Submitted:
31 July 2023
Posted:
01 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1 Patients
2.2 Anamnesis
2.3 Measurements
2.3 Statistical analysis
2.4 Ethical consideration
3. Results
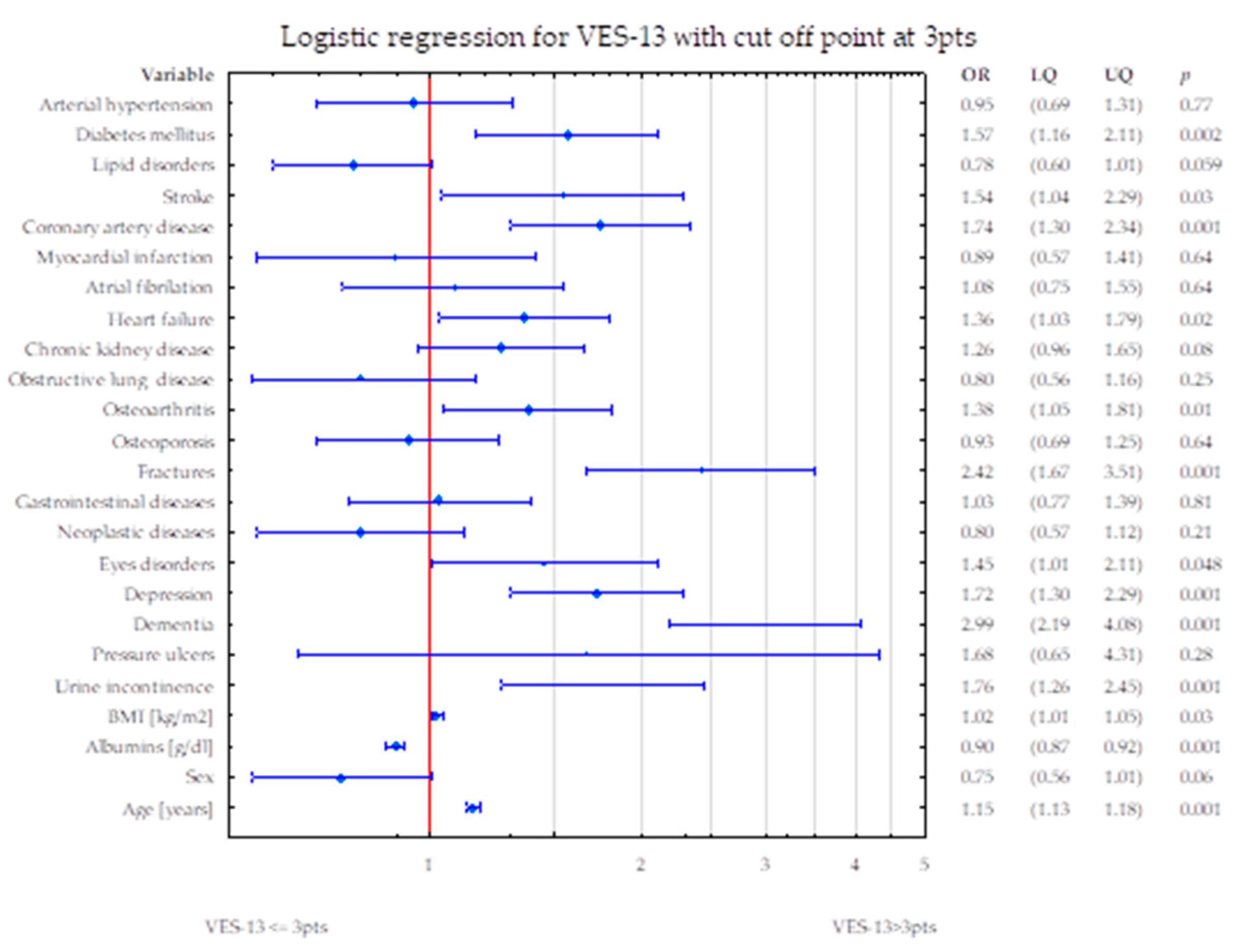
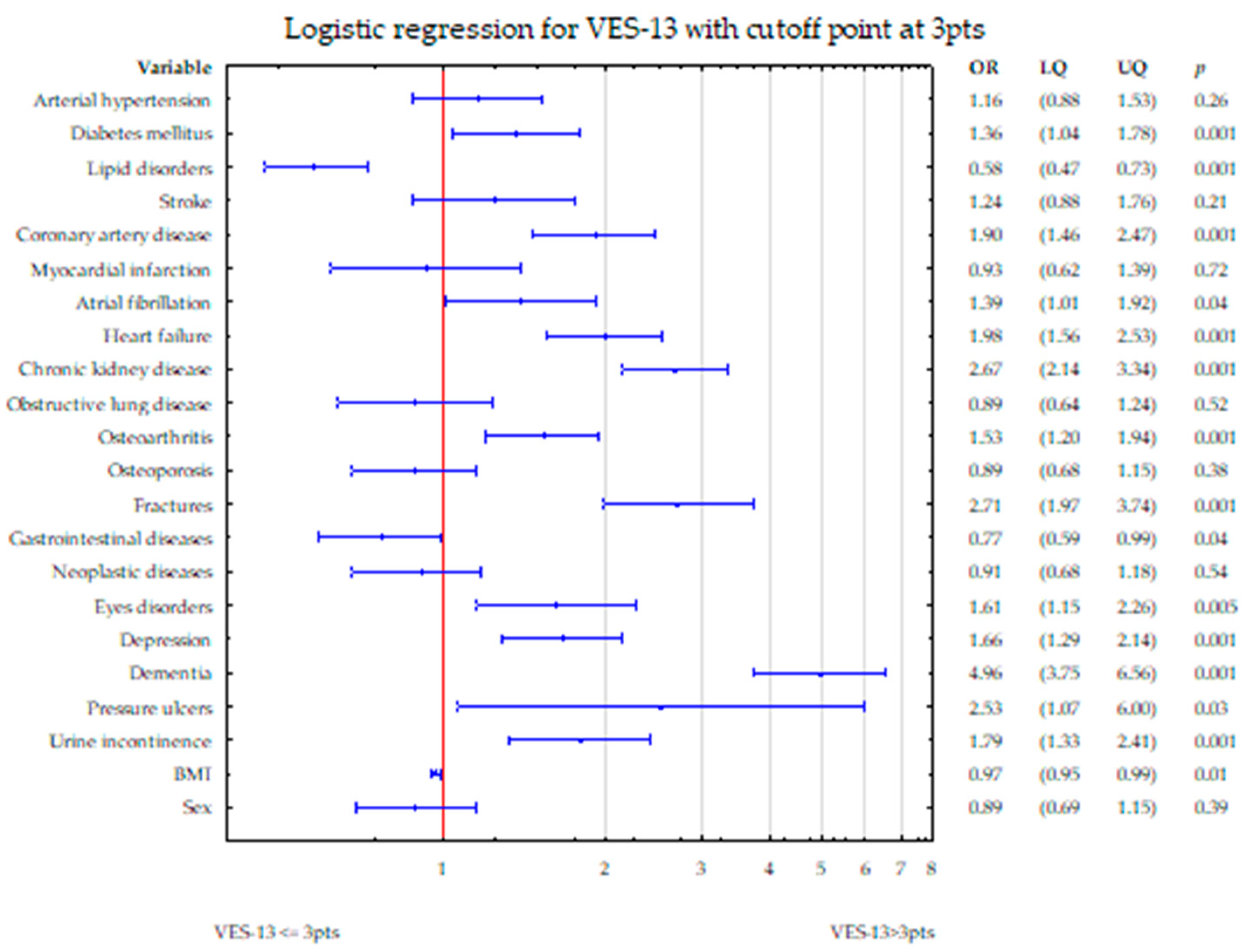
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saliba, D., et al., The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc, 2001. 49(12): p. 1691-9. [CrossRef]
- Kenig, J., et al., Six screening instruments for frailty in older patients qualified for emergency abdominal surgery. Arch Gerontol Geriatr, 2015. 61(3): p. 437-42. [CrossRef]
- Assis, D.L., et al., The role of VES-13 to identify limited life expectancy in older adults in primary healthcare settings. Rev Esc Enferm USP, 2021. 55: p. e03743. [CrossRef]
- Min, L., et al., The vulnerable elders-13 survey predicts 5-year functional decline and mortality outcomes in older ambulatory care patients. J Am Geriatr Soc, 2009. 57(11): p. 2070-6. [CrossRef]
- Silva, S.M., et al., VES-13 and WHOQOL-bref cutoff points to detect quality of life in older adults in primary health care. Rev Saude Publica, 2019. 53: p. 26. [CrossRef]
