Submitted:
24 July 2023
Posted:
26 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experiment
2.1. Ore Samples, Main Reagents and Instruments
2.2. Test method
2.2.1. Flotation Test
2.2.2. Determination of Zeta Potential
2.2.3. Determination of Surface Tension
2.2.4. Infrared Spectrum Test
3. Results and Discussion
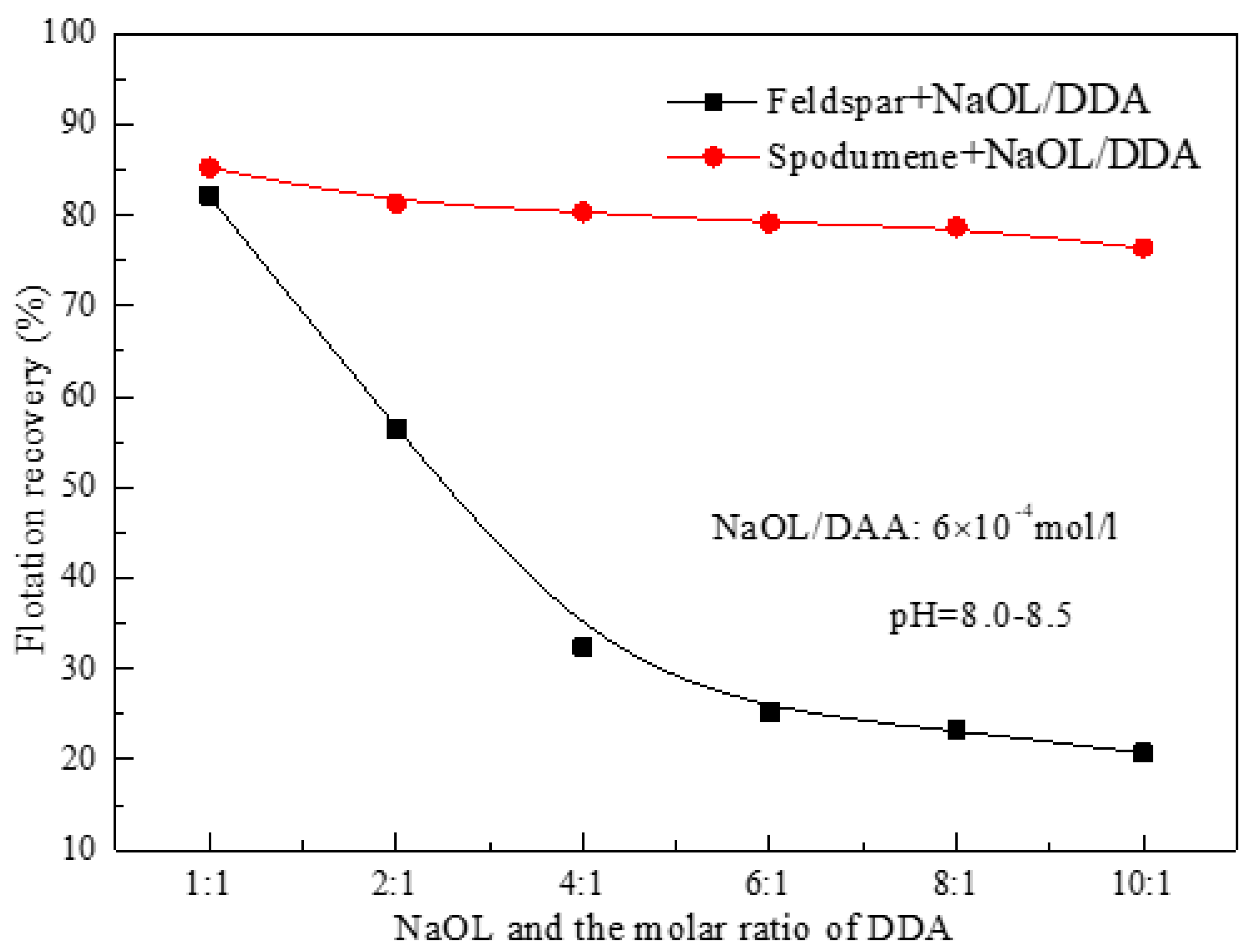
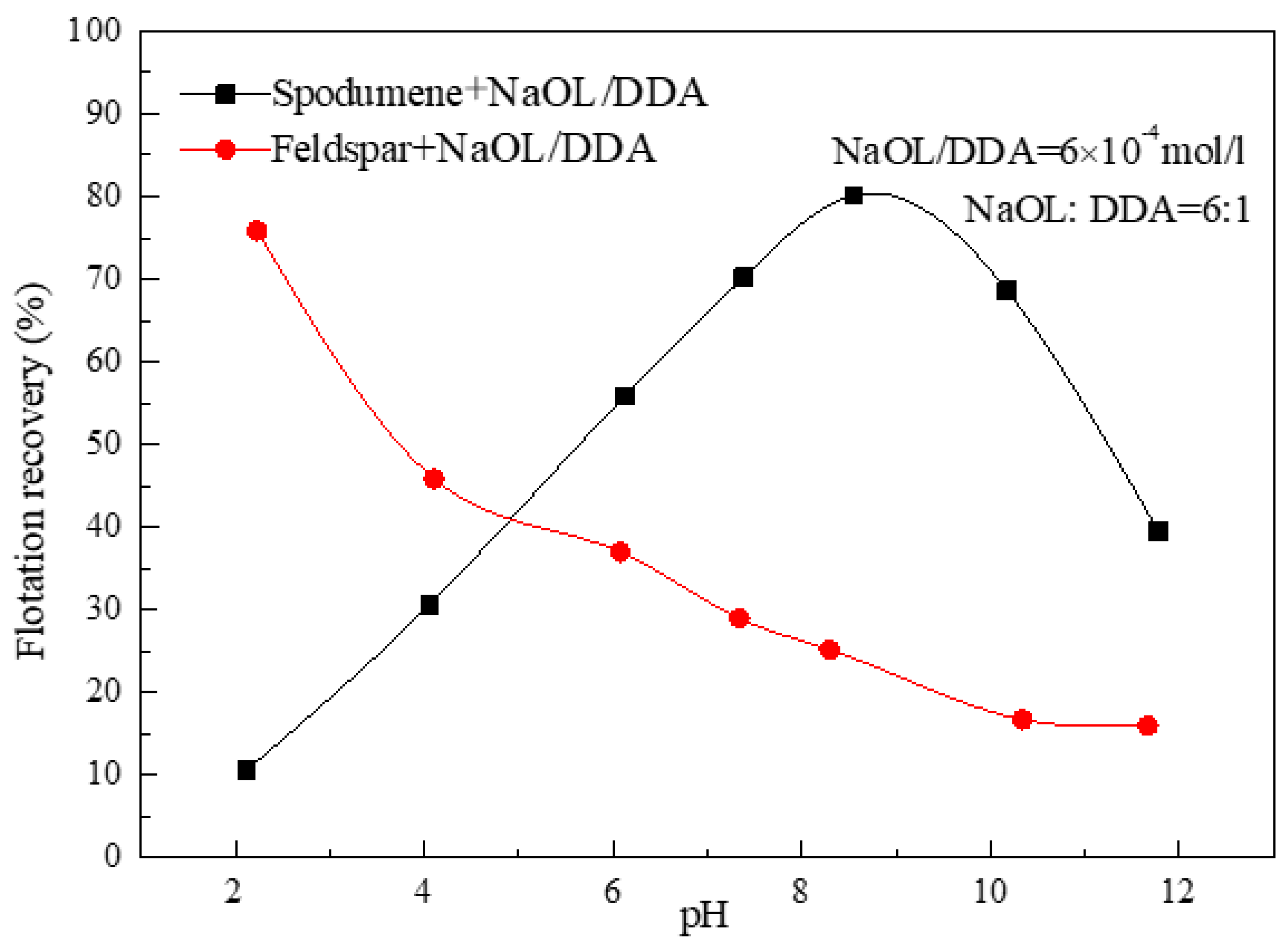
