Submitted:
05 August 2023
Posted:
08 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study protocol.
2.3. Biochemical analyses.
2.4. Statistical methods.
2.5. Ethics
3. Results
3.1. Participants’ somatic and physiological characteristics
3.2. Macronutrients intake.
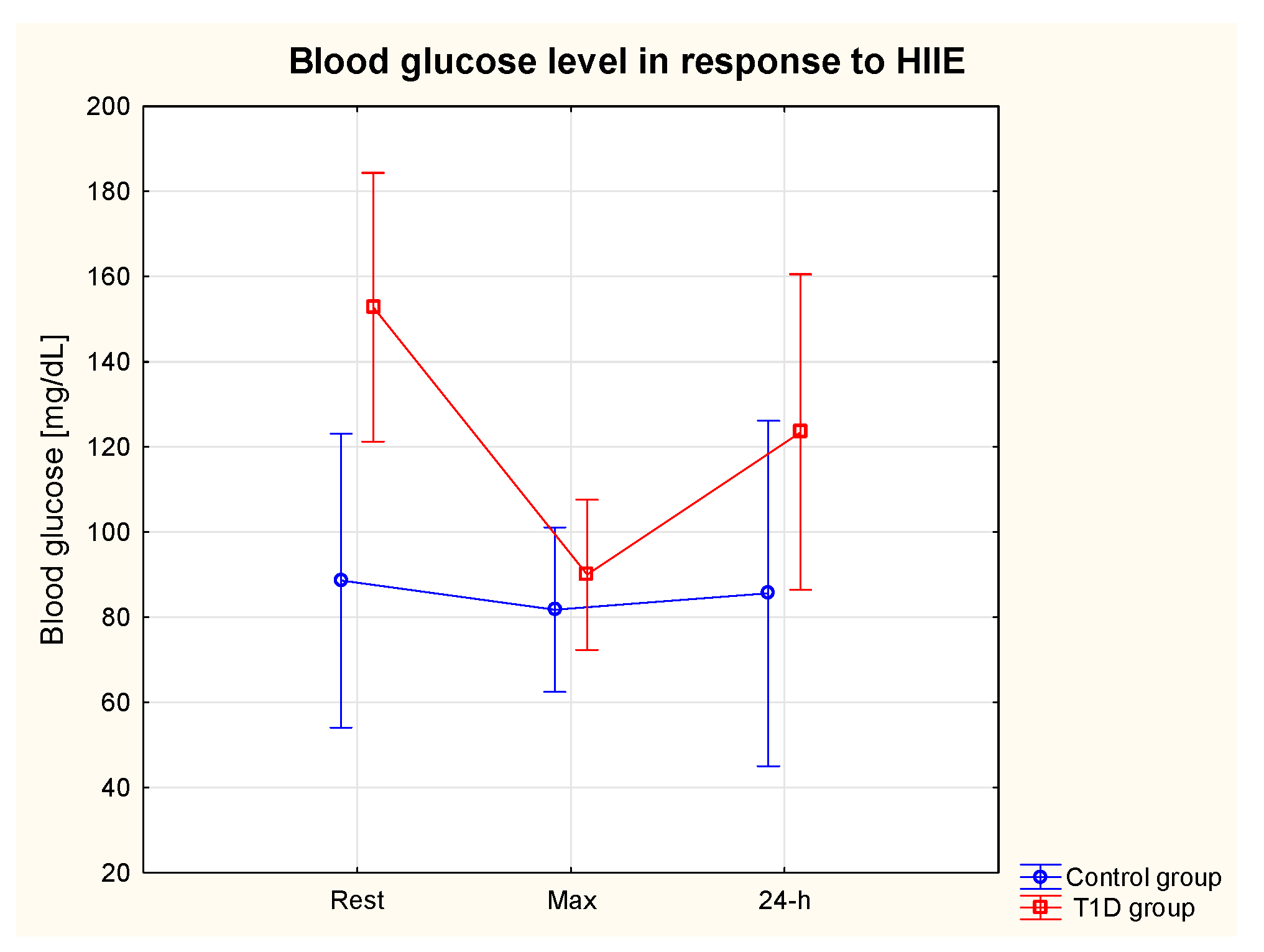
3.3. Glycaemia in response to HIIE
3.4. Glycaemic control in 24-h post-exercise period
3.5. HIF-1α, TNF-α and VEGF in response to HIIE
3.5.1. HIF-1α

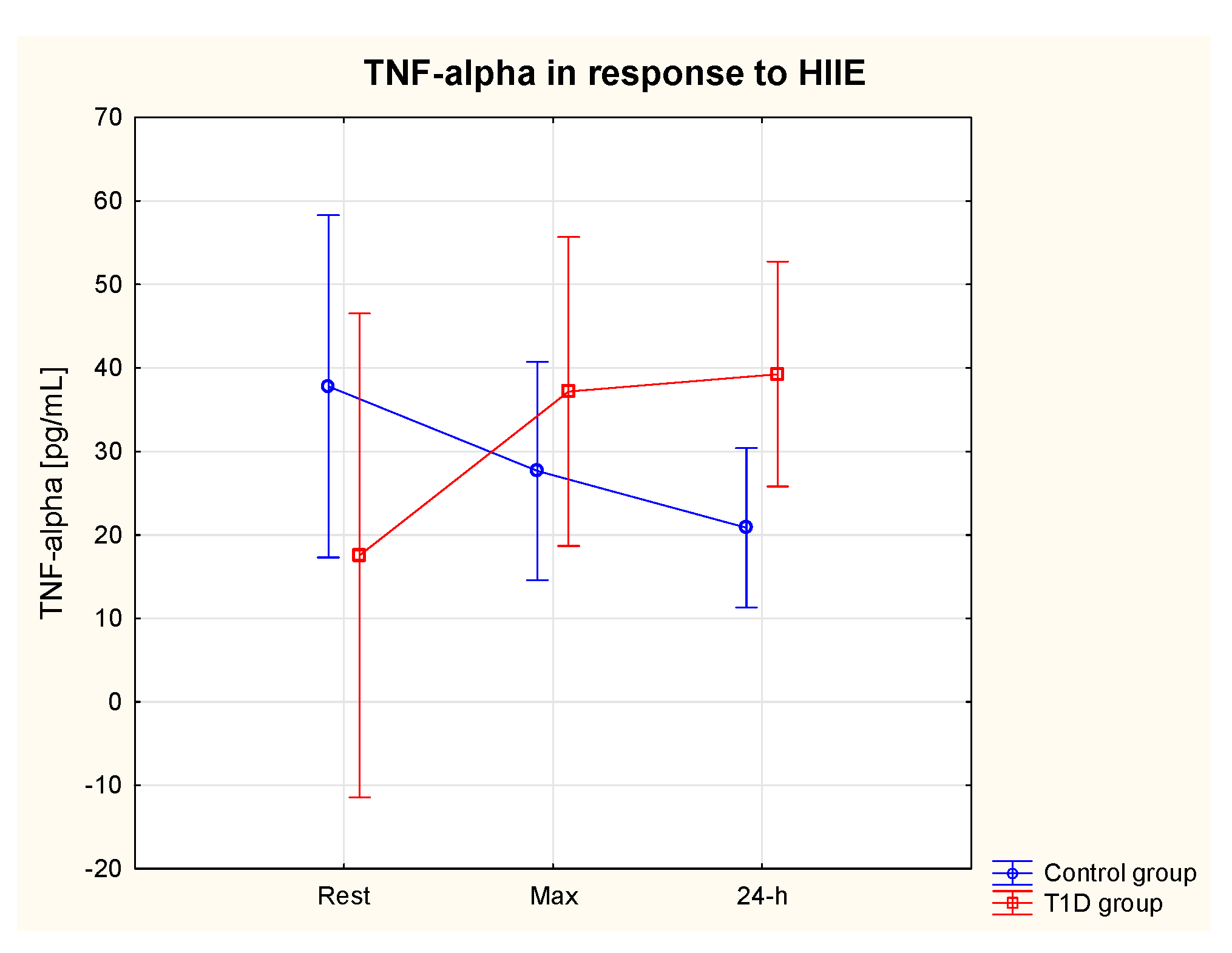
3.5.2. TNF-α
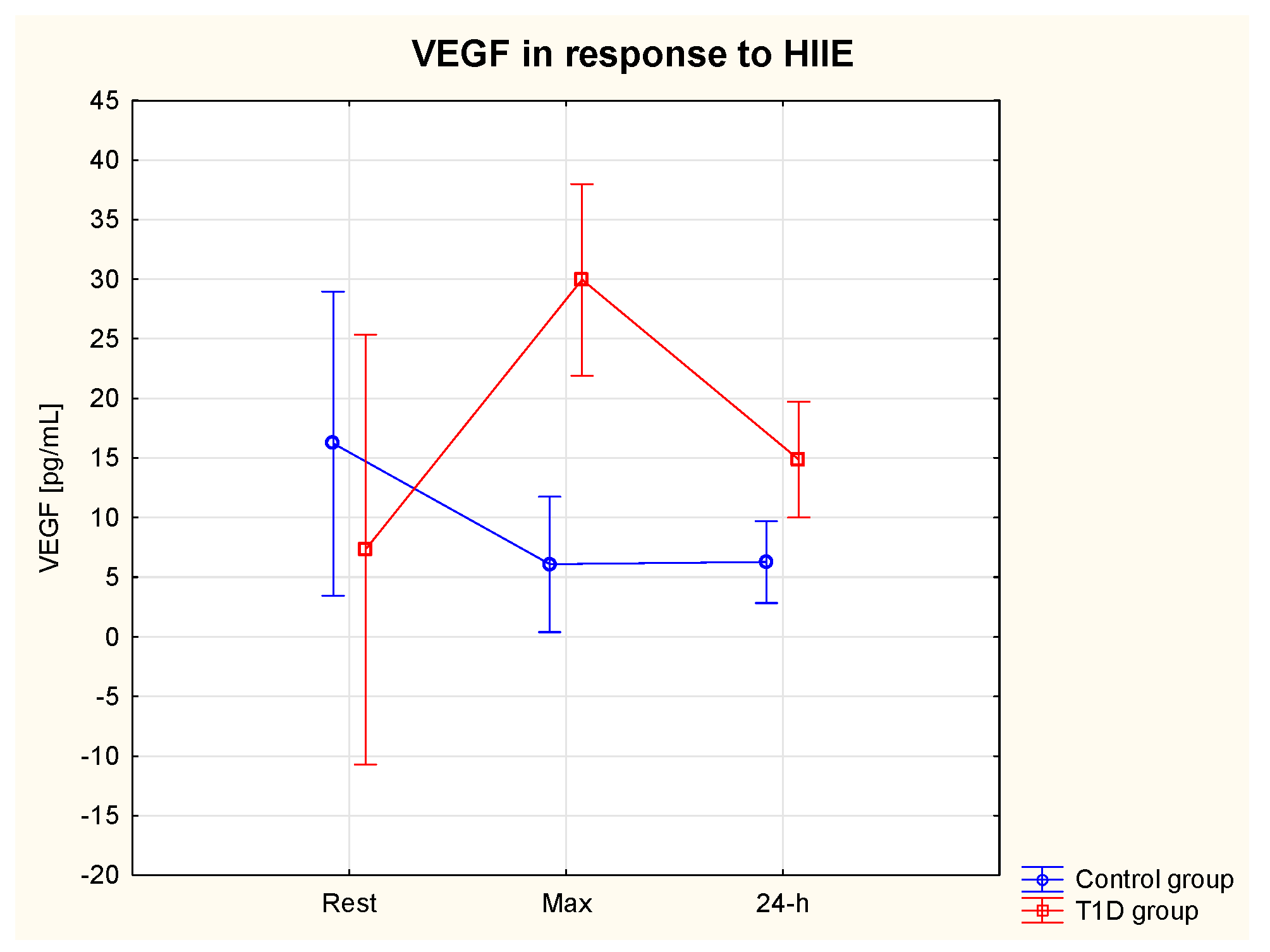
3.5.3. VEGF
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moganti, K.; Li, F.; Schmuttermaier, C.; Riemann, S.; Klüter, H.; Gratchev, A.; Harmsen, M.C.; Kzhyshkowska, J. Hyperglycemia induces mixed M1/M2 cytokine profile in primary human monocyte-derived macrophages. Immunobiology 2017, 222, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Torres-Castro, I.; Arroyo-Camarena, D.; Martínez-Reyes, C.P.; Gómez-Arauz, A.Y.; Dueñas-Andrade, Y.; Hernández-Ruiz, J.; Béjar, Y.L.; Zaga-Clavellina, V.; Morales-Montor, J.; Terrazas, L.I.; et al. Human monocytes and macrophages undergo M1-type inflammatory polarization in response to high levels of glucose. Immunol. Lett. 2016, 176, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Sethi, J.K.; Hotamisligil, G.S. Metabolic Messengers: tumour necrosis factor. Nat. Metab. 2021, 3, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Crespo, J.; Cayón, A.; Fernández-Gil, P.; Hernández-Guerra, M.; Mayorga, M.; Domínguez-Díez, A.; Fernández-Escalante, J.C.; Pons-Romero, F. Gene expression of tumor necrosis factor [alpha ] and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology 2001, 34, 1158–1163. [Google Scholar] [CrossRef]
- Kugelmas, M.; Hill, D.B.; Vivian, B.; Marsano, L.; McClain, C.J. Cytokines and NASH: A pilot study of the effects of lifestyle modification and vitamin E. Hepatology 2003, 38, 413–419. [Google Scholar] [CrossRef]
