1. Introduction
Nanomaterials find extensive utility across diverse domains due to their versatile characteristics encompassing physical-mechanical, electrical, magnetic, chemical, and biological properties. In the realm of medical applications, nanotechnology plays a pivotal role, particularly in the realm of drug delivery systems. These systems are instrumental in enhancing the pharmacokinetics of medications by finely controlling the size distribution of crucial components and finely tuning the drug release and absorption processes.[
1].
Nanotechnology-based drug delivery systems represent a promising and highly interdisciplinary approach within the biomedical sector. They are specifically designed to address several critical challenges in the field, including issues like limited bioavailability caused by poor solubility, inefficient intestinal absorption due to degradation, suboptimal targeting to desired sites, the occurrence of side effects, and inconsistent drug plasma levels [
2].
Nanoparticles have a large porous surface area [
3], which allows surface modifications and surface imaging, giving nanoparticles an advantage for therapeutic uses [
4]. However, unmodified surface nanoparticles immediately make a foreign cell more susceptible to phagocytosis [
5]. As a result, nanoparticles produced from natural or synthetic polymers allow drugs to be delivered to multiple sites of inflammation, expanding the protection of drugs against impairment in the absorption mechanism in the body [
6]. Polymeric nanoparticles are frequently used in the therapeutic sector because they are amenable to modification using organic or inorganic hybrid components. Surface modifications of nanoparticles can be achieved by a covalent bond between synthetic or natural polymers.
Biopolymers derived from proteins or polysaccharides are becoming more popular than synthetic ones due to their biodegradability, biocompatibility, low immunogenicity and antibacterial properties [
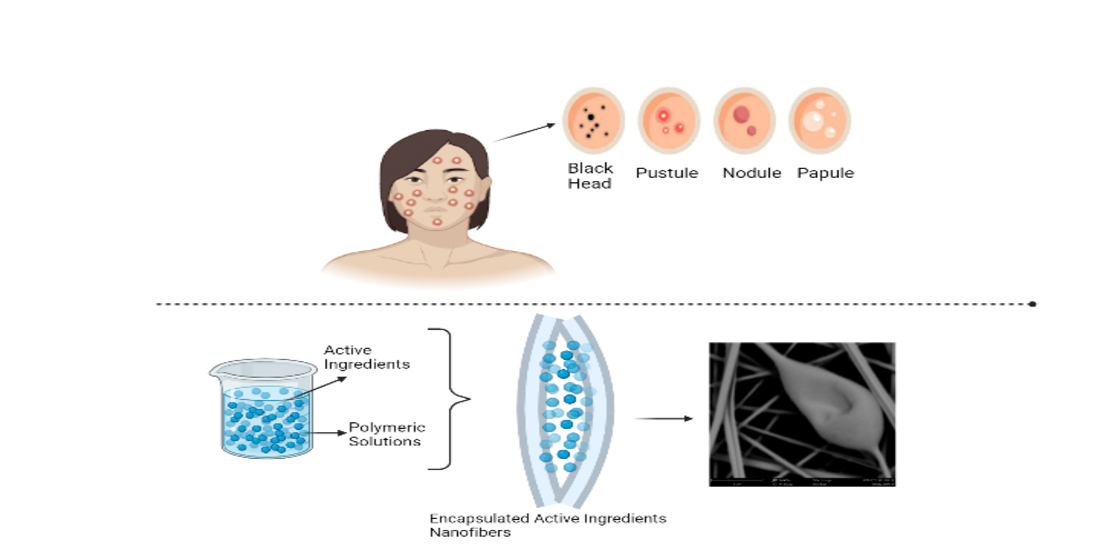
7]. Rapid dissolution and controlled release are crucial for developing innovative drug delivery systems. Conventional methods like tablets and capsules have limitations, such as first-pass metabolism and irritation. To overcome these issues, drugs can be administered directly into the buccal cavity, embedding them in nanofibers. Electrospun nanofibers dissolve quickly with saliva or disintegrate, releasing drugs into the buccal mucosa for rapid absorption. Water-soluble polymers and a large surface area exposure to the solvent enable this. Controlled-release formulations disrupt the drug delivery mechanism within a specified timeframe, improving patient compliance by reducing the risk of dangerous plasma peak concentrations associated with frequent dosing [
8,
9]. The loading of active pharmacological ingredients by electrospinning is an alternative route for such releases because it creates ultra-micro to nanometre diameter fine fibres with an ordered surface shape. These fibres are formed by applying a high electric field to the desired polymer solution or, if the polymer does not have a good solvent, by dissolving the polymer and exposing it to an electric field [
10]. These outstanding properties, e.g., stiffness and resistance to traction) make polymer nanofibers the optimum candidates for many important applications in biomedical areas [
9].
1.1. Drug Delivery into Tissue Engineering
Tissue and organ failure can become a significant problem as the population ages and increases. Surgical treatment, artificial prostheses, mechanical devices and, in some cases, pharmacological treatment [
11] are part of the treatment [
12]. Despite significant progress in the field of organ transplantation in recent years, there remain several challenges and unresolved issues. For instance, organ transplants continue to face a critical shortage of donors, and recipients often require lifelong immunosuppression to prevent rejection. As the population ages and grows, the incidence of tissue and organ failure is expected to rise. To address these challenges, a comprehensive approach to treatment includes surgical repair, the use of prostheses, mechanical devices, and pharmacological interventions [
12]. Aims to repair and/or replace tissues and organs using cells, biomaterials and biologically active chemicals alone or in combination [
13]. Depending on the tissue or organ to be repaired, one of these components will predominate, but often a combination of two or more components is required. The main target of tissue engineering is, therefore, to restore, preserve or improve tissue and organ function [
12]. This multidisciplinary field combines engineering, biology, and medicine. The phrase tissue engineering first appeared in the late 1980s [
14]. For approximately three decades, scientists have been exploring the potential of cell-based therapies to restore functional tissues. Regrettably, the literature has documented limited success in cell-based therapy, primarily due to the low survival rate of transplanted cells during the initial days post-transplantation. To address this issue, researchers have attempted to improve cell survival and differentiation by providing them with growth factors. However, the evidence of effectiveness for this approach remains insufficient.
This suggests that the ideal tissue engineering product should incorporate a biodegradable polymer support structure within which cells with the appropriate phenotype are embedded. Additionally, the inclusion of suitable drugs, such as growth factors to enhance cell survival and differentiation, is crucial.
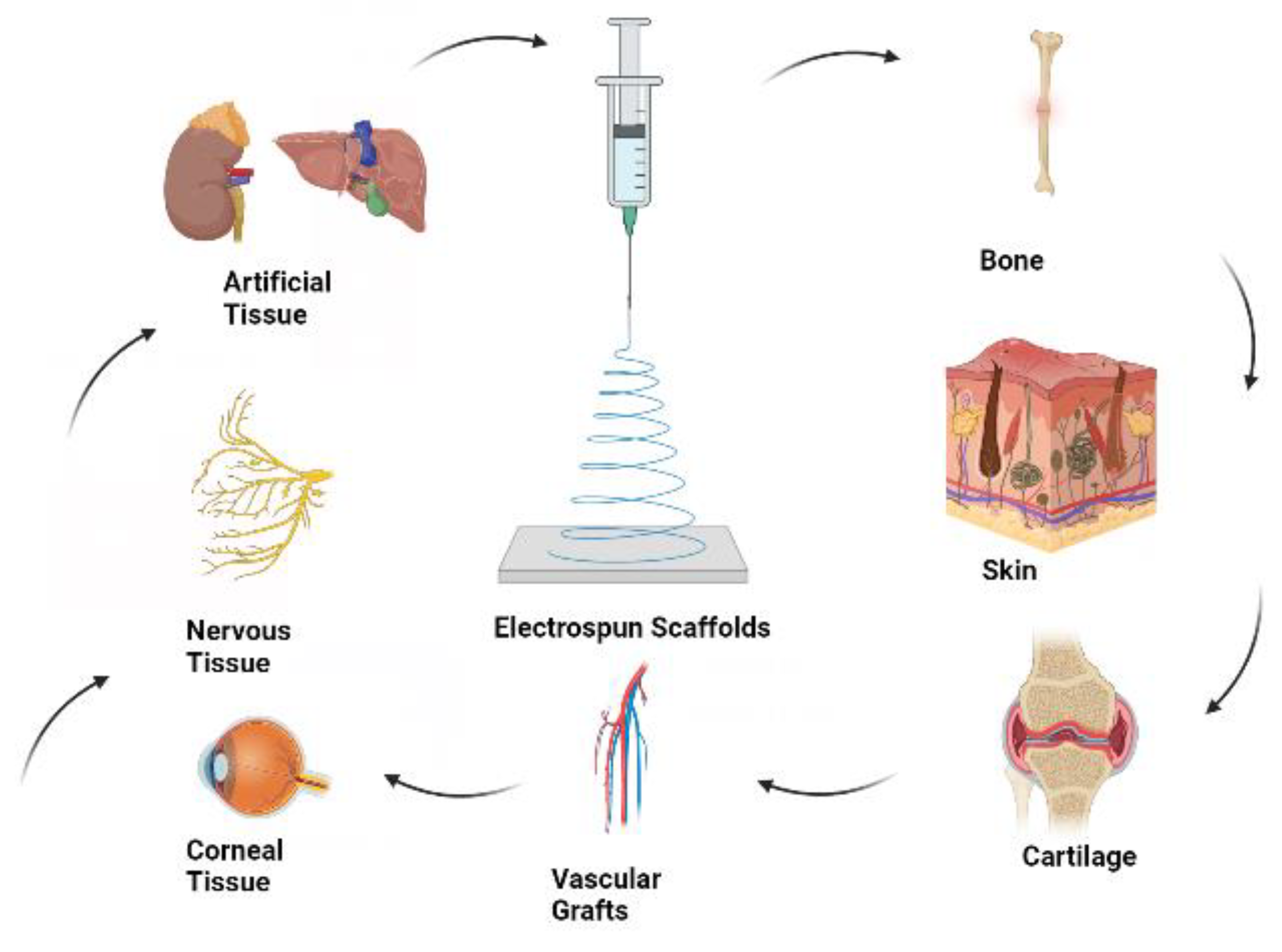
Figure 1 illustrates the fundamental concept of tissue engineering with electrospun scaffolds. The concurrent development of artificial materials using both natural and synthetic components has played a significant role in the early advancement of tissue engineering [
4].
