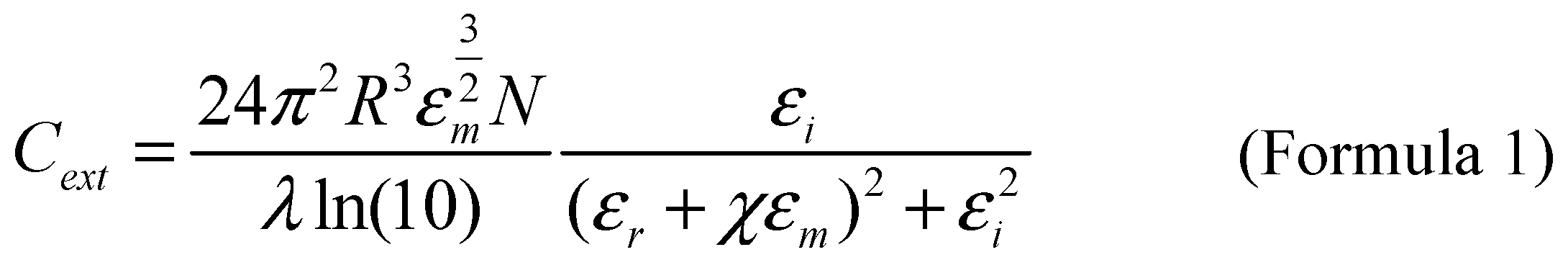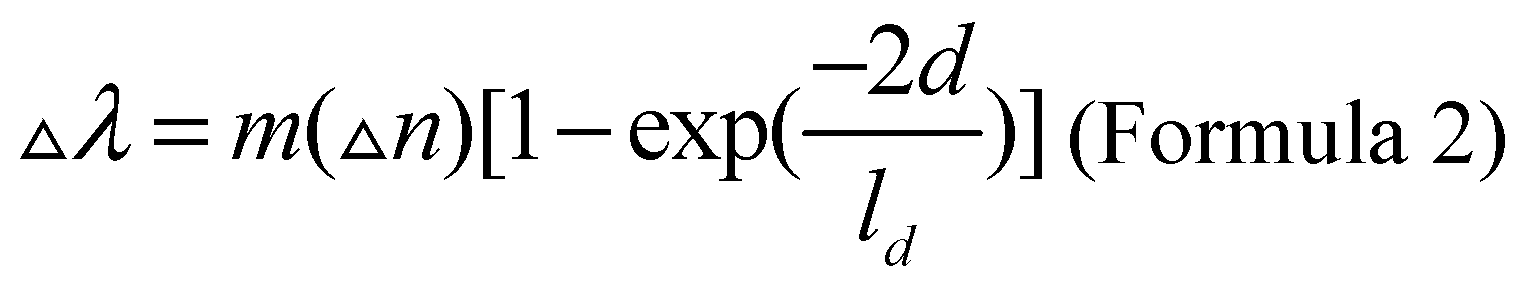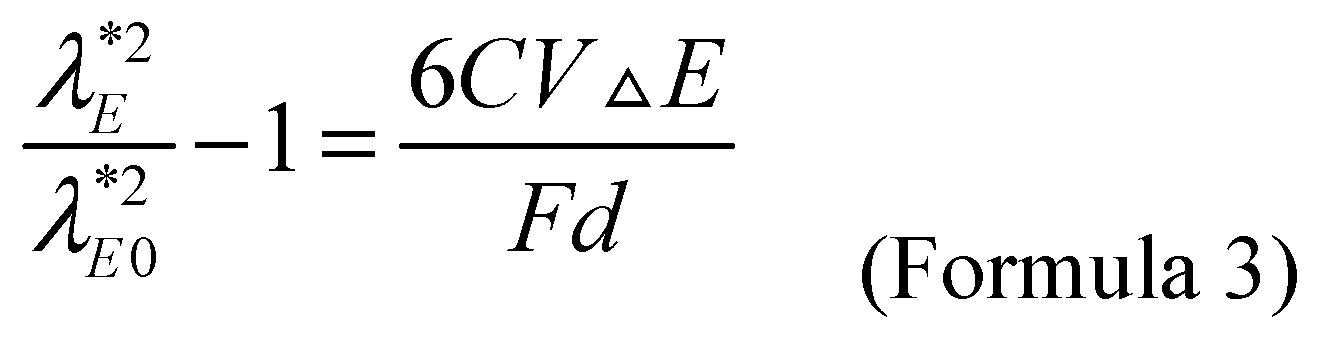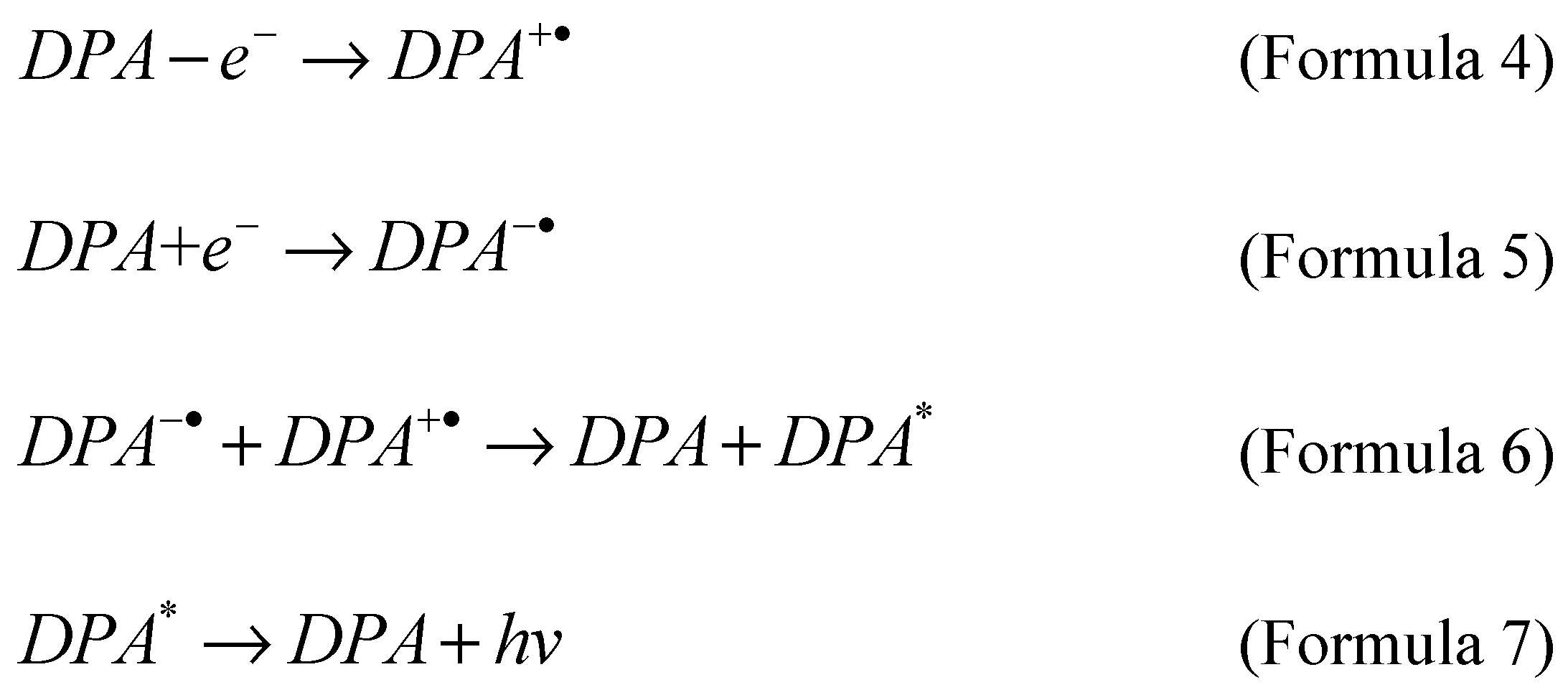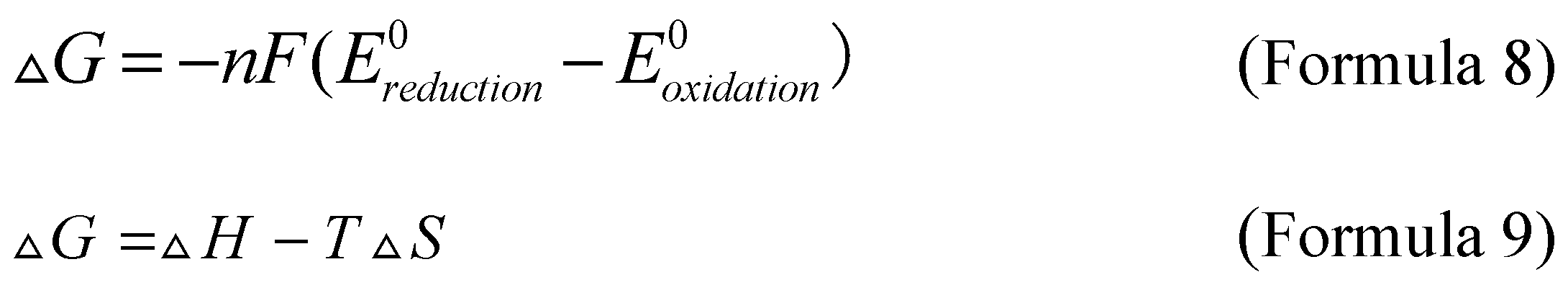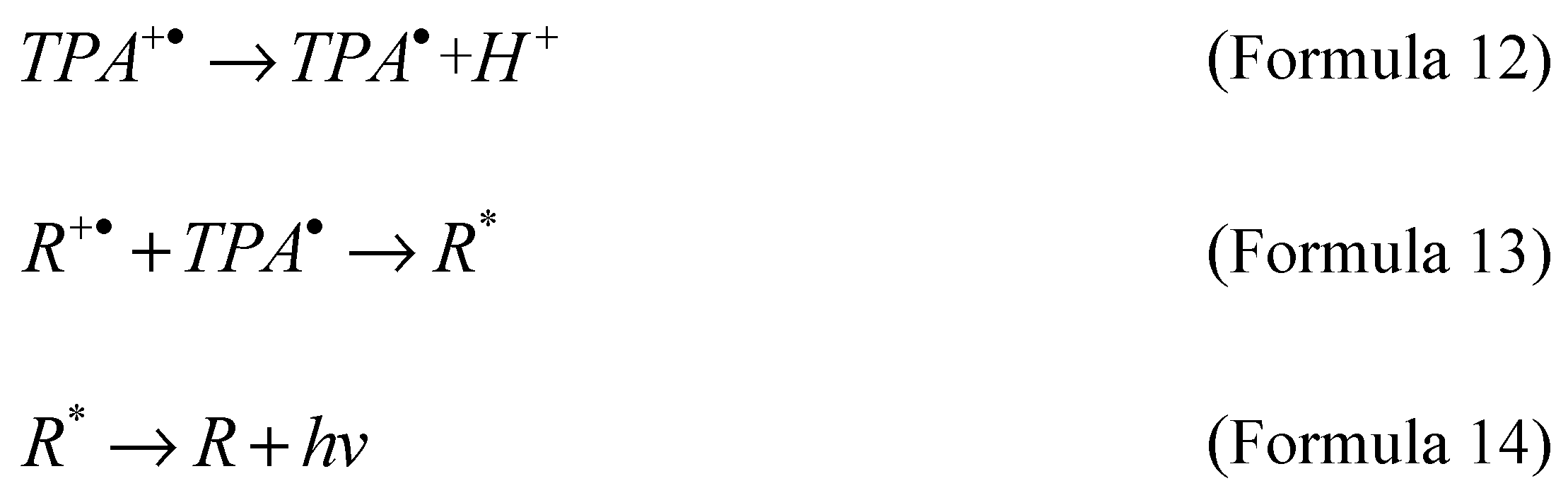1. Introduction
Metal nanoparticles were first applied to the Lycurgus Cup in Rome in the 4th century AD. By embedding gold and silver nanoparticles in glass, they can absorb and scatter specific regions of the visible light spectrum, presenting bright colors [
1]. Due to the optical response of precious metal nanoparticles, the wine glass appears green when light shines from the outside, and red when light shines from the inside. In this process, precious metal nanoparticles can convert the energy of incident photons into collective oscillations of surface electrons, resulting in wavelength selective absorption and high molar extinction coefficient light scattering. At the same time, the coherent oscillation of electrons on the surface of nanoparticles also generates significant electromagnetic field enhancement and radiation attenuation.
The interaction between light and noble metal nanoparticles generates collective oscillation of conduction band electrons, which is called surface plasmon resonance. Only materials with negative real dielectric constant and positive virtual dielectric constant can better support surface plasmon polaritons, and the most commonly used precious metal nanoparticles are gold and silver nanoparticles [
2,
3]. When the incident electromagnetic field matches the electromagnetic field of the oscillating electrons on the surface of the nanoparticles, resonance conditions can be met. This resonance oscillation causes a significant increase in wavelength selectivity of the absorption, scattering, and electromagnetic field on the surface of the nanoparticles [
4,
5]. In the process of surface plasmon resonance, the position of the Formant of the absorption spectrum, scattering spectrum and plasma extinction spectrum (absorption + scattering) of metal nanoparticles will show a corresponding red shift with the increase of refractive index or the decrease of the distance between particles. Based on the above theory, if plasma nanoparticles are modified with a receptor (such as an antibody) that can specifically bind to the target analyte (such as an antigen), then the binding between the analyte and the receptor will