Submitted:
12 July 2023
Posted:
12 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Types of AVTs
| Virus | AVTs | CAS Number | Formula | Chemical Structure | Molecular Weight (MW) (g/mol) |
|---|---|---|---|---|---|
| HIV | abacavir | 136470-78-5 | C14H18N6O | 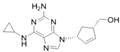 |
286.33 |
| bictegravir sodium | 1807988-02-8 | C21H17F3N3NaO5 | 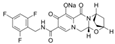 |
471.36 | |
| lamivudine | 131086-21-0 | C8H11N3O3S | 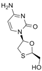 |
229.26 | |
| nevirapine | 129618-40-2 | C15H12N2O4 | 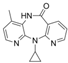 |
266.29 | |
| stavudine | 3056-17-5 | C10H12N2O4 | 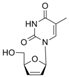 |
224.21 | |
| zidovudine | 30516-87-1 | C10H13N5O4 | 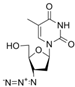 |
267.24 | |
| HSVs | acyclovir | 59277-89-3 | C8H11N5O3 | 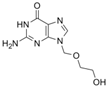 |
225.20 |
| famciclovir | 104227-87-4 | C14H19N5O4 | 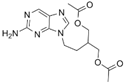 |
321.33 | |
| penciclovir | 39809-25-1 | C10H15N5O3 | 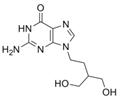 |
253.26 | |
| Influenza | amantadine | 768-94-5 | C10H17N | 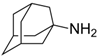 |
151.24 |
| oseltamivir | 196618-13-0 | C16H28N2O4 | 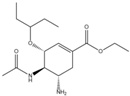 |
312.40 | |
| zanamivir | 139110-80-8 | C12H20N4O7 | 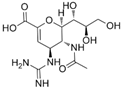 |
332.31 | |
| SARS-CoV-2 | favipiravir | 259793-96-9 | C5H4FN3O2 | 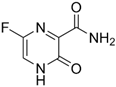 |
157.10 |
| remdesivir | 39809-25-1 | C27H35N6O8P | 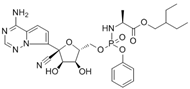 |
602.57 |
3. Occurrence of AVTs in Aqueous Environment
| AVTs | Concentration ng/L(min-max) | Country | References | |
|---|---|---|---|---|
| Influent | Effluent | |||
| acyclovir | 1780–1990 | 27–53 | Germany | [62] |
| lamivudine | 210–720 | ND | ||
| nevirapine | 4.8–21.8 | 7–32 | ||
| oseltamivir | 0–11.9 | 9–16 | ||
| zidovudine | 310–380 | 98–564 | ||
| stavudine | 11.6–22.8 | ND | ||
| acyclovir | ND | ND | [63] | |
| emtricitabine | ND | 130 | ||
