Submitted:
02 August 2023
Posted:
04 August 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
Study Samples
Inclusion Criteria
Exclusion Criteria
Study Procedure
Sample Analyses
Statistical Analyses
Sample Size
Results
Clinical Characteristics
| Non-hospitalized N = 150 n (%) |
Hospitalized N = 72 n (%) |
Total N = 222 n (%) |
|
|---|---|---|---|
| Sex | |||
| Male | 92 (61.3) | 44 (61.1) | 136 (61.3) |
| Female | 58 (38.7) | 28 (38.9) | 86 (38.7) |
| Age (years) | |||
| n | 150 | 72 | 222 |
| Mean (SD) | 36.6 (13.1) | 47.1 (11.7) | 40.0 (13.5) |
| Median | 33.0 | 49.0 | 40.0 |
| Min, max | 20, 78 | 20, 79 | 20, 79 |
| 20 to <40* | 92 (61.3) | 17 (23.6) | 109 (49.1) |
| 40 to <60 | 52 (34.7) | 47 (65.3) | 99 (44.6) |
| ≥60 | 6 (4.0) | 8 (11.1) | 14 (6.3) |
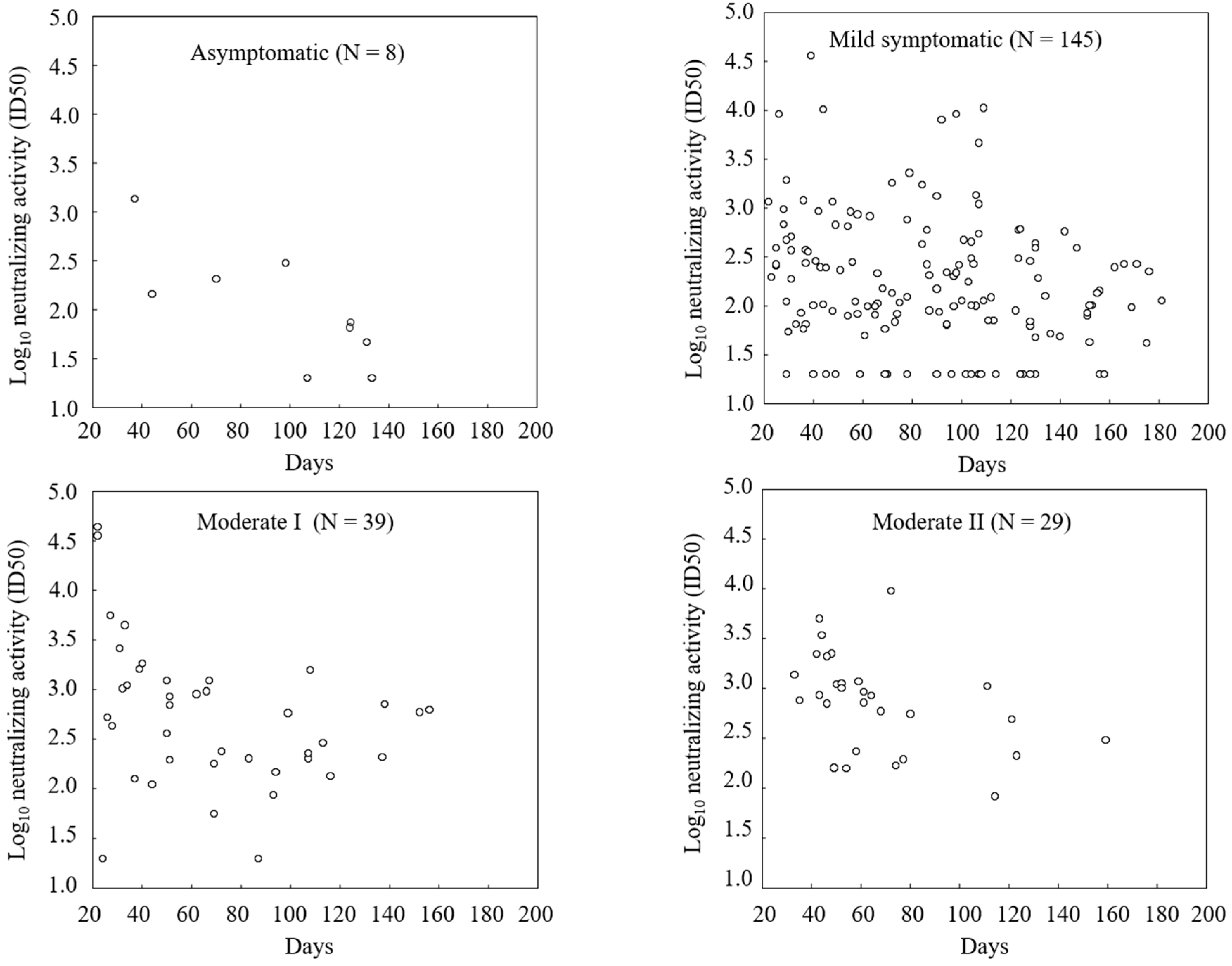
| Severity of SARS-CoV-2 infection | |||
| Asymptomatic | 8 (5.3) | 0 | 8 (3.6) |
| Mild | 141 (94.0) | 5 (6.9) | 146 (65.8) |
| Moderate I | 1 (0.7) | 38 (52.8) | 39 (17.6) |
| Moderate II | 0 | 29 (40.3) | 29 (13.1) |
| Severe | 0 | 0 | 0 |
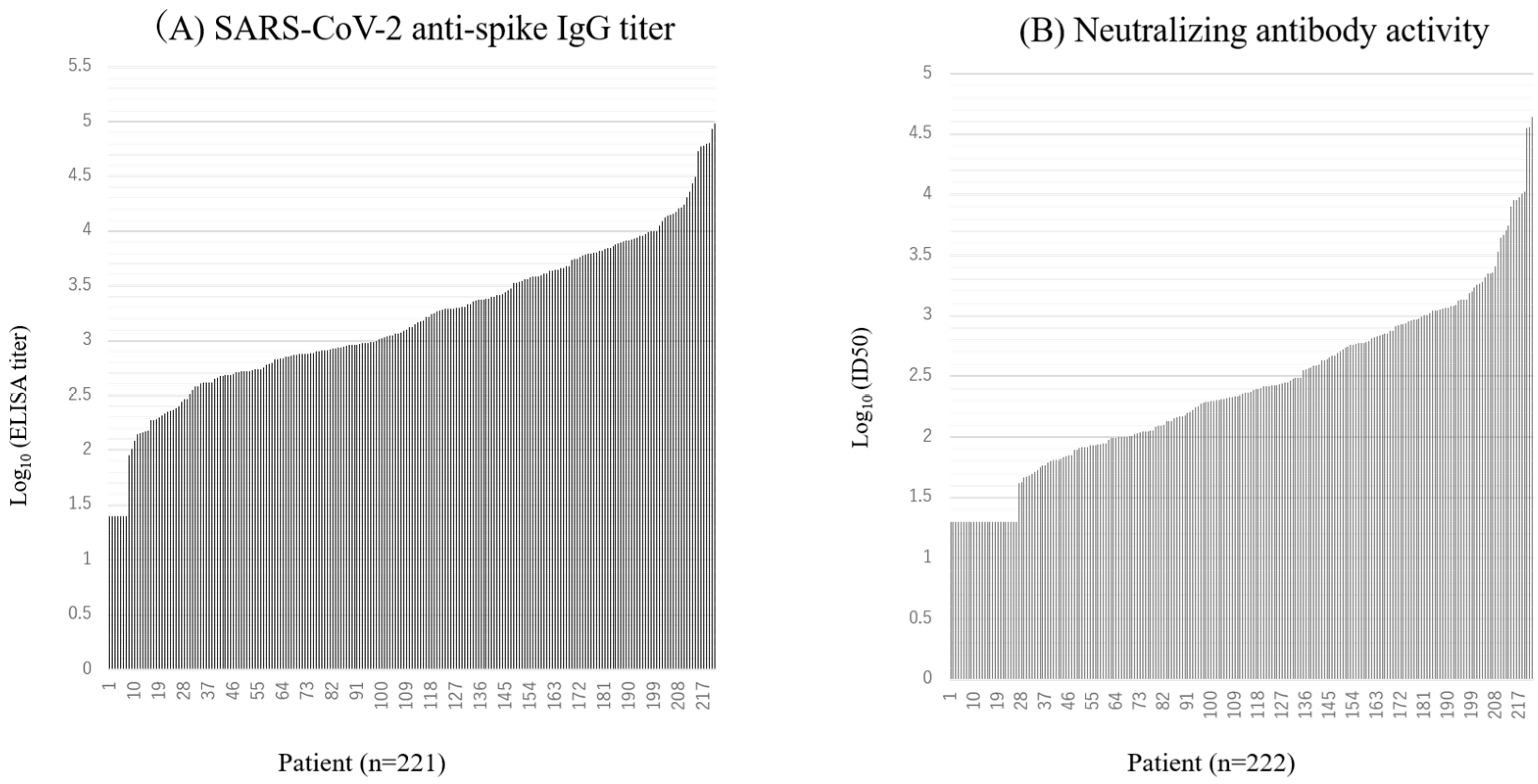
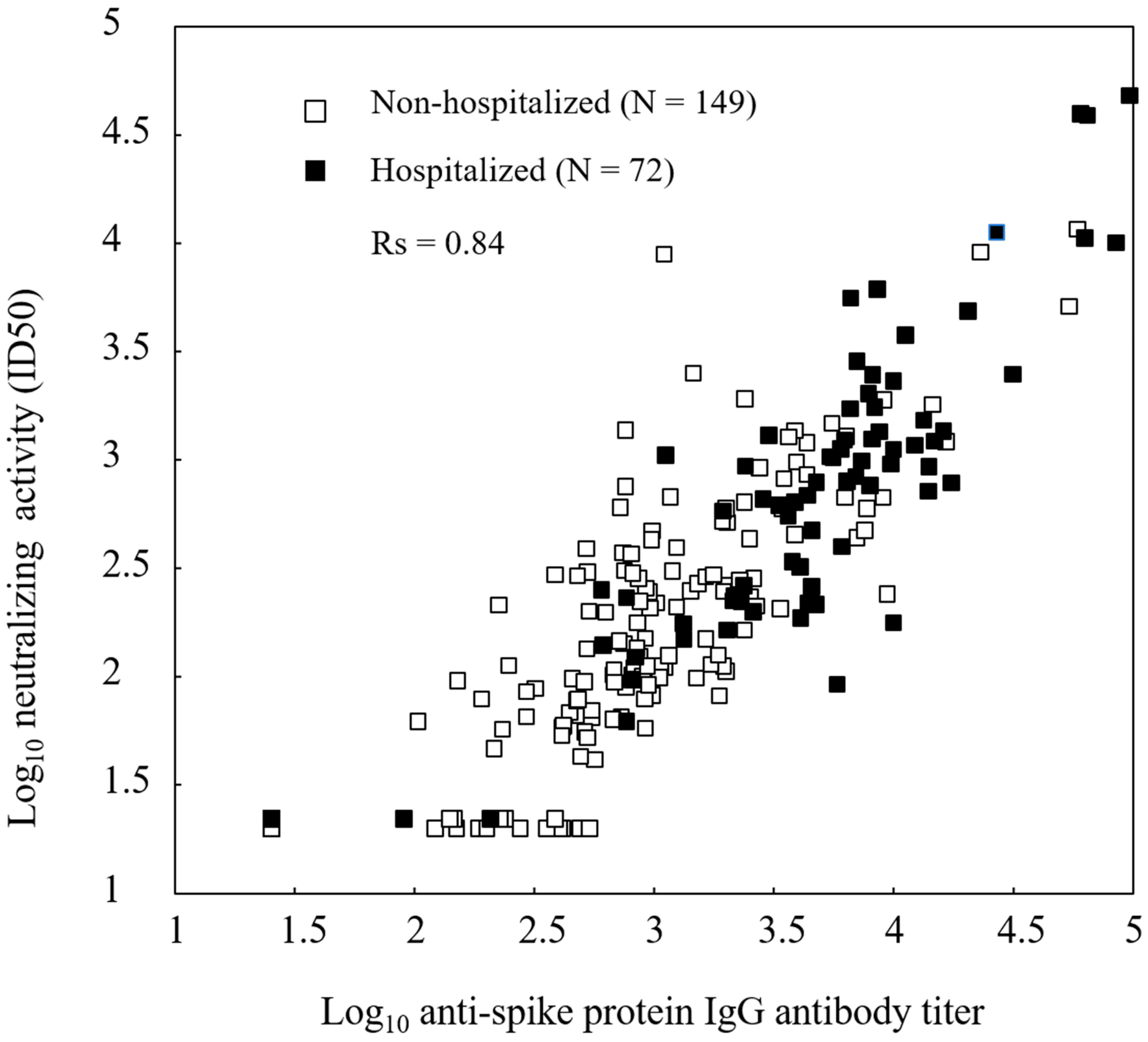
Immunogenicity Assessments
| Antibody titer (ELU/mL): S-ELISA | Neutralizing activity (ID50): PNA | ||||||
|---|---|---|---|---|---|---|---|
| Non-Hospitalized | Hospitalized | Total | Non-Hospitalized | Hospitalized | Total | ||
| All | 941 | 4391 | 1555 | 166 | 607 | 253 | |
| [753, 1176] | [3052, 6317] | [1257, 1923] | [132, 209] | [415, 888] | [204, 313] | ||
| N = 149 | N = 72 | N = 221 | N = 150 | N = 72 | N = 222 | ||
| Gender | Male | 977 | 4062 | 1549.55 | 182 | 638 | 273 |
| [727, 1312] | [2449, 6737] | [1172, 2047] | [133, 247] | [384, 1059] | [206, 361] | ||
