Submitted:
20 July 2023
Posted:
21 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Model Development and Process Simulation
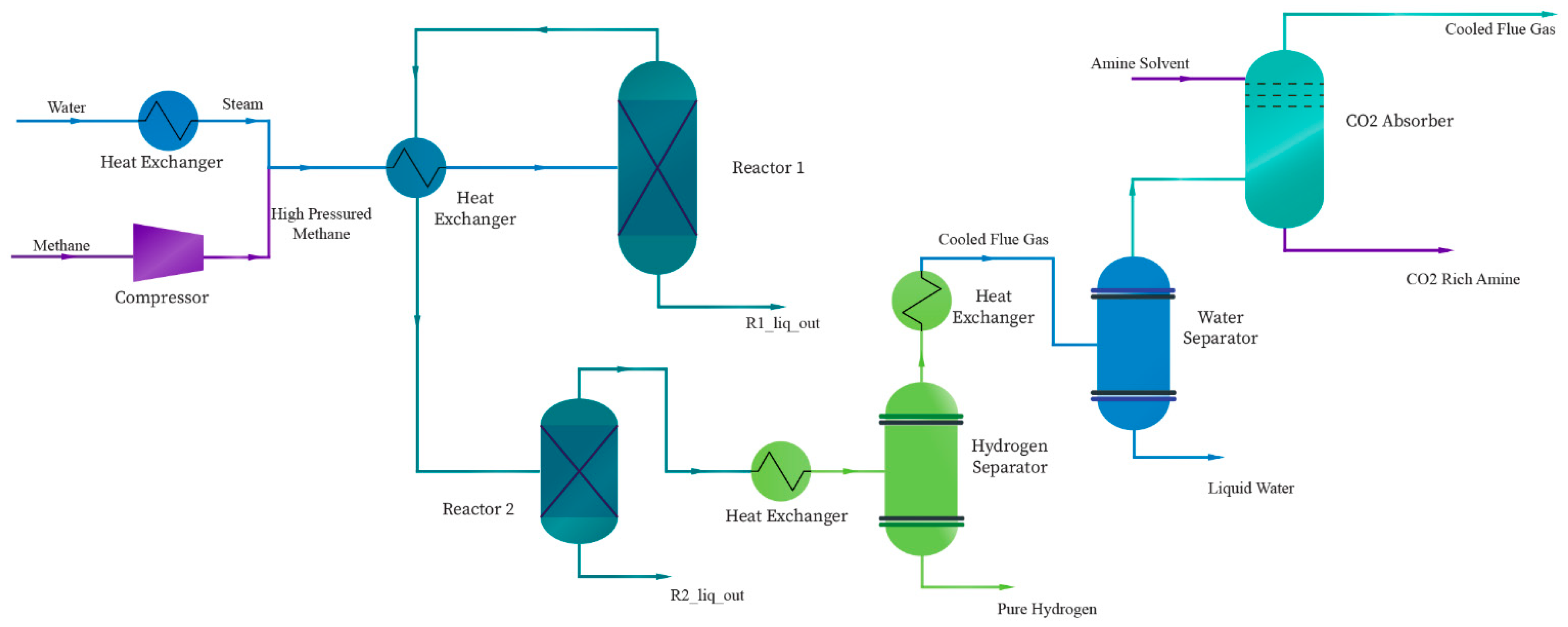
2.1. System Description
2.2. Model Assumptions
- All components possess adiabatic boundaries.
- Kinetic exergy and potential exergy overlooked for all the system components.
- Usage of catalyst is considered.
- Formation of coke is overlooked due to negligible concentration.
- The outlet stream temperature of the reactors is considered as reactor’s temperature.