2. Electrospinning and Application on Drug Delivery Systems
Electrospinning is a technique of fibre production using electrostatic forces to form fibres of 3 nm to 5 μm in size from a polymer solution.
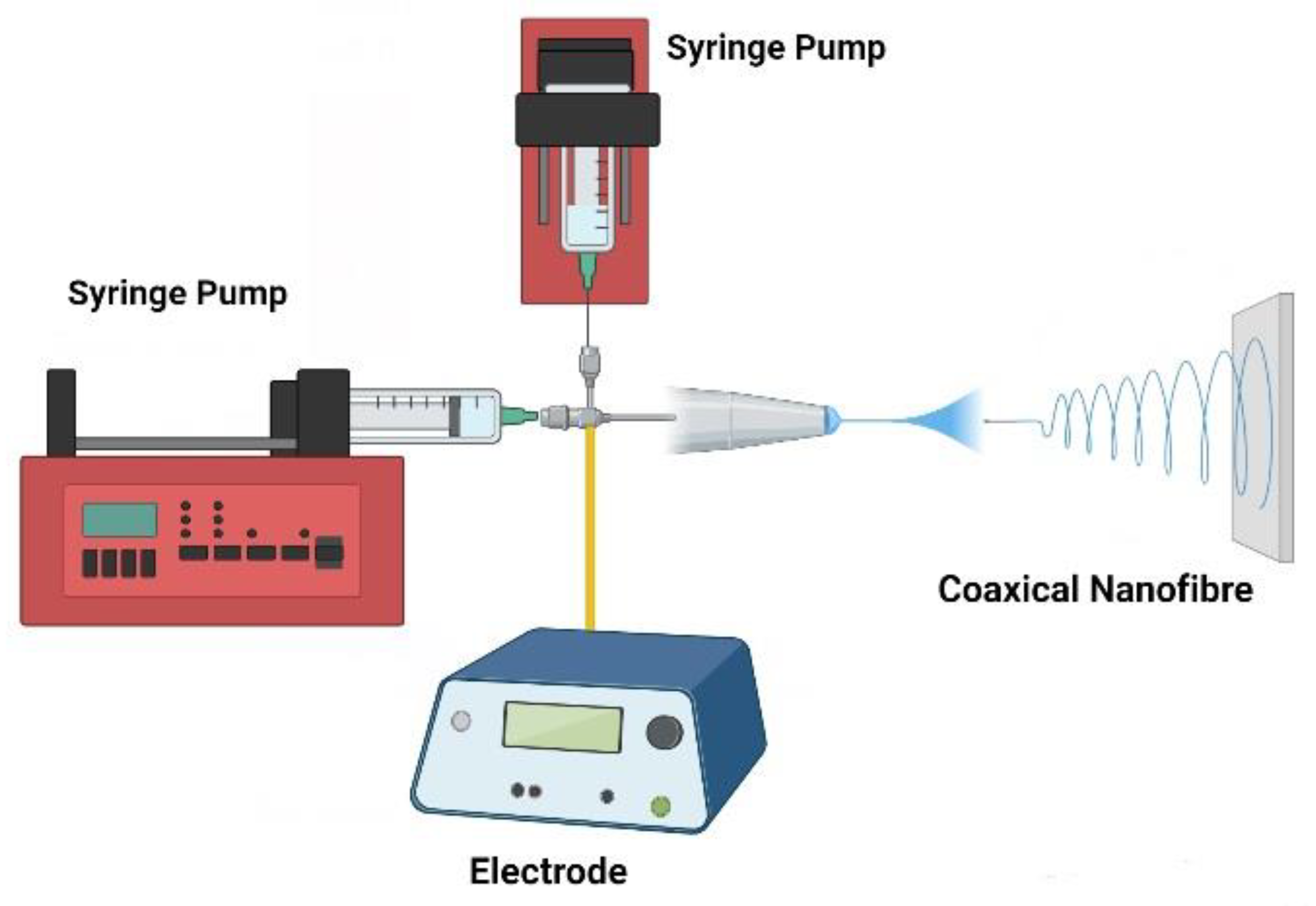
Figure 2 depicts a basic setup for the coaxial electrospinning schema, as well as what sort of chemical and biological components may be present inside the nanofibers. A conductive collector, a polymer syringe pump, and a high-voltage source are the three essential components of this approach [
10].
In drug delivery, a specific quantity of the drug is mixed with a polymeric solution, resulting in the formation of a homogeneous suspension or solution. The choice between suspension or a single component formulation depends on the solubility of the drug. The next step involves utilizing the electrospinning technique to produce nanofibers comprised of this combination of polymer and drug.
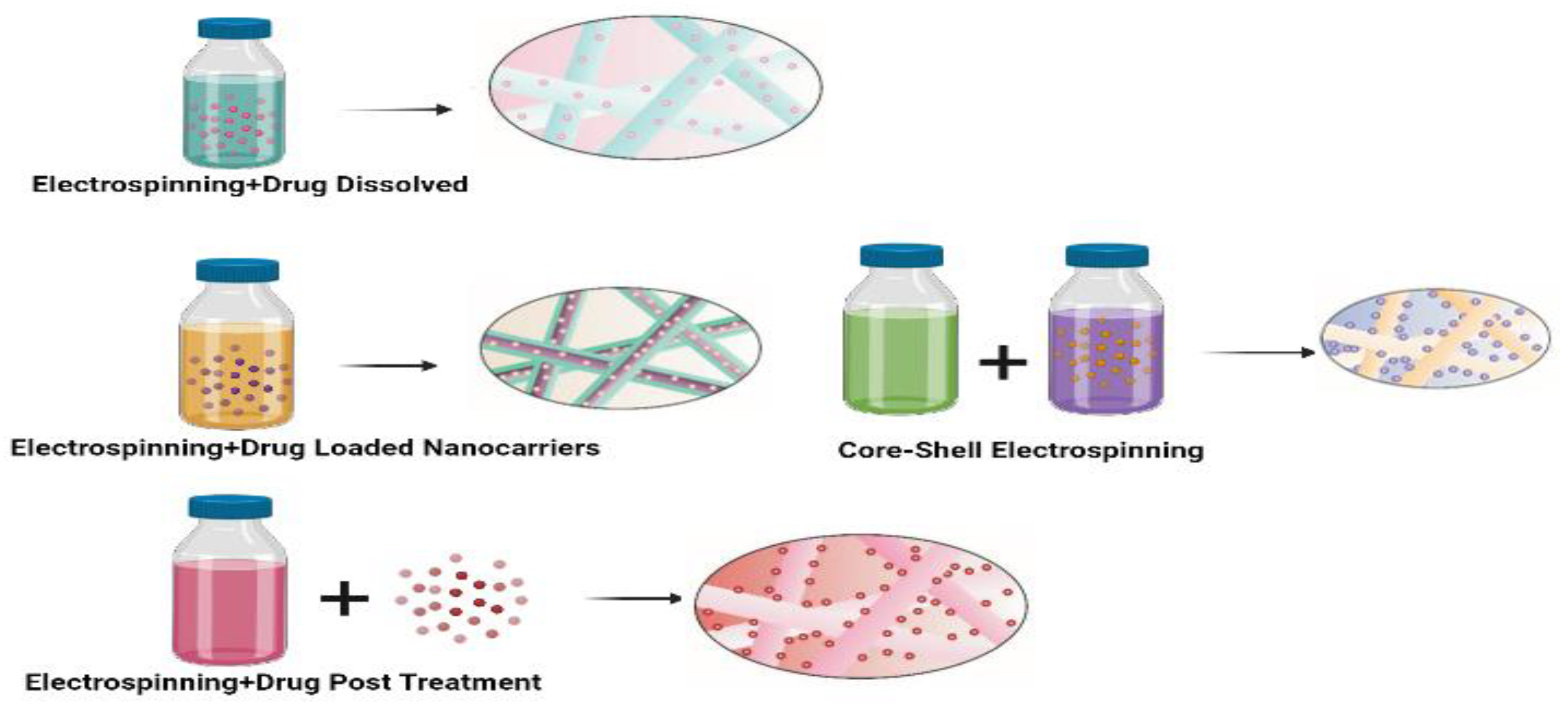
Figure 3 illustrates the structure of nanofibers loaded with both polymer and the drug.
During this process, the solvent is evaporated, leaving behind the nanofibers. Depending on the specific electrospinning method employed, various types of nanofibers can be created. These nanofibers can be composed of bioactive molecules such as drugs, proteins, hormones, or they may contain another polymer, albeit in a smaller proportion compared to the primary polymer, with the majority of the fibre structure being formed by the base polymer [
15]. Nanofibers derived from biopolymers possess the capability to transport bioactive molecules effectively into tissues and cells. Additionally, biopolymer-based nanoparticles can be precisely customized in terms of their size, ensuring prolonged sustainability. This quality makes them an extremely attractive resource for researchers exploring the development of therapeutic interventions.