- Feng, J., et al., Reliability and validity test of VES-13 and analysis of influencing factors for the vulnerable condition of patients with advanced castration-resistant prostate cancer. Pak J Med Sci, 2021. 37(1): p. 137-141. [CrossRef]
- Carneiro, F., et al., Vulnerability in elderly patients with gastrointestinal cancer--translation, cultural adaptation and validation of the European Portuguese version of the Vulnerable Elders Survey (VES-13). BMC Cancer, 2015. 15: p. 723. [CrossRef]
- Ferrero, A., et al., Can Vulnerable Elders Survey-13 predict the impact of frailty on chemotherapy in elderly patients with gynaecological malignancies? Medicine (Baltimore), 2018. 97(39): p. e12298.
- Lowenstein, L.M., et al., Which better predicts mortality among older men, a prostate cancer (PCa) diagnosis or vulnerability on the Vulnerable Elders Survey (VES-13)? A retrospective cohort study. J Geriatr Oncol, 2016. 7(6): p. 437-443. [CrossRef]
- Luciani, A., et al., Estimating the risk of chemotherapy toxicity in older patients with cancer: The role of the Vulnerable Elders Survey-13 (VES-13). J Geriatr Oncol, 2015. 6(4): p. 272-9. [CrossRef]
- Vernon, T.L., 3rd, et al., Implementation of Vulnerable Elders Survey-13 Frailty Tool to Identify At-Risk Geriatric Surgical Patients. J Perianesth Nurs, 2019. 34(5): p. 911-918.e2.