- Tray efficiency in absorption column is assumed to be 100%.
2.3. Model Description
2.4. Fluid Packages
2.4.1. Peng-Robinson
2.4.2. Acid Gas – Chemical solvent
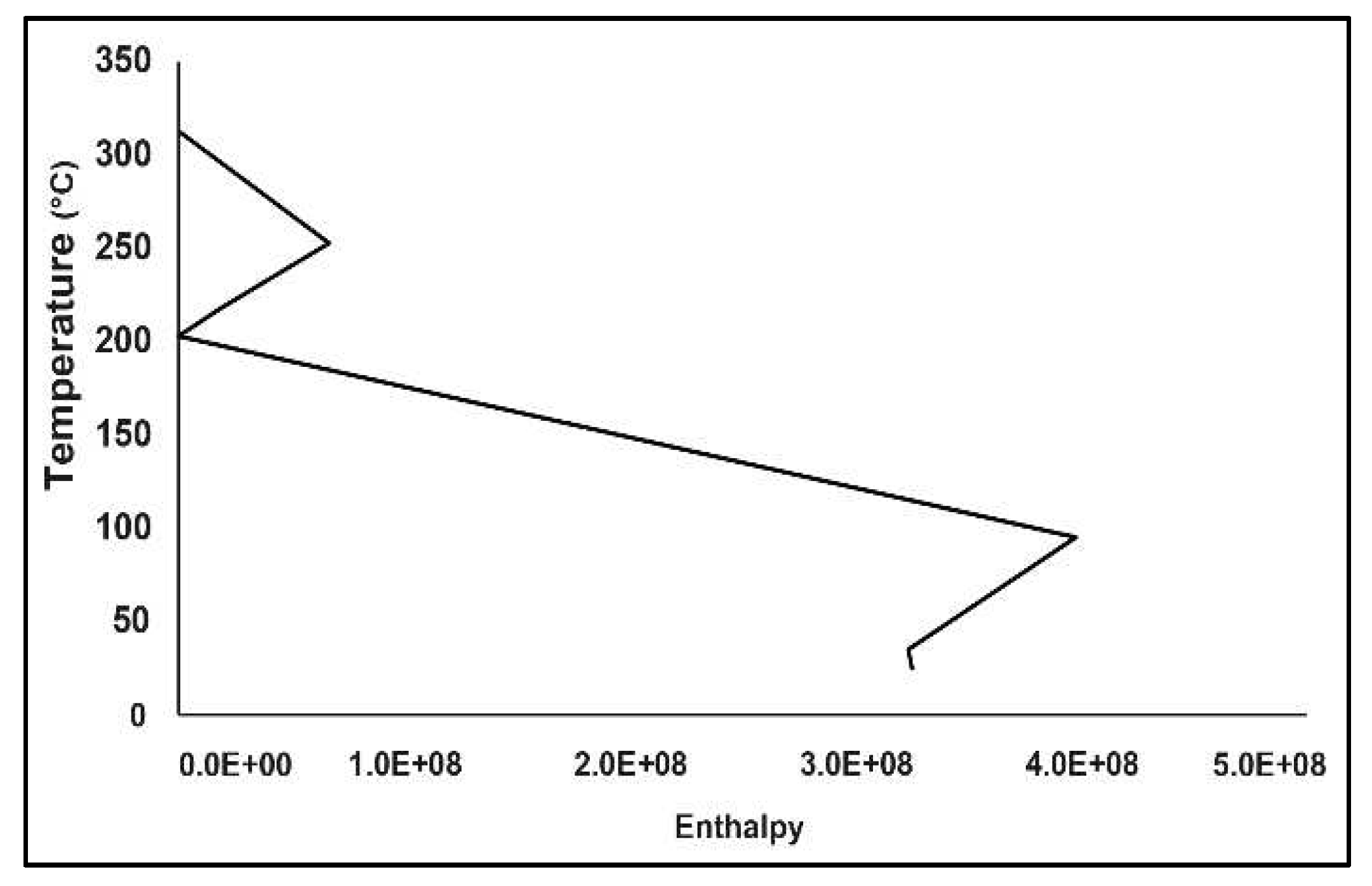
2.5. Pinch Analysis
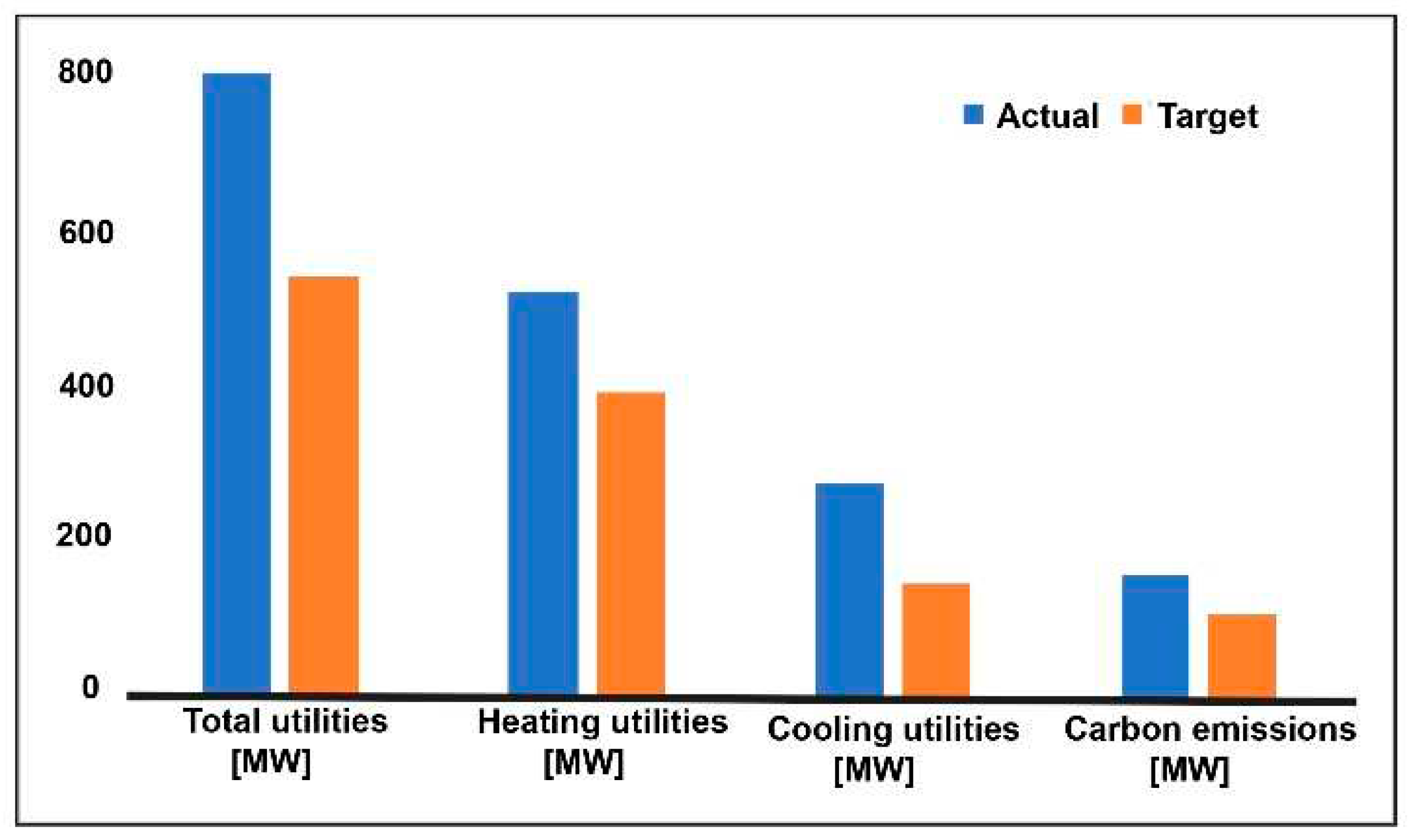
3. Results and Discussion
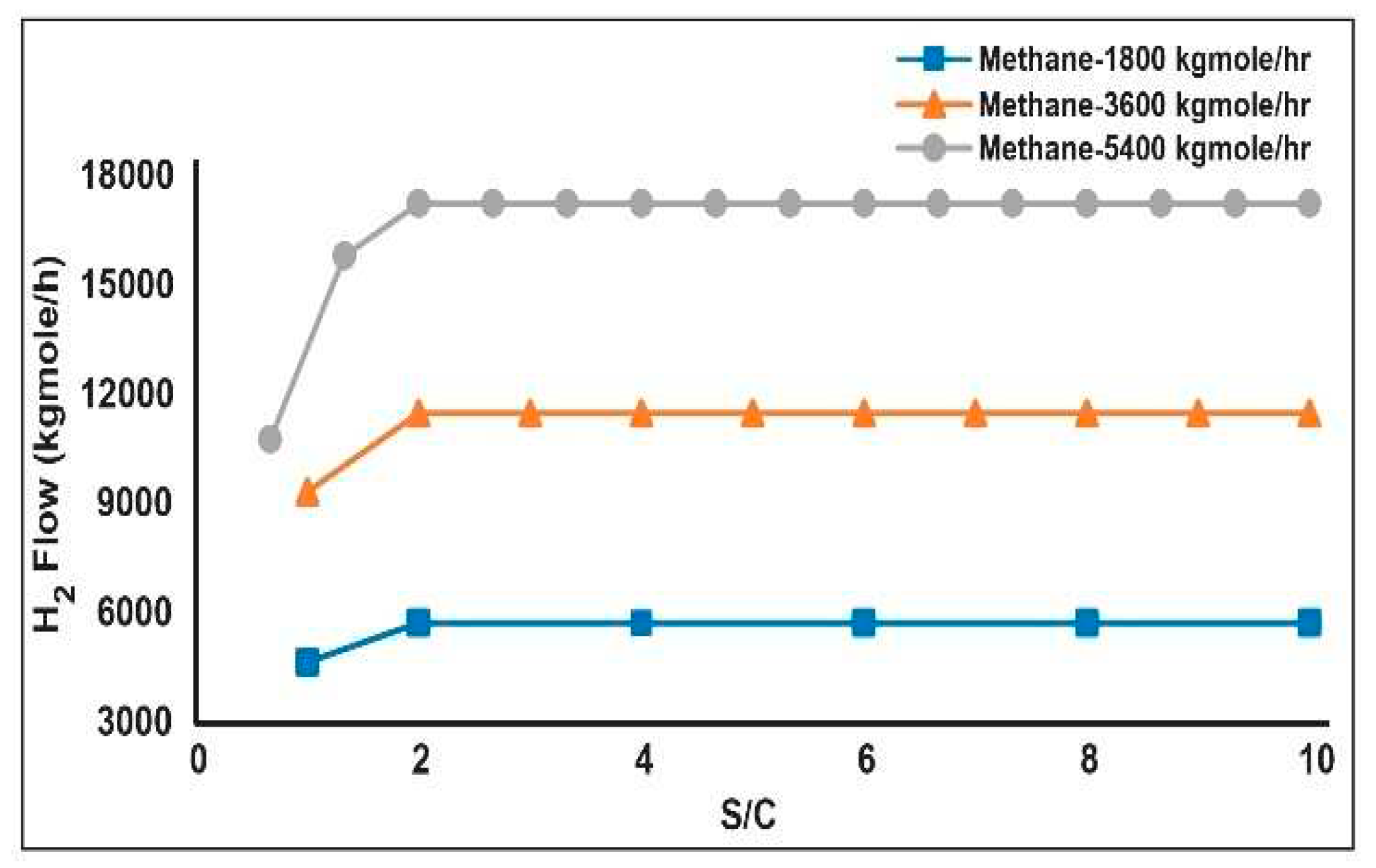
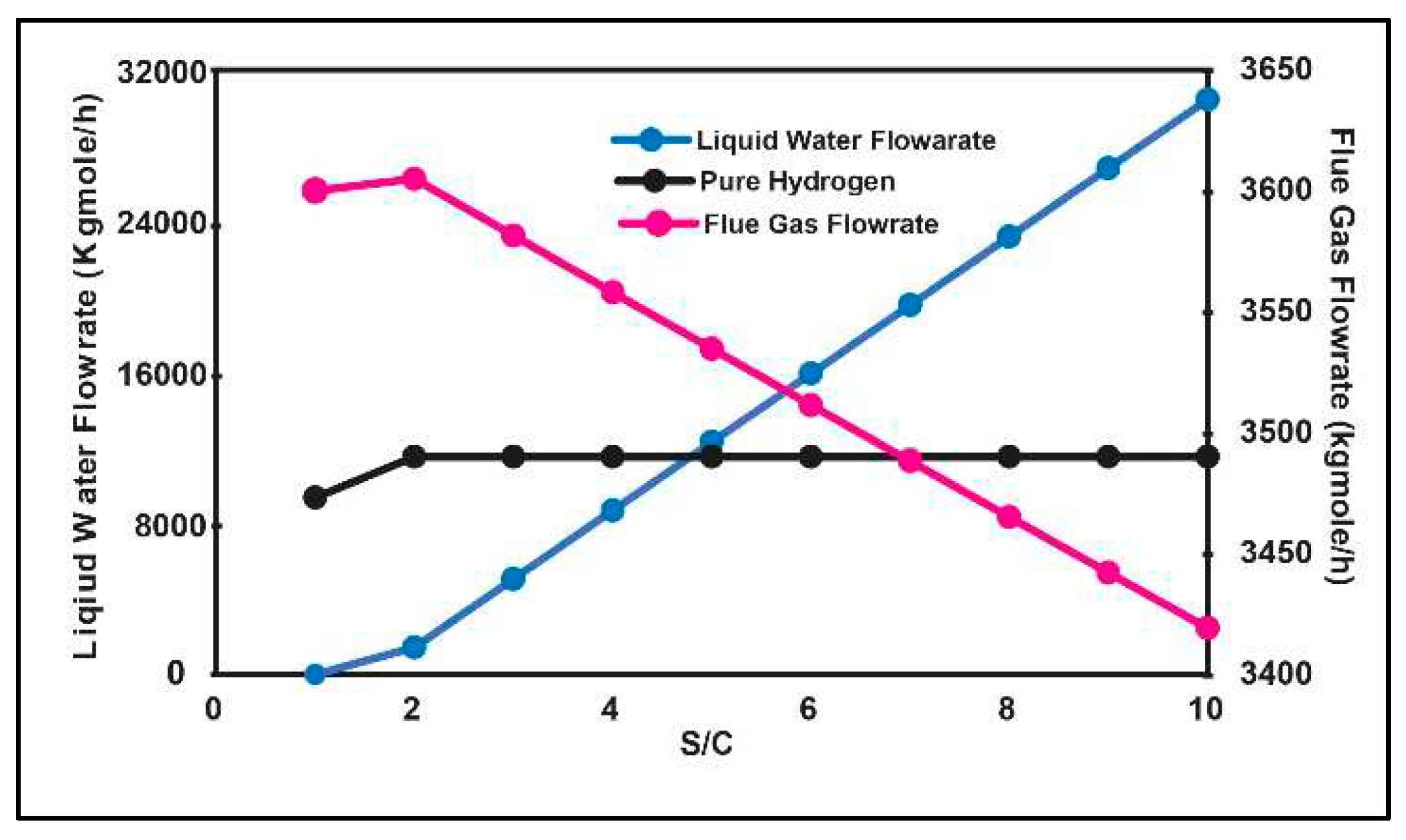
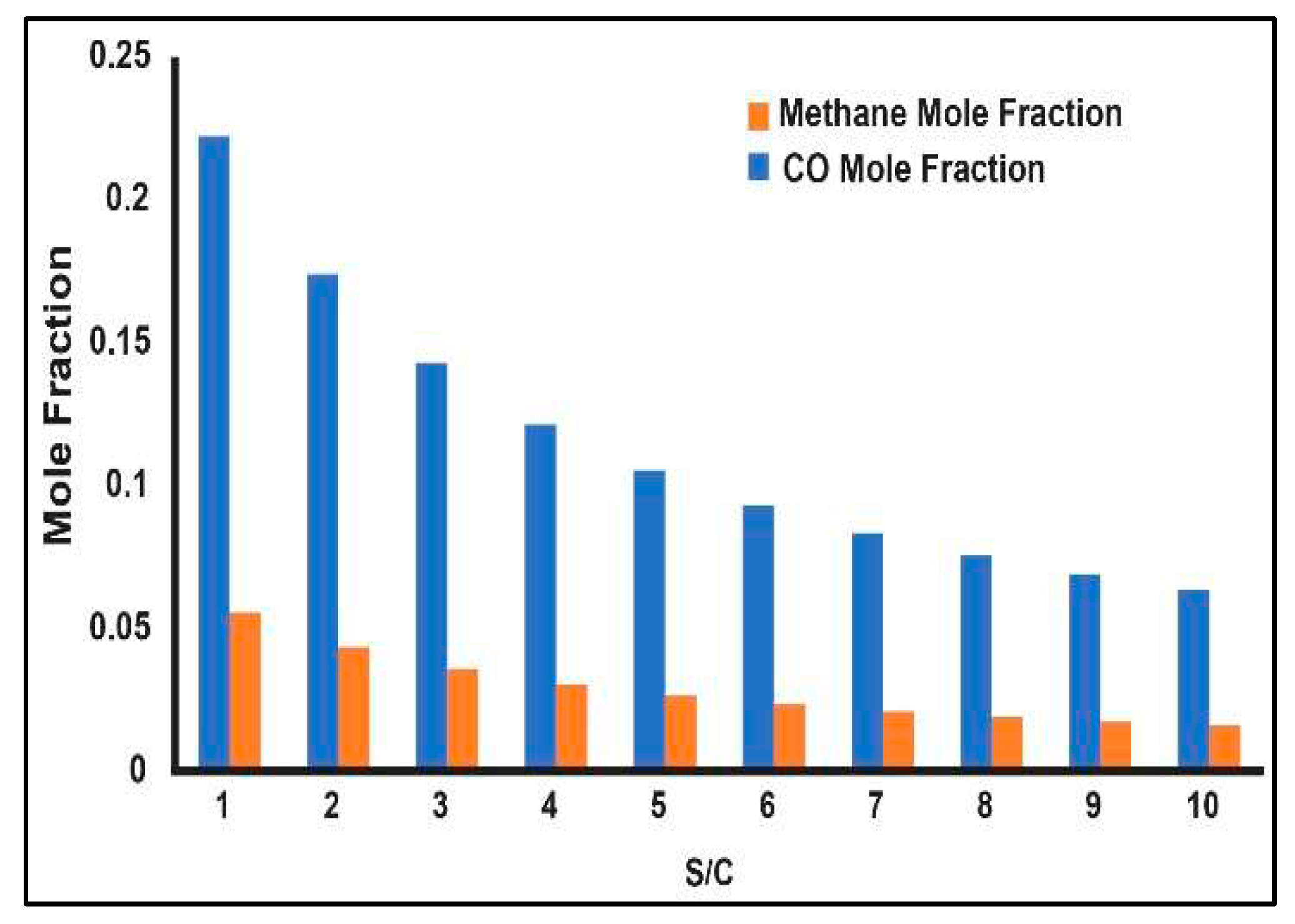
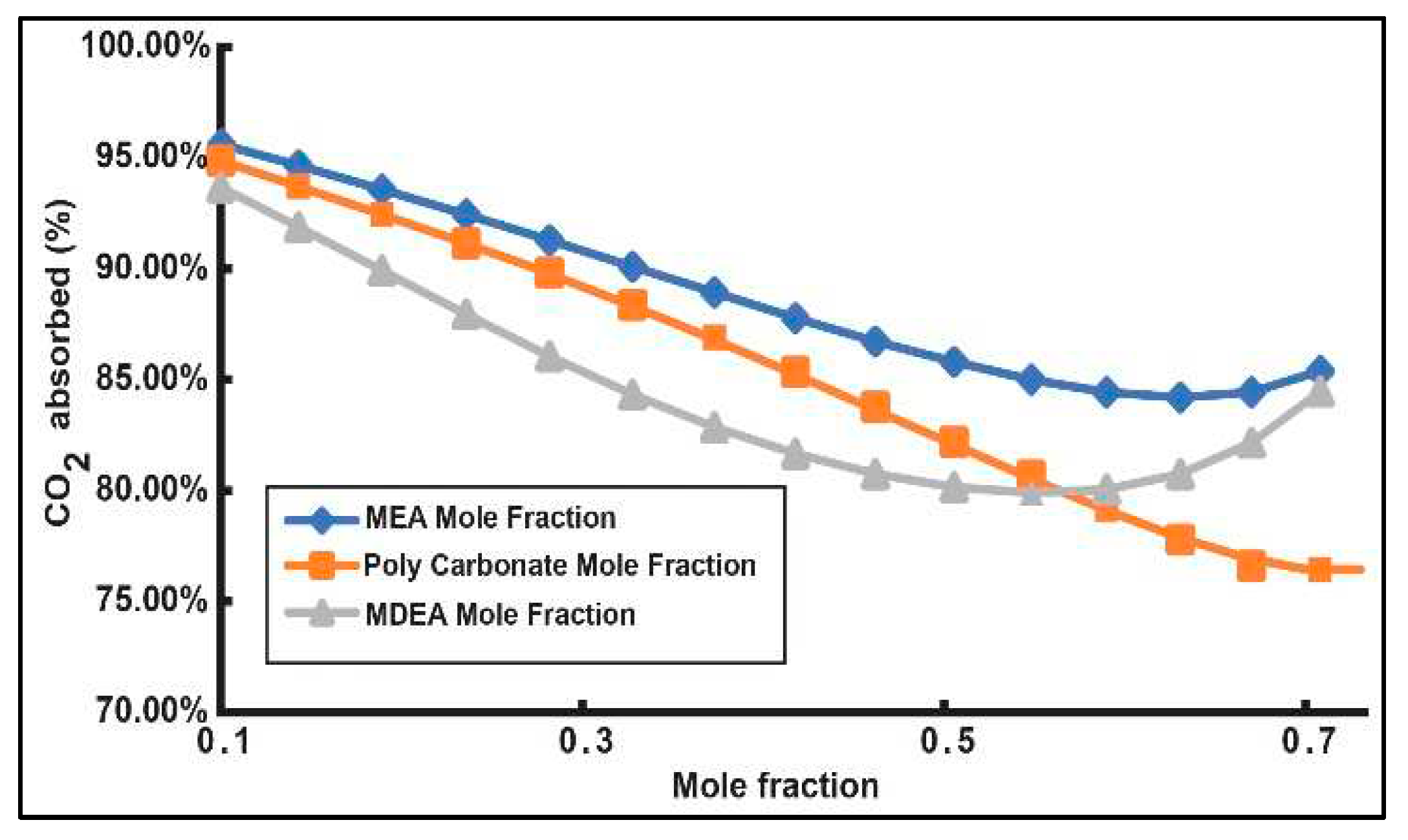
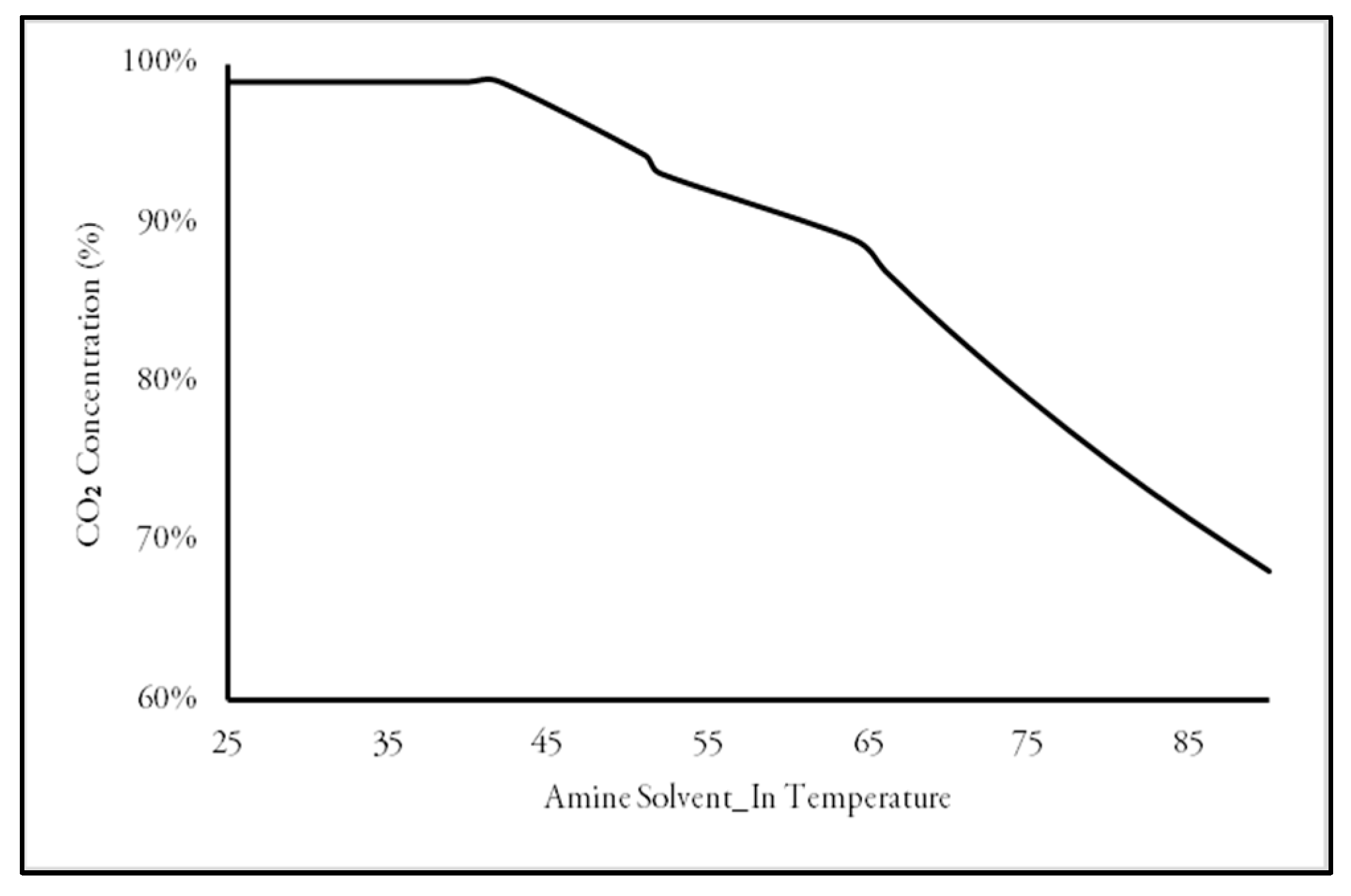
3.1. Production Variation with Change of Process Parameters
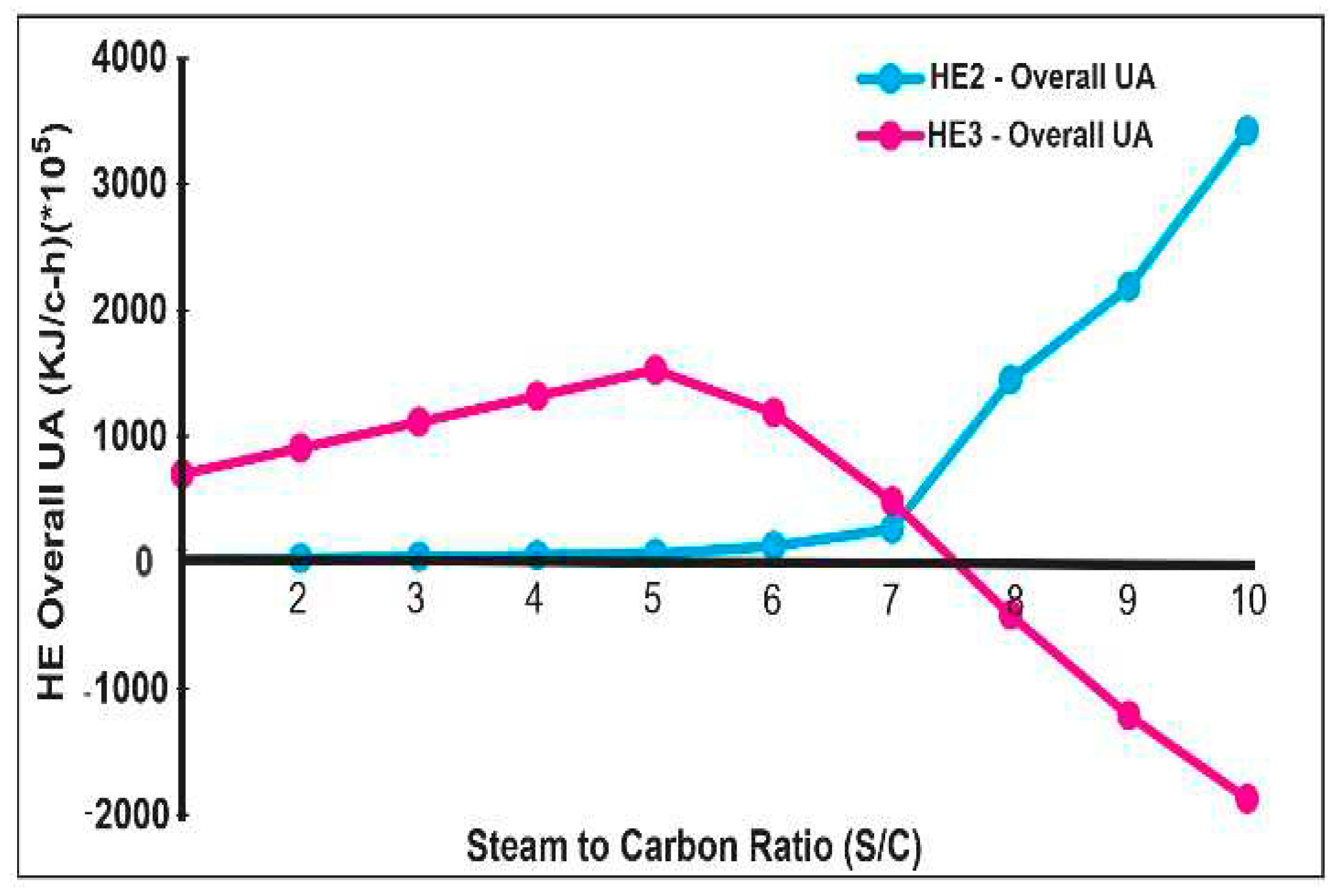
