Submitted:
12 July 2023
Posted:
14 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental part
2.1. Materials
- -
- WIFP-270 of a surface mass of approx. 270 g/m2 (Sieć Badawcza Łukasiewicz - Łódzki Instytut Technologiczny, Łódź, Poland),
- -
- filter non-wovens: FS G-4 of the surface mass of approx. 210 g/m2 and FS F-5 of the surface mass of approx. 240 g/m2 (Filter Service Ltd., Zgierz, Poland),
- -
- polyester non-woven (aqua-jet, Hydronina) having the surface mass of approx. 100 g/m2 (Lentex, Lubliniec, Polska),
- -
- cotton-polyester fabric with 70 wt.% content of PES (Figaro), having a twill weave and the surface mass of approx. 170 g/m2 (Andropol S.A., Andrychów, Poland),
- -
- cotton fabric (Medical) with the plain weave and the surface mass of approx. 150 g/m2 (Andropol S.A., Andrychów, Poland).
2.2. Chemical Reagents
- copper sulfate Cu(SO4)2∙5H2O, pure (Chempur, Piekary Śląskie, Poland);
- Vitrosilicon 137S, a water solution of sodium water glass, having a SiO2:Na2O molar ratio 3.3 (CIECH Vitrosilicon, 68-120 Iłowa, Poland);
- CELLOSIZE HEC QP-40 [(hydroxyethyl)cellulose + sodium acetate] (Amerchol, USA) – thickening agent;
- poly(ethylene glycol) Polikol 400 (PEG400) (PCC Exol, Poland) – a wetting agent;
- poly(ethylene glycol) Pluriol E600 (PEG600) (BASF, Germany) – the wetting agent;
- water dispersion of styrene-acrylic resin Revacryl 247® (Thorex, Łódź, Poland) – a binder;
- water dispersion of acrylic resin – Talens Amsterdam Acrylic Binder 005 (Talens, Niderlands);
- Cinkarna CCA 100 BS, water dispersion of acrylic resin, containing 20-22 % wt.% of nano-TiO2 (~10 nm), (Cinkarna Celje, d.d., Slovenia);
- water dispersion of acrylic resin Dekoral Silver (PPG DECO Sp. z o.o., Wrocław, Poland) – the binder,
- acrylic photocatalytic water dispersion Titanium IN (Pigment, Szczecin, Poland) – the binder,
- silicate photocatalytic water dispersion Titanium FA (Pigment, Szczecin, Poland) – the binder,
- Synexil DN-50, water dispersion of poly(vinyl acetate, PVAc (Synthos S.A., Oświęcim, Poland) – the binder,
- poly(vinyl alcohol) Mowiol 4-98 (Fluka, Germany) with a weight average molecular weight (Mw) ~27 000 g/mol ) – thickening and pro-adhesion agent,
- soluble starch (Chempur, Piekary Śląskie, Poland) – thickening and pro-adhesion agent,
- glycerine, pure (POCh, Gliwice, Poland) – a plasticizer,
- bis(2-etylohexyl adipate) (Adoflex) (Zakłady Azotowe Kędzierzyn S.A., Kędzierzyn-Koźle, Poland) – the plasticizer,
- nanosilica Aerosil 380 (Evonik, Germany) – a stabilizer of dispersions,
- methyl silicone oil containing OH terminal groups, with a viscosity of 500 cP - Polastosil M-200 (Zakład Chemiczny „Silikony Polskie”, Nowa Sarzyna, Poland) – an antifoaming agent,
- enzyme Texazym PES (INOTEX, Dvůr Králové n.L, Czechia);
- syntetic acrylic thickening agent Lutexal Thickener HC (BASF, Niemcy) - for our purpose it was diluted with demineralized water (1:3, w/w),
- copper silicate hydrate CuSiO3∙18.5H2O (Poznań University of Technology, Poznań, Poland) composed of: 35.23 wt.% CuO, 62.16 wt.% SiO2, 18.52 wt.% H2O, 0.02 wt.% Na2O, and 0.01 wt.% K2O), with a particle diameter in a range 1-100 µm [39];
- titanium dioxide TiO2 (TK44) (Poznań University of Technology, Poznań, Poland) with the average particle diameter of 615 nm and their polydispersity of 0.102; it was obtained from the anatase allotrope of TiO2, having a commercial name Tytanpol (Police S.A., Szczecin, Poland), with the average particle diameter of 712-825 nm and the polydispersity of 0.218 – it was modified with 1 wt.% of N-2-aminoethyl-3-aminopropyl(trimethoxy)silane;
- zinc oxide, ZnO (Z11) (obtained from Poznań University of Technology, Poznań, Poland) with the average particle diameter of 396 nm and their polydispersity of 0.161;
- zinc lactate, p.a. (Xenon - Chemists' Cooperative, Rąbień, Poland);
- hybrid oxide ZnO∙SiO2, prepared as it was described in [45a].
2.3. Modification of the surface of nonwovens and polyester fabrics before the dip-coating process
- (1)
- The enzyme treatment was carried out at 35 °C for 30 minutes in a bath containing 1-2 wt.% of an enzyme from the group of esterases Texazym PES, at pH 4.2 (adjusted by the addition of acetic acid). The bath ratio against polyester nonwovens was 10:1.
- (2)
- Alkaline treatment was carried out at 98 °C for 60 min. in the bath containing sodium hydroxide with a concentration of 1.8 g/l, sodium carbonate (3.6 g/l) and a sequestering and wetting agent. The bath ratio was 10:1.
2.3.1. Synthesis of copper silicate hydrate in situ
2.3.2. Description of the dip-coating method of nonwovens and polymer fabrics
| CuSiO3∙xH2O | – | 1-7 wt.%, |
| Cellosize HEC QP-40 | – | 0.9-1.0 wt.%, |
| Polikol 400 (PEG400) | – | 5-10 wt.%, |
| dispersion of chosen acrylic resin (or PVAc) | – | 3-10 wt.%, |
| 2 wt.% water solution of the soluble starch (or PVA) | – | 25 wt.%, |
| nanosilica (Aerosil 380) | – | 0.1-0.2 wt.%, |
| silicone oil (Polastosil M200) | – | 0,1 wt.%, |
| other additives | – | changing amounts. |
2.3.3. Description of the coating method of polymer fabrics and nonwovens
- 3-10 wt.%– reagents with antimicrobial properties (e.g. Cu silicate or/and metal oxide),
- 5-10 wt.% – wetting agent,
- 3-10 wt.% – binders and thickeners.