3.1. Effect of the Ratio of NaOL and DDA and pH Value on the Floatability of Spodumene
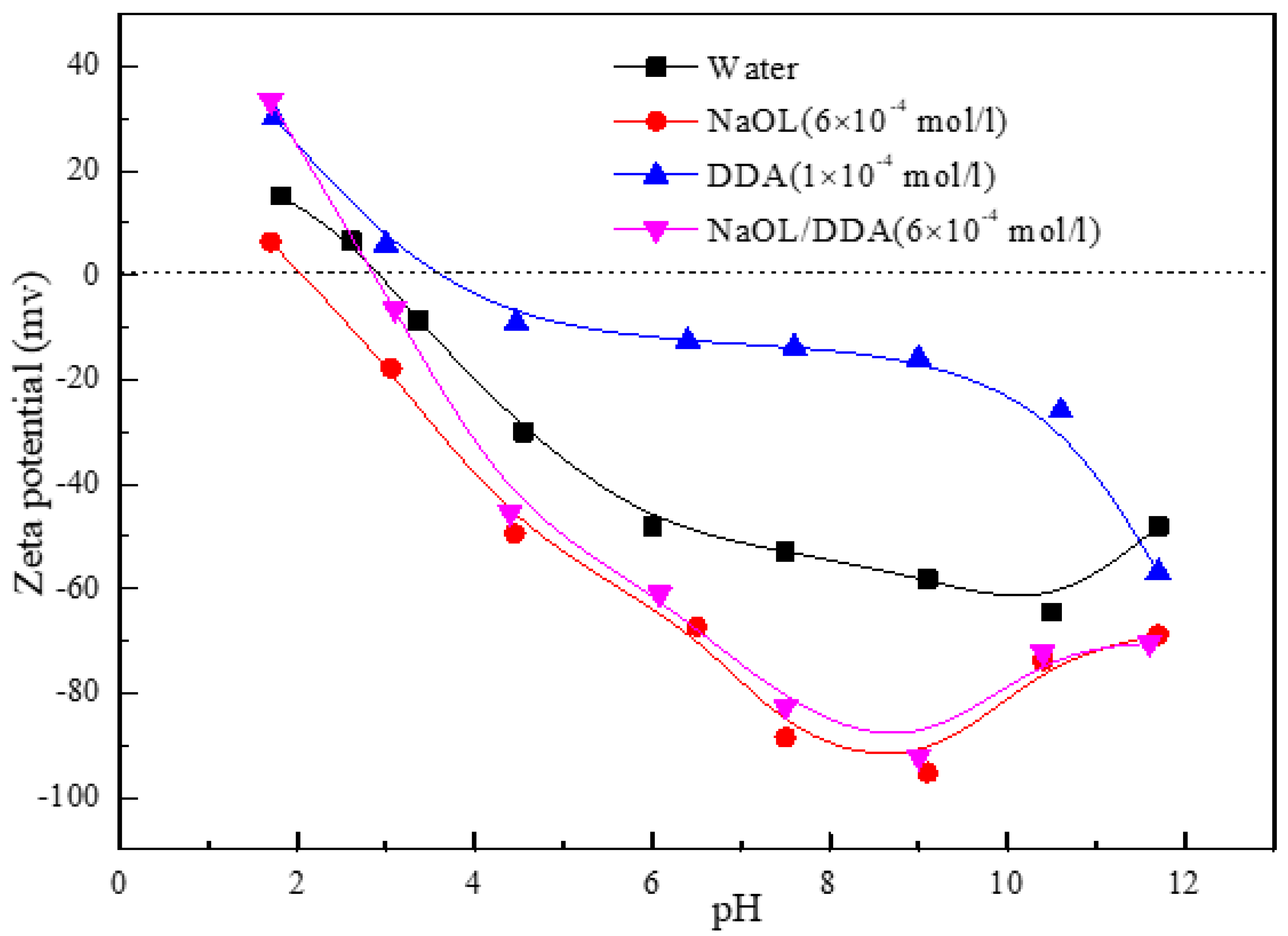
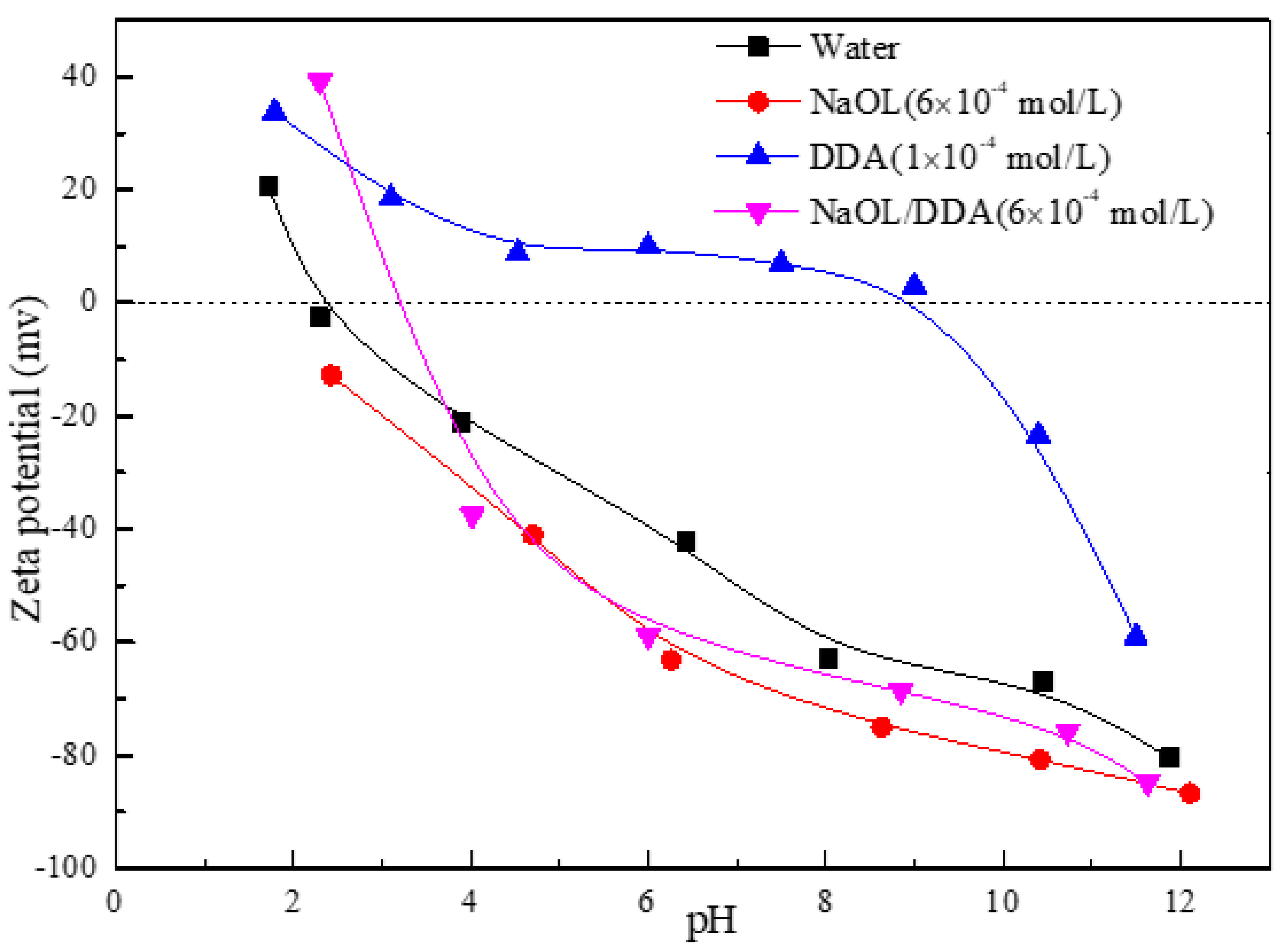
3.2. Effect of Combined Collectors on Zeta Potential of Spodumene Surface
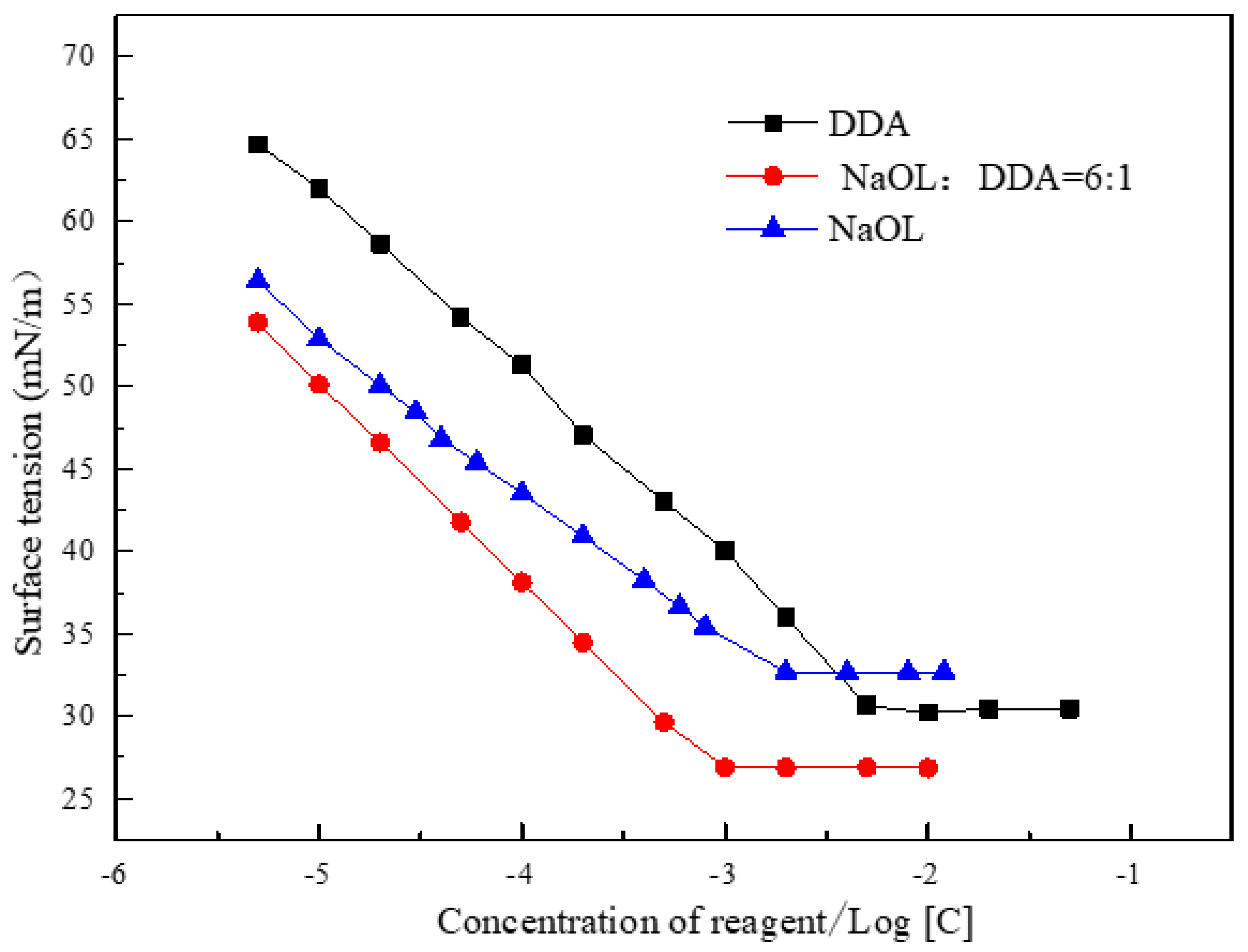
3.3. Analysis of Surface Tension Determination of Combined Collecting Agents and Calculation of Synergistic Parameters
