Submitted:
14 July 2023
Posted:
14 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
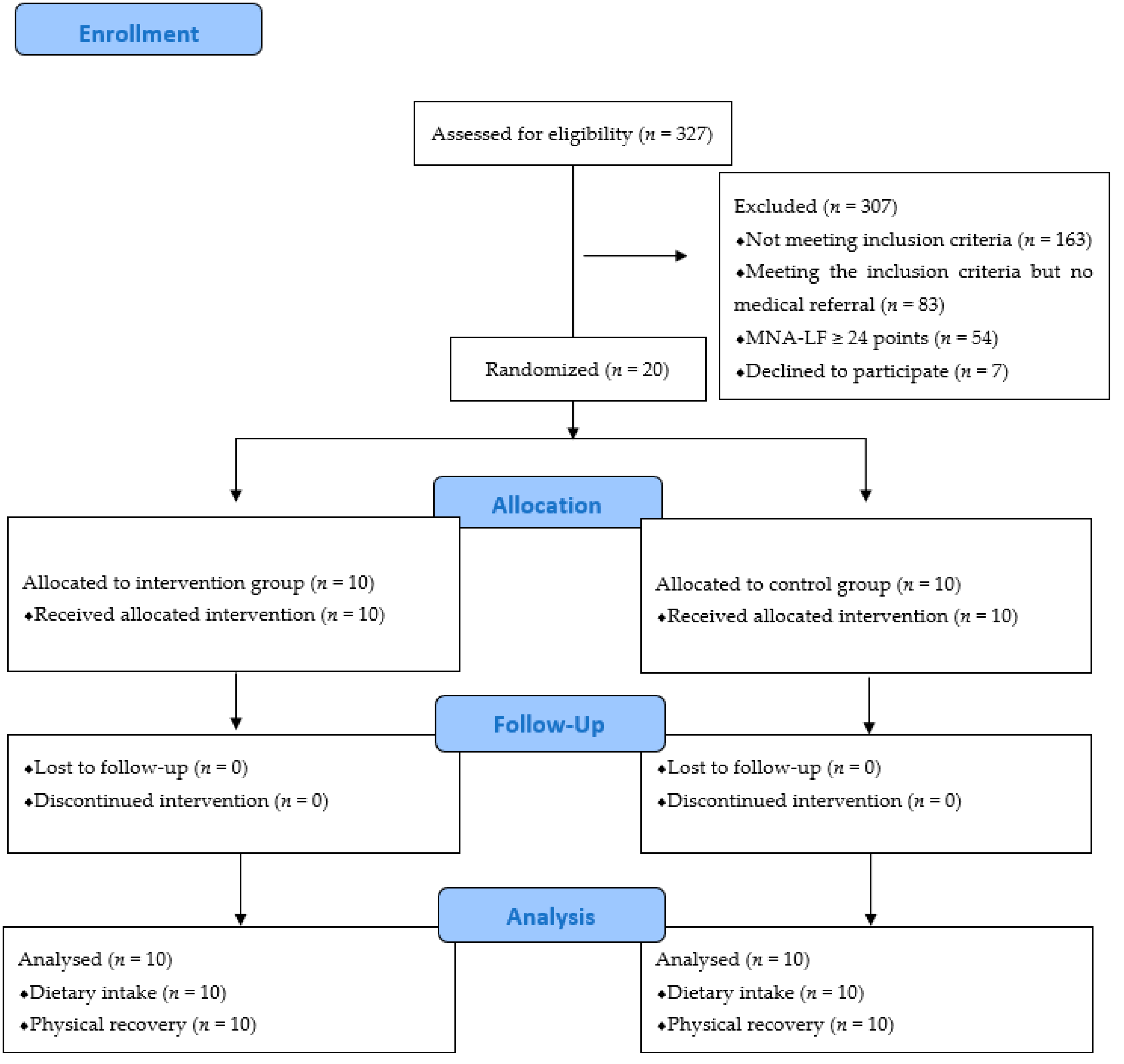
2.1. Study Design and Participants
2.2. Diagnosis of Malnutrition
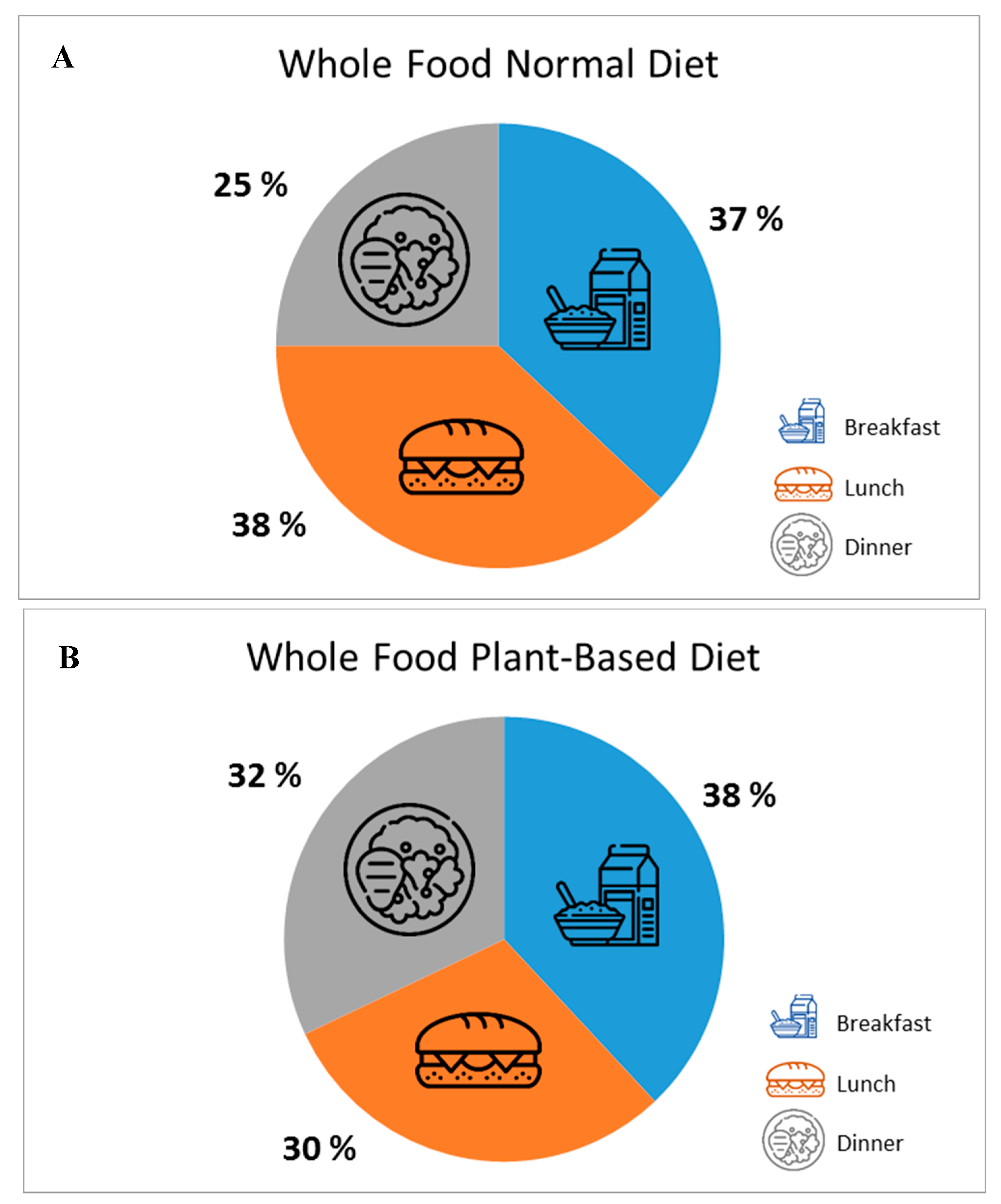