2.3.4. Description of dip-coating and coating experiments (chosen examples)
- Example 1
| Components of dispersions | Water dispersion number | |||||||||||||||||||
| 1 | 2 | 5 | 6 | 7 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | |
| CuSiO3∙xH2O a | 3.0 | 5.0 | 3.0 | 2.0 | 1.0 | 1.0 | 3.0 | 5.0 | 5.0 | 7.0 | .9 | 7,0 | 5.0 | 5.0 | 7.0 | 5.0 | 5.0 | 7.0 | 5.0 | 5.0 |
| Cellosize HEC QP-40 | 1.5 | 1.0 | 1.0 | 0.9 | 0.9 | 0.9 | 0.8 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Polikol 400 (PEG400) | 7.5 | 5.0 | - | - | - | - | 6.0 | 7.5 | - | - | 10.0 | 10.0 | 7.5 | 7.5 | - | 7.5 | 7.5 | 10.0 | 10.0 | 10.0 |
| Pluriol E600 (PEG600) | - | - | 7.5 | 5.0 | 5.0 | 5.0 | - | - | 7.5 | 10.0 | - | - | - | - | 10.0 | - | - | - | - | - |
| Copolymer dispersion Revacryl 247 | - | 5.0 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Cinkarna 100 BS | - | - | - | - | - | - | - | - | - | - | 1.0 b | 2.1 b | 4.2 b | - | - | 5.0 b | 5.0 b | - | - | - |
| Talens Amsterdam Acrylic Binder 005 c | 4.5 | - | 3.0 | 5.0 | 3.0 | 5.0 | 3.0 | 1.0 | 1.0 | 1.0 | - | - | - | - | 1.0 | - | - | - | - | - |
| Acrylic dispersion Dekoral Silver c | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 2.0 | 2.0 | - | - | - |
| Photocatalytic acrylic dispersion Titanium IN c |
- | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 25.0 | - |
