1. Introduction
Ovarian hyperstimulation syndrome (OHSS) is one of the major complications of ovarian stimulation with gonadotropins, particularly in in vitro fertilization (IVF) cycles. Its actual incidence in real world populations has been difficult to define due to poor case ascertainment, but mild OHSS has been estimated to affect one third of cycles [
1]. In many cases OHSS is caused by an excessive response to ovarian stimulation, even if in other cases it may be considered an idiosyncratic reaction to gonadotropins. Spontaneous OHSS cases not related to ovarian stimulation drugs have been also described [
2,
3].
The introduction of GnRH antagonists (GnRH-ant) to suppress the luteinizing hormone (LH) surge in IVF cycles, and of GnRH agonist (GnRH-a) triggering followed by a “freeze all” policy have dramatically reduced if not eliminated the risk of OHSS [
4]. However, the clinical and scientific interest for interventions aimed to prevent and treat OHSS is still high. This reflects that many IVF cycles worldwide are still performed employing the long GnRH-a down-regulation protocol. Furthermore, although the European Society of Human Reproduction and Embryology (ESHRE) guidelines suggest using GnRH-ant for IVF cycles in presumed high-risk patients [
4], OHSS may still occur in presumed normal responders. Because reproductive outcome seems to be better for fresh embryo transfer in patients without polycystic ovary syndrome (PCOS) [
5,
6,
7], this may further influence the choice to avoid elective embryo cryopreservation in clinical practice. Similarly, utilization of a dual trigger (hCG and GnRH-a) and/or intensive luteal phase support (GnRH-a plus low doses of human chorionic gonadotropin (hCG) [
8] may also increase the risk of OHSS by exposure to both exogenous [
9]. Finally, new gonadotropin formulations have been studied in GnRH-a down-regulated IVF cycles [
10,
11,
12], suggesting a new interest for the use of GnRH-a protocols. Collectively these clinical strategies all suggest that OHSS is far from banished from clinical practice and novel interventions will still be required.
Recently, we performed a systematic umbrella review in accordance with the Preferred Reporting Items for Overviews of Reviews (PRIOR) guidelines [
13] with the aim to identify the best evidence-based interventions to prevent or reduce the incidence and severity of OHSS in patients undergoing IVF [
14]. A total of 33 interventions (used in 37 different clinical situations) were analyzed in 28 systematic reviews of RCTs with meta-analysis. Even if the quality assessment of the included studies was high-to-moderate for 25 studies, the certainty of evidence (CoE) was seen to be high-to-moderate only for 6 interventions [
14]. Our analysis confirmed that the use of GnRH-ant should be preferred in presumed high-risk IVF patients, and that GnRH-a triggering with embryo freezing should be mandatory in case of persistent high-risk at the end of ovarian stimulation [
14]. Progestin-primed ovarian stimulation (PPOS) protocol was shown to be a valid option in case of elective embryo transfer or for cancer patients in the context of fertility preservation or for donor patients [
14]. In patients who undergo GnRH-a down-regulation, the use of mild stimulation was shown to be a safe approach, as well as metformin treatment during ovarian stimulation and dopamine agonists administration after ovulation triggering [
14].
Moreover, the specific analysis of systematic reviews of RCTs with meta-analysis in umbrella reviews is not only a strength, but also a limitation of the study because other interesting, and potentially useful interventions were not included in the final analysis. Based on these considerations, the aim of the present study was to systematically review and discuss all interventions for prevention of and reduction in the incidence and severity of OHSS in IVF patients, not supported by systematic reviews of RCTs with meta-analysis, assess their efficacy and grade them according to well-validated levels of clinical evidence.
2. Methods
The protocol of the current review was registered on the PROSPERO website (Protocol study registration: PROSPERO CRD 268626, available at
http://www.crd.york.ac.uk/PROSPERO) and follows the PRISMA 2020 statement [
15] (
http://www.prisma-statement.org) and the Population, Intervention, Comparison, Outcome (PICO) model [
16]. No formal ethical approval was required because the study did not involve humans and/or the use of human tissue and/or hospital records samples, and no personal data were recorded and analyzed.
According to the PICO model [
16], “Population” included women who undergo IVF/ICSI treatment, “Intervention” was considered each strategy used to reduce the risk and the severity of OHSS, “Comparison” included none or another strategy or placebo arm, and “Outcome” were considered primary or secondary outcomes of safety and efficacy, and their importance classified to assess the effect of any intervention (
https://gdt.gradepro.org/app/handbook/handbook.html).
2.1. Literature search
The search was initially performed, using the key words “OVARIAN HYPERSTIMULATION SYNDROME” or “OHSS” in the following electronic databases: PubMed, The Cochrane Library, Web of Science, and on the World Health Organization (WHO) International Trials registry platform, Current Controlled Trials and ClinicalTrial.gov. All publications within the database were considered with no time limits, with the searches re-run prior to final analysis. The first search was performed to identify all potential interventions used/proposed to prevent or reduce the incidence and severity of OHSS. For that search, only comparative/controlled studies in humans published in the English language were included, and no further specific inclusion and exclusion criteria were considered. Subsequently, a further search was performed in the same databases, using each specific intervention previously identified as the key words. For each intervention, we searched before the studies with the highest hierarchy of evidence as defined by the CEBM (
http://www.cebm.ox.ac.uk). Systematic reviews with or without meta-analysis were considered before, followed by RCTs, prospective non-randomized, observational (cohorts, case-control, or cross-sectional) studies, and, finally, case series, experimental/translational studies and expert opinion were searched and included in the final analysis. Among intercepted studies with the same grade of evidence, we included the most recent paper with the lowest risk of bias. Overlapping studies were included only if they had similar quality and were published in the same year or if the selected study did not report data on endpoints considered. Preclinical/experimental studies, sub-analysis, non-comparative studies (not controlled for no intervention, placebo, or other interventions), and network meta-analyses [
17,
18] were excluded from final analysis. The authors also hand-searched the reference lists of the included articles and of previous reviews to find additional data of interest for the aim of the present study. As this was a meta-analysis of published data the original data as not sought from the authors.
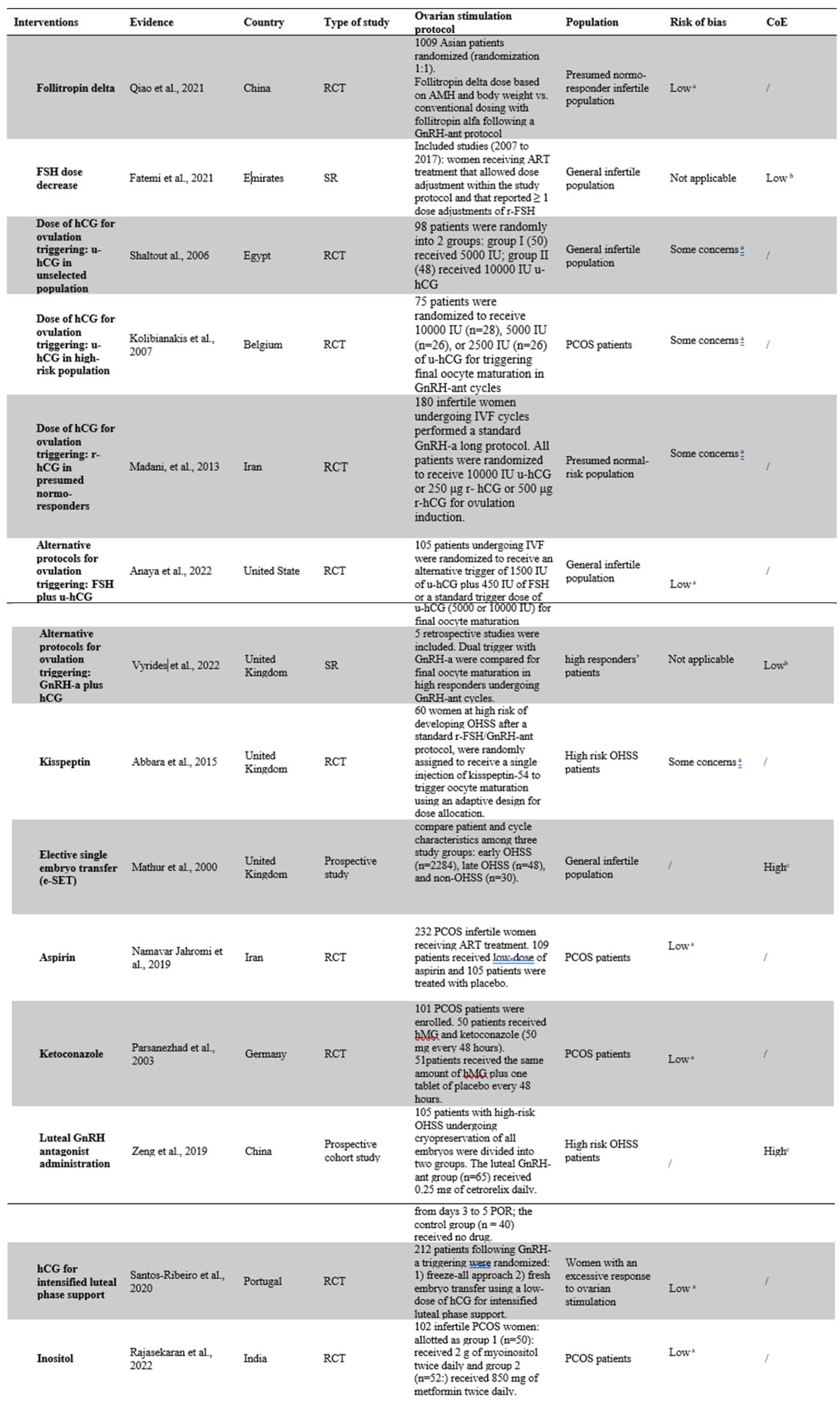
All searches were performed by two authors (FC and AB) and checked by a third (DC). For each intervention a specific table including first Author, year of publication, country, type of study, characteristics of the studied population, diagnostic criteria for OHSS, sample size, protocols used for ovarian stimulation, primary and secondary outcomes, quality of evidence (QoE, according to the risk of bias) and CoE.