- Mohamed-Ali, V.; Armstrong, L.; Clarke, D.; Bolton, C.H.; Pinkney, J.H. Evidence for the regulation of levels of plasma adhesion molecules by proinflammatory cytokines and their soluble receptors in type 1 diabetes. J. Intern. Med. 2001, 250, 415–421. [Google Scholar] [CrossRef]
- Al-Isa, A.N.; Thalib, L.; Akanji, A.O. Circulating markers of inflammation and endothelial dysfunction in Arab adolescent subjects: Reference ranges and associations with age, gender, body mass and insulin sensitivity. Atherosclerosis 2010, 208, 543–549. [Google Scholar] [CrossRef]
- Popov, D. Endothelial cell dysfunction in hyperglycemia: Phenotypic change, intracellular signaling modification, ultrastructural alteration, and potential clinical outcomes. Int. J. Diabetes Mellit. 2010, 2, 189–195. [Google Scholar] [CrossRef]
- Haidari, M.; Zhang, W.; Willerson, J.T.; Dixon, R.A. Disruption of endothelial adherens junctions by high glucose is mediated by protein kinase C-β–dependent vascular endothelial cadherin tyrosine phosphorylation. Cardiovasc. Diabetol. 2014, 13, 105–105. [Google Scholar] [CrossRef]
- Domingueti, C.P.; Dusse, L.M.S.; Carvalho, M.d.G.; de Sousa, L.P.; Gomes, K.B.; Fernandes, A.P. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J. Diabetes its Complicat. 2016, 30, 738–745. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Davis-Smyth, T. The Biology of Vascular Endothelial Growth Factor. Endocr. Rev. 1997, 18, 4–25. [Google Scholar] [CrossRef]
- Melder, R.J.; Koenig, G.C.; Witwer, B.P.; Safabakhsh, N.; Munn, L.L.; Jain, R.K. During angiogenesis, vascular endothelial growth factor regulate natural killer cell adhesion to tumor endothelium. Nat. Med. 1996, 2, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Barleon, B.; Sozzani, S.; Zhou, D.; Weich, H.; Mantovani, A.; Marme, D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 1996, 87, 3336–3343. [Google Scholar] [CrossRef] [PubMed]
- Vordermark, D.; Kraft, P.; Katzer, A.; Bölling, T.; Willner, J.; Flentje, M. Glucose requirement for hypoxic accumulation of hypoxia-inducible factor-1α (HIF-1α). Cancer Lett. 2005, 230, 122–133. [Google Scholar] [CrossRef]