| N = 92 | N = 44 | N = 136 | N = 92 | N = 44 | N = 136 | ||
| Female | 886 | 4963 | 1563 | 144 | 562 | 224 | |
| [627, 1253] | [2923, 8425] | [1119, 2183] | [101, 204] | [306, 1030] | [161, 312] | ||
| N = 58 | N = 28 | N = 85 | N = 58 | N = 28 | N = 86 | ||
| Age | 20 to <40 | 881 | 2932 | 1065 | 150 | 375 | 173 |
| (years) | [696, 1117] | [1376, 6245] | [837, 1356] | [111, 202] | [172, 817] | [131, 229] | |
| N = 91 | N = 17 | N = 108 | N = 92 | N = 17 | N = 109 | ||
| 40 to <60 | 1050 | 6209 | 2442 | 199 | 886 | 405 | |
| [667, 1652] | [4173, 9240] | [1725, 3456] | [134, 295] | [561, 1399] | [291, 563] | ||
| N = 52 | N = 47 | N = 99 | N = 52 | N = 47 | N = 99 | ||
| ≥60 | 983 | 1353 | 1180 | 159 | 183 | 172 | |
| [80, 12015] | [228, 8006] | [339, 4099] | [24, 1032] | [52, 636] | [70, 423] | ||
| N = 6 | N = 8 | N = 14 | N = 6 | N = 8 | N = 14 | ||
| Severity | Asymptomatic | 319 | - | 319 | 105 | - | 105 |
| [116, 877] | - | [116, 877] | [31, 353] | - | [31, 353] | ||
| N = 8 | N = 0 | N = 8 | N = 8 | N = 0 | N = 8 | ||
| Mild | 992 | 4221 | 1043 | 169 | 610 | 176 | |
| [790, 1246] | [77, 229822] | [819, 1327] | [133, 214] | [8, 43608] N = 5 |
[137, 227] | ||
| N = 140 | N = 5 | N = 145 | N = 141 | - | N = 146 | ||
| Moderate I | 3421 | 3241 | 3245 | 591 | 521 | 523 | |
| - | [1877, 5595] | [1908, 5521] | - | [302, 899] | [308, 888] | ||
| N = 1 | N = 38 | N = 39 | N = 1 | N = 38 | N = 39 | ||
| Moderate II | - | 6582 | 6582 | - | 741 | 741 | |
| - | [4840, 8950] | [4840, 8950] | - | [484, 1132] | [484, 1132] | ||
| N = 0 | N = 29 | N = 29 | N = 0 | N = 29 | N = 29 | ||
| Antibody titer and neutralizing activity were expressed as the geometric mean.The 95% confidence interval is presented within brackets.One patient was excluded from the analysis because of an invalid ELISA result. | |||||||