2.3. Nutritional Intervention
2.4. Rehabilitation Program
2.5. Outcomes and Data Collection
2.6. Sample Size
2.7. Statistical Analysis
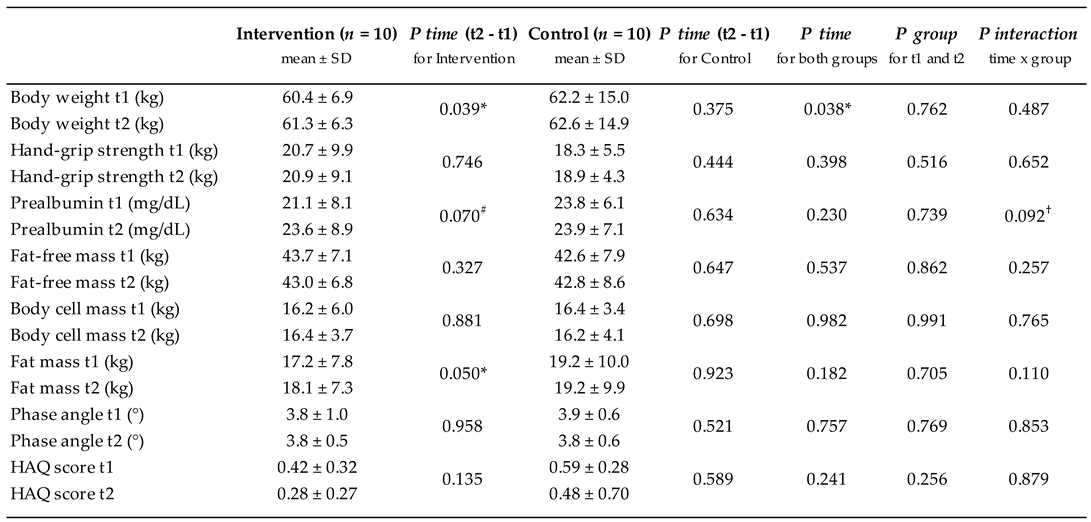
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet 2019, 393, 2636–2646.