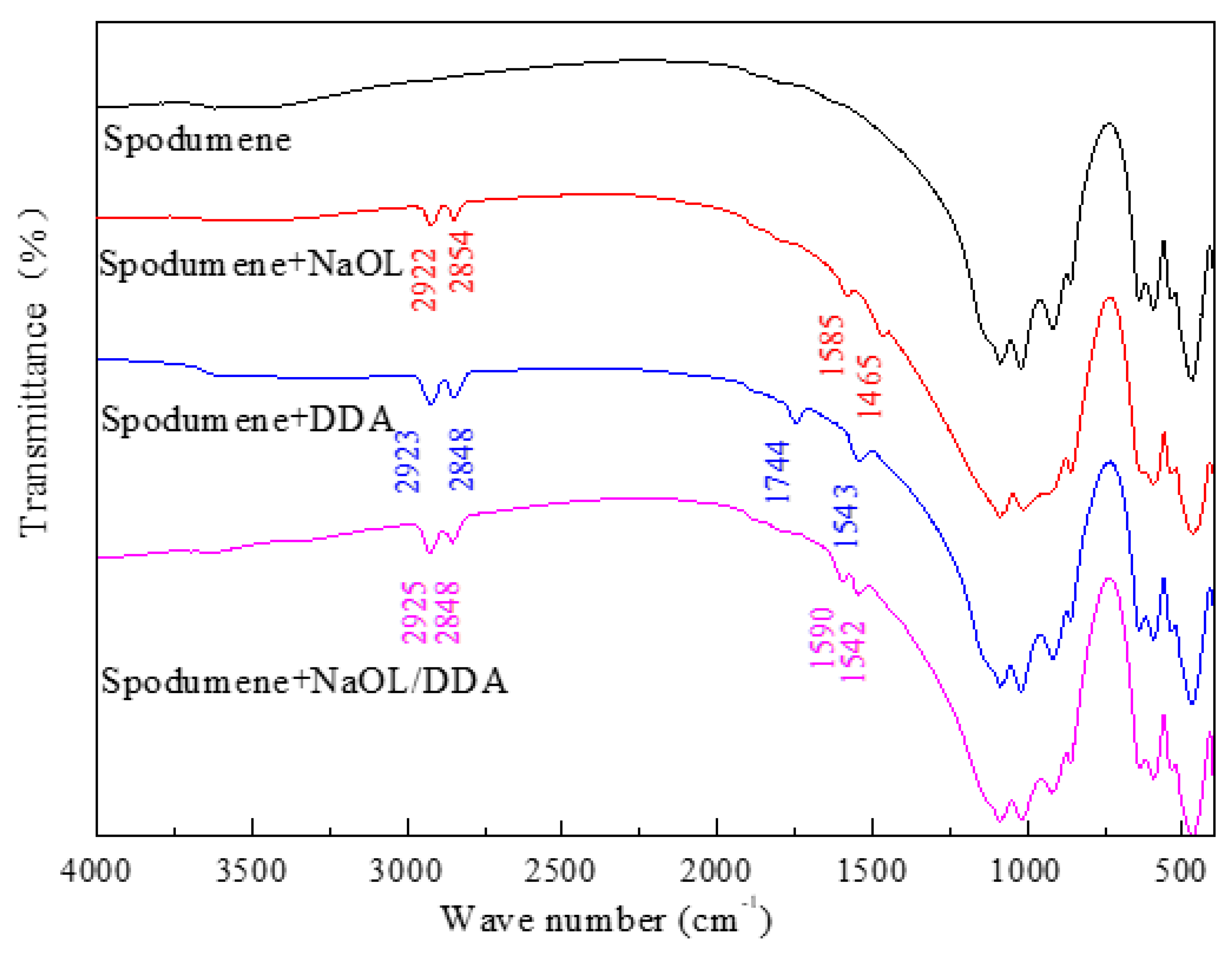
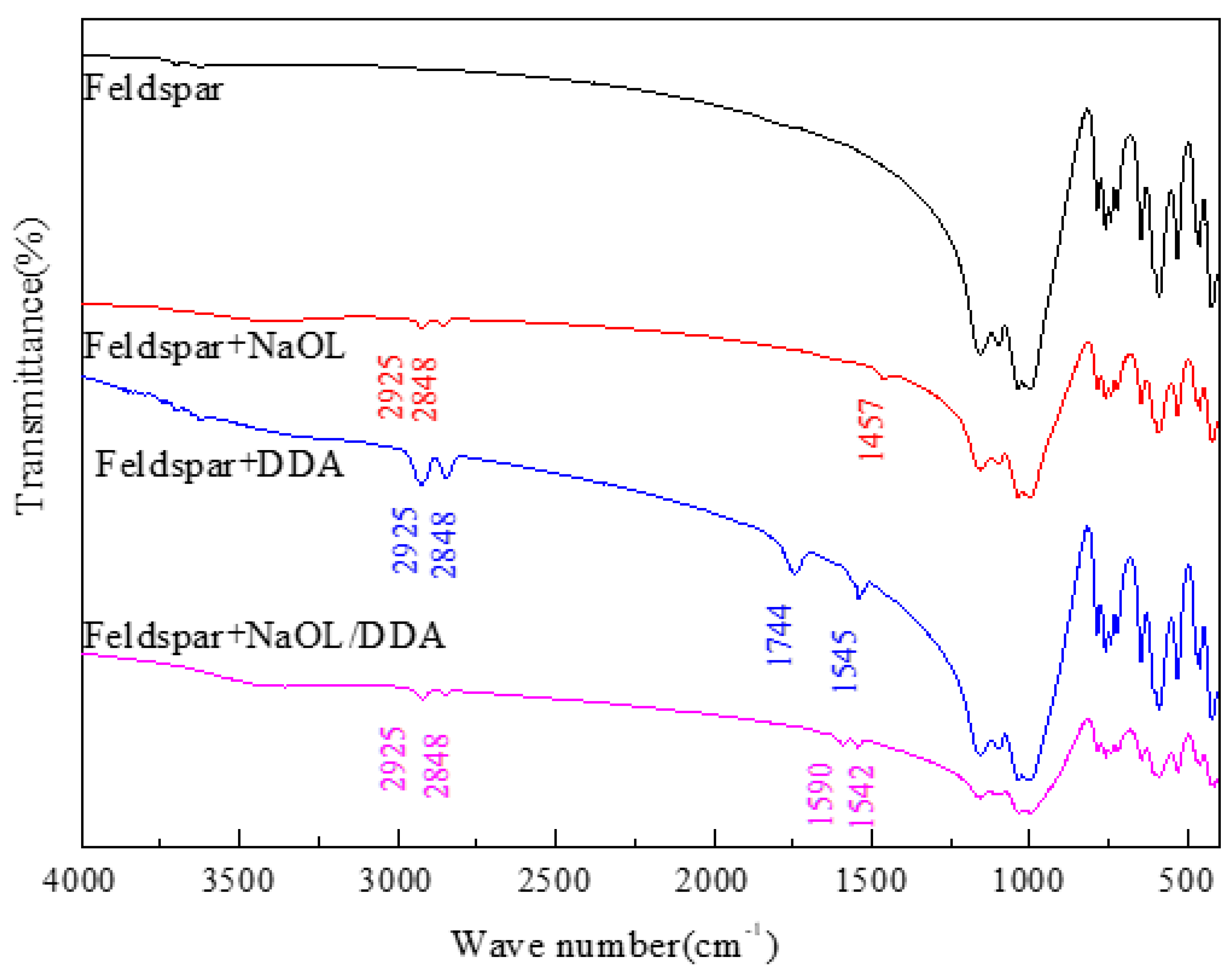
3.4. Infrared Spectrum Analysis of the Product of Combined Collector on Spodumene and Feldspar Surfaces
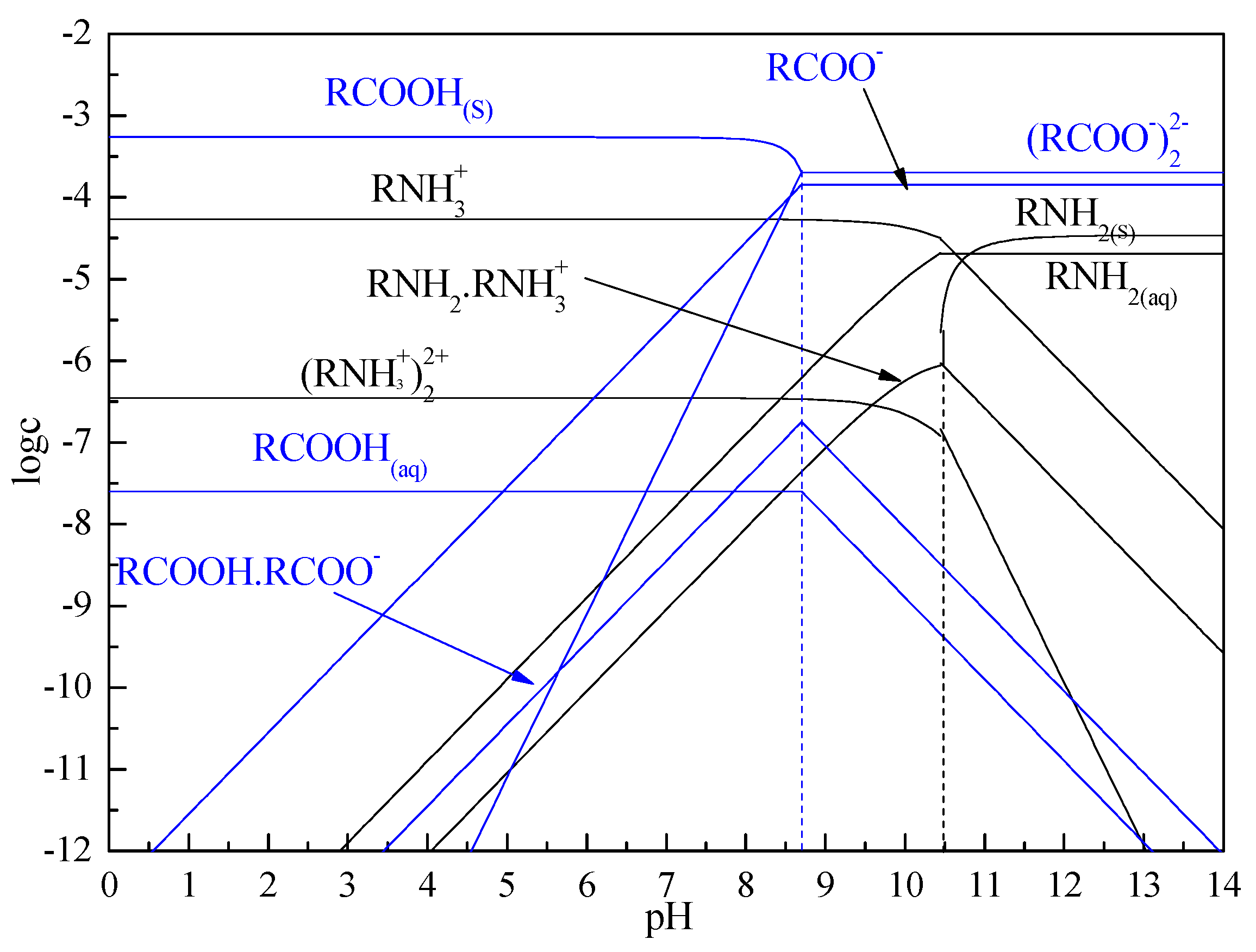
3.5. Solution Chemistry
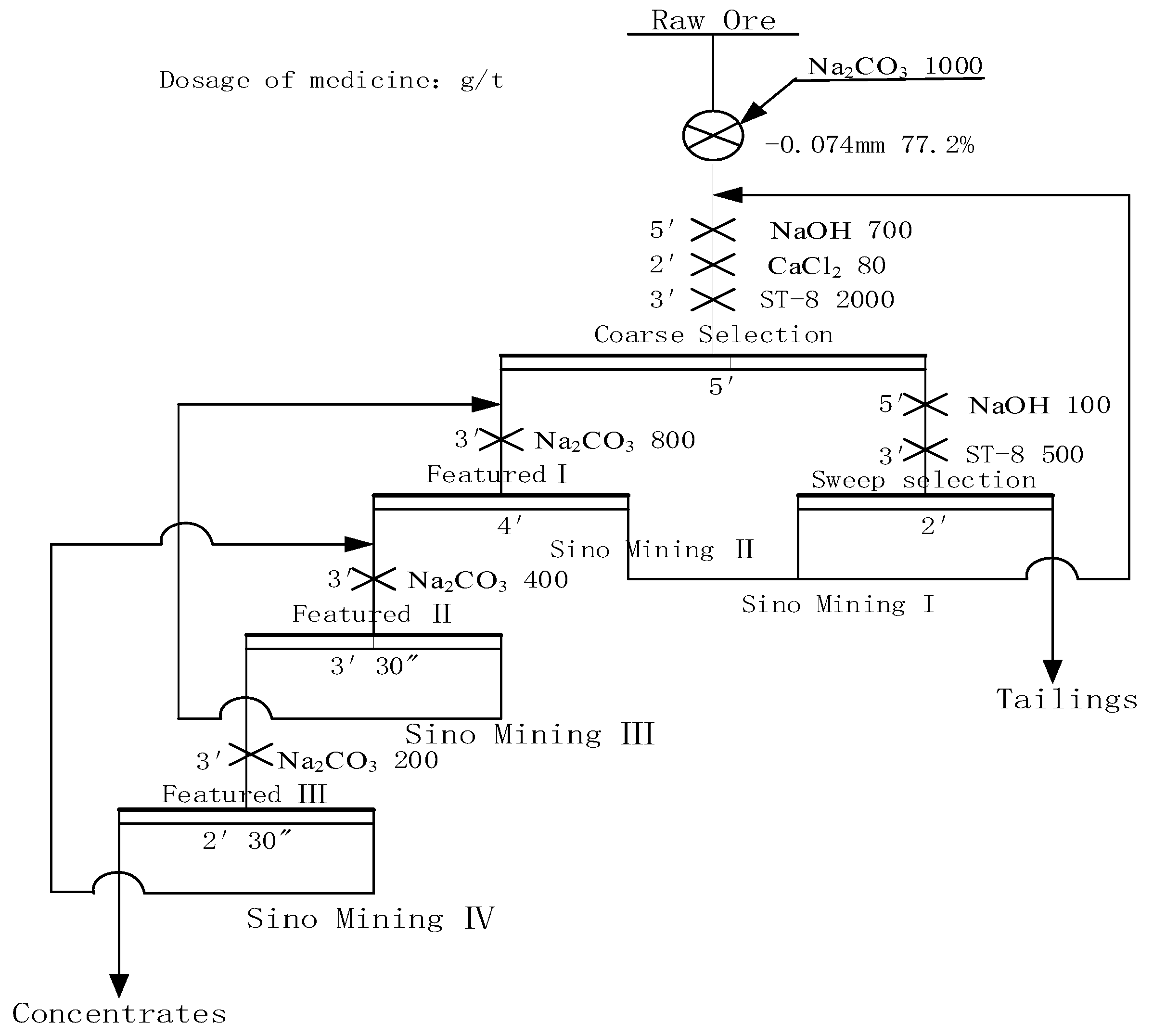
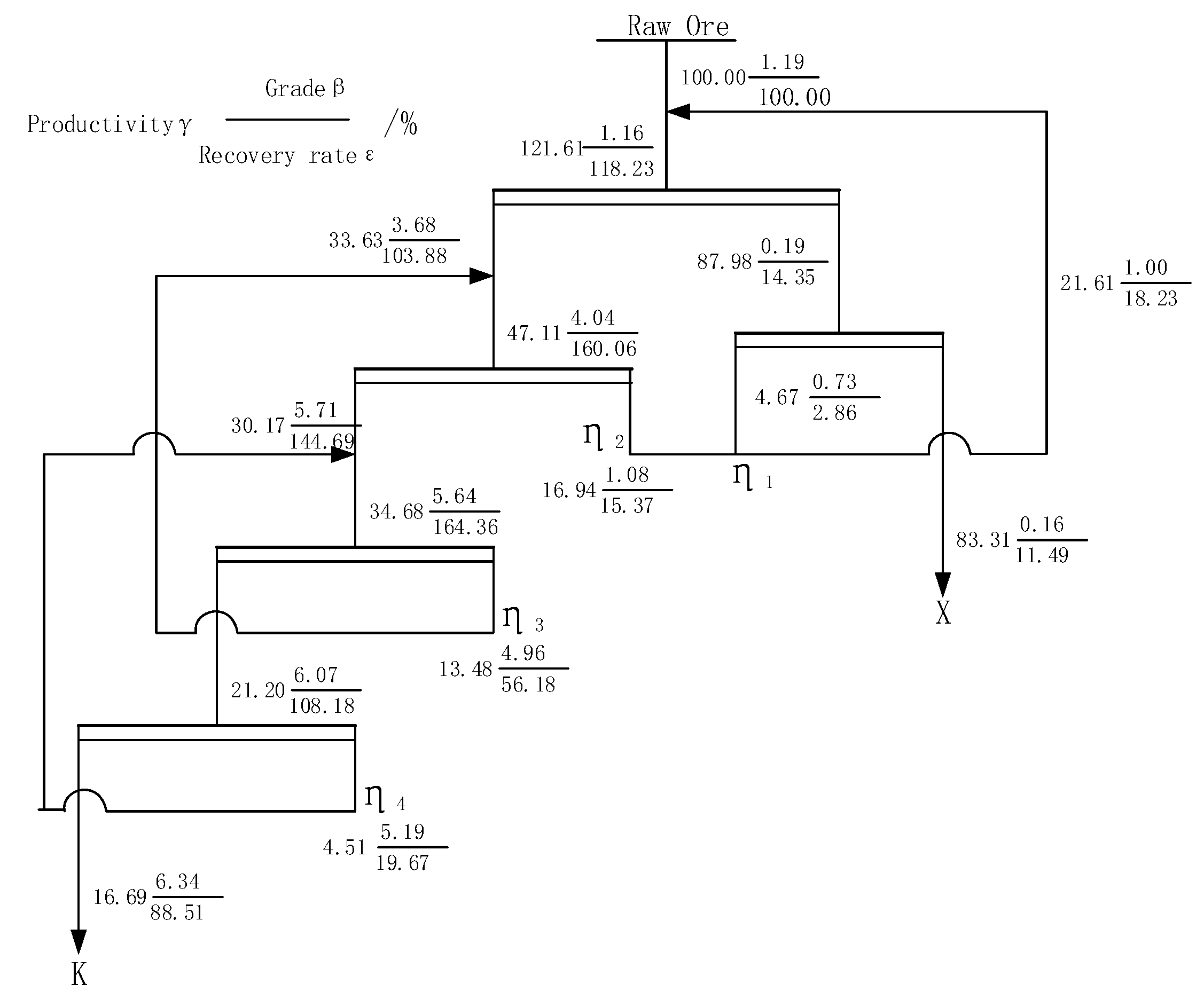
4. Closed Circuit Test
5. Conclusion
- For the flotation separation of single mineral spospoxene and feldspar, the optimal molar combination ratio of anionic collector NaOL and cationic collector DDA is 6:1-10:1, and the optimal pH range for the separation effect is about 8.5
- After the combination collector interacts with spodumene and feldspar, the negative shift degree of spodumene surface zeta potential is much stronger than that of feldspar surface zeta potential. At the same time, the absorption peak intensity after the combination collector interacts with spodumene is much stronger than that after feldspar, indicating that the adsorption capacity of the combination collector on the surface of spodumene and feldspar is greatly different. At the same time, the selectivity of spodumene is stronger than that of feldspar, so that the separation of spodumene and feldspar can be realized.
- The surface tension of the combined collector is lower than that of the anionic collector and the cationic collector used alone, indicating that the combined collector has a strong ability to reduce the surface tension of the gas-liquid interface, so as to improve the surface hydrophobicity of the mineral.
- The absorption peak of the combined collector is similar to that of spodumene and feldspar, but the intensity of the absorption peak after spodumene action is much stronger than that after feldspar action, indicating that the selectivity of the anionic and cationic combination collector for spodumene is stronger than feldspar, so that the separation of spodumene and feldspar can be realized.
