Submitted:
19 July 2023
Posted:
19 July 2023
You are already at the latest version
Abstract
Keywords:
Introduction:
Understanding the composition and diversity of the gut microbiota
Roles of gut microbiota in digestion, nutrient metabolism, and immune regulation:
Factors influencing gut microbiota composition, including diet, lifestyle, and medications:
Gut Microbiota Alterations in COVID-19 Patients:
Mechanisms of Gut Microbiota Dysbiosis in COVID-19
The infiltration of viruses and disruption of the intestinal barrier:
Dysregulation of the immune system:
Effects of antibiotic treatments:
Dietary Modifications and Nutritional Alterations:
Hospitalization and stress:
The Gut-Lung Axis:
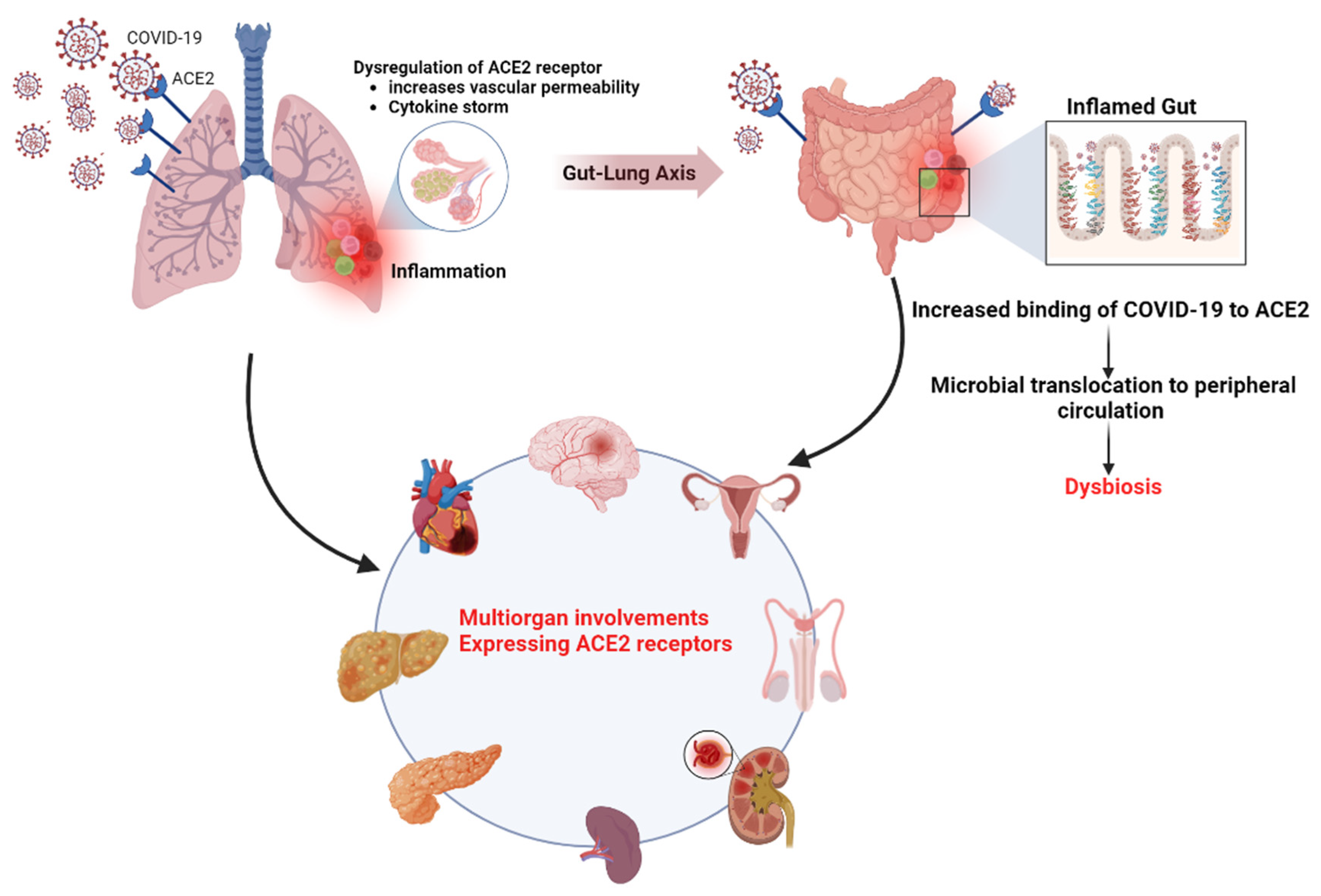
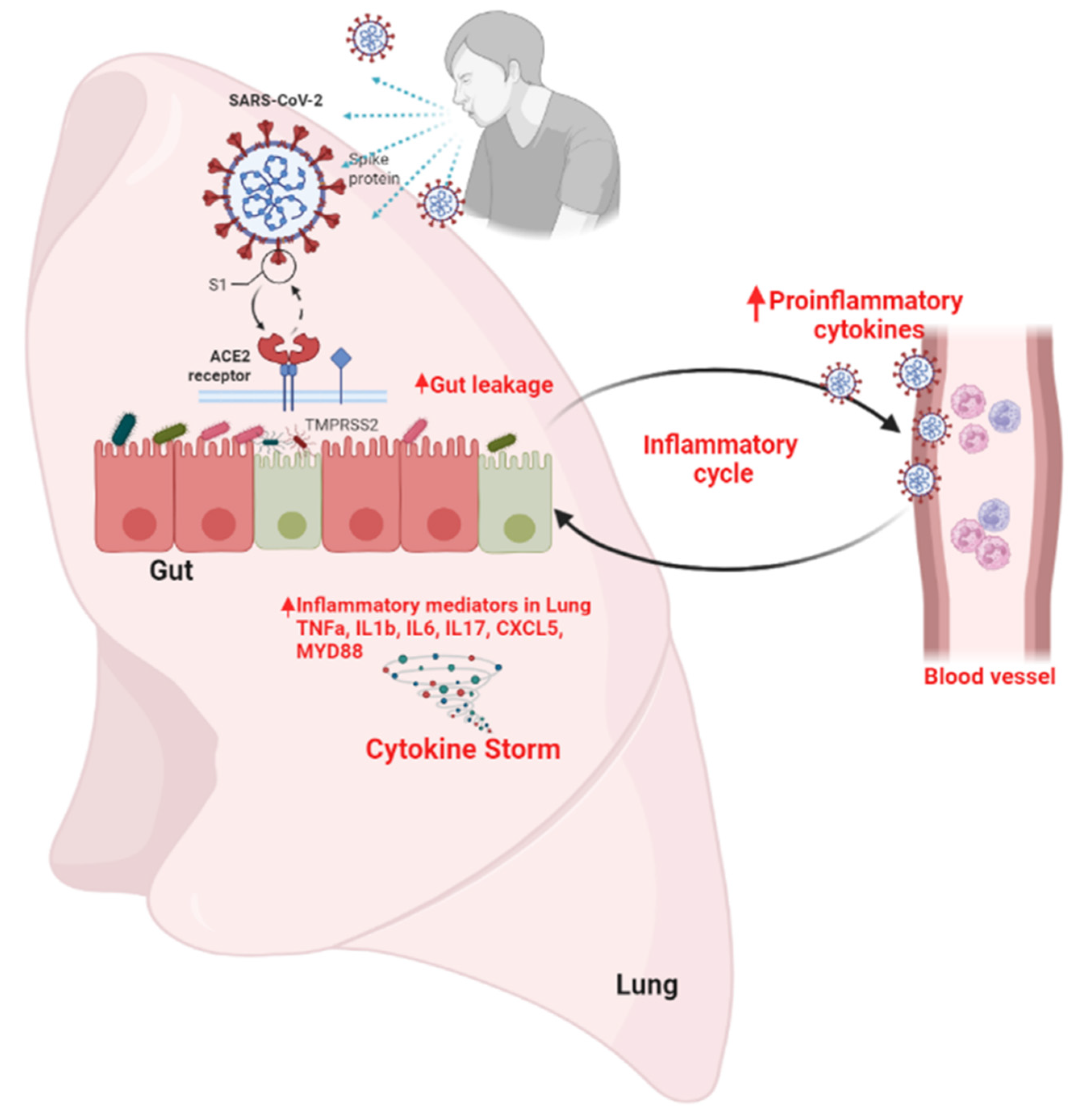
The role of the ACE2 receptor in SARS-CoV-2 infection and gut microbiota dysbiosis
Intestinal Inflammation and Gut Microbiota:
Consequences of intestinal inflammation on gut barrier function and bacterial translocation:
Understanding the interplay between the gut microbiota and the immune system:
Impact on Short-Chain Fatty Acids (SCFAs):
Long-Term Health Consequences of Gut Microbiota Alterations
Therapeutic Strategies and Interventions:
Conclusion:
Funding
Acknowledgments
Conflicts of Interest
References
- Frontiers | SARS-CoV-2: Structure, Biology, and Structure-Based Therapeutics Development. 2023. [CrossRef]
- Aubert O, Y.D. , Zielinski D, Cozzi E, et all. COVID-19 pandemic and worldwide organ transplantation: a population-based study. The Lancet. Public health 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- @sprinklr. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.
