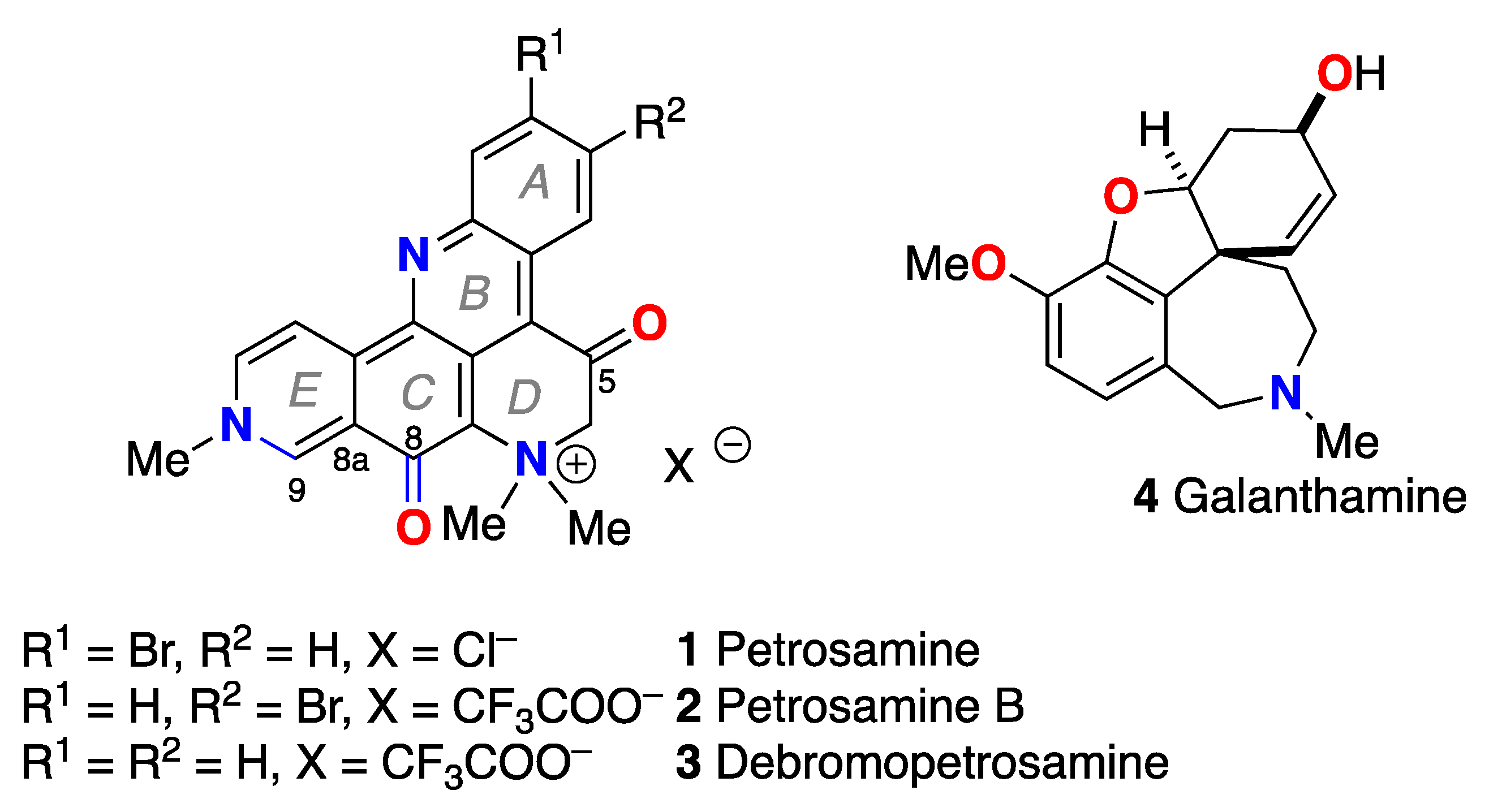
The solvatochromism of
1 and
2 is similar to lower-order merocyanine dyes, the canonical substructures of which can be discerned within the molecular framework of both natural products (
Figure 1). For example, the same conjugated sub-structure in Brooker's merocyanines [
11,
12] [generalized by Brooker as the vinylogous amide; neutral
ia and
ib dipolar (zwitterionic) resonance forms] is embedded in the molecular frameworks of
1 and
2. Brooker merocyanines, for example
5b and
6b (
Figure 1) exhibit strongly positive solvatochromism (hypsochromism, or a blue shift in
lmax in polar solvents). It is not unreasonable to invoke the same spectroscopic and electronic properties of
5 – zwitterionic form with extended conjugation, and stabilization of the ground state by solvation in polar solvents – as necessary and sufficient conditions to explain the solvatochromism of
1–
3.
