Introduction
Abdominal migraine (AM) presents as paroxysmal bouts of intense, acute periumbilical, midline or diffuse abdominal pain lasting no shorter than 1 hour and interfering with normal activities [
1]. The pain can be associated with nausea, vomiting, pallor, anorexia, photophobia and headache, but headache is not an inherent feature of AM; consequently, this disease is considered a subtype of functional abdominal pain disorders that includes functional dyspepsia, irritable bowel syndrome and functional pain not otherwise specified (NOS) [
2]. Therefore, AM as other functional abdominal pain syndromes, may reflect a disruption of the intrinsic gut-brain axis [
3]. From a clinical point of view, abdominal migraine presents functional disturbances in the gastrointestinal (GI) tract, and it is categorized as a functional gastrointestinal disorder (FGID) by the Rome IV classification (H2c) [
6]. The International Classification of Headache Disorders (ICHD) III beta categorizes AM as an “episodic syndrome that may be associated with migraine” (1.6.1.2) [
4]. Although both classifications precisely specify the diagnostic criteria of abdominal migraine, the disease is undiagnosed [
5]. A proper diagnosis is additionally difficult due to a clinical overlap between AM and migraine [
3]. Migraine and AM are characterized by similar patterns of associated recurrent painful conditions, trigger and relieving factors, and associated symptoms during attacks suggesting that they have a common pathogenesis [
6]. Moreover, cyclic vomiting syndrome (CVS), a gastrointestinal episodic syndrome that may be associated with migraine and functional abdominal pain NOS that have much in common in their clinical presentation with AM may additionally challenge the right diagnosis of AM. The persistence of AM in children is reported to be up to approximately 4% [
6]. Although AM is not always associated with headache, it is sometimes considered a pediatric equivalent of migraine, mainly for diagnostic purposes [
7].
Studies on therapeutic interventions in children and adolescents with migraine are limited or inconclusive [
8]. The treatment of migraine in children is usually different from that in adults as drugs targeting calcitonin gene-related peptide (CGRP) and its receptor that revolutionized migraine therapy, are of limited use in children [
9]. Although there is not consistent evidence that monoclonal antibodies against CGRP may affect growth, the lack of evidence results from a lack of studies addressing this problem rather than a lack of this problem. However, the fact that children are in the phase of intense growth is not the only one that must be considered in planning any therapeutic strategy in this group of patients. Metabolic treatment of migraine is reported to be remarkably effective in some studies in adults, but it seems especially suited to migraine in children as the spectrum of accepted antimigraine treatments in children is much narrower than that in adults [
10].
The essential amino acid tryptophan (TRP) and products of its metabolism are reported to be involved in many effects related to the gut-brain axis [
11]. Tryptophan is metabolized in three major pathways: kynurenine (KYN), serotonin (5-hydroxytryptamine, 5-HT) and indole pathways. As TRP is an exogenous amino acid, it must be provided with the diet, and this creates an opportunity to manipulate its concentration and the concentrations of the products of its metabolism through changes in its content in the diet. On the other hand, the concentration of specific products of the KYN pathway may be pharmacologically manipulated within the CNS, creating another opportunity to modulate processes that depend on TRP metabolism [12-15].
As AM is an FGID and a migraine associated syndrome, these aspects should be considered not only in its diagnosis, but also in its treatment. Tryptophan and products of its metabolism are involved in both aspects of AM; therefore, TRP metabolism may be a therapeutic target in this disease [12,16-18].
In this narrative/perspective review we present information on the clinical presentation, pathogenesis and treatment of AM, migraine-related alterations in the functioning of the gut-brain axis, metabolic alterations related to migraine and the KYN pathway of TRP metabolism. We also discuss the emerging problem of obesity in children and its relation to migraine susceptibility and association with the KYN pathway. We provide arguments that metabolic treatments targeting the KYN pathway of TRP metabolism and its products might be subjected to clinical trials of AM. As AM is rare in adults, if not otherwise stated, further considerations will be related to abdominal migraine in children and adolescents.
Abdominal migraine
The average worldwide prevalence of migraine is estimated to be approximately 20% whereas a recent meta-analysis indicated a 9.1% prevalence [
19,
20]. However, no population-based studies have been performed in low and low-middle income countries. In general, it is accepted that pediatric migraine may preferentially affect children with a family history of migraine and that there is a high probability that affected children develop migraine in adulthood [
21]. A school-based study that was performed in two parts separated by 6 years showed that 10.4% of the children, predominantly girls, had migraine when they grew older (1.7% chronic migraine (CM), 8.6% episodic migraine (EM)) [
22]. The occurrence of CM increased with increasing age with risk factors including age, gender, father and sibling headache histories. In adolescents, 18.6% of individuals were diagnosed with migraine (1.5% CM, 17.1% EM) with a predominance of girls without an age difference.
There are some pronounced differences in diagnostic criteria in adult and pediatric migraines. The pediatric patients mostly complain at bilateral headache episodes lasting shorter than typical unilateral episodes in adults [
23,
24]. Moreover, some migraine-associated syndromes, such as AM, CVS, and infantile colic, occur more frequently in children than adults [
25]. Children with migraine are often affected with other comorbidities, including diseases of the neurological, psychiatric and cardiovascular systems, having a great impact on their and their parents’ quality of life [
26]. Pediatric migraine was associated with obesity, low physical activity, atopy, dysfunctional family, and physical or emotional abuse [27-29].
Triggers, diagnosis and clinical presentation
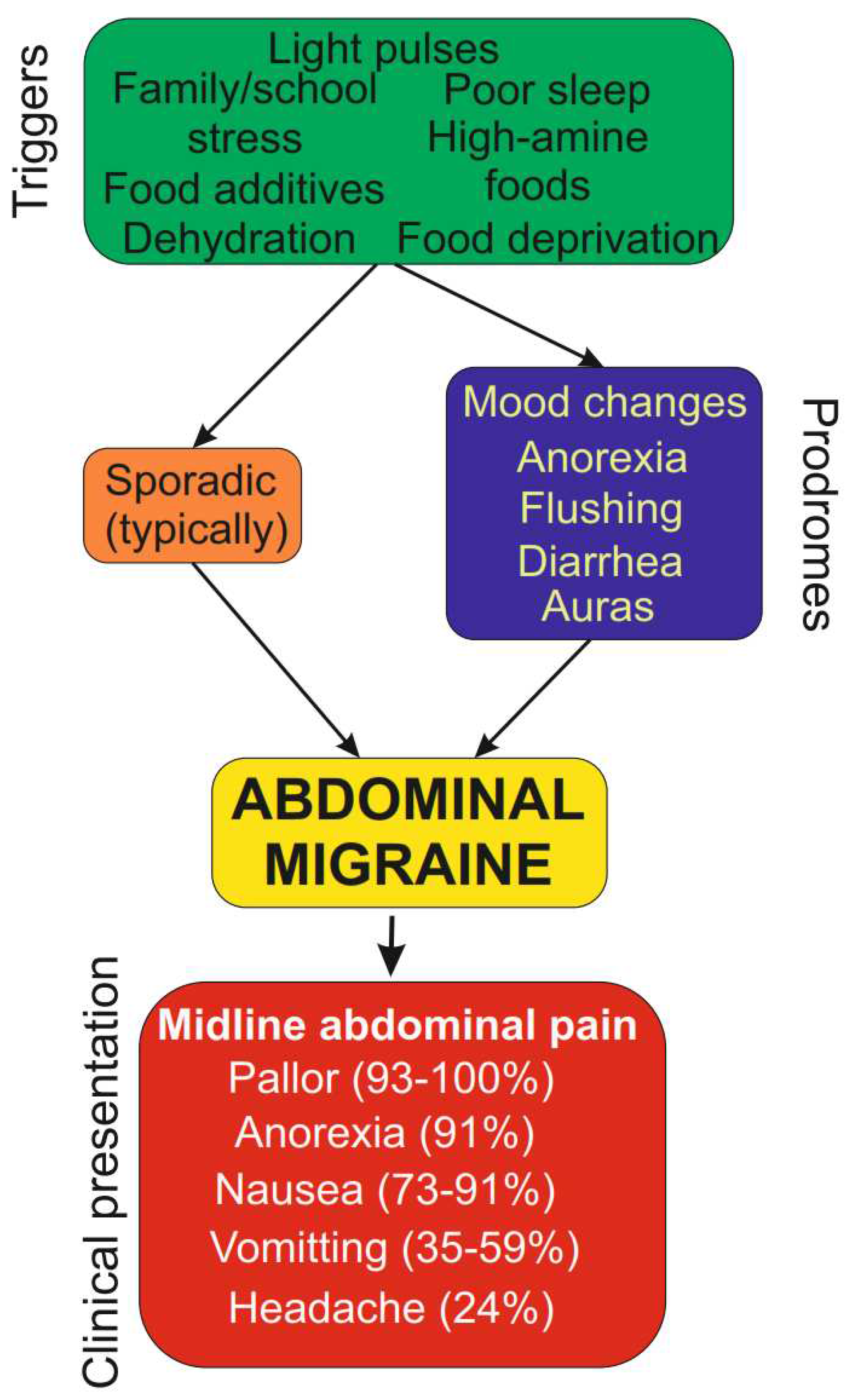
The most commonly recognized triggers of AM are stress, poor sleep, fasting and dehydration, food with a high content of amines, some food additives, bright light, traveling, physical exercise and fatigue [
30,
31]. ICHD-3 beta, and Rome IV criteria slightly differ in the diagnostic criteria and clinically useful characteristics of AM in children are based on the pioneering works on Symon and Russel as well as ICHD-3 beta, and Rome IV criteria and state that the disease is featured by (1) recurrent episodic central abdominal pain lasting 1-72 h occurring at least twice over a 6-month period; (2) pain associated with at least two of the following symptoms anorexia, nausea, vomiting, headache, photophobia, and pallor; and (3) symptoms not assigned to another medical diagnosis [
1,
32]. Therefore, other FDGIs should be excluded in differential AM diagnostic procedures, but exclusion of organic diseases of the GI tract is fundamental for AM diagnosis.
Typical triggers and features of the clinical presentation of AM are presented in
Figure 1.
The treatment of migraine in children is usually different from that in adults as drugs targeting calcitonin gene-related peptide (CGRP) and its receptor, which revolutionized migraine therapy, are of limited use in children [
9]. Headaches are not an inherent symptom of AM, but it is a recurrent, paroxysmal syndrome that might have at least some common etiologies with migraine headache [
7]. Although AM may occur without headaches, they occur in AM relatively frequently. The percentage of coexistence of migraine headaches with abdominal migraine may be as high as 24% with a 10% prevalence of migraine headache in the general population [
6]. When a study population contained pediatric patients with either AM or CVS, the ratio of headache coexistence was as high as 70% [
7]. Equally important is the association between AM and a family history of migraine headaches – a study reported that 90% of AM patients had a family history of migraine headache [
33]. Other studies also reported a high percentage of such coexistence [
7].