- Beaudart C, Sanchez-Rodriguez D, Locquet M, Reginster JY, Lengelé L, Bruyère O. Malnutrition as a strong predictor of the onset of sarcopenia. Nutrients 2019, 11, 2883. [Google Scholar]
- Landi F, Calvani R, Ortolani E, Salini S, Martone AM, Santoro L, Santoliquido A, Sisto A, Picca A, Marzetti E. The association between sarcopenia and functional outcomes among older patients with hip fracture undergoing in-hospital rehabilitation. Osteoporos Int 2017, 28, 1569–1576. [Google Scholar]
- Verstraeten LMG, van Wijngaarden JP, Pacifico J, Reijnierse EM, Meskers CGM, Maier AB. Association between malnutrition and stages of sarcopenia in geriatric rehabilitation inpatients: RESORT. Clin Nutr 2021, 40, 4090–4096. [Google Scholar] [CrossRef] [PubMed]
- Prado CM, Purcell SA, Alish C, Pereira SL, Deutz NE, Heyland DK, Goodpaster BH, Tappenden KA, Heymsfield SB. Implications of low muscle mass across the continuum of care: A narrative review. Ann Med 2018, 50, 675–693. [Google Scholar]
- Ramadi A, Ezeugwu VE, Weber S, Funabashi M, Lima CA, Perracini MR, Beaupre LA. Progressive Resistance Training Program Characteristics in Rehabilitation Programs Following Hip Fracture: A Meta-Analysis and Meta-Regression. Geriatr Orthop Surg Rehabil 2022, 13, 21514593221090799. [Google Scholar]
- Rondanelli M, Cereda E, Klersy C, Faliva MA, Peroni G, Nichetti M, Gasparri C, Iannello G, Spadaccini D, Infantino V, Caccialanza R, Perna S. Improving rehabilitation in sarcopenia: a randomized-controlled trial utilizing a muscle-targeted food for special medical purposes. J Cachexia Sarcopenia Muscle 2020, 11, 1535–1547. [Google Scholar] [CrossRef] [PubMed]
- van Wijngaarden JP, Wojzischke J, van den Berg C, Cetinyurek-Yavuz A, Diekmann R, Luiking YC, Bauer JM. Effects of Nutritional Interventions on Nutritional and Functional Outcomes in Geriatric Rehabilitation Patients: A Systematic Review and Meta-Analysis. J Am Med Dir Assoc 2020, 21, 1207–1215. [Google Scholar]
- Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, Phillips S, Sieber C, Stehle P, Teta D, Visvanathan R, Volpi E, Boirie Y. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Groenendijk I, Kramer CS, den Boeft LM, Hobbelen HSM, van der Putten GJ, de Groot LCPGM. Hip Fracture Patients in Geriatric Rehabilitation Show Poor Nutritional Status, Dietary Intake and Muscle Health. Nutrients 2020, 12, 2528. [Google Scholar]
- Weijzen MEG, Kouw IWK, Geerlings P, Verdijk LB, van Loon LJC. During Hospitalization, Older Patients at Risk for Malnutrition Consume <0.65 Grams of Protein per Kilogram Body Weight per Day. Nutr Clin Pract 2020, 35, 655–663. [Google Scholar]
- Hermanky M, Korninger C, Fuchs D, Strasser B. Effects of a protein optimized diet combined with moderate resistance training on the postoperative course in older patients with hip fracture. Aktuel Ernahrungsmed 2017, 42, 180–187. [Google Scholar]
- Kaiser MJ, Bauer JM, Ramsch C, Uter W, Guigoz Y, Cederholm T, Thomas DR, Anthony P, Charlton KE, Maggio M, Tsai AC, Grathwohl D, Vellas B, Sieber CC; MNA-International Group. Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging 2009, 13, 782–788. [Google Scholar]
- Grote V, Unger A, Böttcher E, Muntean M, Puff H, Marktl W, Mur E, Kullich W, Holasek S, Hofmann P, Lackner HK, Goswami N, Moser M. General and Disease-Specific Health Indicator Changes Associated with Inpatient Rehabilitation. J Am Med Dir Assoc 2020, 21, 2017.e10–2017.e27. [Google Scholar]
- Roza AM, Shizgal HM. The Harris Benedict equation reevaluated: resting energy requirements and the body cell mass. Am J Clin Nutr 1984, 40, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Volkert D, Beck AM, Cederholm T, Cruz-Jentoft A, Goisser S, Hooper L, Kiesswetter E, Maggio M, Raynaud-Simon A, Sieber CC, Sobotka L, van Asselt D, Wirth R, Bischoff SC. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr 2019, 38, 10–47. [Google Scholar]
- Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2018, 48, 16–31.