- Min, L., et al., The vulnerable elders survey-13 predicts hospital complications and mortality in older adults with traumatic injury: a pilot study. J Am Geriatr Soc, 2011. 59(8): p. 1471-6. [CrossRef]
- Assis, F.C., et al., Association of health vulnerability with adverse outcomes in older people with COVID-19: a prospective cohort study. Clinics (Sao Paulo), 2021. 76: p. e3369.
- Balakumar, P., U.K. Maung, and G. Jagadeesh, Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol Res, 2016. 113(Pt A): p. 600-609. [CrossRef]
- Bell, S.P., et al., Development of a multivariable model to predict vulnerability in older American patients hospitalised with cardiovascular disease. BMJ Open, 2015. 5(8): p. e008122. [CrossRef]
- Wang, J., et al., Changes in vulnerability among older patients with cardiovascular disease in the first 90 days after hospital discharge: A secondary analysis of a cohort study. BMJ Open, 2019. 9(1): p. e024766. [CrossRef]
- Kroc, Ł., et al., Validation of the Vulnerable Elders Survey-13 (VES-13) in hospitalized older patients. European Geriatric Medicine, 2016. 7(5): p. 449-453. [CrossRef]
- Oscanoa, T.J., et al., Estimation of the glomerular filtration rate in older individuals with serum creatinine-based equations: A systematic comparison between CKD-EPI and BIS1. Arch Gerontol Geriatr, 2018. 75: p. 139-145. [CrossRef]
- Yesavage, J.A., et al., Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res, 1982. 17(1): p. 37-49. [CrossRef]
- Folstein, M.F., S.E. Folstein, and P.R. McHugh, "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res, 1975. 12(3): p. 189-98. [CrossRef]
- Aprahamian, I., et al., Hypertension and frailty in older adults. J Clin Hypertens (Greenwich), 2018. 20(1): p. 186-192. [CrossRef]
- Richter, D., et al., Frailty in cardiology: definition, assessment and clinical implications for general cardiology. A consensus document of the Council for Cardiology Practice (CCP), Association for Acute Cardio Vascular Care (ACVC), Association of Cardiovascular Nursing and Allied Professions (ACNAP), European Association of Preventive Cardiology (EAPC), European Heart Rhythm Association (EHRA), Council on Valvular Heart Diseases (VHD), Council on Hypertension (CHT), Council of Cardio-Oncology (CCO), Working Group (WG) Aorta and Peripheral Vascular Diseases, WG e-Cardiology, WG Thrombosis, of the European Society of Cardiology, European Primary Care Cardiology Society (EPCCS). Eur J Prev Cardiol, 2022. 29(1): p. 216-227. [CrossRef]
- Benetos, A., M. Petrovic, and T. Strandberg, Hypertension Management in Older and Frail Older Patients. Circ Res, 2019. 124(7): p. 1045-1060. [CrossRef]
- Oliveros, E., et al., Hypertension in older adults: Assessment, management, and challenges. Clin Cardiol, 2020. 43(2): p. 99-107. [CrossRef]
- Odden, M.C., et al., Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med, 2012. 172(15): p. 1162-8.
- Guo, Q., X. Du, and C.S. Ma, Atrial fibrillation and frailty. J Geriatr Cardiol, 2020. 17(2): p. 105-109. [CrossRef]
- Evans, N.R., et al., Frailty and cerebrovascular disease: Concepts and clinical implications for stroke medicine. Int J Stroke, 2022. 17(3): p. 251-259.
- Afilalo, J., et al., Role of Frailty in Patients With Cardiovascular Disease. American Journal of Cardiology, 2009. 103(11): p. 1616-1621. [CrossRef]
- Purser, J.L., et al., Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriatr Soc, 2006. 54(11): p. 1674-81. [CrossRef]
- Proietti, M., et al., Epidemiology and impact of frailty in patients with atrial fibrillation in Europe. Age Ageing, 2022. 51(8). [CrossRef]
- Wilkinson, C., et al., Management of atrial fibrillation for older people with frailty: a systematic review and meta-analysis. Age Ageing, 2019. 48(2): p. 196-203. [CrossRef]
- Proietti, M., et al., Frailty prevalence and impact on outcomes in patients with atrial fibrillation: A systematic review and meta-analysis of 1,187,000 patients. Ageing Res Rev, 2022. 79: p. 101652. [CrossRef]
- Pandey, A., D. Kitzman, and G. Reeves, Frailty Is Intertwined With Heart Failure: Mechanisms, Prevalence, Prognosis, Assessment, and Management. JACC Heart Fail, 2019. 7(12): p. 1001-1011.