- J, M.; T, M. Recent Developments on Therapeutic and Diagnostic Approaches for COVID-19. The AAPS journal 2021, 23. [Google Scholar] [CrossRef]
- Aslan, A.; Aslan, C.; Zolbanin, N.M.; Jafari, R. Acute respiratory distress syndrome in COVID-19: possible mechanisms and therapeutic management. Pneumonia 2021, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Frontiers | Mechanism of Multi-Organ Injury in Experimental COVID-19 and Its Inhibition by a Small Molecule Peptide. 2023. [CrossRef]
- Davis, M.G.; Bobba, A.; Chourasia, P.; Gangu, K.; Shuja, H.; Dandachi, D.; Farooq, A.; Avula, S.R.; Shekhar, R.; Sheikh, A.B. COVID-19 Associated Myocarditis Clinical Outcomes among Hospitalized Patients in the United States: A Propensity Matched Analysis of National Inpatient Sample. Viruses 2022, 14, 2791. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-w.; Ilyas, I.; Weng, J.-p. Endothelial dysfunction in COVID-19: an overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacologica Sinica 2022, 44, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, M.; Emin, M.; Bhutta, A.; Gul, E.H.; Voorhees, E.; Afzal, M.R. Cardiac arrhythmias associated with COVID-19 infection: state of the art review. [CrossRef]
- Increased risk of acute myocardial infarction after COVID-19 recovery: A systematic review and meta-analysis - International Journal of Cardiology. 2023. [CrossRef]
- Javed, N.; Ijaz, Z.; Khair, A.H.; Dar, A.A.; Lopez, E.D.; Abbas, R.; Sheikh, A.B. COVID-19 loss of taste and smell: potential psychological repercussions. The Pan African Medical Journal 2022, 43. [Google Scholar] [CrossRef]
- Tana, C.; Bentivegna, E.; Cho, S.-J.; Harriott, A.M.; García-Azorín, D.; Labastida-Ramirez, A.; Ornello, R.; Raffaelli, B.; Beltrán, E.R.; Ruscheweyh, R.; et al. Long COVID headache. The Journal of Headache and Pain 2022, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Korres, G.; Kitsos, D.K.; Kaski, D.; Tsogka, A.; Giannopoulos, S.; Giannopapas, V.; Sideris, G.; Tyrellis, G.; Voumvourakis, K. The Prevalence of Dizziness and Vertigo in COVID-19 Patients: A Systematic Review. Brain Sciences 2022, 12, 948. [Google Scholar] [CrossRef]
- How does the COVID-19 cause seizure and epilepsy in patients? The potential mechanisms - Multiple Sclerosis and Related Disorders. 2023. [CrossRef]
- Encephalitis as a neurological complication of COVID-19: A systematic review and meta-analysis of incidence, outcomes, and predictors. 2023. [CrossRef]
- COVID-19-associated Guillain-Barre syndrome: Postinfectious alone or neuroinvasive too? 2023. [CrossRef]
- Groff, A.; Kavanaugh, M.; Ramgobin, D.; McClafferty, B.; Aggarwal, C.S.; Golamari, R.; Jain, R. Gastrointestinal Manifestations of COVID-19: A Review of What We Know. 2021. [CrossRef]
- Faour, W.H.; Choaib, A.; Issa, E.; Choueiry, F.E.; Shbaklo, K.; Alhajj, M.; Sawaya, R.T.; Harhous, Z.; Alefishat, E.; Nader, M. Mechanisms of COVID-19-induced kidney injury and current pharmacotherapies. Inflammation Research 2021, 71, 39–56. [Google Scholar] [CrossRef] [PubMed]
- MB, A. Could the menagerie of the gut microbiome really cure cancer? Hope or hype. Journal for immunotherapy of cancer 2019, 7. [Google Scholar] [CrossRef]
- MS, M.; T, H.; R, I.; E, M.-Y.; Y, Y.; H, N.-S.; S, A.; M, K.; M, M.; M, K.; et al. Glucagon-Like Peptide-1 Receptor Agonist Protects Dorsal Root Ganglion Neurons against Oxidative Insult. Journal of diabetes research 2019, 2019. [Google Scholar] [CrossRef]
- Mohabbulla Mohib, M.; Fazla Rabby, S.M.; Paran, T.Z.; Mehedee Hasan, M.; Ahmed, I.; Hasan, N.; Abu Taher Sagor, M.; Mohiuddin, S. Protective role of green tea on diabetic nephropathy—A review. Cogent Biology 2016, 2, 1248166. [Google Scholar] [CrossRef]
- MS, M.; T, H.; Y, Y.; Y, M.; M, K.; S, T.; Y, K.; J, N.; H, K. Glucagon Prevents Cytotoxicity Induced by Methylglyoxal in a Rat Neuronal Cell Line Model. Biomolecules 2021, 11. [Google Scholar] [CrossRef]
- S, T.; FM, A.; Y, H.; R, Y.; I, A.; MS, M.; MM, K.; D, G.; MA, M. Antenatal depression among women with gestational diabetes mellitus: a pilot study. Reproductive health 2022, 19. [Google Scholar] [CrossRef]
- M, M.; T, H.; H, N.-S.; R, I.; N, O.; Y, H.; S, S.; MS, M.; E, A.-H.; M, K.; et al. Deficiency of glucagon gene-derived peptides induces peripheral polyneuropathy in mice. Biochemical and biophysical research communications 2020, 532. [Google Scholar] [CrossRef]
- M, C.; A, R.; A, K. Adiponectin/AdipoRs signaling as a key player in testicular aging and associated metabolic disorders. Vitamins and hormones 2021, 115. [Google Scholar] [CrossRef]
- W, D.; M, C.; S, P.; HA, S.; L, O. Adipocyte CAMK2 deficiency improves obesity-associated glucose intolerance. Molecular metabolism 2021, 53. [Google Scholar] [CrossRef]
- Barua, R.; Mizuno, K.; Tashima, Y.; Ogawa, M.; Takeuchi, H.; Taguchi, A.; Okajima, T. Bioinformatics and Functional Analyses Implicate Potential Roles for EOGT and L-fringe in Pancreatic Cancers. Molecules 2021, 26, 882. [Google Scholar] [CrossRef]
- Choubey, M. Growth Hormone and Insulin-like Growth Factor-I: Novel Insights into the Male Reproductive Health. Available online: https://scholar.google.com/citations?