2.2. Quality assessment and data analysis
All studies included in the final analysis were analyzed for risk of bias, using specific tools according to type of study. In particular, Assessing the Methodological Quality of Systematic Reviews 2 (AMSTAR-2) [
19] (
http://www.amstar.ca), Revised tool for Risk of Bias (rRoB 2) [
20] (
https://www.riskofbias.info/welcome/rob-2-0-tool), Risk Of Bias in Non-randomized Studies – of Intervention (ROBINS-I) [
21], and Newcastle-Ottawa Scale (NOS) [
22] were used respectively, for systematic reviews, RCTs, prospective non-randomized studies, and observational/cohort studies. Case-series were analyzed according to CAse REport (CARE) guidelines [
23] (
http://www.equator-network.org). Concerning the rRoB 2 test, the risk of bias was reported in accordance with the tool as “high”, “low” and “some concerns” [
20].
For each intervention, alone or in combination, a qualitative analysis was performed using the data reported in the original manuscript. For all studies the QoE was calculated after evaluation of the risk of bias. In case of meta-analyses, the CoE was reported as detailed in the original papers.
3. Results
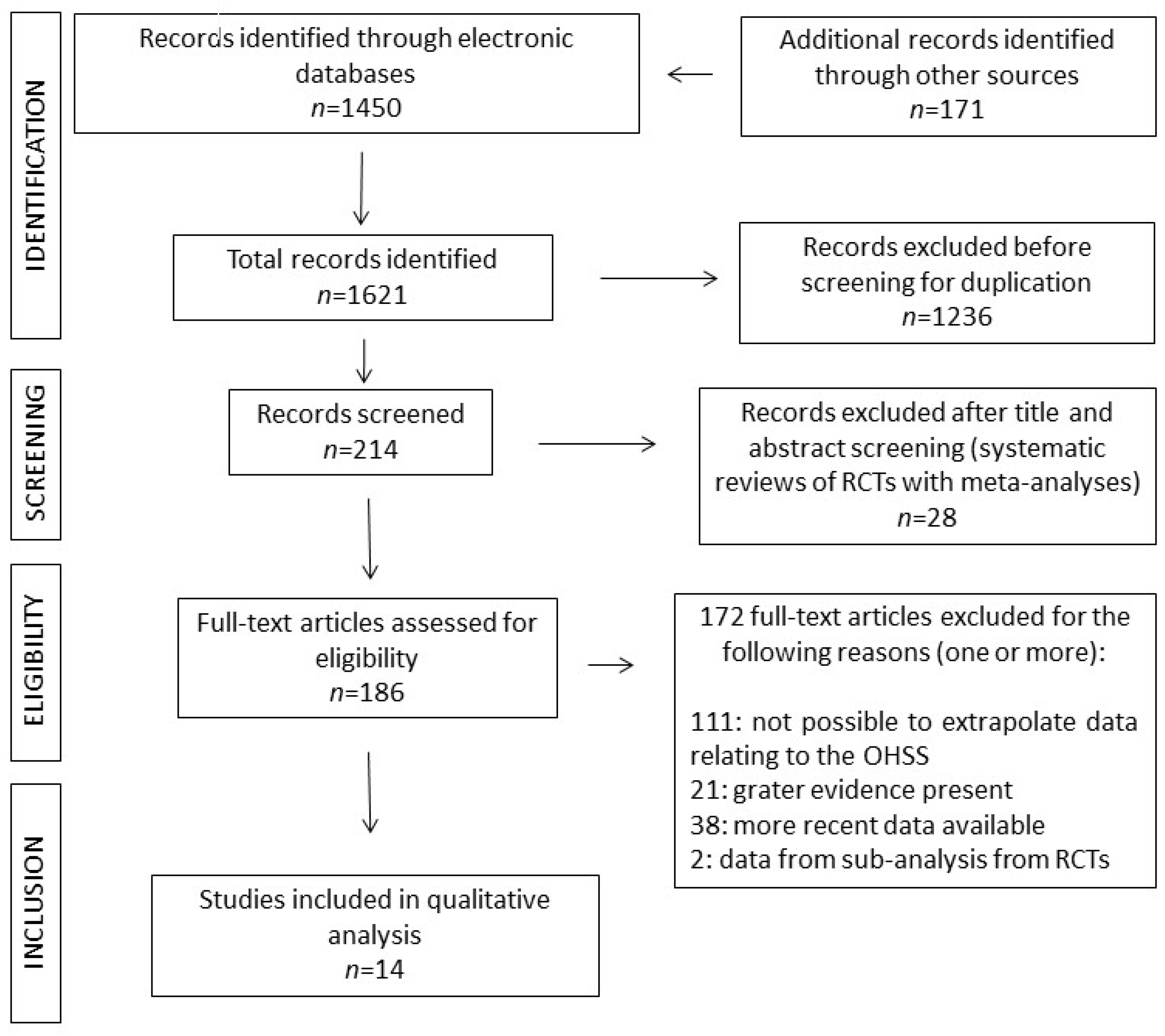
The flow-chart study according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [
15] (
http://www.prisma-statement.org) is reported in
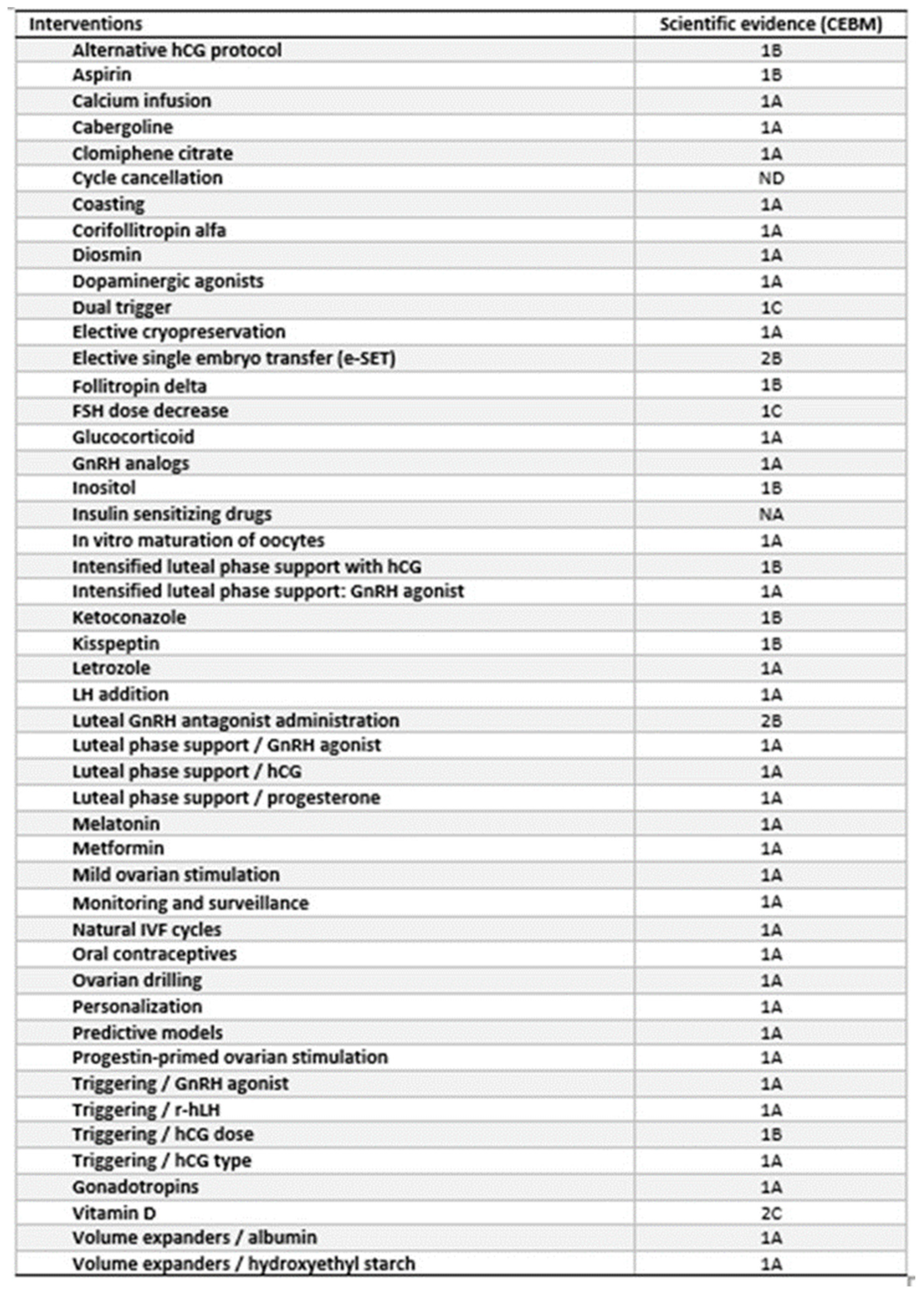
Figure 1. A total of 48 interventions were identified, with fifteen interventions not previously assessed by systematic review and meta-analysis of RCTs. The remaining 33 interventions were supported by type 1A according to the Oxford Centre for Evidence-Based Medicine (CEBM) evidence (
Table 1) and have previously been discussed [
14]. Of the 15 interventions, two were not supported by available data and one (“hCG dose”) was supported by two different studies (as below detailed) (
Table 1) for a total of 14 studies analyzed and discussed (
Figure 1).