The fast wave activity (beta rhythm) in the visual evoked response (VER) to red and white flashes was suggested to support AM diagnosis between attacks [
34]. It was shown that 27 out of 28 children with clinically diagnosed AM demonstrated differences compared with normal controls. Importantly, comparisons with children diagnosed with migraine with or without aura revealed that AM might be a specific form of childhood migraine. That conclusion was drawn based on VER findings of higher amplitude fast wave activity and the presence of paroxysmal sharp wave activity. Furthermore, a decreasing intensity of both clinical and electrophysiological symptoms with age was observed in that study. These conclusions were generally confirmed in a subsequent study with 18 children younger than 10 years with AM and/or CVS [
35].
Treatment
Children with AM often start to outgrowth the disease around puberty and approximately 60% of individuals who experience AM in childhood no longer experience AM episodes in their late teenage years [
5]. Several reports have suggested that children with AM may develop migraine headache in late childhood or adulthood [
32,
36,
37]. Therefore, AM may be considered a kind of migraine prodrome [
32].
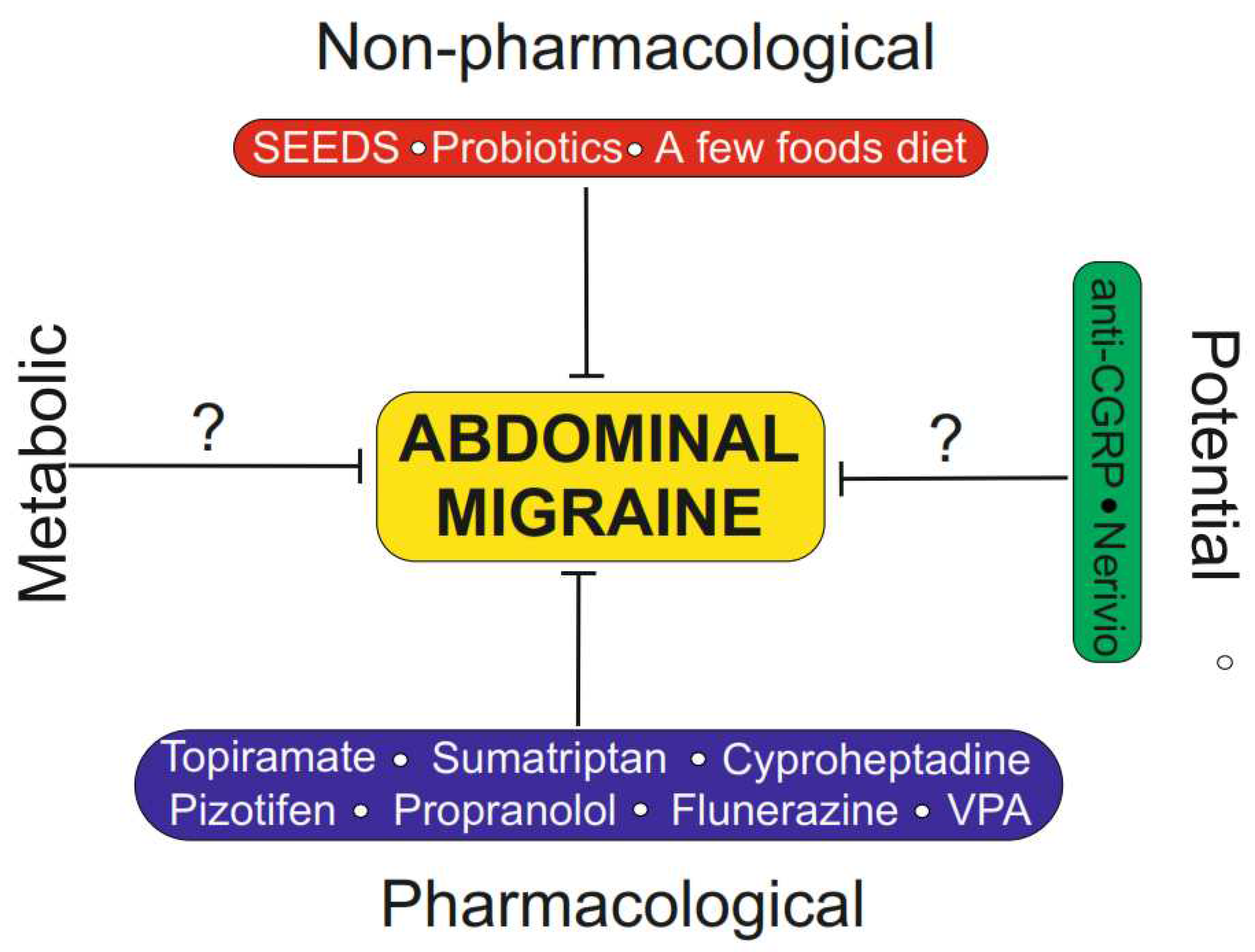
Treatment approaches for AM have not been widely documented in the literature and are based on the experience of physicians, presenting personal rather than evidence-based practice. The efficacy of therapeutic strategies in migraine headache influences the management of AM. In general, pharmacological and non-pharmacological approaches are considered. Both can be further categorized into acute or preventive treatments.
Non-pharmacological treatment of AM is mainly aimed at preventing or ameliorating AM triggers and is based on the STRESS (sleep, exercise, eat, diary, and stress) mnemonic, similar to mnemonic SEEDS (sleep, exercise, eat, diary, and stress), applied in migraine headache [
38,
39] (
Figure 2). Such treatment is justified by the results of studies showing that psychological factors may play a role in the pathogenesis of FGIDs [
40]. It was reported that abdominal pain predominant FGIDs were higher in the group of teenagers aged 13-18 years who experienced sexual or emotional abuse than those who were not abused [
41]. Stress as a risk factor for AM, may play a different role in the pathogenesis of AM than it does in migraine headache. We recently showed that the prevalence of stress associated with social aging has a similar age-dependence as the prevalence of migraine [
42]. It is difficult to assess the immediate consequences of stress experienced by children, and this issue should be further addressed by pediatric psychologists. Therefore, we did not attempt to explore the role of psychological factors in the pathogenesis of AM.
Apart from recommendations on stress avoidance and psychological care, other non-pharmaceutical strategies are considered in AM treatment. An oligoantigenic diet, which eliminates foods than can cause allergic responses, was effective in the treatment of patients with recurrent abdominal pain (RAP) and AM [
43]. Additionally, moderate- to low-quality evidence was found that probiotics were efficient in ameliorating pain in children with RAP [
44]. Many foods are recommended to be excluded from the diet in the prevention of AM and their list significantly overlaps with those for migraine headaches [
1].
To date, the FDA has not approved any pharmacological treatment for AM. Studies on pharmacological intervention in AM are usually performed on small populations of patients [
1,
32]. These studies include propranolol, cyproheptadine, flunarizine and pizotifen in prevention and ibuprofen and sumatriptan in abortive treatment. Children with AM were treated with pizotifen, a potent serotonin and tryptamine antagonist and placebo in a double blind placebo controlled trial [
45]. Seventy percent of the patients reported improvement over 4 months, and they had a reduced number and intensity of AM symptoms. Propranolol caused cessation of abdominal pain in 75% of children with AM as evaluated in a retrospective review [
46]. The same study reported 33% cessations of symptoms in children treated with cyproheptadine, but 50% of patients in this population had a fair response (persistence of symptoms but milder and less frequent). Therefore, both propranolol and cyproheptadine significantly improved the symptoms of AM. Flunerazine, a calcium-blocking agent, given at 7.5 mg/day to children with AM, caused a 61% reduction in the frequency and 51% reduction in the duration of AM attacks [
47]. That study also showed that flunerazine effectively ameliorated symptoms of CVS [
4].
Some drugs were used to abort acute AM attacks. Sumatriptan, a serotonin receptor (1b and 1d) agonist used to treat migraine and cluster headaches, was effective in resolving pain in a 9-year girl diagnosed with AM [
48]. The same study showed that sumatriptan was effective in ameliorating pain in another 9-year girl with childhood functional abdominal pain syndrome (H2d1, Rome III). Dihydroergotamine (DHE) was intravenously given mostly to female adolescent AM patients aged 13 to 19 years, who did not respond to other agents, including amitriptyline, verapamil, topiramate, or depakote [
49]. The AM symptoms were resolved in all patients with the time between relapses ranging from 4 to 12 months. Sodium valproate, an antiepileptic approved for migraine treatment, was also successfully used to ameliorate AM symptoms in several case series studies [
50,
43,
51].
Topiramate is a drug approved by the FDA for epilepsy treatment and migraine prophylaxis that blocks sodium and calcium voltage-gated channels, inhibits glutamate receptors, enhances gamma-aminobutyric acid (GABA) receptors, and inhibits carbonic anhydrase [
52]. Topiramate was effective when administered to children with frequent migraine attacks at a dose of approximately 1.5 mg/kg/day [
53]. The drug reduced mean frequency, severity and duration of headache attacks. An improvement in headache disability, according to the Pediatric Migraine Disability Assessment (PedMIDAS) score was observed in that study [
54]. As a double-blinded prospective clinical trial showed that a supplementation with vitamin D3 increased the efficacy of topiramate in the prevention of pediatric migraine, the use of the vitamin in AM might be justified [
55].
Calcitonin gene-related peptide monoclonal antibodies and gepants made a breakthrough in migraine therapy and raised the question whether such kind of therapy might be applied in children and adolescents [
9]. The next question is whether CGRP may be targeted in AM. In this context it is important that the role of CGRP in the gut-brain-microbiota axis is recognized: CGRP displays an antimicrobial action on the gut microbiota and dysbiosis can increase the release of CGRP [
56]. Increased levels of CGRP were observed in childhood and adolescent migraine patients [57-59]. Although little is known about CGRP metabolism in children, some studies have shown promising results. Rizatriptan is the only FDA approved agonist of serotonin receptors (triptan) for use in children aged 6-17 years, but other triptans have also shown anti-migraine efficacy in children [
60]. No data are available for antagonists of the CGRP receptor (gepants), and for CGRP ligand/receptor antibodies, positive evidence is only reported in a case series [
61]. Therefore, ongoing and prospective clinical trials and experimental studies on the use of the CGRP-targeting drugs in migraine therapy in children and adolescents still need time to provide results that could be implemented in clinical practice. Consequently, the same concerns apply to anti-CGRP therapy in AM.
Metabolic treatment is reported to be effective in some studies in migraine in children and adolescents, but other studies do not report its efficacy. However, there is no evidence of such treatment in AM. This subject will be discussed in further sections.
Figure 2 summarizes present and putative therapeutic approaches in AM.
The gut-brain axis in migraine and functional gastrointestinal diseases
The enteric nervous system (ENS), a part of the peripheral nervous system located within the walls of the GI tract, controls its functions in dependence on and independently of the CNS [
62]. However, the ENS is not fully autonomous, as the neuronal control of GI functions is an integrated network controlled by the CNS. Information flow between the ENS and CNS and between the ENS and sympathetic ganglia occurs in both directions [
62]. Impaired innervation of the GI causes dysfunction manifested by several clinical symptoms, including abdominal pain [
63]. The gut microbiota affects both the ENS and CNS and its disturbances are related to many neurological diseases [
64]. The bidirectional interactions between the CNS, ENS, GI tract and gut microbiome are referred to as the gut-brain axis, which expresses the mutual dependence between functions of the GI tract and CNS.
Abdominal pain, which is a clinical feature of AM, is a gastrointestinal pain that is a kind of visceral pain, common in FGIDs [
65]. Such pain is associated with alterations in brain-gut interactions as it may result from conscious perception of interoceptive information from the GI tract or recall of interoceptive memories of such input [
66].