While a complete description of electronic properties of petrosamine (
1) may be achieved in rigorous quantum mechanical calculations (see below), it is helpful for visualization purposes to consider Lewis bond formalism and resonance structures [
13]. For clarity, only two pairs of resonance forms are depicted for
1 to illustrate the zwitterionic contributions of merocyanine substructures: the non-charged resonance forms
1a,
c, and 'zwitterionic' resonance form
1b,
d. Forms
1a,b depict the shorter bond path (
n = 3, c.f.
ia,
ib,
Figure 4) and dipolar resonance forms
1c,d show a longer bond path in an 'aza-vinylogous amide' (
n = 4). The shortest bond path,
n = 1 (not shown) would involve only the atoms C-8–C8a–C9–N-10), while the longest path,
n = 4, evoking ‘particle-in-a-box’ formalism [
14], best explains the long-wavelength UV-vis bands of
1 giving rise to its colors.
Figure 1.
Canonical resonance forms – neutral (
a) and dipolar (
b) – for merocyanines defined by bond path,
n (see [
11]). (
b) Petrosamine ‘neutral’ and dipolar resonance forms:
1a (
n = 3),
1b (
n= 2),
1c (
n = 4) and
1d (
n = 4). (
c) Brooker merocyanine resonance forms; neutral (
4a,
5a,
6a) and dipolar (
4b,
5b,
6b) forms [
11].
Solvatochromism of Brooker merocyanines has been rationalized [11b], using semi-quantitative valence resonance and frontier orbital theories, as arising from more extensive stabilization of the dipolar form
4a of the ground state relative to the excited state in polar solvents. Accordingly, this differential stabilization gives rise to an increase in the electronic transition energy, ∆
E, due to a larger gap between the HOMO and LUMO of the longest wavelength transitions. The HOMO-LUMO gap is predicted to increase (blue shifted absorption) in those structures with higher contributions from the zwitterionic resonance form
4b, leading to more pronounced solvatochromism. More recent semi-empirical calculations (COSMO, PM3) of a different set of substituted Brooker merocyanines (
5) by Morley and coworkers [
15] supported stabilization of the ground state as mostly responsible for solvatochromism and predicted a larger role for hydrogen bonding in the zwitterionic form
5b over the neutral form
5a.
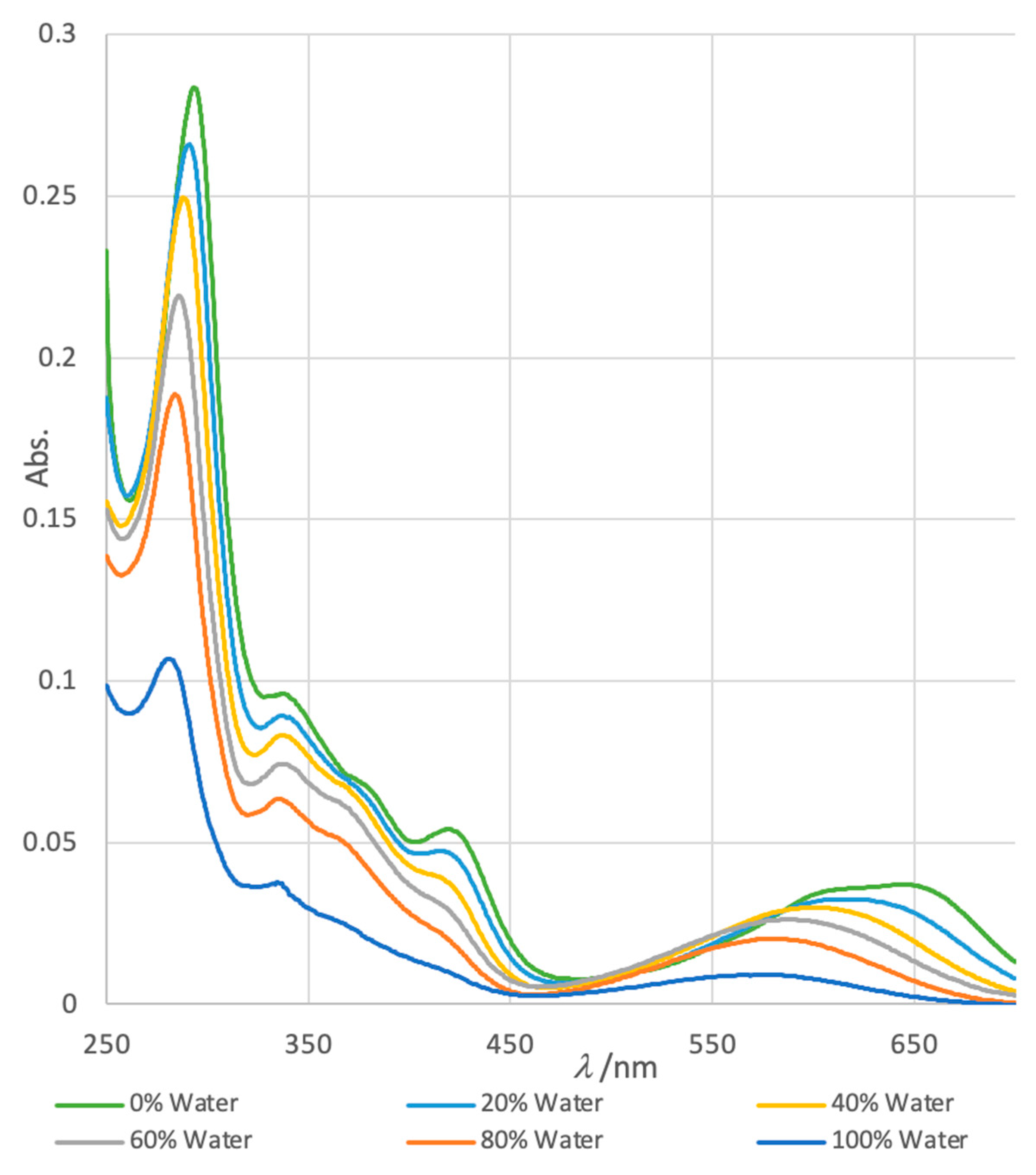
Figure 2.