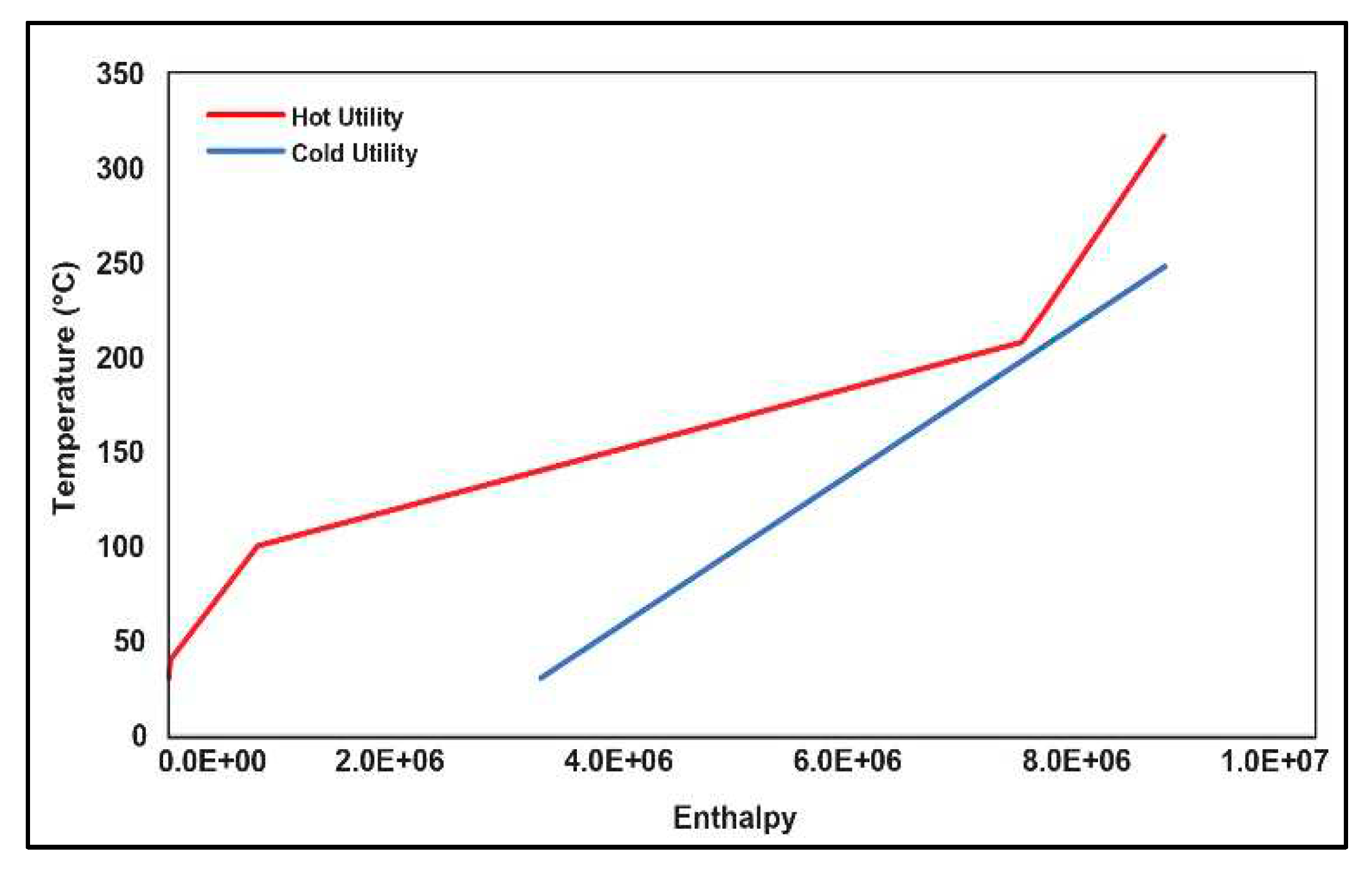
3.2. Pinch Analysis:
- Stream 1, from the initial water to the mixer
- Stream 2, from the output of compressor 1 to the input of compressor 2
- Stream 3, from the output of the hydrogen splitter to the input of the water separator
- Stream 4, from the output steam turbine in the Rankine cycle subsystem to the water pump.
| Stream No | Stream Type | Start Temperature (℃) | Target Temperature (℃) | (kW) |
|---|---|---|---|---|
| 1 | Cold | 30 | 247.7 | |
| 2 | Hot | 222.6 | 30 | |
| 3 | Hot | 316.7 | 40 | |