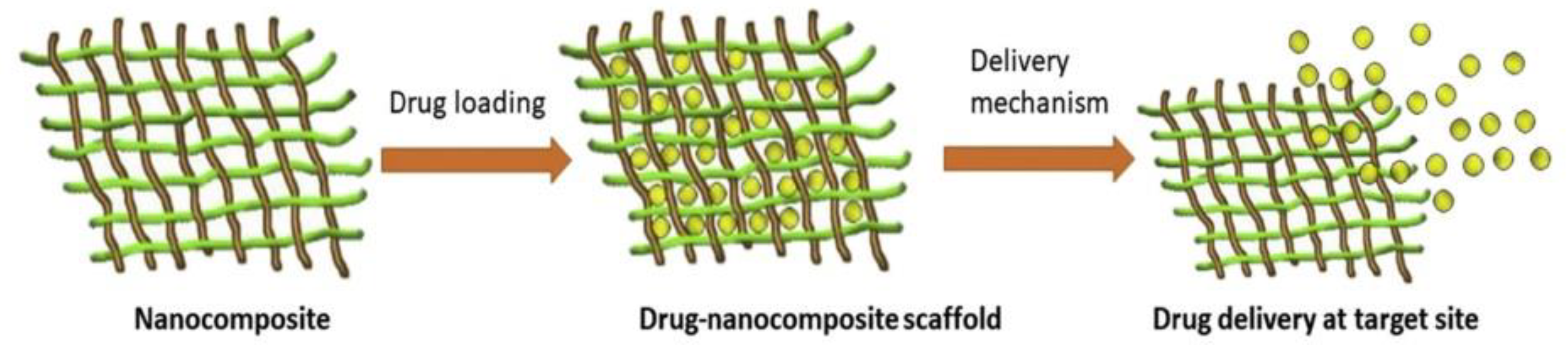
Figure 4 provides a schematic depiction of the drug delivery system and how drugs are loaded into nanocomposites [
16].
The process begins by transferring the polymer solution into a syringe, which is then driven forward by an electric force. At the tip of the needle, a droplet of the polymer solution forms, and a high voltage, typically ranging between 5 and 50 kV, is applied to it. This high voltage electrifies the droplet, evenly distributing induced charges across its surface. When the voltage difference between the electrically conductive liquid and an insulator exceeds a specific threshold, the liquid drop undergoes a transformation. It transitions from a spherical shape to a conical one due to its instability under the electric field [
17]. When the voltage surpasses the critical point, the electric force becomes stronger than the surface tension of the liquid droplet. Depending on the magnitude of the electric field, this can result in the release of one or more jets from the initial point of the droplet. As these jets progress toward a collecting plate, often made of a conductive material like aluminum, the solvent within the jets evaporates. This process leads to the formation of a nonwoven scaffold on the collecting plate [
18,
19].
Research into drug delivery strategies utilizing nanoscale formulations like liposomes, polymer micelles, specific complexes, and nanofibers has gained significant attention in recent years. This heightened interest stems from their potential to enhance therapeutic efficacy while reducing the toxicity associated with traditional pharmaceutical formulations. These drug delivery systems offer several advantages, including high encapsulation efficiency for both rapid and sustained release, applicability in areas such as wound dressings and localized chemotherapy, the capability to load a maximum amount of medication, the ability to simultaneously administer multiple treatments, precise controllability over drug release rates, and cost-effectiveness.
In comparison to other drug delivery systems, electrospinning technology stands out for its versatility in releasing a wide variety of materials and Active Pharmaceutical Ingredients (APIs). What sets electrospinning apart from previous methods is its unique ability to allow researchers to finely regulate the rate of disintegration and drug release [
8,
20].
3. Natural Polymers
Polymers have had a considerable impact on speciality medical applications, and they might still be crucial personnel for biomaterials [
21]. The knowledge of organic reactivity to current biomaterials and a better understanding of human organ structure, function, biomechanics and disease aetiology [
22]. Chemists and chemical compound scientists should continue to collaborate with biologists, medical scientists and engineers to create tailored polymers for biomedical purposes [
23]. In contrast to the previously inert artificial polymers, the focus will be on bioactive, biomimetic and sensitive polymers [
24]. Likewise, because the natural system's relationships with polymers do at the interface [
25], surface-related analysis can still thrive, particularly surface characterisation and surface revision [
26]. The easy integration of biomaterials into the body is promising for improving treatment. [
27,
28]. Natural polymers risk addition operations in the area of bone relief and hard tissue addition [
29]. Basically, the materials used for this purpose must be biocompatible, correspond to the three-dimensional structure of bone and difficult tissues, be able to mimic their physical and mechanical nature, be ready to support appropriate cellular functions and cell mechanism, and be gradually replaced by new tissue [
30]. A number of natural polymers are used, along with extracellular matrix proteins that value collagen; Polysaccharides such as chitosan, alginate, starch and cellulose and glycosaminoglycans such as hyaluronic acid [
31,
32]. Some natural polymers provide a model for biomimetic mineral conditions that are highly desirable to induce rapid bone colonisation [
33]. Both degradable and non-degradable microorganisms usually make up the composite [
34,
35]. The hydroxyapatite present in the composite contains non-stoichiometric nanoscale needle-like particles similar to those found in human bone. The combination of bioactivity and biocompatibility supports the potential of the resulting composite structure for medical science applications [
36].
3.1. Common Biopolymers Used in Drug Delivery Systems
3.1.1. Polysaccharides
Carbohydrates occur in nature mainly as polymers rather than monomers [
37]. In this environment, polysaccharides are the thickest supramolecular emulsion. These polymers harbour ten or more sugar units. They consist of light sugars guaranteed by glycosidic bonds and thus have a molecular abundance of up to a hundred million mass units [
38,
39]. The best-performing polysaccharides are decided by the monomers [
40]. Contains or the style in which they are coupled together.
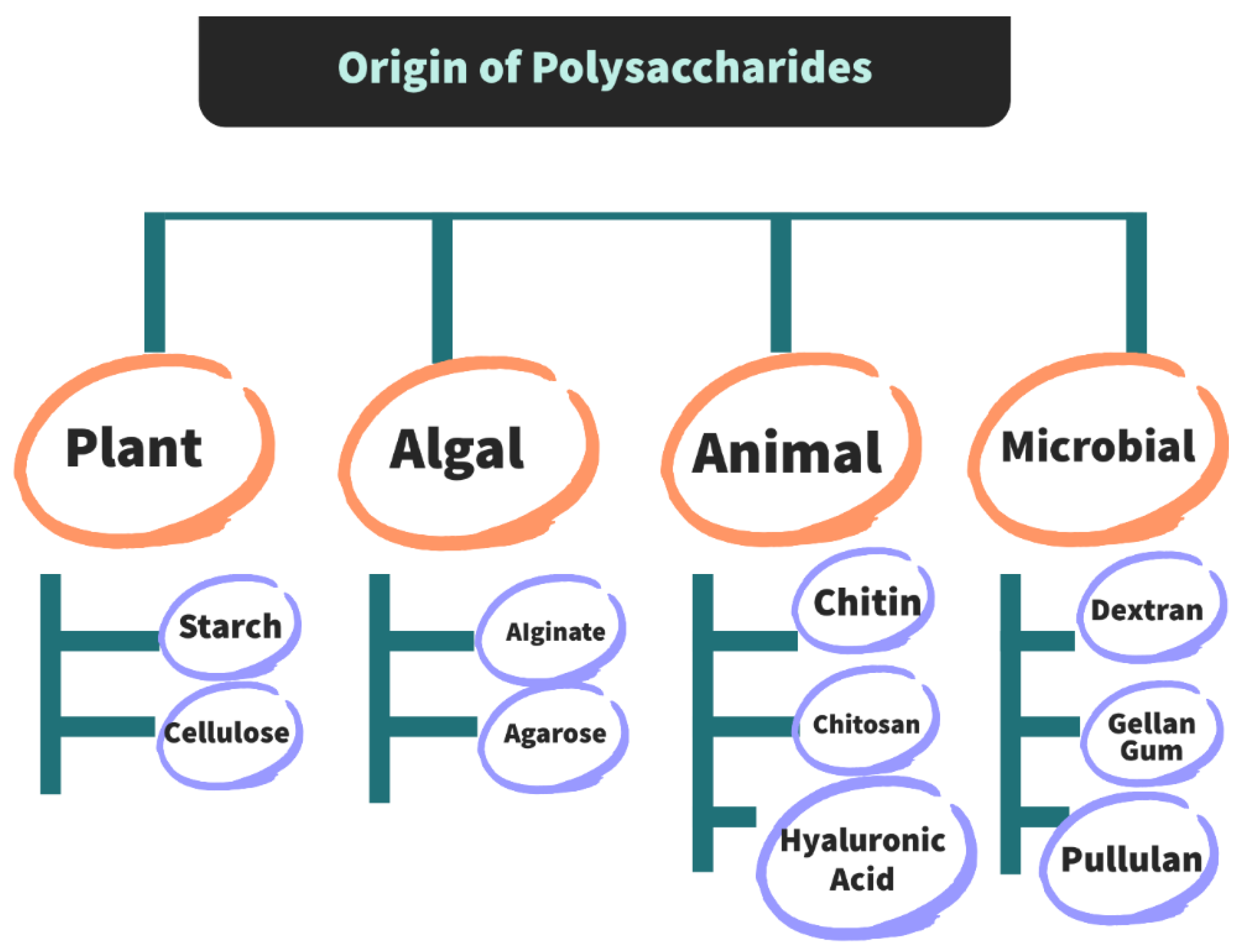
Polysaccharides present in plants, animals and microorganisms are mainly responsible for their structural and functional roles [
41]. The origin of polysaccharides is shown in
Figure 5. These function as storage polysaccharides, while some provide structural support and are known as structural polysaccharides. Structural polysaccharides are mainly based on polysaccharides found in invertebrates. [
42].
Microbial-sourced polysaccharides created by supported developments in biotechnology are widely employed in varied industrial applications due to their superior properties. one of these polysaccharides is pullulan [
43]. The classification according to their origin, functions and presence of monosaccharide units is given in
Table 1.