For each intervention identified, we provided the rationale for its use, the available/intercepted studies, the primary and secondary outcomes, the QoE, and the CoE (for systematic reviews). In
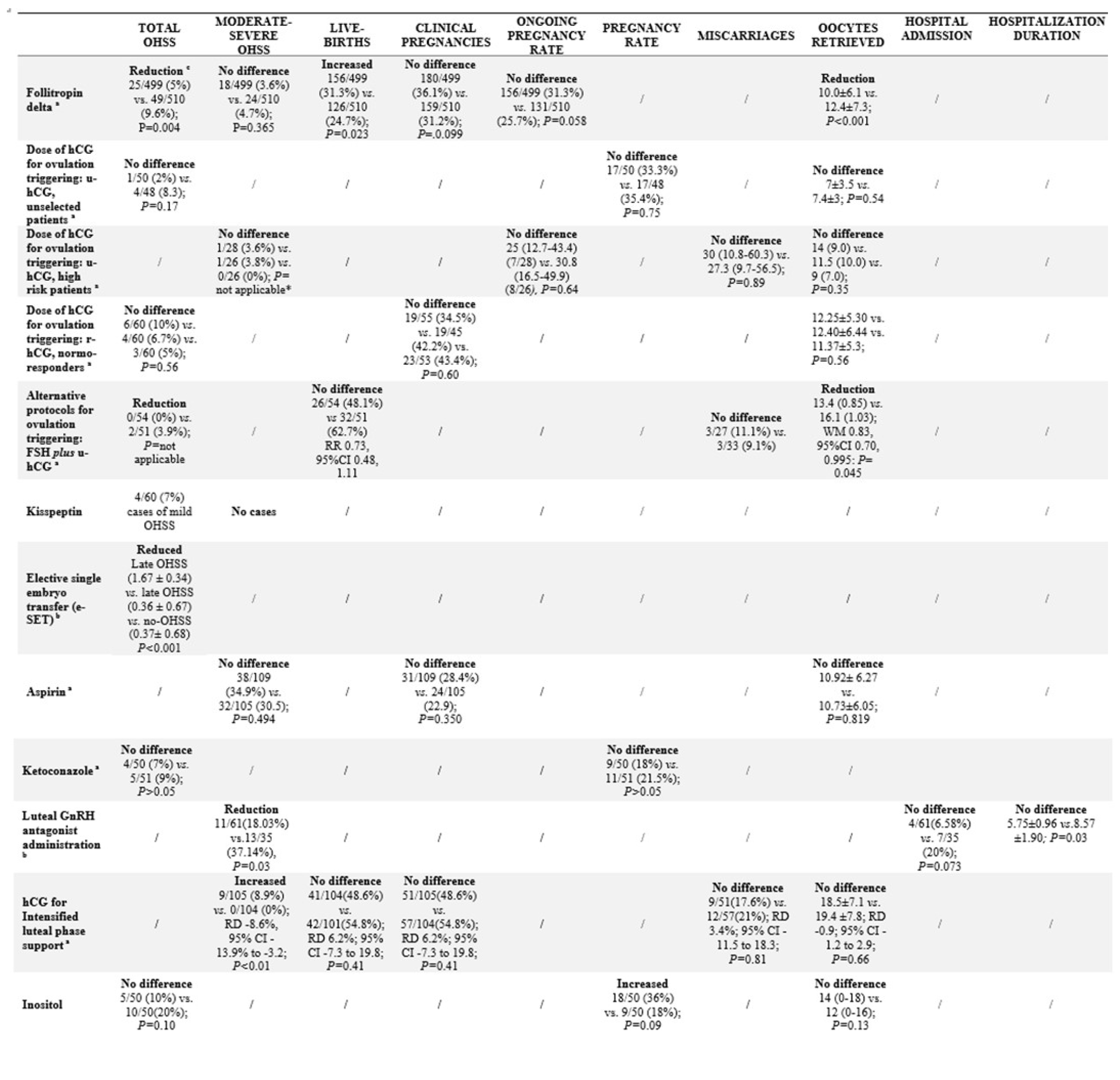
Table 2 the characteristics of the studies included are detailed. In Tables 3 and 4 are summarized the effects of each intervention in specific populations or clinical situations.
Table 2.
Characteristics of the studies included in the final analysis according to the specific intervention.
Table 2.
Characteristics of the studies included in the final analysis according to the specific intervention.
Table 3.
Primary and secondary endpoints for each specific intervention.
Table 3.
Primary and secondary endpoints for each specific intervention.
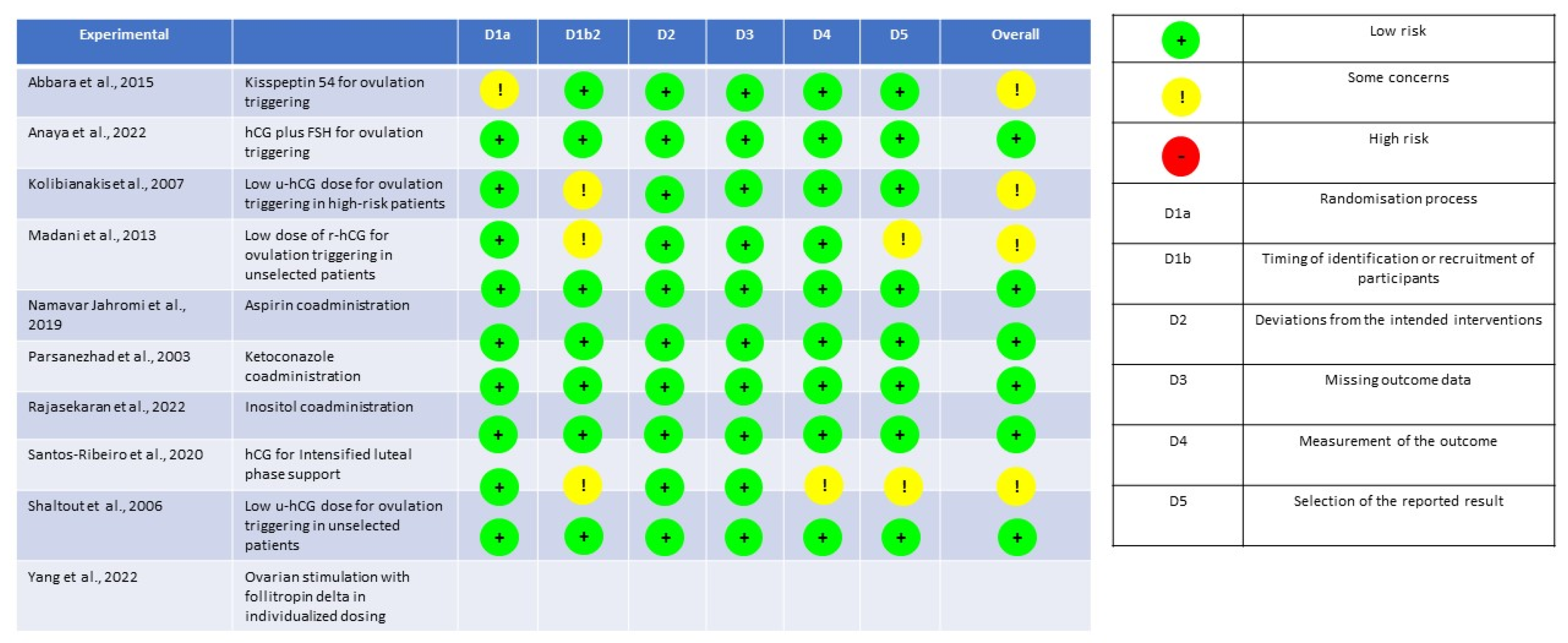
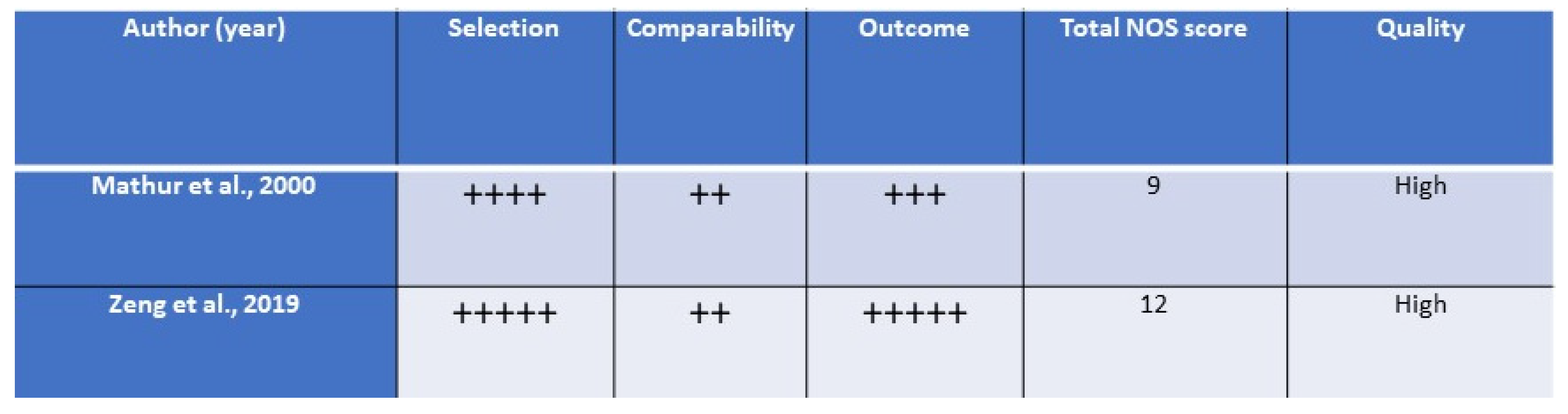
The quality assessment for RCTs and for prospective studies is detailed in the
Figure 2 and
Figure 3, respectively.