- Staab, A.; Löffler, J.; Said, H.M.; Katzer, A.; Beyer, M.; Polat, B.; Einsele, H.; Flentje, M.; Vordermark, D. Modulation of Glucose Metabolism Inhibits Hypoxic Accumulation of Hypoxia-Inducible Factor-1α (HIF-1α). Strahlenther Onkol 2007, 183, 366–373. [Google Scholar] [CrossRef]
- Catrina, S.-B.; Zheng, X. Hypoxia and hypoxia-inducible factors in diabetes and its complications. Diabetologia 2021, 64, 709–716. [Google Scholar] [CrossRef]
- Sada, K.; Nishikawa, T.; Kukidome, D.; Yoshinaga, T.; Kajihara, N.; Sonoda, K.; Senokuchi, T.; Motoshima, H.; Matsumura, T.; Araki, E. Hyperglycemia Induces Cellular Hypoxia through Production of Mitochondrial ROS Followed by Suppression of Aquaporin-1. PLOS ONE 2016, 11, e0158619. [Google Scholar] [CrossRef]
- Echevarría, M.; Muñoz-Cabello, A.M.; Sánchez-Silva, R.; Toledo-Aral, J.J.; López-Barneo, J. Development of Cytosolic Hypoxia and Hypoxia-inducible Factor Stabilization Are Facilitated by Aquaporin-1 Expression. J. Biol. Chem. 2007, 282, 30207–30215. [Google Scholar] [CrossRef]
- Sugano, R.; Matsuoka, H.; Haramaki, N.; Umei, H.; Murase, E.; Fukami, K.; Iida, S.; Ikeda, H.; Imaizumi, T. Polymorphonuclear Leukocytes May Impair Endothelial Function. Arter. Thromb. Vasc. Biol. 2005, 25, 1262–1267. [Google Scholar] [CrossRef]
- Kennedy, L.; Baynes, W. Non-enzymatic glycosylation and the chronic complications of diabetes: an overview. Diabetologia 1984, 26, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Campos, C. Chronic Hyperglycemia and Glucose Toxicity: Pathology and Clinical Sequelae. Postgrad. Med. 2012, 124, 90–97. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee 6. Glycemic Targets: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45 (Suppl. 1), S83–S96. [Google Scholar] [CrossRef]
- World Health Organization, Global Report on Diabetes. 2016. Available online: http://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf?sequence=1&isAllowed=y) (accessed on 26 January 2022).