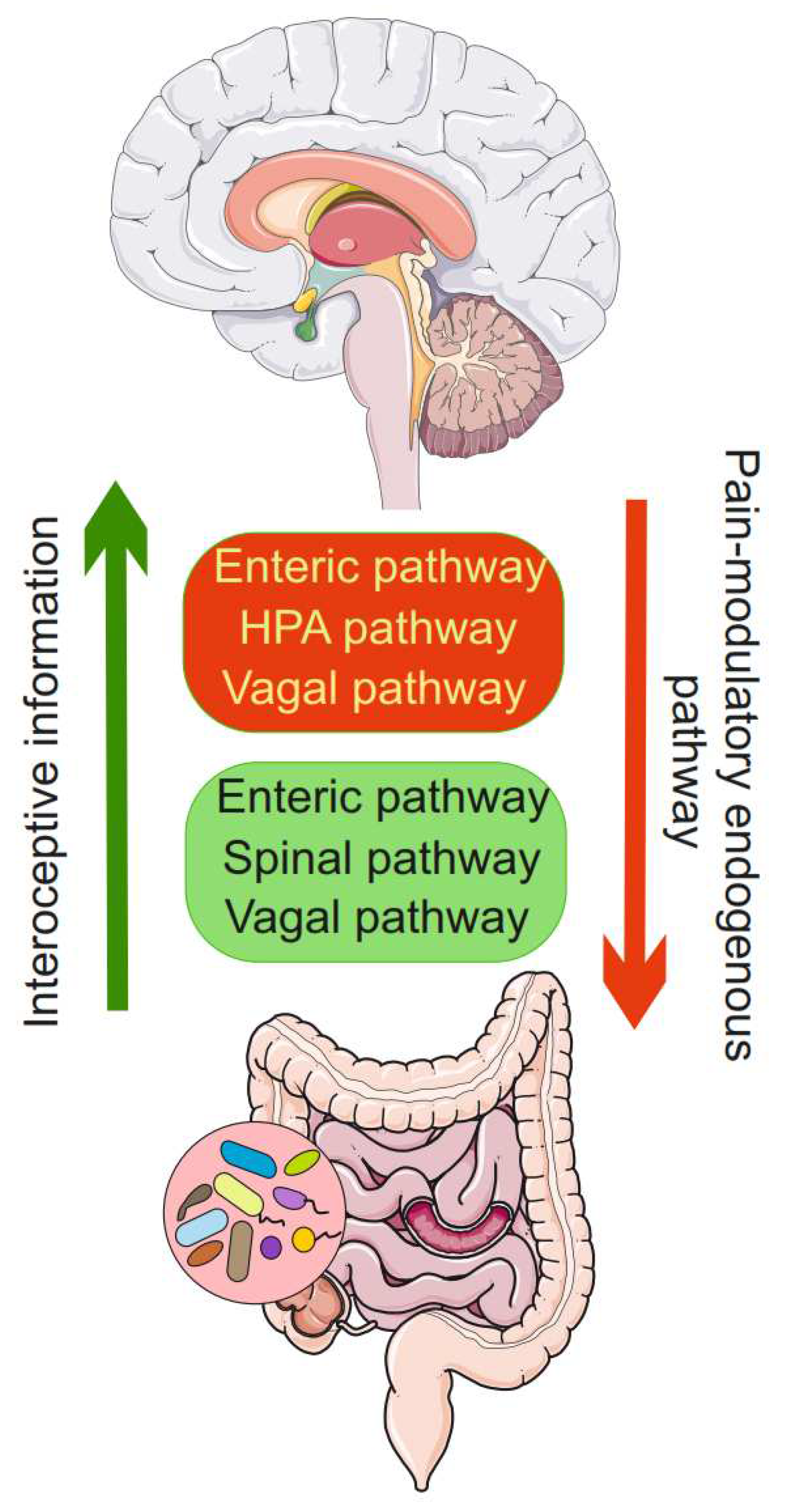
Afferent signals from the lumen of the GI tract are sent by enteric, spinal and vagal afferent pathways to the CNS [
67] (
Figure 3). Proper gut responses to physiological/pathological afferent gut signals are generated by homeostatic reflexes occurring at the level of the ENS, hypothalamic-pituitary (HPA) signaling, the spinal cord and the pontomedullary nuclei and limbic regions [
68]. These reflexes play a role in transmitting vagal visceral inputs to modulate pain and other sensory and emotional experiences [
69]. Visceral pain transmission is modulated by descending corticolimbic impacts that can set the gain and responsiveness of such reflexes and inflict different outlines of motor responses on lower circuits [
70].
Due to the clinical presentation of AM, disturbances in gut-brain axis functioning are an immediate candidate to underline AM pathogenesis. Changes in gastrointestinal (GI) transit resulting in diarrhea or constipation are autonomic symptoms of pre- and post-dorsal phases of migraine attack [
71]. Lower gastric empty times and decreased antral motility were observed in a group of children aged 4-12 years [
72]. Moreover, the more severe the symptoms are, the lower the amplitude of antral contractions, suggesting that slower gut motility may play a role in AM pathogenesis, but several other factors may induce changes in gastric emptying and antral motility. Therefore, it is uncertain, whether the observed changes in GI motility are causative effects or are secondary to AM [
1]. Impaired gastric emptying was observed in pediatric patients with functional dyspepsia [
73,
74].
The blood-brain barrier (BBB) is a physical and functional separator between the gut/microbiota and the brain. As it contains brain microvascular endothelial cells, neurons, microglia, astrocytes, pericytes, and extracellular matrix, it may modulate communication within the gut-brain axis [
75]. The presence of the BBB in the context of the gut-brain axis is an important element that should be considered in projecting therapeutic strategies targeting the disturbances of the axis.
In the context of the functioning of the gut-brain axis it is questionable whether FGIDs exist as individual, distinct and stable syndromes despite clearly distinguishing them in commonly accepted classifications [
66]. Abdominal migraine is not a stable syndrome as in most cases, it either transforms into headache migraine or does not exist in adulthood. Second, it significantly overlaps with other FGIDs, and per ICHD definition, it is associated with migraine and with psychological symptoms and psychiatric syndromes, particularly anxiety, somatization, and depression. As stated above, changes in gastric emptying and antral motility were associated with AM, but such changes were also observed in pediatric patients with functional dyspepsia and in children with gastroesophageal reflux disease [76-79]. Abdominal migraine in adults is rare, but the reported cases are not completely diagnosed to exclude other functional pain-related syndromes [
80]. Therefore, AM can be considered a disturbance in the functioning of the gut-brain axis, whose balance is shifted toward the peripheral components.
Metabolic abnormalities in migraine
Metabolic disturbances are reported to play a role in headache migraine pathogenesis, and they are associated with increased demand and decreased production of energy in migraine [
81]. The brain has high metabolic activity compared with other organs [
82].
Although migraine headache may be associated with the activation of the trigeminovascular system, the main pain-signaling system of the viscera brain, the precise mechanism leading to such activation is poorly known [
83]. However, fasting and skipping meals as well as some specific nutritional compounds are migraine triggers recognized by many patients [
84,
85]. Therefore, a plethora of metabolic triggers may play a role in migraine pathogenesis, which justifies considering metabolic treatment as a therapeutic strategy in this disease [
10]. However, the molecular mechanism behind these metabolic triggers is not fully known and many routes, likely acting in concert, are possible. These assumptions were confirmed in MRI studies showing reduced concentrations of metabolic substrates and products in migraine patients [
86,
87]. Interestingly, MRI studies also revealed that the more pronounced the changes in metabolism in migraine patients, the more severe the disease [
88,
89]. Inborn changes in metabolism and metabolic diseases should be considered in the differential diagnosis of recurrent abdominal pain syndromes, including AM and CVS [
90].
Migraine is associated with the need for energy generation by the affected brain. The brain produces energy mainly by the breakdown of ATP synthesized from glucose in three consecutive stages: glycolysis, the tricarboxylic acid cycle (TCA) and oxidative phosphorylation. This leads to the increased production of inorganic phosphate that may be detected in nuclear magnetic resonance (NMR) studies of the brain. A lower phosphocreatine to creatine ratio and increased concentrations of ADP were observed in
31P-NMR studies in migraine patients, indicating lower energy production by the migraine-affected brain [
91]. Creatine phosphate (phosphocreatine) is an important component in the brain energy metabolism because it participates in ATP recycling [
92]. Moreover, intermediates of glycolysis and TCA are implicated in the biosynthesis of carbohydrates, neurotransmitters, neuromodulators, and amino acids that are essential for the functioning of the gut-brain axis [
93]. Therefore, brain energy metabolism is closely associated with mitochondrial homeostasis as oxidative phosphorylation occurs in mitochondria and consequently, migraine pathogenesis may be underlined by mitochondrial disturbances [
94]. Similar to adult headache migraine, AM prevalence is higher in females than males, suggesting that mitochondrial transmission may contribute to AM pathogenesis. Furthermore, impaired mitochondria may overproduce reactive oxygen and nitrogen species (RONS), increasing oxidative stress in the brain and the GI tract and contributing to central sensitization, which is important in migraine. Moreover, age was correlated with metabolic changes in the critical regions of the brain for migraine to a grater extent than disease duration or the number of monthly migraine days [
95]. However, that observation cannot be directly connected with migraine in children as aging-related changes may not be seen in children, adolescents and even young adults.
Migraine-related alterations in energy metabolism may be, at least in part, determined by the genetic constitution of migraine patients as mutations associated with migraine include genes involved in metabolic reactions [96-99]. Patients with such mutations cannot cope with metabolic challenges transmitted by migraine-affected brains and experience disturbances in cortical homeostasis [
100].
Riboflavin (vitamin B2) is an essential compound for the synthesis of myelin in the nervous system and its deficiency can determine the disruption of myelin lamellae and other "riboflavin transporter deficiencies” as well as further syndromes [
101]. No effect of 50 mg/day riboflavin in children aged 6-13 years in the prevention of migraine attack was observed in a preliminary placebo-controlled, randomized, double-blind, cross-over trial [
102]. Similarly, a double-blind, randomized, placebo-controlled trial showed that high doses (200 mg daily) of riboflavin were not effective in reducing of the number of migraine attacks per day and the severity of the attacks in children [
103]. A high responder rate was observed in that study. However, a subsequent study showed that riboflavin at 200 or 400 mg daily was effective in decreasing the frequency and intensity of migraine attacks in children and adolescents [
104]. Neither of these studies mentioned whether AM patients were included/excluded.
Coenzyme Q10 (CoQ10) is an essential component of the mitochondrial electron transport chain whose deficiency is associated with different disorders and aging [
105]. It is also an antioxidant in cellular membranes and lipoproteins. It was shown that almost 1/3 out of 1550 pediatric and adolescent patients with frequent headaches had CoQ10 levels below the reference value [
106]. Supplementation with CoQ10 in these patients improved their migraine disability assessed with PedMIDAS and reduced headache frequency. In a randomized, double-blinded, placebo-controlled, crossover, add-on study of CoQ10 (100 mg supplement for 224 days) in the prevention of pediatric and adolescent migraine, both the placebo and CoQ10 groups showed reduced migraine frequency, severity and duration over time [
107]. However, CoQ10 treated patients had a greater improvement in frequency from subject-reported baseline starting within 4 weeks after the initiation, but there was no difference in headache outcomes between the CoQ10 and placebo groups on day 224. Generally, those results showed that children and adolescents with migraine improved over time with multidisciplinary, standardized treatment regardless of supplementation with CoQ10 or placebo.