Solvatochromism in normalized electronic UV-vis spectra of petrosamine (1) in H2O-DMSO solvents of variable composition (H2O v/v = 0%, 20%, 40%, 60%, 80% and 100%).
Table 1.
UV-vis properties of lmax11 and lmax21 in petrosamine (1) in DMSO-H2O.1.
| % H2O v/v) |
lmax1 (nm) |
e11
|
lmax2 (nm) |
e21
|
| 0 |
281 |
68,500 |
648 |
24,000 |
| 20 |
282 |
122,252 |
622 |
20,600 |
| 40 |
287 |
141,700 |
604 |
19,400 |
| 60 |
288 |
161,000 |
589 |
16,500 |
| 80 |
290 |
172,000 |
581 |
12,900 |
| 100 |
296 |
184,000 |
570 |
4,700 |
For comparison, we prepared the known merocyanine
6b from 4-methylpyridine by the following sequence adapted from Minch and Sadiq Shah:[
16] methylation (MeI,
iPrOH, reflux), condensation of the resultant pyridinium methiodide with
p-hydroxybenzaldehyde (piperidine, EtOH, reflux), and neutralization of the product
7 (
nBu
4N
+ HO
–) to zwitterionic phenolate
6b. Measurements of the UV-vis spectra of
6b (
nBu
4N
+ salt) in mixed solvents (acetone-H
2O, see Supporting Information) showed a hypsochromic trend similar to that of
1 in DMSO-H
2O. The long-wavelength
(band-2) varied from
lmax(acetone) 591 nm to
lmax (H
2O) 444 nm (∆
lmax –69 nm ) while
e changed only slightly across the range of solvent mixtures [
17].
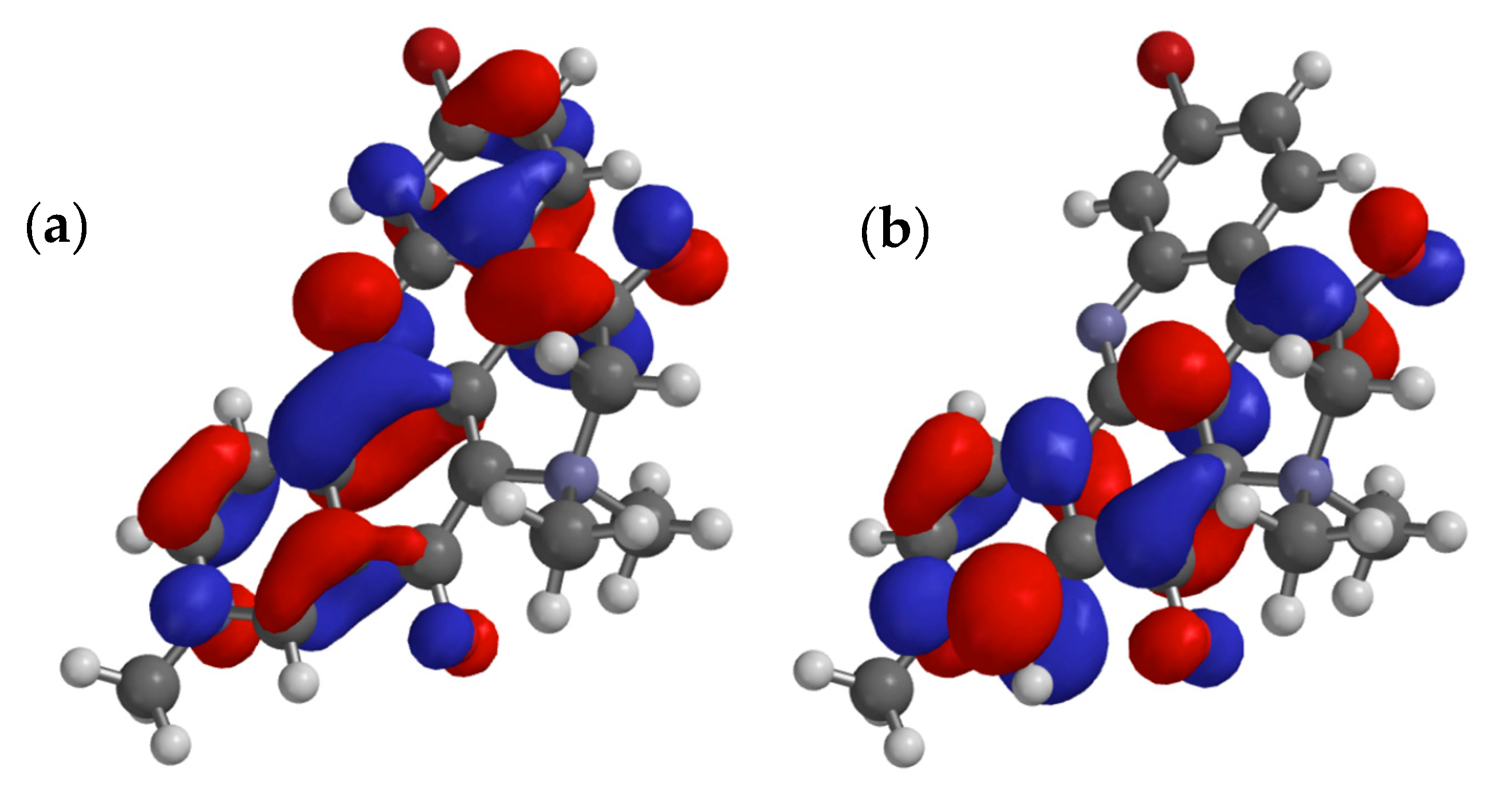
2.1. QM Calculations
The energies of electronic states of
1 were calculated using QM methods (DFT). Starting with the X-ray coordinates of