| 4 | Hot | 207.8 | 99.96 |
3.3. Reformer 1 Yield Variation
| Reformer Yield |
Production (kgmole/hr) |
Flue Gas Formation (kgmole/hr) |
Formation (kgmole/hr) |
Removal (%) in Absorber | Amine Used (kgmole/ hr) |
|---|---|---|---|---|---|
| 80 | 11520 | 8640 | 2880 | 100 | 14450 |
| 70 | 10080 | 9360 | 2520 | 100 | 15000 |
| 60 | 8640 | 10080 | 2160 | 99.99 | 16200 |
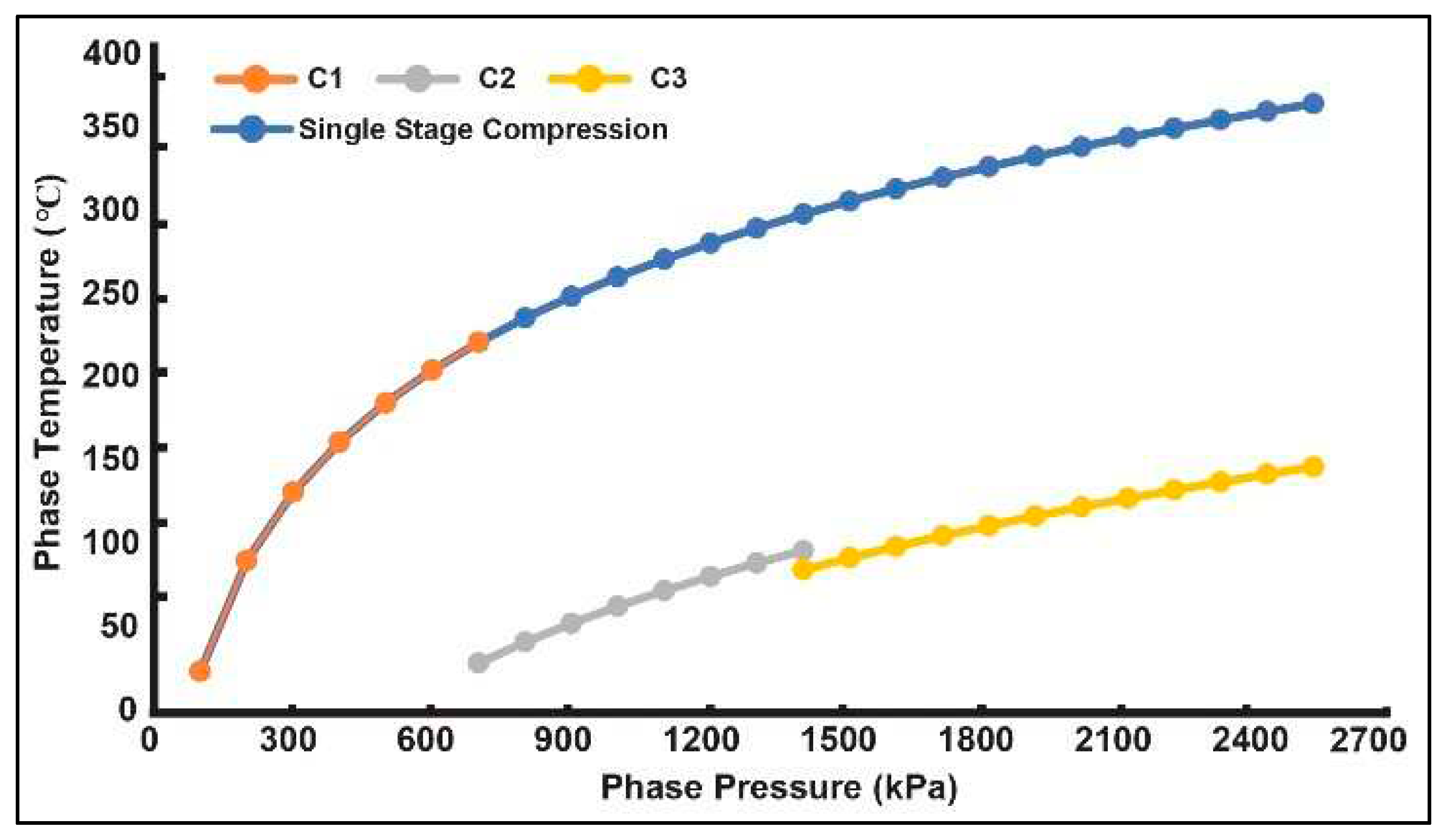
3.3.1. Multi-Stage Compression
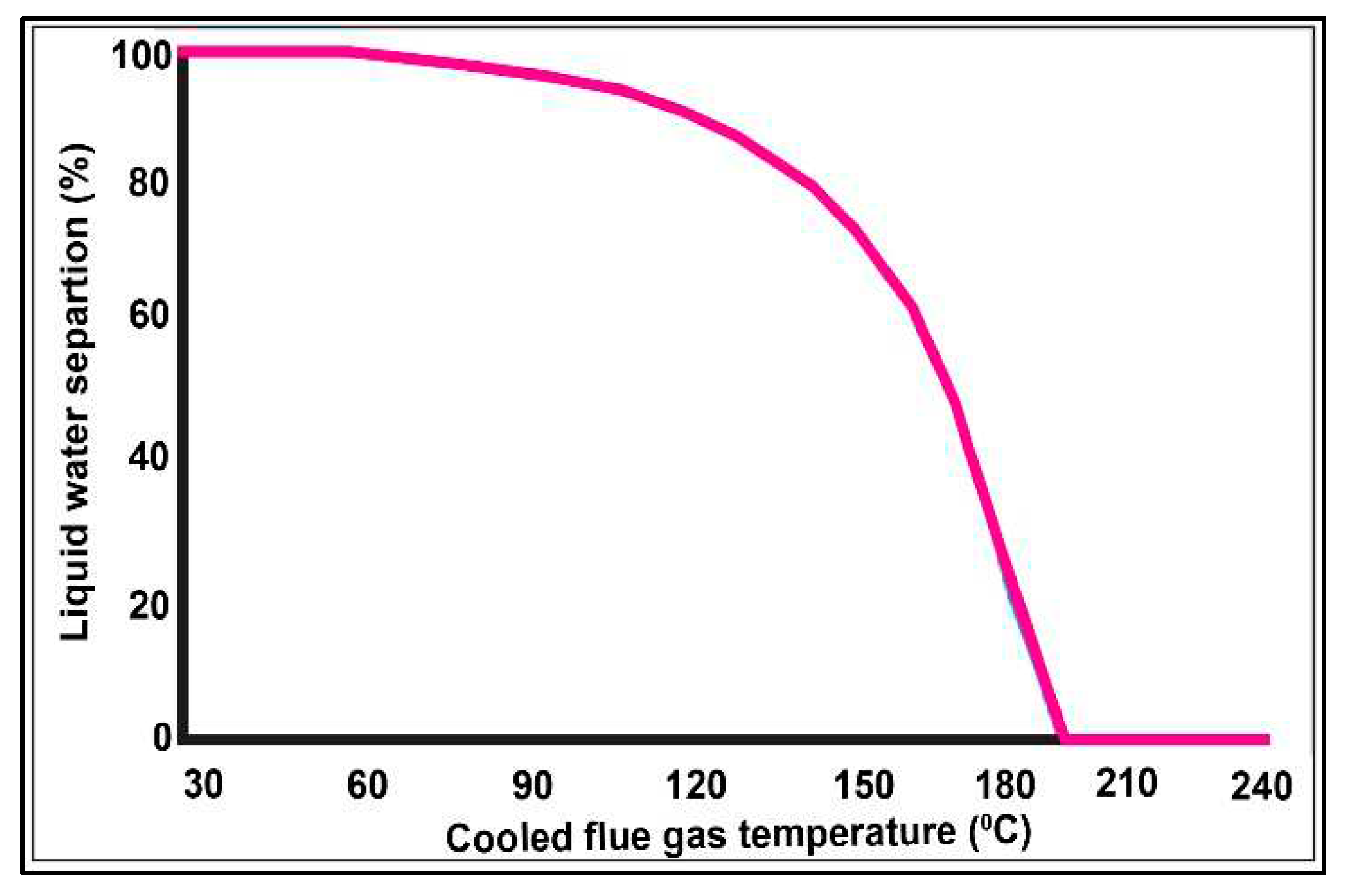
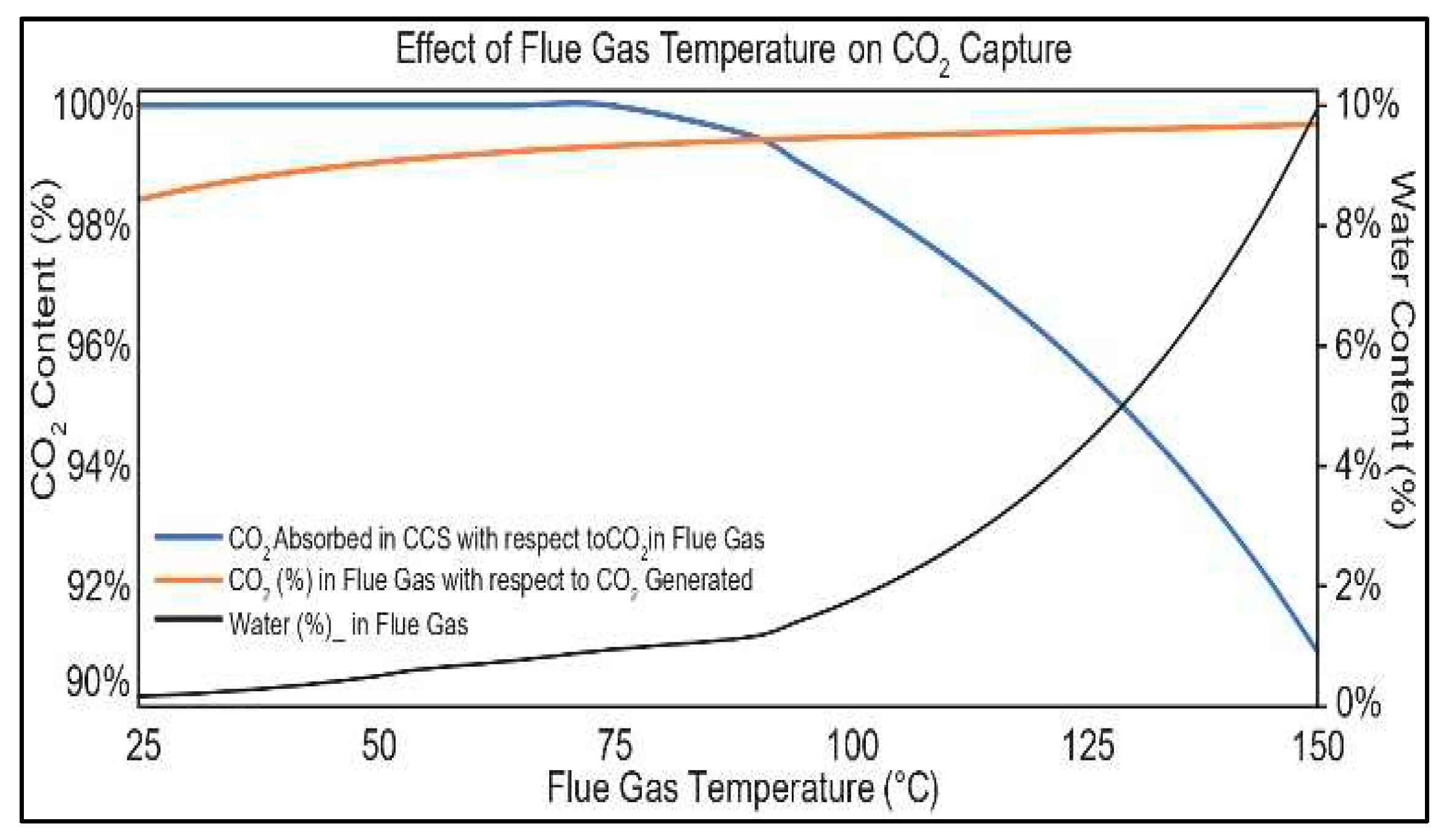
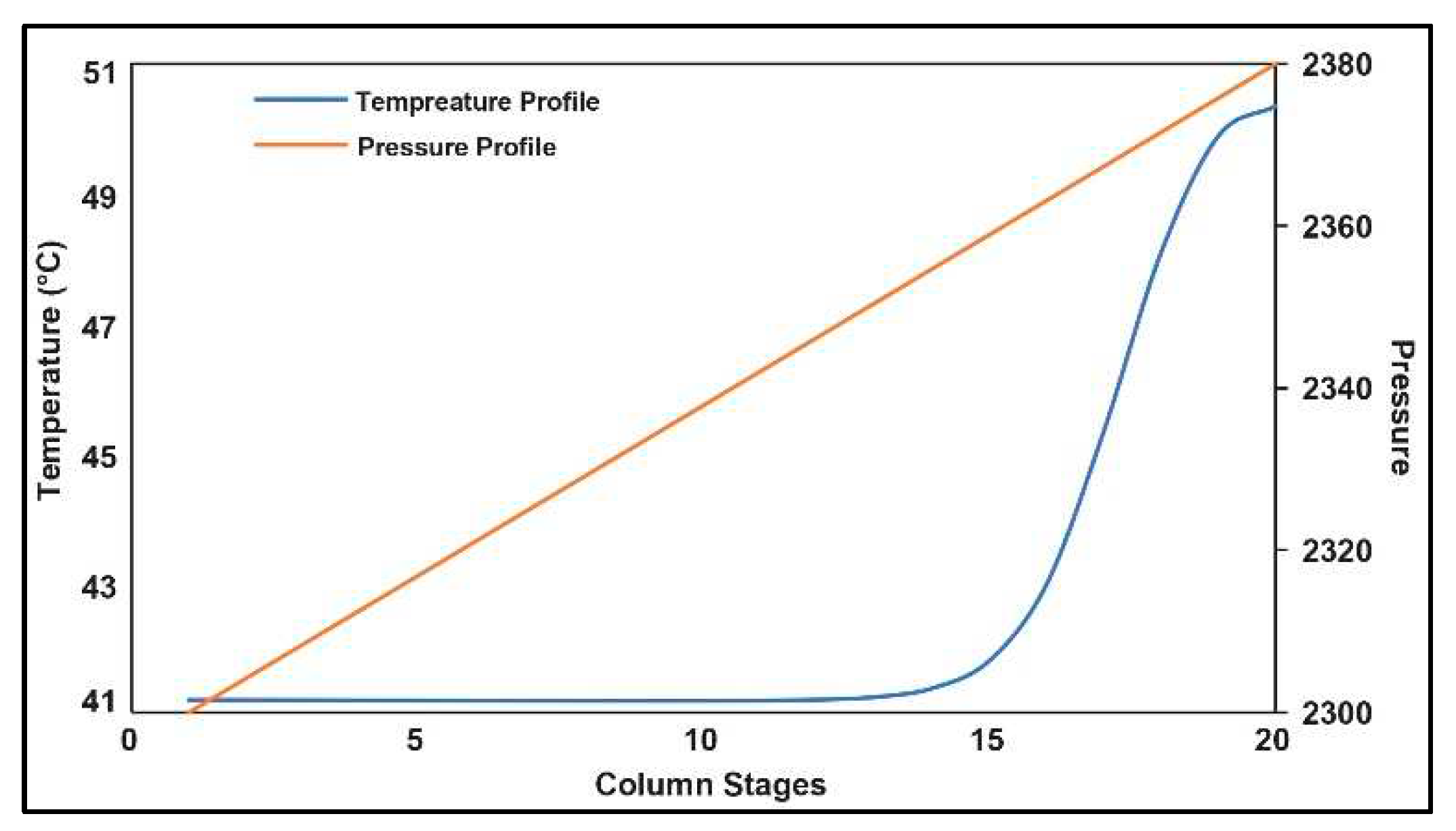
