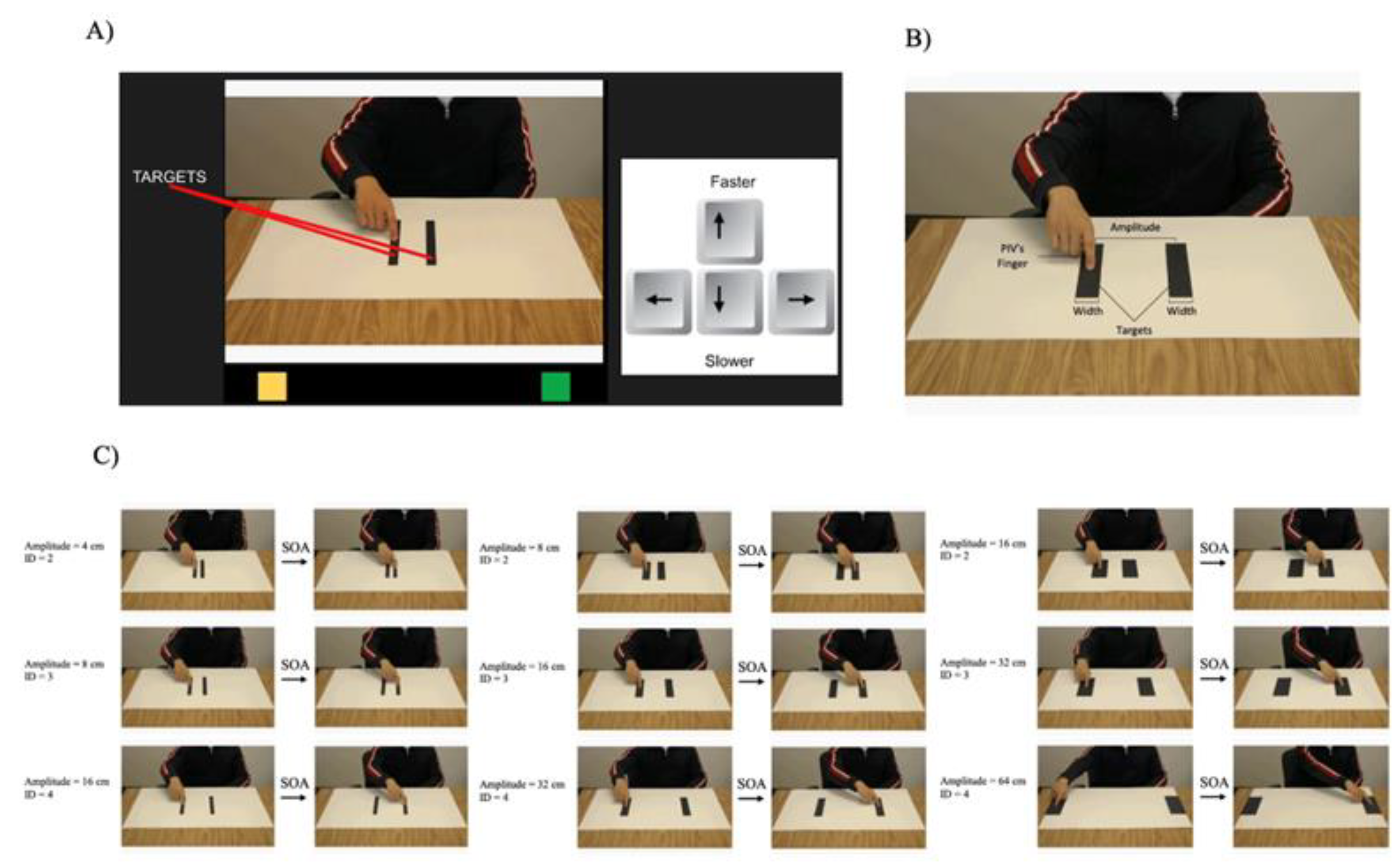
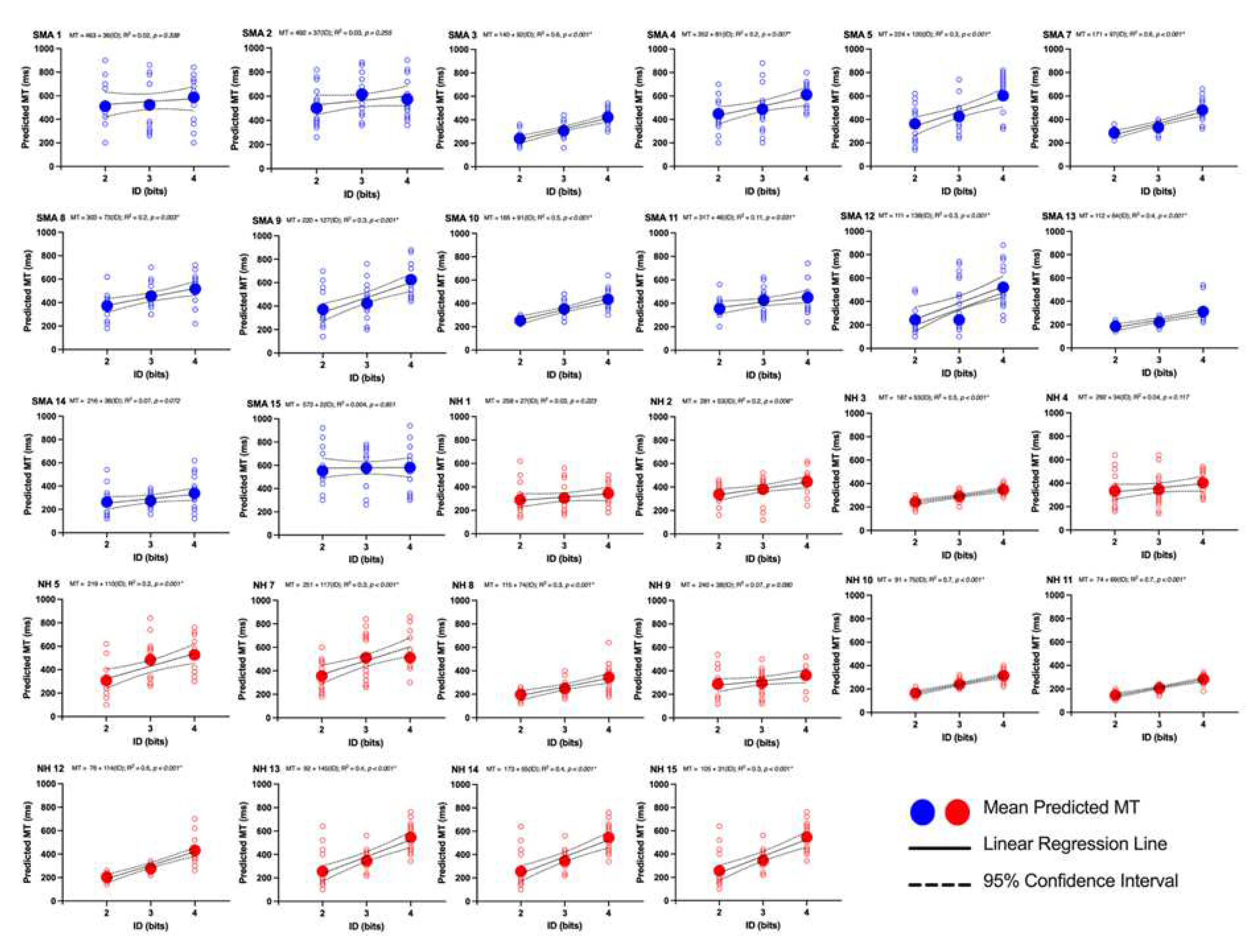
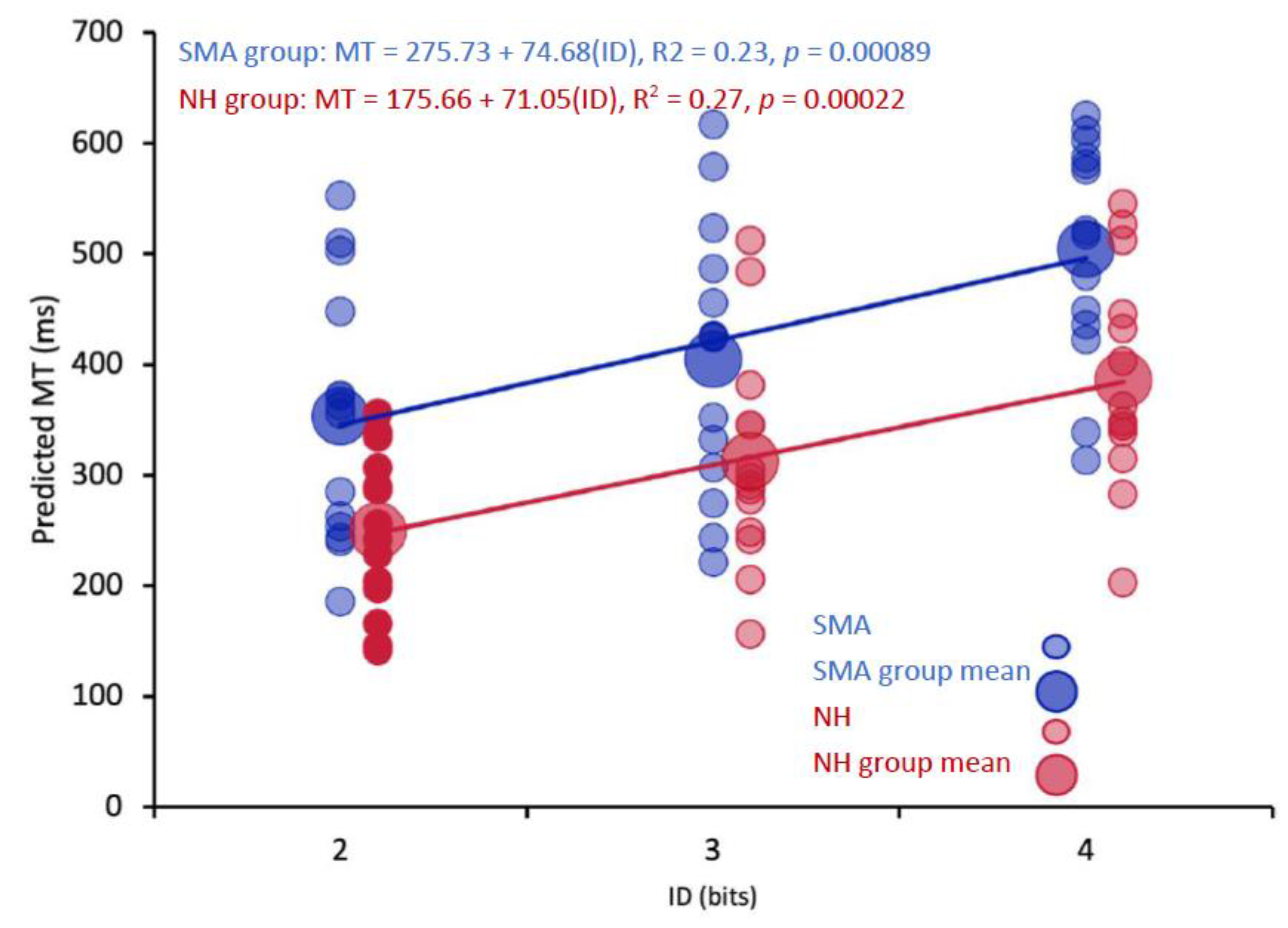
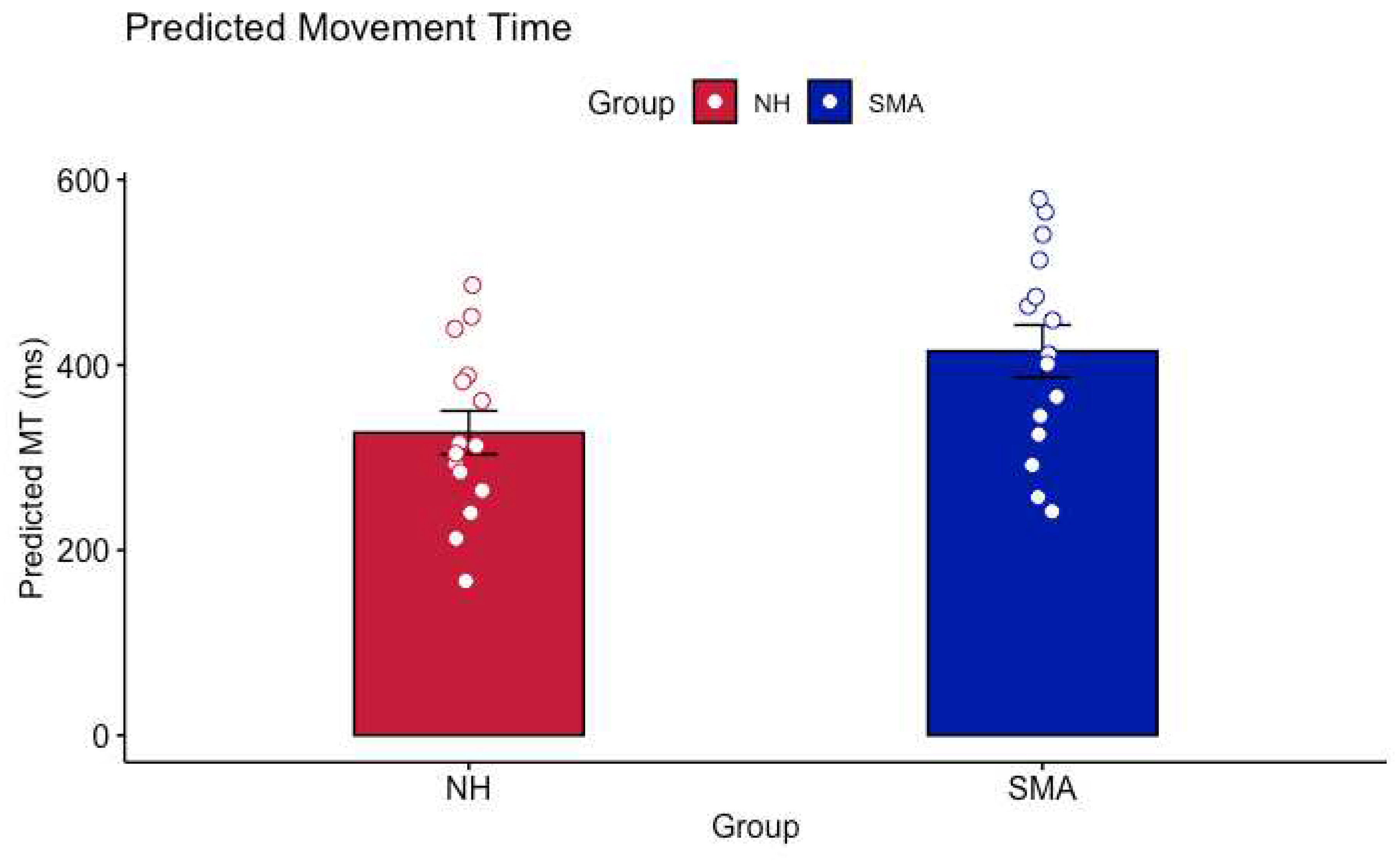
1. Introduction
Making accurate predictions about our own actions and the actions of other individuals is important for effectively interacting with others and our environment. For example, prior to passing an object to a small child, we need to judge if the child can safely hold and maneuver the object. Previous studies have suggested that such action possibility judgements rely on a simulation process that utilizes linked action and perception networks [
1,
2,
3,
4]. Specifically, when asked to make a judgment about another person’s action, the actor first stimulates the action and forms a prediction about their own performance. When predicting for another person, the actor uses their own estimate as a basis, and either adds or subtracts a correction factor based on their perception of the other individual’s characteristics (e.g., [