1, the geometry of the structure was first minimized using MMFF, then further refined by DFT (B3LYP 6-31G*, polarization continuum model = H
2O). The 3D model of
1, overlaid with frontier MOs, is shown in
Figure 3. As expected, the
∆E for the HOMO-LUMO gap is relatively small (∆
E = xx eV). The orbital coefficients and calculated dipole moment of the excited state (
µ = 18.5 D) are consistent with dominant contributions from the resonance form
1b rather than
1a, and the strong donor properties of N-10. At first this may seem anomalous, given the relatively low-field
13C NMR signal measured for C-5 in
1 (
d 187.4 [
1] or 187.2 in
2 [
5]) however, the additional deshielding effect is likely attributed to the inductive effect of the quaternized N-7.
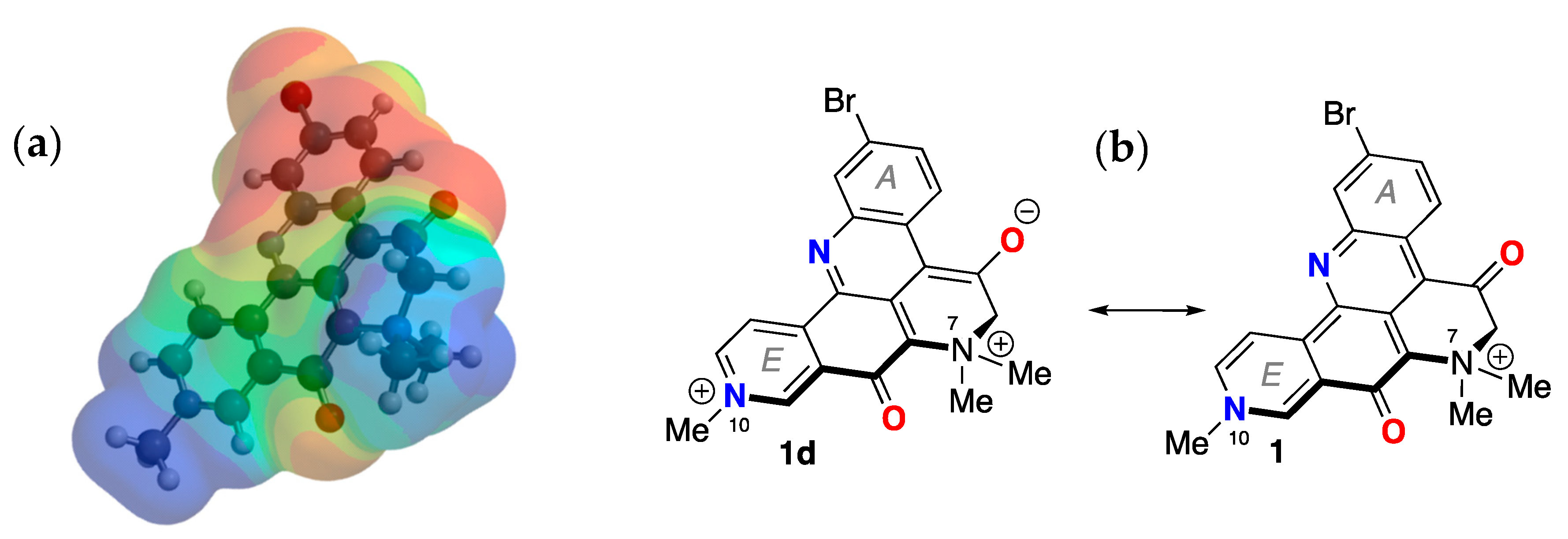
The electrostatic potential map of the minimized structure of
1 (
Figure 4) clearly shows two loci of positive charge: one centered on the quaternized N-10, as expected, in ring D, and a second associated with the N atom in ring E. The latter supports charge separated forms
1b,
d (
Figure 1) in which N-10 participates as a donor group. Consequently, the formal bonding electron pair of N-10 is highly delocalized and can confer only weak basicity to
1. Together with the UV-vis properties of
1, the overall electron delocalization consolidates an electronic structure in which a zwitterionic partial structure strongly lends to charge-separation mostly in the ground state.