lead to an increase in the refractive index around the nanoparticles or a decrease in particle spacing, which will lead to a red shift in the peak position of the plasma extinction spectrum. The concentration of the analyte or the degree of binding reaction can be determined based on the shift of the spectral peak position. Therefore, surface plasmon resonance sensors are widely used in chemical and biological sensing [
6,
7,
8].
In order to understand the factors affecting the increase in absorption and scattering during plasma resonance, Mie theory was proposed. Mie theory is the Analytical expression of Maxwell equations with spherical boundary conditions, which is used to describe the extinction spectrum of a given nano particle. In order to more accurately calculate the dielectric constant at different wavelength values and extend this theory to more complex nanoparticle shapes, a modified wavelength form of Mie theory was proposed [
9,
10,
11]. As shown in Formula 1,
R represents the radius of the particle,
εm is the dielectric constant of the surrounding medium, and
N represents the electron density,
λ Represents the wavelength of incident light,
ε=εr+iεi refers to the complex dielectric constant of a metal,
χ Determined by particle shape. Therefore, factors such as the shape of nanoparticles, the wavelength of incident light, the type of material, and the surrounding medium can all affect the absorption and scattering processes.
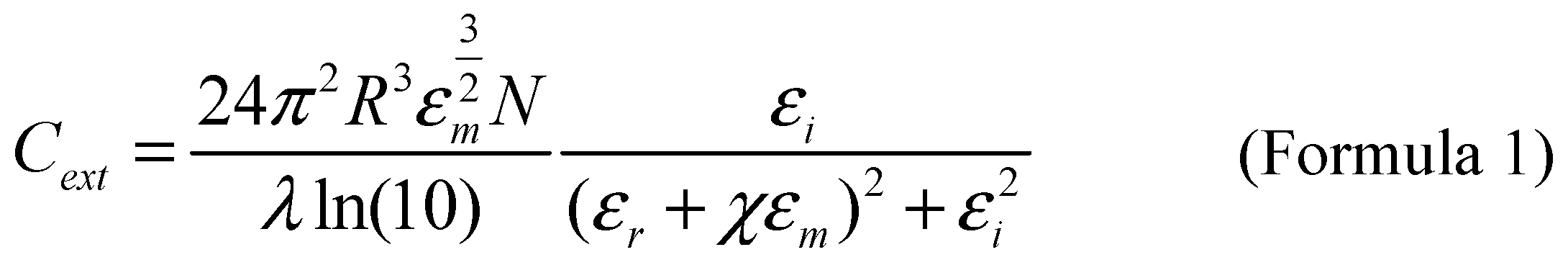
In the field of biosensors, when biological analytes are combined with the surface of nanoparticles, the refractive index of the surface of nanoparticles will change, thus changing the movement of surface plasmon resonance peak position. Among them, silver nanoparticles have the highest negative dielectric constant among all plasma materials and are most sensitive to changes in local refractive index. In addition, asymmetric shaped nanoparticles are more sensitive to changes in surface binding of biomolecules than spherical plasmas. The relationship between the position of the surface plasmon resonance peak and the binding of the adsorbent is described in Formula 2, where m is the refractive index sensitivity, Δn is the refractive index change caused by the adsorbent. d refers to the thickness of the effective adsorption layer, and ld refers to the attenuation length of the electromagnetic field.