- Pedersen, B.K.; Saltin, B. Exercise as medicine–Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. S3), 1–72. [Google Scholar] [CrossRef]
- Reddy, R.; Wittenberg, A.; Castle, J.R.; El Youssef, J.; Winters-Stone, K.; Gillingham, M.; Jacobs, P.G. Effect of Aerobic and Resistance Exercise on Glycemic Control in Adults With Type 1 Diabetes. Can. J. Diabetes 2018, 43, 406–414.e1. [Google Scholar] [CrossRef]
- Colberg, R. Nutrition and Exercise Performance in Adults with Type 1 Diabetes. Can J Diabetes 2020, 44, 750–758. [Google Scholar] [CrossRef]
- Pancheva, R.; Zhelyazkova, D.; Ahmed, F.; Gillon-Keren, M.; Usheva, N.; Bocheva, Y.; Boyadzhieva, M.; Valchev, G.; Yotov, Y.; Iotova, V. Dietary Intake and Adherence to the Recommendations for Healthy Eating in Patients With Type 1 Diabetes: A Narrative Review. Front. Nutr. 2021, 8, 782670. [Google Scholar] [CrossRef]
- Meyer, K.A.; Kushi, L.H.; Jacobs, D.R., Jr.; Slavin, J.; Sellers, T.A.; Folsom, A.R. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am. J. Clin. Nutr. 2000, 71, 921–930. [Google Scholar] [CrossRef]
- Khosravi-Boroujeni, H.; Saadatnia, M.; Shakeri, F.; Keshteli, A.H.; Esmaillzadeh, A. A case-control study on potato consumption and risk of stroke in central Iran. . 2013, 16. [Google Scholar]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef]
- Bohn, B.; Herbst, A.; Pfeifer, M.; Krakow, D.; Zimny, S.; Kopp, F.; Melmer, A.; Steinacker, J.M.; Holl, R.W. Impact of Physical Activity on Glycemic Control and Prevalence of Cardiovascular Risk Factors in Adults With Type 1 Diabetes: A Cross-sectional Multicenter Study of 18,028 Patients. Diabetes Care 2015, 38, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; Zwierzchowska, A.; Jaworska, M.; Solich-Talanda, M.; Mikolajczyk, R.; Zebrowska, A. The effects of physical activity on glycaemic control in children and adolescents with type 1 diabetes mellitus participating in diabetes camp. Balt. J. Heal. Phys. Act. 2018, 10, 151–161. [Google Scholar] [CrossRef]
- Miller, R.G.; Mahajan, H.D.; Costacou, T.; Sekikawa, A.; Anderson, S.J.; Orchard, T.J. A Contemporary Estimate of Total Mortality and Cardiovascular Disease Risk in Young Adults With Type 1 Diabetes: The Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care 2016, 39, 2296–2303. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.; Nirantharakumar, K.; Chimen, M.; Pang, T.T.; Hemming, K.; Andrews, R.C.; Narendran, P. Does Exercise Improve Glycaemic Control in Type 1 Diabetes? A Systematic Review and Meta-Analysis. PLOS ONE 2013, 8, e58861. [Google Scholar] [CrossRef] [PubMed]
- DiPietro, L.; Dziura, J.; Yeckel, C.W.; Neufer, P.D. Exercise and improved insulin sensitivity in older women: evidence of the enduring benefits of higher intensity training. J. Appl. Physiol. 2006, 100, 142–149. [Google Scholar] [CrossRef]
- O’donovan, G.; Kearney, E.M.; Nevill, A.M.; Woolf-May, K.; Bird, S.R. The effects of 24 weeks of moderate- or high-intensity exercise on insulin resistance. Eur. J. Appl. Physiol. 2005, 95, 522–528. [Google Scholar] [CrossRef] [PubMed]
- O'Donovan, G.; Owen, A.; Bird, S.R.; Kearney, E.M.; Nevill, A.M.; Jones, D.W.; Woolf-May, K. Changes in cardiorespiratory fitness and coronary heart disease risk factors following 24 wk of moderate- or high-intensity exercise of equal energy cost. J. Appl. Physiol. 2005, 98, 1619–1625. [Google Scholar] [CrossRef]
- Guiraud, T.; Nigam, A.; Gremeaux, V.; Meyer, P.; Juneau, M.; Bosquet, L. High-Intensity Interval Training in Cardiac Rehabilitation. Sports Med. 2012, 42, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Štajer, V.; Milovanović, I.M.; Todorović, N.; Ranisavljev, M.; Pišot, S.; Drid, P. Let's (Tik) Talk About Fitness Trends. Front. Public Heal. 2022, 10, 899949. [Google Scholar] [CrossRef]
- Moser, O.; Tschakert, G.; Mueller, A.; Groeschl, W.; Pieber, T.R.; Obermayer-Pietsch, B.; Koehler, G.; Hofmann, P. Effects of High-Intensity Interval Exercise versus Moderate Continuous Exercise on Glucose Homeostasis and Hormone Response in Patients with Type 1 Diabetes Mellitus Using Novel Ultra-Long-Acting Insulin. PLOS ONE 2015, 10, e0136489. [Google Scholar] [CrossRef]
- Mitranun, W.; Deerochanawong, C.; Tanaka, H.; Suksom, D. Continuous vs interval training on glycemic control and macro- and microvascular reactivity in type 2 diabetic patients. Scand. J. Med. Sci. Sports 2013, 24, e69–e76. [Google Scholar] [CrossRef]
- Yardley, J.E.; Kenny, G.P.; Perkins, B.A.; Riddell, M.C.; Balaa, N.; Malcolm, J.; Boulay, P.; Khandwala, F.; Sigal, R.J. Resistance Versus Aerobic Exercise. Diabetes Care 2013, 36, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, D.A.; Guy, D.L.A.; Richardson, M.A.; Ertl, A.C.; Davis, S.N. Effects of Low and Moderate Antecedent Exercise on Counterregulatory Responses to Subsequent Hypoglycemia in Type 1 Diabetes. Diabetes 2004, 53, 1798–1806. [Google Scholar] [CrossRef]
- Younk, L.M.; Mikeladze, M.; Tate, D.; Davis, S.N. Exercise-related hypoglycemia in diabetes mellitus. Expert Rev. Endocrinol. Metab. 2011, 6, 93–108. [Google Scholar] [CrossRef]
- Żebrowska, A.; Hall, B.; Kochańska-Dziurowicz, A.; Janikowska, G. The effect of high intensity physical exercise and hypoxia on glycemia, angiogenic biomarkers and cardiorespiratory function in patients with type 1 diabetes. Adv. Clin. Exp. Med. 2018, 27, 207–216. [Google Scholar] [CrossRef]
- Żebrowska, A.; Sikora, M.; Konarska, A.; Zwierzchowska, A.; Kamiński, T.; Robins, A.; Hall, B. Moderate intensity exercise in hypoxia increases IGF-1 bioavailability and serum irisin in individuals with type 1 diabetes. Ther. Adv. Endocrinol. Metab. 2020, 11, 1. [Google Scholar] [CrossRef]
- Hirsch, I.B. Insulin Analogues. New Engl. J. Med. 2005, 352, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Kuipers, H.; Snyder, A.C.; Keizer, H.A.; Jeukendrup, A.; Hesselink, M. A New Approach for the Determination of Ventilatory and Lactate Thresholds. Int. J. Sports Med. 1992, 13, 518–522. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee 5. Facilitating Behavior Change and Well-being to Improve Health Outcomes:Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45, S60–S82. [Google Scholar] [CrossRef]
- Tanita. Understanding your measurements. Available online: https://tanita.eu/understanding-your-measurements (accessed on 13 July 2023).