Figure 3.
Calculated minimized structure and overlaid frontier π-orbitals of petrosamine (1) (DFT B3LYP 6-31G*, polarization continuum model = H2O) (a) HOMO and (b) LUMO.
Figure 3.
Calculated minimized structure and overlaid frontier π-orbitals of petrosamine (1) (DFT B3LYP 6-31G*, polarization continuum model = H2O) (a) HOMO and (b) LUMO.
From an empirical viewpoint, the latter makes sense as the charge-separated forms 1b,d preserve the aromaticity of rings A and E. A result of this delocalization is reduced basicity of N in ring E and, consequently, an expected lower pKa for the conjugate Brønsted acid. Formally, addition of H+ 1 to give a dication should sooner favor the C-8 or C-5 oxygen as an acceptor, rather than N-10 or N-13. In fact, we find no evidence (NMR) for protonation of 1 at pH ~ 2, which attests to the overall poor basicity of 1; an unsurprising finding given the permanent formal charge of +1 in this quaternary ammonium salt in all but the most basic or the most acidic media.
Figure 4.
Minimized energy structure of petrosamine (1, DFT, EDF2 6-31G, dipole moment µ = 18.5 D). (a) electrostatic potential surface and (b) the corresponding molecular framework of 1 (dipolar 1d and ‘charge-minimal’ 1 resonance forms).
Figure 4.
Minimized energy structure of petrosamine (1, DFT, EDF2 6-31G, dipole moment µ = 18.5 D). (a) electrostatic potential surface and (b) the corresponding molecular framework of 1 (dipolar 1d and ‘charge-minimal’ 1 resonance forms).
2.2. pKa of Petrosamine (1). Does the structure of 1 exhibit substantial enol content?
In order to estimate the p
Ka of petrosamine, the UV-vis spectra of
1 were measured in buffered D
2O at different pH. The titration curve of
1 (S1) shows little change in the UV-vis spectra in the range of pH 2 – 10. From the Henderson-Hasselbach relationship (Equation (1))[
18] for Brønsted acid HA, the condition pH = p
Ka is met when concentrations of conjugate species are equal ([HA] = [A
–]). We surmise the p
Ka of
1 lies outside this pH range (p
Ka >10). Indeed, a bathochromic shift in the UV-vis spectrum of
1 is only observed when a methanolic solution is treated with high concentrations of NaOH (2M, pH >13). Conversely, only when a sample of
1 is dissolved in a very strong Brønsted acid (neat CF
3COOH) are conditions met for a reasonable expectation of, diprotonated petrosamine ([
1•2H]
2+). In the event, when
1 was dissolved in neat TFA, the blue-purple color changed to bright yellow. The latter observation contrasts with the supposition drawn by Quinn and coworkers of doubly-protonated petrosamine B ([
2•2H]
2+) from their observation that
2 remains "
bright blue…when dissolved in methanol”, under the relatively benign conditions used in C
18-reversed phase isolation of the alkaloid (5% TFA-MeOH) [
6]. In other words, N-10 in the monocationic molecules
1-
3 is ‘pyridinium-like’ and non-basic (resembling
1d), and the C-8 C=O is insufficiently Brønsted-basic to be substantially protonated under ordinary isolation conditions.
It is evident from the
1H and
13C NMR ( DMSO-
d6) that C-5 in petrosamine (
1) is in the C8 keto form, a conclusion also reached by both the Suwanborirux and coworkers [
5], and independently by Quinn and coworkers for petrosamine B (
2) [
6]. The single crystal X-ray crystallography of
1 is concordant with the C8 keto form. In fact, solid state
1 is best represented by the di-keto tautomer, e.g. interatomic distances C8-O2, 1.256(17) Å; C5-O1, 1.203(15), and C5-C6, 1.495(21) [
1].