Several other supplements, including alpha lipolic acid, magnesium, homocysteine, pyridoxine, folate, cobalamin, carnitine, niacin, thiamine, melatonin and a ketogenic diet were reported to prevent or ameliorate migraine attacks (reviewed in [
108,
10]). All these interventions may be associated with an increase in energy production and reducing in brain energy deficits by improving mitochondrial functions and reducing oxidative stress. They have not been studied in AM cases thus far.
In summary, due to nature of AM, which is characterized by disturbances in GI functioning, it seems suitable for metabolic treatment, which should be preceded by a determination of the metabolic profile of an individual.
The kynurenine pathway of tryptophan metabolism and its role in the pathogenesis of migraine and functional gastrointestinal diseases
Tryptophan is an essential amino acid that must be provided by exogenous sources in humans. In the human body, TRP is metabolized in three major pathways: the 5-HT, KYN, and microbiota-related indole pathways. The major site of TRP metabolism is the GI tract. Under normal conditions, the KYN pathway metabolizes about 90% of TRP in the liver via tryptophan-2,3-dioxygenase (TDO). The products of the KYN pathway may be implicated in the pathogenesis of neurological diseases [
16].
The KYN pathway occurs mainly in epithelial and immune cells, where TRP is metabolized to many products with the involvement of enzymes largely expressed in the liver (
Figure 4) [
109]. KYN and other products of the KYN pathway play an important role in many regulatory functions in the CNS and therefore they may be involved in the pathogenesis of CNS diseases, including neurological disorders [
110]. To date, kynurenic acid (KYNA) has gained the greatest research interest among products of the KYN pat
Some positive results from metabolic treatment of migraine with compounds related to brain energetics do not limit the pathogenesis of migraine to impairments in brain metabolism. This is especially important in AM, in which the gut brain-axis may be functionally impaired at the gut level. Migraine and AM are multifactorial diseases and their treatment should meet their individual features that may include amounts of and proportions between some metabolic pathways.
hway. Some studies report manipulating the amount of KYNA in the brain to prevent and treat CNS disorders [
110]. The interaction of KYNA with 7 nicotinic acetylcholine receptors (7nAchRs), the N-methyl-D-aspartic acid (NMDA) receptor and G protein-coupled receptor 35 (GPR35) underlines the influence of KYNA on brain functions [111-113]. These interactions are also important in migraine pathogenesis as the activation of glutamate receptors may be relevant to central sensitization, which is related to cutaneous allodynia often associated with migraine [
114,
115].
The distribution of the products of the KYN pathway was determined in the serum of CM patients [
116]. Significant reductions in the levels of KYN, KYNA, 3-hydroxykynurenine (3-HK), 3-hydroxyanthranilic acid (3-HAA), 5-hydroxyindolacetic acid (5-HIAA) and quinolinic acid (QUINA) were observed in that study, but the levels of TRP, anthranilic acid (ANA) and xanthurenic acid (XA) were increased. It was concluded that KYN was metabolized to ANA at the expense of KYNA and 3-HK. As KYNA is a competitive antagonist of the glycine site of NMDA receptors that are overactive in migraine, its reduction suggests a protective role of KYNA in migraine [
117,
118]. These results show that the KYN pathway of TRP metabolism may be important in migraine pathogenesis. Several other studies pointed at the role of products of the KYN pathway in effects closely related to migraine, including cortical spreading depolarization (CSD), increased activity of sex hormones and permeability of BBB [119-121].
According to the Rome IV classification, FGIDs in children are categorized into three classes: (1) functional abdominal pain disorders; (2) functional nausea and vomiting disorders; and (3) functional defecation syndromes [
122]. The first class can be further divided into irritable bowel syndrome (IBS), functional dyspepsia and AM. Apart from AM, CVS and infantile colic are gastrointestinal episodic syndromes classified by ICHD-3 beta as variants of migraine. This is not a subject of this work to present association between FGIDs and migraine in children, which is described in many excellent reviews (e.g., [123,3,124-127]). Apart from the Rome and ICHD classifications, medical practice distinguishes some other symptoms that may be related to AM. Medically unexplained symptoms (MUSs) are symptoms that are identified in children and present with headache, fatigue and abdominal pain, but without identifiable infectious or metabolic etiology [
128]. In most MUS cases, the origin of abdominal pain is not identified and usually attributed to psychogenic origin. The term “recurrent abdominal pain” (RAP) describes the presence of at least 3 or more discrete episodes of abdominal pain over a period of at least 3 months, interfering with the normal daily activity of a child [
129]. Therefore, MUS and RAP additionally complicate AM diagnosis, despite their clear distinction, as the symptoms are self-reported by children that may be oversensitive to their abdominal pain. High concentrations of 3-HAA, high 3-HAA:AA ratio, a low AA concentration and a low KYN:TRP ratio were observed in children with RAP as compared with children with asymptomatic intestinal parasitic subclinical infection [
130]. That study also showed changes in the concentration of inflammatory markers, including interleukins 10 and 6, tumor necrosis factor alpha and brain-derived neurotrophic factor.
Most of the research on the role of the KYN pathway in FGIDs focuses on irritable bowel syndrome (IBS). The results of these studies show that IBS patients have a higher KYN/TRP ratio and lower levels of KYNA and display changes in the KYN pathways that are associated with their mental problems [131-135]. Several mechanisms may underlie the link between IBS and the KYN pathway and some of them may also be related to migraine. A differential downstream profile of KYN production was shown in IBS patients after Toll-like receptor activation [
132]. Changes in the TLR profile involved TLR1/2, TLR2, TLR3, TLR5, TLR7, and TLR8. The TLR4 signaling was associated with the mast cell-mediated migraine pain pathway [
136]. In general, migraine presents some clinical features with IBS [
137]. Both are chronic, pain-related disorders that occur with higher prevalence in women than men and are associated with psychological comorbidity and allodynia. However, IBS may display different clinical presentations depending on whether it is associated with diarrhea or constipation [
138]. We showed that limiting TRP intake improved the state of IBS patients with predominant diarrhea evaluated by the Gastrointestinal Symptom Rating Scale with a concomitant increase in KYN and decrease in KYN and QA urinary levels [
139]. Our results are in line with those showing a higher level of TRP in IBS patients with predominant diarrhea [
140].
Obesity in children and its connection with migraine and the kynurenine pathway
Although the prevalence of childhood and adolescent obesity is constant in many high-income countries, it is alarmingly high, e.g., 1 per 5 US children was reported to be obese in 2019 [
141]. Although this problem is not so serious in Europe, it is an important public health issue that affects a large number of children [
142]. Childhood obesity is pursued into adulthood in a high proportion as approximately 85% of obese children become obese adults [143-145]. Moreover, the increasing prevalence of obesity in children is linked with an increased incidence of conditions formerly thought as typical for adulthood, including metabolic syndrome, type 2 diabetes mellitus, non-alcoholic fatty liver disease and depression [
146]. However, obesity cannot be considered as the sole cause and an initiating factor of metabolic syndrome [
147]. On the other hand, as the prevalence of obesity in children increasing worldwide, it is unlikely that the syndrome is largely determined by genetic constitution, and a combination of genetic/epigenetic factors, behavioral risk patterns and environmental and sociocultural influences may affect the body weight regulation system [
148].
Obesity is also linked with migraine, and a lack of such link with tension headache may suggest that migraine can be a specific headache syndrome associated with obesity [
149]. Migraine is among the diseases that can be associated with obesity in children, suggesting that these two diseases may share some elements of pathogenesis [
27]. As there are many nutritional components that are migraine triggers and obesity may be directly linked with central and peripheral pathways regulating feeding, some neurotransmitters such as serotonin, peptides such as orexin, and adipocytokines such as adiponectin and leptin may play a role in both feeding regulation and migraine [
150].
Serotonin may have a special position in the link between feeding control/obesity and migraine. Serotogenic neurons penetrate almost all regions of the CNS, the primary sensory cortex, the thalamus, the trigeminal nuclei and the dorsal horns of the spinal cord [
151]. Serotonin has as many as 15 receptors known to date and their actual number and efficiency along with 5-HT production determine serotonin-related biological effects. However, these effects should not be considered without TRP metabolism and particularly – KYN pathway of TRP metabolism. Serotonin is produced in the 5-HT pathway of TRP metabolism, which transforms only about 5% of total TRP in humans, in contrast to the KYN pathway, which is responsible for almost the rest of the administered TRP. Therefore, any disturbance in the TRP metabolism leading to an alteration in the physiological proportion between the 5-HT and KYN pathways of TRP metabolism may lead to disturbances in feeding control that may result in overweight/obesity as 5-HT signals satiety [
152]. The less 5-HT there is, the weaker the satiety signal. On the other hand, low levels of 5-HT were observed in migraine patients between attacks and high levels of 5-HT were reported during acute headaches [
153,
154]. These results led to the theory that migraine may be syndrome of dysfunction of the serotonergic system with low levels of 5-HT provoking headache attacks with increased 5-HT levels [
155]. This theory was supported by the effective action of antagonists of 5-HT receptors, including triptans [
156]. However, positron emission tomography (PET) imaging of the 5-HT
4 receptor showed that migraine patients had lower levels of this receptor during attacks [
157]. The conclusion was that those patients might have higher levels of 5-HT in some structures of the brain. Therefore, the role of 5-HT in migraine pathogenesis is not completely clear; in particular, the origin of the acute increase in 5-HT levels during attacks is not knownas it was assumed that a higher level between attacks might result from individual susceptibility to migraine [
157].
One of the first studies on the association of obesity with migraine in children and adolescents showed that the prevalence of migraine was 2.5% in normal subjects, 4.4% in overweight subjects and 8.9% in obese subjects [
158]. Several other associations between migraine and obesity in children were reported, but none concerned AM [
27].
Obesity is not only related to TRP metabolism through the 5-HT pathway. Obesity-related inflammation was associated with the TRP metabolism via indoleamine 2,3-dioxygenase (IDO) [
159]. A study with severe obesity (BMI ≥ 97th percentile) patients aged 9-19 years showed that the activity of IDO and its product, KYN, positively correlated with BMI z-score and body fat mass, whereas concentration of 5-HT showed a negative correlation with these parameters [
160]. Therefore, BMI and body fat mass were associated with increased activity of the KYN pathway of TRP metabolism and decreased synthesis of 5-HT in children and adolescents with severe obesity.
Over 500 children aged 8 years were enrolled within the Growing Up in Singapore Toward healthy Outcomes (GUSTO) prospective mother-offspring cohort study [
161]. The levels of the metabolites of the KYN pathway were increased in overweight or obese children as compared with their normal weight counterparts. Specifically, plasma KYN, KYNA, XA, 3-HAA and QUINA showed positive associations with body fat percentage, and systolic blood pressure. All KYN metabolites except 3-hydroxykynurenine (3-HK) were significantly correlated with metabolic syndrome scores. In conclusion, higher plasma concentrations of KYN pathway metabolites are associated with obesity and with an increased risk for metabolic syndrome.