3.1.2. Chitosan
A considerable sum of work has been done researching chitosan and its possible use in tissue engineering and other medicinal applications [
45]. It is generally known for its comparative structure to naturally occurring glycosaminoglycans (GAGs) and its ability to be degraded by enzymes in humans [
46]. Natural biopolymers are frequently found in the exoskeletons of arthropods, the cell walls of animals, and the shells of marine crustaceans. [
47].
Chitosan has been tested to be bio-renewable, biodegradable, bio-adhesive and biocompatible and has been used in wound dressings and healing, drug-loaded delivery systems and various tissue engineering applications [
48]. Chitosan could be a semi-crystalline polymer, and therefore the degree of crystallinity is based on the deacetylation level. Minimum crystallinity is accomplished at intermediate degrees of deacetylation [
46].
The typical course of the healing response involves the formation of traditional connective tissue, which is associated with accelerated angiogenesis, contributing to tissue repair. This immunomodulatory effect is believed to promote the integration of the implanted material by the host. Chitosan, owing to its promising properties, has gained attention in tissue engineering, with a particular focus on tissues such as bone, cartilage, and skin. However, chitosan has also found applications in other tissues like the liver and trachea, serving as a scaffold to support temporary cell functions. Chitosan's versatility makes it suitable for various forms, including porous scaffolds, injectable gels, membranes, tubular systems, and particles, thanks to its ease of processing. Chitosan scaffolds, particularly those designed for bone tissue regeneration, have been extensively studied and demonstrated to enhance bone formation both in vitro and in vivo. This improvement is particularly notable when chitosan is used in combination with other polymers like gelatin and alginates [
49,
50,
51,
52].
Chitosan is a natural polymer commonly used in tissue engineering. Its chemical versatility allows for the predictable manipulation of pore sizes and degradation rates in structures, making it a valuable material in this field. Chitosan also exhibits good biocompatibility, drug delivery capabilities, and the ability to bind to growth factors. These qualities make it a promising candidate for a wide range of tissue engineering applications [
52].
3.1.3. Cellulose
Cellulose is the constituent of plant cell walls. It moreover constitutes the most abundant, renewable polymer resource accessible nowadays, existing basically in lignocellulosic material in forests, with wood being the essential source. This here is the main structure polymer consisting of up to 15,000 D-glucose buildups connected by β (1 →4) glycosidic bond [
52]. The chain structure is stabilised by the totally equatorial conformation of crosslinked glucopyranose residues, minimising its flexibility. Cellulose's unique qualities of mechanical strength and chemical stability result from its ability to hydrogen bond into fibres (microfibrils). It is also leading to insoluble materials with small degradability in vivo [
53]. Cellulose's biodegradability, if it occurs at all, is constrained due to the absence of hydrolases that target the β (1 →4) linkage. The combination of this fact and the difficulty of processing make cellulose unsuitable for tissue engineering [
54].
Cellulose and its variants have been developed. They were employed successfully as biomaterials, and there are some indications that they could be a good material for use in tissue engineering. In orthopaedic applications, it has been demonstrated cellulose sponges may support bone tissue ingrowths, recommending that they could be used in bone tissue engineering [
54,
55]. Cellulose and cellulose acetate scaffolds were also shown to be interesting for cardiac tissue regeneration, as they could advance cardiac cell development and improve cell connection and electrical functioning [
54]. Takata and co-workers compared different controlled bone regeneration membranes and, among other materials, cellulose which also has the capability of inducing cell migration [
56].
3.1.4. Starch
Plants store starch in the form of a network of glycogen in their chloroplasts, mainly found in their leaves, as well as in amyloplasts within storage organs like seeds and tubers. Interestingly, even though there are various sources of native starch in different plants, the majority of starch consumed worldwide is derived from a select few crops. These primary sources include corn, potatoes, wheat, and tapioca. Additionally, smaller quantities of starch come from crops such as rice, sorghum, sweet potatoes, and arrowroot [
57].
This natural polymer has garnered significant attention as a replacement for synthetic polymers across various applications. This popularity primarily stems from several key factors: it is the most cost-effective biopolymer, it is entirely biodegradable, and it is readily accessible. One of the most important characteristics of native starch is its semi-crystalline nature. The degree of crystallinity in native starch can vary based on factors such as moisture content and the source. While both amylose and amylopectin contribute to starch's crystalline structure, amylopectin is typically the dominant component. Despite the branched structure of amylopectin, double helices connect the branches together. Starch granules consist of alternating layers of crystalline and amorphous material [
58].
The biocompatibility and non-immunogenicity of starch-based polymers have been extensively demonstrated through numerous in vitro and in vivo studies. These advantages have led to the exploration of starch-based polymers for a wide range of biomedical applications. Some of the notable applications include partially degradable bone cements and controlled release systems [
59,
60].
3.1.5. Hyaluronan
Hyaluronan, also commonly referred to as hyaluronic acid, is a naturally occurring polymer that is highly hydrophilic. It plays a crucial role in the extracellular matrix (ECM) and various tissues in the body. Hyaluronan is categorized as a glycosaminoglycan, and it is synthesized as a large, negatively charged, linear polysaccharide with varying chain lengths, typically ranging from 2 to 25 meters. It consists of repeating disaccharide units [
61].
Hyaluronan-based polymers, when crosslinked, often form water-insoluble gels or hydrogels, a field that holds significant unexplored potential. Hyaluronic acid and its derivatives exhibit a wide range of characteristics that make them suitable for use as scaffolds in tissue engineering. These materials can take on various forms, including gels, sponges, fibers, films, and microparticles. They meet several requirements necessary for effective tissue engineering scaffolds and also find applications in drug delivery systems [
62].
3.1.6. Proteins
Proteins are organic molecules with a wide range of biological functions, including transport, regulation, protection, structural support, and catalyzing various reactions. They consist of 20 amino acids linked together through amide (or peptide) bonds, forming the main structure of a protein. Amino acids have a central carbon atom linked to amine groups, carboxyl groups, hydrogen atoms, and unique side chains called R groups. R groups are categorized as nonpolar, uncharged, or charged polar groups based on their distribution along the protein backbone. The secondary structure of a protein refers to the initial spatial arrangement of the backbone atoms, without considering the side chain conformations [
13].
Considering the low stabilities of protein conformations, these molecules are effectively susceptible to denaturation by changing the adjustment of the weak interactions that keep up the native conformation. Proteins can be denaturised by a variety of conditions and substances such as heating, extreme pH, chaotropic agents, detergents, adsorption to certain surfaces, etc. [
63].
3.1.7. Collagen
Collagen is a vital macromolecule found in various tissues such as the cornea, blood vessels, skin, cartilage, bone, tendons, and ligaments. It plays a central role in the extracellular matrix (ECM) and is responsible for maintaining the structural integrity of vertebrates and many other organisms. In addition to its structural role, collagen also plays essential functions within the cellular microenvironment and is involved in storing and releasing cell mediators like growth factors. Commercially, collagen is primarily sourced from animal tissues, including porcine and bovine skin, bovine connective tissue, and rat tail, among others. It can be extracted from these tissues using enzymatic treatment and salt/acid extraction methods. However, while collagen holds promise for various applications in tissue engineering, there are limitations to its widespread clinical use. These limitations include its relatively low mechanical properties, the potential for viral contamination, antigenicity concerns, and rapid biodegradation when implanted in the human body [
64].
3.1.8. Elastin
Elastin is another of the critical structural proteins found in the extracellular matrices (ECM) connecting tissue (such as well as blood vessels skin) that must stretch and retract in response to mechanical loading and release. It is mostly present in the vessels' walls, lungs, intestines, skin, and other elastic tissues. In any case, in contrast to type I Collagen, Elastin has less use as a biomaterial. These two main reasons: [
64].
It is found preponderantly within the arteries, lungs, intestines, and skin walls, likewise as different elastic tissues. However, not like-kind I scleroprotein, albuminoid has found very little use as a biomaterial, thanks to 2 main reasons [
59].
Elastin purification is a difficult process. The insoluble nature of elastin also limits its use in conventional matrix manufacturing techniques. [
65].
3.1.9. Silk Fibroin
Fibrin is a protein complex primarily composed of amino acids such as serine, glycine, and alanine, making up approximately 90% of its composition in commercial silkworms. Fibrin plays a crucial role in forming antiparallel crimped layers within silk fibers. Silk scaffolds produced through various methods offer specific silk fibroin concentrations, porous structures, and mechanical properties [
66].
4. Biocompability of Natural Polymers
The growing emphasis on quality of life and well-being has contributed to the advancement of biotechnology. Biomaterials have gained importance in meeting the increasing demands for tissue replacement and regeneration. When biomaterials are implanted, they can elicit immune responses ranging from mild inflammation to significant tissue damage, ultimately leading to implant rejection [
52,
64]
The type of host tissue reaction following implantation of biomaterials depends on the surface physical and chemical properties of the nanofiber and device, as well as the type of tissue involved, the mode of treatment and the overall host cell status [
67].
5. Drugs Loaded into Electrospun Nanofibers
Electrospun fibres are becoming extremely popular due to their significant surface area, the possibility of loading large amounts of drugs, the simultaneous application of various treatments, cost-effectiveness and ease of use. Tissue repair and localised cancer therapies are among the most popular research areas with drug delivery by rapid degradation or controlled release. Some of the drugs used in electrospinning are as follows.
5.1. Anti-Inflammatory Drugs
Pharmaceuticals with anti-inflammatory properties are substances that minimise inflammation-related symptoms and may also have both analgesic and antipyretic effects [
66]. Since they offer practically instant relief and are not easily soluble in water, a number of these pharmacological compounds have been employed in the electrospinning process to create alternative delivery systems. According to studies, electrospinning nanofibrous materials are typically created in conjunction with an anti-inflammatory and another medicinal ingredient (most commonly analgesics) in order to have a better treatment, as can be seen from the graph below. [
9,
69].