- Ranjan, A.; Choubey, M.; Yada, T.; Krishna, A. Nesfatin-1 ameliorates type-2 diabetes-associated reproductive dysfunction in male mice. Journal of Endocrinological Investigation 2019, 43, 515–528. [Google Scholar] [CrossRef] [PubMed]
- M, C.; A, R.; PS, B.; A, K. Protective role of adiponectin against testicular impairment in high-fat diet/streptozotocin-induced type 2 diabetic mice. Biochimie 2020, 168. [Google Scholar] [CrossRef]
- Ranjan, A.; Choubey, M.; Yada, T.; Krishna, A. Immunohistochemical localization and possible functions of nesfatin-1 in the testis of mice during pubertal development and sexual maturation. Journal of Molecular Histology 2019, 50, 533–549. [Google Scholar] [CrossRef] [PubMed]
- M, C.; A, R.; PS, B.; F, B.; A, K. Direct actions of adiponectin on changes in reproductive, metabolic, and anti-oxidative enzymes status in the testis of adult mice. General and comparative endocrinology 2019, 279. [Google Scholar] [CrossRef]
- AT, D.; BJ, M. Microbes, metabolites, and the gut-lung axis. Mucosal immunology 2019, 12. [Google Scholar] [CrossRef]
- Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [CrossRef] [PubMed]
- OC, T.-C.; J, M.; A, G. Colonization and impact of disease and other factors on intestinal microbiota. Digestive diseases and sciences 2007, 52. [Google Scholar] [CrossRef]
- C, L.; K, F.; J, R.; JJ, F.; DN, F.; J, Z.; JI, G.; R, K. Identifying genomic and metabolic features that can underlie early successional and opportunistic lifestyles of human gut symbionts. Genome research 2012, 22. [Google Scholar] [CrossRef]
- MJ, C.; Department of Microbiology, U.C.C. , Ireland.; IB, J.; S, C.; SE, P.; EM, O.C.; S, C.; HM, H.; M, C.; B, L.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2023, 488, 178–184. [Google Scholar] [CrossRef]
- Analyses of the Stability and Core Taxonomic Memberships of the Human Microbiome | PLOS ONE. 2023. [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. 2005. [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome 2019, 7, 1–15. [Google Scholar] [CrossRef]
- HV, L.; A, F.; EJ, K.; AR, N.; MM, L.; JR, K.; JA, H.; D, S.; X, Y.; G, F.; et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PloS one 2012, 7. [Google Scholar] [CrossRef]
- Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits: Cell. 2023. [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nature Communications 2014, 5, 1–11. [Google Scholar] [CrossRef]
- SL, L.; CGM, G.; SA, J. Interactions between gut bacteria and bile in health and disease. Molecular aspects of medicine 2017, 56. [Google Scholar] [CrossRef]
- D, R.; O, W.; E, B.; A, K.; T, K.; D, Z.; PI, C.; A, G.; IN, K.; N, B.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555. [Google Scholar] [CrossRef]
- Gacesa, R.; Kurilshikov, A.; Vich Vila, A.; Sinha, T.; Klaassen, M.A.Y.; Bolte, L.A.; Andreu-Sánchez, S.; Chen, L.; Collij, V.; Hu, S.; et al. Environmental factors shaping the gut microbiome in a Dutch population. Nature 2022, 604, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Frontiers | Is It Time to Begin a Public Campaign Concerning Frailty and Pre-frailty? A Review Article. 2023. [CrossRef]
- B, C.; O, K.; JK, G.; AC, P.; S, S.; RE, L.; AT, G. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519. [Google Scholar] [CrossRef]
- Wu, G.D.; Compher, C.; Chen, E.Z.; Smith, S.A.; Shah, R.D.; Bittinger, K.; Chehoud, C.; Albenberg, L.G.; Nessel, L.; Gilroy, E.; et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. 2016. [CrossRef]
- Jackson, M.A.; Goodrich, J.K.; Maxan, M.-E.; Freedberg, D.E.; Abrams, J.A.; Poole, A.C.; Sutter, J.L.; Welter, D.; Ley, R.E.; Bell, J.T.; et al. Proton pump inhibitors alter the composition of the gut microbiota. 2016. [CrossRef]
- MJ, B. Antibiotic use and its consequences for the normal microbiome. Science (New York, N.Y.) 2016, 352. [Google Scholar] [CrossRef]
- T, Z.; F, Z.; GCY, L.; YK, Y.; AYL, L.; H, Z.; Y, W.; ACK, C.; CP, C.; N, C.; et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159. [Google Scholar] [CrossRef]
- T, Z.; Q, L.; F, Z.; GC, L.; EY, T.; YK, Y.; Z, C.; SS, B.; FK, C.; PK, C.; et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut 2021, 70. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.-Y.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. 2021. [CrossRef]
- S, G.; Y, C.; Z, W.; Y, C.; H, G.; L, L.; F, G.; X, Z.; R, L.; C, H.; et al. Alterations of the Gut Microbiota in Patients With Coronavirus Disease 2019 or H1N1 Influenza. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2020, 71. [Google Scholar] [CrossRef]
