. Sukhorukov, D. V. Volodkin, A. M. Gunther, A. I. Petrov, D. B. Shenoy, H. Muhwald, Porous calciul carbonate Microparticles as templates for encapsulation of bioactive compounds, J. Mater. Chem., 14 (2004) 2073-2085. [CrossRef]
- Kreft, A. M. Javier, G. B. Sukhorukov, W. J. Parak, Polymer microcapsules as mobile local pH-sensors, J. Matter Chem., 17 (2007) 4471-4475. [CrossRef]
- Sadvoy, C. Korzh, M. Escobar, I. Meglinski, Microcapsulated biomarkers for assesment of stress conditions in aquatic organisms in vico, Laser Phys. Lett., 9 (2012) 542-549.
- J. Liu, Y. Huang, A. Kumar, A. Tan, S. Jin, A. Mozhi, X.-Jie Liang, pH-Sensitive nano-systems for drug delivery in cancer therapy, Biotechnol Adv., 32, 4 (2014) 693-710. [CrossRef]
- Salinas, M. Amé, A. G. Bracamonte, Tuning silica nanophotonics based on fluorescence resonance energy transfer for targeted non-classical light delivery applications, J. Nanophoton, 14, 4, 046007 (2020) 1-19. [CrossRef]
- N. Fontaine, A. Picard-Lafond, J. Asselin, D. Boudreau, Thinking outside the shell: novel sensors designed from plasmon-enhanced fluorescent concentric nanoparticles, Analyst, 145 (2020) 5965-5980. [CrossRef]
- J. Asselin, C. Dorais, D. Boudreau, Fluorescencent Nanosensors and uses thereof, United States Patent Application Publication, Pub. No. : US2017/0191935 A1, Pub. Date July 6, 2017.
- J.M. Rabanel, V. Aoun, I. Elkin, M. Mokhtar and P. Hildgen, Drug-Loaded Nanocarriers: Passive Targeting and Crossing of Biological Barriers, Current Medicinal Chemistry, 19 (2012) 3070-3102. [CrossRef]
- Y. Xue, H. Bai, B. Peng, T. Tieu, J. Jiang, Shiping Hao, P. Li, M. Richardson, J. Baell, H. Thissen, A. Cifuentes, L. Li, N. H. Voelcker, Porous Silicon Nanocarriers with Stimulus-Cleavable Linkers for Effective Cancer Therapy, Adv. Healthcare Mater., 2200076 (2022) 1-11. [CrossRef]
- L. Mei , Z. Lu , W. Zhang , Z. Wu , X. Zhang , Y. Wang , Y. Luo , C. Li , Yanxia Jia, Bioconjugated nanoparticles for attachment and penetration into pathogenic bacteria, Biomaterials, 34, 38 (2013) 10328-10337. [CrossRef]
- M. Coccia, Emerging nanotechnological research for future pathways of biomedicine, Int. J. Biomedical Nanoscience and Nanotechnology, 2, 3-4 (2012) 299-319. [CrossRef]
- W. Bao, F. Tian, C. Lyu, B. Liu, B. Li, L. Zhang, X. Liu, F. Li, D. Li, X. Gao, S. Wang, W. Wei, X. Shi, Y. Li, Experimental and theoretical explorations of nanocarriers’ multistep delivery performance for rational design and anticancer prediction, Sci. Adv.; 7 : eaba2458 (2021) 1-15. [CrossRef]
- Parlak, Scott Tom Keene, A. Marais, V. F. Curto, A. Salleo, Molecularly selective nanoporous membrane-based wearable organic electrochemical device for non-invasive cortisol sensing, Sci. Ad ., 4 : eaar2904 (2018) 1-10. [CrossRef]
- Roblyer, Perspective on the increasing role of optical wearables and remote patient monitoring in the COVID-19 era and beyond, Journal of Biomedical Optics, 102703, 25, 10 (2020) 1-9. [CrossRef]
- M. Maksimović, The roles of Nanotechnology and Internet of Nano things in healthcare transformation. Tecnol., .20, 40 (2017) 139-153. [CrossRef]
- K. M. Goodfellow, C. Chakraborty, R. Beams, L. Novotny, A. Nick Vamivakas, Direct On-Chip Optical Plasmon Detection with an Atomically Thin Semiconductor, Nano Lett., 15 (2015) 5477−5481. [CrossRef]
- S. Chen, F. Wang, F. Kuang, S. Kang, H. Liang, Lijing Zheng, L. Guan, Q. Wu, Femtosecond Pulsed Fiber Laser by an Optical Device Based on NaOH-LPE Prepared WSe2 Saturable Absorber, Nanomaterials, 12, 2747 (2022) 1-10. [CrossRef]
- Guillermo Bracamonte, Chapter 28 entitled: “Design of new High Energy near Field Nanophotonic materials for far Field applications”. Book title: Advances in Nanocomposite Materials for Environmental and Energy Harvesting Applications, Part of the Springer Book series: Engineering Materials, ISBN 9783030943189; ISBN 978-3-030-94319-6 (eBook); DOI : 10.1007/978-3-030-94319-6; Series ISSN 1612-1317, ISSN 1868-1212 (electronic); Springer Nature, Switzerland (2022) 859-920. https://link.springer.com/book/9783030943189. [CrossRef]
- D. Brouard, O. Ratelle, A. G.Bracamonte, M. St-Louis, D. Boudreau, Direct molecular detection of SRY gene from unamplified genomic DNA by metal- enhanced fluorescence and FRET, Analytical Methods, 5 (2013) 6896-6899. [CrossRef]
- Salinas, M. Valeria Ame, A. G. Bracamonte*, Synthetic non-classical luminescence generation by Enhanced Silica Nanophotonics based on Nano-Bio-FRET, , RSC Advances, 10 (2020) 20620 – 20637. [CrossRef]
- Yi Fu, Jian Zhang, and Joseph R. Lakowicz, Silver-enhanced fluorescence emission of single quantum dot Nanocomposites, Chem Commun (Camb), 3 (2009) 313-315. [CrossRef]
- M. Purcell, Spontaneous emission probabilities at radio frequencies, Phys. Rev., 69 (1946) 681.