Yu et al. reported using an IBU-loaded PVP K30 electrospinning material (2009). Either solid dispersions or amorphous physical forms of the polymer solutions were employed. The first publication demonstrated that IBU was dispersed as nano solid dispersions in the fibres using X-ray Diffraction (XRD) data, morphological observations, and Diffraction Scattering DSC Calorimetry. The predominant interactions between PVP and IBU were demonstrated by Fourier Transform Infrared Spectroscopy (FTIR) to be hydrogen bonds-mediated. The wetting and disintegration periods of drug delivery membranes with various drug concentrations were almost the same, but the dissolving rate varied significantly because the drug had various physical states. The initial 20 seconds [
70].
PCL was employed as a polymer matrix by Canbolat et al. (2014) to make a drug-loaded NP mechanism. Beta-cyclodextrin (BCD) and the medication were mixed to create an inclusion complex, which was then electrospun. The fibres had a diameter of around 300 nm, as determined by SEM. The polymer matrix for the development of a nano drug delivery system was PCL. Beta-cyclodextrin (BCD) was complexed with the medication to create an inclusion complex, which was then electrospun. The fibres had a diameter of around 300 nm, as determined by SEM. Because the complex improves the medication's solubility, the complexed drug in the fibres released more than the un-complexed drug [
71].
In order to create NP-loaded PVP nanofibers, Wu et al. (2014) demonstrated effective coaxial electrospinning nanofibers. Ethanol serves as both a shell solvent and a component of the PVP solution, making it the sole solvent available. According to Field Emission Scanning Electron Microscopy (FESEM), this method creates exceptional quality nanofibers with a diameter of 270 nm, linear shape, no beads or spindles, and a smooth surface. The amorphous nature of the nanofibers and the homogenous distribution of the drug throughout the polymer were both confirmed by the authors using XRD patterns. Pharmacologically active nanofibers can release the drug quickly and all at once after coming into contact with the dissolution medium, which is far faster than commercially available dispersible tablets, according to in vitro dissolution trials [
72].
5.2. Antimicrobial Drugs
Antimicrobials consist of a large number of compounds with different structures and mechanisms of action against bacteria (antibiotics), viruses (antivirals), fungi (antifungals) and parasites (antiparasitic); however, some generalisations can be made regarding concerns about the use of such drugs [
73,
74]. As an example, GRIS is used in the treatment of dermatophytosis; onychomycosis, nail; onychomycosis, toenail; tinea barbae; tinea capitis and various antifungals belong to the drug class. This medicine has no effect on bacteria or other fungi. Although ringworm lesions rarely do not improve, the strains of these patients are usually still susceptible to GRIS in vitro. The risk during pregnancy cannot be ruled out. It is not a controlled substance under the Controlled Substances Act (CSA) [
75].
Buschle-Diller et al. (2007) reported that electrospun nanofiber TCN produced with chlortetracycline hydrochloride and AMB is another substance with antibacterial properties. These experiments were carried out to investigate the release properties and antimicrobial activity of the model drugs. It was produced by coaxial electrospinning. TCN was released at a higher rate than PCL, while AMB was released more slowly. In dissolution tests, the PCL drug load achieved almost complete release over time, while PLA released only about 10% of the total loaded drugs. The researchers stated that by creating PCL/PLA composite fibres, the surface and release properties could be modified to suit a precise drug-loaded delivery system. The researchers discuss that biocompatible nanofibers that can adapt to the physiological parameters of body tissue have become increasingly important for therapeutic applications over the past decade. Due to their large surface area, absorption and release properties, electrospun fibres provide special advantages, and when loaded with drug substances, a future rapid delivery can be achieved by modifying their delivery properties, making it a versatile technique for loading these compounds [
76].In this study, coaxial electrospinning was used to produce core-shell fibres for a drug delivery application, PLLA was used as the shell structure and TCH as the core material. The authors performed SEM, TEM, DSC and a tensile test to characterise and investigate the usability of the obtained nanofibers as drug delivery vehicles. In vitro drug release behaviour was also studied by UV-VIS spectroscopy. The results of the study showed that the drug delivery device could be suitably obtained for the encapsulation of TCH in PLLA fibres, thereby achieving the prolonged release of TCH [
77].
Huang et al. (2006) argue that despite advances in modern medicine, acquired immunodeficiency syndrome (HIV) continues to impair the health of large numbers of people worldwide, and significant attempts have been made to identify strategies to prevent infection or eliminate the virus after transmission. The authors discuss the possible use of CAP electrospun fibres containing TDF as a method for HIV prevention. It is stated that the fibres are stable in vaginal fluid due to the pH-dependent dissolution of CAP (the pH of healthy genital flora is below 4.5), but when small amounts of sperm cells are added (pH between 7.4 and 8.4), the fibres immediately dissolve, resulting in encapsulated drugs [
78].
Yu et al. (2011) introduced a core-shell-shaped solid dispersion electrospinning method for poorly water-soluble drugs. The authors used sucralose as a sweetener, sodium dodecyl sulphate as a permeation stimulant and PVP, a hydrophilic filament-forming polymer, as a model pharmaceutical matrix. The core-shell nanofibers formed by the authors were made of PVP and ACV for the core and SDS and sucralose for the shell. The authors claimed that a homogeneous structure and an average fibre diameter of 400-90 nm were present. According to the authors, ACV, SDS and sucralose were evenly distributed throughout the PVP matrix. DSC and XRD results confirmed this and showed that second-order interactions were involved. According to in vitro dissolution and permeation studies, solid dispersions of seed coat nanofibers were found to be able to release ACV within one minute with a permeation rate six times higher than that of crude ACV in sublingual mucosa. The researchers stated that this study is an example of the design, production, characterisation and application of a new type of solid dispersion with a wide range of components and structural properties [
79].
Nonbiodegradable drug-loaded nanofibers for topical medication administration and wound healing were created using electrospinning. The research's specific objective was to ascertain whether these techniques might be effective as water-strapped delivery systems. The polymeric substance was polyurethane (PUR), and the model compounds ITR and ketanserin were picked. For each of these medications, the authors used Ketanserin, dimethylacetamide, and dimethylformamide (DMF) to make an amorphous nanodispersion of ITR (DMAc). It was determined that, depending on the structure of the nanofibers and the drug concentration, the gathered fibres release pharmaceuticals at various rates and patterns. With the aid of a specially created release mechanism cantered around a rotating cylinder, the data were gathered. The drug initially had no immediate release, making it suitable for sustained release [
80].
5.3. Anticancer Drugs
Cancer is a category of disorders that causes abnormal cell formation and has the ability to spread to other regions of the body. DOC is a well-known oral cancer mitotic inhibitor. Extravasation, vein irritation, and other chemotherapy side effects are issues. The scientists' goal was to create a mucoadhesive nano-carrier system that remains at the site of application and minimises side effects while increasing the therapeutic potential of the anticancer drug. The electrospinning process was utilised in the research to create DOC-PVAL fibres. To determine their therapeutic potential, the resultant fibres were evaluated using characteristics such as surface shape, drug content, in vitro release, elastic modulus, mucoadhesive and drug permeability, degree of swelling, and anticancer activity against certain cell lines. The findings were promising, and it was determined that the present technique based on polymeric nanofibers might be utilised to successfully administer anticancer medicines locally [
18].
Zhang et al. (2014) Cisplatin is another cancer-treatment chemical that has been reported (CP). CP has been used to treat liver cancer but has the disadvantage of accumulation in this organ and low drug uptake after intravenous administration. The research focuses on making chemotherapy less aggressive for the patient's liver using an electrospun system. The system consists of five layers, with the first, third- and fifth layers being polymer (PLA) and the second and fourth layers being drug. The rationale for this conformation was to have prolonged cisplatin release and prevent local cancer recurrence after surgical resection. In vivo investigations over 24 hours revealed that the multilayer fibre mat had a longer release and much more stable retention in the tissue. In mouse tests, the scientists discovered that liver cancer was delayed, mice lived longer, and there was less damage when compared to other groups receiving alternative therapies. This study shows us the potential of electrospun fibres to have adverse effects on some APIs. In addition, DOX is a chemotherapy drug used in many types of cancer. It can be used in leukaemia, lymphoma, various carcinomas (solid tumours) and soft tissue sarcomas [
81].
The electrospinning of DOX-loaded poly(ethylene glycol) (PEG) fibres has been extensively investigated. The polymers used in the paper (PEG/PLLA) were dissolved in chloroform, forming the oily phase, while DOX was present in the aqueous phase. The aim was to encapsulate the drug into fibres within the oil phase. The diameter of the electrospun fibres ranged from 300 nm to 1 m. The DOX content in the fibres was between 1-5 wt% and was completely encapsulated in the fibres. The release was controlled by diffusion and enzymatic degradation mechanism. The anti-tumour effects of DOX incorporated into PEG-PLLA fibres against glioma cells (C6 cell lines) of mice were evaluated by the MTT method. The results showed that DOX could be released from the fibres without losing its cytotoxicity, and thus the system may be of interest for further studies [
82].
(2013) studied the release behaviour of drug-loaded fibres made via an emulsion. DOX was used as a model drug. Confocal microscopic images of laser scanning showed that the drug was incorporated into PEG/PLLA copolymer nanofibers, forming drug-loaded structural fibres. The drug release behaviour of this system showed a three-stage diffusion-controlled mechanism in which the release rate of the first stage was slower than that of the second stages, but both obeyed Fick's second law. According to the findings, DOX-loaded fibres made by emulsion electrospinning can be a reservoir-type delivery system and the DOX release rate decreases as the DOX concentration in the fibres increases [
83].
