1. Introduction
Advanced urothelial carcinoma poses significant challenges in terms of the diagnosis, treatment, and patient outcomes (1-3). This aggressive form of urothelial carcinoma often presents at an advanced stage, leading to limited treatment options and a poor prognosis. In recent years, molecular biomarkers, such as programmed death-ligand 1 (PD-L1) expression and fibroblast growth factor receptor (FGFR) alterations, have gained importance in identifying potential targets for personalized therapies.
The treatment options for advanced urothelial carcinoma have evolved significantly in recent years. Platinum-based chemotherapy, typically with cisplatin or carboplatin, is the standard of care for eligible patients (4). However, a significant proportion of patients may not be candidates for platinum-based chemotherapy because of renal impairment or a poor performance status. Even when patients are eligible for platinum-based systemic chemotherapy, advanced urothelial carcinomas are refractory to chemotherapy.
For these patients, immune checkpoint inhibitors targeting PD-1/PD-L1 have shown efficacy and have been approved for chemotherapy-resistant cases. However, most cases are refractory even to immune checkpoint inhibitors (5). For these patients, enfortumab vedotin, an antibody-drug conjugate targeting Nectin-4, has shown promising results in clinical trials and has received regulatory approval for subsequent therapy (6, 7).
Enfortumab vedotin is an innovative and promising targeted therapy that has shown significant efficacy in the treatment of advanced urothelial carcinoma in adult patients who have previously received platinum-containing chemotherapy and PD-1 or PD-L1 inhibitors. Based on the encouraging results of EV-201, the EV-301 phase III trial compared enfortumab vedotin with standard chemotherapy (docetaxel, paclitaxel, and vinflumine) in patients with locally advanced or metastatic urothelial carcinoma who had previously received platinum-based chemotherapy and a PD-1/PD-L1 inhibitor (7-9). The trial demonstrated a significantly