5]).
Evidence for the use of simulations in action possibility judgements have emerged from studies examining the ability to predict MT based on movement difficulty [
2,
3,
5]. Specifically, these studies used a Fitts’s Law paradigm that characterizes the relationship between MT and movement difficulty for reciprocal upper-limb reaching movements [
2,
3,
5,
6]. The Fitts’s Law equation which captures the relationship between MT and movement difficulty is: MT = a+b (log2[2A/W]), where “a” and “b” are constants that relate one’s baseline MT (y-intercept) and the change in MT for a given change in movement difficulty (slope of the regression line), respectively. To quantify the index of difficulty (ID) of the movement in bits, the equations uses the term: log2[2A/W] [
7]. The ID is therefore a function of the target width (W) and the movement amplitude which is defined as the distance between the two targets (A). In general, as the width of the target decreases and/or movement amplitude increases, ID, and as a result, MT also increases.
Using the reciprocal aiming Fitts’s Law paradigm, Grosjean et al. (2007) investigated if participants’ action possibility judgements about upper-limb movements followed a Fitts’s Law relationship. In this study, participants observed displays of an arm moving between two targets and estimated the MT. As in the classic Fitts’s law experiment, the distance between the targets as well as the width of the targets were varied. The authors found that the shortest estimated MTs corresponded with the lowest ID and as ID increased, MT increased linearly, showing evidence of a Fitts’s Law relationship. The authors concluded that the observation of a Fitts’s law relationship provided evidence that participants were simulating their reach performance before making action possibility judgements.
The finding suggesting that action possibility judgments involve a simulation of the movement being judged leads to several hypotheses. Firstly, it predicts that the motor system plays a crucial role in forming these judgments, indicating a potential influence of one's own motor capabilities. This is supported by experiments showing that judgments about others' actions are affected by recent motor experience and current body state. For instance, Chandrasekharan et al. (2012) observed that participants' predicted MTs were shorter after they had performed the Fitts's law task. Conversely, the authors also found that predicted MTs were longer when participants performed action possibility judgements while wearing a weight on their wrist (altered body state). These findings demonstrate that recent motor task experience and altered body state can modulate the simulation process and influence the magnitude of the action possibility judgements.