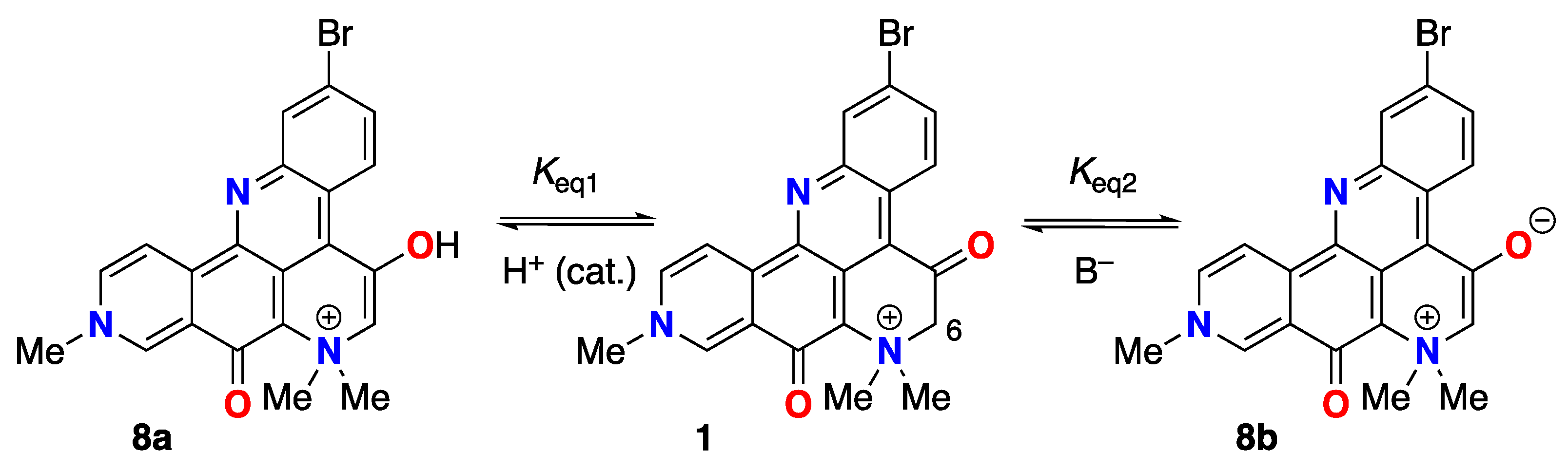
2.3. Kinetic Measurements of Hydrogen-Deuterium Exchange of 1
None of the resonance forms of
1a-
d (Figure 1)– or more precisely, pathways of electron delocalization – explain the complete exchange of the H-6 protons by deuterium when
1 was dissolved in deuteric solvents (D
2O, CD
3OD) [
1]. The latter can only be rationalized by consideration of the possibility of enolization (
Figure 6), either through the positively charged
8a in neutral to weakly acidic pH or the charge-neutral (zwitterionic) enolate
8b under highly basic pH. The kinetic parameters for successive H replacement by D in
1, defined by rate constants
k1 and
k2, are intrinsic properties as opposed to thermodynamic properties that relate to the equilibrium constants
Keq1 and
Keq2; the latter are largely dependent upon the strength and concentration of added base, B
– (
Figure 6).
Figure 6.
Keto-enol tautomerism of 1. Enol 8a (acid -catalyzed), and enolate 8b (base-promoted).
Figure 6.
Keto-enol tautomerism of 1. Enol 8a (acid -catalyzed), and enolate 8b (base-promoted).
In hydroxylic solvents,
1 also resides largely in the di-keto form, however, we found that in both D
2O and CD
3OD, the C-6 methylene protons undergo rapid exchange to give the C-6 CD
2-isotopomer (
1-d2) [
1]; a rate so fast that we were unable to detect the C-6 CH
2 or the intermediate forms within the time frame between sample preparation and measurement of the
1H NMR spectra. This observation was supported by
1H NMR, HSQC and HMBC measurements of
1 in protic solvent (CD
3OH) where the C-6-CH
2 group is still observable (HSQC correlation: H-6 to C-6,
dH 4.64, s;
dC 70.4 ppm). The simplest explanation for both phenomena is catalytic H-D exchange a-to the C-5 C=O group favored by an intermediate; the extensively-conjugated enol tautomer
8a (
Figure 6).
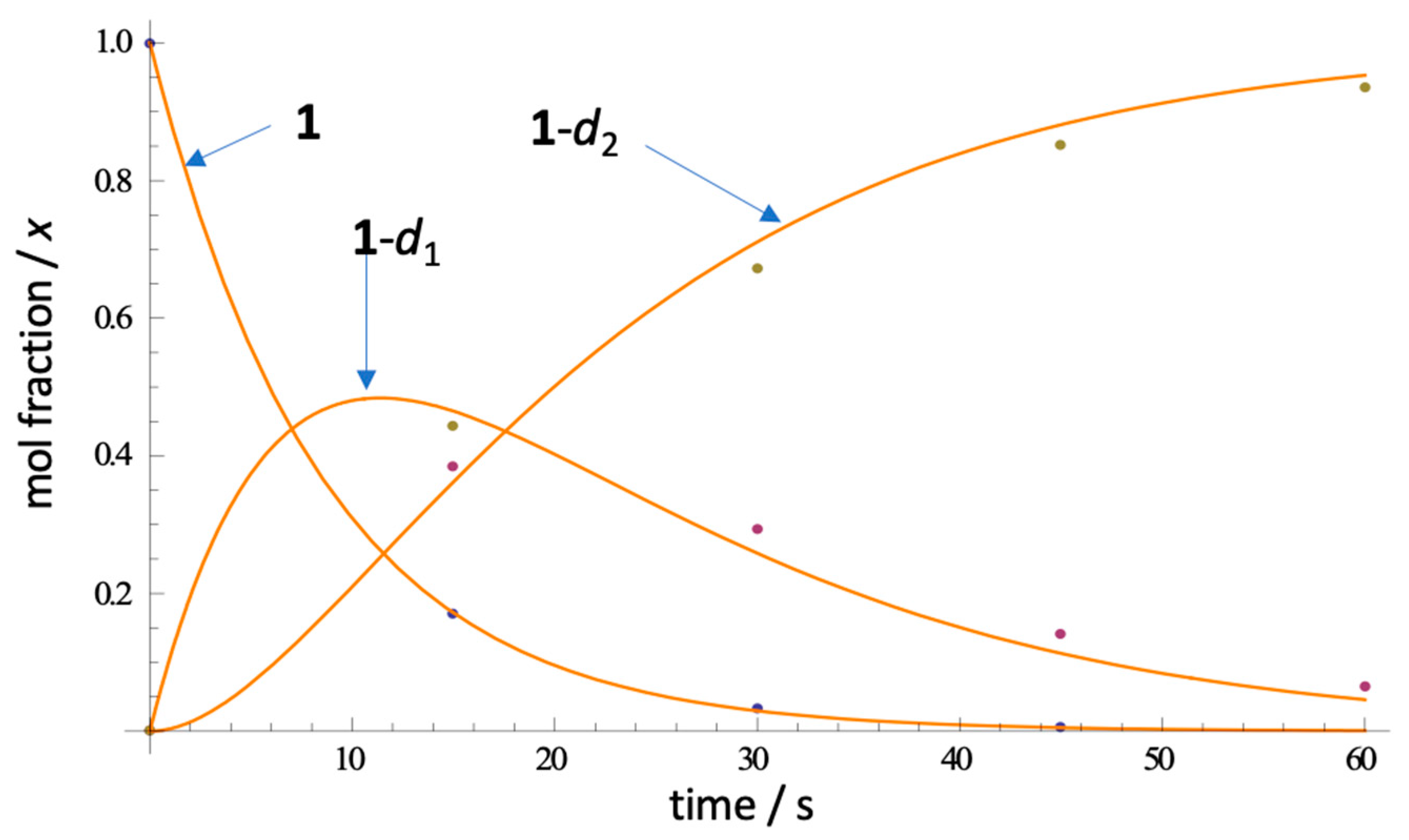
In order to estimate the rate of H-D exchange in 1 and place an upper bound on it, we measured the time-dependent appearance of the CD2-isotopmer by ESI mass spectrometry after rapid dissolution of 1 in CD3OD (Figure 7). Under controlled conditions (23 ˚C, rapid sampling and ESIMS in situ measurement- see Experimental), 1 (C21H1789BrN3O2, m/z 422.0499 [M+]) rapidly disappeared, followed by replacement by ephemeral isotopologue 1-d1 (m/z 423.0561) and finally, convergence upon 1-d2 (m/z 424.0624). Remarkably, complete exchange (>90%) is observed within 90 s of dissolution of 