3.1. Use of follitropin delta for ovarian stimulation
Follitropin delta is a recombinant follicle stimulating hormone (r-FSH) recently developed and expressed only in human retinal fetal cell lines [
24]. Several RCTs have recently analyzed the efficacy and safety of follitropin delta [
25,
26,
27,
28,
29,
30,
31].
The most recent study, with low risk of bias, is a multi-center, assessor-blind RCT conducted on 1009 Asian patients randomized to receive follitropin delta or follitropin alpha in a GnRH-ant IVF protocol [
28]. A significant reduction in the incidence of early OHSS and/or preventive interventions for early OHSS in patients stimulated with follitropin delta were observed in comparison to patients who received follitropin alpha [25/499 (5%) vs. 49/510 (9.6%), respectively; P=0.004]. Therefore, no significant difference in the incidence of moderate/severe OHSS [18/499 (3.6%) vs. 24/510 (4.7%) for follitropin delta vs. follitropin alpha, respectively; P=0.365] was demonstrated [
28]. Concerning reproductive outcomes, follitropin delta in comparison with follitropin alpha was associated to higher live birth rate, not different ongoing and clinical pregnancy rate, but also to a significantly lower number of oocytes retrieved [
28]. The level of evidence was 1B (CEMB). The risk of bias was low (rRoB 2) [
28].
3.2. FSH dose decrease for ovarian stimulation
Gonadotropin dose adjustment is commonly performed in clinical practice to optimize the safety and outcome during ovarian stimulation. Concerning the FSH dose decrease strategy, a systematic review without data synthesis [
32], including 18 studies published form 2007 to 2017 for 6630 IVF cycles in which patients received a gonadotropin ovarian stimulation that allowed dose adjustment within the study protocol and that reported at least one dose adjustments of conventional r-FSH, concluded that decreasing the r-FSH dose during the mid-follicular phase of the ovarian stimulation may reduce the occurrence of OHSS compared to a fixed FSH dosage [
32]. However, most trials evaluating the dose adjustment in predicted hyper-responders were designed to assess an individualized starting dose, thus, confounding the available results. The QoE1 of data was not applicable [
32]. The level of evidence was 1C (CEMB). The QoE2 of data was low (AMSTAR-2) [
32].
3.3. Lower doses of hCG for ovulation triggering
The most commonly used doses of hCG used in IVF until now has been 10000 IU for u-hCG intramuscularly injected, and 250 mcg for r-hCG subcutaneously administered. In consideration of the OHSS pathogenesis, a dose decrease of hCG has been considered as a potential strategy to reduce the OHSS risk [
4].
3.3.1. u-hCG
A double-blind RCT in an unselected population [
33] demonstrated that a single dose of 5000 IU u-hCG compared to 10000 IU u-hCG used for triggering resulted in a non-statistical difference in the incidence of OHSS (2%
vs. 8.3%,
P=0.17). No difference between the two groups regarding oocytes retrieved and pregnancy rate was found [
33]. The level of evidence was 1B (CEMB). The risk of bias reported some concerns (rRoB 2) [
33].
A further RCT [
34] in a selected high-risk population of 80 PCOS patients, who received r-FSH for ovarian stimulation in GnRH-ant IVF cycles, compared the administration of different hCG doses for triggering, i.e., 10000IU vs. 5000IU vs. 2500IU. A dose decrease of u-hCG to trigger final oocyte maturation did not affect the ongoing pregnancy rate, the early pregnancy loss, and the oocyte retrieved [
34]. Concerning OHSS, only two cases of severe OHSS were reported (one patient in 5000 UI group and one patient in 10000 UI group) [
34]. The level of evidence was 1B (CEMB). The risk of bias reported some concerns (rRoB 2) [
34].
3.3.2. r-hCG
Concerning r-hCG, our search did not intercept comparative and/or controlled clinical studies on the use of lower r-hCG doses. An observational study [
35] reported good reproductive outcomes and only one moderate OHSS case in 35 high-responder IVF patients who received 125mcg r-hCG [
35]. A RCT in a total of 180 patients compared 10000 IU u-hCG (n=60) to 500 μg (n=60) and 250 μg (n=60) r-hCG for ovulation triggering [
36]. All the included patients underwent a GnRH-a long down-regulation protocol [
36]. That study reported no statistical difference in OHSS incidence [6/60 (10%)
vs. 4/60 (6.7%)
vs. 3/60 (5%) for 10,000 IU u-hCG
vs. 500 μg r-hCG
vs. 250 μg r-hCG arms, respectively;
P=0.56]. Regarding secondary outcomes, no difference was detected in clinical pregnancy rate nor in number of oocytes retrieved among groups [
36]. The level of evidence was 1B (CEMB). The risk of bias reported some concerns (rRoB 2) [
36].
3.4. Alternative protocols for ovulation triggering
Different alternative protocols for ovulation triggering have been developed to improve the reproductive outcomes of IVF cycles, potentially also modifying the OHSS risk.
3.4.1. u-hCG plus FSH for ovulation triggering
Even if the role of FSH surge for ovulation triggering is not completely understood in humans, experimental and animal data have demonstrated that it increases LH-receptor expression on granulosa cells, promote resumption of meiosis and cumulus expansion [
37,
38,
39,
40,
41], and may trigger ovulation in the absence of LH activity [
42,
43,
44]. Indirect human data from the use of the GnRH-a trigger have demonstrated a significant release of endogenous LH and FSH surges with potential positive effects on biological outcomes (including higher oocyte recovery, maturity, and fertilization) [
45,
46,
47].
A recent double-blind, non-inferiority RCT compared 1500 IU u-hCG plus 450 IU r-FSH (experimental) to 5000 IU or 10000 IU u-hCG in 105 infertile patients scheduled for GnRH-a/GnRH-ant IVF cycles [
48]. No OHSS case in the experimental group compared to two OHSS cases in the standard trigger groups. One patient had mild OHSS, whereas the other had severe OHSS requiring hospitalization for fluid management, anticoagulation, and paracentesis [
48]. No difference was observed in live birth and miscarriage rates, but a slightly significant reduction in retrieved oocyte was found [
48]. The level of evidence was 1B (CEMB). The risk of bias was low (rRoB 2) [
48].
3.4.2. GnRH-a plus hCG vs. GnRH-a vs. hCG
Dual trigger is a strategy initially used as rescue treatment for to improve implantation rates, and the overall reproductive outcomes, in IVF patients who received GnRH-a alone for ovulation triggering [
49,
50,
51]. However, the co-administration of hCG to GnRH-a trigger increases the OHSS risk [
52]. Recently, the use of GnRH-a plus hCG co-administration for final oocyte maturation was explored in patients with a normal ovarian response to improve both oocyte quality and reproductive outcomes compared to hCG trigger alone [
45,
46], and a significant efficacy of the dual triggering with GnRH-a plus hCG (
vs. GnRH-a
vs. hCG) has been also confirmed more recently in a large RCT, including 510 advanced-age IVF patients [
47]. However, no direct data are available from IVF patients with PCOS and/or at high OHSS risk currently.
A recent systematic review without data synthesis [
53], including 5 retrospective studies, found no difference in OHSS risk between the use of dual triggering vs. GnRH-a triggering in four studies, whereas an increased risk of OHSS in patients who received dual triggering was observed in only one. Thus, the authors concluded that the incidence of OHSS was not significantly changed using dual triggering [
53]. Insufficient evidence to support differences in live birth rate, clinical pregnancy, and miscarriage rates was found [
53]. The QoE1 data was not applicable. The level of evidence was 1C (CEMB). The QoE2 data was low (AMSTAR-2) [
53].
3.4.3. Kisspeptin
Kisspeptin is a neuropeptide with a critical role in the function of the hypothalamic-pituitary-gonadal (HPG) axis, stimulating GnRH secretion from the hypothalamus and inducing gonadotropin secretion [
54]. Only a few clinical trials have been published due to kisspeptin not currently being a licensed medication limiting its use in clinical practice. Thus, only one phase 2 RCT in an IVF population of 60 women at high risk of OHSS explored the safety of kisspeptin-54 administration at different dosages [
55]. Kisspeptin-54 was shown to be effective to trigger oocyte maturation without no moderate, severe, or critical OHSS event. In fact, in this study population only 3/60 (5%) cases of mild early OHSS and 1/60 (2%) case with mild late OHSS were reported [
55]. The level of evidence was 1B (CEMB). The risk of bias reported some concerns (rRoB 2) [
55].