- Heyward, V.H. Advanced Fitness Assessment and Exercise Prescription. Med. Sci. Sports Exerc. 1992, 24, 278. [Google Scholar] [CrossRef]
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Normy Żywienia Dla Populacji Polski I Ich Zastosowanie; Narodowy Instytut Zdrowia Publicznego – Państwowy Zakład Higieny: Warszawa, Poland, 2020; ISBN 9788365870285. [Google Scholar]
- Codella, R.; Terruzzi, I.; Luzi, L. Why should people with type 1 diabetes exercise regularly? Acta Diabetol. 2017, 54, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Oja, L.; Piksööt, J. Physical Activity and Sports Participation among Adolescents: Associations with Sports-Related Knowledge and Attitudes. Int. J. Environ. Res. Public Heal. 2022, 19, 6235. [Google Scholar] [CrossRef] [PubMed]
- Adamsen, L.; Andersen, C.; Lillelund, C.; Bloomquist, K.; Møller, T. Rethinking exercise identity: a qualitative study of physically inactive cancer patients’ transforming process while undergoing chemotherapy. BMJ Open 2017, 7, e016689. [Google Scholar] [CrossRef] [PubMed]
- McKevitt, S.; Jinks, C.; Healey, E.L.; Quicke, J.G. The attitudes towards, and beliefs about, physical activity in people with osteoarthritis and comorbidity: A qualitative investigation. Musculoskelet. Care 2021, 20, 167–179. [Google Scholar] [CrossRef]
- Hasan, S.; Shaw, S.M.; Gelling, L.H.; Kerr, C.J.; A Meads, C. Exercise modes and their association with hypoglycemia episodes in adults with type 1 diabetes mellitus: a systematic review. BMJ Open Diabetes Res. Care 2018, 6, e000578. [Google Scholar] [CrossRef]
- Alarcón-Gómez, J.; Chulvi-Medrano, I.; Martin-Rivera, F.; Calatayud, J. Effect of High-Intensity Interval Training on Quality of Life, Sleep Quality, Exercise Motivation and Enjoyment in Sedentary People with Type 1 Diabetes Mellitus. Int. J. Environ. Res. Public Heal. 2021, 18, 12612. [Google Scholar] [CrossRef]
- Petersen, K.F.; Price, T.B.; Bergeron, R. Regulation of Net Hepatic Glycogenolysis and Gluconeogenesis during Exercise: Impact of Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2004, 89, 4656–4664. [Google Scholar] [CrossRef]
- Riddell, M.C.; Gallen, I.W.; Smart, C.E.; Taplin, C.E.; Adolfsson, P.; Lumb, A.N.; Kowalski, A.; Rabasa-Lhoret, R.; McCrimmon, R.J.; Hume, C.; et al. Exercise management in type 1 diabetes: A consensus statement. Lancet Diabetes Endocrinol. 2017, 5, 377–390. [Google Scholar] [CrossRef]
- Yardley, J.E. Fasting May Alter Blood Glucose Responses to High-Intensity Interval Exercise in Adults With Type 1 Diabetes: A Randomized, Acute Crossover Study. Can. J. Diabetes 2020, 44, 727–733. [Google Scholar] [CrossRef]
- Schmidt, M.I.; Hadji-Georgopoulos, A.; Rendell, M.; Margolis, S.; Kowarski, A. The Dawn Phenomenon, an Early Morning Glucose Rise: Implications for Diabetic Intraday Blood Glucose Variation. Diabetes Care 1981, 4, 579–585. [Google Scholar] [CrossRef]
- Edge, J.A. , Matthews, D.R.; Dunger, D.B. The dawn phenomenon is related to overnight growth hormone release in adolescent diabetics. Clin Endocrinol 1990, 33, 729–737. [Google Scholar] [CrossRef]
- Cockcroft, E.J.; Moudiotis, C.; Kitchen, J.; Bond, B.; Williams, C.A.; Barker, A.R. High-intensity interval exercise and glycemic control in adolescents with type one diabetes mellitus: a case study. Physiological reports 2017, 5, e13339. [Google Scholar] [CrossRef]
- Gallen, I.W.; Hume, C.; Lumb, A. Fuelling the athlete with type 1 diabetes. Diabetes, Obes. Metab. 2010, 13, 130–136. [Google Scholar] [CrossRef]
- Chu, L.; Hamilton, J.; Riddell, M.C. Clinical Management of the Physically Active Patient with Type 1 Diabetes. Physician Sportsmed. 2011, 39, 64–77. [Google Scholar] [CrossRef]