| emtricitabine carboxylate |
ND | 120–1000 | ||
| abacavir | 60–140 | ND | [64] | |
| abacavir carboxylate | 180–500 | 100–280 | ||
| emtricitabine | 100–980 | 59–170 | ||
| emtricitabine carboxylate |
24–25 | 140–480 | ||
| acyclovir | 520–4980 | 0–270 | ||
| abacavir | 0–14,000 | ND | South Africa | [65] |
| zidovudine | 6900–53,000 | 87–500 | ||
| nevirapine | 670–2800 | 540–1900 | ||
| lamivudine | 840–2200 | 0–130 | ||
| efavirenz | 24,000–34,000 | 20,000–34,000 | ||
| acyclovir | 0–406 | 0–205 | China | [16] |
| ribavirin | ND | ND | ||
| zidovudine | ND | ND | ||
4. Photocatalytic Degradation of AVTs
4.1. Principle of Photocatalytic Degradation
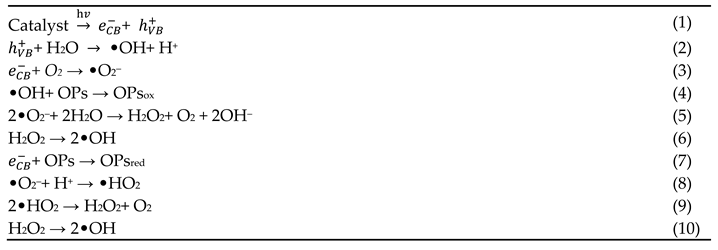
4.2. Semiconductor-Based Photocatalytic Degradation of AVTs
4.2.1. Metal Oxide Semiconductors
| AVTs | Initial Concentration (μM) |
Catalyst | Catalyst Dose (mg/L) |
UV Range (nm) |
Removal (%) | Rate Constant (min‒1) |
References |
|---|---|---|---|---|---|---|---|
| oseltamivir | 24 | P25 | 20 | 365 | 96 | 0.040 | [78] |
| acyclovir | 50 | P25 | 500 | 365 | 100 | - | [75] |
| lamivudine | 100 | P25 | 1000 | 365 | > 95 | 0.0542 | [76] |
| 1-amantadine | 100 | P25 | 1000 | 365 | 100 | 0.076 | [79] |
| 2-amantadine | 100 | P25 | 1000 | 365 | 100 | 0.084 | [79] |
| rimantadine | 100 | P25 | 1000 | 365 | 100 | 0.102 | [79] |
| zanamivir | 0.3 | AEROIXE TiO2 P25 |
17.7 | 380-420 | 100 | - | [80] |
4.2.2. Doped Metal Oxide Semiconductors
4.2.3. Heterojunction Semiconductors
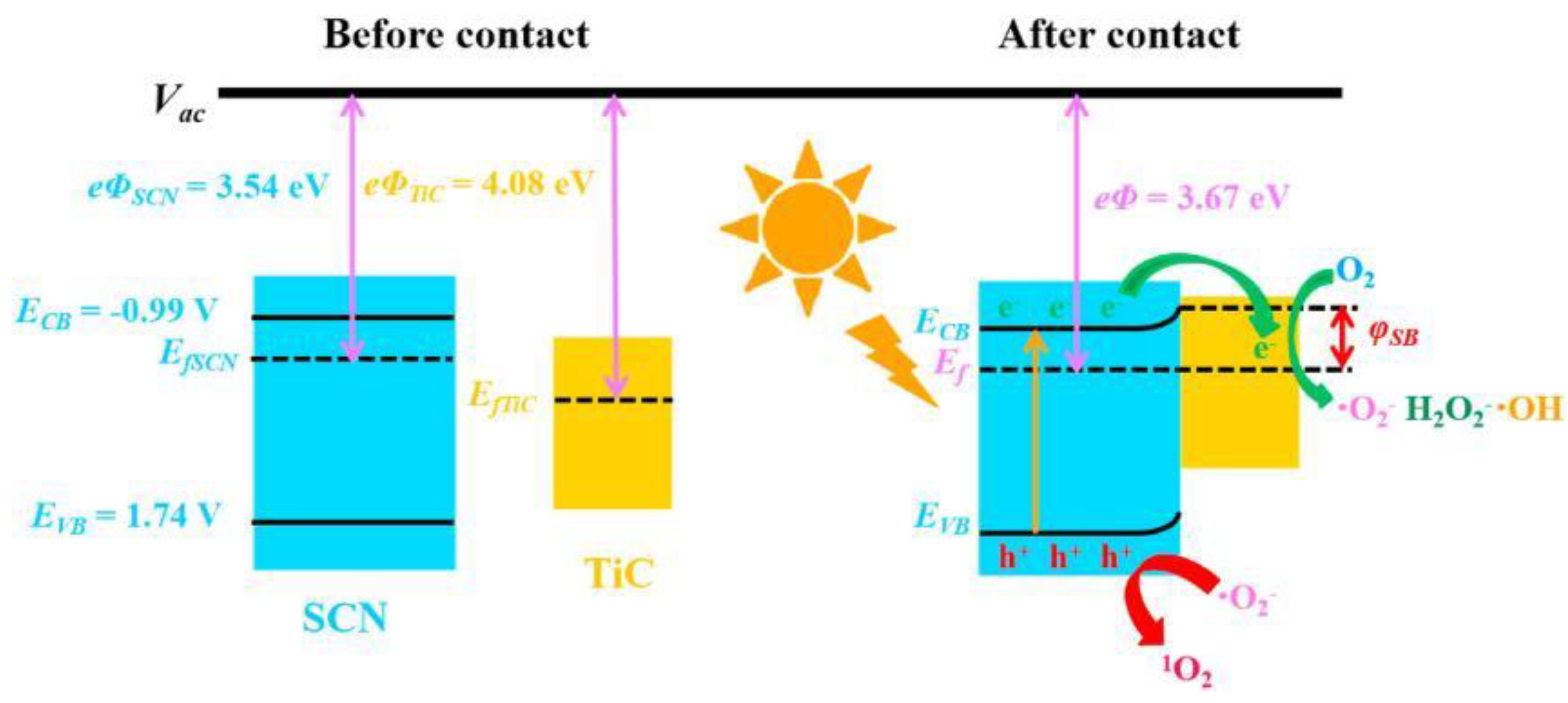
| AVTs | Initial Concentration (μM) |
Catalyst | Catalyst Dose (mg/L) |
UV Range (nm) |
Removal (%) | Rate Constant (min‒1) |
References |
|---|---|---|---|---|---|---|---|
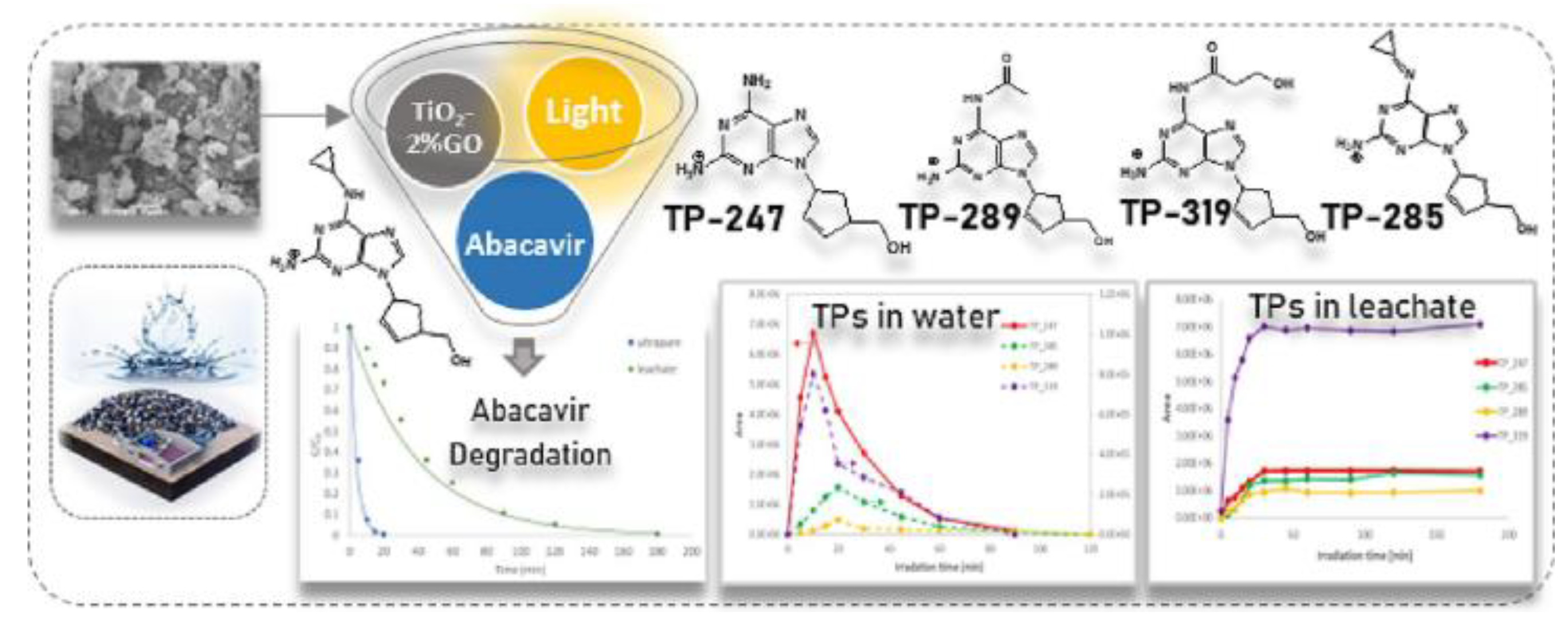
| abacavir | 10 | GO-TiO2 | 100 | solar spectrum | 99.4 | 0.2610 | [91] |
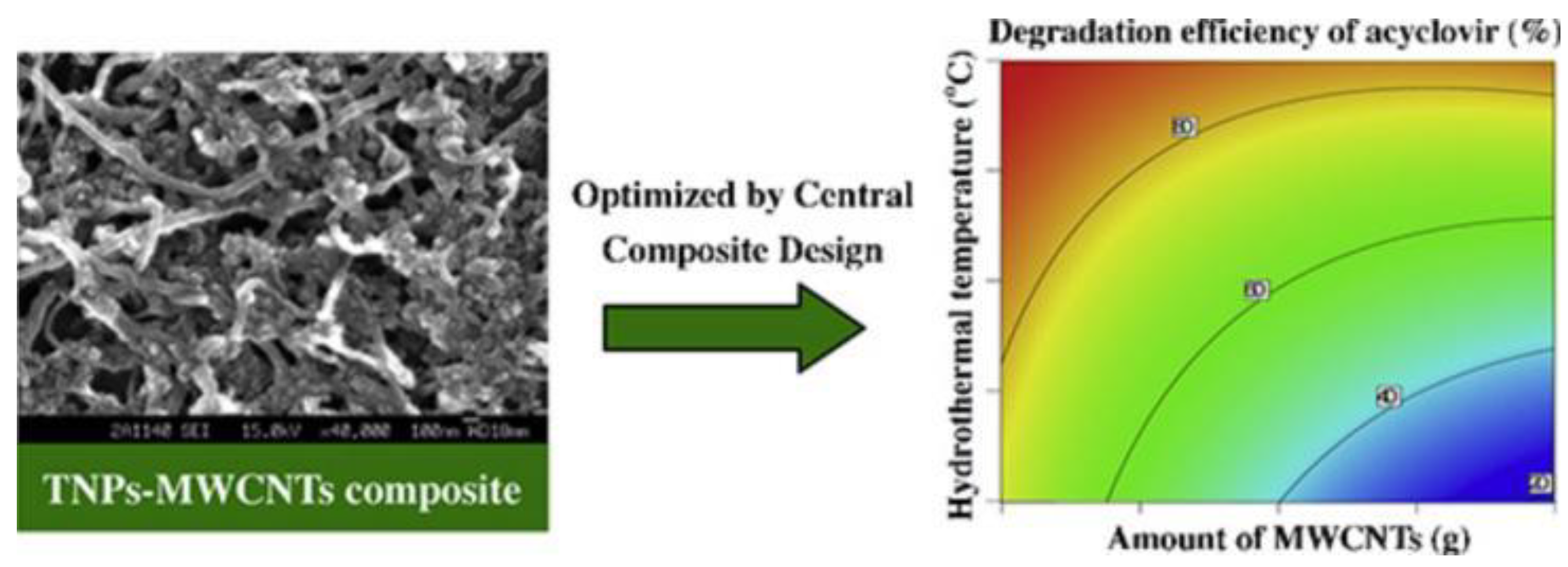
| acyclovir | 10 | TNPs-MWCNTs | 400 | 365 | 98.6 | - | [92] |
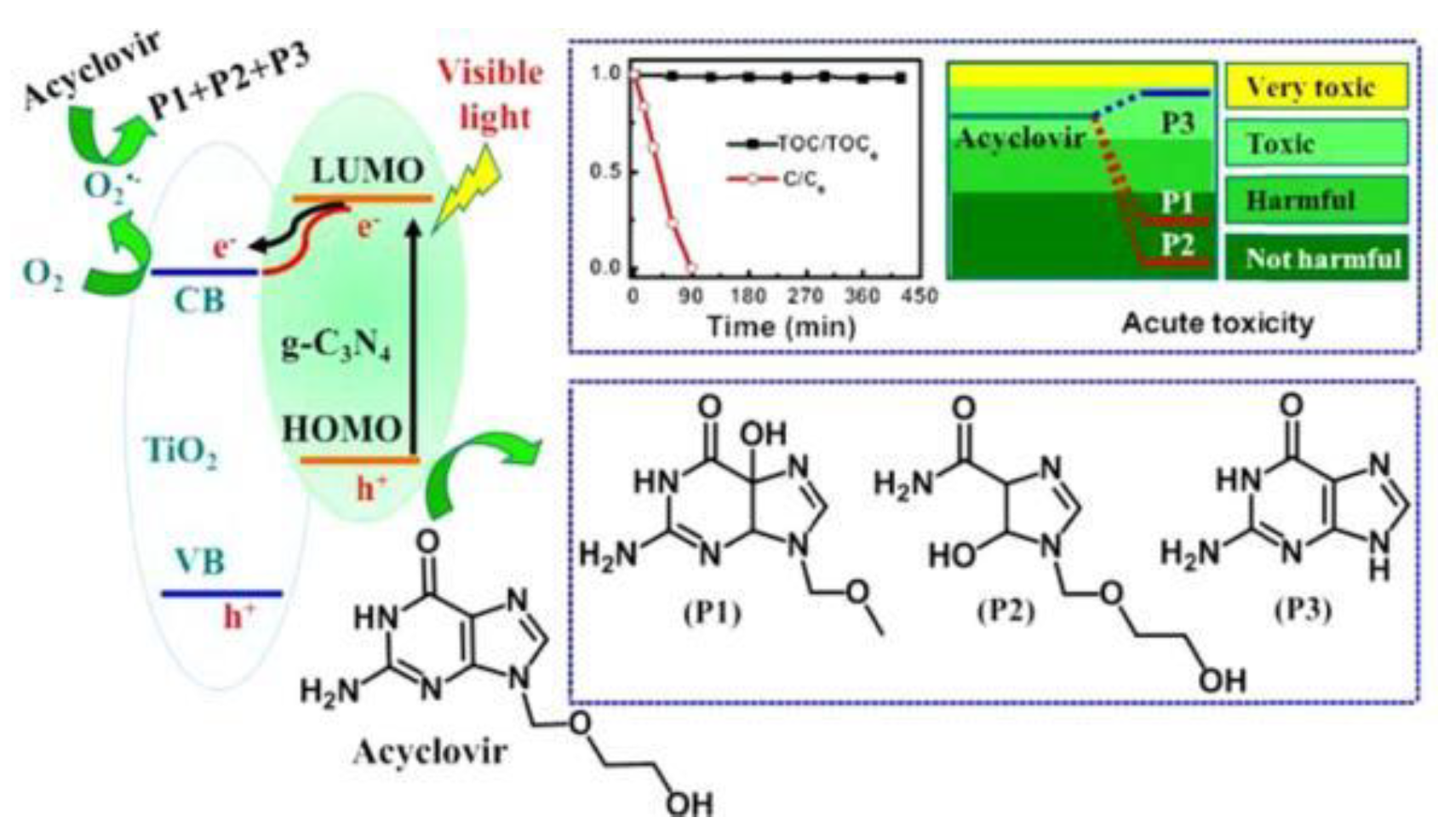
| acyclovir | 10 | g-CN/TiO2 | 300 | > 420 | 100 | 0.0076 | [76] |