3.5. Cycle cancellation
In patients considered at high risk of OHSS, cancellation of the cycles remains an option [
4]. A cycle may be cancelled before ovulation triggering in GnRH-a cycles (withholding hCG) or in GnRH-ant cycles when elective cryopreservation is not possible. Our systematic research did not intercept specific and formal documents analyzing the efficacy of the cycle cancellation as strategy for preventing OHSS.
3.6. Elective single embryo transfer (e-SET)
The risk and severity of OHSS are closely related to luteal hCG levels, which are significantly higher in multiple implantation pregnancies. However, direct data supporting the e-SET as a strategy to reduce the OHSS risk are not available. Indirect evidence from a large prospective study [
56] showed a close and significant association between the number of gestational sacs (±standard deviation) and the occurrence of late OHSS (1.67 ± 0.34), with early OHSS (0.36 ± 0.67) or no OHSS (0.37± 0.68) [
56]. The level of evidence was 2B (CEMB). The QoE1of the data was high (NOS) [
56].
3.7. Aspirin
Aspirin inhibits the activity of the cyclooxygenase-1 enzyme, which results in a decrease in platelet activity and a reduction in the risk of blood clotting, altering the pathological cascade caused by vascular endothelial growth factor (VEGF) [
57]. Different RCTs were intercepted from literature [
58,
59,
60].
The most recent study is a double-blind placebo-controlled RCT [
60] demonstrated no difference in the incidence of moderate-to-severe OHSS between low-dose aspirin (100 mg daily)
vs. placebo in 214 infertile PCOS patients scheduled for GnRH-a IVF programs [38/109 (34.9%)
vs. 32/105 (30.5), respectively;
P=0.494]. No difference was found in the number of oocytes retrieved and the clinical pregnancy rate [
60]. The level of evidence was 1B (CEMB). The risk of bias was low (rRoB 2) [
60].
3.8. Ketoconazole
Ketoconazole is an inhibitor of the steroidogenic enzyme P450 in the adrenal cortex and gonads and is a potential modulator of the ovarian response to gonadotropin [
53].
Two RCTs were found in our research [
61,
62]. The highest quality RCT, a double-blind, placebo-controlled study [
62], showed that ketoconazole administration did not prevent OHSS [4/50 (7%)
vs. 5/51 (9%), for ketoconazole
vs. placebo group, respectively;
P>0.05] in PCOS patients. No differences in pregnancy rates were detected between arms who receive and did not receive ketoconazole [
62]. The level of evidence was 1B (CEMB). The risk of bias was low (rRoB 2) [
62].
3.9. Luteal GnRH-ant administration
The administration of GnRH-ant during the luteal phase was studied as a potential intervention to prevent early OHSS and reduce the severity of OHSS [
63]. GnRH-ant injections suppress the release of LH by the pituitary, inducing a significant reduction in circulating VEGF [
64].
A prospective study in a total of 105 patients at high-risk of OHSS concluded that GnRH-ant administration for three days was effective in reducing the moderate-to-severe OHSS incidence, and in inducing a faster regression of the OHSS symptoms [11/61 (18.03%)
vs.13/35 (37.14%),
P=0.03] [
65]. No data on reproductive outcomes are available and no difference in the hospital admission and average of hospitalization duration were seen [
65]. The level of evidence was 2B (CEMB). The quality of the data was high (NOS) [
65].
3.10. hCG administration for intensified luteal phase support
Small doses of hCG, as luteal phase support, were tested in high responders who received ovulation triggering with GnRH-a in GnRH-ant IVF cycles context [
66,
67,
68,
69]. A recent RCT [
70] in 212 infertile IVF patients who received GnRH-a for triggering compared fresh transfer to a freeze-all policy. In the fresh transfer group patients were administered a bolus of 1500 IU hCG on the day of oocyte retrieval in addition to oral estradiol and vaginal micronized progesterone for luteal phase support [
70]. Moderate-severe OHSS occurred only in the low-dose hCG group [9/105 (8.9%)
vs. 0/104 (0%); risk difference (RD) -8.6%, 95%CI -13.9% to -3.2,
P<0.01] [
70]. No difference between the two groups was found in clinical pregnancy, live birth, miscarriage, or oocyte retrieval rates [
62]. The level of evidence was 1B (CEMB). The risk of bias was low (rRoB 2) [
70].
3.11. Inositol
Inositol is a compound of biological origin, involved in numerous biological processes including cellular signaling [
71]. Positive effects have been demonstrated with the use of inositol supplementation in women with PCOS [
72]. Several RCTs related to the efficacy of inositol were intercepted [
73,
74,
75].
The most recent double-blind RCT [
75] in a total of 102 infertile PCOS IVF patients, compared the effect of 3-month myoinositol and metformin pretreatment. No statistically significant difference in OHSS incidence was found between the two groups [5/50 (10%)
vs. 10/50 (20%) for myoinositol and metformin group, respectively;
P=0.10]. A significantly higher clinical pregnancy rate was found in myoinositol-treated patients, whereas no difference was observed in terms of oocytes retrieved [
75]. The level of evidence was 1B (CEMB). The risk of bias was low (rRoB 2) [
75].
3.12. Insulin sensitizing drugs (ISDs)
All data on the effects of ISDs regarding OHSS risk involve the administration of metformin. No data on other ISDs, including rosiglitazone, pioglitazone, sulfonylurea, peroxisome proliferator-activated receptor agonists, liraglutide, semaglutide, glucagon, α-glucosidase inhibitors, sodium glucose cotransporter (SGLT)-2 inhibitors were intercepted about the effect on OHSS risk in IVF patients. The effect of metformin on the risk of OHSS has recently been reported elsewhere [
14].
4. Discussion
The current study was aimed to identify interventions to prevent or reduce the OHSS risk with clinical evidence lower than type 1A according to the CEBM hierarchy of evidence. In fact, in a recent umbrella review [
14], only systematic reviews of RCTs with meta-analysis were included, and many interesting and potentially useful treatment regimens were excluded. In the present paper 15 further interventions were identified and discussed evaluating the risk/benefit ratio. Each intervention was analyzed considering the best evidence available according to the CEBM hierarchy (highest), the risk of bias (lowest), and the year of publication (more recent). Although we used the CEBM system to grade the evidence, which has not been updated since 2009, it represents a simple and reproducible tool to evaluate the available scientific literature (
http://www.cebm.ox.ac.uk). In this regard, many interventions for minimizing OHSS risk were supported by systematic reviews without meta-analysis or RCTs.
Our findings suggest that follitropin delta in GnRH-ant IVF cycles may be efficacious in terms of reduction of early OHSS risk without a negative effect on reproductive outcomes, and with an improvement in the live birth rate [
28]. These results on OHSS risk are in accordance with other two large multicenter RCTs [
26,
29]. The first was an assessor-blinded, noninferiority RCT that demonstrated a reduction also of the moderate-to-severe OHSS incidence and/or of their preventive interventions in 1329 Caucasian patients with polycystic ovaries using an individualized follitropin delta administration in comparison to conventional follitropin alpha [
26]. The second was another assessor-blind, non-inferiority RCT trial, performed on 347 Japanese IVF patients, that reported an overall risk of OHSS (early and late OHSS) and a moderate-severe OHSS risk reduced to approximately half with the use of individualized follitropin delta administration in comparison to standard regimen [
29]. Of note, the use of individualized follitropin delta dosing regimen achieves significantly and clinically higher live birth rates compared to the women who received a conventional follitropin alfa regimen with a relative increase of more than 25% [
28], and these findings are partially in agreement with other RCTs that showed no clinically significant difference in reproductive outcomes between two protocols [
26], and a better live birth rate per started cycle with individualized follitropin delta protocol [
29]. Of note, a secondary analysis of two clinical trials, designed to assess the OHSS risk in 1326 patients who received sequential ovarian stimulation cycles with follitropin delta, demonstrated that follitropin delta, administered in individualized dosing in comparison to conventional follitropin alfa protocol, significantly reduced the risk of moderate-to-severe OHSS and of preventive interventions [
27]. The greatest benefit was observed in patients in the highest anti-Müllerian hormone quartile. Unfortunately, controlled data on the safety and efficacy of follitropin delta in GnRH-a IVF cycles and presumed hyper-responder patients are needed [
76].
Surprisingly, only few and confounded data are available about the use of gonadotropin dose decrease, a strategy well supported by common sense and used in up to 41% of IVF cycles in the United States [
77]. On the other hand, many data have been published about the tailoring / personalization of the starting dose of gonadotropins during the last years with single or multiple parameters, combined also in specific algorithms [
14], and the dose adjustment of the initial dose of gonadotropin is frequently included in the conventional arm (
vs. individualized arm) [
32]. In the systematic review by Fatemi et al. [
32] only three studies reported direct comparisons of outcomes between constant dose vs. dose adjustment groups [
26,
78,
79], and the many confounders and biases did not permit solid conclusion about the incidence of dose adjustment in routine clinical practice and its impact on clinical outcomes.