improved overall survival (OS) with enfortumab vedotin compared to chemotherapy, establishing it as a superior treatment option. The median OS in the enfortumab vedotin group was 12.9 months, compared to 9.0 months in the chemotherapy group (hazard ratio [HR]: 0.70). Furthermore, enfortumab vedotin showed a higher Objective response rate (ORR) of 40.6% than chemotherapy (17.9%).
However, despite the proven effectiveness of enfortumab vedotin in cases of urothelial carcinoma, the EV-301 study only included 86 Japanese patients (50 in the chemotherapy group and 36 in the enfortumab vedotin group), and there are no large real-world data in terms of enfortumab vedotin.
The present study therefore investigated the effectiveness of enfortumab vedotin as a post-pembrolizumab treatment for Japanese patients with advanced or metastatic urothelial carcinoma using a large medical insurance database (7, 10).
2. Materials and Methods
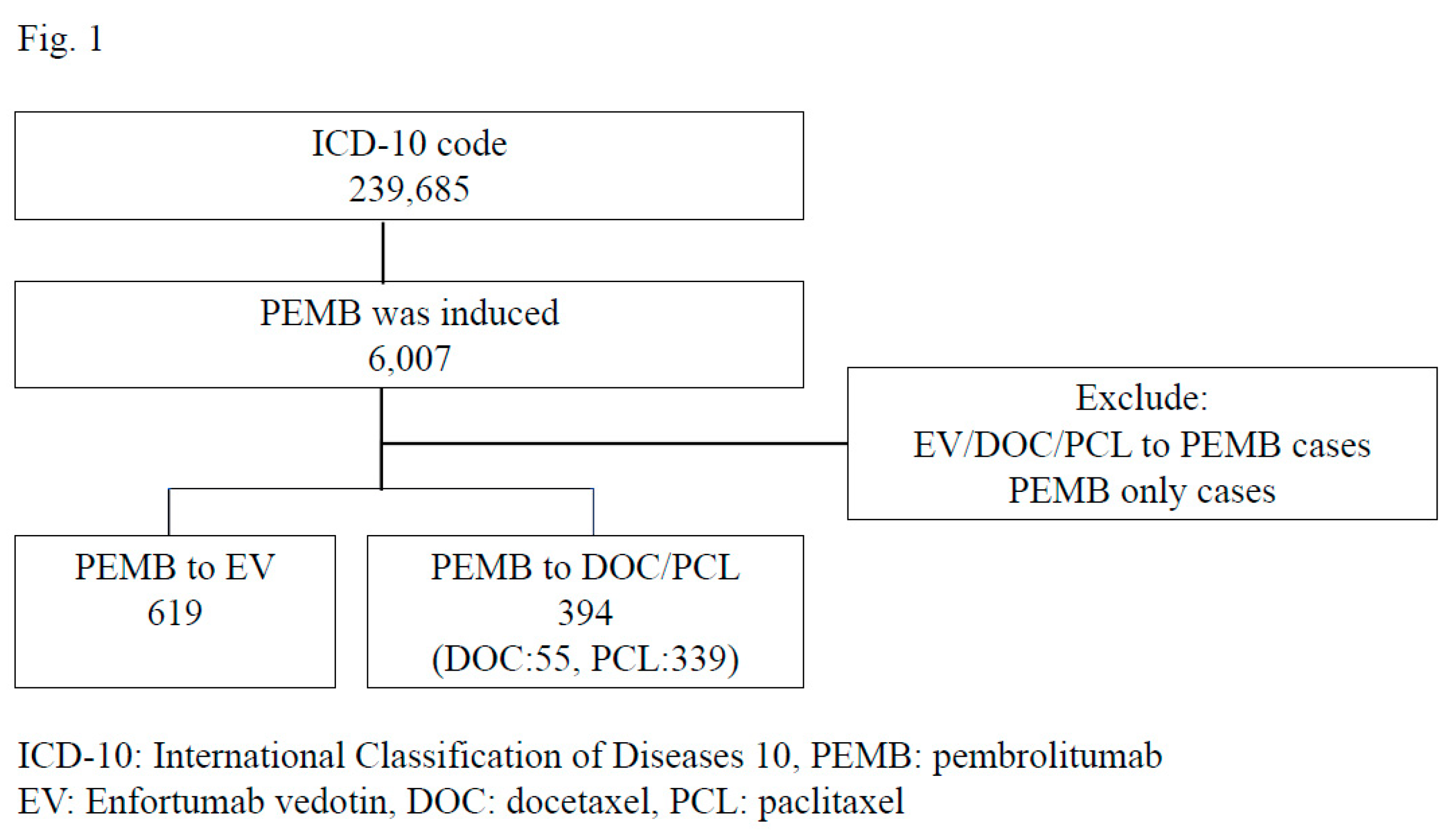
This study used the database of healthcare fees, which covers approximately 25.6% of Diagnosis Procedure Combination (DPC) hospitals in Japan (11). These data were obtained from the Medical Data Vision (Tokyo, Japan). From April 2008 to December 2022, we identified patients who were diagnosed with urothelial carcinoma using ICD-10 codes. Data of approximately 239,685 urothelial cancer patients were extracted from this database, and 6,007 were found to have been treated with pembrolizumab. The age, cancer location, types of previous chemotherapy, types of post-chemotherapy, and prognosis of these patients were analyzed.
A total of 6,007 patients were treated with pembrolizumab as second-line treatment. To analyze the efficacy of enfortumab vedotin compared to paclitaxel or docetaxel, cases treated with pembrolizumab monotherapy were excluded in this study. Ultimately, 619 patients received enfortumab vedotin after pembrolizumab, and 394 patients, including 55 docetaxel and 339 paclitaxel patients who received docetaxel or paclitaxel after pembrolizumab, were analyzed in this study.