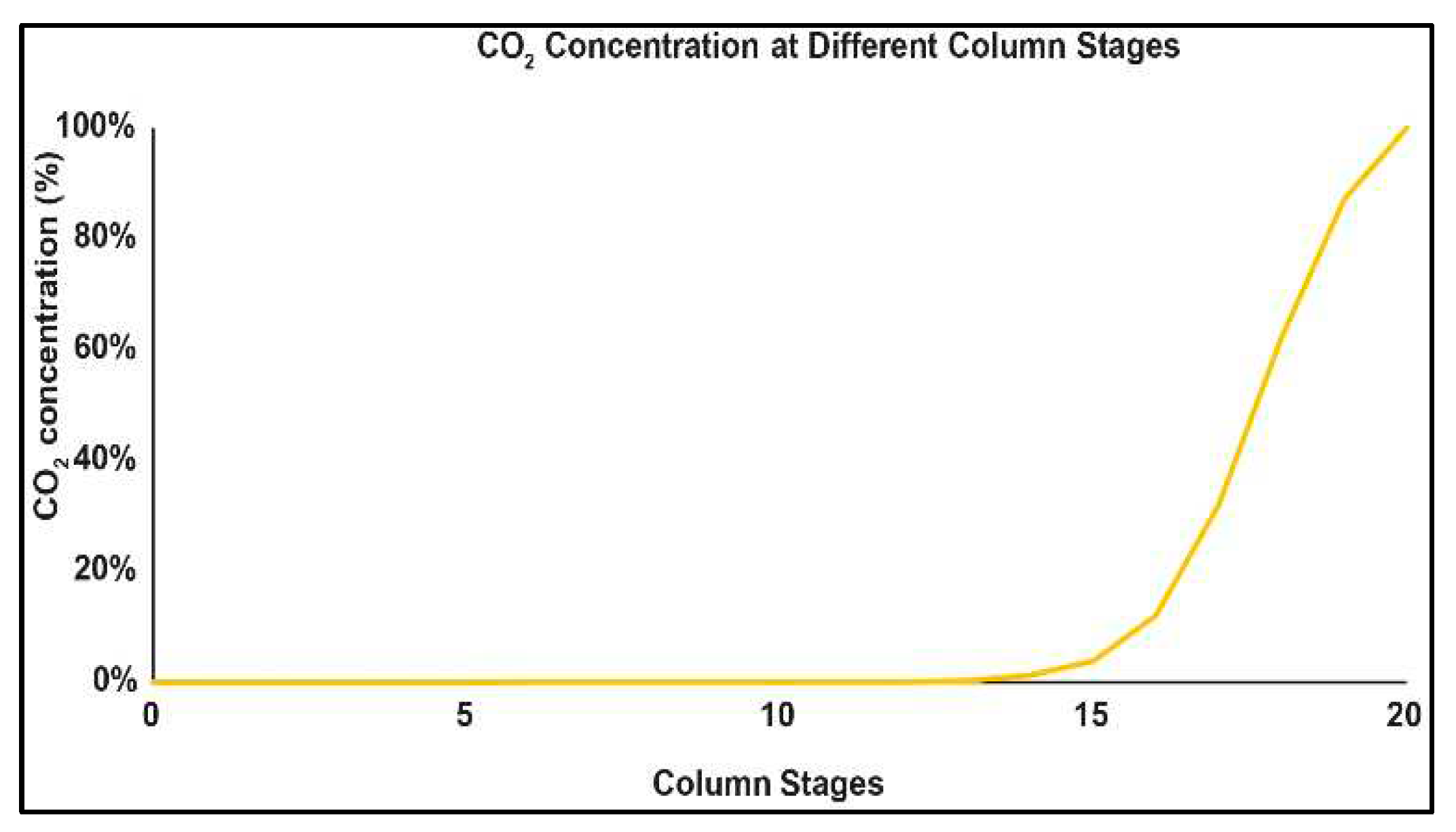
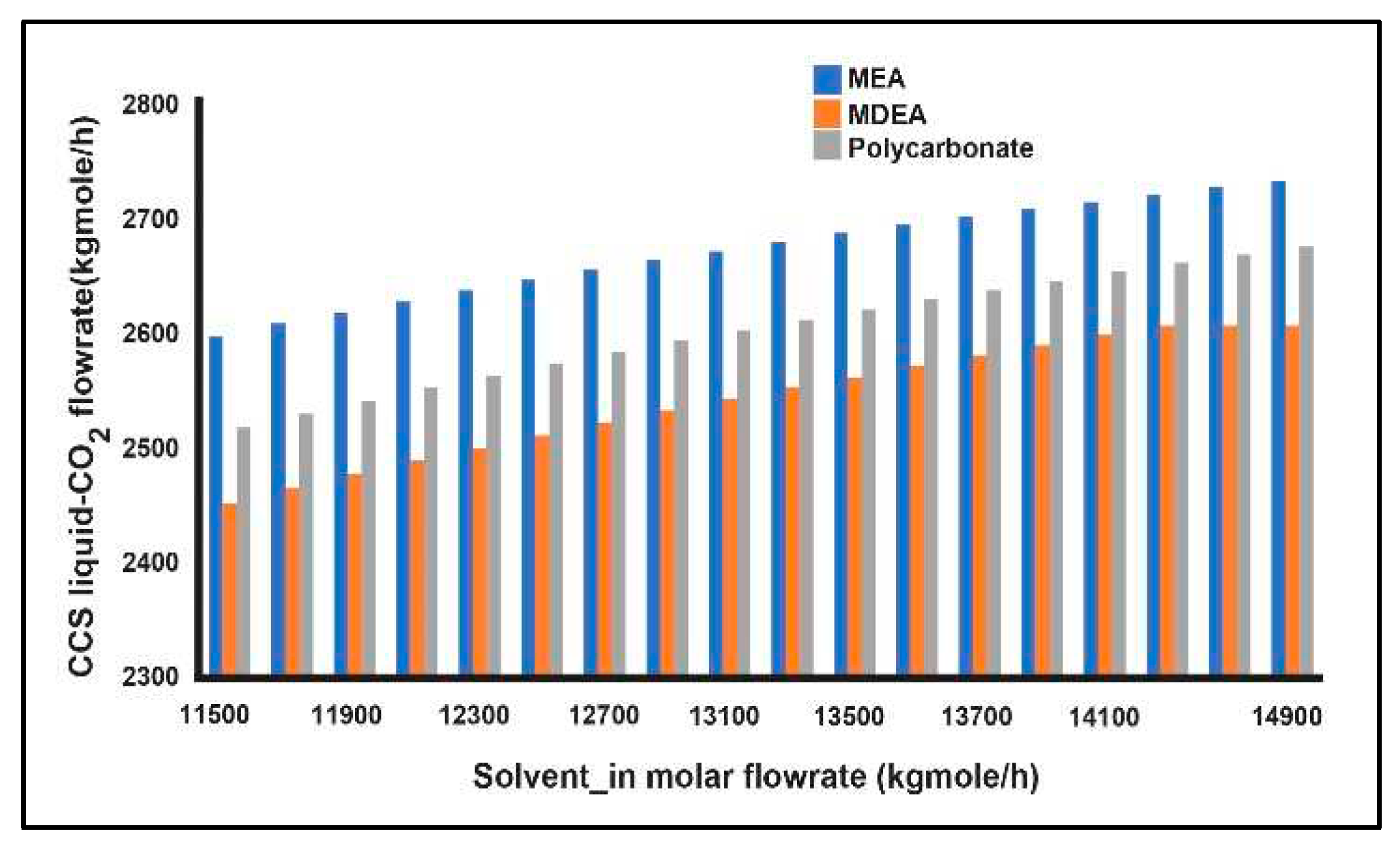
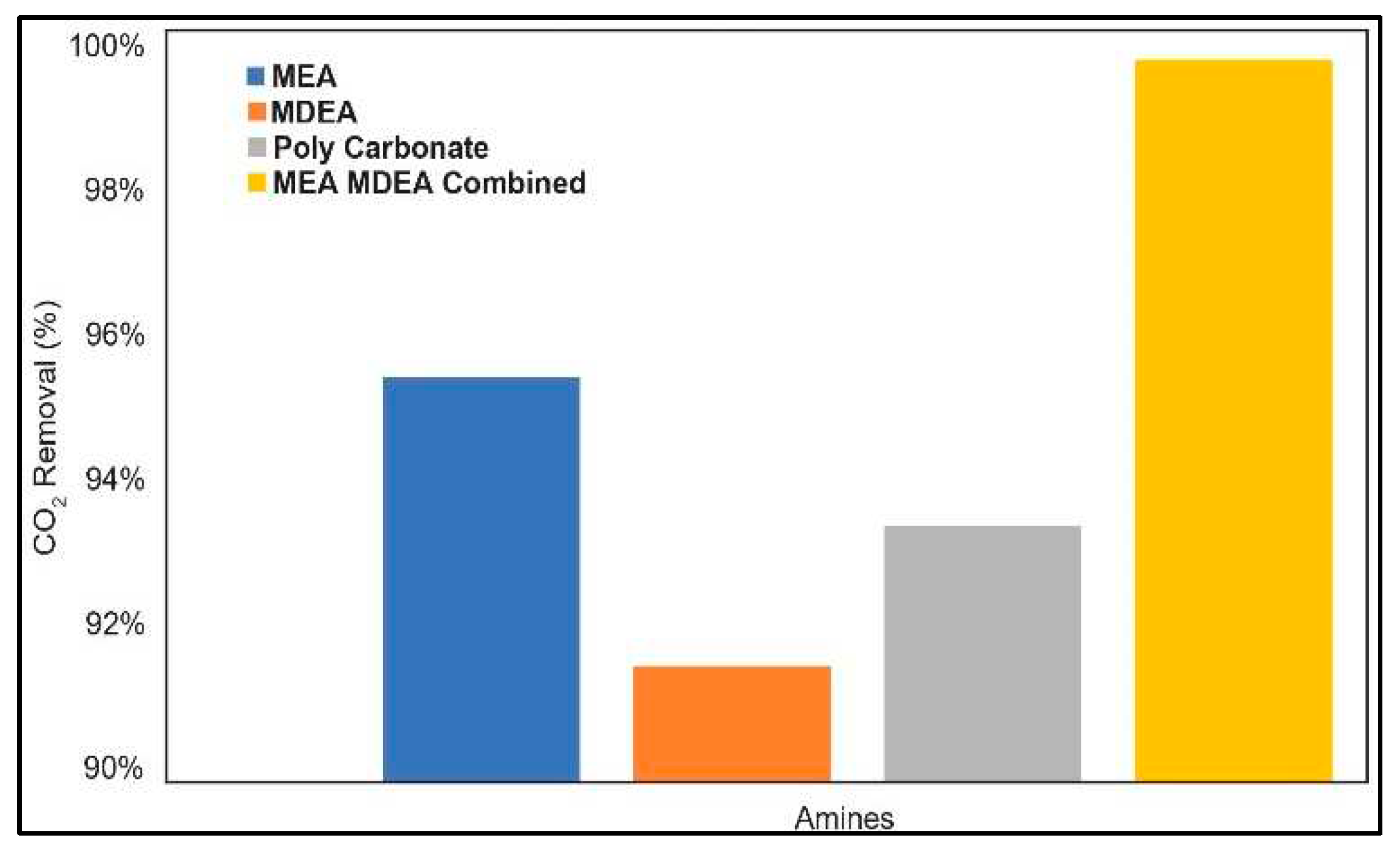
3.5. Carbon Capture System (CCS)
3.6. ASPEN Energy Analyzer
4. Conclusion
Author Contributions
Funding
Conflicts of Interest
Appendix A
| SMR | Steam Methane Reforming |
| LHV | Lower Heating Value |
| MDEA | Methyl Diethanolamine |
| DEA | Diethanolamine |
| MEA | Mono Ethanolamine |
| CCS | Carbon Capture and Storage |
| PCC | Post Combustion Capture |
| PA | Pinch Analysis |
| WGS | Water Gas Shift |
| COP | Climate Change Conference of Parties |
| DGA | Diglycolamine |
| DIPA | Di-isopropanolamine |
| PZ | Piperazine |
| TEA | Triethanolamine |
References
- Asadi, J.; Kazempoor, P. Techno-economic analysis of membrane-based processes for flexible CO2 capturing from power plants. Energy Convers Manag. 2021, 246, 114633. [Google Scholar] [CrossRef]
- Hossain, M.R.; Khatun, A.A.; Ahmed, M.F.; Faroque, M.O.; Sobahan, M.A. Experimental Behavior of Bituminous Mixes with Waste Concrete Aggregate Experimental Behavior of Bituminous Mixes with Waste Concrete Aggregate. 2020, 9, 31–50.