Discussion
Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of interest
Ethics Statement
Abbreviations
| 50% inhibitory dilution | ID50 |
| Confidence interval | CI |
| Coronavirus disease 2019 | COVID-19 |
| PhenoSense SARS-CoV-2 neutralizing antibody assay | PNA |
| Severe acute respiratory syndrome coronavirus 2 | SARS-CoV-2 |
| SARS-COV-2 spike enzyme-linked immunosorbent assay | S-ELISA |
| University Hospital Medical Information Network | UMIN |
References
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. China novel coronavirus investigating and research team. N Engl J Med. 2020;382(8):727-733. doi: 10.1056/NEJMoa2001017. [CrossRef]
- Goto A, Go H, Miyakawa K, Yamaoka Y, Ohtake N, Kubo S, Jeremiah SS, Mihara T, Senuki K, Miyazaki T, Ikeda S, Ogura T, Kato H, Matsuba I, Sanno N, Miyakawa M, Ozaki H, Kikuoka M, Ohashi Y, Ryo A, Yamanaka T. Sustained neutralizing antibodies 6 months following infection in 376 Japanese COVID-19 survivors. Front Microbiol. 2021;12:661187. doi: 10.3389/fmicb.2021.661187. [CrossRef]
- Miyakawa K, Kubo S, Stanleyraj Jeremiah S, Go H, Yamaoka Y, Ohtake N, Kato H, Ikeda S, Mihara T, Matsuba I, Sanno N, Miyakawa M, Shinkai M, Miyazaki T, Ogura T, Ito S, Kaneko T, Yamamoto K, Goto A, Ryo A. Persistence of Robust Humoral Immune Response in Coronavirus Disease 2019 Convalescent Individuals Over 12 Months After Infection. Open Forum Infect Dis. 2021;9(2):ofab626. doi: 10.1093/ofid/ofab626. [CrossRef]