2.1. Statistical analyses
Participants’ characteristics and scores were analyzed using the Mann-Whitney U test. The OS and cancer-specific survival (CSS) were calculated using the Kaplan-Meier curve, and the log-rank test was used for the comparison. A multivariate analysis was used to compare risk factors for the OS and CSS. These tests were conducted using the GraphPad Prism software program (GraphPad Software, La Jolla, CA, USA). Statistical significance was set at P <0.05.
3. Results
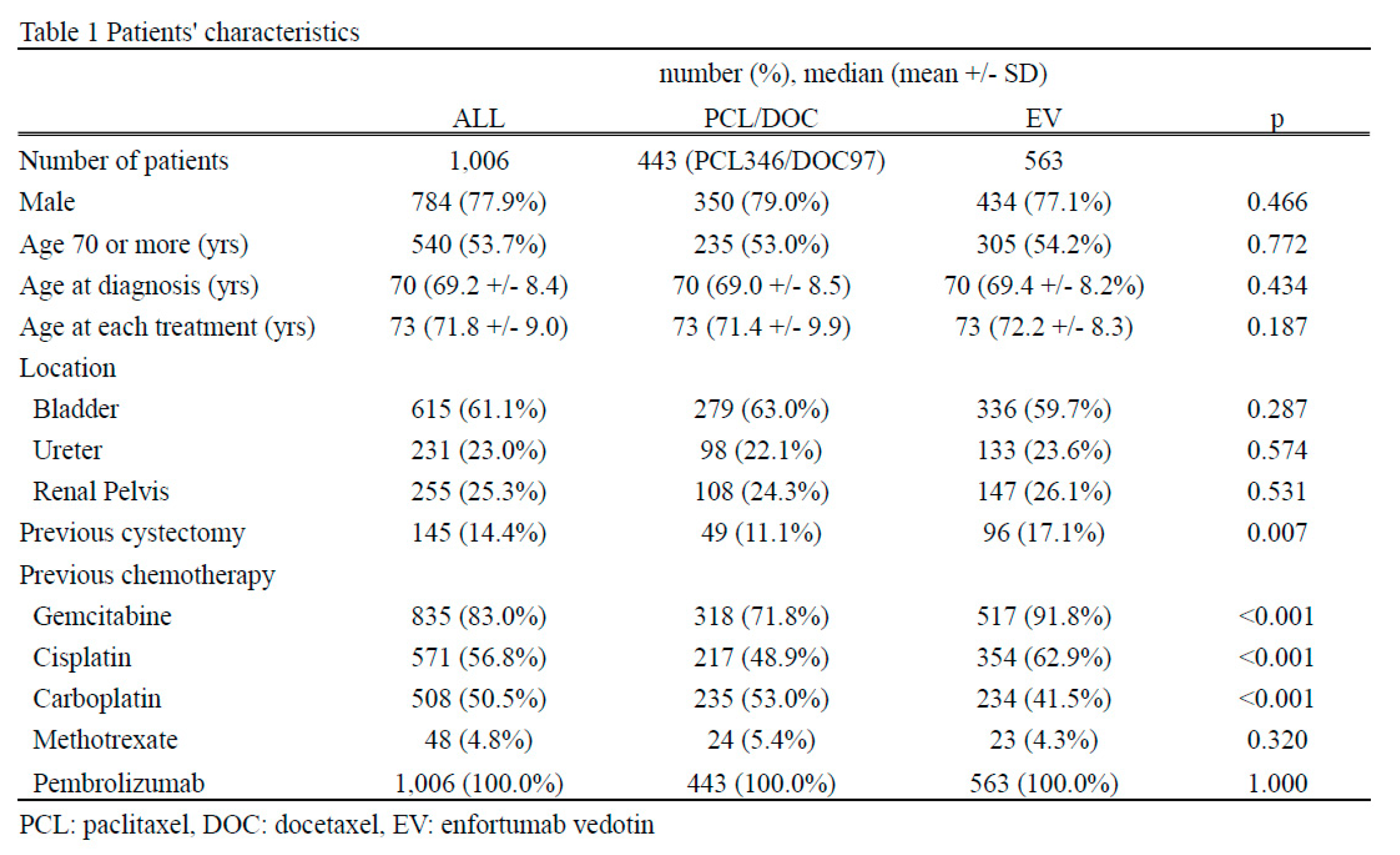
A total of 1,006 patients, including 444 paclitaxel/docetaxel cases and 563 enfortumab vedotin cases, were enrolled in this study [Figure 1]. No gender or age differences were observed (males: 79.0% in the paclitaxel/docetaxel group and 77.1% in the enfortumab vedotin group, p=0.446; age [median {mean ± standard deviation}]: 73 [71.4 ± 9.9] years old in the paclitaxel/docetaxel group and 73 [72.2 ± 8.3] years old in the enfortumab vedotin group, p=0.187). There were also no marked differences in the location of urothelial carcinoma: 279 (63.0%) cases in the paclitaxel/docetaxel group and 336 (59.7%) in the enfortumab vedotin group observed in the bladder (p=0.287); 98 (22.1%) cases in the paclitaxel/docetaxel group and 133 (23.6%) in the enfortumab vedotin group observed in the ureter (p=0.574), and 108 (24.3%) cases in the paclitaxel/docetaxel group and 147 (26.1%) in the enfortumab