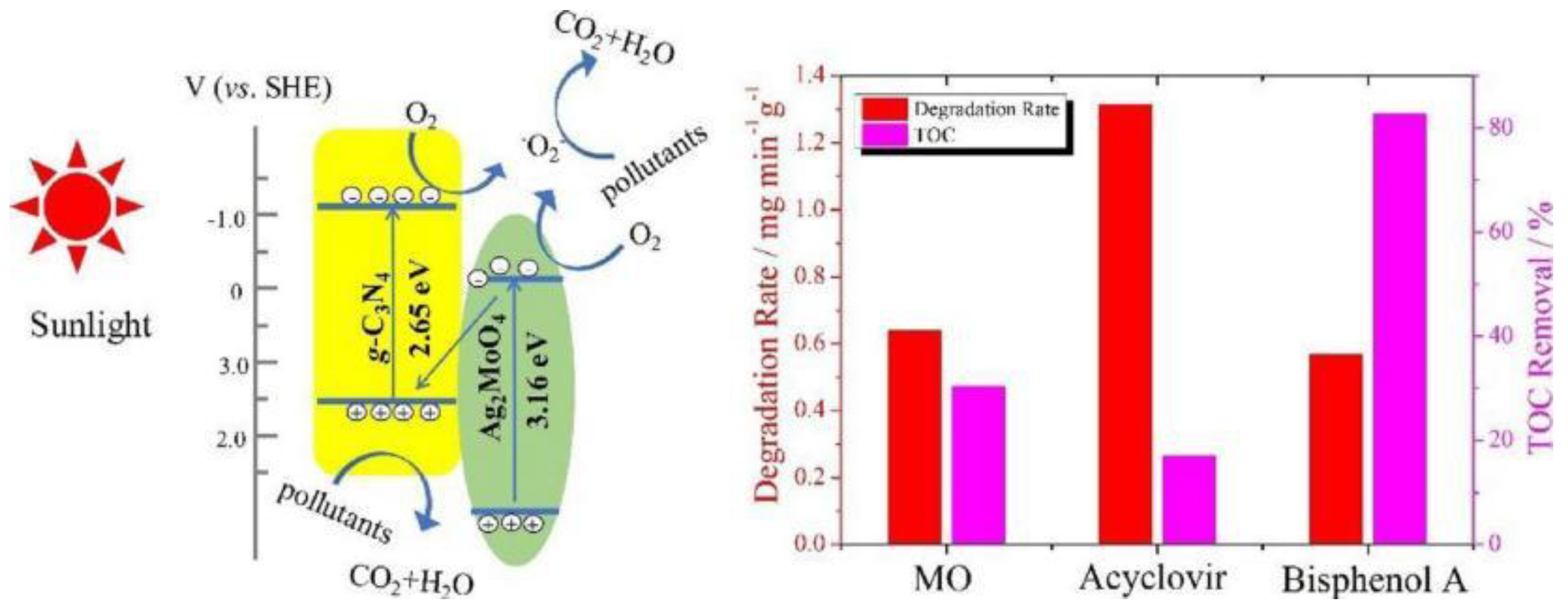
| acyclovir | 10 | Ag2MoO4/g-C3N4 | 250 | > 420 | 100 | - | [95] |
| arbidol hydrochloride | 10 | Ti3C2 MXene/g-C3N4 | 100 | > 420 | 99.2 | 0.0295 | [96] |
| zidovudine | 10 | CuSm0.06Fe1.94O4@g-C3N4 | 1200 | > 420 | 71.5 | 0.0081 | [98] |
| acyclovir | 10 | Bi4VO8Cl | 50 | 200-780 | 100 | - | [99] |
| ribavirin | 10 | Bi4VO8Cl | 50 | 200-780 | 100 | - | [99] |
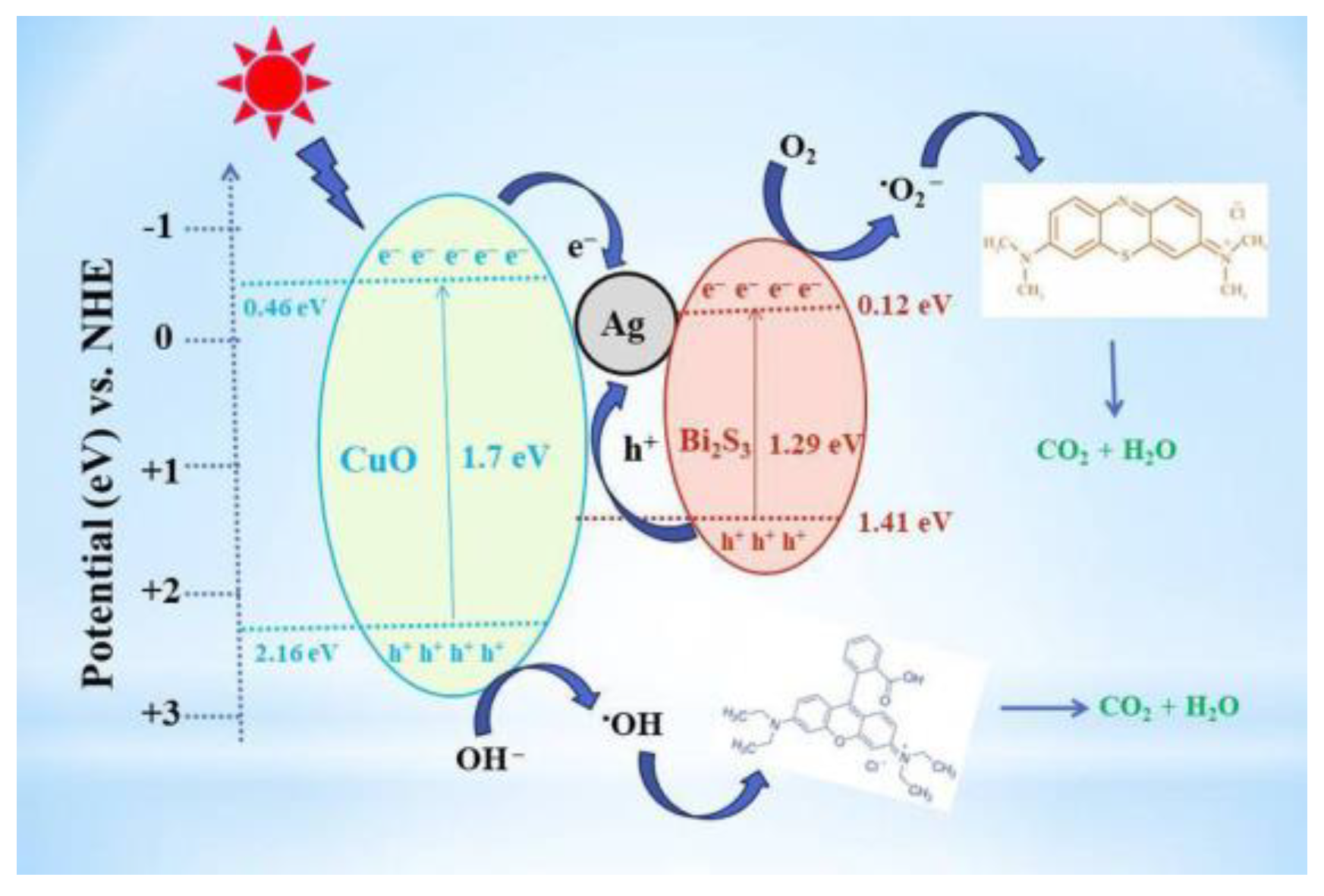
| stavudine | 10 | CuO@Ag@Bi2S3 | 20 | 365 | 92.1 | - | [100] |
| zidovudine | 10 | CuO@Ag@Bi2S3 | 20 | 365 | 87.4 | - | [100] |
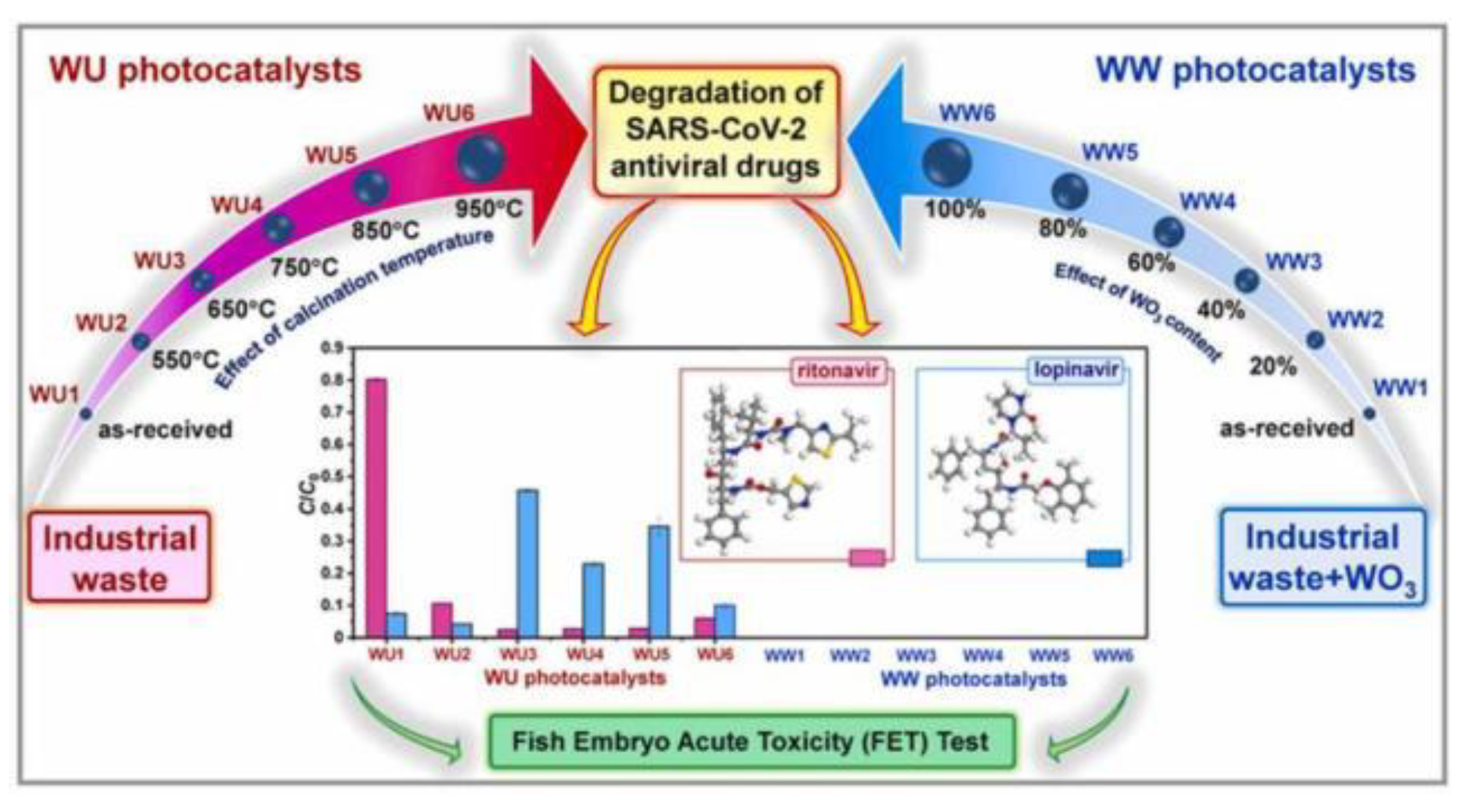
| lopinavir | 10 | ammonium molybdate (WU and WWphotocatalysts) |
400 | 500-550 | 95 | - | [102] |
| ritonavir | 10 | ammonium molybdate (WU and WWphotocatalysts) |
400 | 500-550 | 95 | - | [102] |
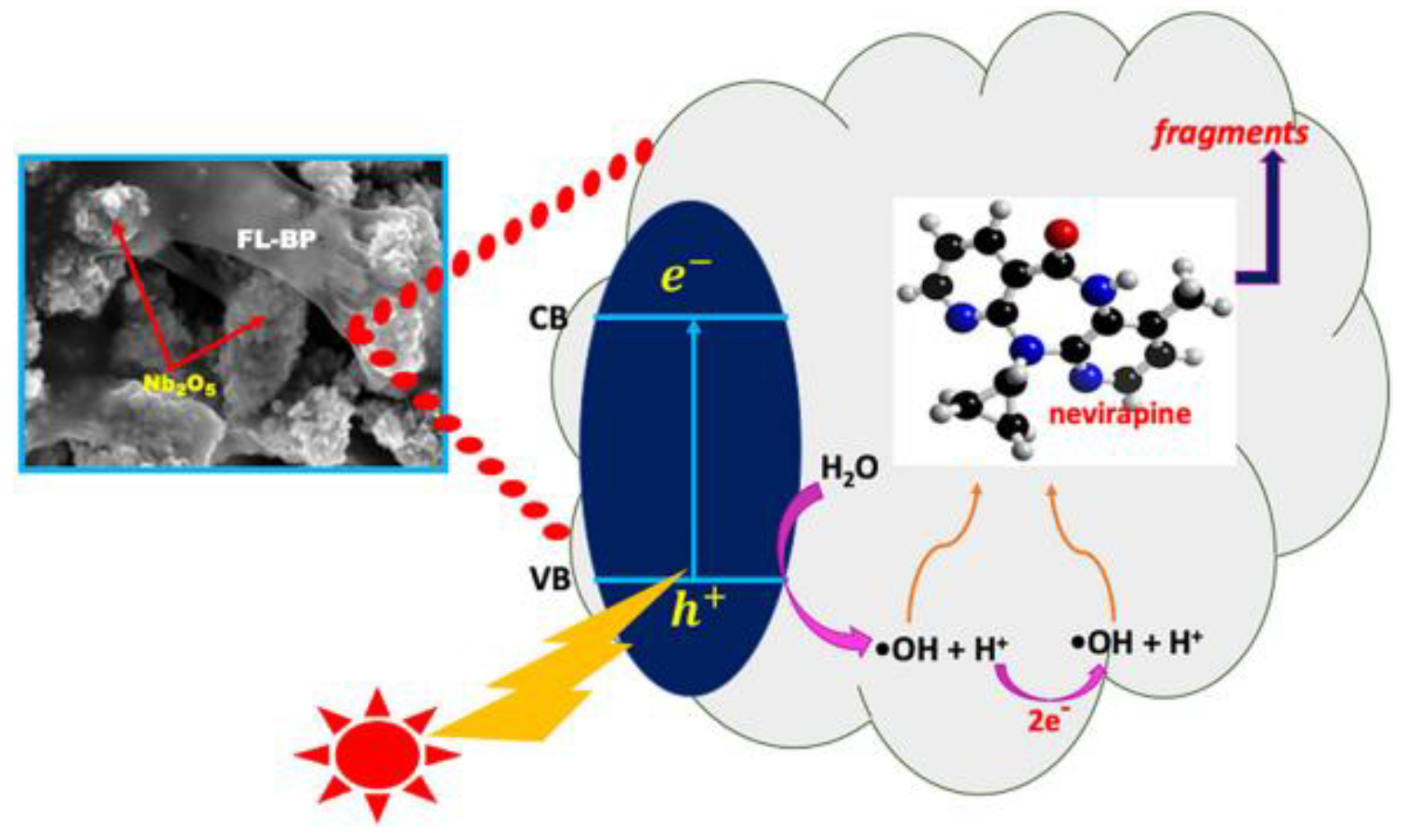
| nevirapine | 5 | FL-BP@Nb2O5 | 100 | > 420 | 68 | 0.0152 | [103] |
5. Challenges and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bjorkstrom, N.K.; Strunz, B.; Ljunggren, H.G. Natural killer cells in antiviral immunity. Nat Rev Immunol 2022, 22, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Beck, B.R.; Shin, B.; Choi, Y.; Park, S.; Kang, K. Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target interaction deep learning model. Comput Struct Biotec 2020, 18, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Vahidnia, F.; Stramer, S.L.; Kessler, D.; Shaz, B.; Leparc, G.; Krysztof, D.E.; Glynn, S.A.; Custer, B. Recent viral infection in US blood donors and health-related quality of life (HRQOL). Qual Life Res 2017, 26, 349–357. [Google Scholar] [CrossRef]
- Dhama, K.; Patel, S.K.; Pathak, M.; Yatoo, M.I.; Tiwari, R.; Malik, Y.S.; Singh, R.; Sah, R.; Rabaan, A.A.; Bonilla-Aldana, D.K.; et al. An update on SARS-CoV-2/COVID-19 with particular reference to its clinical pathology, pathogenesis, immunopathology and mitigation strategies. Travel Med Infect Di 2020, 37. [Google Scholar] [CrossRef]