| Photocatalytic silicate dispersion Titanium FA c | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 25.0 |
| Zinc lactate cz. | - | - | - | - | - | - | - | - | - | - | - | - | - | 5.0 | - | - | - | - | - | - |
| Glycerine | - | - | 0.5 | 0.4 | 0.4 | 0.4 | 0.5 | 1.0 | 1.0 | 1.0 | 0.5 | 0.5 | 0.5 | 0.5 | 1.0 | 0,5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Synexil DN-50 | - | - | - | - | - | - | 1.0 | 4.0 | 4.0 | 4.0 | 2.50 | 2.0 | 2.0 | 2.0 | 4.0 | 2.0 | - | - | - | - |
| Bis(2-ethylhexyl adipate) (Adoflex) | - | - | - | - | - | - | 0.5 | 2.0 | 2.0 | 2,0 | 1.25 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | - | - | - | - |
| 2 wt.% water solution of soluble starch | - | - | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 |
| 5 wt.% water solution of PVA | - | 10.0 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Nanosilica (Aerosil 380) | - | - | - | - | - | 0.1 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Silicone oil (Polastosil M200) | - | - | - | - | - | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Demineralized water | 83.5 | 74.0 | 60.0 | 61.5 | 64.5 | 62.5 | 60.0 | 53.2 | 53.2 | 48.7 | 48.5 | 43.2 | 37.7 | 51.7 | 49.7 | 33.9 | 34.9 | 31.2 | 33.2 | 33.2 |
- Example 2
- Example 3
- Example 4
- Example 5
- Example 6
2.3.5. Evaluation of antimicrobial activity of polymeric nonwovens modified with aqueous dispersions containing copper silicate
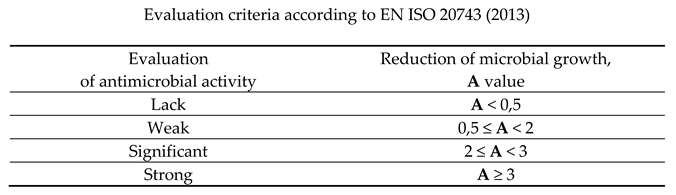