- The Intergovernmental Panel on Climate Change (IPCC). Global Warming of 1.5 oC. Published 2018. Available online: https://www.ipcc.ch/sr15/ (accessed on 25 December 2022).
- Fetisov, V.; Gonopolsky, A.M.; Zemenkova, M.Y.; et al. On the Integration of CO2 Capture Technologies for an Oil Refinery. Energies (Basel). 2023, 16, 865. [Google Scholar] [CrossRef]
- Felseghi, R.A.; Carcadea, E.; Raboaca, M.S.; TRUFIN, C.N.; Filote, C. Hydrogen Fuel Cell Technology for the Sustainable Future of Stationary Applications. Energies (Basel). 2019, 12, 4593. [Google Scholar] [CrossRef]
- Khojasteh Salkuyeh, Y.; Saville, B.A.; MacLean, H.L. Techno-economic analysis and life cycle assessment of hydrogen production from natural gas using current and emerging technologies. Int J Hydrogen Energy. 2017, 42, 18894–18909. [Google Scholar] [CrossRef]
- International Energy Agency. Putting CO2 to Use- Creating Value from Emissions; 2019. [Google Scholar]
- RENEE CHO. Capturing Carbon’s Potential: These Companies Are Turning CO2 into Profits. State of Planet, Columbia Climate School. May 29, 2019. 29 May.
- Ahmed, F.; Hutton-prager, B. Influence of Bulk and Surface Interactions from Thick, Porous, Soil-based Substrates on the Spreading Behavior of Different Viscosity Oils. Environmental Challenges. 2021, 3, 100045. [Google Scholar] [CrossRef]
- Chakraborty, S.C.; Qamruzzaman, M.; Zaman, M.W.U.; et al. Metals in e-waste: Occurrence, fate, impacts and remediation technologies. Process Safety and Environmental Protection 2022. [Google Scholar] [CrossRef]
- Jolaoso, L.; Zaman, S.F. Catalytic Ammonia Decomposition for Hydrogen Production: Utilization of Ammonia in a Fuel Cell. In: Inamuddin, Boddula, R.; Asiri, A.M.; eds. Sustainable Ammonia Production. Springer International Publishing; 2020; pp. 81-105. [CrossRef]
- U.S. Department of Energy. Hydrogen Production and Distribution | Alternative Fuels Data Center. Available online: https://afdc.energy.gov/fuels/hydrogen_production.html (accessed on 31 December 2022).
- Clean Hydrogen Partnership, European Union. Hydrogen Roadmap Europe: A Sustainable Pathway for the European Energy Transition. Published 2019. Available online: https://www.h2haul.eu.
- Fuel Cell & Hydrogen Energy Association (FCHEA). US. Road Map to a US Hydrogen Economy: Reducing Emissions and Driving Growth Across the Nation. Published 2019. Available online: www.fchea.org.
- Gummer, J. Hydrogen in a Low-Carbon Economy. Committee on Climate Change. Published 2018. Available online: https://www.thecec.org.uk/wp-content/%20uploads/2018/11/Hydrogen-in-a-low-carbon-economy.
- Bruce, S. National Hydrogen Roadmap- Pathways to an Economically Sustainable Hydrogen Industry in Australia. AUSTRALIA’S NATIONAL SCIENCE AGENCY. Published 2018. Available online: https://www.csiro.au.
- BMWi, Federal Ministry of Education and Research. The National Hydrogen Strategy: The Federal Government. Published 2021. Available online: https://www.bmbf.de/files/bmwi_Nationale%20Wasserstoffstrategie_Eng_s01.pdf.
- IEA. The Future of Hydrogen: Seizing Today’s Opportunities. International Energy Agency. Published 2019. Available online: https://www.iea.org.
- Amran, U.I.; Ahmad, A.; Othman, M.R. Kinetic based simulation of methane steam reforming and water gas shift for hydrogen production using aspen plus. Chem Eng Trans. 2017, 56, 1681–1686. [Google Scholar]
- Zamaniyan, A.; Behroozsarand, A.; Ebrahimi, H. Modeling and simulation of large scale hydrogen production. J Nat Gas Sci Eng. 2010, 2, 293–301. [Google Scholar] [CrossRef]
- Department of Energy. Fact of the month May 2018: 10 million metric tons of hydrogen produced annually in the United States. Published May 2018. Available online: https://www.energy.gov/eere/fuelcells/fact-month-may-2018-10-million-metric-tons-hydrogen-produced-annually-united-states (accessed on 31 December 2022).
- Kumar, A.; Singh, R.; Sinha, A.S.K. Catalyst modification strategies to enhance the catalyst activity and stability during steam reforming of acetic acid for hydrogen production. Int J Hydrogen Energy. 2019, 44, 12983–13010. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis – A review. Mater Sci Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chinese Journal of Catalysis. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Beswick, R.R.; Yan, Y. A green hydrogen economy for a renewable energy society. Curr Opin Chem Eng. 2021, 33, 100701. [Google Scholar] [CrossRef]
- Jolaoso, L.A.; Asadi, J.; Duan, C.; Kazempoor, P. A novel green hydrogen production using water-energy nexus framework. Energy Convers Manag. 2023, 276. [Google Scholar] [CrossRef]
- Pruvost, F.; Cloete, S.; Arnaiz del Pozo, C.; Zaabout, A. Blue, green, and turquoise pathways for minimizing hydrogen production costs from steam methane reforming with CO2 capture. Energy Convers Manag. 2022, 274, 116458. [Google Scholar] [CrossRef]
- National Energy Technology Laboratory (NETL), Mission Execution and Strategic Analysis (MESA). COST AND PERFORMANCE BASELINE FOR FOSSIL ENERGY PLANTS VOLUME 1: BITUMINOUS COAL AND NATURAL GAS TO ELECTRICITY; 2019. [Google Scholar]
- Jiang, Y.; Mathias, P.M.; Freeman, C.J.; et al. Techno-economic comparison of various process configurations for post-combustion carbon capture using a single-component water-lean solvent. International Journal of Greenhouse Gas Control. 2021, 106, 103279. [Google Scholar] [CrossRef]
- DISSANAYAKE, D. Partial oxidation of methane to carbon monoxide and hydrogen over a Ni/Al2O3 catalyst. J Catal. 1991, 132, 117–127. [Google Scholar] [CrossRef]
- Alejo, L.; Lago, R.; Peña, M.A.; Fierro, J.L.G. Partial oxidation of methanol to produce hydrogen over CuZn-based catalysts. Appl Catal A Gen. 1997, 162, 281–297. [Google Scholar] [CrossRef]
- Ge, Z.; Guo, S.; Guo, L.; Cao, C.; Su, X.; Jin, H. Hydrogen production by non-catalytic partial oxidation of coal in supercritical water: Explore the way to complete gasification of lignite and bituminous coal. Int J Hydrogen Energy. 2013, 38, 12786–12794. [Google Scholar] [CrossRef]
- ONOZAKI, M.; WATANABE, K.; HASHIMOTO, T.; SAEGUSA, H.; KATAYAMA, Y. Hydrogen production by the partial oxidation and steam reforming of tar from hot coke oven gas. Fuel. 2006, 85, 143–149. [Google Scholar] [CrossRef]
- George Thomas, George Parks. Potential Roles of Ammonia in a Hydrogen Economy.; 2006.
- Barelli, L.; Bidini, G.; Gallorini, F.; Servili, S. Hydrogen production through sorption-enhanced steam methane reforming and membrane technology: A review. Energy. 2008, 33, 554–570. [Google Scholar] [CrossRef]
- Nahar, G.; Mote, D.; Dupont, V. Hydrogen production from reforming of biogas: Review of technological advances and an Indian perspective. Renewable and Sustainable Energy Reviews. 2017, 76, 1032–1052. [Google Scholar] [CrossRef]
- Pandey, B.; Prajapati, Y.K.; Sheth, P.N. Recent progress in thermochemical techniques to produce hydrogen gas from biomass: A state of the art review. Int J Hydrogen Energy. 2019, 44, 25384–25415. [Google Scholar] [CrossRef]
- Mishra, P.; Krishnan, S.; Rana, S.; Singh, L.; Sakinah, M.; Ab Wahid, Z. Outlook of fermentative hydrogen production techniques: An overview of dark, photo and integrated dark-photo fermentative approach to biomass. Energy Strategy Reviews. 2019, 24, 27–37. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Fermentative hydrogen production using various biomass-based materials as feedstock. Renewable and Sustainable Energy Reviews. 2018, 92, 284–306. [Google Scholar] [CrossRef]
- Kadier, A.; Kalil, M.S.; Abdeshahian, P.; et al. Recent advances and emerging challenges in microbial electrolysis cells (MECs) for microbial production of hydrogen and value-added chemicals. Renewable and Sustainable Energy Reviews. 2016, 61, 501–525. [Google Scholar] [CrossRef]
- Basheer, A.A.; Ali, I. Water photo splitting for green hydrogen energy by green nanoparticles. Int J Hydrogen Energy. 2019, 44, 11564–11573. [Google Scholar] [CrossRef]
- Velazquez Abad, A.; Dodds, P.E. Production of Hydrogen. In Encyclopedia of Sustainable Technologies; Elsevier, 2017; pp. 293–304. [Google Scholar] [CrossRef]
- Kothari, R.; Buddhi, D.; Sawhney, R.L. Comparison of environmental and economic aspects of various hydrogen production methods. Renewable and Sustainable Energy Reviews. 2008, 12, 553–563. [Google Scholar] [CrossRef]
- Syed, M.B. Technologies for renewable hydrogen production. In Bioenergy Resources and Technologies; Elsevier, 2021; pp. 157–198. [Google Scholar] [CrossRef]
- van Twigg, M. Catalyst Handbook; Routledge, 2018. [Google Scholar] [CrossRef]
- National Oceanic and Atmospheric Administration (NOAA). Ocean Acidification. U.S. Department of Commerce.