vedotin observed in the renal pelvis (p=0.531). A certain number of cases were duplicated for each urothelial cancer location. The enfortumab vedotin group received cystectomy more frequently than the paclitaxel/docetaxel group (49 [11.1%] in the paclitaxel/docetaxel group and 96 [17.1%] in the enfortumab vedotin group, p=0.007). This study included patients who had received pembrolizumab. A total of 318 (71.8%) patients in paclitaxel/docetaxel received and 517 (91.8%) in enfortumab vedotin received gemcitabine (p<0.001). A total of 217 (48.9%) in the paclitaxel/docetaxel group and 354 (62.9%) in the enfortumab vedotin had received cisplatin (P <0.001). A total of 235 (53.0%) patients in paclitacel/docetaxel and 234 (41.5%) in the enfortumab vedotin group had received carboplatin (p<0.001). A total of 24 (5.4%) in the paclitaxel/docetaxel group and 23 (4.3%) in the enfortumab vedotin group had received methotrexate (p=0.320) [Table 1].
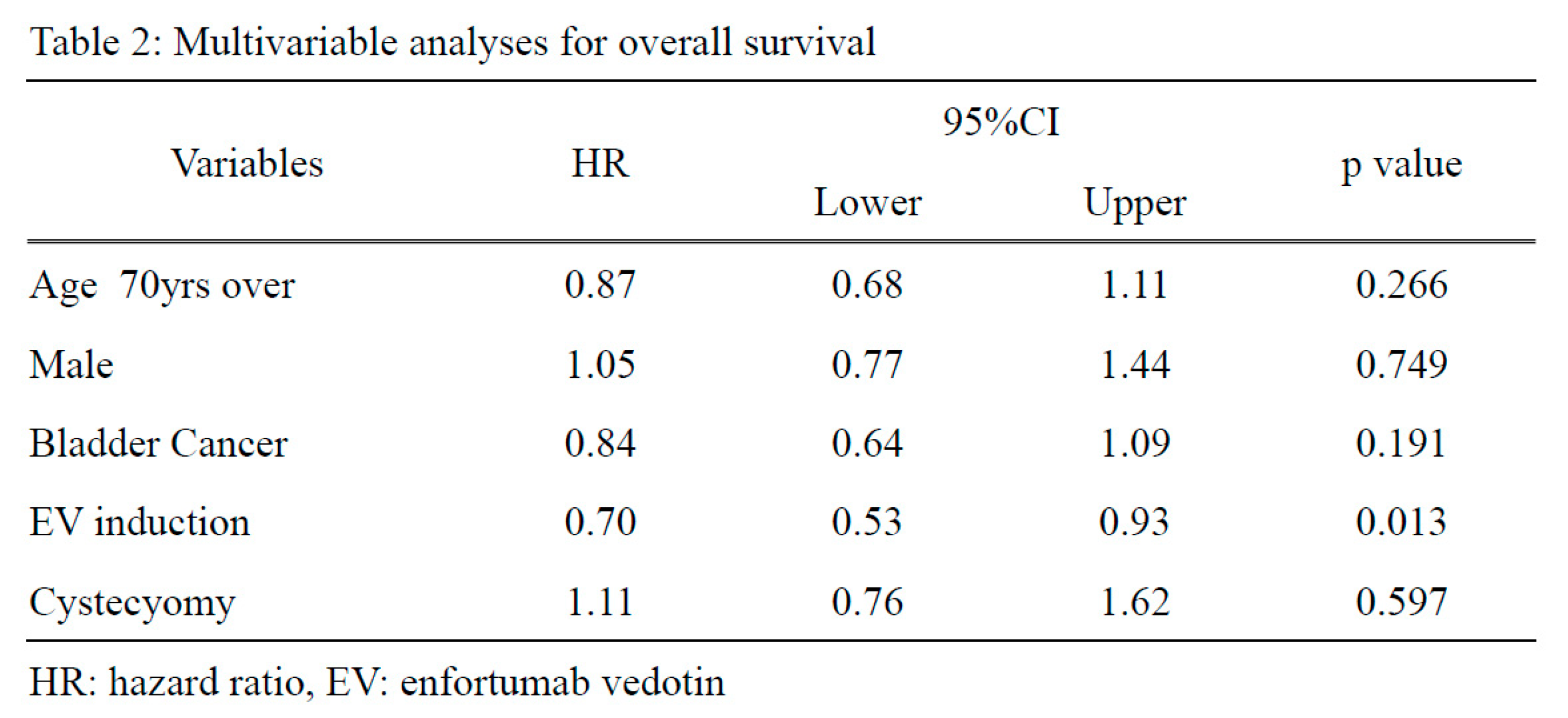
Regarding the prognosis, the enfortumab vedotin group showed a longer OS than the paclitaxel/docetaxel group (p=0.013, HR: 0.71) [Figure 2]. In the multivariate analysis, enfortumab vedotin induction was an independent risk factor for the OS (p=0.013, HR: 0.70) [Table 2], whereas no significant differences were observed in the CSS [supplementary Table 1 and supplementary Figure 1].
4. Discussion