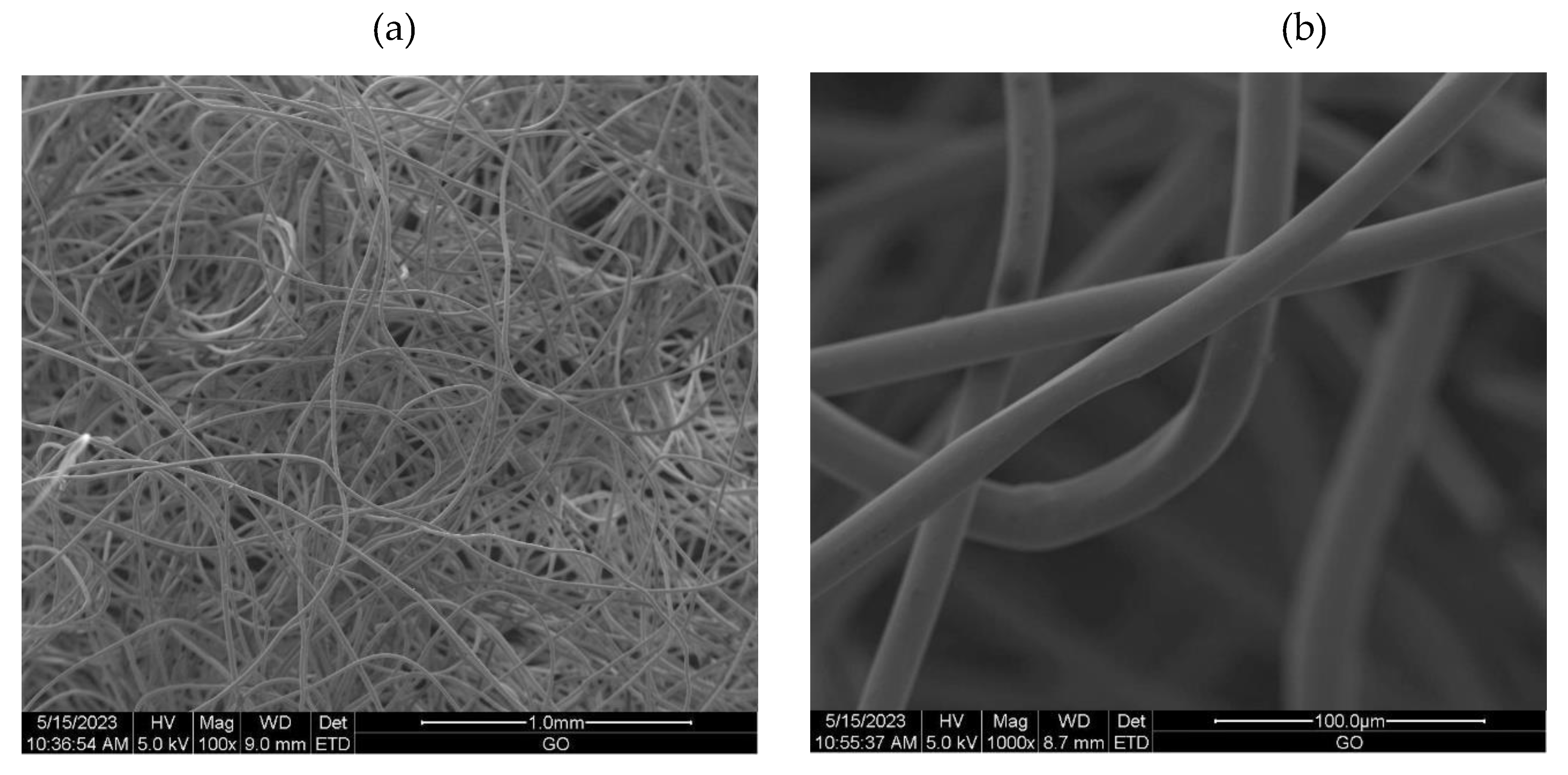
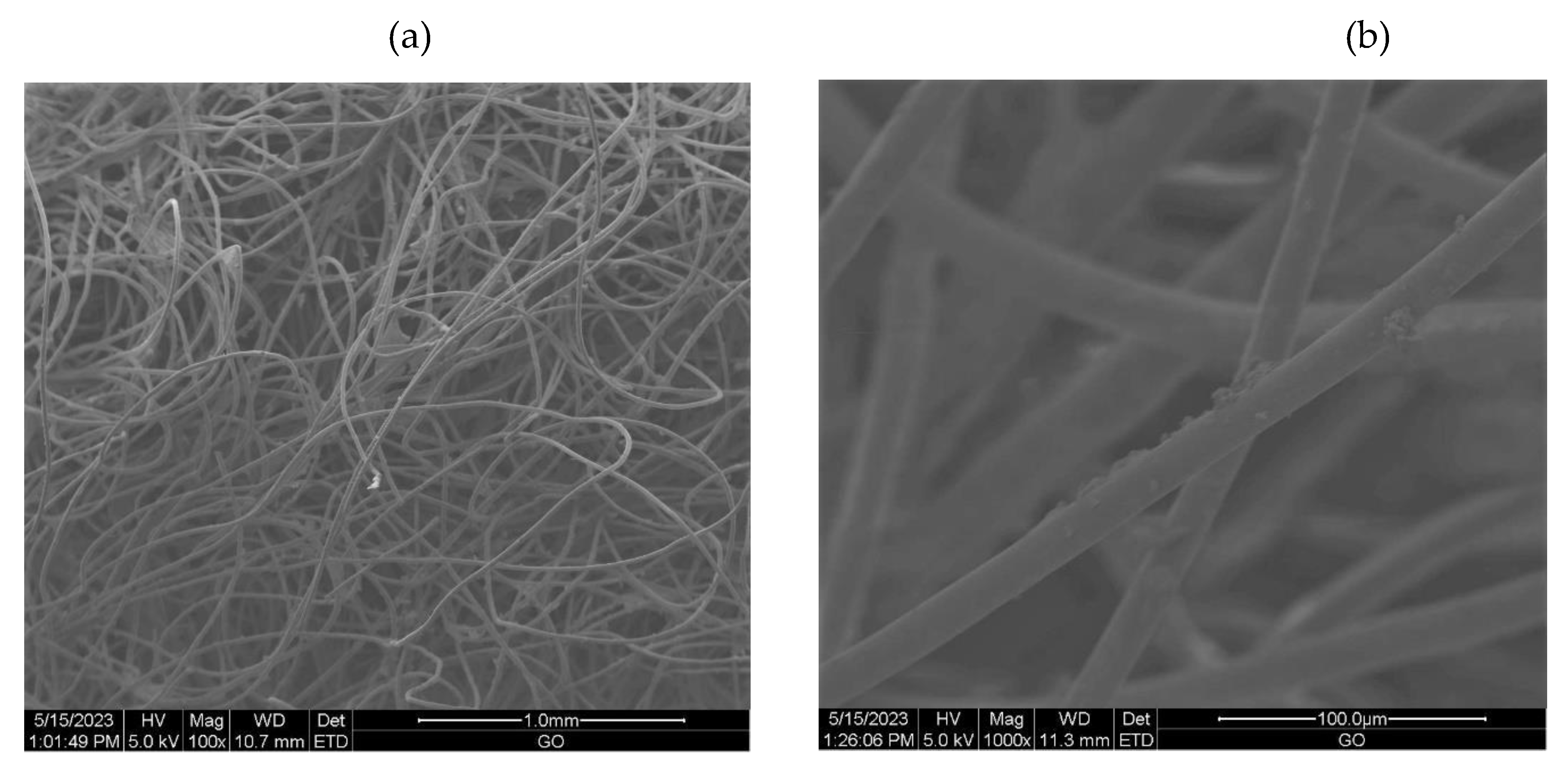
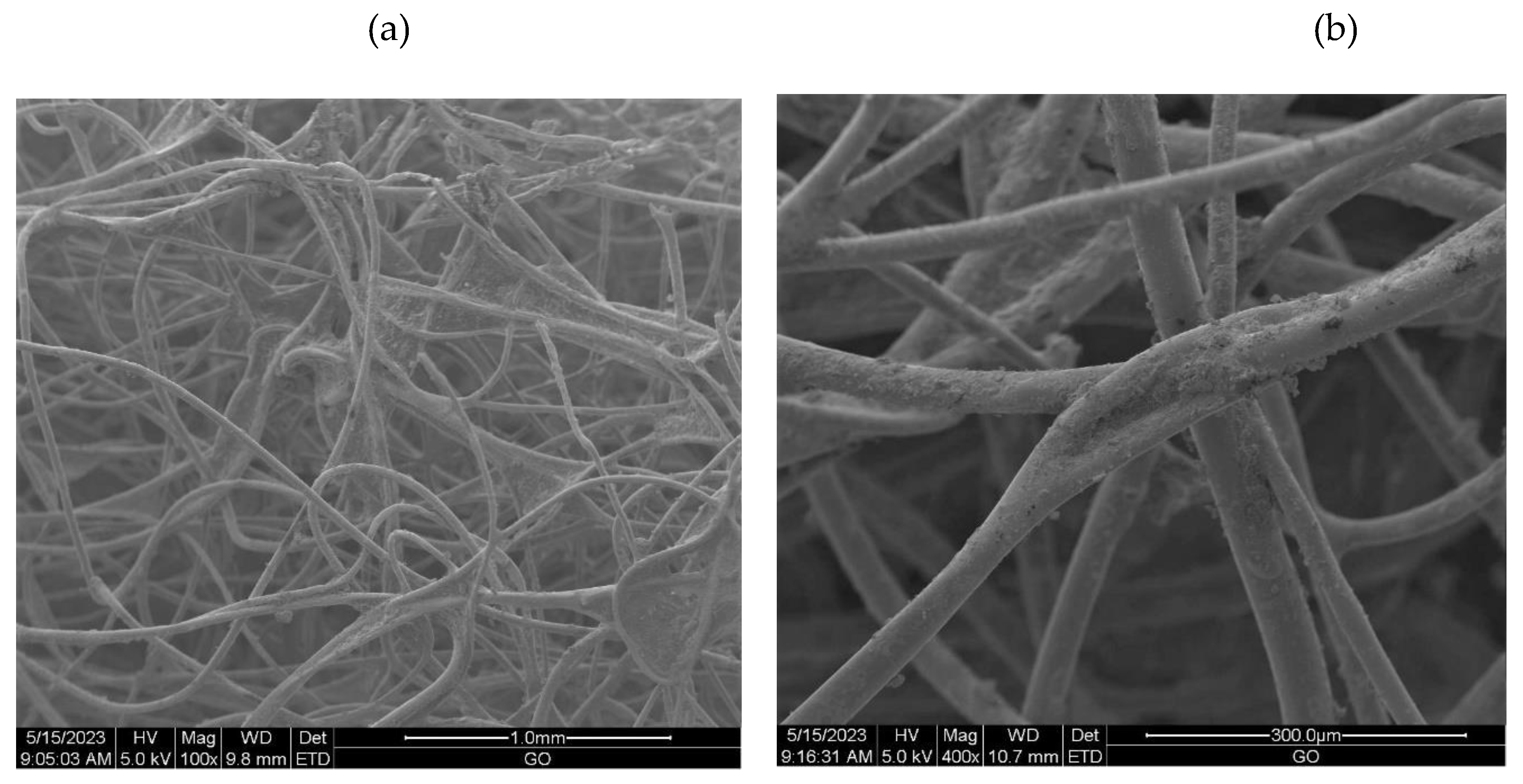
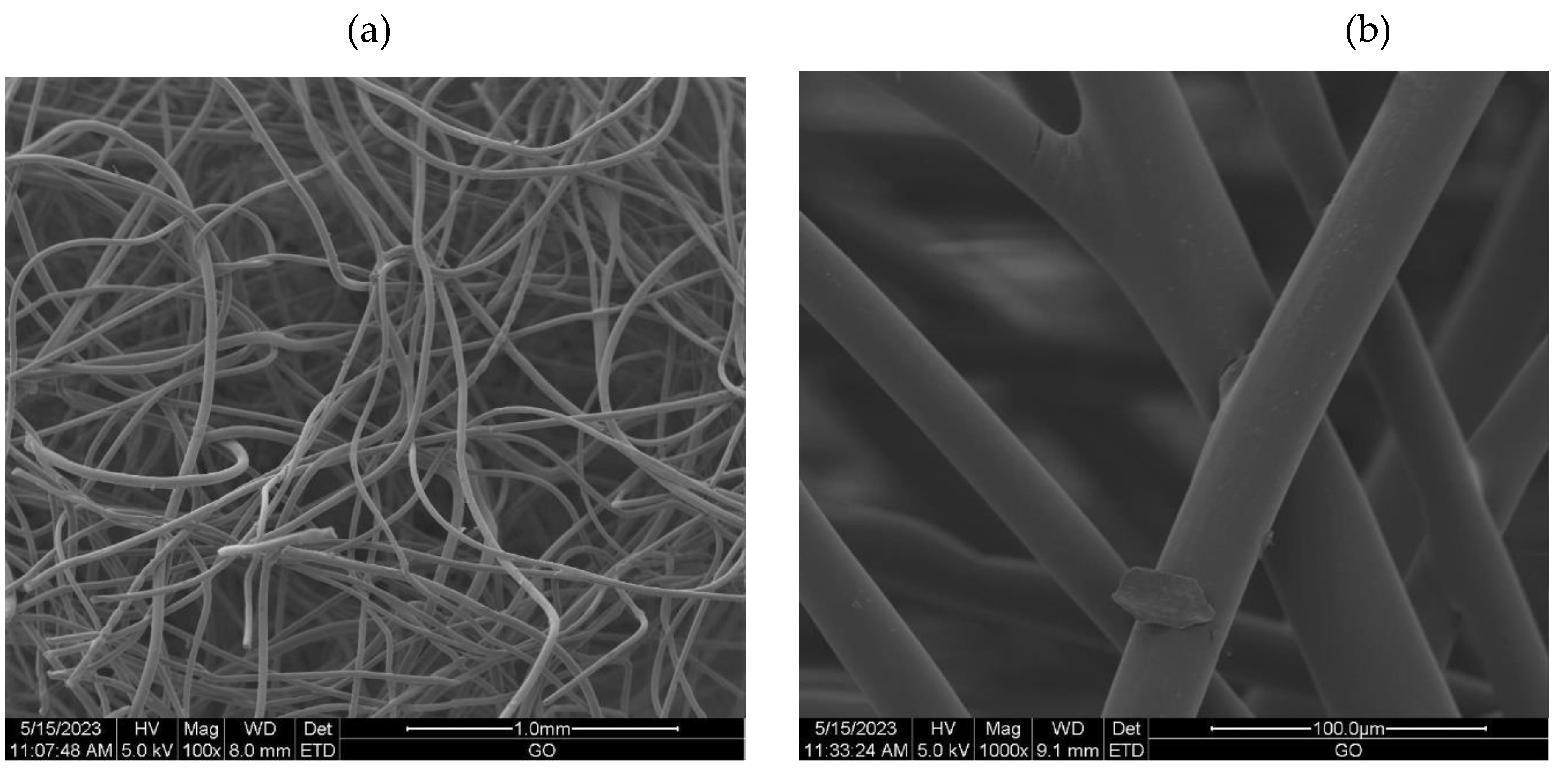
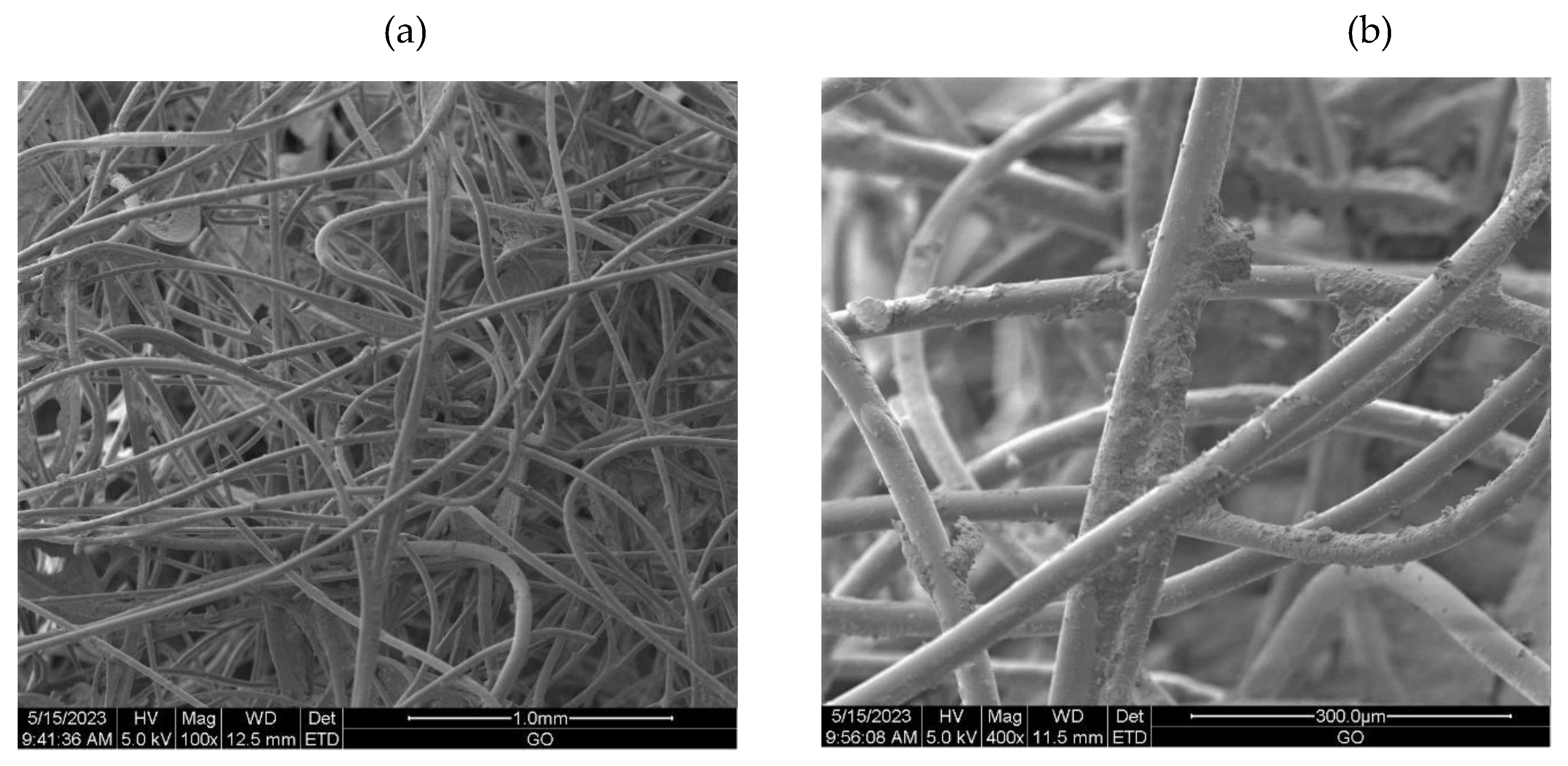
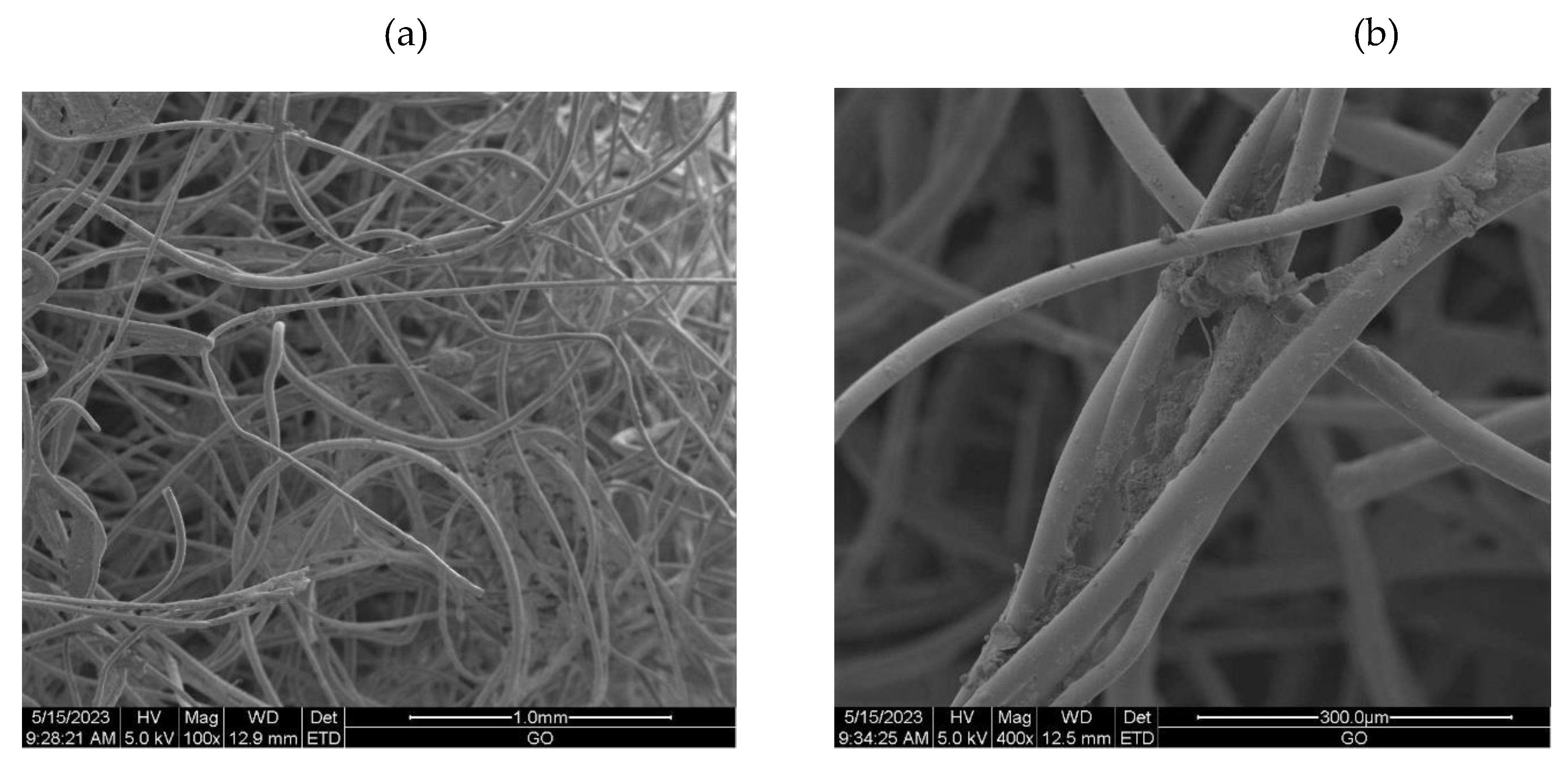
2.3.6. Scanning Electron Microscopy (SEM)
3. Research results and discussion
- -
- copper silicate CuSiO3∙xH2O (which is a synthetic version of minerals: chrysicolla and dioptase),
- -
- titanium dioxide, zinc oxide, zinc silicate ZnO∙SiO2 and zinc lactate
- -
- or their mixtures.
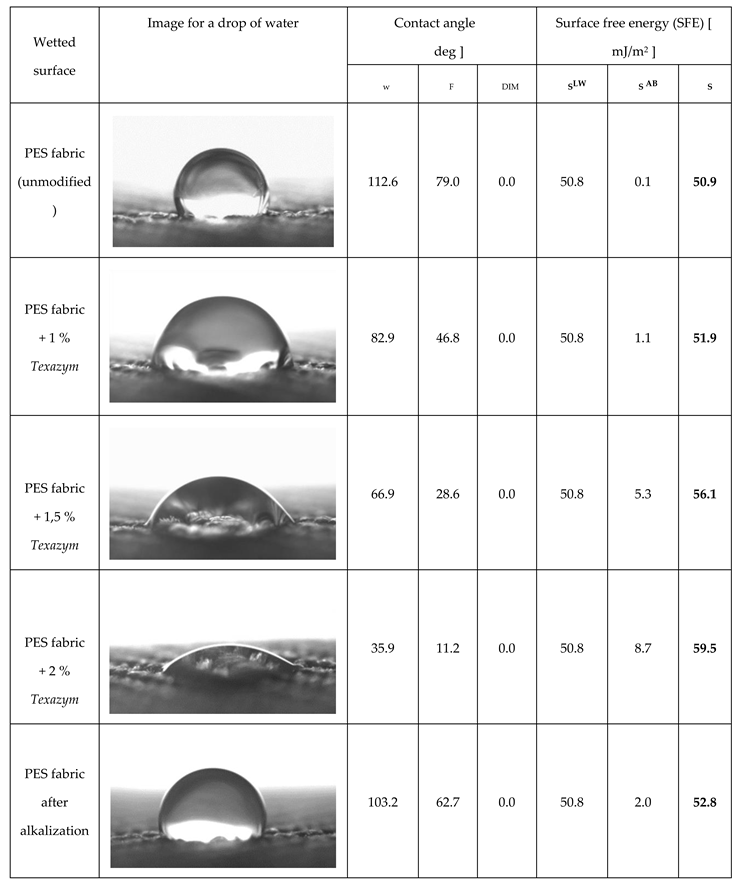
3.1. An improvement of a surface wettability and hydrophilicity of textile materials
3.2. Evaluation of the results of antimicrobial modification of the properties of nonwovens and fabrics, modified with aqueous dispersions containing copper silicate
3.2.1. The scanning electron microscopy (SEM) of non-woven samples
3.3. Evaluation of the results of the antimicrobial modification of the properties of fabrics (and nonwovens) with copper silicate and composite hybrid oxide ZnO∙ SiO2 by the dip-coating and coating methods
- 1.
-
the cotton fabric Medical, polyester fabric, the cotton-polyester fabric Figaro, and also polyester non-woven fabric Hydronina, biofunctionalized by the dip-coating method with the dispersions containing 6.0 wt.% CuSiO3∙xH2O (or the composite hybrid oxide ZnO∙SiO2) exhibited:(a) against the gram-negative bacteria Escherichia coli