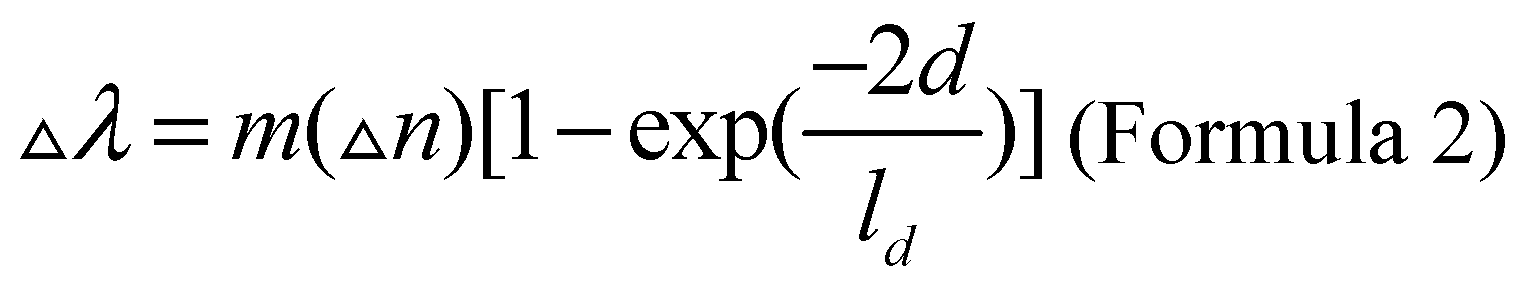
The refractive index sensitivity is usually obtained by calculating the slope of surface plasmon resonance frequency and refractive index curve under a small range of refractive index using the Drude model of electronic structure [
12,
13]. Research shows that the influence of the electromagnetic field attenuation length on the surface plasmon resonance peak shift is mainly due to the sensitivity to the shape of nanoparticles. The electromagnetic field attenuation length of many nanoparticles is similar to that of proteins in size (5~10 nm), which enables nanoparticles to sensitively sense the binding of biomolecules to their surfaces [
14,
15,
16,
17]. Therefore, the two key variables that determine the shift of the plasma formant are the refractive index difference of the absorbance relative to the solution (
Δn) and the degree of surface binding between the analyte and the nanoparticles (
d). Due to the sensitivity of refractive index being the key to detecting target biomolecules, many studies in recent years have attempted to make the nanoparticle matrix highly sensitive to changes in refractive index. The relevant simulation results show that when the surface plasmon resonance peak of the material moves from the blue wavelength to the red wavelength, the sensitivity of the refractive index increases in a linear way[
18,
19,
20]. By creating larger and/or asymmetric nanoparticles, surface plasmon resonance peaks can be raised to longer wavelengths. In addition, when two plasmas approach each other, the electromagnetic field between them will generate a certain degree of mutual coupling, which can also cause significant wavelength shift [
21,
22]. Because the presence of nearby plasma active substances often has a greater impact on surface plasmon resonance, many sensing studies use plasma coupling as a technical means to improve the response of detection signals.
2. Electrochemical coupled surface plasmon resonance
As people pay more and more attention to the application of nanostructures in charge transfer and storage, it is crucial to control the charge transfer at the nanoscale through the physical and chemical process in the reaction [
23,
24,
25]. Drude describes metals as plasma of free electrons, which forms the physical basis for their optical and electrical properties. For some metals, quantum effects are ignored when the size exceeds 10 nm, and the classic Drude model can provide quantitative detection results in optical and electrical systems. However, in nanometers (less than 1 μm) structurally, the stimulated luminescence of plasma and the influence of the environment have a certain impact on conductivity, which has a high surface sensitivity, that is, they have a strong response to environmental changes that occur on the surface, leading to the development of new unlabeled biosensors. Typically, these sensors do not require sensitive labels, such as fluorescent groups, radioactive labels, or quantum dots, mainly based on electrical or optical signal transduction [
26,
27]. On this basis, electrochemical coupled optical technology has been developed, and with the development of nanomaterials, electrochemical coupled optical detection has become more inclined towards simplification of operation, stable curing of testing, and miniaturization of systems.
Surface plasmon resonance is an optical phenomenon generated by the collective movement of electrons on the surface of metal nanoparticles, and the existence of electric field will cause the deposition of electroactive molecules on the surface of metal nanoparticles, which will lead to the movement of the plasma formant [
28,
29,
30,
31]. Therefore, when the electrochemical signal acts on the plasma nanoparticles, the positive displacement of the potential reduces the density of free electrons in the nanoparticles, leading to a red shift in the peak position of the extinction spectrum, resulting in the electrochemical coupling of surface plasmon resonance effect.
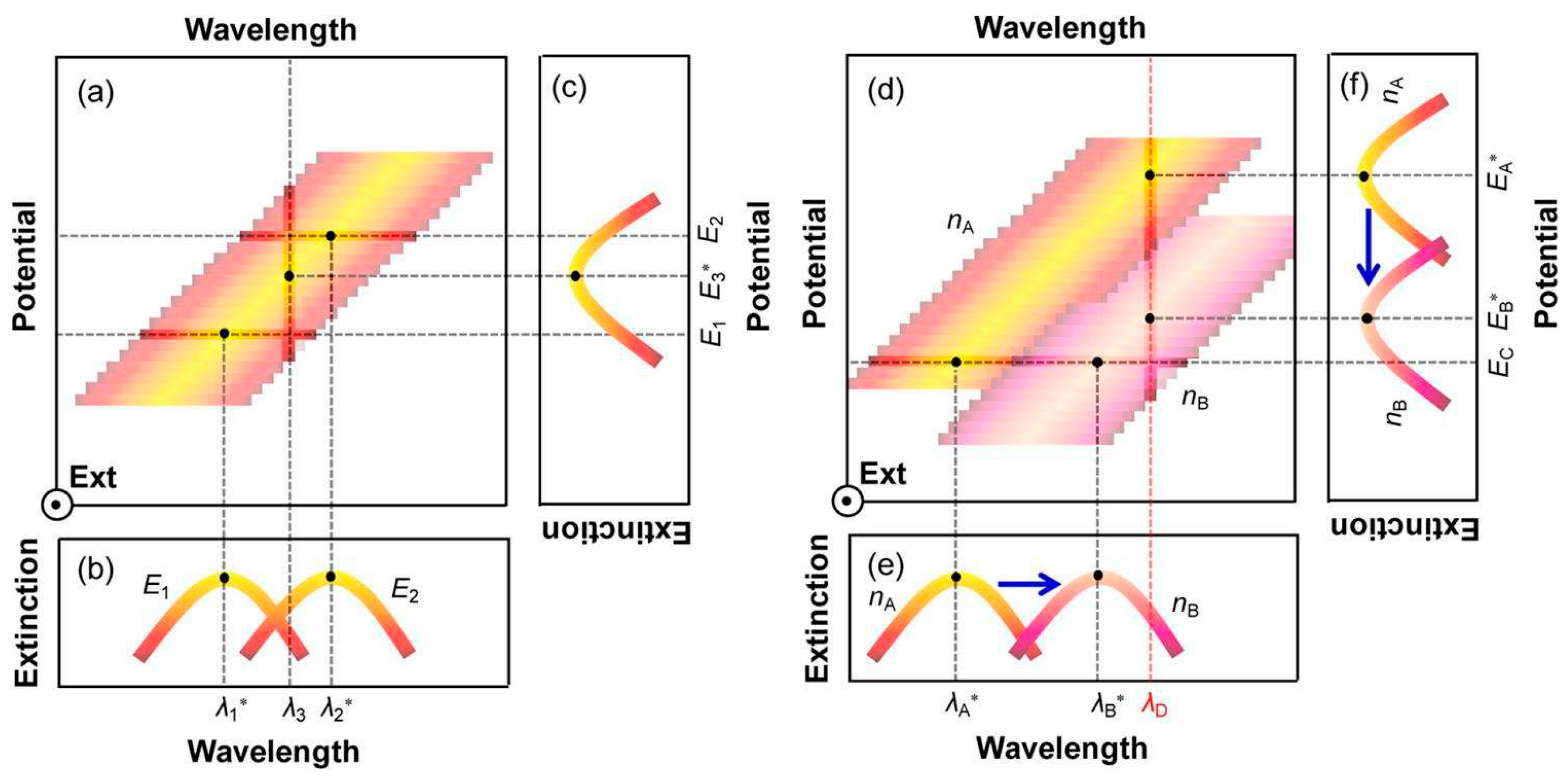
Figure 1a shows the relationship between the wavelength, potential, and extinction spectrum of plasma nanoparticles. The two curves in
Figure 1b (wavelength extinction spectrum) are the cross-sectional views of
Figure 1a at potentials
E1 and
E2. It can be clearly seen that when the potential increases from
E1 and
E2, the peak position changes from
λ1* move to
λ2*. On the other hand,
Figure 1c (potential extinction spectrum) shows that at a wavelength of
λ3, a new formant can be obtained in the extinction spectrum through potential scanning, and the peak position voltage is
E3*. The optical properties of plasma nanoparticles also depend on the refractive index changes of the surrounding medium, resulting in a red shift at the peak position in the wavelength extinction spectrum as the refractive index increases (
Figure 1d).
Figure 1e shows a cross-sectional view at a potential equal to
Ec, showing the peak position from
nA to
nB as the refractive index increases
λA* Move to
λB*. Meanwhile,
Figure 1f shows that at wavelengths of
λD, the cross-sectional view at D shows that as the refractive index increases from
nA to
nB, the peak potential shifts from
EA* negative to
EB*. Overall, the increase in refractive index change
nB-nA will lead to an increase in negative potential shift
-(EB*-EA*) and a positive wavelength shift of
λB*-λA*.
The relationship between applied potential
E=E0+ΔE and wavelength is given by Formula 3, where
C,
V,
F, and
d, are the capacitance, molar volume, faraday constant and diameter of metal nanoparticles, respectively. It is reported that the
C value of the citrate coated gold nanoparticles with
d=3.7~40.8 nm is about 70 μF/cm
2 when compared with the silver/silver chloride/saturated potassium chloride electrode (at the potential from -0.4 V to +0.6 V) [
32]. The V value of gold nanoparticles is 10.2 cm
3/mol. Within the range of actual refractive index, the peak potential
Eλ* almost linear depends on the change of refractive index. It can be seen from the slope of the approximate line that the theoretical refractive index sensitivity
SE-n of the electrochemical coupling surface plasmon resonance sensor for gold nanoparticles with a diameter of 13 nm is about 10.3 V/RIU. Both electrochemistry and surface plasmon resonance realize sensing detection by sensing the relative change of reaction interface. Due to the potential sensitivity of noble metal plasma surface, electrochemical signals can be introduced into surface plasmon resonance to realize electro-optic coupling applications.