Enfortumab vedotin uses a unique mechanism of action that combines the specificity of an antibody-drug conjugate (ADC) with the potent cytotoxicity of a microtubule-disrupting agent (4, 12). The key components of this therapy are an anti-Nectin-4 monoclonal antibody and the cytotoxic agent monomethyl auristatin E (MMAE). Nectin-4 is overexpressed in urothelial carcinoma cells, making it an ideal therapeutic target (13). The anti-Nectin-4 monoclonal antibody in enfortumab vedotin specifically binds to Nectin-4 on the cancer cell surface, allowing selective delivery of the cytotoxic agent to the tumor site. Once bound to Nectin-4, enfortumab vedotin is internalized by cancer cells via receptor-mediated endocytosis. This process allows the ADC to enter the intracellular compartment, where it undergoes proteolytic cleavage, releasing MMAE (12). MMAE is a potent inhibitor of microtubule polymerization and is crucial for cell division and growth. By disrupting microtubules, MMAE interferes with the ability of cancer cells to divide and proliferate, leading to cell cycle arrest and, ultimately, cell death. Furthermore, MMAE induces apoptosis, a programmed cell death mechanism, by triggering various cellular signals that activate the intrinsic apoptotic pathway (4). This process involves the release of cytochrome c from the mitochondria, activation of caspases, and the subsequent degradation of cellular components, ultimately resulting in cancer cell death.