5.4. Cardiovascular Drugs
Cardiovascular diseases are a range of diseases affecting the cardiovascular system and blood vessels, including cerebrovascular diseases, coronary heart disease, peripheral arteriopathies, rheumatic heart disease, and congenital heart defects. Nicorandil (NICO) is a vasodilator drug used to treat angina pectoris; it is the principal therapeutic agent with the ability to hyperpolarise muscle tissues and is a potent coronary vasodilator that has been shown to be helpful in the treatment of all forms of angina pectoris, including advanced coronary artery lesions. Some diseases, such as arteriosclerosis, coronary arteriosclerosis and aortic stenosis, can cause them. Angina is usually caused by vasospasm of the coronary arteries [
84].
5.5. Antihistamine Drugs
Nanofibers have been combined with antihistamine drugs. One research focused on the use of chlorpheniramine maleate (CPM). CPM was applied to glutinous rice starch (GRS) with PVA electrospun fibres to demonstrate the concept of a drug delivery carrier and drug release control from nanofibers[
85]. LOR is used to treat allergies such as hay fever (allergic rhinitis) and multifactorial chronic urticaria, in addition to various skin allergies. LOR is effective for both nasal and ocular allergic rhinitis symptoms such as sneezing, runny nose, itching or burning eyes [
86]. The authors observed that with low polymer concentration, low feed rate and high applied voltage (30% PVP concentration in ethanol, 1:4 drug-to-polymer ratio, 10 kV voltage and 1 ml/h feed rate), smaller diameter and more homogeneous nanofibers were formed. According to the results of the report, this pharmaceutics component is released faster and dissolved more quickly as the fibre diameter and drug amount decrease [
85].
DPH, another antihistamine, reduces or restricts histamine effects on smooth muscle and immune cells because it acts as an antagonist of muscarinic receptors and adrenergic receptors. This drug is normally used for insomnia, cold symptoms and nausea [
87,
88]. DPH nanofibers were produced by electrospinning directly on a polymeric support film composed of HPMC and glycerol to develop a mucoadhesive system. Performance variables were evaluated: disintegration time, work of adhesion, adhesion strength, the position under the curve (at 1 min) and position under the permeability curve (at 3 min); these last two were used to calculate the filling volume, HPMC and glycerol concentration, which are independent variables of the application. The researchers investigated the physical, chemical and mechanical properties by the following methods: Rheology, FTIR, strength determination, mucoadhesion and nano-tensile tests. The data obtained from the physical-mechanical characterisation confirmed that it is suitable for application in the delivery of drug-loaded systems [
87].
5.6. Gastrointestinal Drugs
Gastrointestinal disease is the study of normal function and disease of the oesophagus, stomach, small intestine, colon, pancreas, gallbladder, bile ducts and liver. In addition, it is a collective term for all diseases that directly damage the digestive tract [
89]. Metoclopramide (MET) is a prokinetic drug that has an in vitro effect on the contractility of colonic smooth muscle. It has been reported that HCl is loaded into PVA/PCL core-shell fibres and these fibres exhibit an initial burst of approximately 55% of the total release. It has been suggested that the bursting effect is mostly due to the presence of micron- or nano-sized pores in the PCL shell [
90].
5.7. Palliative Drugs
Palliative medicine is a disciplined approach to specialised therapeutic care for people with serious illnesses. It focuses on providing patients with relief from the symptoms, pain, physical stress and emotional stress of a serious illness, regardless of diagnosis. The aim of this treatment is to improve the quality of life of both the patient and their family [
91]. Donepezil-loaded nanofibers were used in a study. HCl was prepared as a dosage form for oral dissolution. In this research, the authors aimed to create a removable scaffold by ultrafast oscillatory electrospinning using PVAL and donepezil as matrix polymers. The drug to be loaded is HCl. The authors found that low molecular weight PVAL gave the best results. They also found diameters between 100 nm and 300 nm by SEM. In vitro tests showed that due to the large surface area of the nanofibers, the loaded drug was released in less than 30 seconds, regardless of the drug content in the fibres. According to the authors' comparison of scaffolds and conventional tablets, electrospinning technology is a potential strategy for the production of alternative effective dosage forms, especially for patients with swallowing difficulties, children and the elderly [
92].
5.8. Contraceptive Drugs
Oral contraceptives have been used to prevent pregnancy. Oestrogen and progestin are two female sex hormones that, in combination, prevent ovulation. They also change the lining of the uterus (womb) to prevent the development of pregnancy and change the cervical mucosa to prevent sperm entry [
86]. (2014) fabricated electrospun PVAL nanofibers with various microscale shapes for the delivery of levonorgestrel, a progestin. The authors investigated the composite materials and studied the release kinetics of the drug in addition to measuring its cytotoxicity. In their results, they report processing settings for electrospinning of PVAL/levonorgestrel solution as well as for electrospinning of free surfaces of medical tissues characterised by regulated microarchitecture in addition to significant drug loading up to 20 wt%. The in vitro release of levonorgestrel was found to be influenced by fabric thickness, composite microarchitecture and drug content, thus achieving the researcher's objectives and optimising the electrospinning process [
93].
6. Conclusions
This study examines the many ways to produce nano-biopolymer-based drug carriers, including existing technologies such as electrospinning and the many types of drug species used in this field. Crosslinking and swelling properties can be tailored by changing the ratio of biomaterials to modifying materials. Although much research on drug delivery systems using natural nanomaterials has been published recently, some critical issues need to be addressed. First, most of the studies are in the early stages of in vitro, and it is critical that the methods are tailored to meet different demands. Different nanomaterials from different origins are likely to alter degradation and drug-loaded release kinetics. Such issues can be addressed by using genetically recombinant nanomaterials for drug-loaded delivery systems instead of using natural ones. These genetically modified and surface-modified nanomaterials can improve the therapeutic efficiency, specificity and functionality of drug-loaded delivery systems, such as tumour targeting and non-toxicity. Protein therapies have similar challenges, such as limited bioavailability and metabolic liability. Peptides are readily degradable in the gastrointestinal tract; therefore, drug delivery via oral administration is limited, and proteins have less ability to cross the epithelial barrier. Orally administered drug-loaded delivery systems are the most commonly used, but they present a number of barriers, including pill swallowing difficulties and the overall toxicity of nanocomposites, especially in elderly and paediatric populations. Moreover, utmost care needs to be taken to hide cost-effectiveness, therapeutic advantages and unpleasant flavours associated with oral delivery. Some anticancer therapies require rapid release, but the high swelling and loading properties of crosslinked biopolymers prevent this. Consequently, there is an important need to achieve improved drug release that is sensitive to environmental changes in the release site in the future. Electrospinning is the most known technology for the production of continuous nanofibers with a simple configuration. However, in recent years it has attracted great interest due to its potential in biomedical and other nanotechnical applications. High surface-to-volume ratio, ease of use and cost-effectiveness are attractive features for biomedical applications. Electrospinning to load drugs into hydrophilic fibres is particularly important to enhance the dissolution of poorly water-soluble drugs and the biodegradability of the sample. Immediate dissolution formulations for buccal absorption of the drug are produced with this technique for rapid drug absorption and to avoid first-pass metabolism or degradation in gastric fluids. With the creation of electrospinning methods such as coaxial electrospinning and the availability of a wide range of materials (including organic, synthetic and semi-synthetic polymers), many drugs have been electrospun into ultra-fine fibres with controlled diameters and morphologies. Advanced electrospinning arrangements enable the creation of hydrophilic drug delivery systems involving macromolecules such as proteins and DNA. Most of the research on the release of antibiotics and drugs (psychoactive, antineoplastic, etc.) has been performed in vitro. In vivo, in-depth systemic studies, particularly on the kinetics and dynamics of drug release in vivo and the effects of drug dosage and release kinetics on therapeutic efficacy and bio-distribution of released drugs, are required before any clinical marketing can be considered. Complete studies on the toxic effects of polymeric carriers, as well as their distribution and elimination processes, are also required. So far, many pharmaceutical drugs have been loaded onto nanofibers, but these studies are limited to the loading and characterisation of nanofibers. The lack of accurate dosage seems to be a common issue in most papers. It can be concluded that this drawback is the strongest weakness of electrospinning: it is difficult to load nanofibers with the desired concentration of drugs for application in clinical trials in humans. Also, because most pharmaceuticals have a disagreeable taste, it is critical to sweeteners in drug-loaded nanofibers, especially for applications on fast-dissolving systems designed for oral delivery of drugs. However, with the exception of reports on the loading of sugar in core-shell nanofibers, the efficacy of this process is not widely reported. This review proposes to proceed with the investigation to optimise the incorporation of interesting drugs into nanofibers but assist clinical consideration, considering patient acceptance of the administration form. Scaling to mass preparations of drug-loaded electrospun mats is additionally an issue that must be regarded. Finally, investigations on electrospinning's various applications will continue due to its flexibility, cost-effectiveness, and demonstrated effectiveness in a wide range of biological applications. simple to utilise and easy to manufacture in any research facility, even with low financial support.
Acknowledgement
This review work was supported by the Ministry of Education, Youth and Sports of the Czech Republic and the European Union European Structural and Investment Funds Operational Program Research, Development and Education project "Modular platform for autonomous chassis of specialised electric vehicles for freight and equipment transportation" (Reg. No. CZ.02.1.01/0.0/0.0/16_025/0007293). The authors also acknowledge the support of the project "Textile structures combining virus protection and comfort", Reg. No. CZ.01.1.02/0.0/0.0/20_321/0024467.