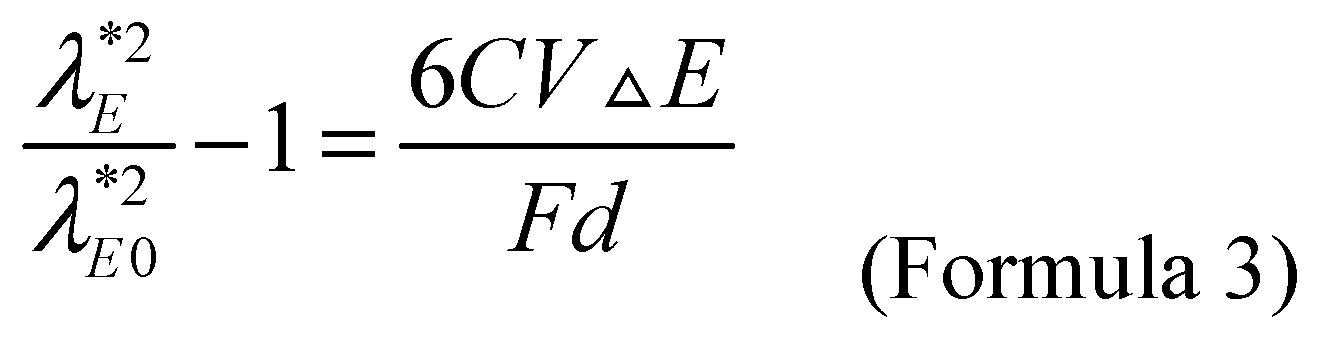
Continuous, sensitive, and real-time analysis of complex unknown samples is a fundamental requirement widely used in biosensing detection. Although many existing biosensing technologies have shown extremely high sensitivity in laboratory environments, they are still subject to interference from high-strength non-specific reactions in complex samples in practical applications due to the hybrid effect caused by non-specific adsorption on the sensor surface [
33]. A potential solution to address this challenge is to integrate compatible biosensing methods into an analytical tool that can measure and analyze more supplementary parameters. The integration of electrochemistry and surface plasmon resonance sensing technology provides a very attractive method for converting biochemical response results into a variety of measurable analytical signals.
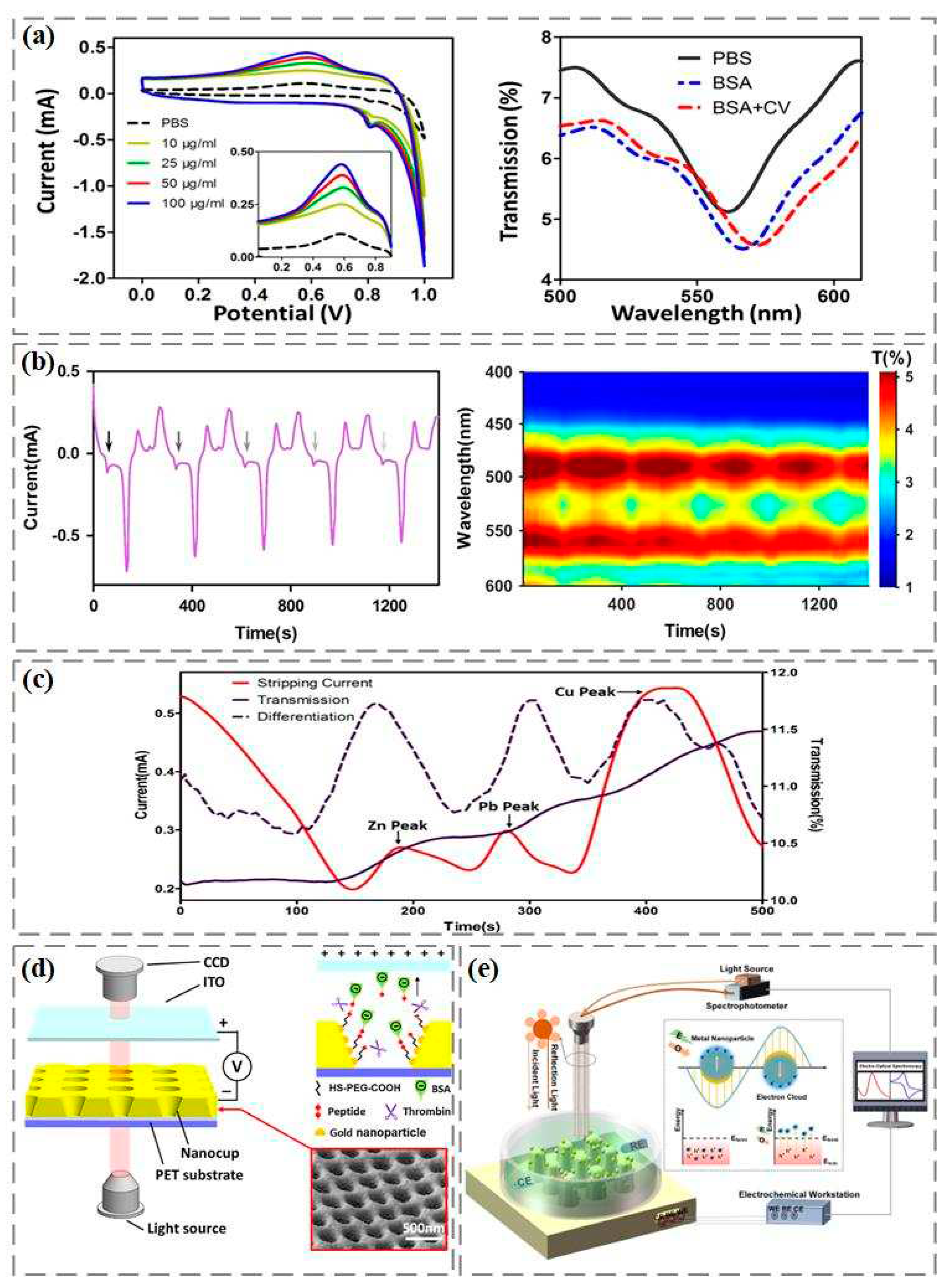
Research shows that the redox current in the electrochemical reaction can cause the shift of the resonance wavelength in the spectrum to increase the surface plasmon resonance detection signal. Zhang et al., based on Cyclic voltammetry coupled with surface plasmon resonance technology, realized the highly sensitive detection of bovine serum albumin [
34]. As shown in
Figure 2a, the oxidation reduction potential of bovine serum albumin appears at 0.6 V. With the increase of bovine serum albumin concentration, the reduction current increases, which further causes the movement of surface plasmon resonance wave valley and enhances the signal of bovine serum albumin detection. Li et al. activated dopamine itself to generate redox current by cyclic voltammetry, and simultaneously recorded the change of electrode surface refractive index using spectrum, which can achieve highly sensitive and specific dopamine detection [
35]. As shown in
Figure 2b, the arrow indicates the oxidation current position of dopamine, with the first oxidation peak occurring at 55 seconds and an interval of 280 seconds from the next oxidation peak. In order to better observe the changes in the intensity and peak position of the transmission spectrum during cyclic voltammetry scanning, the color contour line map calibrates and presents each transmission spectrum as a time function. The intensity of the transmission spectrum fluctuates periodically with the appearance of the dopamine oxidation current peak, and reaches the maximum of the spectral intensity at the current peak. The peak position shows an obvious periodic red shift.
In addition, anodic stripping voltammetry can determine multiple metal ions at one time by combining voltammetry technology with constant potential electrolytic enrichment. However, due to the interference of background current, the oxidation reduction of oxygen and the precipitation of hydrogen generate additional current, which limits the detection of trace metal ions. The interference of background current can be effectively removed through the coupling of anodic stripping voltammetry and surface plasmon resonance, which greatly improves the sensitivity and signal-to-noise ratio of metal ion detection [
36]. As shown in the mixed detection of zinc, lead, and copper in
Figure 2c, there is not much change in the spectral transmittance during the dissolution stage. Since the difference in transmissivity reflects the change rate of the number of electroplating metal nanoparticles remaining on the device surface, which is related to the dissolution current in anodic stripping voltammetry detection, the first order difference between transmissivity and time can be made through the difference between every two sampling data points in the transmissivity time curve, which can better realize the distinction of metal ions.