In the present study, 563 patients were treated with enfortumab vedotin after pembrolizumab treatment, and their prognosis was compared with that of 463 patients treated with paclitaxel or docetaxel. To our knowledge, this is the largest Japanese report to date. The EV-301 study included 301 patients in the enfortumab vedotin group and 307 in the chemotherapy group. Among them, the Japanese cohort included 36 patients treated with enfortumab vedotin and 50 patients treated with chemotherapy (paclitaxel and docetaxel). Vinflumin was not induced in the Japanese cohort because it is not yet approved in Japan. One of the characteristics of our clinical trial is that only patients with a good performance status were included; thus, these results do not always reflect real-world results. Furthermore, because of differences in post-treatment courses due to economic conditions in each country, it is difficult to evaluate the results in terms of the OS for a specific country. In Japan, all patients are covered by the medical insurance system and can receive full treatment options; thus, real-world data are required. In this study, we demonstrated the usefulness of enfortumab vedotin compared with systemic taxane chemotherapy after pembrolizumab treatment in real-world Japanese patients.
The enfortumab vedotin group in our study showed a longer OS than the paclitaxel/docetaxel group. The median survival for the enfortumab vedotin group was not reached due to the short observation period. The median survival of the paclitaxel/docetaxel group was similar to that of the chemotherapy group in the EV-301 study (this study paclitaxel/docetaxel cohort: 13.9 months, EV-301 study chemotherapy cohort: 9.97 months, EV-301 study chemotherapy Japanese cohort: 10.6 months), and the HR was 0.74 in the EV-301 study and 0.70 in the present study (7, 10). These real-world data showed results similar to those of EV-301 clinical trials.
We detected a significant difference in the OS but not in the CSS in the present study. This may be due to the small number of patients diagnosed as dying from cancer in the database analysis. In the current cohort, there were 251 (33.3%) deaths and 153 (18.0%) cancer deaths. We suspect that the difference between the overall incidence of death and cancer death would be smaller in urothelial carcinoma patients receiving enfortumab vedotin or paclitaxel/docetaxel induction after pembrolizumab treatment. It is highly likely that the insurance database recorded cancer-related deaths. Further studies with more detailed databases and longer observation periods are required.
In the present study, the patients were compared with those in the chemotherapy group, as in the EV-301 study. In Japan, neither paclitaxel nor docetaxel are currently approved by insurance for urothelial carcinoma; therefore, using paclitaxel/docetaxel is an off-label specification (14). The number of patients in the chemotherapy group was thus relatively small. Notably, before the insurance indication for enfortumab vedotin was approved in Japan, there was no established treatment option after pembrolizumab treatment, and paclitaxel/docetaxel was used in the off-label setting.
Our study did not include patients who had been treated with avelumab. We excluded these cases because the insurance approval of avelumab in Japan based on the JAVELIN Bladder 100 study results was in 2021; therefore, a sufficient observation period could not have been obtained, and the profile of urothelial carcinoma would have been different from that of the pembrolizumab group because the JAVELIN Bladder 100 study included only complete response, partial response, and stable disease cases after 4-6 courses of platinum-based chemotherapy (15). The profile of urothelial carcinoma in the post-avelumab setting would be different from that in the post-pembrolizumab setting.
Several limitations associated with the present study warrant mention. First, this was a retrospective study, and because of our use of an insurance system database analysis, there was little patient information. However, we noted no significant difference in the time from the diagnosis to the start of treatment in each group, nor was there any significant difference in the age either; therefore, comparing the OS between paclitaxel/docetaxel and enfortumab vedotin was not considered problematic. Second, this study did not examine side effects; enfortumab vedotin is known to cause hematological toxicity, hyperglycemia, skin rash, and other side effects. Although there was some difference in the OS, the side effects should be examined in different ways in the future.
In conclusion, enfortumab vedotin prolonged the OS of Japanese patients with advanced or metastatic urothelial carcinoma compared to paclitaxel or docetaxel after pembrolizumab treatment.