Extending the idea that current body state can influence action predictions, Manson et al., 2014 [
3] examined if changes in motor capabilities as a result of neurological injury could influence one’s ability to perform action possibility judgments. In their study, participants with limited upper-limb function due to cervical spinal cord injury (cervical SCI) and control participants with typical upper limb function performed action possibility judgements using the Fitts’s law paradigm. Critically, participants made judgements for both themselves and for a neurologically healthy young adult. The authors found that participants with cervical SCI had significantly longer predicted MTs compared to controls when predicting for themselves; however, there were no differences between participants with cervical SCI and the control group when predicting for the healthy young adult. Furthermore, the authors found that the predicted MTs for both groups followed a Fitts’s law relationship in both judgment tasks.
The authors forwarded two possible hypotheses for their results. First, that individuals with cervical SCI were able form action possibility judgments for the healthy young adult by utilizing intact central action-perception networks that were developed pre-injury. This hypothesis is based on previous studies showing that perception of other’s actions are heavily influenced by prior motor experience (e.g., [
8,
9]). The second hypothesis was that individuals with cervical SCI first simulated their own perceived performance using their unimpaired central action-perception networks, and then modified action possibility judgements based on the perceived differences between themselves and the healthy young adult. This latter, and preferred hypothesis, was based on the finding that pattern of action possibility judgements (i.e., the slope of the relationship between ID and MT) were not different between the self and other action possibility judgement tasks.
The goal of the present study was to challenge the idea that prior motor experience is critical for the action simulation process by assessing whether individuals with limited-to-no upper-limb motor function from birth are capable of forming action possibility judgments for others. To do this, we compared the action possibility judgements of participants with Spinal Muscle Atrophy (SMA) to neurologically-healthy age, and sex-matched controls using the Fitts’s Law paradigm. SMA (specifically type 1, 2, and 3 SMA) is a severely disabling genetic neuromuscular disease that primarily affects motor neurons in the spinal cord, leading to muscle loss and weakness in the upper and lower-limbs (see: [