In our previous work, we constructed an electrophoretic enhanced surface plasmon resonance sensing system. The gold nanocup array is used as an electro-optic sensor, and the transparent indium tin oxide electrode is used as a positive electrode [
37]. Direct current is applied between the gold nanocup array sensor and the indium tin oxide electrode. As shown in
Figure 2d, using the "sandwich sandwich" strategy, polyethylene glycol with thiol groups is fixed on the surface of the gold nanocup array device through gold sulfur bonds, forming a good biosensitive film. Then, the thrombin specific shear peptide is fixed on the polyethylene glycol biosensitive membrane through amide action. Finally, the end of the peptide chain was modified with negatively charged bovine serum albumin. When thrombin is introduced into the surface of the gold nanocup array sensor, the peptide cleavage reaction of thrombin is carried out at a specific position of the polypeptide. With the help of the electric field between the indium tin oxide electrode and the gold nanocup array sensor, the shear peptide and electronegativity bovine serum albumin can be separated. Due to the change of the refractive index of the sensor surface, the formant in the transmission spectrum shifted, and the construction of the sensor for determining the catalytic activity of thrombin was completed. In addition, we also modified gold nanoparticles and silver nanoparticles on the nanocone array by electron beam evaporation and electrochemical reduction deposition, and then carried out linear scanning voltammetry and surface plasmon resonance sensing on the gold/silver nanocone array sensor to record dynamic electro-optic spectrum signals for sialic acid detection (
Figure 2e) [
38]. The charge distribution induced by plasma is concentrated on the surface of metal nanoparticles, leading to the excitation of hot electrons and hot holes. If the electron energy exceeds the Fermi energy level of the material, the photoexcited electrons will generate light emission through electron-electron or electron-phonon collisions. Then light absorption and surface plasmon resonance will be generated on the nano sensor, accompanied by electromagnetic attenuation on the femtosecond scale, and energy will be transferred to hot electrons in a non-radiative manner.
Due to the high requirement for relative balance between electrodes in electrochemical measurements, it is often necessary to keep the analyte stationary as much as possible during detection. Any relative movement between the analyte solution and the surface of the sensing electrode will cause severe fluctuations in the voltammetry curve. Baseline drift during the detection process may cause vertical fluctuations in the potential, while impurities in the analyte (such as buffer solutions and dissolved gases) may also cause horizontal fluctuations in the potential. However, the use of surface plasmon resonance technology can achieve real-time analysis of the target under dynamic conditions, which greatly improves the robustness of the detection. Therefore, the electrochemical coupled surface plasmon resonance detection has a strong anti-interference ability on the impact of temperature, buffer solution and analogues, and has a good application prospect in the dynamic detection conditions with high integration, such as the miniaturized mobile sensing platform.
3. Electrochemiluminescence
Electrochemiluminescence is a reaction on the electrode surface under the condition of electrolytic cell, which is mainly controlled by the change of electrode potential, and emits light signals of specific wavelength with the process of excited state electron transfer [
37,
38]. In a sense, it is an ideal combination of electrochemistry and optics because it not only maintains high sensitivity and wide dynamic range from traditional chemiluminescence, but also demonstrates the simplicity, stability, and convenience of electrochemical methods. As a light emission technology, electrochemiluminescence has unique advantages compared to other luminescence methods such as photoluminescence and chemiluminescence. Especially compared to chemiluminescence, electrochemiluminescence has superior temporal and spatial precise control characteristics for light emission, which can significantly improve the signal-to-noise ratio in the detection process. In addition, compared to photoluminescence, which needs to withstand the influence of non-selective photoexcitation induced background, electrochemiluminescence does not require the use of external light sources and has zero background interference characteristics, avoiding disturbance caused by light scattering. Therefore, electrochemiluminescence has become a powerful analytical technique, often used for sensing detection of trace target analytes, and plays an important role in fields such as medical diagnosis, food safety, and biochemical warfare agent detection [
39,
40,
41].
Electrochemiluminescence is generated by the recombination of electrogenerated free radicals, and its mechanism can be divided into two categories based on the source of free radicals: annihilation mechanism and coreactant mechanism. For the former, free radicals are generated by a single emitter, while the latter involves a set of bimolecular electrochemical reactions between the luminescent body and the coreactant.
(1) Annihilation mechanism
The annihilation reaction involves the formation of electrochemical intermediates on the electrode surface, and then the intermediates interact with each other and undergo the formation of ground state and electronic excited state. Finally, the excited state emits light due to relaxation. A typical example is 9,10-biphenylanthracene [
42,
43]. Diphenylanthracene (DPA) undergoes oxidation (Formula 4) and reduction (Formula 5) reactions on the electrode surface, respectively. Then the oxidation intermediate (DPA
+·) and the reduction intermediate (DPA
-·) undergo an annihilation reaction and generate an excited state (DPA
*) (Formula 6). The last unstable Excited state (DPA
*) generates a luminous signal (Formula 7) in the process of returning to the ground state (DPA).
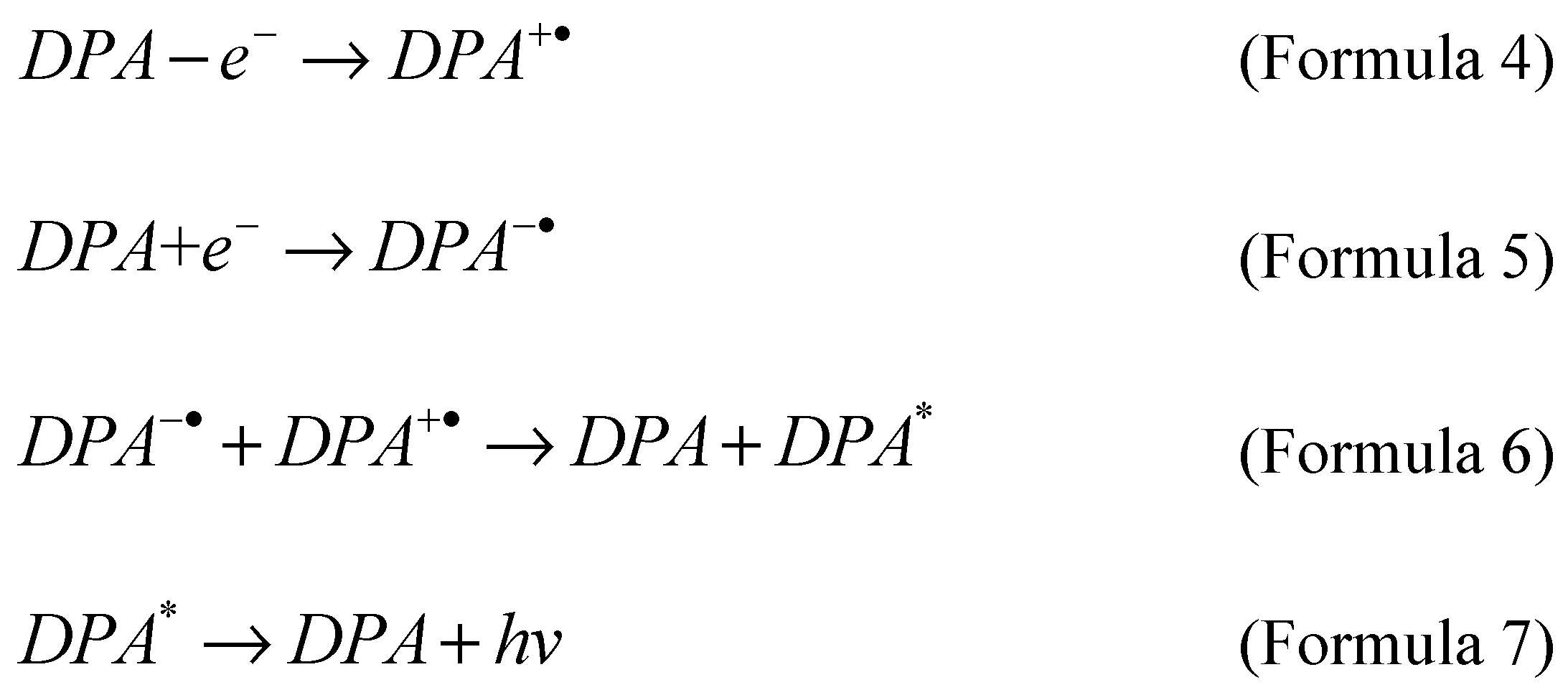
Gibbs free energy related to the annihilation process is obtained by calculating the oxidation and reduction potential of Formula 4 and Formula 5, as shown in Formula 8, where ΔG represents Gibbs free energy, F is the Faraday constant, Ereduction0 and Eoxidation0 are reduction potential and oxidation potential, respectively. Formula 9 provides the calculation formula for Gibbs free energy related enthalpy.