In summary, overweight or obese children, display metabolic disturbances that may predispose them to AM as these disturbances are typical for both migraine and FGIDs. The amount and proportions of the products of the KYN pathway of TRP metabolism are changed in these children and modulation of the KYN pathway may be considered to reduce overweight/obesity and ameliorate AM in thic group of children.
Conclusions and perspectives
The correct diagnosis of AM can be difficult, as it is characterized by several symptoms overlapping with other diseases. Abdominal migraine can be regarded as an FGID according to the Rome IV classification, but ICHD III classifies it as a syndrome that may be associated with migraine, although AM does not have to be linked with headache. However, the diagnostic criteria provided by Rome IV and ICHD III are slightly different, additionally complicating a right AM diagnosis. The pathogenesis of AM is poorly understood, impeding progress in its diagnosis and treatment. Abdominal migraine almost exclusively affects children and adolescents which implies difficulties in its diagnosis based on self-reported symptoms and also in recruiting subjects for controlled clinical trials. Moreover, there is no reasonable animal model of AM and due to the specificity of the disease, there is little hope to establish such a model at all. Therapy of abdominal migraine is mainly based on the individual experience of physicians and is not always evidence-based; thus far the FDA has not approved any pharmacological treatment for AM. These facts indicate that AM is an undiagnosed and an undertreated disease, although AM may be a serious problem for affected children and their families, significantly interfering with their everyday activity.
As AM is characterized by elements typical of FIGDs and migraine pathogenesis and studies on AM are scarce, we often related to the results obtained either in migraine or FGIDs studies. It cannot be excluded that due to difficulties in AMD diagnosis in children, some study populations in both migraine and FGIDs research included cases of AM. On the other hand, focusing on AM as a disease of children and adolescents may lead to overlooking its rare cases in adults [
30].
We and others showed that the KYN pathway of TRP metabolism played a role in the pathogenesis of FGIDs and mood disorders accompanying FGDIs. We also showed that the changes in the concentration of the products of the KYN pathway might modulate pharmacological treatment of FGIDs. This, along with reports on the involvement of products of KYN metabolism in migraine and obesity in children, allowed us to hypothesize that the KYN pathway may be involved in AM pathogenesis. The levels of the products of the KYN pathway can be changed with the adjustment of TRP content in the diet, as we showed in clinical trials on the role of TRP intake in the pathogenesis of the GI tract and mood disorders [
162,
163,
139,
164,
165]. Surely, the TRP content in the diet does not be the only determinant of the concentration and activity of the product of TRP metabolism. The main product of the other TRP metabolic pathway, 5-HT, has a broad impact as a neurotransmitter and neuromodulator and is implicated in many physiological processes and pathological conditions within the CNS, including migraine, although only approximately 1% of dietary TRP is used for 5-HT synthesis in the brain [
166]. Therefore, it is justified to study the 5’-HT pathway of TRP metabolism in AM.
Considering AM as an impairment in the functioning of the gut-brain axis, the gut microbiota should be taken into consideration, especially because disturbances in the gut microbiota can be directly related to the pathogenesis of FGIDs [
167]. Recently, it was shown that children with migraine displayed differences in the gut microbiota as compared with their peers without migraine [
168]. Again, AM may be more closely related to changes in the gut microbiome than migraine due to its closer connections with the gut microbiota, typical for FGIDs. Therefore, this aspect of AM should be explored in the therapeutic context.
In summary, we suggest that placebo-controlled clinical trials in AM with dietary interventions changing the products of the KYN pathway of TRP metabolism are justified because:
1. Abdominal migraine has the features of both FGIDs and migraine, whose pathogeneses include changes in the KYN pathway.
2. Metabolic treatment, shown to be effective in migraine cases, is especially suited for children, as this group of patients should be treated with drugs only when symptoms are severe and frequent enough to strongly interfere with their everyday activities.
Such clinical trials might be accompanied by pharmacological interventions modulating the KYN pathway in children with severe symptoms of AM. Molecular and cellular studies on the role of the KYN pathway in AM pathogenesis, should clarify the mechanisms underlying the clinical symptoms of AM. Such preclinical studies are needed to establish details of KYN pathway modulation in clinical trials.
Author Contributions
Conceptualization, J.B., M.F.; writing—original draft preparation, J.B., A.J., C.C., and J.C.; writing—review and editing, J.B. All authors have read and agreed to the submitted version of the manuscript.
Funding
This research received no external funding.
Acknowledgements
The authors thank Monika Kicinska for technical assistance.
Competing interest
The authors declare no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Abbreviations
3-HAA: 3-hydroxyanthranilic acid; 3-HK: 3-hydroxykynirenine; 5-HIAA; 5-hydroxyindolacetic acid; 5-HT: 5-hydroxytryptamine, serotonin; 7nAchR: 7 nicotinic acetylcholine receptor; AM: abdominal migraine; ANA: anthranilic acid; BBB: blood-brain barrier; CGRP: calcitonin gene-related peptide; CoQ10: coenzyme Q10; CSD: cortical spreading depolarization; CVS: cyclic vomiting syndrome; FGID: functional gastrointestinal disorder; CM: chronic migraine; CNS: central nervous system; EM: episodic migraine; ENS: enteric nervous system; FGID: functional gastrointestinal disorder; GI: gastrointestinal; GPR35: G protein-coupled receptor 35; IBS: irritable bowel syndrome; IDO: indoleamine 2,3-dioxygenase; KYN: kynurenine; KYNA: kynurenic acid; MUS; medically unexplained symptom; NMDA: N-methyl-D-aspartic acid; NMR: nuclear magnetic resonance; NOS: not otherwise specified; PedMIDAS: Pediatric Migraine Disability Assessment tool; QUINA: quinolinic acid; RAP: recurrent abdominal pain; RONS: reactive oxygen and nitrogen species; TCA: tricarboxylic acid cycle; TLR: toll-like receptor; TRP: tryptophan; VER: visual evoked response; XA: xanthurenic acid.
References
- Azmy DJ, Qualia CM (2020) Review of abdominal migraine in children. Gastroenterol Hepatol (N Y) 16:632-639. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8132691/pdf/GH_16_632.pdf.
- Koppen IJ, Nurko S, Saps M, Di Lorenzo C, Benninga MA (2017) The pediatric rome iv criteria: What's new? Expert Rev Gastroenterol Hepatol 11:193-201. [CrossRef]
- Lenglar TL, Caula C, Moulding T, Lyles A, Wohrer D, Titomanlio L (2021) Brain to belly: Abdominal variants of migraine and functional abdominal pain disorders associated with migraine. J Neurogastroenterol Motil 27:482-494. [CrossRef]
- The international classification of headache disorders, 3rd edition (beta version) (2013). Cephalalgia 33:629-808. [CrossRef]
- Mani J, Madani S (2018) Pediatric abdominal migraine: Current perspectives on a lesser known entity. Pediatric Health Med Ther 9:47-58. [CrossRef]
- Abu-Arafeh I, Russell G (1995) Prevalence and clinical features of abdominal migraine compared with those of migraine headache. Arch Dis Child 72:413-417. [CrossRef]
- Al-Twaijri WA, Shevell MI (2002) Pediatric migraine equivalents: Occurrence and clinical features in practice. Pediatr Neurol 26:365-368. [CrossRef]
- Di Lorenzo C, Colletti RB, Lehmann HP, Boyle JT, Gerson WT, Hyams JS, Squires RH, Jr., Walker LS, Kanda PT (2005) Chronic abdominal pain in children: A technical report of the american academy of pediatrics and the north american society for pediatric gastroenterology, hepatology and nutrition. J Pediatr Gastroenterol Nutr 40:249-261. [CrossRef]
- Szperka CL, VanderPluym J, Orr SL, Oakley CB, Qubty W, Patniyot I, Lagman-Bartolome AM, Morris C, Gautreaux J, Victorio MC, Hagler S, Narula S, Candee MS, Cleves-Bayon C, Rao R, Fryer RH, Bicknese AR, Yonker M, Hershey AD, Powers SW, Goadsby PJ, Gelfand AA (2018) Recommendations on the use of anti-cgrp monoclonal antibodies in children and adolescents. Headache 58:1658-1669. [CrossRef]
- Lisicki M, Schoenen J (2020) Metabolic treatments of migraine. Expert Rev Neurother 20:295-302. [CrossRef]
- Gao K, Mu CL, Farzi A, Zhu WY (2020) Tryptophan metabolism: A link between the gut microbiota and brain. Adv Nutr 11:709-723. [CrossRef]
- Bosi A, Banfi D, Bistoletti M, Giaroni C, Baj A (2020) Tryptophan metabolites along the microbiota-gut-brain axis: An interkingdom communication system influencing the gut in health and disease. Int J Tryptophan Res 13:1178646920928984. [CrossRef]
- Erhardt S, Olsson SK, Engberg G (2009) Pharmacological manipulation of kynurenic acid: Potential in the treatment of psychiatric disorders. CNS Drugs 23:91-101. [CrossRef]
- Reinhard JF, Jr. (2004) Pharmacological manipulation of brain kynurenine metabolism. Ann N Y Acad Sci 1035:335-349. [CrossRef]