- D, D.; A, M. Gut microbiota and Covid-19- possible link and implications. Virus research 2020, 285. [Google Scholar] [CrossRef]
- Liu, Q.; Su, Q.; Zhang, F.; Tun, H.M.; Mak, J.W.Y.; Lui, G.C.-Y.; Ng, S.S.S.; Ching, J.Y.L.; Li, A.; Lu, W.; et al. Multi-kingdom gut microbiota analyses define COVID-19 severity and post-acute COVID-19 syndrome. Nature Communications 2022, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bernard-Raichon, L.; Venzon, M.; Klein, J.; Axelrad, J.E.; Zhang, C.; Sullivan, A.P.; Hussey, G.A.; Casanovas-Massana, A.; Noval, M.G.; Valero-Jimenez, A.M.; et al. Gut microbiome dysbiosis in antibiotic-treated COVID-19 patients is associated with microbial translocation and bacteremia. Nature Communications 2022, 13, 1–13. [Google Scholar] [CrossRef]
- Tsounis, E.P.; Triantos, C.; Konstantakis, C.; Marangos, M.; Assimakopoulos, S.F. Intestinal barrier dysfunction as a key driver of severe COVID-19. http://www.wjgnet.com/. [CrossRef]
- Sun, Z.; Song, Z.-G.; Liu, C.; Tan, S.; Lin, S.; Zhu, J.; Dai, F.-H.; Gao, J.; She, J.-L.; Mei, Z.; et al. Gut microbiome alterations and gut barrier dysfunction are associated with host immune homeostasis in COVID-19 patients. BMC Medicine 2022, 20, 1–13. [Google Scholar] [CrossRef]
- Z, S.; ZG, S.; C, L.; S, T.; S, L.; J, Z.; FH, D.; J, G.; JL, S.; Z, M.; et al. Gut microbiome alterations and gut barrier dysfunction are associated with host immune homeostasis in COVID-19 patients. BMC medicine 2022, 20. [Google Scholar] [CrossRef]
- Frontiers | The COVID-19 Cytokine Storm; What We Know So Far. 2023. [CrossRef]
- Gonçalves, P.; Innate Immunity Unit, I.P. , Paris, France; Institut National de la Santé et de la Recherche Médicale (INSERM) U1223, P., France; Araújo, J.R.; Molecular Microbial Pathogenesis Unit, I.P., Paris, France; Institut National de la Santé et de la Recherche Médicale (INSERM) U1202, P., France; Di Santo, J.P.; Institut National de la Santé et de la Recherche Médicale (INSERM) U1223, P., France. A Cross-Talk Between Microbiota-Derived Short-Chain Fatty Acids and the Host Mucosal Immune System Regulates Intestinal Homeostasis and Inflammatory Bowel Disease. Inflammatory Bowel Diseases 2023, 24, 558–572. [Google Scholar] [CrossRef]
- J, T.; C, M.; M, P.; AN, T.; CR, M.; L, M. The role of short-chain fatty acids in health and disease. Advances in immunology 2014, 121. [Google Scholar] [CrossRef]
- EE, H.; MR, M.; KJ, L. HDAC Inhibitors as Epigenetic Regulators of the Immune System: Impacts on Cancer Therapy and Inflammatory Diseases. BioMed research international 2016, 2016. [Google Scholar] [CrossRef]
- AS, H.; M, T.; O, R.; SA, H.; TW, S. GPCR-Mediated Signaling of Metabolites. Cell metabolism 2017, 25. [Google Scholar] [CrossRef]
- M, L.; BCAM, v.E.; GTM, W.; J, G.; G, F.; PAJ, H. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. European journal of pharmacology 2018, 831. [Google Scholar] [CrossRef]
- Y, L.; Z, Z.; Y, L.; W, H.; M, Z.; X, Z.; W, J. Angiotensin-converting enzyme inhibition attenuates lipopolysaccharide-induced lung injury by regulating the balance between angiotensin-converting enzyme and angiotensin-converting enzyme 2 and inhibiting mitogen-activated protein kinase activation. Shock (Augusta, Ga.) 2015, 43. [Google Scholar] [CrossRef]
- Pro-Inflammatory Activated Kupffer Cells by Lipids Induce Hepatic NKT Cells Deficiency through Activation-Induced Cell Death | PLOS ONE. 2023. [CrossRef]
- MF, D.L.C.; T, D.; P, L.; A, B.; JP, G.; J, D. Resilience of the dominant human fecal microbiota upon short-course antibiotic challenge. Journal of clinical microbiology 2005, 43. [Google Scholar] [CrossRef]
- L, D.; S, H.; ML, S.; DA, R. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS biology 2008, 6. [Google Scholar] [CrossRef]
- F, R.; AA, O.; M, D.; N, I.; H, G.; B, D.; P, L.; PL, P.; R, G.; È, B.; et al. The initial state of the human gut microbiome determines its reshaping by antibiotics. The ISME journal 2016, 10. [Google Scholar] [CrossRef]
- G, D.; JC, L.; C, R.; F, A.; P, H.; S, M.; N, D.; NP, D.; A, P.; J, A.; et al. Culturomics and pyrosequencing evidence of the reduction in gut microbiota diversity in patients with broad-spectrum antibiotics. International journal of antimicrobial agents 2014, 44. [Google Scholar] [CrossRef]
- Modi, S.R.; Collins, J.J.; Relman, D.A. Antibiotics and the gut microbiota. 2014. [CrossRef]
- Global antibiotic use during the COVID-19 pandemic: analysis of pharmaceutical sales data from 71 countries, 2020–2022 - eClinicalMedicine. 2023. [CrossRef]