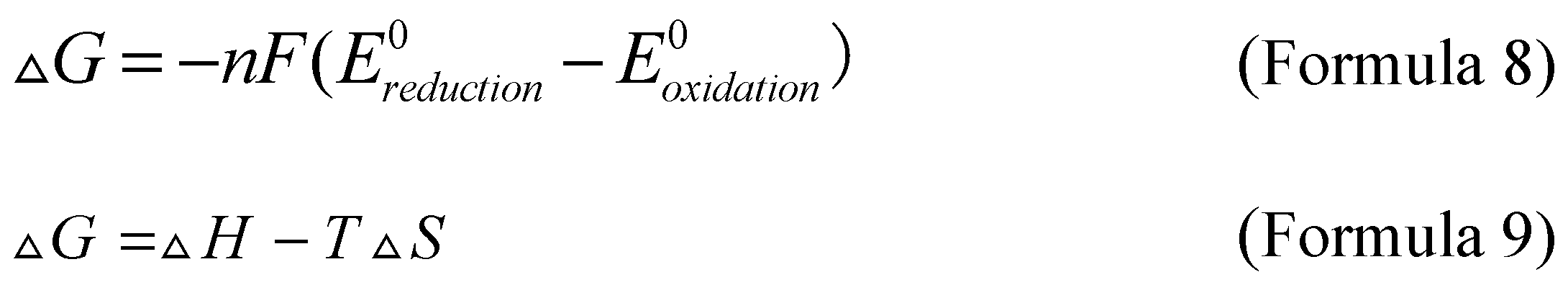
If the enthalpy exceeds the minimum value of energy required from the ground state to the excited state, the reaction is defined as "energy sufficient" or follows the "singlet state", and biphenylanthracene is a reaction conforming to the "singlet state". On the contrary, if the enthalpy is lower than the minimum value of the energy required to move from the ground state to the excited state, but still exceeds the triplet state energy, triplet state-triplet state annihilation (TTA) will occur. A typical example of triplet state annihilation electrochemiluminescence is ruthenium bipyridine derivatives. In addition, the annihilation reaction can also form excited dimers and excited complexes. In this case, the main advantage of the annihilation process is that it only requires luminescent substances, solvents, and supporting electrolytes to produce luminescence.
(2) Coreactant mechanism
Coreactant electrochemiluminescence is usually generated by applying an electric potential to the electrode, and the luminescent material is either deposited on the electrode surface or in a solution system, accompanied by the presence of coreactant, resulting in luminescence phenomenon. When a potential (positive or negative) is applied to the electrode, the luminescent material and the coreactant undergo simultaneous oxidation or reduction reactions, forming free radicals and intermediates. Then, the intermediate state will interact with the oxidized or reduced emitters to generate the excited state with the formation of highly active oxidized or reduced substances, and then the excited state will emit light signals when returning to the steady state. In typical electrochemiluminescence reactions based on ruthenium bipyridyl emitters, as shown in Formula 10 to Formula 14, Tripropylamine (TPA) is the main co reagent in the process of coreactant luminescence. Through electron transfer or chemical reaction, tripropylamine deporoton product (TPA·) reacts with the emitter to generate an excited state (R*), and the unstable excited state (R*) is generated along with the luminescence signal in the process of returning to the ground state (R).

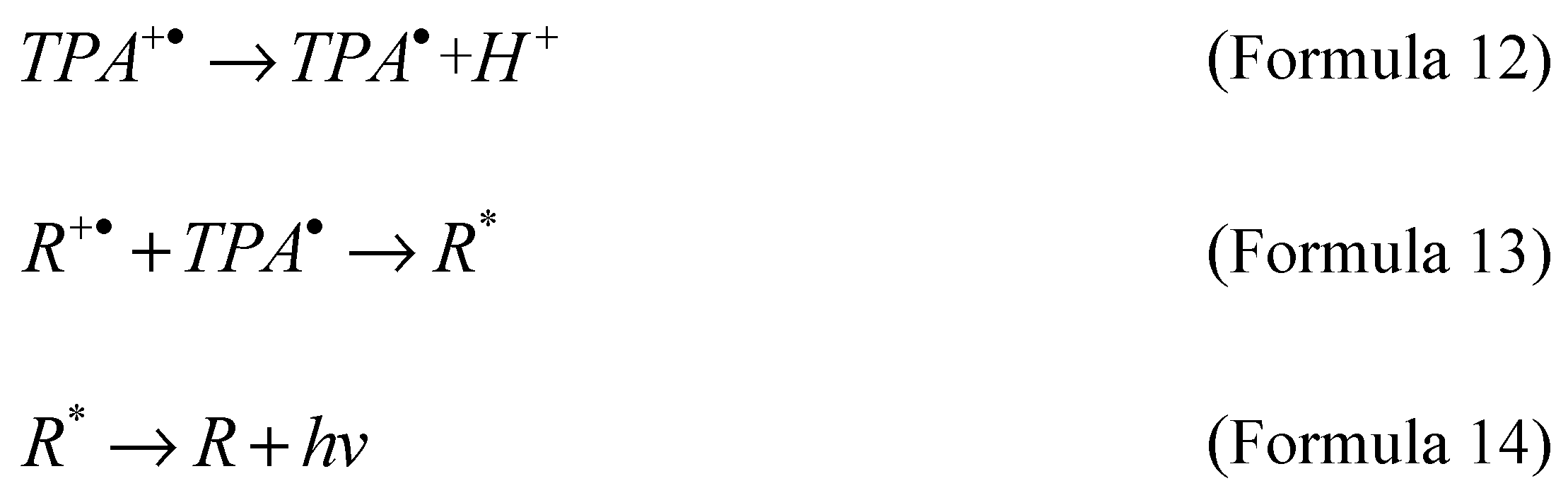
Other coreactant include oxalate and pyruvic acid salt ions operating in the redox mode, and persulfate, hydrazine and hydrogen peroxide operating in the redox mode. Oxidation-reduction type electrochemiluminescence usually occurs under positive potential conditions, and the release reaction of oxygen in aqueous solution is slow, with little impact on electrochemiluminescence [
44]. On the contrary, many reduction oxidation type electrochemiluminescence require very negative potential conditions, which generate a large amount of hydrogen gas and cause immediate decomposition of electrogenerated intermediates, making it difficult to achieve stable reduction oxidation type electrochemiluminescence in a liquid phase environment [
45]. Therefore, electrochemiluminescence based on co reactants is particularly useful when it is necessary to avoid oxygen dissolution, which is of great significance for the detection of analytical samples without deoxygenation. It should be noted that as long as the electrolytic cell keeps working, the light-emitting body can be recycled near the electrode, and the co reagent is constantly consumed in the whole reaction process, so it is only necessary to ensure that there is sufficient co reagent in the reaction system during the reaction process to ensure the continuous and stable luminescence. Appropriate coreactant can easily oxidize or reduce, and then undergo a rapid chemical reaction to form an intermediate with sufficient oxidation or reduction ability to stimulate the formation of the excited state of the emitter, thus promoting the generation of luminescence.
Electrochemical luminescent materials can generally be divided into three categories: inorganic, organic, and nanomaterials. The abundant luminescent materials play a very important role in the development of electrochemiluminescence [
46,
47]. Specifically, ruthenium tripyridine (Ru(bpy)
32+), as an inorganic emitter and the most successful emitter, has a very wide range of applications, mainly due to its strong luminescence and solubility in aqueous or non-aqueous solvents, and its reversible electron transfer ability at easily available potential [
48]. Considering the electrochemical and spectroscopic properties required for electrochemiluminescence exhibited by many metal complexes, such as iridium (Ir), gold (Au), silver (Ag), copper (Cu), platinum (Pt), aluminum (Al), cadmium (Cd), chromium (Cr), molybdenum (Mo), tungsten (W), europium (Eu), osmium (Os), palladium (Pd), thallium (Tl), rhenium (Re), terbium (Tb), silicon (Si) and mercury (Hg) have also been reported for electrochemiluminescence detection [
49].
In organic systems, diphenylanthracene and its derivatives have attracted widespread attention in the field of electrochemiluminescence due to their significant luminescent properties. In addition, studies have found that fluorene, thiophene triazole, and their derivatives can also generate electrochemiluminescence reactions [
50,
51]. The optical, electrical, electrochemical, and luminescent properties of nanoparticles (including polymer and metal nanoparticles) make them an attractive material. Among them, silica nanoparticles have been proven to be a good electrochemical luminescent material due to their easy surface chemical properties for modification and functionalization [
52].
Subsequently, semiconductor nanocrystals or quantum dots received widespread attention due to their excellent luminescent properties and applications in many important fields [
53,
54,
55]. Quantum dots have high fluorescence quantum yield, stability against photobleaching and size controlled luminescence characteristics, which make quantum dots as a new nano material used for sensing analysis [
56,
57,
58]. Among them, silicon nanocrystalline quantum dots were first reported to be used for electrochemiluminescence research [
59], and then various quantum dots, including cadmium sulfide (CdS), cadmium selenide (CdSe), cadmium telluride (CdTe), zinc sulfide (ZnS), silver selenide (Ag
2Se), were also reported for electrochemiluminescence detection [
60]. In addition, other hybrid nanomaterials with various compositions, sizes and shapes, such as metal nanoclusters [
61], carbon nanodots [
62], carbon nitride [
63], graphene and its composites [
64] are also used as electrochemiluminescence materials.
Due to its inherent sensitivity, negligible background, and simple controllability, electrochemiluminescence has been recognized as a powerful analytical technique. In the past few decades, various bioassay methods have been used for electrochemiluminescence detection of target analytes. For the construction of sensitive electrochemiluminescence sensing detection methods, they can generally be divided into five categories [
65]. Firstly, the analyte can inhibit or enhance the electrochemiluminescence reaction by means of energy transfer or electron transfer. Secondly, through redox or surface bonding/detachment, the enhancement or decomposition of the electrochemiluminescence is realized. Thirdly, by producing or consuming coreactant through enzyme-linked immunosorbent reaction, changes in electrochemiluminescence signals can be achieved. Fourthly, an electrochemiluminescence sensing strategy for signal cutoff is achieved through the spatial hindrance caused by biological cognitive reactions or target induced deposition. Fifthly, an efficient electrochemiluminescence resonance energy transfer sensing strategy is achieved by overlapping the spectra of the receptor and donor. Based on these methods, electrochemiluminescence technology is widely used in metal ion detection [
66,
67], immunoassay [
68], gene sensing [
69,
70,
71], early cancer diagnosis [
72] and other fields.