References
- Bruschi ML, editor. 2 - Modification of drug release. Strateg. Modify Drug Release Pharm. Syst., Woodhead Publishing; 2015, p. 15–28. [CrossRef]
- Kukoyi, AR. Economic Impacts of Natural Polymers. In: Olatunji O, editor. Nat. Polym. Ind. Tech. Appl., Cham: Springer International Publishing; 2016, p. 339–62. [CrossRef]
- Mauricio MD, Guerra-Ojeda S, Marchio P, Valles SL, Aldasoro M, Escribano-Lopez I, et al. Nanoparticles in Medicine: A Focus on Vascular Oxidative Stress. Oxid Med Cell Longev 2018, 2018:e6231482. [CrossRef]
- Nitta SK, Numata K. Biopolymer-Based Nanoparticles for Drug/Gene Delivery and Tissue Engineering. Int J Mol Sci 2013, 14, 1629–54. [CrossRef]
- Sahai N, Ahmad N, Gogoi M. Nanoparticles Based Drug Delivery for Tissue Regeneration Using Biodegradable Scaffolds: a Review. Curr Pathobiol Rep 2018, 6, 219–24. [CrossRef]
- Yang Y-Y, Wang Y, Powell R, Chan P. Polymeric Core-Shell Nanoparticles for Therapeutics. Clin Exp Pharmacol Physiol 2006, 33, 557–62. [CrossRef]
- Gopi S, Amalraj A, Sukumaran NP, Haponiuk JT, Thomas S. Biopolymers and Their Composites for Drug Delivery: A Brief Review. Macromol Symp 2018, 380, 1800114. [CrossRef]
- Huang Z-M, Zhang Y-Z, Kotaki M, Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol 2003, 63, 2223–53. [CrossRef]
- Torres-Martinez EJ, Cornejo Bravo JM, Serrano Medina A, Pérez González GL, Villarreal Gómez LJ. A Summary of Electrospun Nanofibers as Drug Delivery System: Drugs Loaded and Biopolymers Used as Matrices. Curr Drug Deliv 2018, 15, 1360–74. [CrossRef]
- Villarreal-Gómez LJ, Cornejo-Bravo JM, Vera-Graziano R, Grande D. Electrospinning as a powerful technique for biomedical applications: a critically selected survey. J Biomater Sci Polym Ed 2016, 27, 157–76. [CrossRef]
- Biological, Chemical, and Electronic Applications of Nanofibers - Nguyen - 2013 - Macromolecular Materials and Engineering - Wiley Online Library n.d. https://onlinelibrary.wiley.com/doi/abs/10.1002/mame.201200143 (accessed October 4, 2022).
- Han F, Wang J, Ding L, Hu Y, Li W, Yuan Z, et al. Tissue Engineering and Regenerative Medicine: Achievements, Future, and Sustainability in Asia. Front Bioeng Biotechnol 2020, 8, 83. [CrossRef]
- Bakhshandeh B, Zarrintaj P, Oftadeh MO, Keramati F, Fouladiha H, Sohrabi-Jahromi S, et al. Tissue engineering; strategies, tissues, and biomaterials. Biotechnol Genet Eng Rev 2017, 33, 144–72. [CrossRef]
- Vacanti, J. Tissue engineering and regenerative medicine: from first principles to state of the art. J Pediatr Surg 2010, 45, 291–4. [Google Scholar] [CrossRef]
- Fernández de la Mora, J. The Fluid Dynamics of Taylor Cones. Annu Rev Fluid Mech 2007, 39, 217–43. [Google Scholar] [CrossRef]
- Jacob J, Haponiuk JT, Thomas S, Gopi S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater Today Chem 2018, 9, 43–55. [CrossRef]
- Chou S-F, Carson D, Woodrow KA. Current strategies for sustaining drug release from electrospun nanofibers. J Control Release Off J Control Release Soc 2015, 220, 584–91. [CrossRef]
- Singh H, Sharma R, Joshi M, Garg T, Goyal AK, Rath G. Transmucosal delivery of Docetaxel by mucoadhesive polymeric nanofibers. Artif Cells Nanomedicine Biotechnol 2015, 43, 263–9. [CrossRef]
- Tucker N, Stanger J, Staiger M, Razzaq H, Hofman K. The History of the Science and Technology of Electrospinning from 1600 to 1995. J Eng Fibers Fabr 2012, 7. [CrossRef]
- Production of membranes from nanofibrous polysaccharides 2022. https://dspace.tul.cz/handle/15240/160908 (accessed May 25, 2022).
- Auría-Soro C, Nesma T, Juanes-Velasco P, Landeira-Viñuela A, Fidalgo-Gomez H, Acebes-Fernandez V, et al. Interactions of Nanoparticles and Biosystems: Microenvironment of Nanoparticles and Biomolecules in Nanomedicine. Nanomaterials 2019, 9, 1365. [CrossRef]
- Biomaterials: Design, Development and Biomedical Applications - ScienceDirect 2022. https://www.sciencedirect.com/science/article/pii/B9780323328890000029 (accessed May 25, 2022).
- Ebnesajjad S, editor. Handbook of biopolymers and biodegradable plastics: properties, processing and applications. Oxford ; Waltham, MA: Elsevier/William Andrew; 2013.
- Jung K, Corrigan N, Wong EHH, Boyer C. Bioactive Synthetic Polymers. Adv Mater 2022, 34, 2105063. [CrossRef]
- Deming TJ, Klok H-A, Armes SP, Becker ML, Champion JA, Chen EY-X, et al. Polymers at the Interface with Biology. Biomacromolecules 2018, 19, 3151–62. [CrossRef]
- Van Den Bulcke AI, Bogdanov B, De Rooze N, Schacht EH, Cornelissen M, Berghmans H. Structural and Rheological Properties of Methacrylamide Modified Gelatin Hydrogels. Biomacromolecules 2000, 1, 31–8. [CrossRef]
- Kutz, M. Applied Plastics Engineering Handbook n.d.:661.
- Polymeric Biomaterial - an overview | ScienceDirect Topics n.d. https://www.sciencedirect.com/topics/materials-science/polymeric-biomaterial (accessed October 5, 2022).
- Gong T, Xie J, Liao J, Zhang T, Lin S, Lin Y. Nanomaterials and bone regeneration. Bone Res 2015, 3, 15029. [CrossRef]
- Tran V, Wen X. Rapid prototyping technologies for tissue regeneration. Rapid Prototyp. Biomater., Elsevier; 2014, p. 97–155. [CrossRef]
- Lanza RP, Langer RS, Vacanti J, editors. Principles of tissue engineering. Fourth edition. Amsterdam: Academic Press, an imprint of Elsevier; 2014.
- O’Brien, FJ. Biomaterials & scaffolds for tissue engineering. Mater Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Filippi M, Born G, Chaaban M, Scherberich A. Natural Polymeric Scaffolds in Bone Regeneration. Front Bioeng Biotechnol 2020, 8, 474. [CrossRef]
- Kjeldsen A, Price M, Lilley C, Guzniczak E, Archer I. A review of standards for biodegradable plastics n.d.:33.
- Im SH, Kim CY, Jung Y, Jang Y, Kim SH. Biodegradable vascular stents with high tensile and compressive strength: a novel strategy for applying monofilaments via solid-state drawing and shaped-annealing processes. Biomater Sci 2017, 5, 422–31. [CrossRef]
- Vert M, Doi Y, Hellwich K-H, Hess M, Hodge P, Kubisa P, et al. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl Chem 2012, 84, 377–410. [CrossRef]
- Jain E, Damania A, Kumar A. Biomaterials for liver tissue engineering. Hepatol Int 2014, 8, 185–97. [CrossRef]
- Saberi-Riseh R, Moradi-Pour M, Mohammadinejad R, Thakur VK. Biopolymers for Biological Control of Plant Pathogens: Advances in Microencapsulation of Beneficial Microorganisms. Polymers 2021, 13, 1938. [CrossRef]
- Schmidt T, Hillebrand A, Wurbs D, Wahler D, Lenders M, Schulze Gronover C, et al. Molecular Cloning and Characterization of Rubber Biosynthetic Genes from Taraxacum koksaghyz. Plant Mol Biol Report 2010, 28, 277–84. [CrossRef]
- Zeng P, Li J, Chen Y, Zhang L. The structures and biological functions of polysaccharides from traditional Chinese herbs. Prog. Mol. Biol. Transl. Sci., vol. 163, Elsevier; 2019, p. 423–44. [CrossRef]
- Costa OYA, Raaijmakers JM, Kuramae EE. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front Microbiol 2018, 9, 1636. [CrossRef]
- Guo MQ, Hu X, Wang C, Ai L. Polysaccharides: Structure and Solubility. In: Xu Z, editor. Solubility Polysacch., InTech; 2017. [CrossRef]
- Moysidou C-M, Barberio C, Owens RM. Advances in Engineering Human Tissue Models. Front Bioeng Biotechnol 2021, 8, 620962. [CrossRef]
- Davis TA, Volesky B, Mucci A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res 2003, 37, 4311–30. [CrossRef]
- Cheung R, Ng T, Wong J, Chan W. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar Drugs 2015, 13, 5156–86. [CrossRef]
- Cheung R, Ng T, Wong J, Chan W. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar Drugs 2015, 13, 5156–86. [CrossRef]
- Di Martino A, Sittinger M, Risbud MV. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–90. [CrossRef]
- Zeng P, Li J, Chen Y, Zhang L. The structures and biological functions of polysaccharides from traditional Chinese herbs. Prog. Mol. Biol. Transl. Sci., vol. 163, Elsevier; 2019, p. 423–44. [CrossRef]
- Märtson M, Viljanto J, Laippala P, Saukko P. Connective Tissue Formation in Subcutaneous Cellulose Sponge Implants in the Rat. Eur Surg Res 1998, 30, 419–25. [CrossRef]
- Entcheva E, Bien H, Yin L, Chung C-Y, Farrell M, Kostov Y. Functional cardiac cell constructs on cellulose-based scaffolding. Biomaterials 2004, 25, 5753–62. [CrossRef]
- Märtson M, Viljanto J, Hurme T, Laippala P, Saukko P. Is cellulose sponge degradable or stable as implantation material? An in vivo subcutaneous study in the rat. Biomaterials 1999, 20, 1989–95. [CrossRef]
- Miyamoto T, Takahashi S, Ito H, Inagaki H, Noishiki Y. Tissue biocompatibility of cellulose and its derivatives. J Biomed Mater Res 1989, 23, 125–33. [CrossRef]
- Märtson M, Viljanto J, Hurme T, Laippala P, Saukko P. Is cellulose sponge degradable or stable as implantation material? An in vivo subcutaneous study in the rat. Biomaterials 1999, 20, 1989–95. [CrossRef]
- Entcheva E, Bien H, Yin L, Chung C-Y, Farrell M, Kostov Y. Functional cardiac cell constructs on cellulose-based scaffolding. Biomaterials 2004, 25, 5753–62. [CrossRef]
- Singh H, Sharma C, Dhasmana A, Gupta S, Purohit SD, Mishra N, et al. Bone Regeneration: Emerging Paradigms and Existing Snags 2018.