- De Clercq, E. Three decades of antiviral drugs. Nat Rev Drug Discov 2007, 6, 941–941. [Google Scholar] [CrossRef]
- Spruance, S.L.; Stewart, J.C.B.; Freeman, D.J.; Brightman, V.J.; Cox, J.L.; Wenerstrom, G.; Mckeough, M.B.; Rowe, N.H. Early Application of Topical 15-Percent Idoxuridine in Dimethyl-Sulfoxide Shortens the Course of Herpes-Simplex Labialis - a Multicenter Placebo-Controlled Trial. J Infect Dis 1990, 161, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Tahir, I.M.; Shah, S.M.A.; Mahmood, Z.; Altaf, A.; Ahmad, K.; Munir, N.; Daniyal, M.; Nasir, S.; Mehboob, H. Antiviral potential of medicinal plants against HIV, HSV, influenza, hepatitis, and coxsackievirus: A systematic review. Phytother Res 2018, 32, 811–822. [Google Scholar] [CrossRef]
- Bian, L.L.; Wang, Y.P.; Yao, X.; Mao, Q.Y.; Xu, M.; Liang, Z.L. Coxsackievirus A6: a new emerging pathogen causing hand, foot and mouth disease outbreaks worldwide. Expert Rev Anti-Infe 2015, 13, 1061–1071. [Google Scholar] [CrossRef]
- Kausar, S.; Khan, F.S.; Rehman, M.I.M.U.; Akram, M.; Riaz, M.; Rasool, G.; Khan, A.H.; Saleem, I.; Shamim, S.; Malik, A. A review: Mechanism of action of antiviral drugs. Int J Immunopath Ph 2021, 35. [Google Scholar] [CrossRef]
- Bilal, M.; Adeel, M.; Rasheed, T.; Zhao, Y.P.; Iqbal, H.M.N. Emerging contaminants of high concern and their enzyme-assisted biodegradation - A review. Environ Int 2019, 124, 336–353. [Google Scholar] [CrossRef]
- Nannou, C.; Ofrydopoulou, A.; Evgenidou, E.; Heath, D.; Heath, E.; Lambropoulou, D. Analytical strategies for the determination of antiviral drugs in the aquatic environment. Trends Environ Anal 2019, 24. [Google Scholar] [CrossRef]
- Tarpani, R.R.Z.; Azapagic, A. A methodology for estimating concentrations of pharmaceuticals and personal care products (PPCPs) in wastewater treatment plants and in freshwaters. Sci Total Environ 2018, 622, 1417–1430. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.P.; Yu, X.P.; Yu, F.R.; Huang, X. Occurrence, sources and fate of pharmaceuticals and personal care products and artificial sweeteners in groundwater. Environ Sci Pollut R 2021, 28, 20903–20920. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Jin, X.W.; Feng, C.L.; Wang, Z.J.; Wu, F.C.; Johnson, A.C.; Xiao, H.X.; Hollert, H.; Giesy, J.P. Ecological risk assessment of fifty pharmaceuticals and personal care products (PPCPs) in Chinese surface waters: A proposed multiple-level system. Environ Int 2020, 136. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, J.; Klaper, R. Environmental concentrations of the selective serotonin reuptake inhibitor fluoxetine impact specific behaviors involved in reproduction, feeding and predator avoidance in the fish Pimephales promelas (fathead minnow). Aquat Toxicol 2014, 151, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.Z.; Wang, C.W.; Zhang, K.; Wang, Z.F.; Huang, Q.X.; Yu, Y.Y.; Ou, W.H. Profile and behavior of antiviral drugs in aquatic environments of the Pearl River Delta, China. Sci Total Environ 2014, 466, 755–761. [Google Scholar] [CrossRef]
- Kosma, C.I.; Nannou, C.I.; Boti, V.I.; Albanis, T.A. Psychiatrics and selected metabolites in hospital and urban wastewaters: Occurrence, removal, mass loading, seasonal influence and risk assessment. Sci Total Environ 2019, 659, 1473–1483. [Google Scholar] [CrossRef]
- Ferrando-Climent, L.; Reid, M.J.; Rodriguez-Mozaz, S.; Barcelo, D.; Thomas, K.V. Identification of markers of cancer in urban sewage through the use of a suspect screening approach. J Pharmaceut Biomed 2016, 129, 571–580. [Google Scholar] [CrossRef]
- Morales-Paredes, C.A.; Rodriguez-Diaz, J.M.; Boluda-Botella, N. Pharmaceutical compounds used in the COVID-19 pandemic: A review of their presence in water and treatment techniques for their elimination. Sci Total Environ 2022, 814. [Google Scholar] [CrossRef]
- Midassi, S.; Bedoui, A.; Bensalah, N. Efficient degradation of chloroquine drug by electro-Fenton oxidation: Effects of operating conditions and degradation mechanism. Chemosphere 2020, 260. [Google Scholar] [CrossRef]