Due to the urgent need for rapid diagnosis, point of care testing (POCT) has been proposed and attracted widespread attention, mainly using portable, low-cost, and user-friendly devices to provide rapid analysis and real-time monitoring of health status [
73,
74,
75]. Research has shown that the design of real-time detection systems based on electrochemiluminescence technology mainly includes two key components: electrochemiluminescence excitation and luminescent detectors.
(1) Electrochemiluminescence excitation
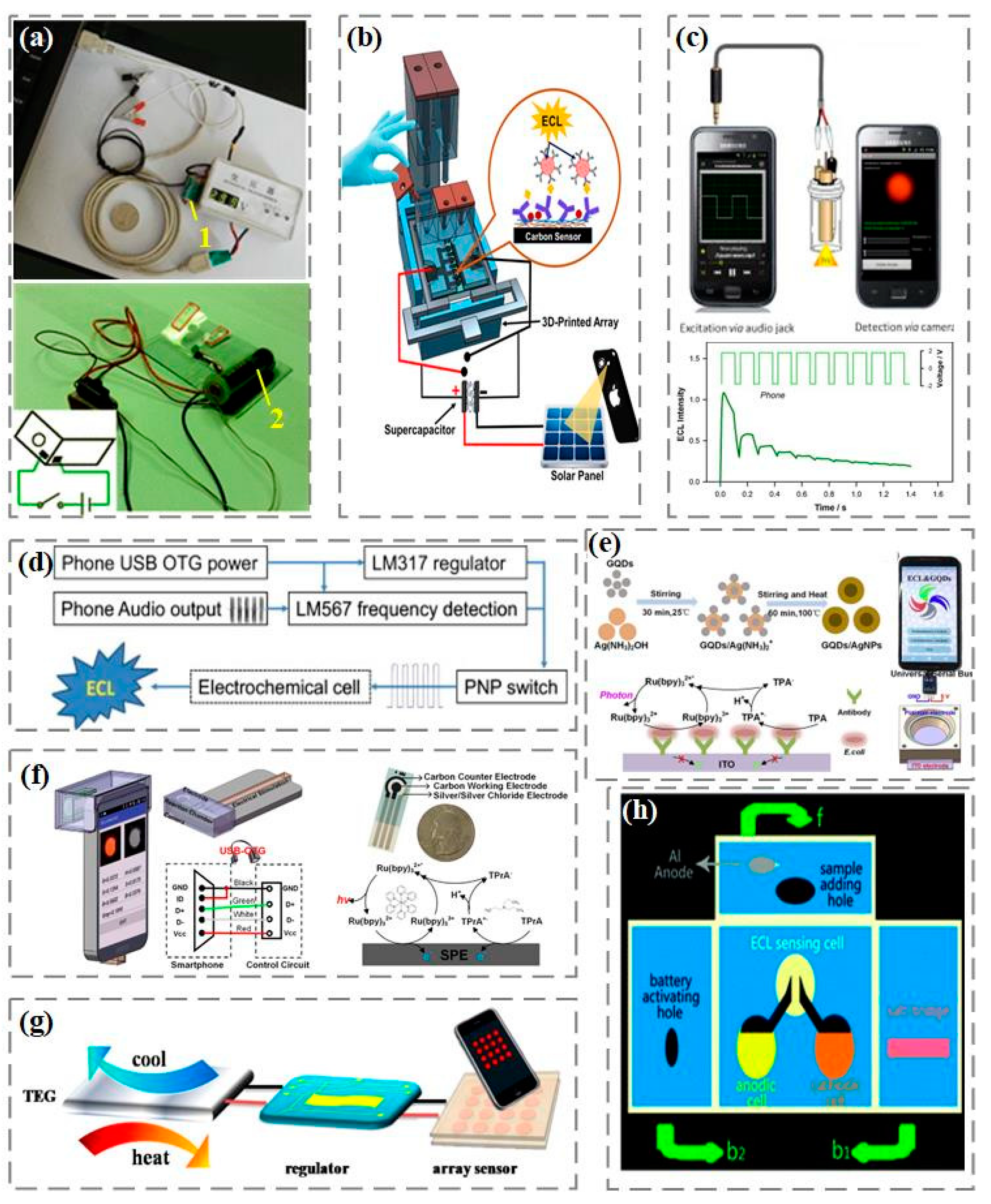
In traditional electrochemiluminescence analysis, the luminescence signal is excited and controlled through an electrochemical workstation. Due to the high cost of electrochemical workstations, developing alternative electrochemiluminescence excitation for real-time detection is a top priority. Among them, portable and low-cost rechargeable batteries, due to their advantages of high efficiency, stable voltage output, large capacity, and low self-discharge rate, have become a good choice for electrical excitation in the manufacturing of portable electrochemical luminescent devices for real-time detection (
Figure 3a) [
76,
77]. In addition, as shown in
Figure 3b, rechargeable supercapacitors have also been reported by Kadimisetty et al. to provide voltage excitation for electrochemiluminescence [
78]. In addition to charging batteries, Delaney et al. proposed an electrochemiluminescence excitation method that does not require the use of a potentiostat [
79]. As shown in
Figure 3c, voltage excitation comes from the audio port of the smartphone and is modulated through the audio output to obtain a square wave signal with a positive interval of 0.1 s and a negative interval of 0.04 s, which is used to stimulate the generation of electrochemiluminescence signals. Afterwards, they further designed a novel and universal electrochemiluminescence excitation based on the standard USB On-The-Go (USB-OTG) specification [
80]. As shown in
Figure 3d, the luminescence excitation voltage can be driven from any USB-OTG certified smartphone USB port and modulated through an audio interface to output to the electrochemiluminescence reaction cell. In our previous work, using graphene quantum dots/silver nanoparticles to amplify and enhance the electrochemiluminescence signal, and combining with the electrochemiluminescence system based on smartphone to detect Escherichia coli (
Figure 3e). In the electrochemiluminescence system, tripyridine ruthenium is used as the luminescent body, tripropylamine as the coreactant, and graphene quantum dots/silver nanoparticles as the enhancer to stabilize and enhance the luminescence response. Using the universal serial bus USB-OTG on the smartphone to provide voltage excitation, the camera captures light and achieves concentration detection of Escherichia coli from 10 cfu/mL to 10
7 cfu/mL [
81]. In addition, we developed a silica nanopore enhanced electrochemiluminescence system on smartphone, using the universal serial bus USB-OTG as the electrical stimulus and the camera to capture the luminescence (
Figure 3f). Using positively charged tripyridine ruthenium as the luminescent material in the electrochemiluminescence reaction, the signal amplification in the electrochemiluminescence detection was achieved by enhancing the luminescence signal response of the nanopores with negative charge characteristics on the inner wall of the pores [
82]. The smartphone detection system also uses inexpensive, flexible, and disposable screen-printed electrodes, which are convenient for on-site biochemical detection applications.
To further simplify the design of electrochemiluminescence excitation, a portable thermally powered electrochemiluminescence visualization sensor was proposed by Hao et al. [
83]. The sensor consists of a micro power supply and an easily prepared transistor array (
Figure 3g). The unique power supply structure provides a valuable reference for the miniaturization of electrochemical light-emitting devices, and facilitates subsequent on-site operation and real-time detection. On this basis, Zhang et al. developed a stable, environmentally friendly, metal free, self-powered three-dimensional microfluidics electrochemiluminescence biosensor platform based on the origami principle [
84]. As shown in
Figure 3h, the platform assembles the energy part and the sensing part on a three-dimensional paper chip. The microfluidics origami electrochemiluminescence device has broad application prospects in portable, green, low-cost and disposable detection equipment.