- Halib N, Perrone F, Cemazar M, Dapas B, Farra R, Abrami M, et al. Potential Applications of Nanocellulose-Containing Materials in the Biomedical Field. Mater Basel Switz 2017, 10. [CrossRef]
- Hedley CL, Bogracheva TY, Wang TL. A Genetic Approach to Studying the Morphology, Structure and Function of Starch Granules using Pea as a Model. Starch - Stärke 2002, 54, 235–42. [CrossRef]
- Beery KE, Ladisch MR. Chemistry and properties of starch based desiccants. Enzyme Microb Technol 2001, 28, 573–81. [CrossRef]
- Mano JF, Silva GA, Azevedo HS, Malafaya PB, Sousa RA, Silva SS, et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J R Soc Interface 2007, 4, 999–1030. [CrossRef]
- Gomes ME, Godinho JS, Tchalamov D, Cunha AM, Reis RL. Alternative tissue engineering scaffolds based on starch: processing methodologies, morphology, degradation and mechanical properties. Mater Sci Eng C 2002, 20, 19–26. [CrossRef]
- Menzel EJ, Farr C. Hyaluronidase and its substrate hyaluronan: biochemistry, biological activities and therapeutic uses. Cancer Lett 1998, 131, 3–11. [CrossRef]
- Ehlers E-M, Behrens P, Wünsch L, Kühnel W, Russlies M. Effects of hyaluronic acid on the morphology and proliferation of human chondrocytes in primary cell culture. Ann Anat - Anat Anz 2001, 183, 13–7. [CrossRef]
- Moysidou C-M, Barberio C, Owens RM. Advances in Engineering Human Tissue Models. Front Bioeng Biotechnol 2021, 8, 620962. [CrossRef]
- Simionescu DT, Lu Q, Song Y, Lee J, Rosenbalm TN, Kelley C, et al. Biocompatibility and remodeling potential of pure arterial elastin and collagen scaffolds. Biomaterials 2006, 27, 702–13. [CrossRef]
- Bakhshandeh B, Zarrintaj P, Oftadeh MO, Keramati F, Fouladiha H, Sohrabi-Jahromi S, et al. Tissue engineering; strategies, tissues, and biomaterials. Biotechnol Genet Eng Rev 2017, 33, 144–72. [CrossRef]
- Lee KY, Mooney DJ. Alginate: Properties and biomedical applications. Prog Polym Sci 2012, 37, 106–26. [CrossRef]
- Deming TJ, Klok H-A, Armes SP, Becker ML, Champion JA, Chen EY-X, et al. Polymers at the Interface with Biology. Biomacromolecules 2018, 19, 3151–62. [CrossRef]
- Buer, JK. Origins and impact of the term “NSAID. ” Inflammopharmacology 2014, 22, 263–7. [Google Scholar] [CrossRef]
- Yu D-G, Branford-White C, White K, Li X-L, Zhu L-M. Dissolution Improvement of Electrospun Nanofiber-Based Solid Dispersions for Acetaminophen. AAPS PharmSciTech 2010, 11, 809–17. [CrossRef]
- Yu DG, Shen XX, Branford-White CJ, White KN, Zhu LM, Bligh SWA. Oral fast-dissolving drug delivery membranes prepared from electrospun polyvinylpyrrolidone ultrafine fibers. Nanotechnology 2009, 20, 055104.
- Canbolat MF, Celebioglu A, Uyar T. Drug delivery system based on cyclodextrin-naproxen inclusion complex incorporated in electrospun polycaprolactone nanofibers. Colloids Surf B Biointerfaces 2014, 115, 15.
- Németh C, Gyarmati B, Gacs J, Salakhieva DV, Molnár K, Abdullin T, et al. Fast dissolving nanofibrous matrices prepared by electrospinning of polyaspartamides. Eur Polym J 2020, 130, 109624. [CrossRef]
- Chavarria-Bolaños D, Granados-Hernandez M, Serrano J, Suárez Franco JL, Guarino V, Pérez M. Introduction to electrofluidodynamic techniques. PART II. cell-to-cell / material interactions, 2018.
- Collignon P, Powers JH, Chiller TM, Aidara-Kane A, Aarestrup FM. World Health Organization ranking of antimicrobials according to their importance in human medicine: A critical step for developing risk management strategies for the use of antimicrobials in food production animals. Clin Infect Dis Off Publ Infect Dis Soc Am 2009, 49, 132–41. [CrossRef]
- Lopez FL, Shearman GC, Gaisford S, Williams GR. Amorphous Formulations of Indomethacin and Griseofulvin Prepared by Electrospinning. Mol Pharm 2014, 11.
- Buschle-Diller G, Cooper J, Xie Z, Wu Y, Waldrup J, Ren X. Release of antibiotics from electrospun bicomponent fibers. Cellulose 2007, 14, 553–62. [CrossRef]
- He C, Huang Z, Han X, Liu L, Zhang H, Chen L. Coaxial Electrospun Poly(L-Lactic Acid) Ultrafine Fibers for Sustained Drug Delivery. J Macromol Sci Part B 2006, 45, 515–24. [CrossRef]
- Huang Z-M, He C-L, Yang A, Zhang Y, Han X-J, Yin J, et al. Encapsulating drugs in biodegradable ultrafine fibers through co-axial electrospinning. J Biomed Mater Res A 2006, 77, 169–79. [CrossRef]
- Yu D-G, Zhu L-M, Branford-White CJ, Yang J-H, Wang X, Li Y, et al. Solid dispersions in the form of electrospun core-sheath nanofibers. Int J Nanomedicine 2011, 6, 3271–80. [CrossRef]
- Verreck G, Chun I, Rosenblatt J, Peeters J, Dijck AV, Mensch J, et al. Incorporation of drugs in an amorphous state into electrospun nanofibers composed of a water-insoluble, nonbiodegradable polymer. J Control Release Off J Control Release Soc 2003, 92, 349–60. [CrossRef]
- Ma Y, Wang X, Zong S, Zhang Z, Xie Z, Huang Y, et al. Local, combination chemotherapy in prevention of cervical cancer recurrence after surgery by using nanofibers co-loaded with cisplatin and curcumin. RSC Adv 2015, 5, 106325–32. [CrossRef]
- Xu X, Yang L, Xu X, Wang X, Chen X, Liang Q, et al. Ultrafine medicated fibers electrospun from W/O emulsions. J Control Release Off J Control Release Soc 2005, 108, 33–42. [CrossRef]
- Vrbata P, Berka P, Stránská D, Doležal P, Musilová M, Čižinská L. Electrospun drug loaded membranes for sublingual administration of sumatriptan and naproxen. Int J Pharm 2013, 457, 168–76. [CrossRef]
- Jaiturong P, Sirithunyalug B, Eitsayeam S, Asawahame C, Tipduangta P, Sirithunyalug J. Preparation of glutinous rice starch/polyvinyl alcohol copolymer electrospun fibers for using as a drug delivery carrier. Asian J Pharm Sci 2018, 13, 239–47. [CrossRef]
- Torres-Martínez EJ, Lizeth Pérez-González G, Serrano-Medina A, Grande D, Vera-Graziano R, Cornejo-Bravo M, et al. Drugs Loaded into Electrospun Polymeric Nanofibers for Delivery. J Pharm Pharm Sci 2019, 22, 313–31. [CrossRef]
- Davies, JE. The pharmacological basis of therapeutics. Occup Environ Med 2007, 64:e2. [CrossRef]
- Pérez-González GL, Villarreal-Gómez LJ, Serrano-Medina A, Torres-Martínez EJ, Cornejo-Bravo JM. Mucoadhesive electrospun nanofibers for drug delivery systems: applications of polymers and the parameters’ roles. Int J Nanomedicine 2019, 14, 5271–85. [CrossRef]
- Zitek T, Gates M, Pitotti C, Bartlett A, Patel J, Rahbar A, et al. A Comparison of Headache Treatment in the Emergency Department: Prochlorperazine Versus Ketamine. Ann Emerg Med 2018, 71, 369–377e1. [CrossRef]
- Hoffmann EM, Breitenbach A, Breitkreutz J. Advances in orodispersible films for drug delivery. Expert Opin Drug Deliv 2011, 8, 299–316. [CrossRef]
- Jaber BM, Petroianu GA, Rizvi SA, Borai A, Saleh NA, Hala SM, et al. Protective effect of metoclopramide against organophosphate-induced apoptosis in the murine skin fibroblast L929. J Appl Toxicol JAT 2018, 38, 329–40. [CrossRef]
- Fallowfield LJ, Jenkins VA, Beveridge HA. Truth may hurt but deceit hurts more: communication in palliative care. Palliat Med 2002, 16, 297–303. [CrossRef]
- Nagy ZsK, Nyul K, Wagner I, Molnar K, Marosi Gy. Electrospun water soluble polymer mat for ultrafast release of Donepezil HCl. Express Polym Lett 2010, 4, 763–72. [CrossRef]
- Blakney AK, Krogstad EA, Jiang YH, Woodrow KA. Delivery of multipurpose prevention drug combinations from electrospun nanofibers using composite microarchitectures. Int J Nanomedicine 2014, 9, 2967–78. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).