- Schwarcz R, Pellicciari R (2002) Manipulation of brain kynurenines: Glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther 303:1-10. [CrossRef]
- Cervenka I, Agudelo LZ, Ruas JL (2017) Kynurenines: Tryptophan's metabolites in exercise, inflammation, and mental health. Science (New York, NY) 357. [CrossRef]
- Drummond PD (2006) Tryptophan depletion increases nausea, headache and photophobia in migraine sufferers. Cephalalgia 26:1225-1233. [CrossRef]
- Razeghi Jahromi S, Togha M, Ghorbani Z, Hekmatdoost A, Khorsha F, Rafiee P, Shirani P, Nourmohammadi M, Ansari H (2019) The association between dietary tryptophan intake and migraine. Neurol Sci 40:2349-2355. [CrossRef]
- Steiner TJ, Stovner LJ (2023) Global epidemiology of migraine and its implications for public health and health policy. Nature Reviews Neurology 19:109-117. [CrossRef]
- Wöber-Bingöl C (2013) Epidemiology of migraine and headache in children and adolescents. Curr Pain Headache Rep 17:341. [CrossRef]
- Kienbacher C, Wöber C, Zesch HE, Hafferl-Gattermayer A, Posch M, Karwautz A, Zormann A, Berger G, Zebenholzer K, Konrad A, Wöber-Bingöl C (2006) Clinical features, classification and prognosis of migraine and tension-type headache in children and adolescents: A long-term follow-up study. Cephalalgia 26:820-830. [CrossRef]
- Ozge A, Saşmaz T, Buğdaycı R, Cakmak SE, Kurt A, Kaleağası SH, Siva A (2013) The prevalence of chronic and episodic migraine in children and adolescents. Eur J Neurol 20:95-101. [CrossRef]
- Abu-Arafeh I, Callaghan M (2004) Short migraine attacks of less than 2 h duration in children and adolescents. Cephalalgia 24:333-338. [CrossRef]
- McAbee GN, Morse AM, Assadi M (2016) Pediatric aspects of headache classification in the international classification of headache disorders-3 (ichd-3 beta version). Curr Pain Headache Rep 20:7. [CrossRef]
- Gelfand AA (2015) Episodic syndromes that may be associated with migraine: A.K.A. "The childhood periodic syndromes". Headache 55:1358-1364. [CrossRef]
- Bellini B, Arruda M, Cescut A, Saulle C, Persico A, Carotenuto M, Gatta M, Nacinovich R, Piazza FP, Termine C, Tozzi E, Lucchese F, Guidetti V (2013) Headache and comorbidity in children and adolescents. J Headache Pain 14:79. [CrossRef]
- Farello G, Ferrara P, Antenucci A, Basti C, Verrotti A (2017) The link between obesity and migraine in childhood: A systematic review. Ital J Pediatr 43:27. [CrossRef]
- Tietjen GE, Brandes JL, Peterlin BL, Eloff A, Dafer RM, Stein MR, Drexler E, Martin VT, Hutchinson S, Aurora SK, Recober A, Herial NA, Utley C, White L, Khuder SA (2010) Childhood maltreatment and migraine (part ii). Emotional abuse as a risk factor for headache chronification. Headache 50:32-41. [CrossRef]
- Youssef PE, Mack KJ (2020) Episodic and chronic migraine in children. Dev Med Child Neurol 62:34-41. [CrossRef]
- Cervellin G, Lippi G (2015) Abdominal migraine in the differential diagnosis of acute abdominal pain. Am J Emerg Med 33:864.e863-865. [CrossRef]
- Winner P (2016) Abdominal migraine. Semin Pediatr Neurol 23:11-13. [CrossRef]
- Dignan F, Abu-Arafeh I, Russell G (2001) The prognosis of childhood abdominal migraine. Arch Dis Child 84:415-418. [CrossRef]
- Bentley D, Kehely A, al-Bayaty M, Michie CA (1995) Abdominal migraine as a cause of vomiting in children: A clinician's view. J Pediatr Gastroenterol Nutr 21 Suppl 1:S49-51. [CrossRef]
- Mortimer MJ, Good PA (1990) The ver as a diagnostic marker for childhood abdominal migraine. Headache 30:642-645. [CrossRef]
- Good PA (1995) Neurologic investigations of childhood abdominal migraine: A combined electrophysiologic approach to diagnosis. J Pediatr Gastroenterol Nutr 21 Suppl 1:S44-48. [CrossRef]
- Lagman-Bartolome AM, Lay C (2015) Pediatric migraine variants: A review of epidemiology, diagnosis, treatment, and outcome. Curr Neurol Neurosci Rep 15:34. [CrossRef]
- Lanzi G, Balottin U, Fazzi E, Rosano FB (1983) The periodic syndrome in pediatric migraine sufferers. Cephalalgia 3 Suppl 1:91-93. [CrossRef]
- Bougea A, Spantideas N, Chrousos GP (2018) Stress management for headaches in children and adolescents: A review and practical recommendations for health promotion programs and well-being. J Child Health Care 22:19-33. [CrossRef]
- Robblee J, Starling AJ (2019) Seeds for success: Lifestyle management in migraine. Cleve Clin J Med 86:741-749. [CrossRef]
- Lee SY, Ryu HS, Choi SC, Jang SH (2020) A study of psychological factors associated with functional gastrointestinal disorders and use of health care. Clin Psychopharmacol Neurosci 18:580-586. [CrossRef]
- Devanarayana NM, Rajindrajith S, Perera MS, Nishanthanie SW, Karunanayake A, Benninga MA (2014) Association between functional gastrointestinal diseases and exposure to abuse in teenagers. J Trop Pediatr 60:386-392. [CrossRef]
- Fila M, Pawlowska E, Szczepanska J, Blasiak J (2023) Different aspects of aging in migraine. Aging and disease. [CrossRef]
- Russell G, Abu-Arafeh I, Symon DN (2002) Abdominal migraine: Evidence for existence and treatment options. Paediatr Drugs 4:1-8. [CrossRef]
- Newlove-Delgado TV, Martin AE, Abbott RA, Bethel A, Thompson-Coon J, Whear R, Logan S (2017) Dietary interventions for recurrent abdominal pain in childhood. Cochrane Database Syst Rev 3:Cd010972. [CrossRef]
- Symon DN, Russell G (1995) Double blind placebo controlled trial of pizotifen syrup in the treatment of abdominal migraine. Arch Dis Child 72:48-50. [CrossRef]
- Worawattanakul M, Rhoads JM, Lichtman SN, Ulshen MH (1999) Abdominal migraine: Prophylactic treatment and follow-up. J Pediatr Gastroenterol Nutr 28:37-40. [CrossRef]
- Kothare SV (2005) Efficacy of flunarizine in the prophylaxis of cyclical vomiting syndrome and abdominal migraine. Eur J Paediatr Neurol 9:23-26. [CrossRef]
- Kakisaka Y, Wakusawa K, Haginoya K, Saito A, Uematsu M, Yokoyama H, Sato T, Tsuchiya S (2010) Efficacy of sumatriptan in two pediatric cases with abdominal pain-related functional gastrointestinal disorders: Does the mechanism overlap that of migraine? J Child Neurol 25:234-237. [CrossRef]
- Raina M, Chelimsky G, Chelimsky T (2013) Intravenous dihydroergotamine therapy for pediatric abdominal migraines. Clin Pediatr (Phila) 52:918-921. [CrossRef]
- Corbo J (2003) The role of anticonvulsants in preventive migraine therapy. Curr Pain Headache Rep 7:63-66. [CrossRef]
- Tan V, Sahami AR, Peebles R, Shaw RJ (2006) Abdominal migraine and treatment with intravenous valproic acid. Psychosomatics 47:353-355. [CrossRef]
- Pearl NZ, Babin CP, Catalano NT, Blake JC, Ahmadzadeh S, Shekoohi S, Kaye AD (2023) Narrative review of topiramate: Clinical uses and pharmacological considerations. Adv Ther. [CrossRef]
- Hershey AD, Powers SW, Vockell AL, LeCates S, Kabbouche M (2002) Effectiveness of topiramate in the prevention of childhood headaches. Headache 42:810-818. [CrossRef]
- Hershey AD, Powers SW, Vockell AL, LeCates SL, Segers A, Kabbouche MA (2004) Development of a patient-based grading scale for pedmidas. Cephalalgia 24:844-849. [CrossRef]
- Kotb Elmala M, Suliman HA, Al-Shokary AH, Ibrahim AO, Kamal NM, Elshorbagy HH, Nasef KA, El Din Fathallah MG (2022) The impact of vitamin d(3) supplementation to topiramate therapy on pediatric migraine prophylaxis. J Child Neurol 37:833-839. [CrossRef]
- Arzani M, Jahromi SR, Ghorbani Z, Vahabizad F, Martelletti P, Ghaemi A, Sacco S, Togha M (2020) Gut-brain axis and migraine headache: A comprehensive review. J Headache Pain 21:15. [CrossRef]
- Fan PC, Kuo PH, Chang SH, Lee WT, Wu RM, Chiou LC (2009) Plasma calcitonin gene-related peptide in diagnosing and predicting paediatric migraine. Cephalalgia 29:883-890. [CrossRef]
- Fan PC, Kuo PH, Lee MT, Chang SH, Chiou LC (2019) Plasma calcitonin gene-related peptide: A potential biomarker for diagnosis and therapeutic responses in pediatric migraine. Front Neurol 10:10. [CrossRef]
- Gallai V, Sarchielli P, Floridi A, Franceschini M, Codini M, Glioti G, Trequattrini A, Palumbo R (1995) Vasoactive peptide levels in the plasma of young migraine patients with and without aura assessed both interictally and ictally. Cephalalgia 15:384-390. [CrossRef]
- Wang G, Tan T, Liu Y, Hong P (2020) Drugs for acute attack of pediatric migraine: A network meta-analysis of randomized controlled trials. Clin Neurol Neurosurg 195:105853. [CrossRef]
- Evers S (2022) Cgrp in childhood and adolescence migraine: (patho)physiological and clinical aspects. Curr Pain Headache Rep 26:475-480. [CrossRef]
- Fleming MA, 2nd, Ehsan L, Moore SR, Levin DE (2020) The enteric nervous system and its emerging role as a therapeutic target. Gastroenterol Res Pract 2020:8024171. [CrossRef]
- Furness JB, Callaghan BP, Rivera LR, Cho HJ (2014) The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Advances in experimental medicine and biology 817:39-71. [CrossRef]
- Gwak MG, Chang SY (2021) Gut-brain connection: Microbiome, gut barrier, and environmental sensors. Immune Netw 21:e20. [CrossRef]
- Drewes AM, Olesen AE, Farmer AD, Szigethy E, Rebours V, Olesen SS (2020) Gastrointestinal pain. Nat Rev Dis Primers 6:1. [CrossRef]
- Mayer EA, Tillisch K (2011) The brain-gut axis in abdominal pain syndromes. Annu Rev Med 62:381-396. [CrossRef]
- Bielefeldt K, Christianson JA, Davis BM (2005) Basic and clinical aspects of visceral sensation: Transmission in the cns. Neurogastroenterol Motil 17:488-499. [CrossRef]