- -
- strong antibacterial properties (an antibacterial activity coefficient A reached the values in a range: 3.1-6.2 for the samples modified with CuSiO3∙xH2O and 3.7-6.0 - for the samples modified with ZnO∙SiO2),
- -
- strong and significant bacteriostatic properties (a bacteriostatic coefficient S reached the values in the range: 3.0-6.7 - for the samples modified with CuSiO3∙xH2O and 2.1-6.6 - for the samples modified with ZnO∙SiO2),
- -
- good bactericidal properties (a bacterial growth reduction factor R was: 77.5-96.8% - for the samples modified with CuSiO3∙xH2O, and 72.3-97.0% - for the samples modified with ZnO∙SiO2);(b) against gram-positive bacteria Staphylococcus aureus
- -
- strong or significant antibacterial properties (the antibacterial activity coefficient A reached the values in the range: 2.5-6.2 for the samples modified with CuSiO3∙xH2O and 2.8-4.8 - for the samples modified with ZnO∙SiO2),
- -
- strong and significant bacteriostatic properties (the bacteriostatic coefficient S reached the values in the range: 2.6-6.5 for samples modified with CuSiO3∙xH2O, and 3.0-5.2 - for the samples modified with ZnO∙SiO2)
- -
- and good and significant bactericidal properties (the bacterial growth reduction factor R was: 89.6-99.0% - for the samples modified with CuSiO3∙xH2O, and 70.8% - for the sample modified with ZnO∙SiO2).