- Davis, M.R., et al., Gender differences in the prevalence of frailty in heart failure: A systematic review and meta-analysis. Int J Cardiol, 2021. 333: p. 133-140. [CrossRef]
- Mielke, N., et al., Gender differences in frailty transition and its prediction in community-dwelling old adults. Sci Rep, 2022. 12(1): p. 7341. [CrossRef]
- Yanase, T., et al., Frailty in elderly diabetes patients. Endocr J, 2018. 65(1): p. 1-11. [CrossRef]
- Assar, M.E., O. Laosa, and L. Rodríguez Mañas, Diabetes and frailty. Curr Opin Clin Nutr Metab Care, 2019. 22(1): p. 52-57.
- Umegaki, H., Sarcopenia and frailty in older patients with diabetes mellitus. Geriatr Gerontol Int, 2016. 16(3): p. 293-9. [CrossRef]
- Chao, C.T., J. Wang, and K.L. Chien, Both pre-frailty and frailty increase healthcare utilization and adverse health outcomes in patients with type 2 diabetes mellitus. Cardiovasc Diabetol, 2018. 17(1): p. 130. [CrossRef]
- Lee, Y., et al., Frailty and body mass index as predictors of 3-year mortality in older adults living in the community. Gerontology, 2014. 60(6): p. 475-82. [CrossRef]
- Jayanama, K., et al., Relationship of body mass index with frailty and all-cause mortality among middle-aged and older adults. BMC Med, 2022. 20(1): p. 404. [CrossRef]
- Bhardwaj, P.V., et al., The Association Between Body Mass Index, Frailty and Long-Term Clinical Outcomes in Hospitalized Older Adults. Am J Med Sci, 2021. 362(3): p. 268-275. [CrossRef]
- Cook, M.J., et al., Increased Frailty in Individuals With Osteoarthritis and Rheumatoid Arthritis and the Influence of Comorbidity: An Analysis of the UK Biobank Cohort. Arthritis Care Res (Hoboken), 2022. 74(12): p. 1989-1996. [CrossRef]
- O'Brien, M.S. and J.J. McDougall, Age and frailty as risk factors for the development of osteoarthritis. Mech Ageing Dev, 2019. 180: p. 21-28. [CrossRef]
- Salaffi, F., S. Farah, and M. Di Carlo, Frailty syndrome in rheumatoid arthritis and symptomatic osteoarthritis: an emerging concept in rheumatology. Acta Biomed, 2020. 91(2): p. 274-296. [CrossRef]
- Ardoino, I., et al., Pain and Frailty in Hospitalized Older Adults. Pain Ther, 2020. 9(2): p. 727-740. [CrossRef]
- Sternberg, S.A., et al., Frailty and osteoporosis in older women--a prospective study. Osteoporos Int, 2014. 25(2): p. 763-8. [CrossRef]
- Ma, S.L., et al., Self-reported frailty is associated with low calcaneal bone mineral density in a multiracial population of community-dwelling elderly. Osteoporos Int, 2009. 20(11): p. 1837-46. [CrossRef]
- Court-Brown, C.M. and M.M. McQueen, Global Forum: Fractures in the Elderly. J Bone Joint Surg Am, 2016. 98(9): p. e36. [CrossRef]
- Curtis, E., et al., Frailty score on admission predicts mortality and discharge disposition in elderly trauma patients over the age of 65 y. J Surg Res, 2018. 230: p. 13-19. [CrossRef]
- Yan, B., et al., Prognostic significance of frailty in older patients with hip fracture: a systematic review and meta-analysis. Int Orthop, 2022. 46(12): p. 2939-2952. [CrossRef]
- Cabral, J.F., et al., Vulnerability and associated factors among older people using the Family Health Strategy. Cien Saude Colet, 2018. 24(9): p. 3227-3236.
- Soysal, P., et al., Relationship between depression and frailty in older adults: A systematic review and meta-analysis. Ageing Res Rev, 2017. 36: p. 78-87. [CrossRef]
- Vaughan, L., A.L. Corbin, and J.S. Goveas, Depression and frailty in later life: a systematic review. Clin Interv Aging, 2015. 10: p. 1947-58. [CrossRef]
- Waite, S.J., et al., Sarcopenia and frailty in individuals with dementia: A systematic review. Arch Gerontol Geriatr, 2021. 92: p. 104268. [CrossRef]
- Arai, H., S. Satake, and K. Kozaki, Cognitive Frailty in Geriatrics. Clin Geriatr Med, 2018. 34(4): p. 667-675. [CrossRef]
- Sugimoto, T., H. Arai, and T. Sakurai, An update on cognitive frailty: Its definition, impact, associated factors and underlying mechanisms, and interventions. Geriatr Gerontol Int, 2022. 22(2): p. 99-109. [CrossRef]
- Cornish, L., Prevention of pressure ulcers in older people with frailty. Nurs Older People, 2022. [CrossRef]
- Jaul, E., Assessment and management of pressure ulcers in the elderly: current strategies. Drugs Aging, 2010. 27(4): p. 311-25.