10]. Symptoms appear from 4-18 months of age and last throughout adulthood. The life expectancy for the most severe forms of SMA (Type 1) is 2 years, whereas people with type 2 and type 3 SMA can live into adulthood. SMA is a rare disease and is estimated to occur in approximately 1 in 6,000 to 1 in 10,000 live births, depending on the geographical region [
11].
It was hypothesized that, if previous motor experience forms the basis of action possibility judgements, then both the predicted MTs and pattern of action possibility judgements would be different in participants with SMA than controls. In contrast, if action possibility judgements are based a more cognitive representation of one’s performance (whether it be real or perceived), then we expect that only the predicted MTs and not the pattern of action possibility judgements would be different between participants with SMA and controls. This second hypothesis considers the importance of prior motor experience (and recent practice) in the modification of the accuracy of judgments, but not the pattern of predictions (see [
5,
6]). Overall, the results of the present study support the latter hypothesis and suggests that prior motor experience may lead to more accurate judgements but may not impact the overall pattern of predictions.
4. Discussion
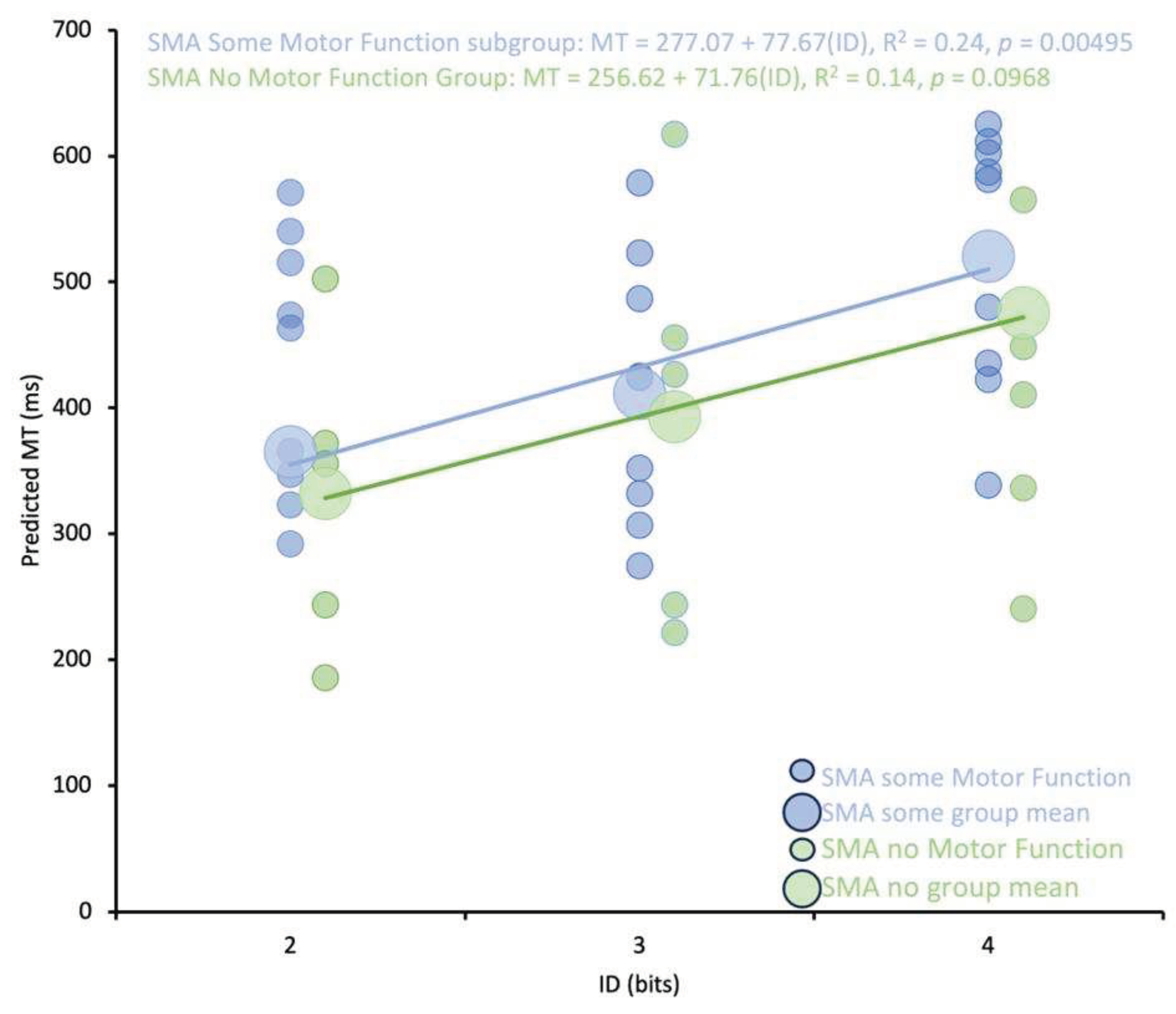
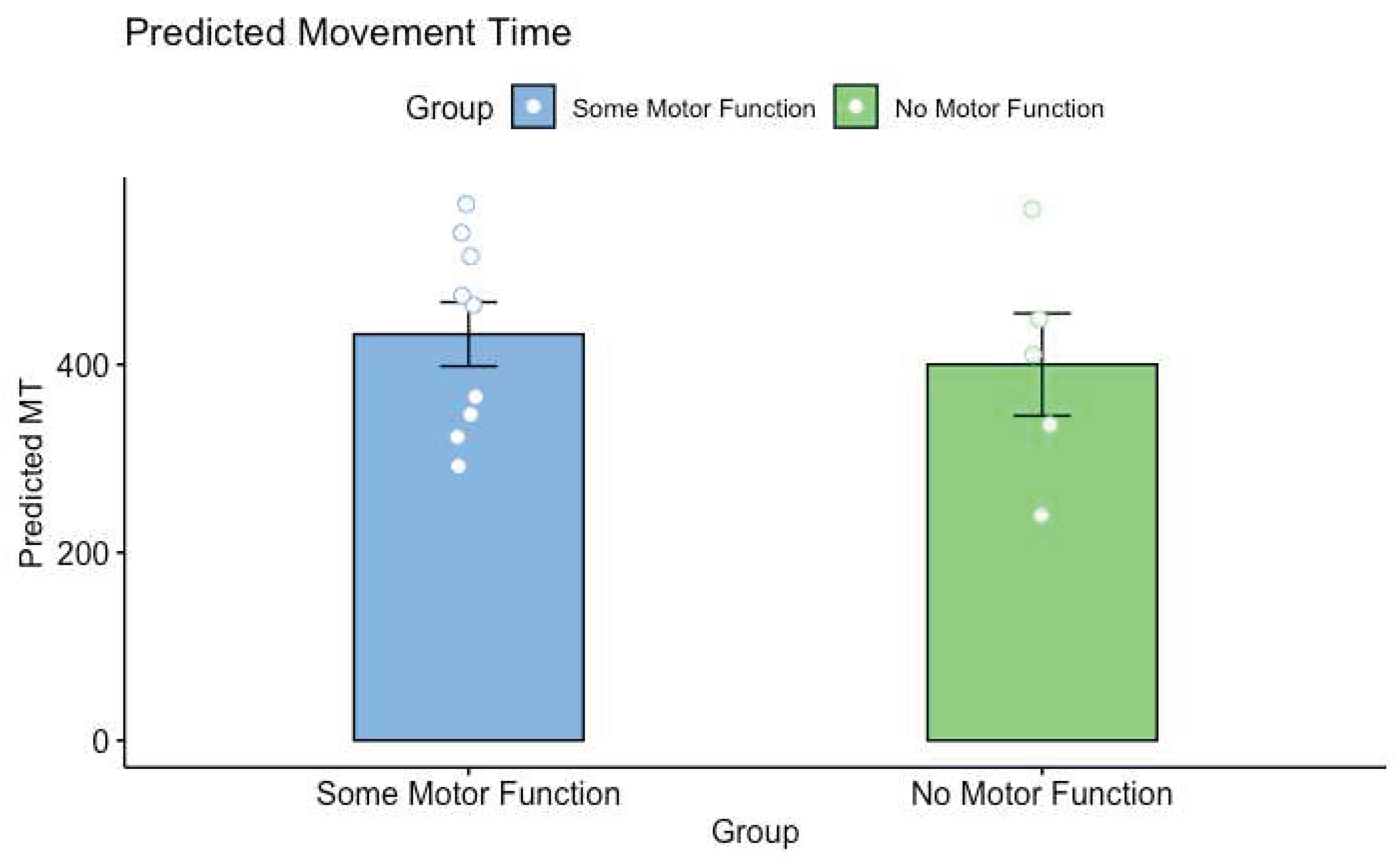
The purpose of this study was to investigate the impact of limited motor experience on the perception of others’ actions. Participants with SMA and NH participants predicted the shortest possible time that a healthy young adult male could perform a reciprocal aiming task. It was found that participants with SMA had significantly longer predicted MTs than NH controls. Critically, analyses of the regression lines at the group level revealed that the differences in MT predictions were driven by higher baseline predictions (i.e., the y-intercepts were higher in the SMA group) rather than differences in the pattern of predictions (i.e., the slope of the regression lines). Thus, the relationship between movement difficulty and predicted MT was not different between participants with SMA and NH controls. Further analyses revealed that there were no differences in predicted MTs between participants with SMA with some motor function and those with no motor function as categorized by the SMAHI. This result suggests that action-perception was not reliably altered by the severity of SMA symptoms.