International guidelines [
4] suggest the use of low-doses of hCG for triggering ovulation in case of high OHSS risk, and this is particularly true for GnRH-a IVF cycles. Moreover, our analysis revealed a reduction in OHSS incidence in high-risk patients only with the low-doses of u-hCG administrated in GnRH-ant cycles, However, it is interesting to note that the lower doses of r-hCG (250µg) are widely used into clinical practice as standard treatment but the larger 10000IU dose of u-hCG is still commonly administrated. Interesting data regard the use of very low u-hCG dose plus high-dose r-FSH bolus in IVF patients not at high-risk for OHSS, even if that presumed efficacy on OHSS incidence needs to be confirmed in other settings, on larger study samples and in high-risk populations. Efficacy and safety data on kisspeptin, and its analogs, as ovulation trigger, are still limited to experimental setting. On the other hand, inositol pretreatment may be also effective in reducing the risk of OHSS and GnRH-ant administration during the luteal phase may reduce the severity and the duration of OHSS. Finally, our data, in agreement with a recent network meta-analysis [
80], does not support the use of aspirin and ketoconazole for OHSS prevention in IVF.
The present review has several strengths. These include the extensive literature search of specific potential interventions with an impact on OHSS, the use of the PICO model [81] and a careful quality assessment performed for each intervention with specific tools according to study design. We do, however, acknowledge several limitations including the low quality of several studies included in the analysis. In addition, almost all studies here discussed were not designed and powered to detect differences in OHSS incidence, data on maternal mortality and morbidity were poorly reported, and many studies not even to highlight differences in terms of live births. In many studies the risk of OHSS was reported as secondary outcomes and not tested in population at high risk of OHSS. For example, in the Anaya’s study, the IVF patients considered to be at highest risk for OHSS, i.e., serum estradiol levels higher than 5000 pg/mL on the day of trigger, were excluded form randomization for safety concerns, limiting the clinical application of the trial findings to at-risk patients [
48]. Finally, another limitation may be due to the evaluation of the RCTs with the use of rRob2. We feel that this tool is not so sensible to discriminate the best study quality in consideration that it includes only three possible categories. This may have introduced a significant confounder due to an incomplete interception of more relevant data.
5. Conclusion
The present systematic review identified several treatments/strategies which are potentially effective in reducing the incidence and severity of OHSS, even if not supported by highest clinical evidence. Importantly, further research is certainly needed to demonstrate their effectiveness and safety in clinical practice.
Author Contributions
SP conceptualized and designed the study and drafted the article. FC and AB acquired the main data and additional references, tabulated data, performed the study quality assessment, and drafted the article. DC checked the searches and drafted the article. SN and PH improved the interpretation of data, and critically revised the article. All authors have provided their final approval of the version to be published and agree to be accountable for all aspects of the work especially regarding its accuracy and integrity.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Conflicts of Interest
SMN has participated in Advisory Boards and received speakers or consultancy fees from Access Fertility, Beckman Coulter, Ferring, Finox, Merck, Modern Fertility MSD, Roche Diagnostics and The Fertility Partnership. PH received speakers or consultancy fees from IBSA, Merck, Gedeon Richter, Besins Healthcare. SP, FC, AB and DC have no conflict of interest to declare.
References
- Green-top guideline No.5: The management of ovarian hyperstimulation syndrome. https://www.rcog.org.uk/media/or1jqxbf/gtg_5_ohss.pdf (11 January 2023 date last accessed).
- Humaidan, P.; Nelson, S.M.; Devroey, P.; Coddington, C.C.; Schwartz, L.B.; Gordon, K.; Frattarelli, J.L.; Tarlatzis, B.C.; Fatemi, H.M.; Lutjen, P, et al. Ovarian hyperstimulation syndrome: review and new classification criteria for reporting in clinical trials. Hum Reprod 2016, 31, 1997–2004. [Google Scholar] [CrossRef]
- Dey, A.K.; Dubey, A.; Mittal, K.; Kale, S. Spontaneous ovarian hyperstimulation syndrome - understanding the dilemma. Gynecol Endocrinol 2015, 31, 587–589. [Google Scholar] [CrossRef]
- Ovarian Stimulation TEGGO.; Bosch, E.; Broer, S.; Griesinger, G.; Grynberg, M.; Humaidan, P.; Kolibianakis, E.; Kunicki, M.; La Marca, A.; Lainas, G.; Le Clef, N. et al. ESHRE guideline: ovarian stimulation for IVF/ICSI. Hum Reprod Open 2020, hoaa009. [CrossRef]
- Zaat, T.; Zagers, M.; Mol, F.; Goddijn, M.; van Wely, M, Mastenbroek, S. Fresh versus frozen embryo transfers in assisted reproduction. Cochrane Database Syst Rev 2021, 2, CD011184. [Google Scholar] [CrossRef]
- Roque, M.; Haahr, T.; Geber, S.; Esteves, S.C.; Humaidan, P. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: a systematic review and meta-analysis of reproductive outcomes. Hum Reprod Update 2019, 25, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Shi, Y.; Sun, Y.; Zhang, B.; Liang, X.; Cao, Y.; Yang, J.; Liu, J.; Wei, D.; Weng, N.; Tian, L.; Hao, C.; Yang, D.; Zhou, F.; Shi, J.; Xu, Y.; Li, J.; Yan, J.; Qin, Y.; Zhao, H.; Zhang, H.; Legro, RS. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N Engl J Med 2016, 375, 523–533. [Google Scholar] [CrossRef]
- Hu, K.L.; Wang, S.; Ye, X.; Zhang, D.; Hunt, S. GnRH agonist and hCG (dual trigger) versus hCG trigger for follicular maturation: a systematic review and meta-analysis of randomized trials. Reprod Biol Endocrinol 2021, 19, 78. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, P.G.; Bosdoum, J.K.; Lainas, G.T.; Lainas, T.G.; Grimbizis, G.F.; Kolibianakis, E.M. How frequent is severe ovarian hyperstimulation syndrome after GnRH agonist triggering in high-risk women? A systematic review and meta-analysis. Reprod Biomed Online 2021, 42, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Lunenfeld, B.; Bilger, W.; Longobardi, S.; Kirsten, J.; D'Hooghe, T.; Sunkara, SK. Decision points for individualized hormonal stimulation with recombinant gonadotropins for treatment of women with infertility. Gynecol Endocrinol 2019, 35, 1027–1036. [Google Scholar] [CrossRef]
- Cozzolino, M.; Vitagliano, A.; Cecchino, G.N.; Ambrosini, G.; Garcia-Velasco, J.A. Corifollitropin alfa for ovarian stimulation in in vitro fertilization: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril 2019, 111, 722–733. [Google Scholar] [CrossRef]
- Fernández Sánchez, M.; Višnová, H.; Larsson, P.; Yding Andersen, C.; Filicori, M.; Blockeel, C.; Pinborg, A.; Khalaf, Y.; Mannaerts, B; Rainbow Study Group. A randomized, controlled, first-in-patient trial of choriogonadotropin beta added to follitropin delta in women undergoing ovarian stimulation in a long GnRH agonist protocol. Hum Reprod 2022, 37, 1161–1174. [Google Scholar] [CrossRef]
- Gates, M.; Gates, A.; Pieper, D.; Fernandes, RM.; Tricco, AC.; Moher, D.; Brennan, S.E.; Li, T.; Pollock, M.; Lunny,C.; et al. Reporting guideline for overviews of reviews of healthcare interventions: development of the PRIOR statement. BMJ 2022, 9, 378:e070849. [Google Scholar] [CrossRef] [PubMed]
- Palomba, S.; Costanzi, F.; Nelson, S.M.; Caserta, D.; Humaidan, P. Interventions to prevent or reduce the incidence and severity of ovarian hyperstimulation syndrome: a systematic umbrella review of the best clinical evidence. Reprod Biol Endocrinol 2023, 21, 67. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Frandsen, T.F.; Bruun Nielsen, M.F.; Lindhardt, C.L.; Eriksen, M.B. Using the full PICO model as a search tool for systematic reviews resulted in lower recall for some PICO elements. J Clin Epidemiol 2020, 127, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Puhan, MA.; Vedula, S.S.; Singh, S.; Dickersin, K. Ad Hoc Network Meta-analysis Methods Meeting Working Group. Network meta-analysis-highly attractive but more methodological research is needed. BMC Med 2011, 9, 79. [Google Scholar] [CrossRef]