- 2.
- For all samples of the PES fabric and PES nonwoven Hydronina, modified by coating method with the paste containing approx. 10 wt.% CuSiO3∙xH2O, the growth reduction factor of Candida albicans R reached the values in the range: 97.9-99.6%, the antibacterial activity coefficient A was in the range: 4.8-5.6, the bacteriostatic coefficient S had the same value 4.8-5.6, and the bactericidal coefficient L was in the range: 1.6-2.4.
- 3.
- The obtained composite-polymer textile materials also showed a good inhibitory effect on the development of the mold fungus Chaetomium globosum. The samples of the textile materials coated with CuSiO3 hydrate, and especially the polyester fabric subjected to biomodification with 7.0 wt.% CuSiO3∙xH2O, showed a clear effect of antifungal activity against the fungus Chaetomium globosum, which grew on the surface of the samples only in the range of 0-25%.
- 4.
- The new biofunctionalized textile materials obtained by coating method (mainly cotton, cotton-polyester and polyester fabrics) with pastes containing: (a) CuSiO3 or (b) CuSiO3 + ZnO, or (c) CuSiO3 + TiO2 particles introduced onto the surface and incortporated into their structures also showed good barrier properties against UV radiation (UPF > 50), and the lowest transmittance (T average was 2.5-3.5), which was characteristic for the textile products subjected to the initial alkaline or biochemical (enzymatic) modifications, followed by the biofunctionalization with mixtures containing a total of 10 wt.% of CuSiO3∙xH2O and TiO2 (or ZnO) in a weight ratio of 7:3 or 1:1.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- T.J. Berger, J.A. Spadaro, R. Bierman, S.E. Chapin, R.O. Becker, Antifungal properties of electrically generated metallic-ions, Antimicrobial Agents and Chemotherapy, 10, 1976, 856-860. [CrossRef]
- J.M. Hunter, Geophagy in Africa and the United-States, Geographical Reviews, 63, 1973, 170-195.
- M.J. Domek, M.W. Lechevallier, S.C. Cameron, G.A. McFeters, Evidence for the role of copper in the injury process of Coliform bacteria in drinking water, Applied and Environmental Microbiology, 48, 1984, 289-293. [CrossRef]
- B. Bagchi, S. Kara, S.K. Deyb, S. Bhandarya, D. Roya, T.K. Mukhopadhyayc, S. Dasa, P. Nandy, In situ synthesis and antibacterial activity of copper nanoparticle loaded natural montmorillonite clay based on contact inhibition and ion release, Colloilds and Surfaces B: Biointerfaces, 108, 2013, 358-365.
- X. Xu, Q. Yang, Y. Wang, H. Yu, X. Chen, X. Jing, Biodegradable electrospun poly(L-lactide) fibers containing antibacterial silver nanoparticles, European Polymer Journal, 42, 2006, 2081-2087. [CrossRef]
- K. Zou, Q. Liu, J. Chen, J. Du, Silver-decorated biodegradable polymer vesicles with excellent antibacterial efficacy, Polymer Chemistry, 5, 2014, 405-411. [CrossRef]
- D.L. Van Hyning, Yarns and fabrics having a wash-durable antimicrobial silver particulate finish, Pat. US 7232777, 2007.
- E. Rybicki, B. Filipowska, A. Walawska, M. Kozicki, E. Matyjas-Zgondek, Sposób nadawania płaskim wyrobom włókienniczym właściwości antybakteryjnych i antygrzybicznych, Pat PL 214689 B1, 2013.
- H.J., Lee, S.Y. Yeo, S.H. Jeong, Antibacterial effect of nanosized silver colloidal solution on textile fabrics, Journal of Material Science, 38, 2003, 2199-2204. [CrossRef]
- J. Yan, J. Cheng, Antimicrobial yarn having nanosilver particles and methods for manufacturing the same, Pat. US 6 979 491, 2005.
- J. Bucheńska, S. Słomkowski, J. Tazbir, D. Timler, E. Sobolewska, A. Karaszewska, Sposób nadawania włóknom poliestrowym właściwości antybakteryjnych, Pat PL 196 213 B1, 2007.
- E. Rybicki, B. Filipowska, A. Walawska, J. Grad, E. Wilk, Z. Żakowska, H. Stobińska, J. Rosiak, Sposób nadawania wyrobom włókienniczym właściwości antybakteryjnych lub terapeutycznych, Pat PL 200 059 B1, 2008.
- K.D. Min, J.H .Youk, Y.J. Kwark, W.H. Park, Preparation of inorganic silica nanofibers containing silver nanoparticles, Fibers and Polymers, 8, 2007, 591-600. [CrossRef]
- H.T. Au, L.N. Pham, T.H.T. Vu, J.S. Park, Fabrication of an antibacterial non-woven mat of a poly(lactic acid)/chitosan blend by electrospinning, Macromolecular Research, 20, 2012, 51-58. [CrossRef]
- G. Borkow, J. Gabbay, Putting copper into action: copper-impregnated products with potent biocidal activities, FASEB Journal, 18, 2004, 1728. [CrossRef]
- G. Borkow, R.W. Sidwell, D.F. Smee, D.L. Barnard, J.D. Morrey, H.H. Lara-Villegas, Y. Shemer-Avni, J. Gabbay, Neutralizing viruses in suspensions by copper oxide-based filters, Antimicrobial Agents and Chemotherapy, 51, 2007, 2605-2607. [CrossRef]
- G. Ren, D. Hu, E.W.C. Cheng, M.A. Vargas-Reusc, P. Reipd, R.P. Allaker, Characterisation of copper oxide nanoparticles for antimicrobial applications, International Journal of Antimicrobial Agents, 33, 2009, 587-590. [CrossRef]
- R.V. Ravishankar, B.A. Jamuna, Nanoparticles and their potential application as antimicrobials, Formatex, 2011, 197-209.
- Weinberg, A. Lazary, A. Jefidoff, J.J. Vatine, G. Borkow, N. Ohana, Safety of using diapers containing copper oxide in chronic care elderly patients, The Open Biology Journal, 6, 2013, 1-7. [CrossRef]
- R. Rani, H. Kumar, R.K. Salar, S.S. Purewal, Antibacterial activity of copper oxide nanoparticles against gram negative bacterial strain synthesized by reverse micelle technique, International Journal of Pharmaceutical Research and Developments, 6, 2014, 72-78.