Overall, our findings provide evidence that central action-perception networks may still be functional in people with limited previous motor experience. Furthermore, simulations using these networks were likely still employed by participants with SMA when making action possibility judgements for others. Finally, our results suggest that although action predictions are informed by previous motor experience, this information may be more important for the fine-tuning of judgements. The following discussion will focus on the impact of limited motor experience on the development of action-perception networks and the formation of action possibility judgements.
In contrast to previous studies (e.g., [
3,
16]), participants with limited motor capabilities, as a result of SMA, predicted significantly longer MTs than control participants when making action possibility judgements for a neurologically healthy individual. One possible explanation for this finding is that the limited motor experience of participants with SMA could have impaired the development of the action-perception networks that are used to form predictions for others. This hypothesis is supported by the findings of Manson et al., (2014) [
3] who demonstrated that participants with limited upper-limb function (as a result of cervical SCI), but previous motor experience (i.e., full arm function prior to injury) were able to adjust their predictions when forming action possibility judgements for healthy individuals.
The importance of previous motor experience in the formation of action-perception networks has also received support from both behavioural and neuroimaging experiments (see: [
8,
9,
17,
18]). For example, Stapel and colleagues used gaze behaviour to determine if children (and adults) with different levels of motor experience could predict the timing and trajectory of different types of locomotion (e.g., crawling, walking), and object translation. The authors found that participants were better at forming predictions for movements that were in participants’ motor repertoires. For example, infants who were proficient crawlers, but not proficient walkers, were better at predicting the timing of crawling than walking (see [
18]).
Although the abovementioned studies provide evidence that previous motor experience likely contributed to the differences in predicted MTs between SMA and control groups, two of our findings suggest that previous motor experience may not be necessary to form reasonable action possibility judgements. First, the predicted MTs of both the SMA and control groups followed a Fitts’s Law relationship where predicted MTs were significantly positively correlated with movement difficulty. Second, comparisons of the slopes of the regression lines between groups of participants revealed that the pattern of predictions, that is, the impact of increases in difficulty on the changes in predicted MTs was no different between groups.