- Khaw, K.-T.; Wareham, N.; Luben, R.; Bingham, S.; Oakes, S.; Welch, A.; Day, N. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of European Prospective Investigation of Cancer and Nutrition (EPIC-Norfolk). BMJ 2001, 322, 15–15. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Lee, D.; Ahmed, A.; Cheung, A.; A Khan, T.; Blanco, S.; Mejia; Mirrahimi, A.; A Jenkins, D.J.; Livesey, G.; et al. Effect of low glycaemic index or load dietary patterns on glycaemic control and cardiometabolic risk factors in diabetes: systematic review and meta-analysis of randomised controlled trials. BMJ 2021, 374, n1651. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Kendall, C.W.C.; Augustin, L.S.A.; Mitchell, S.; Sahye-Pudaruth, S.; Mejia, S.B.; Chiavaroli, L.; Mirrahimi, A.; Ireland, C.; Bashyam, B.; et al. Effect of Legumes as Part of a Low Glycemic Index Diet on Glycemic Control and Cardiovascular Risk Factors in Type 2 Diabetes Mellitus. JAMA Intern. Med. 2012, 172, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Giacco, R.; Parillo, M.; A Rivellese, A.; Lasorella, G.; Giacco, A.; D'Episcopo, L.; Riccardi, G. Long-term dietary treatment with increased amounts of fiber-rich low-glycemic index natural foods improves blood glucose control and reduces the number of hypoglycemic events in type 1 diabetic patients. Diabetes Care 2000, 23, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.I.; De Leeuw, I.; Hermansen, K.; Karamanos, B.; Karlström, B.; Katsilambros, N.; Riccardi, G.; Rivellese, A.A.; Rizkalla, S.; Slama, G.; et al. Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2004, 14, 373–394. [Google Scholar] [CrossRef]
- Khosravi-Boroujeni, H.; Mohammadifard, N.; Sarrafzadegan, N.; Sajjadi, F.; Maghroun, M.; Khosravi, A.; Alikhasi, H.; Rafieian, M.; Azadbakht, L. Potato consumption and cardiovascular disease risk factors among Iranian population. Int. J. Food Sci. Nutr. 2012, 63, 913–920. [Google Scholar] [CrossRef]
- Blaak, E.E.; Antoine, J.-M.; Benton, D.; Björck, I.; Bozzetto, L.; Brouns, F.; Diamant, M.; Dye, L.; Hulshof, T.; Holst, J.J.; et al. Impact of postprandial glycaemia on health and prevention of disease. Obes. Rev. 2012, 13, 923–984. [Google Scholar] [CrossRef]
- Gray, A.; Threlkeld, R.J. Nutritional Recommendations for Individuals with Diabetes. [Updated 2019 Oct 13]. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Blackman, M.R., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2790; Available online: https://www.ncbi.nlm.nih.gov/books/NBK279012/.
- Franz, M.J.; Boucher, J.L.; Evert, A.B. Evidence-based diabetes nutrition therapy recommendations are effective: the key is individualization. Diabetes, Metab. Syndr. Obesity: Targets Ther. 2014, 7, 65–72. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Dietary Reference Values for Nutrients Summary Report. EFSA Support. Public. 2017, 14, e15121. [CrossRef]
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E.; Predimed Investigators. Benefits of the Mediterranean Diet: Insights From the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 5. Lifestyle Management: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42 (Suppl. 1), S46–S60. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, F.Q.; Gannon, M.C. Metabolic response of people with type 2 diabetes to a high protein diet. Nutr. Metab. 2004, 1, 6–6. [Google Scholar] [CrossRef] [PubMed]
- Turton, J.L.; Struik, N.A.; Riley, M.; Brinkworth, G.D. Adults with and without type 1 diabetes have similar energy and macronutrient intakes: an analysis from the Australian Health Survey 2011-2013. Nutr. Res. 2020, 84, 25–32. [Google Scholar] [CrossRef]
- National Kidney Foundation. KDOQI clinical practice guidelines for diabetes and chronic kidney disease. Am J Kidney Dis 2012, 49 (Suppl. 2), S1–S179. [Google Scholar]