- de Nies, L.; Galata, V.; Martin-Gallausiaux, C.; Despotovic, M.; Busi, S.B.; Snoeck, C.J.; Delacour, L.; Budagavi, D.P.; Laczny, C.C.; Habier, J.; et al. Altered infective competence of the human gut microbiome in COVID-19. Microbiome 2023, 11, 1–16. [Google Scholar] [CrossRef]
- J, W. Murine gut microbiota-diet trumps genes. Cell host & microbe 2015, 17. [Google Scholar] [CrossRef]
- C, D.F.; D, C.; M, D.P.; M, R.; JB, P.; S, M.; S, C.; G, P.; P, L. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proceedings of the National Academy of Sciences of the United States of America 2010, 107. [Google Scholar] [CrossRef]
- T, Y.; FE, R.; MJ, M.; I, T.; MG, D.-B.; M, C.; M, M.; G, H.; RN, B.; AP, A.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486. [Google Scholar] [CrossRef]
- J, O.; F, C.; EG, Z.; JP, D.; M, W.; K, N.; HR, G.; SJ, O.K. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. The American journal of clinical nutrition 2013, 98. [Google Scholar] [CrossRef]
- LA, D.; CF, M.; RN, C.; DB, G.; JE, B.; BE, W.; AV, L.; AS, D.; Y, V.; MA, F.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505. [Google Scholar] [CrossRef]
- ED, S.; JL, S. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell metabolism 2014, 20. [Google Scholar] [CrossRef]
- Dhar, D. Frontiers | Impending Mental Health Issues During Coronavirus Disease 2019 – Time for Personalized Nutrition Based on the Gut Microbiota to Tide Over the Crisis? 2023. [CrossRef]
- M, L.; JJ, V.; MT, B. Anxiogenic effect of subclinical bacterial infection in mice in the absence of overt immune activation. Physiology & behavior 1998, 65. [Google Scholar] [CrossRef]
- LE, G.; RP, G.; N, O.; R, R.; N, B.; M, L. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain, behavior, and immunity 2005, 19. [Google Scholar] [CrossRef]
- EPA, R.; K, L.; WA, B.; EFM, W. Disturbed intestinal integrity in patients with COPD: effects of activities of daily living. Chest 2014, 145. [Google Scholar] [CrossRef]
- Frontiers | Diet, Microbiota and Gut-Lung Connection. 2023. [CrossRef]
- CY, S.; CH, Y.; WY, Y.; HZ, Y. Gut-Lung Microbiota in Chronic Pulmonary Diseases: Evolution, Pathogenesis, and Therapeutics. The Canadian journal of infectious diseases & medical microbiology = Journal canadien des maladies infectieuses et de la microbiologie medicale 2021, 2021. [CrossRef]
- Schuijt, T.J.; Lankelma, J.M.; Scicluna, B.P.; Melo, F.d.S.e.; Roelofs, J.J.T.H.; Boer, J.D.d.; Hoogendijk, A.J.; Beer, R.d.; Vos, A.d.; Belzer, C.; et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. 2016. [CrossRef]
- R, P.; MJ, P.; JL, F.; CP, V.; S, F.; M, D.; A, H.; CS, J.; J, W.; R, L.; et al. Plasma microbiome in COVID-19 subjects: an indicator of gut barrier defects and dysbiosis. bioRxiv : the preprint server for biology. [CrossRef]
- K, K.; Y, I.; JM, P. Multiple functions of angiotensin-converting enzyme 2 and its relevance in cardiovascular diseases. Circulation journal : official journal of the Japanese Circulation Society 2013, 77. [Google Scholar] [CrossRef]
- Frontiers | Body Localization of ACE-2: On the Trail of the Keyhole of SARS-CoV-2. 2023. [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nature Reviews Molecular Cell Biology 2021, 23, 3–20. [Google Scholar] [CrossRef]
- The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients - Chen - 2020 - Journal of Medical Virology - Wiley Online Library. 2023. [CrossRef]
- Qian, Q.; Department of Colorectal and Anal Surgery, Z.H.o.W.U. , Wuhan, China; Clinical Center of Intestinal and Colorectal Diseases of Hubei Province, W., China; Hubei Key Laboratory of Intestinal and Colorectal Diseases (Zhongnan Hospital of Wuhan University), W., China; Colorectal and Anal Disease Research Center of Medical School (Zhongnan Hospital of Wuhan University), W., China; Quality Control Center of Colorectal and Anal Surgery of Health Commission of Hubei Province, W., China; Fan, L.; Department of Pathology, H.C.H., Wuhan, China; Liu, W.; Department of Colorectal and Anal Surgery, Z.H.o.W.U., Wuhan, China; et al. Direct Evidence of Active SARS-CoV-2 Replication in the Intestine. Clinical Infectious Diseases 2023, 73, 361–366. [Google Scholar] [CrossRef]
- T, H.; T, P.; A, R.; J, T.; H, I.; M, P.; V, S.; T, H.; R, H.; S, L.; et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012, 487. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, S.; Liu, M.; Zhao, Z.; Xu, Y.; Wang, P.; Lin, M.; Xu, Y.; Huang, B.; Zuo, X.; et al. ACE2 expression by colonic epithelial cells is associated with viral infection, immunity and energy metabolism. 2020. [CrossRef]
- Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization - Gastroenterology. 2023. [CrossRef]
- Ferreira, C.; Viana, S.D.; Reis, F. Gut Microbiota Dysbiosis–Immune Hyperresponse–Inflammation Triad in Coronavirus Disease 2019 (COVID-19): Impact of Pharmacological and Nutraceutical Approaches. Microorganisms 2020, 8, 1514. [Google Scholar] [CrossRef]
- DC, B.; SR, C. Inflammatory bowel disease: cause and immunobiology. Lancet (London, England) 2007, 369. [Google Scholar] [CrossRef]
- Zhang, H.; DiBaise, J.K.; Zuccolo, A.; Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittmann, B.E.; et al. Human gut microbiota in obesity and after gastric bypass. 2009. [CrossRef]
- Kim, Y.; Division of Gastroenterology and Hepatology, D.o.I.M., University of Michigan; Kamada, N.; Division of Gastroenterology and Hepatology, D.o.I.M., University of Michigan; Laboratory of Microbiology and Immunology, W.I.F.R.C. , Osaka University, Suita, Osaka 565-0871, Japan. The role of the microbiota in myelopoiesis during homeostasis and inflammation. International Immunology 2023, 35, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Y, T.; L, R.; W, N.; Y, H. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Alimentary pharmacology & therapeutics 2020, 51. [Google Scholar] [CrossRef]
- Pan, L.M., PhD1; Mu, M.M., 4; Yang, P.M.; Sun, Y.M.; Wang, R.M.; Yan, J.M.; Li, P.M.; Hu, B.M., PhD10; Wang, J.M.; Hu, C.M.; et al. Clinical Characteristics of COVID-19 Patients With Digestive... : Official journal of the American College of Gastroenterology | ACG. 2023. [CrossRef]
- G, C.; A, P.; VI, A.; B, T.; EA, P.; SP, S.; D, K.; A, H.; A, A. High Prevalence of Concurrent Gastrointestinal Manifestations in Patients With Severe Acute Respiratory Syndrome Coronavirus 2: Early Experience From California. Gastroenterology 2020, 159. [Google Scholar] [CrossRef]
- JW, B.; J, L.; D, J.; DE, F. Prevalence and risk factors for gastrointestinal symptoms after recovery from COVID-19. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 2022, 34. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Department of Internal Medicine, V.M.C., Richmond; Wang, J.; Department of Internal Medicine, V.M.C., Richmond; Yannie, P.J.; Hunter Homes McGuire VA Medical Center, R., Virginia; Ghosh, S.; Department of Internal Medicine, V.M.C., Richmond; Hunter Homes McGuire VA Medical Center, R. , Virginia. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. Journal of the Endocrine Society 2023, 4. [Google Scholar] [CrossRef]
- Gionchetti, P.; Lammers, K.M.; Rizzello, F.; Campieri, M. Probiotics and barrier function in colitis. 2005. [CrossRef]
- Bander, Z.A.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. International Journal of Environmental Research and Public Health 2020, 17, 7618. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. https://doi.org/10.4161/gmic.19320. [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Research 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, X.; Wang, M.; He, Z.; Li, H.; Wang, J.; Li, Q. Gut microbiota influence immunotherapy responses: mechanisms and therapeutic strategies. Journal of Hematology & Oncology 2022, 15, 1–20. [Google Scholar] [CrossRef]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. https://doi.org/10.1080/21688370.2017.1373208. [CrossRef]
- YP, S.; A, B.; RL, F. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Frontiers in endocrinology 2020, 11. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nature Medicine 2014, 20, 159–166. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Bhattacharya, M.; Dhama, K.; Lee, S.-S. Altered gut microbiota patterns in COVID-19: Markers for inflammation and disease severity. http://www.wjgnet.com/. [CrossRef]
- F, Z.; Y, W.; T, Z.; YK, Y.; Q, L.; L, Z.; H, Z.; W, L.; W, X.; GCY, L.; et al. Prolonged Impairment of Short-Chain Fatty Acid and L-Isoleucine Biosynthesis in Gut Microbiome in Patients With COVID-19. Gastroenterology 2022, 162. [Google Scholar] [CrossRef]
- Iatcu, C.O.; Steen, A.; Covasa, M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients 2021, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.-E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Medicine 2016, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Richard, W. Stephens, L.A., Mihai Covasa. Gut Microbiota: From Microorganisms to Metabolic Organ Influencing Obesity - Stephens - 2018 - Obesity - Wiley Online Library. 2023. [CrossRef]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef]