- Ode H, Nakata Y, Nagashima M, Hayashi M, Yamazaki T, Asakura H, Suzuki J, Kubota M, Matsuoka K, Matsuda M, Mori M, Sugimoto A, Imahashi M, Yokomaku Y, Sadamasu K, Iwatani Y. Molecular epidemiological features of SARS-CoV-2 in Japan, 2020-1. Virus Evol. 2022;8(1):veac034. doi: 10.1093/ve/veac034. [CrossRef]
- Seow J, Graham C, Merrick B, Acors S, Pickering S, Steel KJA, Hemmings O, O’Byrne A, Kouphou N, Galao RP, Betancor G, Wilson HD, Signell AW, Winstone H, Kerridge C, Huettner I, Jimenez-Guardeño JM, Lista MJ, Temperton N, Snell LB, Bisnauthsing K, Moore A, Green A, Martinez L, Stokes B, Honey J, Izquierdo-Barras A, Arbane G, Patel A, Tan MKI, O’Connell L, O’Hara G, MacMahon E, Douthwaite S, Nebbia G, Batra R, Martinez-Nunez R, Shankar-Hari M, Edgeworth JD, Neil SJD, Malim MH, Doores KJ. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020 Dec;5(12):1598-1607. doi: 10.1038/s41564-020-00813-8. Epub 2020 Oct 26. PMID: 33106674; PMCID: PMC7610833. [CrossRef]
- Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183(4):996-1012.e19. doi: 10.1016/j.cell.2020.09.038. [CrossRef]
- Weisberg SP, Connors TJ, Zhu Y, Baldwin MR, Lin WH, Wontakal S, Szabo PA, Wells SB, Dogra P, Gray J, Idzikowski E, Stelitano D, Bovier FT, Davis-Porada J, Matsumoto R, Poon MML, Chait M, Mathieu C, Horvat B, Decimo D, Hudson KE, Zotti FD, Bitan ZC, La Carpia F, Ferrara SA, Mace E, Milner J, Moscona A, Hod E, Porotto M, Farber DL. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat Immunol. 2021 Jan;22(1):25-31. doi: 10.1038/s41590-020-00826-9. Epub 2020 Nov 5. PMID: 33154590; PMCID: PMC8136619. [CrossRef]
- Trinité B, Tarrés-Freixas F, Rodon J, Pradenas E, Urrea V, Marfil S, Rodríguez de la Concepción ML, Ávila-Nieto C, Aguilar-Gurrieri C, Barajas A, Ortiz R, Paredes R, Mateu L, Valencia A, Guallar V, Ruiz L, Grau E, Massanella M, Puig J, Chamorro A, Izquierdo-Useros N, Segalés J, Clotet B, Carrillo J, Vergara-Alert J, Blanco J. SARS-CoV-2 infection elicits a rapid neutralizing antibody response that correlates with disease severity. Sci Rep. 2021;11(1):2608. doi: 10.1038/s41598-021-81862-9. [CrossRef]