- Mayer EA, Naliboff BD, Craig AD (2006) Neuroimaging of the brain-gut axis: From basic understanding to treatment of functional gi disorders. Gastroenterology 131:1925-1942. [CrossRef]
- Porreca F, Ossipov MH, Gebhart GF (2002) Chronic pain and medullary descending facilitation. Trends Neurosci 25:319-325. [CrossRef]
- Berntson GG, Sarter M, Cacioppo JT (2003) Ascending visceral regulation of cortical affective information processing. Eur J Neurosci 18:2103-2109. [CrossRef]
- Aurora SK, Papapetropoulos S, Kori SH, Kedar A, Abell TL (2013) Gastric stasis in migraineurs: Etiology, characteristics, and clinical and therapeutic implications. Cephalalgia 33:408-415. [CrossRef]
- Devanarayana NM, Rajindrajith S, Benninga MA (2016) Abdominal migraine in children: Association between gastric motility parameters and clinical characteristics. BMC Gastroenterol 16:26. [CrossRef]
- Devanarayana NM, Rajindrajith S, Perera MS, Nishanthanie SW, Benninga MA (2013) Gastric emptying and antral motility parameters in children with functional dyspepsia: Association with symptom severity. J Gastroenterol Hepatol 28:1161-1166. [CrossRef]
- Shava U, Srivastava A, Mathias A, Kumar N, Yachha SK, Gambhir S, Poddar U (2021) Functional dyspepsia in children: A study of pathophysiological factors. J Gastroenterol Hepatol 36:680-686. [CrossRef]
- Parker A, Fonseca S, Carding SR (2020) Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 11:135-157. [CrossRef]
- Chitkara DK, Camilleri M, Zinsmeister AR, Burton D, El-Youssef M, Freese D, Walker L, Stephens D (2005) Gastric sensory and motor dysfunction in adolescents with functional dyspepsia. J Pediatr 146:500-505. [CrossRef]
- Cucchiara S, Bortolotti M, Colombo C, Boccieri A, De Stefano M, Vitiello G, Pagano A, Ronchi A, Auricchio S (1991) Abnormalities of gastrointestinal motility in children with nonulcer dyspepsia and in children with gastroesophageal reflux disease. Dig Dis Sci 36:1066-1073. [CrossRef]
- Miller J, Khlevner J, Rodriguez L (2021) Upper gastrointestinal functional and motility disorders in children. Pediatr Clin North Am 68:1237-1253. [CrossRef]
- Riezzo G, Cucchiara S, Chiloiro M, Minella R, Guerra V, Giorgio I (1995) Gastric emptying and myoelectrical activity in children with nonulcer dyspepsia. Effect of cisapride. Dig Dis Sci 40:1428-1434. [CrossRef]
- Woodruff AE, Cieri NE, Abeles J, Seyse SJ (2013) Abdominal migraine in adults: A review of pharmacotherapeutic options. Ann Pharmacother 47:e27. [CrossRef]
- Borkum JM (2021) Brain energy deficit as a source of oxidative stress in migraine: A molecular basis for migraine susceptibility. Neurochem Res 46:1913-1932. [CrossRef]
- Rolfe DF, Brown GC (1997) Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiological reviews 77:731-758. [CrossRef]
- Ashina M, Hansen JM, Do TP, Melo-Carrillo A, Burstein R, Moskowitz MA (2019) Migraine and the trigeminovascular system—40 years and counting. The Lancet Neurology 18:795-804. [CrossRef]
- Gazerani P (2020) Migraine and diet. Nutrients 12. [CrossRef]
- Gazerani P (2021) A bidirectional view of migraine and diet relationship. Neuropsychiatr Dis Treat 17:435-451. [CrossRef]
- Barbiroli B, Montagna P, Cortelli P, Funicello R, Iotti S, Monari L, Pierangeli G, Zaniol P, Lugaresi E (1992) Abnormal brain and muscle energy metabolism shown by 31p magnetic resonance spectroscopy in patients affected by migraine with aura. Neurology 42:1209-1214. [CrossRef]
- Montagna P, Cortelli P, Barbiroli B (1994) Magnetic resonance spectroscopy studies in migraine. Cephalalgia 14:184-193. [CrossRef]
- Lodi R, Iotti S, Cortelli P, Pierangeli G, Cevoli S, Clementi V, Soriani S, Montagna P, Barbiroli B (2001) Deficient energy metabolism is associated with low free magnesium in the brains of patients with migraine and cluster headache. Brain Res Bull 54:437-441. [CrossRef]
- Reyngoudt H, Paemeleire K, Descamps B, De Deene Y, Achten E (2011) 31p-mrs demonstrates a reduction in high-energy phosphates in the occipital lobe of migraine without aura patients. Cephalalgia 31:1243-1253. [CrossRef]
- Irwin S, Barmherzig R, Gelfand A (2017) Recurrent gastrointestinal disturbance: Abdominal migraine and cyclic vomiting syndrome. Curr Neurol Neurosci Rep 17:21. [CrossRef]
- Younis S, Hougaard A, Vestergaard MB, Larsson HBW, Ashina M (2017) Migraine and magnetic resonance spectroscopy: A systematic review. Curr Opin Neurol 30:246-262. [CrossRef]
- Kekelidze T, Khait I, Togliatti A, Benzecry JM, Wieringa B, Holtzman D (2001) Altered brain phosphocreatine and atp regulation when mitochondrial creatine kinase is absent. Journal of neuroscience research 66:866-872. [CrossRef]
- Belenguer P, Duarte JMN, Schuck PF, Ferreira GC (2019) Mitochondria and the brain: Bioenergetics and beyond. Neurotoxicity Research 36:219-238. [CrossRef]
- Fila M, Pawłowska E, Blasiak J (2019) Mitochondria in migraine pathophysiology - does epigenetics play a role? Arch Med Sci 15:944-956. [CrossRef]
- Lisicki M, D'Ostilio K, Coppola G, Parisi V, de Noordhout AM, Magis D, Schoenen J, Scholtes F, Versijpt J (2019) Age related metabolic modifications in the migraine brain. Cephalalgia 39:978-987. [CrossRef]
- Eising E, de Vries B, Ferrari MD, Terwindt GM, van den Maagdenberg AM (2013) Pearls and pitfalls in genetic studies of migraine. Cephalalgia 33:614-625. [CrossRef]
- Eising E, Huisman SMH, Mahfouz A, Vijfhuizen LS, Anttila V, Winsvold BS, Kurth T, Ikram MA, Freilinger T, Kaprio J, Boomsma DI, van Duijn CM, Järvelin MR, Zwart JA, Quaye L, Strachan DP, Kubisch C, Dichgans M, Davey Smith G, Stefansson K, Palotie A, Chasman DI, Ferrari MD, Terwindt GM, de Vries B, Nyholt DR, Lelieveldt BPF, van den Maagdenberg A, Reinders MJT (2016) Gene co-expression analysis identifies brain regions and cell types involved in migraine pathophysiology: A gwas-based study using the allen human brain atlas. Human genetics 135:425-439. [CrossRef]
- Kaur S, Ali A, Siahbalaei Y, Ahmad U, Pandey AK, Singh B (2019) Could rs4379368 be a genetic marker for north indian migraine patients with aura?: Preliminary evidence by a replication study. Neuroscience letters 712:134482. [CrossRef]
- Zaki EA, Freilinger T, Klopstock T, Baldwin EE, Heisner KR, Adams K, Dichgans M, Wagler S, Boles RG (2009) Two common mitochondrial DNA polymorphisms are highly associated with migraine headache and cyclic vomiting syndrome. Cephalalgia 29:719-728. [CrossRef]
- Lisicki M, D'Ostilio K, Coppola G, Scholtes F, Maertens de Noordhout A, Parisi V, Schoenen J, Magis D (2018) Evidence of an increased neuronal activation-to-resting glucose uptake ratio in the visual cortex of migraine patients: A study comparing (18)fdg-pet and visual evoked potentials. J Headache Pain 19:49. [CrossRef]
- Plantone D, Pardini M, Rinaldi G (2021) Riboflavin in neurological diseases: A narrative review. Clin Drug Investig 41:513-527. [CrossRef]
- Bruijn J, Duivenvoorden H, Passchier J, Locher H, Dijkstra N, Arts WF (2010) Medium-dose riboflavin as a prophylactic agent in children with migraine: A preliminary placebo-controlled, randomised, double-blind, cross-over trial. Cephalalgia 30:1426-1434. [CrossRef]
- MacLennan SC, Wade FM, Forrest KM, Ratanayake PD, Fagan E, Antony J (2008) High-dose riboflavin for migraine prophylaxis in children: A double-blind, randomized, placebo-controlled trial. J Child Neurol 23:1300-1304. [CrossRef]
- Condò M, Posar A, Arbizzani A, Parmeggiani A (2009) Riboflavin prophylaxis in pediatric and adolescent migraine. J Headache Pain 10:361-365. [CrossRef]
- Aaseth J, Alexander J, Alehagen U (2021) Coenzyme q(10) supplementation - in ageing and disease. Mech Ageing Dev 197:111521. [CrossRef]
- Hershey AD, Powers SW, Vockell AL, Lecates SL, Ellinor PL, Segers A, Burdine D, Manning P, Kabbouche MA (2007) Coenzyme q10 deficiency and response to supplementation in pediatric and adolescent migraine. Headache 47:73-80. [CrossRef]
- Slater SK, Nelson TD, Kabbouche MA, LeCates SL, Horn P, Segers A, Manning P, Powers SW, Hershey AD (2011) A randomized, double-blinded, placebo-controlled, crossover, add-on study of coenzyme q10 in the prevention of pediatric and adolescent migraine. Cephalalgia 31:897-905. [CrossRef]
- Fila M, Chojnacki C, Chojnacki J, Blasiak J (2021) Nutrients to improve mitochondrial function to reduce brain energy deficit and oxidative stress in migraine. Nutrients 13. [CrossRef]
- Badawy AA (2017) Kynurenine pathway of tryptophan metabolism: Regulatory and functional aspects. Int J Tryptophan Res 10:1178646917691938. [CrossRef]
- Fukuwatari T (2020) Possibility of amino acid treatment to prevent the psychiatric disorders via modulation of the production of tryptophan metabolite kynurenic acid. Nutrients 12. [CrossRef]
- Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX (2001) The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: Physiopathological implications. J Neurosci 21:7463-7473. [CrossRef]
- Kessler M, Terramani T, Lynch G, Baudry M (1989) A glycine site associated with n-methyl-d-aspartic acid receptors: Characterization and identification of a new class of antagonists. J Neurochem 52:1319-1328. [CrossRef]
- Wang J, Simonavicius N, Wu X, Swaminath G, Reagan J, Tian H, Ling L (2006) Kynurenic acid as a ligand for orphan g protein-coupled receptor gpr35. The Journal of biological chemistry 281:22021-22028. [CrossRef]
- Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH (2000) An association between migraine and cutaneous allodynia. 6: Ann Neurol 47.