- Haneishi, Y.; Furuya, Y.; Hasegawa, M.; Picarelli, A.; Rossi, M.; Miyamoto, J. Inflammatory Bowel Diseases and Gut Microbiota. International Journal of Molecular Sciences 2023, 24, 3817. [Google Scholar] [CrossRef] [PubMed]
- Colucci, R.; Moretti, S. Implication of Human Bacterial Gut Microbiota on Immune-Mediated and Autoimmune Dermatological Diseases and Their Comorbidities: A Narrative Review. Dermatology and Therapy 2021, 11, 363–384. [Google Scholar] [CrossRef] [PubMed]
- Vijay, A.; Valdes, A.M. Role of the gut microbiome in chronic diseases: a narrative review. European Journal of Clinical Nutrition 2021, 76, 489–501. [Google Scholar] [CrossRef]
- Lee, K.H.; Song, Y.; Wu, W.; Yu, K.; Zhang, G. The gut microbiota, environmental factors, and links to the development of food allergy. Clinical and Molecular Allergy 2020, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Plunkett, C.H.; Department of Pathology, T.U.o.C. , Chicago, IL 60637; and; Nagler, C.R.; Department of Pathology, T.U.o.C., Chicago, IL 60637; and; Committee on Immunology, T.U.o.C., Chicago, IL 60637. The Influence of the Microbiome on Allergic Sensitization to Food. The Journal of Immunology 2023, 198, 581–589. [Google Scholar] [CrossRef]
- Mangiola, F.; Ianiro, G.; Franceschi, F.; Fagiuoli, S.; Gasbarrini, G.; Gasbarrini, A. Gut microbiota in autism and mood disorders. http://www.wjgnet.com/. [CrossRef]
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.-F. Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis. Journal of Neuroinflammation 2019, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.P. , ∗; Barata, P.P., MD,; Fernandes, R.P. The influence of gut microbiota in cardiovascular diseases—a... : Porto Biomedical Journal. 2023. [CrossRef]
- Buford, T.W. (Dis)Trust your gut: the gut microbiome in age-related inflammation, health, and disease. Microbiome 2017, 5, 1–11. [Google Scholar] [CrossRef]
- McFarland, L.V. Use of probiotics to correct dysbiosis of normal microbiota following disease or disruptive events: a systematic review. 2014. [CrossRef]
- AC, C.; TJ, F.; HH, H.; JF, C.; YW, K.; YY, H.; SY, T.; SF, W.; HC, L.; YT, Y. A multi-strain probiotic blend reshaped obesity-related gut dysbiosis and improved lipid metabolism in obese children. Frontiers in nutrition 2022, 9. [Google Scholar] [CrossRef]
- Tsai, Y.-L.; Lin, T.-L.; Chang, C.-J.; Wu, T.-R.; Lai, W.-F.; Lu, C.-C.; Lai, H.-C. Probiotics, prebiotics and amelioration of diseases. Journal of Biomedical Science 2019, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Md. Ehsan Uddin Talukder, F.M. Md. Ehsan Uddin Talukder, F.M., Rashu Barua, Samsad Sultana, Farhana Yesmin, Mohammad Sayedul Islam, Robiul Hasan Bhuiyan. In vitro Assessment of Cytotoxic Activity of Hybrid Variety of Momordica charantia (Bitter Gourd). 2023. [CrossRef]
- Barua, R.; Department of Biochemistry and Molecular Biology, U.o.C. , Chittagong-4331, Bangladesh.; Sultana, S.; Department of Biochemistry and Molecular Biology, U.o.C., Chittagong-4331, Bangladesh.; Talukder, M.E.U.; Department of Biochemistry and Molecular Biology, U.o.C., Chittagong-4331, Bangladesh.; Chakma, K.; Department of Biochemistry and Molecular Biology, U.o.C., Chittagong-4331, Bangladesh abd Tohoku University Graduate School of Medicine, Sendai 980-8575, Japan; Hasan, C.M.M.; Department of Biochemistry and Molecular Biology, U.o.C., Chittagong-4331, Bangladesh.; et al. Antioxidant and Cytotoxic Activity of Crude Flavonoid Fraction from the Fruits of Hybrid Variety of Momordica charantia (Bitter Gourd). Journal of Pharmaceutical Research International. [CrossRef]
- Rashu Barua, M.E.U.T. , Mohammad Sayedul Islam, Farhana Yesmin, Kanchan Chakma, Md Golam Kabir, Robiul Hasan Bhuiyan. Nutritional Analysis and Phytochemical Evaluation of Bitter Gourd (Momordica Charantia) from Bangladesh. 2020. [CrossRef]
- Akter, S.; Choubey, M.; Mohib, M.M.; Arbee, S.; Sagor, M.A.T.; Mohiuddin, M.S. Stem Cell Therapy in Diabetic Polyneuropathy: Recent Advancements and Future Directions. Brain Sciences 2023, 13, 255. [Google Scholar] [CrossRef]
- CR, K.; S, K.; P, K.; L, L.; D, R.; A, A.; T, M.; G, W. Update on Fecal Microbiota Transplantation 2015: Indications, Methodologies, Mechanisms, and Outlook. Gastroenterology 2015, 149. [Google Scholar] [CrossRef]
- Diana, P. Bojanova, S.R.B. Fecal Transplants: What Is Being Transferred? 2023. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