- Rath, S.; Pereira, L.A.; Dal Bosco, S.M.; Maniero, M.G.; Fostier, A.H.; Guimaraes, J.R. Fate of ivermectin in the terrestrial and aquatic environment: mobility, degradation, and toxicity towards Daphnia similis. Environ Sci Pollut R 2016, 23, 5654–5666. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Liu, H.; Li, B.Q.; Zhang, B.J.; Wu, Y.X.; Hu, H.P.; Yu, D.Y.; Huang, S.B. Efficient photocatalytic ozonation of azithromycin by three-dimensional g-C3N4 nanosheet loaded magnetic Fe-MCM-48 under simulated solar light. Appl Catal B-Environ 2023, 324. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem Rev 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Hu, X.L.; Li, G.S.; Yu, J.C. Design, Fabrication, and Modification of Nanostructured Semiconductor Materials for Environmental and Energy Applications. Langmuir 2010, 26, 3031–3039. [Google Scholar] [CrossRef]
- Din, M.I.; Khalid, R.; Hussain, Z. Recent Research on Development and Modification of Nontoxic Semiconductor for Environmental Application. Sep Purif Rev 2021, 50, 244–261. [Google Scholar] [CrossRef]
- Nannou, C.; Ofrydopoulou, A.; Evgenidou, E.; Heath, D.; Heath, E.; Lambropoulou, D. Antiviral drugs in aquatic environment and wastewater treatment plants: A review on occurrence, fate, removal and ecotoxicity. Sci Total Environ 2020, 699. [Google Scholar] [CrossRef]
- Arruda, V.R.; Rossi, C.L.; Nogueira, E.; AnnicchinoBizzacchi, J.M.; Costa, F.F.; Costa, S.C.B. Cytomegalovirus infection as cause of severe thrombocytopenia in a nonimmunosuppressed patient. Acta Haematol-Basel 1997, 98, 228–230. [Google Scholar] [CrossRef]
- Boppana, S.B.; Pass, R.F.; Britt, W.J.; Stagno, S.; Alford, C.A. Symptomatic Congenital Cytomegalovirus-Infection - Neonatal Morbidity and Mortality. Pediatr Infect Dis J 1992, 11, 93–99. [Google Scholar] [CrossRef]
- Choi, Y.; Choi, B.Y.; Kim, S.I.; Choi, J.; Kim, J.; Park, B.Y.; Kim, S.M.; Kim, S.W.; Choi, J.Y.; Song, J.Y.; et al. Effect of characteristics on the clinical course at the initiation of treatment for human immunodeficiency virus infection using dimensionality reduction. Sci Rep-Uk 2023, 13. [Google Scholar] [CrossRef]