- The closed circuit test results show that when the grade of Li2O is 1.19%, the final flotation index can be 6.34% with Li2O grade and 88.51% Li2O recovery after a closed circuit process of one roughing, three selections and one sweeping sequence return.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dazhe, L. The use of lithium and its resource development. Chinese Journal of Safety Science 2004, 76-80+98. [CrossRef]
- Ting, C.; Zihua, K. Progress of lithium resources and development technology in China. Guangdong Trace Element Science 2007, 6–9. [Google Scholar] [CrossRef]
- Wenlong, Z.; Wanfu, H. Overview of domestic and foreign lithium mineral resources and their beneficiation process. Modern Mining 2010, 26, 1–4. [Google Scholar]
- Xuejing; Shanying, H. Analysis of the current situation and prospect of lithium industry in China. Chemical Progress 2011, 30, 782–787. [Google Scholar] [CrossRef]
- Zhiyong, G. Fundamental study on the relationship between crystal anisotropy and flotation behavior of three calcium-bearing minerals. PhD, Central South University, 2013.
- Yu Fushun, W.Y. Theory and practice of lithium pyroxene ore flotation; Changsha: Central South University Press: 2015; p. 141.
- Moon, K.S.; Fuerstenau, D.W. Surface crystal chemistry in selective flotation of spodumene (LiAl[SiO3]2) from other aluminosilicates. International Journal of Mineral Processing 2003, 72, 11–24. [Google Scholar] [CrossRef]
- Yang, B.; Wanshun, C.; Weixiang, W.; Hongyun, A.; Fushun, Y.; Pingke, Y. Synergistic mechanism of combined anionic and cationic collecting agents at the gas-liquid interface of lithium mica flotation. Mineral Conservation and Utilization 2023, 43, 44–49. [Google Scholar] [CrossRef]
- Yaoyang, R.; Chuanli, W.; Huihua, L.; Wei, X.; Anyu, T.; Ru'an, C.; Bonar. , D. Flotation separation of quartz and dolomite from phosphate ore with cotton oil acid soap complex collector. Chemical Minerals and Processing 2022, 51, 9–14. [Google Scholar] [CrossRef]
- Zhengwu, W.; Ganzuo, L.; Juncheng, L.; Daren, G.; Anjing, L. Further study on the theory of compounding and synergism of non-ideal binary surfactants. Journal of Chemical Physics 2001, 426–432. [Google Scholar]
- Liu, L.; Yuhua, W.; Guangli, Z.; Fushun, Y.; School, G.D.; Dongfang, L.; Xiayu, Z. Application and mechanism of mixed collector flotation of lithium pyroxene. Chinese Journal of Nonferrous Metals 2020, 30, 675–683. [Google Scholar]
- Liu, R.; Wei, S.; Mu, F.; Zhi, M.; Jiao, J. Mechanism of combined collector flotation of lithium pyroxene. Chinese Journal of Nonferrous Metals 2018, 28, 612–617. [Google Scholar] [CrossRef]
- Liu, R.; Wei, S.; Mu, F.; Zhi, M.; Jiao, J. Study on the mechanism of action of a new collecting agent for flotation of lithium pyroxene. Non-ferrous metals (mineral processing part) 2018, 87-90+98.
- Kaiqian, S. Enhanced flotation separation and mechanism of action of combined anionic and cationic collecting agents on lithium pyroxene ore. Master, Southwest University of Science and Technology, 2021.
- Mu, F.; Wei, S.; Liu, R.; Zhi, M. Study on the synergistic effect of combined collecting agents in lithium pyroxene flotation. Non-ferrous metals (mineral processing part) 2015, 96-100.
- Vidyadhar, A.; Rao, K.H.; Chernyshova, I.V. Mechanisms of amine–feldspar interaction in the absence and presence of alcohols studied by spectroscopic methods. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2003, 214, 127–142. [Google Scholar] [CrossRef]
- Vidyadhar, A.; Rao, K.H.; Chernyshova, I.V.; Pradip; Forssberg, K. S.E. Mechanisms of Amine–Quartz Interaction in the Absence and Presence of Alcohols Studied by Spectroscopic Methods. Journal of Colloid and Interface Science 2002, 256, 59–72. [Google Scholar] [CrossRef]
- Fengchun, L.; Jiadi, L. Separation of quartz-feldspar by flotation with mixed anionic and cationic trapping agents. China Mining 2000, 62–63. [Google Scholar]
- Kou, J.; Tao, D.; Xu, G. A study of adsorption of dodecylamine on quartz surface using quartz crystal microbalance with dissipation. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2010, 368, 75–83. [Google Scholar] [CrossRef]
- Haiqiang, F.; Yuhua, W. A review of lithium pyroxene flotation collecting agents and their conformational relationships. Rare Metals 2022, 46, 1083–1096. [Google Scholar] [CrossRef]
- Jia, T.; Longhua, X.; Wei, D.; Fa-Cheng, Y.; Houqin, W.; Jing, L.; Zhen, W. Mixed-collector flotation separation of lithium pyroxene from feldspar and its mechanism. Journal of Central South University (Natural Science Edition) 2018, 49, 511–517. [Google Scholar]
- Wang Dianzuo, H.Y. Flotation solution chemistry; Changsha: Hunan Science and Technology Press: 1988; p. 343.
| Chemical Composition | Li2O | Na2O | K2O | SiO2 | Al2O3 | Fe2O3 |
|---|---|---|---|---|---|---|
| spodumene | 7.86 | 0.15 | 0.043 | 62.477 | 27.434 | 0.133 |
| albite | — | 11.60 | 0.144 | 66.432 | 20.584 | 0.253 |
| Element | Li2O | P2O5 | MnO | Cs2O | Rb2O | Na2O | MgO | K2O | Fe2O3 |
| Content (%) | 1.19 | 0.30 | 0.15 | 0.012 | 0.12 | 3.95 | 0.022 | 2.27 | 0.80 |
| Element | Al2O3 | SiO2 | CaO | BeO* | Sn* | Ta2O5* | Nb2O5* | Ga* | TFe |
| Content (%) | 16.85 | 74.36 | 0.23 | 338 | 98.4 | 68. 5 | 90.1 | 29.4 | 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