- Christofilos, S.I.; Tsikopoulos, K.; Tsikopoulos, A.; Kitridis, D.; Sidiropoulos, K.; Stoikos, P.N.; Kavarthapu, V. Network meta-analyses: methodological prerequisites and clinical usefulness. World J Methodol 2022, 12, 92–98. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions; or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán. M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 12, 355:i4919. [Google Scholar] [CrossRef]
- Wells, G.A.; Wells, G.; Shea, B.; Shea, B.; O'Connell, D.; Peterson, J.; Welch Losos, M.; Tugwell, P.; Ga, SW.; Zello, G.A.; Petersen, J. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2014. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D; CARE Group. The CARE guidelines: consensus-based clinical case report guideline development. J Diet Suppl 2013, 10, 381–390. [Google Scholar] [CrossRef]
- Lunenfeld, B.; Bilger, W.; Longobardi, S.; Kirsten, J.; D'Hooghe, T.; Sunkara, SK. Decision points for individualized hormonal stimulation with recombinant gonadotropins for treatment of women with infertility. Gynecol Endocrinol 2019, 35, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zhang, Y.; Liang, X.; Song, X.; Wei, Z.; Liu, J.; Yang, Y.; Tan, J.; Zhang, Q.; Sun, Y.; Wang, W.; Qian, W.; Jin, L.; Wang, S.; Xu, Y.; Yang, J.; Goethberg, M.; Mannaerts, B.; Wu, W:; Zheng, Z, Qiao, J. Comparative clinical outcome following individualized follitropin delta dosing in Chinese women undergoing ovarian stimulation for in vitro fertilization /intracytoplasmic sperm injection. Reprod Biol Endocrinol 2022, 20, 147. [Google Scholar] [CrossRef]
- Nyboe Andersen, A.; Nelson, S.M.; Fauser, B.C.; García-Velasco, J.A.; Klein, B.M.; Arce, J. C; ESTHER-1 study group. Individualized versus conventional ovarian stimulation for in vitro fertilization: a multicenter, randomized, controlled, assessor-blinded, phase 3 noninferiority trial. Fertil Steril 2017, 107, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, M.; Visnova, H.; Yuzpe, A.; Klein, BM.; Mannaerts, B.; Arce JC; ESTHER-1 and ESTHER-2 Study Group. Individualization of the starting dose of follitropin delta reduces the overall OHSS risk and/or the need for additional preventive interventions: cumulative data over three stimulation cycles. Reprod Biomed Online 2019, 38, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Zhang, Y.; Liang, X.; Ho, T.; Huang, H.Y.; Kim, S.H.; Goethberg, M.; Mannaerts, B.; Arce, J.C. A randomised controlled trial to clinically validate follitropin delta in its individualised dosing regimen for ovarian stimulation in Asian IVF/ICSI patients. Hum Reprod 2021, 36, 2452–2462. [Google Scholar] [CrossRef]
- Ishihara, O.; Arce, J.C. Japanese Follitropin Delta Phase 3 Trial (STORK) Group. Individualized follitropin delta dosing reduces OHSS risk in Japanese IVF/ICSI patients: a randomized controlled trial. Reprod Biomed Online 2021, 42, 909–918. [Google Scholar] [CrossRef]
- Ishihara, O.; Klein, B.M.; Arce, J.C. Japanese Follitropin Delta Phase 2 Trial Group. Randomized, assessor-blind, antimüllerian hormone-stratified.; dose-response trial in Japanese in vitro fertilization/intracytoplasmic sperm injection patients undergoing controlled ovarian stimulation with follitropin delta. Fertil Steril 2021, 115, 1478–1486. [Google Scholar] [CrossRef]
- Ishihara, O.; Nelson, S.M.; Arce, J.C. Comparison of ovarian response to follitropin delta in Japanese and White IVF/ICSI patients. Reprod Biomed Online 2022, 44, 177–184. [Google Scholar] [CrossRef]
- Fatemi, H.; Bilger, W.; Denis, D.; Griesinger, G.; La Marca, A.; Longobardi, S.; Mahony, M.; Yin, X.; D'Hooghe, T. Dose adjustment of follicle-stimulating hormone (FSH) during ovarian stimulation as part of medically-assisted reproduction in clinical studies: a systematic review covering 10 years (2007-2017). Reprod Biol Endocrinol 2021, 19, 68. [Google Scholar] [CrossRef]
- Shaltout, A.; Eid, M.; Shohayeb, A. Does triggering ovulation by 5000 IU of uhCG affect ICSI outcome? Middle East Fertility Society Journal 2006, 11, 99–103. [Google Scholar]
- Kolibianakis, E.M.; Papanikolaou, E.G.; Tournaye, H.; Camus, M.; Van Steirteghem, A.C.; Devroey, P. Triggering final oocyte maturation using different doses of human chorionic gonadotropin: a randomized pilot study in patients with polycystic ovary syndrome treated with gonadotropin-releasing hormone antagonists and recombinant follicle-stimulating hormone. Fertil Steril 2007, 88, 1382–1388. [Google Scholar] [CrossRef]
- Tiboni, G.M.; Colangelo, E.C.; Ponzano, A. Reducing the trigger dose of recombinant hCG in high-responder patients attending an assisted reproductive technology program: an observational study. Drug Des Devel Ther 2016, 10, 1691–1694. [Google Scholar] [CrossRef] [PubMed]
- Madani, T.; Mohammadi Yeganeh, L.; Ezabadi, Z.; Hasani, F.; Chehrazi, M. Comparing the efficacy of urinary and recombinant hCG on oocyte/follicle ratio to trigger ovulation in women undergoing intracytoplasmic sperm injection cycles: a randomized controlled trial. J Assist Reprod Genet 2013, 30, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Tapanainen, J.S.; Lapolt, P.S.; Perlas, E.; Hsueh, AJ. Induction of ovarian follicle luteinization by recombinant follicle-stimulating hormone. Endocrinology 1993, 133, 2875–2880. [Google Scholar] [CrossRef]
- Zelinski-Wooten, M.B.; Hutchison, J.S.; Hess, D.L.; Wolf, D.P.; Stouffer, R.L. Follicle stimulating hormone alone supports follicle growth and oocyte development in gonadotrophin-releasing hormone antagonist-treated monkeys. Hum Reprod 1995, 10, 1658–1666. [Google Scholar] [CrossRef]
- Andersen, C.Y.; Leonardsen, L.; Ulloa-Aguirre, A.; Barrios-De-Tomasi, J.; Moore, L.; Byskov, A.G. FSH-induced resumption of meiosis in mouse oocytes: effect of different isoforms. Mol Hum Reprod 1999, 5, 726–731. [Google Scholar] [CrossRef]
- Andersen, C.Y. Effect of FSH and its different isoforms on maturation of oocytes from pre-ovulatory follicles. Reprod Biomed Online 2002, 5, 232–239. [Google Scholar] [CrossRef]
- Franciosi, F.; Manandhar, S.; Conti, M. FSH regulates mRNA translation in mouse oocytes and promotes developmental competence. Endocrinology 2016, 157, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Rice, V.C.; Zusmanis, K.; Malter, H.; Mitchell-Leef, D. Pure FSH alone induces ovulation and subsequent pregnancy in the mouse resulting in fetal development. Life Sci 1993, 53, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.N.; Greenwald, G.S. Human chorionic gonadotropin or human recombinant follicle-stimulating hormone (FSH)-induced ovulation and subsequent fertilization and early embryo development in hypophysectomized FSH-primed mice. Endocrinology 1993, 132, 2009–2016. [Google Scholar] [CrossRef]
- Zelinski-Wooten, M.B.; Hutchison, J.S.; Hess, D.L.; WoIf, D.P.; Stouffer, R.L. A bolus of recombinant human follicle stimulating hormone at midcycle induces periovulatory events following multiple follicular development in macaques. Hum Reprod 1998, 13, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.H.; Wu, F.S.; Lee, R.K.; Li, S.H.; Lin, S.Y.; Hwu, Y.M. Dual trigger with combination of gonadotropin-releasing hormone agonist and human chorionic gonadotropin significantly improves the live-birth rate for normal responders in GnRH-antagonist cycles. Fertil Steril 2013, 100, 1296–302. [Google Scholar] [CrossRef]
- Haas, J.; Bassil, R.; Samara, N.; Zilberberg, E.; Mehta, C.; Orvieto, R.; Casper, R.F. GnRH agonist and hCG (dual trigger) versus hCG trigger for final follicular maturation: a double-blinded, randomized controlled study. Hum Reprod 2020, 35, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yang, X.; Wang, Y.; Xi, J.; Pan, H.; Wang, M.; Zhou, Y.; Xiao, Y. Ovulation triggering with hCG alone, GnRH agonist alone or in combination? A randomized controlled trial in advanced-age women undergoing IVF/ICSI cycles. Hum Reprod 2022, 37, 1795–1805. [Google Scholar] [CrossRef]
- Anaya, Y.; Cakmak, H.; Mata, D.A.; Letourneau, J.; Zhang, L.; Lenhart, N.; Juarez-Hernandez, F.; Jalalian, L.; Cedars, M.I.; Rosen, M. Triggering with 1500 IU of human chorionic gonadotropin plus follicle-stimulating hormone compared to a standard human chorionic gonadotropin trigger dose for oocyte competence in in vitro fertilization cycles: a randomized, double-blinded, controlled noninferiority trial. Fertil Steril 2022, 118, 266–278. [Google Scholar] [CrossRef]
- Shapiro, B.S.; Daneshmand, S.T.; Garner, F.C.; Aguirre, M.; Hudson, C. Comparison of “triggers” using leuprolide acetate alone or in combination with low-dose human chorionic gonadotropin. Fertil Steril 2011, 95, 2715–2717. [Google Scholar] [CrossRef] [PubMed]