- De Clercq, E.; Li, G.D. Approved Antiviral Drugs over the Past 50 Years. Clin Microbiol Rev 2016, 29, 695–747. [Google Scholar] [CrossRef]
- Tompa, D.R.; Immanuel, A.; Srikanth, S.; Kadhirvel, S. Trends and strategies to combat viral infections: A review on FDA approved antiviral drugs. Int J Biol Macromol 2021, 172, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.Z. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci Trends 2020, 14, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Zhan, P.; Pannecouque, C.; De Clercq, E.; Liu, X.Y. Anti-HIV Drug Discovery and Development: Current Innovations and Future Trends. J Med Chem 2016, 59, 2849–2878. [Google Scholar] [CrossRef] [PubMed]
- Saag, M.S.; Gandhi, R.T.; Hoy, J.F.; Landovitz, R.J.; Thompson, M.A.; Sax, P.E.; Smith, D.M.; Benson, C.A.; Buchbinder, S.P.; del Rio, C.; et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults 2020 Recommendations of the International Antiviral Society-USA Panel. Jama-J Am Med Assoc 2020, 324, 1651–1669. [Google Scholar] [CrossRef]
- Friedman, W.H. Antiretroviral drug access and behavior change. J Dev Econ 2018, 135, 392–411. [Google Scholar] [CrossRef]
- Ncube, S.; Madikizela, L.M.; Chimuka, L.; Nindi, M.M. Environmental fate and ecotoxicological effects of antiretrovirals: A current global status and future perspectives. Water Res 2018, 145, 231–247. [Google Scholar] [CrossRef]
- Russo, D.; Siciliano, A.; Guida, M.; Andreozzi, R.; Reis, N.M.; Li Puma, G.; Marotta, R. Removal of antiretroviral drugs stavudine and zidovudine in water under UV254 and UV254/H2O2 processes: Quantum yields, kinetics and ecotoxicology assessment. J Hazard Mater 2018, 349, 195–204. [Google Scholar] [CrossRef]
- Allahverdiyev, A.; Bağırova, M.; Yaman, S.; Koc, R.C.; Abamor, E.Ş.; Ateş, S.C.; Baydar, S.Y.; Elçiçek, S.; Oztel, O.N. Development of New Antiherpetic Drugs Based on Plant Compounds. 2013.