The finding that predicted MTs linearly increased with ID suggests that functional action-perception networks could have been developed in participants with SMA even though they had limited motor experience. Seminal studies that employed the Fitts’s Law paradigm have argued that the presence of a significant correlation between MT and ID (i.e., the Fitts’s Law relationship) indicates that linked action-perception networks were engaged for action possibility judgements (see: [
2,
5,
6,
19,
20]). Furthermore, the absence of a Fitts’s law relationship has been associated with dysfunction in central action-perception networks (see [
19,
20]). Eskanazi and colleagues used the Fitts’s paradigm to investigate if predicted MTs would be impaired in a participant with a frontal brain lesion (i.e., a stroke induced lesion that affected the left inferior, middle, and superior frontal gyri, see [
19]). The author’s found that the participant’s predicted MTs were correlated with movement amplitude, but not scale to the movement’s ID. The absence of a Fitts’s Law relationship provided evidence that the impaired brain regions, which have also been associated with action-perception in the Fitts’s task and other contexts, were not engaged during predictions (see: [
20,
21]. Considering the aforesaid findings, the presence of a Fitts’s Law relationship in the current study lends support to the idea that participants with SMA used functional cortical action-perception networks to make predictions for others.
The hypothesis that functional action-perception networks were employed by participants with SMA to make action possibility judgements is also supported by the finding that there were no reliable differences in the pattern of predictions (i.e., the slopes of the regression lines) between participants with SMA and NH controls. Thus, for both groups, an increase in movement difficulty resulted in a similar increase in predicted MT (see
Figure 3). This pattern of results is similar to those described by Manson et al., (2014) where the authors found that there were no differences in the slopes of the regression lines when participants with cervical-SCI and controls predicted MTs for themselves and a neurologically healthy adult. Critically, there were also no differences in slopes between the cervical SCI and control group when predicting for the neurologically healthy adult. Based on these results, the authors concluded that both groups of participants engaged in a simulation process using linked action-perception networks when predicting the actions of others. Predicted MTs were therefore hypothesized to be derived based on an adjustment that considered the perceived differences between one’s own capabilities and the perceived capabilities of the other actor (see also [
15]). Based the results of the present study, it is hypothesized that participants with limited motor experience as a result of SMA engaged in an action-simulation process that was similar to NH controls. That is, participants with SMA simulated the movement using intact central simulation networks, and then adjusted their judgement based on the perceived differences in capabilities between their simulation and the characteristics of the actor.
The idea that participants with limited previous motor experience could simulate actions that they could not perform themselves contrasts with literature presented earlier about the importance of motor capabilities in the formation of action-perception networks [
8,
9,
17,
18]. However, beyond the results of the present study, further support for this hypothesis can be drawn from studies examining action perception in children with cerebral palsy (e.g., [
22] see also [
23] for a brief overview). For example, Dinomais and colleagues found that temporo-frontal and parieto-occipital brain regions that are active during action observation in adults were similarly activated when children and adolescents with congenital unilateral hemiparesis observed arm movements. Importantly, activation in these action-perception networks was present when children viewed movements of limbs that corresponded to both their unimpaired and impaired limbs. This finding aligns well studies showing that people born without limbs (congenital amputees) also engage motor, and more widespread visual networks when observing other’s limb movements predicting the actions of other’s [
24,
25,
26]. Although no studies have examined action observation in SMA, previous work has shown that visuo-spatial cognitive function is preserved, and can be further enhanced through early locomotor experience, in children with SMA (see [
27,
28]). Although further neuroimaging work is needed, it could be that participants with SMA employed central action perception networks to simulate actions and form action possibility judgements.
Although our results suggest that previous motor experience may not be critical for the formation of action possibility judgements (see also [
25,
26]). Our data do suggests that motor experience could be used to refine the accuracy of action possibility judgements. In studies by Welsh et al., 2013 [
5], and Wong et al., 2013 [
6], the authors found that participants predictions about their own MTs were more closely matched to their actual MTs after physically performing the movement themselves. The idea that previous motor experience can be used to refine judgements could also partially account for the contrasting results between our study and Manson et al., 2014 [
3]. In their study, participants performed the task prior to completing both self-and other action possibility judgements. Although participants with cervical SCI in Manson et al., 2014 only had partial limb function, and performed the task slower than control participants, perhaps recent task experience allowed them to refine their judgement for others. If this is the case, the results of the present study could be initial evidence that passive movement therapy (perhaps with robotic guidance see: [
29,
30] could impact action-perception in people with limited motor capabilities due to SMA.
One limitation of the present study is that we did not collect data about the specific diagnosis of participants (i.e., the type of SMA), or if they were on medication at the time of the experiment. Although the SMAHI and pre-experiment screening questions ensured they met fit the criteria for limited previous motor experience, it is unknown if being on medication could have influenced action possibility judgements. Finally, another limitation of the current study is the exclusion of the self judgement task (see [
3,
16,
19]). While the inclusion of this condition could have provided concrete data on how participants with SMA perceived their own ability, initial pilot tests revealed that participants with SMA consistently chose the slowest possible times for every ID when asked to estimate “how fast they could perform the action possibility judgement task.”