- Veronese, N., et al., Association between urinary incontinence and frailty: a systematic review and meta-analysis. Eur Geriatr Med, 2018. 9(5): p. 571-578. [CrossRef]
- John, G., et al., Urinary Incontinence as a Predictor of Death: A Systematic Review and Meta-Analysis. PLoS One, 2016. 11(7): p. e0158992. [CrossRef]
- Chowdhury, R., et al., Frailty and chronic kidney disease: A systematic review. Arch Gerontol Geriatr, 2017. 68: p. 135-142. [CrossRef]
- Lahousse, L., et al., Risk of Frailty in Elderly With COPD: A Population-Based Study. J Gerontol A Biol Sci Med Sci, 2016. 71(5): p. 689-95. [CrossRef]
- Yee, N., et al., Frailty in Chronic Obstructive Pulmonary Disease and Risk of Exacerbations and Hospitalizations. Int J Chron Obstruct Pulmon Dis, 2020. 15: p. 1967-1976. [CrossRef]
- Bandidwattanawong, C. and G. Kerkarchachai, The benefits of G8 and VES-13 geriatric screening tools for older patients with advanced lung cancer. J Geriatr Oncol, 2022. 13(8): p. 1256-1259. [CrossRef]
- Ushida, K., et al., Hospital Frailty Risk Score Predicts Outcomes in Chronic Obstructive Pulmonary Disease Exacerbations. Arch Gerontol Geriatr, 2022. 100: p. 104658. [CrossRef]
- Kameyama, H., et al., Efficacy of preoperative frailty assessment in patients with gastrointestinal disease. Geriatr Gerontol Int, 2021. 21(3): p. 327-330. [CrossRef]
- Augschoell, J., et al., PPT and VES-13 in elderly patients with cancer: evaluation in multidimensional geriatric assessment and prediction of survival. J Geriatr Oncol, 2014. 5(4): p. 415-21. [CrossRef]
- Cavdar, E., et al., Prospective comparison of the value of CARG, G8, and VES-13 toxicity tools in predicting chemotherapy-related toxicity in older Turkish patients with cancer. J Geriatr Oncol, 2022. 13(6): p. 821-827. [CrossRef]
- Shang, X., et al., Associations of vision impairment and eye diseases with frailty in community-dwelling older adults: a nationwide longitudinal study in China. Br J Ophthalmol, 2022. [CrossRef]
- Varadaraj, V., et al., Near Vision Impairment and Frailty: Evidence of an Association. Am J Ophthalmol, 2019. 208: p. 234-241. [CrossRef]
- Zhao, Y., et al., Combined Vision and Hearing Impairment is Associated with Frailty in Older Adults: Results from the West China Health and Aging Trend Study. Clin Interv Aging, 2022. 17: p. 675-683. [CrossRef]
- Kroc, Ł., et al., Comparison of Nutrition Risk Screening 2002 and Subjective Global Assessment Form as Short Nutrition Assessment Tools in Older Hospitalized Adults. Nutrients, 2021. 13(1). [CrossRef]
- Zhang, Z., et al., Evaluation of Blood Biomarkers Associated with Risk of Malnutrition in Older Adults: A Systematic Review and Meta-Analysis. Nutrients, 2017. 9(8). [CrossRef]
| Parameter/disease | Women n=2036 |
Men n=824 |
p-value |
|---|---|---|---|
| Age (mean±SD, median (quartiles)) | 81.7±7.9 83 (77-87) |
81.8±8.3 83 (76-88) |
p=0.4(U) |
| BMI, m/kg² (mean±SD, median (quartiles)) | 26.2±5.4 25.4 (22.3-29.2) |
26.0±4.6 25.5 (23.0-28.1) |