- Food and Nutrition Board. Proteins and Amino Acids. In Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005; pp. 589–768. [Google Scholar]
- Huang, L.E.; Arany, Z.; Livingston, D.M.; et al. Activation of hypoxia-inducible transcription factor depends primarily upon re-dox-sensitive stabilization of its alpha subunit. J Biol Chem 1996, 271, 32253–32259. [Google Scholar] [CrossRef] [PubMed]
- Żebrowska, A.; Jastrzębski, D.; Sadowska-Krępa, E.; Sikora, M.; Di Giulio, C. Comparison of the Effectiveness of High-Intensity Interval Training in Hypoxia and Normoxia in Healthy Male Volunteers: A Pilot Study. BioMed Res. Int. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Xing, J.; Lu, J. HIF-1α Activation Attenuates IL-6 and TNF-α Pathways in Hippocampus of Rats Following Transient Global Ischemia. Cell. Physiol. Biochem. 2016, 39, 511–520. [Google Scholar] [CrossRef]
- Li, G.; Lu, W.-H.; Ai, R.; Yang, J.-H.; Chen, F.; Tang, Z.-Z. The relationship between serum hypoxia-inducible factor 1α and coronary artery calcification in asymptomatic type 2 diabetic patients. Cardiovasc. Diabetol. 2014, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Rusdiana, R.; Moradi, A.; Widjaja, S.; Sari, M.; Hidayat, H.; Savira, M.; Amelia, R.; Rusmalawaty, R. The Effect of Hypoxia Inducible Factor -1 Alpha and Vascular Endothelial Growth Factor Level in Type 2 Diabetes Microvascular Complications and Development. Med Arch. 2022, 76, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Gu, Z.; Wang, G.; Zhao, T. The Possible Mechanisms Underlying the Impairment of HIF-1α Pathway Signaling in Hyperglycemia and the Beneficial Effects of Certain Therapies. Int. J. Med Sci. 2013, 10, 1412–1421. [Google Scholar] [CrossRef]
- Catrina, S.-B.; Okamoto, K.; Pereira, T.; Brismar, K.; Poellinger, L. Hyperglycemia Regulates Hypoxia-Inducible Factor-1α Protein Stability and Function. Diabetes 2004, 53, 3226–3232. [Google Scholar] [CrossRef]
- Thangarajah, H.; Vial, I.N.; Grogan, R.H.; Yao, D.; Shi, Y.; Januszyk, M.; Galiano, R.D.; Chang, E.I.; Galvez, M.G.; Glotzbach, J.P.; et al. HIF-1α dysfunction in diabetes. 2010, 9, 75–79. Cell cycle 2010, 9, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, A.; Tarnawski, A.S. Critical Role of Hypoxia Sensor - HIF-1α in VEGF Gene Activation. Implications for Angiogenesis and Tissue Injury Healing. Curr. Med. Chem. 2012, 19, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.D.; Hudlicka, O. Modulation of physiological angiogenesis in skeletal muscle by mechanical forces: Involvement of VEGF and metalloproteinases. Angiogenesis 2003, 6, 1–14. [Google Scholar] [CrossRef]
- Shoag, J.; Arany, Z. Regulation of Hypoxia-Inducible Genes by PGC-1α. Arter. Thromb. Vasc. Biol. 2010, 30, 662–666. [Google Scholar] [CrossRef]
- Martin, A.; Komada, M.R.; Sane, D.C. Abnormal angiogenesis in diabetes mellitus. Med. Res. Rev. 2003, 23, 117–145. [Google Scholar] [CrossRef]
- Tooke, J.E. Microvasculature in diabetes. Cardiovasc Res 1996, 32, 764–71. [Google Scholar] [CrossRef]
- Waltenberger, J. Impaired collateral vessel development in diabetes: potential cellular mechanisms and therapeutic implications. Cardiovasc. Res. 2001, 49, 554–560. [Google Scholar] [CrossRef]
- Petersen, A.M.W.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef]
- Giraudo, E.; Primo, L.; Audero, E.; Gerber, H.-P.; Koolwijk, P.; Soker, S.; Klagsbrun, M.; Ferrara, N.; Bussolino, F. Tumor Necrosis Factor-α Regulates Expression of Vascular Endothelial Growth Factor Receptor-2 and of Its Co-receptor Neuropilin-1 in Human Vascular Endothelial Cells. J. Biol. Chem. 1998, 273, 22128–22135. [Google Scholar] [CrossRef] [PubMed]