- Di Vincenzo O, Marra M, Di Gregorio A, Pasanisi F, Scalfi L. Bioelectrical impedance analysis (BIA)-derived phase angle in sarcopenia: A systematic review. Clin Nutr 2021, 40, 3052–3061. [Google Scholar] [CrossRef] [PubMed]
- Norman K, Herpich C, Müller-Werdan U. Role of phase angle in older adults with focus on the geriatric syndromes sarcopenia and frailty. Rev Endocr Metab Disord 2022, 1–9.
- Bruce B, Fries JF. The Stanford Health Assessment Questionnaire: dimensions and practical applications. Health Qual Life Outcomes 2003, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health 2008, 31, 180–191. [CrossRef] [PubMed]
- Volkert D, Beck AM, Cederholm T, Cruz-Jentoft A, Goisser S, Hooper L, Kiesswetter E, Maggio M, Raynaud-Simon A, Sieber CC, Sobotka L, van Asselt D, Wirth R, Bischoff SC. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr 2019, 38, 10–47. [Google Scholar]
- Deutz NEP, Ashurst I, Ballesteros MD, Bear DE, Cruz-Jentoft AJ, Genton L, Landi F, Laviano A, Norman K, Prado CM. The underappreciated role of low muscle mass in the management of malnutrition. J Am Med Dir Assoc 2019, 20, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Liao CD, Chen HC, Huang SW, Liou TH. The role of muscle mass gain following protein supplementation plus exercise therapy in older adults with sarcopenia and frailty risks: A systematic review and meta-regression analysis of randomized trials. Nutrients 2019, 11, 1713. [Google Scholar] [CrossRef] [PubMed]
- Gomes F, Baumgartner A, Bounoure L, Bally M, Deutz NE, Greenwald JL, Stanga Z, Mueller B, Schuetz P. Association of Nutritional Support With Clinical Outcomes Among Medical Inpatients Who Are Malnourished or at Nutritional Risk: An Updated Systematic Review and Meta-analysis. JAMA Netw Open 2019, 2, e1915138. [Google Scholar]
- Bhasin S, Apovian CM, Travison TG, Pencina K, Moore LL, Huang G, Campbell WW, Li Z, Howland AS, Chen R, Knapp PE, Singer MR, Shah M, Secinaro K, Eder RV, Hally K, Schram H, Bearup R, Beleva YM, McCarthy AC, Woodbury E, McKinnon J, Fleck G, Storer TW, Basaria S. Effect of Protein Intake on Lean Body Mass in Functionally Limited Older Men: A Randomized Clinical Trial. JAMA Intern Med 2018, 178, 530–541. [Google Scholar]
- Tieland M, Dirks ML, van der Zwaluw N, Verdijk LB, van de Rest O, de Groot LC, van Loon L. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 2012, 13, 713–9. [Google Scholar]
- Churchward-Venne TA, Tieland M, Verdijk LB, Leenders M, Dirks ML, de Groot LC, van Loon LJ. There Are No Nonresponders to Resistance-Type Exercise Training in Older Men and Women. J Am Med Dir Assoc 2015, 16, 400–411. [Google Scholar]
- Dodds RM, Syddall HE, Cooper R, Benzeval M, Deary IJ, Dennison EM, Der G, Gale CR, Inskip HM, Jagger C, Kirkwood TB, Lawlor DA, Robinson SM, Starr JM, Steptoe A, Tilling K, Kuh D, Cooper C, Sayer AA. Grip strength across the life course: normative data from twelve British studies. PLoS One 2014, 9, e113637. [Google Scholar]
- Papadopoulou SK, Tsintavis P, Potsaki P, Papandreou D. Differences in the Prevalence of Sarcopenia in Community-Dwelling, Nursing Home and Hospitalized Individuals. A Systematic Review and Meta-Analysis. J Nutr Health Aging 2020, 24, 83–90. [Google Scholar]