- Liu YJ, Li YL, Fang ZH, Liao HL, Zhang YY, Lin J, Liu F, Shen JF (2022) Nmdars mediate peripheral and central sensitization contributing to chronic orofacial pain. Front Cell Neurosci 16:999509. [CrossRef]
- Curto M, Lionetto L, Negro A, Capi M, Fazio F, Giamberardino MA, Simmaco M, Nicoletti F, Martelletti P (2015) Altered kynurenine pathway metabolites in serum of chronic migraine patients. J Headache Pain 17:47. [CrossRef]
- Benbow T, Teja F, Sheikhi A, Exposto FG, Svensson P, Cairns BE (2022) Peripheral n-methyl-d-aspartate receptor activation contributes to monosodium glutamate-induced headache but not nausea behaviours in rats. Sci Rep 12:13894. [CrossRef]
- Kemp JA, Foster AC, Leeson PD, Priestley T, Tridgett R, Iversen LL, Woodruff GN (1988) 7-chlorokynurenic acid is a selective antagonist at the glycine modulatory site of the n-methyl-d-aspartate receptor complex. Proceedings of the National Academy of Sciences of the United States of America 85:6547-6550. [CrossRef]
- Chauvel V, Vamos E, Pardutz A, Vecsei L, Schoenen J, Multon S (2012) Effect of systemic kynurenine on cortical spreading depression and its modulation by sex hormones in rat. Exp Neurol 236:207-214. [CrossRef]
- Oláh G, Herédi J, Menyhárt A, Czinege Z, Nagy D, Fuzik J, Kocsis K, Knapp L, Krucsó E, Gellért L, Kis Z, Farkas T, Fülöp F, Párdutz A, Tajti J, Vécsei L, Toldi J (2013) Unexpected effects of peripherally administered kynurenic acid on cortical spreading depression and related blood-brain barrier permeability. Drug Des Devel Ther 7:981-987. [CrossRef]
- Tuka B, Nyári A, Cseh EK, Körtési T, Veréb D, Tömösi F, Kecskeméti G, Janáky T, Tajti J, Vécsei L (2021) Clinical relevance of depressed kynurenine pathway in episodic migraine patients: Potential prognostic markers in the peripheral plasma during the interictal period. J Headache Pain 22:60. [CrossRef]
- Hyams JS, Di Lorenzo C, Saps M, Shulman RJ, Staiano A, van Tilburg M (2016) Functional disorders: Children and adolescents. Gastroenterology. [CrossRef]
- Gelfand AA, Goadsby PJ, Allen IE (2015) The relationship between migraine and infant colic: A systematic review and meta-analysis. Cephalalgia 35:63-72. [CrossRef]
- Qubty W, Gelfand AA (2016) The link between infantile colic and migraine. Curr Pain Headache Rep 20:31. [CrossRef]
- Trivić I, Hojsak I (2018) Initial diagnosis of functional gastrointestinal disorders in children increases a chance for resolution of symptoms. Pediatr Gastroenterol Hepatol Nutr 21:264-270. [CrossRef]
- van Hemert S, Breedveld AC, Rovers JM, Vermeiden JP, Witteman BJ, Smits MG, de Roos NM (2014) Migraine associated with gastrointestinal disorders: Review of the literature and clinical implications. Front Neurol 5:241. [CrossRef]
- Zhang D, Zhang Y, Sang Y, Zheng N, Liu X (2019) The relationship between infant colic and migraine as well as tension-type headache: A meta-analysis. Pain Res Manag 2019:8307982. [CrossRef]
- Eminson DM (2007) Medically unexplained symptoms in children and adolescents. Clinical Psychology Review 27:855-871. [CrossRef]
- Apley J, Naish N (1958) Recurrent abdominal pains: A field survey of 1,000 school children. Arch Dis Child 33:165-170. [CrossRef]
- Myint K, Jacobs K, Myint A-M, Lam SK, Lim YA-L, Boey CC-M, Hoe SZ, Guillemin GJ (2021) Psychological stresses in children trigger cytokine- and kynurenine metabolite-mediated abdominal pain and proinflammatory changes. Frontiers in Immunology 12. [CrossRef]
- Burr RL, Gu H, Cain K, Djukovic D, Zhang X, Han C, Callan N, Raftery D, Heitkemper M (2019) Tryptophan metabolites in irritable bowel syndrome: An overnight time-course study. J Neurogastroenterol Motil 25:551-562. [CrossRef]
- Clarke G, McKernan DP, Gaszner G, Quigley EM, Cryan JF, Dinan TG (2012) A distinct profile of tryptophan metabolism along the kynurenine pathway downstream of toll-like receptor activation in irritable bowel syndrome. Front Pharmacol 3:90. [CrossRef]
- Kennedy PJ, Allen AP, O'Neill A, Quigley EM, Cryan JF, Dinan TG, Clarke G (2015) Acute tryptophan depletion reduces kynurenine levels: Implications for treatment of impaired visuospatial memory performance in irritable bowel syndrome. Psychopharmacology (Berl) 232:1357-1371. [CrossRef]
- Keszthelyi D, Troost FJ, Jonkers DM, Kruimel JW, Leue C, Masclee AA (2013) Decreased levels of kynurenic acid in the intestinal mucosa of ibs patients: Relation to serotonin and psychological state. J Psychosom Res 74:501-504. [CrossRef]
- Li P, Zheng J, Bai Y, Wang D, Cui Z, Li Y, Zhang J, Wang Y (2020) Characterization of kynurenine pathway in patients with diarrhea-predominant irritable bowel syndrome. Eur J Histochem 64. [CrossRef]
- Ramachandran R, Wang Z, Saavedra C, DiNardo A, Corr M, Powell SB, Yaksh TL (2019) Role of toll-like receptor 4 signaling in mast cell-mediated migraine pain pathway. Mol Pain 15:1744806919867842. [CrossRef]
- Chang FY, Lu CL (2013) Irritable bowel syndrome and migraine: Bystanders or partners? J Neurogastroenterol Motil 19:301-311. [CrossRef]
- Viera AJ, Hoag S, Shaughnessy J (2002) Management of irritable bowel syndrome. 1: Am Fam Physician 66, 1867.
- Chojnacki C, Poplawski T, Blonska A, Konrad P, Chojnacki J, Blasiak J (2023) The usefulness of the low-fodmap diet with limited tryptophan intake in the treatment of diarrhea-predominant irritable bowel syndrome. Nutrients 15. [CrossRef]
- Christmas DM, Badawy AA, Hince D, Davies SJ, Probert C, Creed T, Smithson J, Afzal M, Nutt DJ, Potokar JP (2010) Increased serum free tryptophan in patients with diarrhea-predominant irritable bowel syndrome. Nutr Res 30:678-688. [CrossRef]
- Sanyaolu A, Okorie C, Qi X, Locke J, Rehman S (2019) Childhood and adolescent obesity in the united states: A public health concern. Glob Pediatr Health 6:2333794x19891305. [CrossRef]
- Spinelli A, Buoncristiano M, Kovacs VA, Yngve A, Spiroski I, Obreja G, Starc G, Pérez N, Rito AI, Kunešová M, Sant'Angelo VF, Meisfjord J, Bergh IH, Kelleher C, Yardim N, Pudule I, Petrauskiene A, Duleva V, Sjöberg A, Gualtieri A, Hassapidou M, Hyska J, Burazeri G, Petrescu CH, Heinen M, Takacs H, Zamrazilová H, Bosi TB, Sacchini E, Pagkalos I, Cucu A, Nardone P, Gately P, Williams J, Breda J (2019) Prevalence of severe obesity among primary school children in 21 european countries. Obes Facts 12:244-258. [CrossRef]
- Dietz WH (1998) Health consequences of obesity in youth: Childhood predictors of adult disease. 5: Pediatrics 101.
- Kelsey MM, Zaepfel A, Bjornstad P, Nadeau KJ (2014) Age-related consequences of childhood obesity. Gerontology 60:222-228. [CrossRef]
- Rolland-Cachera MF, Deheeger M, Maillot M, Bellisle F (2006) Early adiposity rebound: Causes and consequences for obesity in children and adults. Int J Obes (Lond) 30 Suppl 4:S11-17. [CrossRef]
- Weiss R, Bremer AA, Lustig RH (2013) What is metabolic syndrome, and why are children getting it? Ann N Y Acad Sci 1281:123-140. [CrossRef]
- Engin A (2017) The definition and prevalence of obesity and metabolic syndrome. Advances in experimental medicine and biology 960:1-17. [CrossRef]
- Lister NB, Baur LA, Felix JF, Hill AJ, Marcus C, Reinehr T, Summerbell C, Wabitsch M (2023) Child and adolescent obesity. Nat Rev Dis Primers 9:24. [CrossRef]
- Bigal ME, Lipton RB (2006) Obesity is a risk factor for transformed migraine but not chronic tension-type headache. Neurology 67:252-257. [CrossRef]
- Verrotti A, Di Fonzo A, Agostinelli S, Coppola G, Margiotta M, Parisi P (2012) Obese children suffer more often from migraine. Acta Paediatr 101:e416-421. [CrossRef]
- Törk I (1990) Anatomy of the serotonergic system. Ann N Y Acad Sci 600:9-34; discussion 34-35. [CrossRef]
- van Galen KA, ter Horst KW, Serlie MJ (2021) Serotonin, food intake, and obesity. Obesity Reviews 22:e13210. [CrossRef]
- Ferrari MD, Odink J, Tapparelli C, Van Kempen GM, Pennings EJ, Bruyn GW (1989) Serotonin metabolism in migraine. Neurology 39:1239-1242. [CrossRef]
- Hamel E (2007) Serotonin and migraine: Biology and clinical implications. Cephalalgia 27:1293-1300. [CrossRef]
- Deen M, Christensen CE, Hougaard A, Hansen HD, Knudsen GM, Ashina M (2016) Serotonergic mechanisms in the migraine brain – a systematic review. Cephalalgia 37:251-264. [CrossRef]
- Humphrey PP (1991) 5-hydroxytryptamine and the pathophysiology of migraine. J Neurol 238 Suppl 1:S38-44. [CrossRef]
- Deen M, Hansen HD, Hougaard A, Nørgaard M, Eiberg H, Lehel S, Ashina M, Knudsen GM (2018) High brain serotonin levels in migraine between attacks: A 5-ht4 receptor binding pet study. NeuroImage: Clinical 18:97-102. [CrossRef]
- Pinhas-Hamiel O, Frumin K, Gabis L, Mazor-Aronovich K, Modan-Moses D, Reichman B, Lerner-Geva L (2008) Headaches in overweight children and adolescents referred to a tertiary-care center in israel. Obesity (Silver Spring) 16:659-663. [CrossRef]
- Moyer BJ, Rojas IY, Kerley-Hamilton JS, Hazlett HF, Nemani KV, Trask HW, West RJ, Lupien LE, Collins AJ, Ringelberg CS, Gimi B, Kinlaw WB, Tomlinson CR (2016) Inhibition of the aryl hydrocarbon receptor prevents western diet-induced obesity. Model for ahr activation by kynurenine via oxidized-ldl, tlr2/4, tgfβ, and ido1. Toxicology and Applied Pharmacology 300:13-24. [CrossRef]
- Lischka J, Schanzer A, Baumgartner M, de Gier C, Greber-Platzer S, Zeyda M (2022) Tryptophan metabolism is associated with bmi and adipose tissue mass and linked to metabolic disease in pediatric obesity. Nutrients 14:286. https://www.mdpi.com/2072-6643/14/2/286.
- Tan KM-L, Tint M-T, Kothandaraman N, Yap F, Godfrey KM, Lee YS, Tan KH, Gluckman PD, Chong Y-S, Chong MFF, Eriksson JG, Cameron-Smith D (2022) Association of plasma kynurenine pathway metabolite concentrations with metabolic health risk in prepubertal asian children. International Journal of Obesity 46:1128-1137. [CrossRef]
- Chojnacki C, Gąsiorowska A, Popławski T, Błońska A, Konrad P, Zajdler R, Chojnacki J, Blasiak J (2023) Reduced intake of dietary tryptophan improves beneficial action of budesonide in patients with lymphocytic colitis and mood disorders. Nutrients 15. [CrossRef]
- Chojnacki C, Gąsiorowska A, Popławski T, Konrad P, Chojnacki M, Fila M, Blasiak J (2023) Beneficial effect of increased tryptophan intake on its metabolism and mental state of the elderly. Nutrients 15. [CrossRef]
- Chojnacki C, Popławski T, Chojnacki J, Fila M, Konrad P, Blasiak J (2020) Tryptophan intake and metabolism in older adults with mood disorders. Nutrients 12. [CrossRef]
- Chojnacki C, Popławski T, Konrad P, Fila M, Błasiak J, Chojnacki J (2022) Antimicrobial treatment improves tryptophan metabolism and mood of patients with small intestinal bacterial overgrowth. Nutr Metab (Lond) 19:66. [CrossRef]
- Sandyk R (1992) L-tryptophan in neuropsychiatric disorders: A review. Int J Neurosci 67:127-144. [CrossRef]
- Wei L, Singh R, Ro S, Ghoshal UC (2021) Gut microbiota dysbiosis in functional gastrointestinal disorders: Underpinning the symptoms and pathophysiology. JGH Open 5:976-987. [CrossRef]
- Bai J, Shen N, Liu Y (2023) Associations between the gut microbiome and migraines in children aged 7-18 years: An analysis of the american gut project cohort. Pain Manag Nurs 24:35-43. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).