- Klein SL, Pekosz A, Park HS, Ursin RL, Shapiro JR, Benner SE, Littlefield K, Kumar S, Naik HM, Betenbaugh MJ, Shrestha R, Wu AA, Hughes RM, Burgess I, Caturegli P, Laeyendecker O, Quinn TC, Sullivan D, Shoham S, Redd AD, Bloch EM, Casadevall A, Tobian AA. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. Clin Invest. 2020;130(11):6141-6150. doi: 10.1172/JCI142004. [CrossRef]
- Mehew J, Johnson R, Roberts D, Harvala H. Convalescent plasma for COVID-19: male gender, older age and hospitalisation associated with high neutralising antibody levels, England. Euro Surveill. 2020;25(45):2001754. doi: 10.2807/1560-7917.ES.2020.25.45.2001754. [CrossRef]
- Kageyama T, Ikeda K, Tanaka S, Taniguchi T, Igari H, Onouchi Y, Kaneda A, Matsushita K, Hanaoka H, Nakada TA, Ohtori S, Yoshino I, Matsubara H, Nakayama T, Yokote K, Nakajima H. Antibody responses to BNT162b2 mRNA COVID-19 vaccine in 2,015 healthcare workers in a single tertiary referral hospital in Japan. Clin Microbiol Infect. 2021;27(12):1861.e1-1861.e5. doi: 10.1016/j.cmi.2021.07.042. [CrossRef]
- Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20(7):442-447. doi: 10.1038/s41577-020-0348-8. [CrossRef]
- Garcia-Beltran WF, Lam EC, Astudillo MG, Yang D, Miller TE, Feldman J, Hauser BM, Caradonna TM, Clayton KL, Nitido AD, Murali MR, Alter G, Charles RC, Dighe A, Branda JA, Lennerz JK, Lingwood D, Schmidt AG, Iafrate AJ, Balazs AB. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021;184(2):476-488.e11. doi: 10.1016/j.cell.2020.12.015. [CrossRef]