- M. Humara, S. J.J.Kwoka, M. Choi, A. Yetisen, S.Cho, S.-HyunYun, Toward biomaterial-based implantable photonic device, Nanophotonics,6, 2 (2017) 414–434. [CrossRef]
- Z. Wang, H. Bai, W. Yu, Z. Gao, W. Chen, Z. Yang, C. Zhu, Y. Huang, F. Lv, S. Wang, Flexible bioelectronic device fabricated by conductive polymer–based living material, Sci. Adv. 8, eabo1458 (2022) 1-10. [CrossRef]
- S. Salminger , A. Sturma , C. Hofer , M. Evangelista , M. Perrin , K. D. Bergmeister , A. D. Roche , T. Hasenoehrl , H. Dietl , D. Farina and O. C. Aszmann, Long-term implant of intramuscular sensors and nerve transfers for wireless control of robotic arms in above-elbow amputees, Science Robotics , 4, 32, eaaw6306 (2019) 1-8. [CrossRef]
- C. Larivière-Loiselle, E. Bélanger, Pierre Marquet, Polychromatic digital holographic microscopy: a quasicoherent-noise-free imaging technique to explore the connectivity of living neuronal networks, Neurophotonics, 040501, 4 (2020) 1-17. [CrossRef]
- Etsuo A. Susaki et al., Versatile whole-organ/body staining and imaging based on electrolyte-gel properties of biological tissues, Nature Communications, 11, 1982 (2020) 1-10. [CrossRef]
- NandorLudvig, Rationale of replacing the upper part of the human skull with a biocompatible,re-chargeable re-fillable and re-cleanable electrical/molecular device to safely and effectively treat and/or cure severe, currently intractable brain disorders, AcademiaLetters, Article3355 (2021) 1-6. [CrossRef]
- Guillermo Bracamonte, Neurophotonics by controlled signal tracking from chemical structures, and Biostructures towards the Nanoscale and beyond, Frontiers Drug Chemistry Clinical Res., 5 (2022) 1-8. [CrossRef]
- M. Schulz , S. Probst, S. Calabrese , A. R. Homann , N. Borst, M. Weiss, F. von Stetten, R. Zengerle, Nils Paust, Versatile Tool for Droplet Generation in Standard Reaction Tubes by Centrifugal Step Emulsification, Molecules, 25, 1914 (2020) 1-11. [CrossRef]
- U. R. Dahiya, G. Dutt Gupt, R. S. Dhaka, D. Kalyanasundaram, Functionalized Co2FeAl Nanoparticles for Detection of SARS CoV-2 Based on Reverse Transcriptase Loop-Mediated Isothermal Amplification, ACS Appl Nano Mater., 4, 6 (2021) 5871–5882. [CrossRef]
- Guillermo Bracamonte, Microarrays towards Nanoarrays and the Future next generation of sequencing methodologies (NGS), Sensing and Bio-Sensing Research, Elsevier, 37, 100503 (2022) 1-14. [CrossRef]
- Luna R. Gomez Palacios, A. G. Bracamonte, Development of Nano-, Microdevices for the next generation of Biotechnology, Wearables and miniaturized Instrumentation, RSC Adv., 12 (2022) 12806–12822. [CrossRef]
- Luna R. Gomez Palacios, Carina Salinas, Alicia V. Veglia, Maria Valeria Ame, A.Guillermo Bracamonte, Self-assembly dynamics and effect on synthetic nanobio-optical properties by hybrid monocolored silica nanoparticle labeling of Escherichia coli. Journal of Nanophotonics, SPIE, JNP 22039G, 16, 3, 036005 (2022) 1-10. [CrossRef]
- Luna R. Gomez Palacios, A.Guillermo Bracamonte, Generation of Bioimaging towards design of hybrid micro-machines and micro-swimmers, J. Chem. Res. Adv. (JCRA), 3, 1 (2022) 22-27.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).