(2) Luminous detector
The collection of luminescent signals is another important component of electrochemical luminescent sensing systems. The traditional luminous signal acquisition is completed by Photomultiplier tube, mainly because of its high sensitivity. With the increasing demand for portable and user-friendly electrochemiluminescence detectors in real-time detection, a number of alternative optical sensors have emerged, such as charge coupled devices (CCD), complementary metal oxide semiconductor devices (CMOS), and photodiodes [
85]. In order to reduce testing costs, numerous studies have reported the use of digital cameras as electrochemical luminescent detectors. As shown in
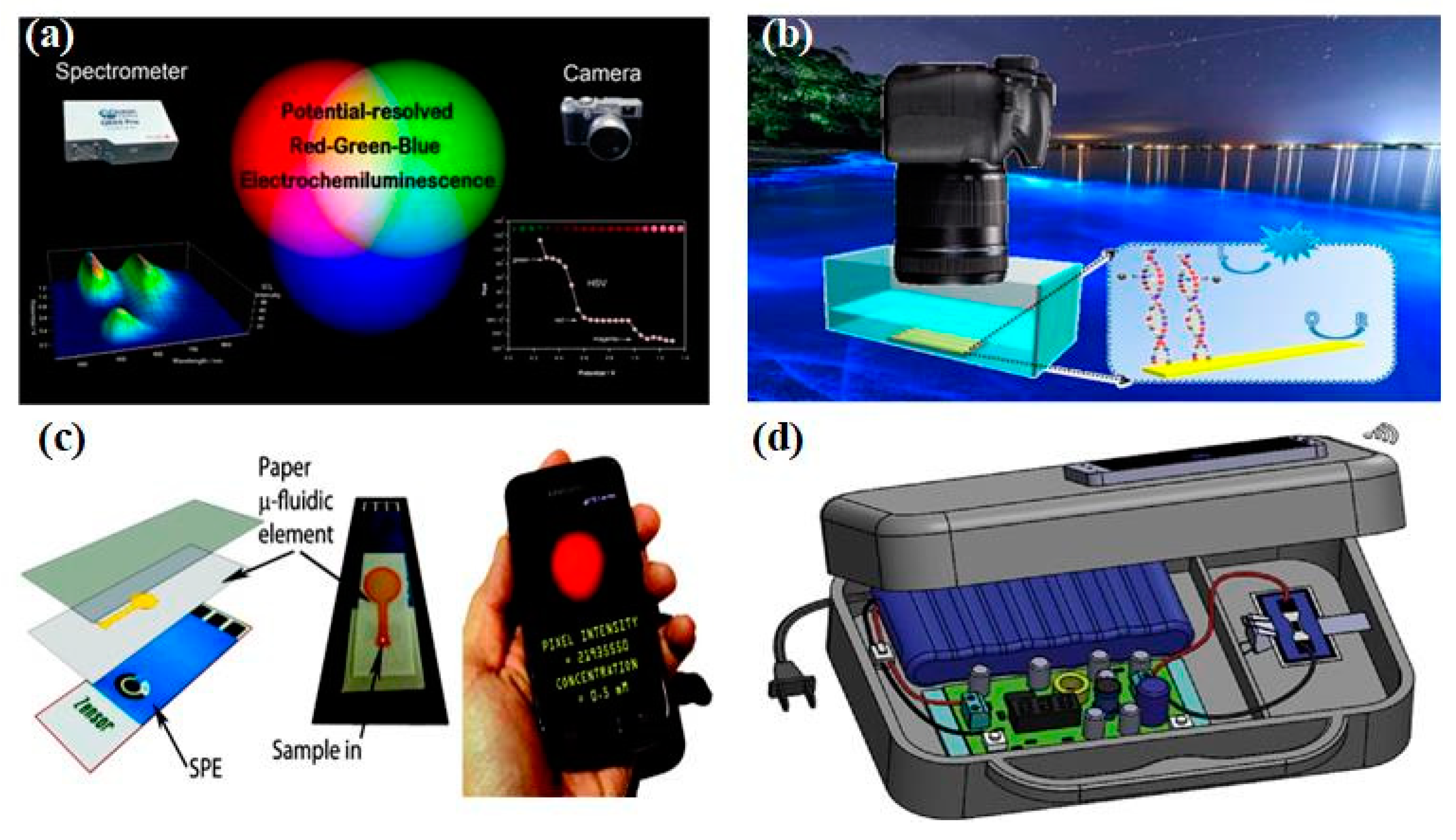
Figure 4a, Doeven et al. utilized the excitation and emission characteristics of electrochemiluminescence materials, combined with the inherent color selectivity of traditional digital cameras, to effectively distinguish red, green, and blue emitters in the three-dimensional space of electrochemiluminescence intensity, applied potential, and emission wavelength, creating a new multi-channel electrochemiluminescence detection strategy suitable for developing low-cost and portable clinical diagnostic equipment [
86]. In addition, Khoshfetrat et al. used digital cameras to visualize electrochemiluminescence signals and developed a radio iluminescence DNA array for visual genotyping of different single-nucleotide polymorphism (Fig. 4b) [
87].
Smartphone, due to their high imaging and computing power, as well as open-source operating systems, are playing an increasingly crucial role in sensor analysis and medical monitoring [
90]. The huge user base of smartphone has further promoted the rapid development of embedded hardware and software, as well as high-end imaging and sensing technologies in mobile phones. This has made smartphone gradually become a promising intelligent platform for developing various biological sensor analysis devices, which can be used for fast, real-time, and on-site detection, greatly simplifying design and reducing the cost of detection systems [
91,
92]. The portability and ubiquitous availability of smartphone provide great convenience for the development of portable electrochemical luminescent devices. As shown in
Figure 4c, the combination of paper-based microfluidics electrodes and the electrochemiluminescence detection system based on smartphone can build a sensing analysis platform without traditional photodetectors [
88]. In addition, Chen et al. proposed a handheld bipolar electrochemiluminescence system, which uses smartphone to read electrochemiluminescence signals (
Figure 4d) [
89]. In addition, the development of bipolar electrochemistry, microfluidics chips, wireless power supply and data transmission, as well as optical detection technology, has made the performance of electrochemiluminescence sensors unprecedented improved towards real-time detection applications. Undoubtedly, the significant progress and breakthroughs in real-time detection in the coming years will also greatly promote the development of these emerging technologies. Based on the above progress, it can be found that integrating various processes of electrochemiluminescence (including sample collection, separation, and detection) into an "all-in-one machine", while cleverly combining different technologies and elements to manufacture integrated electrochemiluminescence sensors, and ensuring the high specificity, sensitivity, and repeatability of sensors in real-time detection is still a direction that needs continuous efforts and exploration.
4. Prospect
Although this article has conducted relevant research on nano biosensing motion detection based on electrochemical coupled optical technology, there are still some problems that need to be solved to further promote the application of electrochemical coupled optical technology in biosensing motion detection. First, the strictness of anti-interference requirements limited to optical sensing has greatly increased the complexity of optical path construction, limiting the realization of electrochemical coupling surface plasmon resonance on smartphone. In addition, currently only achieved single biological modification of nanoarray devices, only utilizing the improvement of sensing sensitivity brought by their nanostructures, and did not fully utilize the advantages of their array structures. Further exploration is needed to achieve multi-channel detection through modification of different sensitive layers, enriching the output signals of nanoarray sensors. Secondly, this review has demonstrated the excellent electrochemical/optical sensing properties of nanomaterials. Then the biosensor characteristics of metal carbide nanomaterials with two-dimensional layered structure such as graphite oxide derivatives and MXene need to be further studied to broaden the types of detection probes and promote the development of biosensors. However, the limited sensing characteristics brought by nanomaterials cannot fully meet the demand for biosensing specificity detection. Therefore, further research is needed to improve the sensing selectivity of nanomaterials by coupling them with biological sensitive layers such as proteins, peptides, or DNA. Finally, this study conducted a series of detailed studies on electrochemiluminescence detection based on smartphone. The sensing system is sufficiently complete to meet the relevant biological sensing detection needs, but there are still many aspects that can be improved in combination with the development of electrochemiluminescence technology. Although the luminescent material is not consumed in the reaction, it is difficult to recycle and reuse. Solid state luminescence not only facilitates detection operations but also reduces testing costs by fixing and modifying the luminescent material on the electrode surface, which is conducive to mobile detection applications. Although current researches utilize mobile phone cameras to dynamically capture luminous videos, the luminous processing is still based on luminous imaging and does not directly analyze the optical signal. Therefore, real-time processing of luminous signals using smartphone light sensors and other components is a further area that needs improvement. In the future, there is still a long way to go for related in-depth research, such as integrating more precise sensors on smartphone or developing more precise detection technologies. To achieve quantitative analysis of fingerprint substances and promote mobile biosensing detection during the unlocking process of mobile phones. Given the excellent performance of electrochemiluminescence in imaging analysis, further exploration can be made in the spatial distribution of luminescence, such as multicolor luminescence. At the same time, the convenience of image processing using smartphone can enable multi-channel biosensing detection applications on multicolor luminescent materials.
The ideas for future work are:
(1) Research on multi-channel biosensing detection based on nanoarray sensors
Precious metal nano array sensors were prepared to realize electrochemical coupling surface plasmon resonance detection. And modify multiple biosensitive layers on it. Build a multi-channel electrochemical coupled optical detection platform based on nanoarray sensors. Realize simultaneous detection of multiple target analytes.
(2) Research on sensing detection based on nanomaterial composite biosensitive layer
Study the electrochemical/optical sensing characteristics of metal carbide materials with two-dimensional layered structure. Preparation of composite probes for nanomaterials and biosensitive layers. To achieve specific biosensing detection of target analytes.
(3) Research on biosensing detection based on quantum dot solid state electrochemiluminescence
Build a dynamic real-time electrochemiluminescence analysis system based on smartphone light sensors. And on this system, study the electrochemiluminescence related characteristics of new quantum dot nanomaterials, and complete the construction of solid-state electrochemiluminescence sensing components. Implement relevant biosensing detection applications.
(4) Research on multicolor luminescent biosensing detection based on smartphone
Preparation of potential resolved multicolor electrochemiluminescence sensitive probes and construction of multi-channel biosensing components. Build an electrochemiluminescence platform based on smartphone and conduct multimodal analysis of luminescence signals. Furthermore, mobile sensing detection of complex biological samples can be achieved.