- P. Pallavicini, G. Dacarro, L. Cucca, F. Denat, P. Grisoli, M. Patrini, N. Sok, A. Taglietti, A monolayer of a Cu2+-tetraazamacrocyclic complex on glass as the adhesive layer for silver nanoparticles grafting, in the preparation of surface-active antibacterial materials, New Journal of Chemistry, 35, 2011, 1198-1201. [CrossRef]
- J. Gabbay, J. Mishal, E. Magen, R. Zatcoff, Y. Shemer-Avni, G. Borkow, Copper oxide impregnated textiles with potent biocidal activities, Journal of Industrial Textiles, 35, 2006, 323-335. [CrossRef]
- D. Deng, Y. Cheng, Y. Jin, T. Qi, F. Xiao, Antioxidative effect of lactic acid-stabilized copper nanoparticles prepared in aqueous solution, Journal of Materials Chemistry, 22, 2012, 23989-23995. [CrossRef]
- I.W. Shim, W.T. Noh, J. Kwon, J.Y. Cho, K.S. Kim, D.H. Kang, Preparation of copper nanoparticles in cellulose acetate polymer and the reaction chemistry of copper complexes in the polymer, Bulletin of Korean Chemical Society, 23, 2002, 563-566. [CrossRef]
- N. Cioffi, L. Torsi, N. Ditaranto, G. Tantillo, L. Ghibelli, L. Sabbatini, T. Bleve-Zacheo, M. D’Alessio, P.G. Zambonin, E. Traversa, Copper nanoparticle/polymer composites with antifungal and bacteriostatic properties, Chemistry of Materials, 17, 2005, 5255-5262. [CrossRef]
- J. Konieczny, Z. Rdzawski, Antibacterial properties of copper and its alloys, Archives of Materials Science and Engineering, 56, 2012, 53-60.
- C.C. Trapalis, M. Kokkoris, G. Perdikakis, G. Kordas, Study of antibacterial composite Cu/SiO2 thin coatings, Journal of Sol-Gel Science and Technology, 26, 2003, 1213-1218. [CrossRef]
- Singh, V. Krishna, A. Angerhofer, B. Do, G. MacDonald, B. Moudgi, Copper coated silica nanoparticles for odor removal, Langmuir, 26, 2010, 15837-15844. [CrossRef]
- N. Zhang, Y. Gao, H. Zhang, X. Feng, H. Cai, Y. Liu, Preparation and characterization of core–shell structure of SiO2@Cu antibacterial agent, Colloids and Surfaces B: Biointerfaces, 81, 2010, 537–543. [CrossRef]
- P. Maniprasad, S. Santra, Novel copper (Cu) loaded core-shell silica nanoparticles with improved Cu bioavailability: synthesis, characterization and study of antibacterial properties, Journal of Biomedical Nanotechnology, 8, 2012, 558-566.
- S. Varghese, S.O. ElFakhri, D.W Sheel, P. Sheel, F.J.E. Bolton, H.A. Foster, Antimicrobial activity of novel nanostructured Cu-SiO2 coatings prepared by chemical vapour deposition against hospital related pathogens, AMB Express, 3, 2013, 53.
- V. Grumezescu, C.M. Chifiriuc, A.M. Holban, P. Stoica, A.M. Grumezescu, G. Voicu, G. Socol, K.S. Huang, C. Bletou, R. Radulescu, Antimicrobial and biocompatibility assay of newly fabricated materials based copper or zinc alginate and SiO2 network, Digest Journal of Nanomaterials and Biostructures, 8, 2013, 869-876.
- M.S. Xia, C.H. Hu, Z.R. Xu, Y. Ye, Y.H. Zhou and L. Xiong, Effects of Copper-bearing Montmorillonite (Cu-MMT) on Escherichia coli and Diarrhea on Weanling Pigs, Asian-Australasian Journal of Animal Sci., 17, 1712-1716 (2004). [CrossRef]
- S.I. Hossain, E.A. Kukushkina, M. Izzi, M.C. Sportelli, R.A. Picca, N. Ditaranto and N. Cioffi, A Review on Montmorillonite-Based Nanoantimicrobials: State of the Art, Nanomaterials, 2023, 13, 848. [CrossRef]
- Esteban-Cubillo, C. Pecharromán, E. Aguilar, J. Santarén, J.S. Moya, Antibacterial activity of copper monodispersed nanoparticles into sepiolite, Journal of Materials Science, 41, 2006, 5208-5212. [CrossRef]
- S. Kar, B. Bagchi, B. Kundu, S. Bhandary, R. Basu, P. Nandy, S. Das, Synthesis and characterization of Cu/Ag nanoparticle loaded mullite nanocomposite system: A potential candidate for antimicrobial and therapeutic applications, Biochimica et Biophysica Acta, 1840, 2014, 3264-3276. [CrossRef]
- R. Górecki,.W. Danielski-Busch, J. Stępowski, T. Jakubowski, Emulsja do pojemników szkółkarskich i doniczek, Pat PL 196994 (2008).
- X. Wu, L. Ye, K. Liu, W. Wang, J. Wei, F. Chen, C. Liu, Antibacterial properties of mesoporous copper-doped silica xerogels, Biomedical Materials, 4, 2009, 045008. [CrossRef]
- M. Nowacka, A. Modrzejewska-Sikorska, Ł. Chrzanowski, D. Ambrożewicz, T. Rozmanowski, K. Myszka, K. Czaczyk, K. Bula, T. Jesionowski, Electrokinetic and bioactive properties of CuO∙SiO2 oxide composites, Bioelectrochemistry, 87, 2012, 50-57. [CrossRef]
- Kloziński, P. Jakubowska, D. Ambrożewicz, T. Jesionowski, Thermal Properties of Polyolefin Composites with Copper Silicate, AIP Conference Proceedings 1664, 060016 (2015); https://doi.org/10.1063/1.4918434. [CrossRef]
- M. Young, S. Santra, Copper (Cu)-silica nanocomposite containing valence-engineered Cu: A new strategy for improving the antimicrobial efficacy of Cu biocides, Journal of Agricultural and Food Chemistry, 62, 2014, 6043-6052. [CrossRef]
- J. Sójka-Ledakowicz, J. Olczyk, A. Walawska, I. Kamińska, B. Gutarowska, Z. Żakowska, E. Kozanecka, Nowe materiały włókiennicze o właściwościach barierowych przed promieniowaniem nadfioletowym i drobnoustrojami. Cz. I, Przegląd Włókienniczy - Włókno, Odzież, Skóra, nr 4, 43-45 (2010).
- J. Sójka-Ledakowicz, J. Olczyk, A. Walawska, I. Kamińska, B. Gutarowska, Z. Żakowska, E. Kozanecka, Nowe materiały włókiennicze o właściwościach barierowych przed promieniowaniem nadfioletowym i drobnoustrojami. Cz. II, Przegląd Włókienniczy - Włókno, Odzież, Skóra, nr 5, 40-43 (2010).
- T. Jesionowski, A. Kołodziejczak-Radzimska, F. Ciesielczyk, J. Sójka-Ledakowicz, J. Olczyk, J. Sielski, Synthesis of Zinc Oxide in an Emulsion System and its Deposition on PES Nonwoven Fabrics, Fibres & Textiles in Eastern Europe, 2011, 19(2), 70-75.
- J. Sójka-Ledakowicz, J. Olczyk, A. Walawska, A. Laurentowska, A. Kołodziejczak-Radzimska, T. Jesionowski, Modyfikacja wyrobów włókienniczych przy wykorzystaniu tlenku cynku o cząstkach nanometrycznych oraz kompozytu tlenkowego ZnO-SiO2, Przemysł Chemiczny, 89(12), 1648-1652 (2010); (b) A. Laurentowska, T. Jesionowski, ZnO-SiO2 Oxide Composites Synthesis During Precipitation From Emulsion System, Physicochem. Probl. Miner. Process., 2012, 48(1), 63–76.
- J. Sójka-Ledakowicz, J. Chruściel, M. Kudzin, M. Kiwała, (a) Antimicrobial Functionalization of Textile Materials with Copper Silicate, Fibers and Textiles in Eastern Europe, 24, 5(119), 151-156 (2016); (b) A method for biofunctionalization of textile materials, Patent EP 3067445 A1 (2017); (c) Sposób biofunkcjonalizacji materiałów włókienniczych, Patent PL 231089 (2019).