- Renjie Shao, Aage Stangeland. Amines Used in CO2 Capture - Health and Environmental Impacts.; 2009.
- Mathews, J.A. Carbon-negative biofuels. Energy Policy. 2008, 36, 940–945. [Google Scholar] [CrossRef]
- Wilberforce, T.; Olabi, A.G.; Sayed, E.T.; Elsaid, K.; Abdelkareem, M.A. Progress in carbon capture technologies. Science of The Total Environment. 2021, 761, 143203. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int J Hydrogen Energy. 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Budinis, S.; Krevor, S.; Dowell, N.; Brandon, N.; Hawkes, A. An assessment of CCS costs, barriers and potential. Energy Strategy Reviews. 2018, 22, 61–81. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R. Catalytic Steam Reforming. 1984; 1-117. [CrossRef]
- Christensen, K.O.; Chen, D.; Lødeng, R.; Holmen, A. Effect of supports and Ni crystal size on carbon formation and sintering during steam methane reforming. Appl Catal A Gen. 2006, 314, 9–22. [Google Scholar] [CrossRef]
- Collodi, G.; Azzaro, G.; Ferrari, N.; Santos, S. Techno-economic Evaluation of Deploying CCS in SMR Based Merchant H2 Production with NG as Feedstock and Fuel. Energy Procedia. 2017, 114, 2690–2712. [Google Scholar] [CrossRef]
- Meerman, J.C.; Hamborg, E.S.; van Keulen, T.; Ramírez, A.; Turkenburg, W.C.; Faaij, A.P.C. Techno-economic assessment of CO2 capture at steam methane reforming facilities using commercially available technology. International Journal of Greenhouse Gas Control. 2012, 9, 160–171. [Google Scholar] [CrossRef]
- Hacarlioglu, P.; Gu, Y.; Oyama, S.T. Studies of the Methane Steam Reforming Reaction at High Pressure in a Ceramic Membrane Reactor. Journal of Natural Gas Chemistry. 2006, 15, 73–81. [Google Scholar] [CrossRef]
- Oh, S.Y.; Binns, M.; Cho, H.; Kim, J.K. Energy minimization of MEA-based CO2 capture process. Appl Energy. 2016, 169, 353–362. [Google Scholar] [CrossRef]
- Hanak, D.P.; Biliyok, C.; Yeung, H.; Białecki, R. Heat integration and exergy analysis for a supercritical high-ash coal-fired power plant integrated with a post-combustion carbon capture process. Fuel. 2014, 134, 126–139. [Google Scholar] [CrossRef]
- Asadi, J.; Jolaoso, L.; Kazempoor, P. Efficiency And Flexibility Improvement of Amine-Based Post Combustion CO2 Capturing System (CCS) in Full and Partial Loads. In Proceedings of the ASME 2022 16th International Conference on Energy Sustainability ES 2022; 2022. [Google Scholar] [CrossRef]
- Choi, J.; Cho, H.; Yun, S.; et al. Process design and optimization of MEA-based CO2 capture processes for non-power industries. Energy. 2019, 185, 971–980. [Google Scholar] [CrossRef]
- Chai, S.Y.W.; Ngu, L.H.; How, B.S. Review of carbon capture absorbents for CO 2 utilization. Greenhouse Gases: Science and Technology. 2022, 12, 394–427. [Google Scholar] [CrossRef]
- Yu, C.H.; Huang, C.H.; Tan, C.S. A Review of CO2 Capture by Absorption and Adsorption. Aerosol Air Qual Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Wang, M.; Lawal, A.; Stephenson, P.; Sidders, J.; Ramshaw, C. Post-combustion CO2 capture with chemical absorption: A state-of-the-art review. Chemical Engineering Research and Design. 2011, 89, 1609–1624. [Google Scholar] [CrossRef]
- Budinis, S.; Krevor, S.; Dowell, N.M.; Brandon, N.; Hawkes, A. An assessment of CCS costs, barriers and potential. Energy Strategy Reviews. 2018, 22, 61–81. [Google Scholar] [CrossRef]
- Rossiter, A.P.; Jones, B.P. ENERGY MANAGEMENT AND EFFICIENCY FOR THE PROCESS INDUSTRIES; American Institute of Chemical Engineers and John Wiley & Sons, Inc, 2015. [Google Scholar]
- Jaber, O.; Naterer, G.F.; Dincer, I. Natural gas usage as a heat source for integrated SMR and thermochemical hydrogen production technologies. Int J Hydrogen Energy. 2010, 35, 8569–8579. [Google Scholar] [CrossRef]
- Hamdy, L.B.; Goel, C.; Rudd, J.A.; Barron, A.R.; Andreoli, E. The application of amine-based materials for carbon capture and utilisation: An overarching view. Mater Adv. 2021, 2, 5843–5880. [Google Scholar] [CrossRef]
- Aspen Technology. Inc. Aspen Tech®. Top 10 Questions About Acid Gas Removal Optimization With Aspen HYSYS®. Published 2018. Available online: https://www.aspentech.com (accessed on 31 December 2022).
- Yang, G.; Fan, Z.; Li, X. Determination of confined fluid phase behavior using extended Peng-Robinson equation of state. Chemical Engineering Journal. 2019, 378, 122032. [Google Scholar] [CrossRef]
- Aminian, A.; Celný, D.; Mickoleit, E.; Jäger, A.; Vinš, V. Ideal Gas Heat Capacity and Critical Properties of HFE-Type Engineering Fluids: Ab Initio Predictions of Cpig, Modeling of Phase Behavior and Thermodynamic Properties Using Peng–Robinson and Volume-Translated Peng–Robinson Equations of State. Int J Thermophys. 2022, 43, 87. [Google Scholar] [CrossRef]
- Maciá, E.; Marie Dubois, J.; Ann Thiel, P. Quasicrystals. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA, 2008. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, X.; Yang, F.; Ge, T.; Wang, R. Easily-synthesized and low-cost amine-functionalized silica sol-coated structured adsorbents for CO2 capture. Chemical Engineering Journal. 2021, 425, 131409. [Google Scholar] [CrossRef]
- Bollas, G.M.; Chen, C.C.; Barton, P.I. Refined electrolyte-NRTL model: Activity coefficient expressions for application to multi-electrolyte systems. AIChE Journal. 2008, 54, 1608–1624. [Google Scholar] [CrossRef]
- Aspen Technology Inc. AspenHYSYS. Aspen HYSYS V12.1 User Guide; 2017. [Google Scholar]
- Priya, G.S.K.; Bandyopadhyay, S. Multiple objectives Pinch Analysis. Resour Conserv Recycl. 2017, 119, 128–141. [Google Scholar] [CrossRef]
- Santanu Bandyopadhyay. Mathematical Foundation of Pinch Analysis. Chem Eng Trans. 2015, 45, 1753–1758. [Google Scholar]
- ProSim S& S in, P.S. PINCH ANALYSIS. Available online: https://www.prosim.net/en/engineering-services/pinch-analysis/ (accessed on 31 December 2022).
- Fahim, M.A.; Alsahhaf, T.A.; Elkilani, A. Hydrogen Production. In Fundamentals of Petroleum Refining; Elsevier, 2010; pp. 285–302. [Google Scholar] [CrossRef]
- Song, K.; Lee, C.J.; Jeon, J.; Han, C. Dynamic simulation of natural gas liquefaction process. 2012, 882-886. [CrossRef]
- Alexander, H. Penn. Reactions in HYSYS. Rice University Chemical Engineering Department.
- Kamaruddin, M.; Hamid, A. HYSYS ® : An Introduction to Chemical Engineering Simulation For UTM Degree++ Program. Available online: http://www.fkkksa.utm.my/staff/kamaruddin.
- Masiren, E.E.; Harun, N.; W. Ibrahim, W.H.; Adam, F. Effect of Temperature on Diffusivity of Monoethanolamine (MEA) on Absorption Process for CO2 Capture. International Journal of Engineering Technology and Sciences. 2016, 3, 43–51. [Google Scholar] [CrossRef]
- Wang, M.; Wang, M.; Rao, N.; Li, J.; Li, J. Enhancement of CO 2 capture performance of aqueous MEA by mixing with [NH 2 e-mim][BF 4]. RSC Adv. 2018, 8, 1987–1992. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Fu, K.; Idem, R.; Tontiwachwuthikul, P. Review on current advances, future challenges and consideration issues for post-combustion CO2 capture using amine-based absorbents. Chin J Chem Eng. 2016, 24, 278–288. [Google Scholar] [CrossRef]
- ’Plaza, J.M. Modeling of Carbon Dioxide Absorption using Aqueous Monoethanolamine, Piperazine and Promoted Potassium Carbonate. Doctoral dissertation. Published online May 2012.
- van Straelen, J.; Geuzebroek, F.; Goodchild, N.; Protopapas, G.; Mahony, L. CO2 capture for refineries, a practical approach. Energy Procedia. 2009, 1, 179–185. [Google Scholar] [CrossRef]
- Bahadori, A. Natural Gas Sweetening. In Natural Gas Processing; Elsevier, 2014; pp. 483–518. [Google Scholar] [CrossRef]
- Earthworks. Hydrogen Sulfide. Published 2023. Available online: https://earthworks.org/issues/hydrogen-sulfide/.
- Wang, M.; Rahimi, M.; Kumar, A.; Hariharan, S.; Choi, W.; Hatton, T.A. Flue gas CO2 capture via electrochemically mediated amine regeneration: System design and performance. Appl Energy. 2019, 255, 113879. [Google Scholar] [CrossRef]
- Jiang, N.; Shen, Y.; Liu, B.; et al. CO2 capture from dry flue gas by means of VPSA, TSA and TVSA. Journal of CO2 Utilization. 2020, 35, 153–168. [Google Scholar] [CrossRef]
- Knudsen, J.N.; Jensen, J.N.; Vilhelmsen, P.J.; Biede, O. Experience with CO2 capture from coal flue gas in pilot-scale: Testing of different amine solvents. Energy Procedia. 2009, 1, 783–790. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