- Maeda K, Higashi-Kuwata N, Kinoshita N, Kutsuna S, Tsuchiya K, Hattori SI, Matsuda K, Takamatsu Y, Gatanaga H, Oka S, Sugiyama H, Ohmagari N, Mitsuya H. Neutralization of SARS-CoV-2 with IgG from COVID-19-convalescent plasma. Sci Rep. 2021;11(1):5563. doi: 10.1038/s41598-021-84733-5. [CrossRef]
- Pierce CA, Preston-Hurlburt P, Dai Y, Aschner CB, Cheshenko N, Galen B, Garforth SJ, Herrera NG, Jangra RK, Morano NC, Orner E, Sy S, Chandran K, Dziura J, Almo SC, Ring A, Keller MJ, Herold KC, Herold BC. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci Transl Med. 2020 Oct 7;12(564):eabd5487. doi: 10.1126/scitranslmed.abd5487. Epub 2020 Sep 21. PMID: 32958614; PMCID: PMC7658796. [CrossRef]
- Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Subbarao K, Kent SJ, Triccas JA, Davenport MP. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021 Jul;27(7):1205-1211. doi: 10.1038/s41591-021-01377-8. [CrossRef]
- McMahan K, Yu J, Mercado NB, Loos C, Tostanoski LH, Chandrashekar A, , Liu J, Peter L, Atyeo C, Zhu A, Bondzie EA, Dagotto G, Gebre MS, Jacob-Dolan C, Li Z, Nampanya F, Patel S, Pessaint L, Van Ry A, Blade K, Yalley-Ogunro J, Cabus M, Brown R, Cook A, Teow E, Andersen H, Lewis MG, Lauffenburger DA, Alter G, Barouch DH. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590(7847):630-634. doi: 10.1038/s41586-020-03041-6. [CrossRef]
- Preliminary data reported at the New Coronavirus Infectious Disease Control Advisory Board held at the Ministry of Health, Labor and Welfare on April 13, 2022 (in Japanese). Available from: https://www.mhlw.go.jp/content/10900000/000929082.pdf.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