p=0.92(U) |
| Body mass, kg (mean±SD, median (quartiles)) | 64.4±14.3 63 (54.5-72) |
74.8±14.5 73 (65-81) |
p<0.0001(U) |
| Albumins, g/l (mean±SD, median (quartiles)) | 39.7±5.7 41 (36.9-43.7) |
39±6.2 40.3 (35.9-43.2) |
p=0.002 (U) |
| VES-13, points (mean±SD, median (quartiles)) | 6.78±2.8 8 (4-10) |
6.4±2.9 7 (4-9) |
p=0.002(U) |
| Arterial hypertension, n, % | 1618 (79.5%) | 633 (76.8%) | p=0.117(chi2) |
| Diabetes mellitus, n, % | 507 (24.9%) | 248 (30.1%) | p=0.004(chi2) |
| Lipid disorders, n, % | 919 (45.1%) | 284 (34.5%) | p<0.0001(chi2) |
| Stroke, n, % | 322 (15.8%) | 141 (17.1%) | p=0.4(chi2) |
| Coronary artery disease, n, % | 766 (37.6%) | 335 (40.7%) | p=0.13(chi2) |
| Myocardial infarction, n, % | 186 (9.1%) | 128 (15.5%) | p<0.0001(chi2) |
| Atrial fibrillation, n, % | 385 (18.9%) | 212 (25.7%) | p<0.0001(chi2) |
| Heart failure, n, % | 964 (47.4%) | 397 (48.2%) | p=0.69(chi2) |
| Chronic kidney disease, n, % | 1331 (66.1%) | 519 (63.6%) | p=0.22(chi2) |
| Obstructive lung diseases, n, % | 250 (12.3%) | 119 (14.4%) | p<0.0001(chi2) |
| Osteoarthritis, n, % | 767 (37.7%) | 212 (25.7%) | p<0.0001(chi2) |
| Osteoporosis, n, % | 667 (32.8%) | 93 (11.3%) | p<0.0001(chi2) |
| Fractures, n, % | 464 (22.8%) | 109 (13.2%) | p<0.0001(chi2) |
| Gastrointestinal diseases, n, % | 439 (21.5%) | 183 (22.2%) | p=0.83(chi2) |
| Neoplastic diseases, n, % | 293 (14.4%) | 153 (18.6%) | p=0.06(chi2) |
| Eyes disorders, n, % | 302 (14.8%) | 101 (12.3%) | p=0.2(chi2) |
| Depression, n, % | 678 (33.3%) | 187 (22.7%) | p<0.0001(chi2) |
| Dementia, n, % | 866 (42.5%) | 313 (38%) | p=0.3(chi2) |
| Pressure ulcers, n, % | 118 (5.8%) | 54 (6.6%) | p=0.96(chi2) |
| Urinary incontinence, n, % | 443 (21.7%) | 114 (13.8%) | p<0.0001(chi2) |
| Disease | Gender | VES-13 | p value | |||
| in patients with the presence of particular disease | in patients without particular disease | |||||
| mean±SD | median (quartiles) | mean±SD | median (quartiles) | |||
| Arterial hypertension | Women | 6.9±2.8 | 8.0 (4.0-10.0) | 6.2±2.9 | 7.0 (3.0-8.0) | p<0.0001(U) |
| Men | 6.4±2.8 | 7.0 (3.0-9.0) | 6.6±3.0 | 7.0 (4.0-10.0) | p=0.17(U) | |
| Diabetes mellitus | Women | 7.1±2.7 | 8.0 (4.0-10.0) | 6.7±2.9 | 7.0 (4.0-10.0) | p=0.01(U) |
| Men | 6.5±2.8 | 7.0 (4.0-9.0) | 6.4±2.9 | 7.0 (3.0-9.0) | p=0.44(U) | |
| Lipid disorders | Women | 6.1±2.9 | 6.0 (3.0-8.0) | 7.3±2.7 | 8.0 (5.0-10.0) | p<0.0001(U) |
| Men | 5.6±2.9 | 5.5 (3.0-8.0) | 6.8±2.8 | 7.0 (4.0-10.0) | p<0.0001(U) | |
| Stroke | Women | 7.4±2.5 | 8.0 (6.0-10.0) | 6.6±2.9 | 7.0 (4.0-10.0) | p<0.0001(U) |
| Men | 6.9±2.6 | 7.0 (4.0-9.0) | 6.3±2.9 | 7.0 (3.0-9.0) | p=0.04(U) | |
| Coronary artery disease | Women | 7.4±2.6 | 8.0 (6.0-10.0) | 6.4±2.9 | 7.0 (3.0-9.0) | p<0.0001(U) |
| Men | 6.8±2.8 | 7.0 (4.0-10.0) | 6.1±2.9 | 7.0 (3.0-8.0) | p=0.0003(U) | |
| Myocardial infarction | Women | 7.3±2.7 | 8.0 (5.0-10.0) | 6.7±2.9 | 7.0 (4.0-10.0) | p=0.014(U) |