- A Febbraio, M.; Steensberg, A.; Starkie, R.L.; McConell, G.K.; A Kingwell, B. Skeletal muscle interleukin-6 and tumor necrosis factor-α release in healthy subjects and patients with type 2 diabetes at rest and during exercise. Metabolism 2003, 52, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Quarta, S.; Massaro, M.; Carluccio, M.A.; Calabriso, N.; Bravo, L.; Sarria, B.; García-Conesa, M.-T. An Exploratory Critical Review on TNF-α as a Potential Inflammatory Biomarker Responsive to Dietary Intervention with Bioactive Foods and Derived Products. Foods 2022, 11, 2524. [Google Scholar] [CrossRef]
- Kurtovic, N.; Halilovic, E. Serum Levels of Tumor Necrosis Factor - alpha in Patients With Psoriasis. Mater. Socio Medica 2022, 34, 40–43. [Google Scholar] [CrossRef]
- Feuerer, M.; Herrero, L.; Cipolletta, D.; Naaz, A.; Wong, J.; Nayer, A.; Lee, J.; Goldfine, A.B.; Benoist, C.; Shoelson, S.; et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009, 15, 930–939. [Google Scholar] [CrossRef]
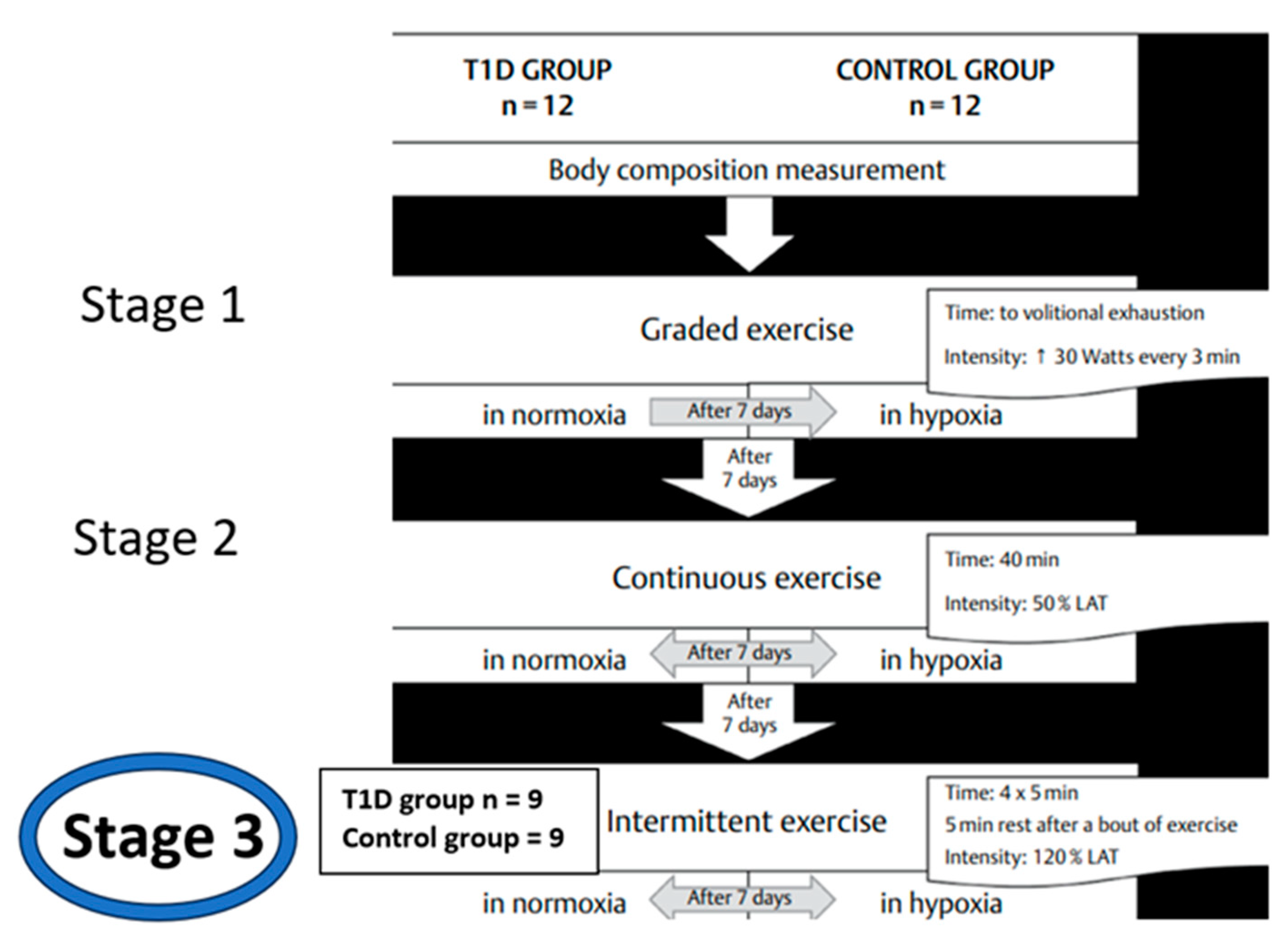
| T1D group n = 9 (female n = 2) | Control group n = 9 (female n = 3) | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | ±SD | Min | max | Mean | ±SD | min | max | |
| Age | 30.2 | 10.9 | 18.0 | 43.0 | 22.4 | 1.4 | 21.0 | 26.0 |
| Body mass [kg] | 74.8 | 14.3 | 51.1 | 99.1 | 70.1 | 9.2 | 58.3 | 91.5 |
| Body height [m] | 1.8 | 0.1 | 1.6 | 1.9 | 1.7 | 0.1 | 1.6 | 1.8 |
| BMI | 23.8 | 3.6 | 18.1 | 29.9 | 23.5 | 2.5 | 19.9 | 27.9 |
| %BF | 17.5 | 8.3 | 6.5 | 33.5 | 18.7 | 6.3 | 10.1 | 26.4 |
| FFM [kg] | 61.6 | 12.6 | 41.6 | 75.3 | 56.9 | 8.3 | 43.4 | 70.0 |
| V̇O₂peak (ml/kg/min) | 39.0 | 8.2 | 31.0 | 48.0 | 47.8 | 11.1 | 30.0 | 69.0 |
| T1D duration [years] | 12.3 | 9.1 | 4.0 | 27.0 | n.a. | n.a. | n.a. | n.a. |
| HbA1C [%] | 7.3 | 0.8 | 6.1 | 8.8 | n.m. | n.m. | n.m. | n.m. |
| Energy intake [kcal/day] | 2153.7 | 739.1 | 1076 | 3243 | 2778.6 | 769.1 | 2174.7 | 4408.9 |
| CHO intake [g/day] | 241.3 | 98.2 | 113.7 | 365.5 | 353.2 | 146.9 | 250.4 | 663.6 |
| CHO %energy intake | 54.0 | 10.3 | 41.2 | 70.8 | 60.4 | 6.9 | 48.5 | 70.0 |
| Protein intake [g/day] | 116.2 | 58.4 | 51.2 | 224.7 | 111.4 | 27.6 | 82.1 | 159.0 |
| Protein intake [g/kg/day] | 1.6 | 0.8 | 0.7 | 2.8 | 1.6 | 0.4 | 1.2 | 2.2 |
| Protein %energy intake | 26.6 | 9.8 | 13.5 | 42.3 | 20.2 | 5.6 | 13.9 | 30.8 |
| Fat intake [g/day] | 86.2 | 45.0 | 25.8 | 152.7 | 109.2 | 22.6 | 76.7 | 152.1 |
| Fat %energy intake | 19.4 | 7.1 | 10.3 | 29.5 | 19.4 | 2.2 | 16.1 | 22.9 |
| Variables | T1D group | |
|---|---|---|
| Mean | SD | |
| Fasting glycaemia day 1 [mg/dL] | 164.5 | 78.5 |
| Fasting glycaemia day 2 [mg/dL] | 139.0 | 9.9 |
| Insulin day 1 [units/day] | 35.3 | 10.9 |
| Insulin day 2 [units/day] | 34.5 | 13.5 |
| Digestible CHO day 1 | 157.0 | 104.2 |
| Digestible CHO day 2 | 190.5 | 57.7 |
| Variables | T1D group | Control group | T1D group | Control group | |||||
|---|---|---|---|---|---|---|---|---|---|
| %change: rest vs max | %change: rest vs 24-h | %change: rest vs max | %change: rest vs 24-h | ||||||
| Mean | ±SD | Mean | ±SD | ||||||
| HIF-1α rest [ng/mL] | 657.0*** | 210.4 | 28.3 | 11.5 | 34.4%↓ | 39.1%↓ | 278.9%↑ | 242.7%↑ | |
| HIF-1α max [ng/mL] | 430.0 | 163.3 | 107.0 | 56.9 | |||||
| HIF-1α 24-h [ng/mL] | 399.5 | 297.7 | 96.8 | 76.9 | |||||
| TNF-α rest [pg/mL] | 17.6 | 19.4 | 37.8 | 13.0 | 111.0%↑ | 123.1%↑ | 26.3%↓ | 44.3%↓ | |
| TNF-α max [pg/mL] | 37.2 | 9.3 | 27.7 | 10.3 | |||||
| TNF-α 24-h [pg/mL] | 39.3 | 16.5 | 20.9 | 9.5 | |||||
| VEGF rest [pg/mL] | 7.3 | 4.6 | 16.2 | 4.6 | 310.3%↑ | 103.4%↑ | 62.9%↓ | 61.4%↓ | |
| VEGF max [pg/mL] | 30.0* | 25.3 | 6.1 | 3.4 | |||||
| VEGF 24-h [pg/mL] | 14.9 | 3.1 | 6.3 | 3.20 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