5. Conclusions
Enfortumab vedotin prolonged the overall survival in Japanese patients with advanced or metastatic urothelial carcinoma compared with paclitaxel or docetaxel after pembrolizumab treatment.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org. supplementary Table 1 supplementary Figure 1.
Author Contributions
TK, AH, KY, and HU drafted the manuscript. TK, KU, HI, JT, TT, HH, KO, KM, and HU performed experiments.
Institutional Review Board Statement
We received approval from the Institutional Review Board of Yokohama City University Medical Center.
Informed Consent Statement
N/A due to the anonymous nature of the database
Data Availability Statement
The raw data to create tables and figures are available upon request.
Acknowledgments
There are no applicable grant numbers associated with this study.
Conflicts of Interest
We declare no conflicts of interests.
References
- Alimohamed N, Grewal S, Wirtz HS, Hepp Z, Sauvageau S, Boyne DJ, et al. Understanding Treatment Patterns and Outcomes among Patients with De Novo Unresectable Locally Advanced or Metastatic Urothelial Cancer: A Population-Level Retrospective Analysis from Alberta, Canada. Curr Oncol. 2022;29(10):7587-97. [CrossRef]
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108. [CrossRef]
- Alfred Witjes J, Lebret T, Comperat EM, Cowan NC, De Santis M, Bruins HM, et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur Urol. 2017;71(3):462-75. [CrossRef]
- Maas M, Stuhler V, Walz S, Stenzl A, Bedke J. Enfortumab vedotin - next game-changer in urothelial cancer. Expert Opin Biol Ther. 2021;21(7):801-9. [CrossRef]
- Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med. 2017;376(11):1015-26. [CrossRef]
- Koshkin VS, Henderson N, James M, Natesan D, Freeman D, Nizam A, et al. Efficacy of enfortumab vedotin in advanced urothelial cancer: Analysis from the Urothelial Cancer Network to Investigate Therapeutic Experiences (UNITE) study. Cancer. 2022;128(6):1194-205. [CrossRef]
- Powles T, Rosenberg JE, Sonpavde GP, Loriot Y, Duran I, Lee JL, et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N Engl J Med. 2021;384(12):1125-35. [CrossRef]
- Rosenberg JE, O'Donnell PH, Balar AV, McGregor BA, Heath EI, Yu EY, et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. J Clin Oncol. 2019;37(29):2592-600. [CrossRef]
- Yu EY, Petrylak DP, O'Donnell PH, Lee JL, van der Heijden MS, Loriot Y, et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV-201): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):872-82. [CrossRef]
- Matsubara N, Yonese J, Kojima T, Azuma H, Matsumoto H, Powles T, et al. Japanese subgroup analysis of EV-301: An open-label, randomized phase 3 study to evaluate enfortumab vedotin versus chemotherapy in subjects with previously treated locally advanced or metastatic urothelial carcinoma. Cancer Med. 2023;12(3):2761-71. [CrossRef]
- Kawahara T, Miyoshi Y, Ninomiya S, Sato M, Takeshima T, Hasumi H, et al. Administration of radium-223 and the prognosis in Japanese bone metastatic castration-resistant prostate cancer patients: A large database study. Int J Urol. 2022;29(9):1079-84. [CrossRef]
- Challita-Eid PM, Satpayev D, Yang P, An Z, Morrison K, Shostak Y, et al. Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res. 2016;76(10):3003-13. [CrossRef]
- Zhang Y, Zhang J, Shen Q, Yin W, Huang H, Liu Y, et al. High expression of Nectin-4 is associated with unfavorable prognosis in gastric cancer. Oncol Lett. 2018;15(6):8789-95. [CrossRef]
- Raggi D, Miceli R, Sonpavde G, Giannatempo P, Mariani L, Galsky MD, et al. Second-line single-agent versus doublet chemotherapy as salvage therapy for metastatic urothelial cancer: a systematic review and meta-analysis. Ann Oncol. 2016;27(1):49-61. [CrossRef]
- Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2020;383(13):1218-30. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).