| Men | 6.7±2.8 | 7.0 (4.0-9.5) | 6.4±2.9 | 7.0 (3.0-9.0) | p=0.3(U) | |
| Atrial fibrillation | Women | 7.6±2.6 | 8.0 (6.0-10.0) | 6.6±2.9 | 7.0 (4.0-9.0) | p<0.0001(U) |
| Men | 7.2±2.8 | 8.0 (5.0-10.0) | 6.1±2.8 | 7.0 (3.0-8.0) | p<0.0001(U) | |
| Heart failure | Women | 7.4±2.6 | 8.0 (5.0-10.0) | 6.2±2.9 | 7.0 (3.0-9.0) | p<0.0001(U) |
| Men | 7.1±2.7 | 8.0 (5.0-10.0) | 5.8±2.9 | 6.0 (3.0-8.0) | p<0.0001(U) | |
| Chronic kidney disease | Women | 7.2±2.7 | 7.0 (4.0-10.0) | 6.0±3.0 | 7.0 (3.0-8.0) | p<0.0001(U) |
| Men | 6.8±2.8 | 7.0 (4.0-10.0) | 5.7±2.9 | 6.0 (3.0-8.0) | p<0.0001(U) | |
| Obstructive lung diseases | Women | 7.0±2.7 | 8.0 (4.0-10.0) | 6.7±2.9 | 8.0 (4.0-10.0) | p=0.24(U) |
| Men | 6.6±2.8 | 7.0 (4.0-9.0) | 6.4±2.9 | 7.0 (4.0-9.0) | p=0.58(U) | |
| Osteoarthritis | Women | 6.8±2.7 | 8.0 (4.0-10.0) | 6.7±2.9 | 8.0 (4.0-10.0) | p=0.68(U) |
| Men | 6.5±2.8 | 7.0 (4.0-9.0) | 6.4±2.9 | 7.0 (3.5-9.0) | p=0.57(U) | |
| Osteoporosis | Women | 6.4±2.9 | 7.0 (4.0-9.0) | 7.0±2.8 | 8.0 (4.0-10.0) | p=0.00002(U) |
| Men | 6.8±2.8 | 8.0 (4.0-10.0) | 6.4±2.9 | 7.0 (3.0-9.0) | p=0.15(U) | |
| Fractures | Women | 7.3±2.7 | 8.0 (4.0-10.0) | 6.6±2.9 | 7.0(4.0-10.0) | p<0.0001(U) |
| Men | 7.2±2.6 | 8.0 (5.0-10.0) | 6.3±2.9 | 7.0(3.0-9.0) | p=0.0017(U) | |
| Gastrointestinal diseases | Women | 6.4±2.8 | 7.0 (3.0-9.0) | 6.9±2.8 | 8.0 (4.0-10.0) | p=0.0004(U) |
| Men | 6.0±2.8 | 6.0 (3.0-8.0) | 6.5±2.9 | 7.0 (4.0-9.0) | p=0.04(U) | |
| Neoplastic diseases | Women | 6.4±2.9 | 7.0 (4.0-9.0) | 6.8±2.8 | 8.0 (4.0-10.0) | p=0.04(U) |
| Men | 6.8±2.8 | 8.0 (4.0-9.0) | 6.3±2.9 | 7.0 (3.0-9.0) | p=0.07(U) | |
| Eyes disorders | Women | 7.0±2.7 | 8.0 (4.0-10.0) | 6.7±2.9 | 8.0 (4.0-10.0) | p=0.14(U) |
| Men | 7.0±2.6 | 7.0 (5.0-9.0) | 6.3±2.9 | 7.0 (3.0-9.0) | p=0.37(U) | |
| Depression | Women | 7.2±2.6 | 8.0 (6.0-10.0) | 6.5±2.9 | 7.0 (4.0-10.0) | p<0.0001(U) |
| Men | 7.0±2.7 | 8.0 (4.0-9.0) | 6.2±2.9 | 7.0 (3.0-9.0) | p=0.002(U) | |
| Dementia | Women | 8.0±2.3 | 8.0 (7.0-10.0) | 5.9±2.9 | 6.0 (3.0-8.0) | p<0.0001(U) |
| Men | 7.7±2.5 | 8.0 (7.0-10.0) | 5.6±2.8 | 5.0 (3.0-8.0) | p<0.0001(U) | |
| Pressure ulcers | Women | 8.5±2.0 | 9.0 (8.0-10.0) | 6.7±2.8 | 7.0 (4.0-10.0) | p<0.0001(U) |
| Men | 8.4±1.8 | 8.5 (7.0-10.0) | 6.3±2.9 | 7.0 (3.0-9.0) | p<0.0001(U) | |
| Urinary incontinence | Women | 6.9±2.6 | 8.0 (4.0-9.0) | 7.0±2.9 | 8.0 (4.0-10.0) | p=0.53(U) |
| Men | 7.0±2.4 | 7.0 (5.0-9.0) | 6.3±3.0 | 7.0 (3.0-9.0) | p=0.033(U) | |
| Disease | Gender | Age | p value | |||
| in patients with the presence of particular disease | in patients without particular disease | |||||
| mean±SD | median (quartiles) | mean±SD | median (quartiles) | |||
| Arterial hypertension | Women | 82.3±7.5 | 83 (78-87) | 79.3±8.8 | 80 (72-86) | p<0.0001 (U) |
| Men | 81.8±8.2 | 83 (76-88) | 81.8±8.5 | 83 (76-88) | p=0.81 (U) | |
| Diabetes mellitus | Women | 81.9±7.4 | 83 (77-87) | 81.6±8.1 | 83 (77-87) | p=0.9 (U) |
| Men | 80.8±8.2 | 82 (74-87) | 82.3±8.3 | 84 (78-88) | p=0.03 (U) | |