- J. Sójka-Ledakowicz, J.J. Chruściel, M.H. Kudzin, J. Olczyk, M. Kiwała, T. Jesionowski, Hybrid modifiers for antimicrobial functionalization of textile materials, 6th International Conference on Multifunctional, Hybrid and Nanomaterials, 11-15 March 2019, Sitges, Spain (poster P1-066).
- M. Łatwińska, J. Sójka-Ledakowicz, J. Chruściel, M. Piórkowski, PLA and PP Composite Nonwoven with Antimicrobial Activity for Filtration Applications, International Journal of Polymer Science, Article ID 2510372 (2016).
- G.E. Luckachan, C.K.S. Pillai, Biodegradable polymers – A review on recent trends and emerging perspectives, Journal of Polymers and the Environment, 19, 2011, 637-676. [CrossRef]
- R. Auras, L.-T. Lim, S.E.M. Selke, H. Tsuji, Poly(lactic acid): synthesis, structures, properties, processing and applications, John Willey & Sons, Inc., Hoboken, New Jersey, 2010.
- D. Garlotta, A literature review of poly(lactic acid), Journal of Polymers and the Environment, 9, 2001, 63-84. [CrossRef]
- B.W. Chieng, N.A. Ibrahim, W.M.Z.W. Yunus, M.Z. Hussein, Plasticized poly(lactic acid) with low molecular weight poly(ethylene glycol): mechanical, thermal, and morphology properties, Journal of Applied Polymer Science, 130, 2013, 4576-4580. [CrossRef]
- R.T.H. Chan, H. Marçal, R.A. Russell, P.J. Holden, L.J.R. Foster, Application of polyethylene glycol to promote cellular biocompatibility of polyhydroxybutyrate films, International Journal of Polymer Science, Article ID 473045, 2011. [CrossRef]
- J. Olczyk, J. Sójka-Ledakowicz, A. Walawska, A. Antecka, K. Siwińska-Ciesielczyk, J. Zdarta, T. Jesionowski, Antimicrobial Activity and Barrier Properties against UV Radiation of Alkaline and Enzymatically Treated Linen Woven Fabrics Coated with Inorganic Hybrid Material, Molecules, 2020, 25, 5701. [CrossRef]
- M.H. Kudzin, M. Boguń, Z. Mrozińska, A. Kaczmarek, Physical Properties, Chemical Analysis, and Evaluation of Antimicrobial Response of New Polylactide/Alginate/Copper Composite Materials, Mar. Drugs, 2020, 18, 660. [CrossRef]
- L.E. Román, E.D. Gomez, J.L. Solís and M.M. Gómez, Antibacterial Cotton Fabric Functionalized with Copper Oxide Nanoparticles, Molecules, 2020, 25, 5802. [CrossRef]
- X. Hanga, H. Pengb, H. Songc, Z. Qib, X Miaoa, W. Xu, Antiviral activity of cuprous oxide nanoparticles againstHepatitis C Virus in vitro, Journal of Virological Methods, 222 (2015) 150–157.
- N. van Doremalen, T. Bushmaker, D.H. Morris, M.G. Holbrook, A. Gamble, B.N. Williamson, A. Tamin, J.L. Harcourt, N.J. Thornburg, S.I. Gerber, J.O. Lloyd-Smith, E. de Wit, Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1, N. Engl. J. Med. 2020, 382, 1564–1567.
- S. Behzadinasab, A. Chin, M. Hosseini, L. Poon, W.A. Ducker, A surface coating that rapidly inactivates SARS-CoV-2. ACS Appl. Mater. Interfaces, 2020, 12, 34723–34727.
- S. Raha, R. Mallick, S. Basak, A.K. Duttaroy, Is copper beneficial for COVID-19 patients ?, Medical Hypotheses 142 (2020) 109814. [CrossRef]

| No. | Type of nonwoven fabric sample |
Escherichia coli (ATCC 25922) |
Staphylococcus aureus (ATCC 6538) |
Candida albicans (ATCC 10231) |
| 1. | PES WIFP-270 + 1.0% CuSiO3 | 89.7 | 43.0 | 0.0 |
| 2. | PES FS G-4 + 1.0% CuSiO3 | 97.4 | 16.8 | 0.0 |
| 3. | PES FS F-5 + 1.0% CuSiO3 | 99.1 | 20.6 | 1.4 |
| 4. | PLA-350 + 1.0% CuSiO | > 99.4 | 28.0 | 0.0 |
| 5. | PES WIFP-270 + 2.0% CuSiO3 | 99.6 | 59.1 | 26.8 |
| 6. | PES WIFP-270 + 3.0% CuSiO3 | 99.8 | 66.9 | 27.8 |
| 7. | PES WIFP-270 + 5.0% CuSiO3 | > 99.8 | 87.6 | 30.6 |
| 8. | PES FS F-5 + 5.0% CuSiO3 | 99.4 | 98.7 | 35.4 |
| 9. | PLA-350 + 5.0% CuSiO3 | > 99.8 | 97.0 | 30.8 |
| 10. | PES WIFP-270 + 7.0% CuSiO3 | > 99.98 | 94.4 | 37.6 |
| 11. | PES FS F-5 + 7.0% CuSiO3 | > 99.98 | 99.5 | 49.5 |
| 12. | PLA-350 + 7.0% CuSiO3 | > 99.98 | > 99.9 | 38.6 |
| 13. | PES WIFP-270 + 6.9% CuSiO3 + 1.0% TiO2 | > 99.97 | 92.2 | 48.5 |
| 14. | PES WIFP-270 + 7.0% CuSiO3 + 2.1% TiO2 | > 99.97 | 99.7 | 58.8 |
| 15. | PES WIFP-270 + 5.0% CuSiO3 + 4.2% TiO2 | > 99.97 | 99.5 | 80.9 |
| 16. | PES WIFP-270 + 5.0% CuSiO3 + 5.0% TiO2 | > 99.99 | 99.4 | 87.5 |
| 17. | PES FS F-5 + 5.0% CuSiO3 + 5.0% TiO2 | 99.06 | 98.1 | 93.9 |
| 18. | PES FS F-5 + 5.0% CuSiO3 + 5.0% TiO2 + 2% Dekoral Silver |
95.63 |
94.6 |
92.7 |
| 19. | PES WIFP-270 + 5.0% CuSiO3 + 5.0% TiO2 + 2% Dekoral Silver |
97.08 |
92.6 |
98.0 |
| 20. | PES WIFP-270 + 5.0% CuSiO3 + 25% Ti-IN | > 99.87 | 97.7 | 89.2 |
| 21. | PES FS F-5 + 5.0% CuSiO3 + 25% Ti-IN | > 99.97 | 94.8 | 95.0 |
|
Sample No. |
Sample type |
CuSiO3 ∙xH2O |
PEG 600 |
Revacryl 247 |
Lutexal HC |
Microbiological activity against the fungus Candida albicans (ATCC 10321) |
|||
| [ wt.% ] | A | S | L | R [%] | |||||
|
1 |
PES nonwoven fabric (Hydronina) |
10.36 |
5.18 |
10.36 |
1.67 |
5.6 |
5.6 |
2.4 |
99.6 |
| 2 | PES fabric (untreated) |
10.36 | 5.18 | 10.36 | 1.67 | 5.1 | 5.0 | 1.8 | 98.7 |
| 3 | PES fabric alkalized |
10.36 |
5.18 |
10.36 |
1.67 |
4.8 |
4.8 |
1.6 |
97.9 |
|
4 |
PES fabric + Texazym (1.5 wt.%) |
10.21 |
5.11 |
10.21 |
1.72 |
5.5 |
5.4 |
2.2 |
99.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