1. From triplicate measurements, and rapid sampling (first measurement at t = 15 sec), we were able to fit the kinetic deuterium exchange data to a first order rate law and estimated the apparent first and second H-D exchange rate constants to be (Table 2), k1= 0.1394(7) s–1 and k2 = 0.0765(47) s–1 (best R2; see Supporting Information). As expected, k2 is about half of k1, consistent with the expected rate laws, and negates involvement of a substantial kinetic isotope effect for the exchange reaction.
Figure 7.
Representative ESIMS measurements of the rate of H-D exchange of petrosamine (1 in CD3OD, 23 ˚C) and fitted curves (non-linear regression). See Supp. Info. for rate law and k determinations.
Figure 7.
Representative ESIMS measurements of the rate of H-D exchange of petrosamine (1 in CD3OD, 23 ˚C) and fitted curves (non-linear regression). See Supp. Info. for rate law and k determinations.
The exchange of
1 to
1-
d2, in the absence of added acid, appears to be much faster than H-D exchange rates of other phenones, e.g. propiophenone (p
Ka 24.4, DMSO [
19] a-tetralone (p
Ka 24.7 [
20,
21]. The rates of acid-promoted keto-enol equilibration of acetophenone (p
Ka 18.4±0.03) have been measured. For example, the rate of ketonization of acetophenone enol (1-phenylethen-1-ol) is linearly dependent upon [H
3O
+] with a catalytic efficiency determined to be
kH+ = (1.25±0.02) x 10
3 M
–1s
–1 [
22]. We conclude that tautomerism of
1 too must be very fast even in the absence of added acid. Either the enol
8a or enolate
8b, although undetectable as a discrete species in the time frame of
1H NMR, present a pathway to the acid-base equilibria of
1 and
1-
d2, but enolization of the keto form is likely dominant.
Table 2.
Rate constants k1 and k2 for H-D exchange in 1 in CD3OD (23˚ C, see Figure 5).1.
Table 2.
Rate constants k1 and k2 for H-D exchange in 1 in CD3OD (23˚ C, see Figure 5).1.
| Reaction |
k1 (s–1) |
k2 (s–1) |
|
1 –> 1-d1
|
0.1394(7) |
– |
|
1-d1 –> 1-d2
|
– |
0.0765(47) |
| |
|
|
Some amount of discussion has been given on the keto-enol state of
1. The NMR data for
1 reported by Suwainborirux and coworkers support the C5 keto form in DMSO-
d6 [
5] and – in agreement with Molinski and Faulkner, the enol form in D
2O or CD
3OD [
1] – but Quinn and coworkers find, “
no evidence for this keto-enol isomerism” in
2. [1] Likely, the
Keq of keto-enol tautomerism lies on some continuum, dependent upon solvent dialectric and H-bond donor ability.