| Lipid disorders | Women | 79.9±7.9 | 81 (74-86) | 83.2±7.6 | 84 (79-88) | p<0.0001 (U) |
| Men | 80.1±8.6 | 82 (74-86) | 82.8±8.0 | 84 (79-88) | p<0.0001 (U) | |
| Stroke | Women | 82.7±7.5 | 84 (79-88) | 81.5±7.9 | 83 (77-87) | P=0.02 (U) |
| Men | 81.1±8.3 | 83 (75-88) | 82.0±8.3 | 83 (77-88) | p=0.3 (U) | |
| Coronary artery disease | Women | 83.4±6.8 | 84 (79-88) | 80.7±8.3 | 82 (75-87) | p<0.0001 (U) |
| Men | 83.7±7.7 | 85 (80-89) | 80.6±8.4 | 82 (75-87) | p<0.0001 (U) | |
| Myocardial infarction | Women | 83.1±7.3 | 85 (79-88) | 81.6±7.9 | 83 (77-87) | p=0.007 (U) |
| Men | 83.5±7.3 | 85 (79.5-88) | 81.5±8.4 | 83 (76-88) | p=0.02 (U) | |
| Atrial fibrillation | Women | 84.2±6.6 | 85 (80-89) | 81.1±8.1 | 82 (76-87) | p<0.0001 (U) |
| Men | 84.2±7.6 | 86 (81-89) | 81.0±8.4 | 82 (75-87) | p<0.0001 (U) | |
| Heart failure | Women | 83.8±6.9 | 85 (80-88) | 79.8±8.3 | 81 (74-86) | p<0.0001 (U) |
| Men | 83.6±7.8 | 85 (80-89) | 80.1±8.3 | 81 (74-86) | p<0.0001 (U) | |
| Chronic kidney disease | Women | 83.8±6.7 | 84 (80-88) | 77.6±8.4 | 78 (71-84) | p<0.0001 (U) |
| Men | 84.5±6.6 | 85 (81-89) | 77.3±9.0 | 78 (70-84) | p<0.0001 (U) | |
| Obstructive lung diseases | Women | 81.9±7.0 | 82.5 (78-87) | 81.7±8.0 | 83 (77-87) | p=0.9 (U) |
| Men | 82.2±7.5 | 83 (78-87) | 81.8±8.4 | 83 (76-88) | p=0.9 (U) | |
| Osteoarthritis | Women | 81.9±7.4 | 83 (78-87) | 81.6±8.2 | 83 (76-88) | p=0.68 (U) |
| Men | 82.0±8.4 | 84 (78-88) | 81.8±8.2 | 83 (76-88) | p=0.9 (U) | |
| Osteoporosis | Women | 80.9±7.9 | 82 (76-87) | 82.1±7.9 | 83 (77-88) | p=0.001 (U) |
| Men | 83.5±7.3 | 84 (79-89) | 81.6±8.4 | 83 (76-88) | p=0.06 (U) | |
| Fractures | Women | 82.6±7.3 | 84 (78-88) | 81.4±8.0 | 83 (76.5-87) | p=0.009 (U) |
| Men | 83.3±9.6 | 84 (78-91) | 81.6±8.1 | 83 (76-88) | p=0.02 (U) | |
| Gastrointestinal diseases | Women | 79.8±8.4 | 81 (74-86) | 82.2±7.7 | 83 (78-88) | p<0.0001 (U) |
| Men | 81.5±7.6 | 83 (77-87) | 81.9±8.5 | 83 (76-88) | p=0.5 (U) | |
| Neoplastic diseases | Women | 81.0±8.0 | 82 (76-87) | 81.8±7.9 | 83 (77-87) | p=0.07 (U) |
| Men | 83.0±7.9 | 84 (80-88) | 81.6±8.4 | 83 (76-88) | p=0.06 (U) | |
| Eyes disorders | Women | 82.6±7.3 | 83 (79-88) | 81.6±8.0 | 83 (77-87) | p=0.2 (U) |
| Men | 83.7±7.4 | 85 (81-89) | 81.6±8.4 | 83 (76-88) | p=0.01 (U) | |
| Depression | Women | 81.8±7.3 | 83 (78-87) | 81.7±8.2 | 83 (77-88) | p=0.7 (U) |
| Men | 81.9±7.6 | 83 (78-87) | 81.8±8.5 | 83 (76-88) | p=0.8 (U) | |
| Dementia | Women | 84.6±6.4 | 85 (81-89) | 79.5±8.2 | 81 (73-86) | p<0.0001 (U) |
| Men | 84.1±7.2 | 85 (80-89) | 80.4±8.6 | 82 (74-87) | p<0.0001 (U) | |
| Pressure ulcers | Women | 84.4±7.9 | 85 (80-90) | 81.5±7.9 | 83 (77-87) | p=0.0002 (U) |
| Men | 83.7±6.9 | 85 (82-88) | 81.7±8.4 | 83 (76-88) | p=0.07 (U) | |
| Urinary incontinence | Women | 81.8±7.6 | 83 (77-87) | 81.6±8.0 | 83 (77-87) | p=0.9 (U) |
| Men | 82.5±7.8 | 84 (78-88) | 81.7±8.3 | 83 (76-88) | p=0.3 (U) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