- Bianchi L, Ferrucci L, Cherubini A, Maggio M, Bandinelli S, Savino E, Brombo G, Zuliani G, Guralnik JM, Landi F, Volpato S. The Predictive Value of the EWGSOP Definition of Sarcopenia: Results from the InCHIANTI Study. J Gerontol A Biol Sci Med Sci 2016, 71, 259–64. [Google Scholar] [CrossRef] [PubMed]
- Tan VMH, Pang BWJ, Lau LK, Jabbar KA, Seah WT, Chen KK, Ng TP, Wee SL. Malnutrition and Sarcopenia in Community-Dwelling Adults in Singapore: Yishun Health Study. J Nutr Health Aging 2021, 25, 374–381. [Google Scholar]
- Lengelé L, Bruyère O, Beaudart C, Reginster JY, Locquet M. Impact of Malnutrition Status on Muscle Parameter Changes over a 5-Year Follow-Up of Community-Dwelling Older Adults from the SarcoPhAge Cohort. Nutrients 2021, 13, 407. [Google Scholar]
- Pourhassan M, Rommersbach N, Lueg G, Klimek C, Schnatmann M, Liermann D, Janssen G, Wirth R. The Impact of Malnutrition on Acute Muscle Wasting in Frail Older Hospitalized Patients. Nutrients 2020, 12, 1387. [Google Scholar] [CrossRef] [PubMed]
- Rossi AP, Zanandrea V, Zoico E, Zanardo M, Caliari C, Confente S, Gabriele S, Mazzali G, Fantin F, Zamboni M. Inflammation and nutritional status as predictors of physical performance and strength loss during hospitalization. Eur J Clin Nutr 2016, 70, 1439–1442. [Google Scholar] [CrossRef] [PubMed]
- Cereda E, Pedrolli C, Klersy C, Bonardi C, Quarleri L, Cappello S, Turri A, Rondanelli M, Caccialanza R. Nutritional status in older persons according to healthcare setting: A systematic review and meta-analysis of prevalence data using MNA. Clin Nutr 2016, 35, 1282–1290. [Google Scholar]
- Jung YW, Hong N, Kim CO, Kim HC, Youm Y, Choi J-, Rhee Y. The diagnostic value of phase angle, an integrative bioelectrical marker, for identifying individuals with dysmobility syndrome: the Korean Urban-Rural Elderly study. Osteoporos Int 2021, 32, 939–949. [Google Scholar]
- Uemura K, Yamada M, Okamoto H. Association of bioimpedance phase angle and prospective falls in older adults. Geriatr Gerontol Int 2019, 19, 503–507. [Google Scholar]
- Uemura K, Doi T, Tsutsumimoto K, Nakakubo S, Kim MJ, Kurita S, Ishii H, Shimada H. Predictivity of bioimpedance phase angle for incident disability in older adults. J Cachexia Sarcopenia Muscle 2020, 11, 46–54. [Google Scholar]
- Mertz KH, Reitelseder S, Bechshoeft R, Bulow J, Højfeldt G, Jensen M, Schacht SR, Lind MV, Rasmussen MA, Mikkelsen UR, Tetens I, Engelsen SB, Nielsen DS, Jespersen AP, Holm L. The effect of daily protein supplementation, with or without resistance training for 1 year, on muscle size, strength, and function in healthy older adults: A randomized controlled trial. Am J Clin Nutr 2021, 113, 790–800. [Google Scholar]
| Intervention (n = 10) | Control (n = 10) | P | |
|---|---|---|---|
| Age (years) | 75.0 ± 6.5 | 74.2 ± 6.8 | 0.792 |
| Sex (male/female) | 1/9 | 2/8 | 0.531 |
| Height (cm) | 163.0 ± 8.3 | 162.6 ± 6.8 | 0.907 |
| Body weight (kg) | 60.4 ± 6.9 | 62.2 ± 15.0 | 0.735 |
| BMI (kg/m2) | 22.9 ± 4.0 | 23.5 ± 5.9 | 0.792 |
| RMR (kcal/d) | 1198 ± 99 | 1232 ± 174 | 0.604 |
| MNA (score) | 21.4 ± 2.4 | 20.9 ± 1.3 | 0.575 |
| Mobility aids (yes/no) | 4/6 | 4/6 | 1.000 |
| Medical diagnosis for admission (n) Arthrosis Diseases of the musculoskeletal system Lower limb injuries |
1 2 7 |
1 1 8 |
0.819 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