The observed 1H and 13C NMR spectra of petrosamine (1), and the C6 exchange to CD2 in CD3OD/NaOD, support the enol form in hydroxylic solvents. It’s likely fast exchange hybrid structure of 1 and 8a,b with an equilibrium constant Keq1 largely in favor of 1 in DMSO-d6, moving to the dominant 8a in hydroxylic solvents. The enolate 8b may be favored as the catalytically important intermediate, for reasons of charge neutralization, but insufficient data can support this hypothesis with more certainty. In either case – enol or enolate intermediate – we surmise the electron-withdrawing quaternized N+Me2 group in 1 and the related petrosamines, 2, 3, plays an significant role in accelerating the rates of H-D exchange and lowering the pKa of the C-6 CH2 group.
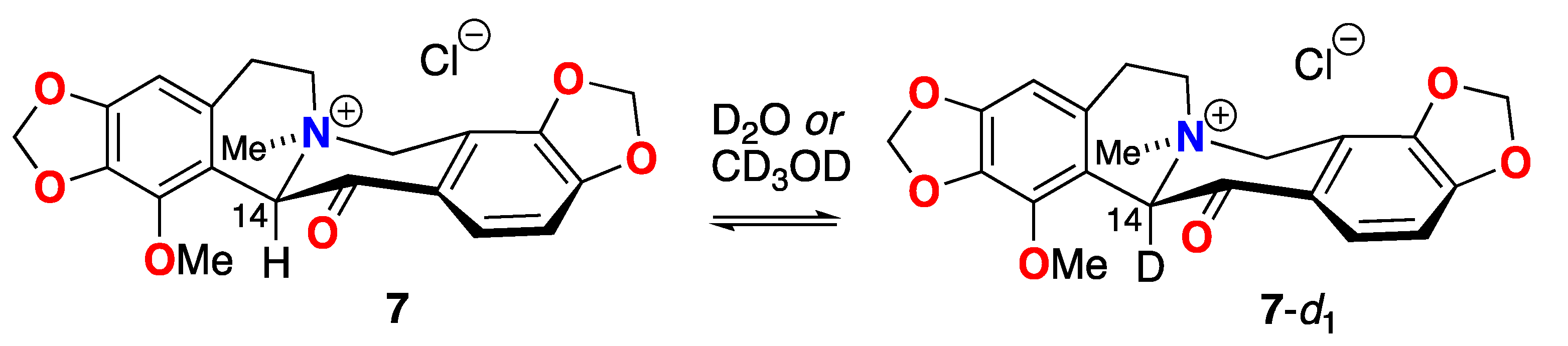
To the best of our knowledge, rapid H-D exchange of a substituted b-quaternary ammonium ketone within a natural product has been observed only in one other instance, coulteroberbinone (
7,
Figure 8 [
23]), an
N-quaternary ammonium isoquinoline alkaloid from the leaves of
Romneya trichocalyx. The authors note the C-14 C-H in
7, assigned to the α-proton between the carbonyl and quaternized nitrogen (
dH 5.64, s), underwent rapid proton-deuterium exchange in D
2O or CD
3OD to C-D (
7-
d1) under ambient conditions (supported by
1H NMR and ESIMS data).
Figure 8.
Rapid H-D exchange of coulterberbinone (7) in deuteric solvent under ambient conditions.
Figure 8.
Rapid H-D exchange of coulterberbinone (7) in deuteric solvent under ambient conditions.
Two major factors most likely explain the relatively low p
Ka of
1 and
7: the electron withdrawing (inductive) effect of the -N
+Me
2 quaternary ammonium group (N-7) and stabilization of enol
5 (or enolate
6) through extensive conjugation of the heteroaromatic core, not unlike the stabilization of the enolates of alkyl phenones, e.g. acetophenone, determined by UV-vis (
pK
a = 18.4). Indirect determinations of the p
Ka of the enol of acetophenone has also be obtained from kinetics of reactions of acetophenone: e.g. a-chlorination [
24], and aminolysis of the corresponding enol acetate [
25]. In contrast,
1 appears to undergo rapid enolization in the
absence of added Brønsted acid, suggesting the possibility that this tautomerization that may even be autocatalytic.
Is the enol
8a of petrosamine (
1) present in substantial concentrations? From the
1H NMR spectrum of
1, (DMSO-
d6) we detect no signals attributable to
8a. It can be ascertained from
1H NMR that
Keq1 (
Figure 7) is very small (estimated from the limits of integration,
Keq1 < 0.05) and the equilibrium of tautomers lies well towards the keto form
1. As noted above, in highly-basic aqueous solutions of
1, the charge-minimal enolate
8b begins to appear in substantially concentrations placing a lower-boundary of p
Ka ~15 for
1. In the absence of base, the mechanism of H-D exchange
1 to
1-
d2 in CD
3OD most likely engages substantial equilibrium concentrations
8a to allow rapid exchange of the CH
2 to CD
2 in deuteric solvents within less than 2 minutes at 23 ˚C. Estimation of an accurate p
KA of
1 is not accessible from measurements in aqueous solvents and securing the same will likely require measurements in a suitable aprotic solvent (e.g. titration in DMSO [
20,
26][2]).