- González, V.G.; Triana, A.M.; García, I.S.; Nieto, S.O.; Urrutia, M.C.; García, I.C.; Gastañaga-Holguera, T. Dual trigger vs. conventional trigger outcomes in in vitro fertilization. Systematic review and meta-analysis. JBRA Assist Reprod 2023, 27, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, X.; Guo, L.; Chang, H.M.; Shu, J.; Leung, P.C.K. Outcomes comparison of IVF/ICSI among different trigger methods for final oocyte maturation: A systematic review and meta-analysis. FASEB J, 2021, 35, e21696. [Google Scholar] [CrossRef] [PubMed]
- Mourad, S.; Brown, J.; Farquhar, C. Interventions for the prevention of OHSS in ART cycles: an overview of Cochrane reviews. Cochrane Database Syst Rev 2017, 1, CD012103. [Google Scholar] [CrossRef]
- Vyrides, A.A.; Mahdi, E.E.; Lamnisos, D.; Giannakou, K. Dual trigger with gonadotropin releasing hormone agonist and human chorionic gonadotropin of fresh autologous cycles in high responders: a systematic review. J Reprod Infertil 2022, 23, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Abbara, A.; Eng, P.C.; Phylactou, M.; Clarke, S.A.; Richardson, R.; Sykes, C.M.; Phumsatitpong, C.; Mills, E.; Modi, M.; Izzi-Engbeaya, C.; et al. Kisspeptin receptor agonist has therapeutic potential for female reproductive disorders. J Clin Invest 2020, 130, 6739–6753. [Google Scholar] [CrossRef]
- Abbara, A.; Jayasena, C.N.; Christopoulos, G.; Narayanaswamy, S.; Izzi-Engbeaya, C.; Nijher, G.M.; Comninos, A.N.; Peters, D.; Buckley, A.; Ratnasabapathy, R et al. Efficacy of kisspeptin-54 to trigger oocyte maturation in women at high risk of ovarian hyperstimulation syndrome (OHSS) during in vitro fertilization (IVF) therapy. J Clin Endocrinol Metab 2015, 100, 3322–3331. [Google Scholar] [CrossRef] [PubMed]
- Mathur, R.S.; Akande, A.V.; Keay, S.D.; Hunt, L.P.; Jenkins, JM. Distinction between early and late ovarian hyperstimulation syndrome. Fertil Steril 2000, 73, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Vane, JR.; Botting, RM. The mechanism of action of aspirin. Thromb Res 2003, 110, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Moini, A.; Zafarani, F.; Haddadian, S.; Ahmadi, J.; Honar, H.; Riazi, K. Effect of low-dose aspirin therapy on implantation rate in women undergoing in-vitro fertilization cycles. Saudi Med J 2007, 28, 732–736. [Google Scholar] [PubMed]
- Várnagy, A.; Bódis, J.; Mánfai, Z.; Wilhelm, F.; Busznyák, C.; Koppán, M. Low-dose aspirin therapy to prevent ovarian hyperstimulation syndrome. Fertil Steril 2010, 93, 2281–2284. [Google Scholar] [CrossRef]
- Namavar Jahromi, B.; Zolghadri, J.; Rahmani, E.; Alipour, S.; Anvar, Z.; Zarei, A.; Keramati, P. Effect of low-dose aspirin on the development of ovarian hyperstimulation syndrome and outcomes of assisted reproductive techniques in the women with PCOS, a randomized double-blinded clinical trial. Taiwan J Obstet Gynecol 2019, 58, 255–260. [Google Scholar] [CrossRef]
- Owj, M.; Tehrani-Nejad, E.S.; Amirchaghmaghi, E.; Baghestani, A.R.; Ahmadi, J. The role of ketoconazole in the prevention of ovarian hyperstimulation syndrome in patients with polycystic ovary syndrome during assisted reproductive technology cycles. Saudi Med J 2005, 26, 1584–1587. [Google Scholar] [PubMed]
- Parsanezhad, M.E.; Alborzi, S.; Pakniat, M.; Schmidt, E.H. A double-blind, randomized, placebo-controlled study to assess the efficacy of ketoconazole for reducing the risk of ovarian hyperstimulation syndrome. Fertil Steril 2003, 80, 1151–1155. [Google Scholar] [CrossRef]
- Lainas, G.T.; Kolibianakis, E.M.; Sfontouris, I.A.; Zorzovilis, I.Z.; Petsas, G.K.; Tarlatzi, T.B.; Tarlatzis, B.C.; Lainas, T.G. Outpatient management of severe early OHSS by administration of GnRH antagonist in the luteal phase: an observational cohort study. Reprod Biol Endocrinol 2012, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Lainas, G.T.; Kolibianakis, E.M.; Sfontouris, I.A.; Zorzovilis, I.Z.; Petsas, G.K.; Lainas, T.G.; Tarlatzis, B.C. Serum vascular endothelial growth factor levels following luteal gonadotrophin-releasing hormone antagonist administration in women with severe early ovarian hyperstimulation syndrome. BJOG 2014, 121, 848–855. [Google Scholar] [CrossRef]
- Zeng, C.; Shang, J.; Jin, AM.; Wu, PL.; Li, X.; Xue, Q. The effect of luteal GnRH antagonist on moderate and severe early ovarian hyperstimulation syndrome during in vitro fertilization treatment: a prospective cohort study. Arch Gynecol Obstet 2019, 300, 223–233. [Google Scholar] [CrossRef]
- Humaidan, P.; Bredkjaer, H.E.; Bungum, L.; Bungum, M.; Grøndahl, M.L.; Westergaard, L.; Andersen, C.Y. GnRH agonist (buserelin) or hCG for ovulation induction in GnRH antagonist IVF/ICSI cycles: a prospective randomized study. Hum Reprod 2005, 20, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Humaidan, P.; Polyzos, N.P.; Alsbjerg, B.; Erb, K.; Mikkelsen, A.L.; Elbaek, H.O.; Papanikolaou, E.G.; Andersen, C.Y. GnRHa trigger and individualized luteal phase hCG support according to ovarian response to stimulation: two prospective randomized controlled multi-centre studies in IVF patients. Hum Reprod 2013, 28, 2511–2521. [Google Scholar] [CrossRef]
- Andersen, C.Y.; Elbaek, H.O.; Alsbjerg, B.; Laursen, R.J.; Povlsen, B.B.; Thomsen, L.; Humaidan, P. Daily low-dose hCG stimulation during the luteal phase combined with GnRHa triggered IVF cycles without exogenous progesterone: a proof of concept trial. Hum Reprod 2015, 30, 2387–2395. [Google Scholar] [CrossRef]
- Svenstrup, L.; Möller, S.; Fedder, J.; Pedersen, D.E.; Erb, K.; Andersen, C.Y.; Humaidan, P. Does the HCG trigger dose used for IVF impact luteal progesterone concentrations? a randomized controlled trial. Reprod Biomed Online 2022, 45, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Santos-Ribeiro, S.; Mackens, S.; Popovic-Todorovic, B.; Racca, A.; Polyzos, N.P.; Van Landuyt, L.; Drakopoulos, P.; de Vos, M.; Tournaye, H.; Blockeel, C. The freeze-all strategy versus agonist triggering with low-dose hCG for luteal phase support in IVF/ICSI for high responders: a randomized controlled trial. Hum Reprod 2020, 35, 2808–2818. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol Rev 2016, 96, 1261–1296. [Google Scholar] [CrossRef]
- Greff, D.; Juhász, A.E.; Váncsa, S.; Váradi, A.; Sipos, Z.; Szinte, J.; Park, S; Hegyi, P.; Nyirády, P.; Ács, N.; Várbíró, S.; Horváth, E.M. Inositol is an effective and safe treatment in polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Reprod Biol Endocrinol 2023, 21, 10. [Google Scholar] [CrossRef]
- Mendoza, N.; Diaz-Ropero, MP.; Aragon, M.; Maldonado, V.; Llaneza, P.; Lorente, J.; Mendoza-Tesarik, R.; Maldonado-Lobon, J.; Olivares, M.; Fonolla, J. Comparison of the effect of two combinations of myo-inositol and D-chiro-inositol in women with polycystic ovary syndrome undergoing ICSI: a randomized controlled trial. Gynecol Endocrinol 2019, 35, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Mahey, R.; Kachhawa, G.; Khadgawat, R.; Vanamail, P.; Kriplani, A. Comparison of metformin plus myoinositol vs metformin alone in PCOS women undergoing ovulation induction cycles: randomized controlled trial. Gynecol Endocrinol 2019, 35, 511–514. [Google Scholar] [CrossRef]
- Rajasekaran, K.; Malhotra, N.; Mahey, R.; Khadgawat, R.; Kalaivani, M. Myoinositol versus metformin pretreatment in GnRH-antagonist cycle for women with PCOS undergoing IVF: a double-blinded randomized controlled study. Gynecol Endocrinol 2022, 38, 140–147. [Google Scholar] [CrossRef]
- Sánchez, M.F.; Larsson, P.; Serrano, M.F.; Bosch, E.; Velasco, J.A.G.; López, E.S.; Mannaerts, B. Live birth rates following individualized dosing algorithm of follitropin delta in a long GnRH agonist protocol. Reprod Biol Endocrinol 2023, 21, 45. [Google Scholar] [CrossRef]
- Broekmans, F.J. Individualization of FSH doses in assisted reproduction: facts and fiction. Front Endocrinol (Lausanne) 2019, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Nakhuda, G.S.; Douglas, N.C.; Thornton, M.H.; Guarnaccia, M.M.; Lobo, R.; Sauer, M.V. Anti-Mullerian hormone testing is useful for individualization of stimulation protocols in oocyte donors. Reprod Biomed Online 2010, 20, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Yovich, J.; Stanger, J.; Hinchliffe, P. Targeted gonadotrophin stimulation using the PIVET algorithm markedly reduces the risk of OHSS. Reprod Biomed Online 2012, 24, 81–92. [Google Scholar] [CrossRef]
- Wu, D.; Shi, H.; Yu, Y.; Yu, T.; Zhai, J. Comparison of the effectiveness of various medicines in the prevention of ovarian hyperstimulation syndrome: a network meta-analysis of randomized controlled trials. Front Endocrinol (Lausanne) 2022, 13, 808517. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).