- Faccin-Galhardi, L.C.; Ray, S.; Lopes, N.; Ali, I.; Espada, S.F.; dos Santos, J.P.; Ray, B.; Linhares, R.E.C.; Nozawa, C. Assessment of antiherpetic activity of nonsulfated and sulfated polysaccharides from Azadirachta indica. Int J Biol Macromol 2019, 137, 54–61. [Google Scholar] [CrossRef]
- Novakova, L.; Pavlik, J.; Chrenkova, L.; Martinec, O.; Cerveny, L. Current antiviral drugs and their analysis in biological materials - Part II: Antivirals against hepatitis and HIV viruses. J Pharmaceut Biomed 2018, 147, 378–399. [Google Scholar] [CrossRef]
- Greeley, Z.W.; Giannasca, N.J.; Porter, M.J.; Margulies, B.J. Acyclovir, cidofovir, and amenamevir have additive antiviral effects on herpes simplex virus TYPE 1. Antivir Res 2020, 176. [Google Scholar] [CrossRef]
- O'Brien, J.J.; Campoli-Richards, D.M. Acyclovir. An updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs, 3.
- Celebioglu, A.; Uyar, T. Electrospun formulation of acyclovir/cyclodextrin nanofibers for fast-dissolving antiviral drug delivery. Mat Sci Eng C-Mater 2021, 118. [Google Scholar] [CrossRef]
- Saifi, Z.; Rizwanullah, M.; Mir, S.R.; Amin, S. Bilosomes nanocarriers for improved oral bioavailability of acyclovir: A complete characterization through in vitro, ex-vivo and in vivo assessment. J Drug Deliv Sci Tec 2020, 57. [Google Scholar] [CrossRef]
- Litster, A.L.; Lohr, B.R.; Bukowy, R.A.; Thomasy, S.M.; Maggs, D.J. Clinical and antiviral effect of a single oral dose of famciclovir administered to cats at intake to a shelter. Vet J 2015, 203, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Rezk, M.S.; El Nashar, R.M. Dissolution testing and potentiometric determination of famciclovir in pure, dosage forms and biological fluids. Bioelectrochemistry 2013, 89, 26–33. [Google Scholar] [CrossRef] [PubMed]
- FathimaRizwana, B.; Prasana, J.C.; Muthu, S.; Abraham, C.S. Wavefunction analysis, charge transfer and molecular docking studies on famciclovir and entecavir: Potential anti-viral drugs. Chemical Data Collections 2020, 26, 100353. [Google Scholar]
- Suttapanit, K.; Boriboon, J.; Sanguanwit, P. Risk factors for non-invasive ventilation failure in influenza infection with acute respiratory failure in emergency department. Am J Emerg Med 2021, 45, 368–373. [Google Scholar] [CrossRef]
- Chan, Y.H.; Ng, S.W.; Mehta, M.; Anand, K.; Singh, S.K.; Gupta, G.; Chellappan, D.K.; Dua, K. Advanced drug delivery systems can assist in managing influenza virus infection: A hypothesis. Med Hypotheses 2020, 144. [Google Scholar] [CrossRef]
- Hsu, P.H.; Chiu, D.C.; Wu, K.L.; Lee, P.S.; Jan, J.T.; Cheng, Y.S.E.; Tsai, K.C.; Cheng, T.J.; Fang, J.M. Acylguanidine derivatives of zanamivir and oseltamivir: Potential orally available prodrugs against influenza viruses. Eur J Med Chem 2018, 154, 314–323. [Google Scholar] [CrossRef]
- Chughtai, A.A.; Tan, T.C.; Hitchen, E.M.; Kunasekaran, M.P.; Macintyre, C.R. Association of influenza infection and vaccination with cardiac biomarkers and left ventricular ejection fraction in patients with acute myocardial infarction. International Journal of Cardiology. Heart & Vasculature 2020, 31. [Google Scholar]
- Hu, Y.M.; Musharrafieh, R.; Ma, C.L.; Zhang, J.T.; Smee, D.F.; DeGrado, W.F.; Wang, J. An M2-V27A channel blocker demonstrates potent in vitro and in vivo antiviral activities against amantadine-sensitive and-resistant influenza A viruses. Antivir Res 2017, 140, 45–54. [Google Scholar] [CrossRef]
- Kode, S.S.; Pawar, S.D.; Tare, D.S.; Keng, S.S.; Mullick, J. Amantadine resistance markers among low pathogenic avian influenza H9N2 viruses isolated from poultry in India, during 2009-2017. Microb Pathogenesis 2019, 137. [Google Scholar] [CrossRef] [PubMed]
- Naveja, J.J.; Madariaga-Mazon, A.; Flores-Murrieta, F.; Granados-Montiel, J.; Maradiaga-Cecena, M.; Alaniz, V.D.; Maldonado-Rodriguez, M.; Garcia-Morales, J.; Senosiain-Pelaez, J.P.; Martinez-Mayorga, K. Union is strength: antiviral and anti-inflammatory drugs for COVID-19. Drug Discov Today 2020, 26, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Shin, J.S.; Park, S.J.; Jung, E.; Park, Y.G.; Lee, J.; Kim, S.J.; Park, H.J.; Lee, J.H.; Park, S.M.; et al. Antiviral activity and safety of remdesivir against SARS-CoV-2 infection in human pluripotent stem cell-derived cardiomyocytes. Antivir Res 2020, 184. [Google Scholar] [CrossRef] [PubMed]
- Frediansyah, A.; Tiwari, R.; Sharun, K.; Dhama, K.; Harapan, H. Antivirals for COVID-19: A critical review. Clinical Epidemiology and Global Health 2020, 9, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Acquavia, M.A.; Foti, L.; Pascale, R.; Nicolò, A.; Brancaleone, V.; Cataldi, T.R.I.; Martelli, G.; Scrano, L.; Bianco, G. Detection and quantification of Covid-19 antiviral drugs in biological fluids and tissues. Talanta 2020, 224, 121862–121862. [Google Scholar] [CrossRef]
- Madelain, V.; Duthey, A.; Mentré, F.; Jacquot, F.; Solas, C.; Lacarelle, B.; Vallvé, A.; Barron, S.; Barrot, L.; Munweiler, S.; et al. Ribavirin does not potentiate favipiravir antiviral activity against Ebola virus in non-human primates. Antivir Res 2020, 104758. [Google Scholar] [CrossRef]
- Agrawal, U.; Raju, R.; Udwadia, Z.F. Favipiravir: A new and emerging antiviral option in COVID-19. Medical Journal, Armed Forces India 2020, 76, 370–376. [Google Scholar] [CrossRef]
- Reddy Vegivinti, C.T.; Pederson, J.M.; Saravu, K.; Gupta, N.; Barrett, A.; Davis, A.R.; Kallmes, K.M.; Evanson, K.W. Remdesivir therapy in patients with COVID-19: A systematic review and meta-analysis of randomized controlled trials. Annals of Medicine and Surgery 2021, 62, 43–48. [Google Scholar] [CrossRef]
- Jain, S.; Kumar, P.; Vyas, R.K.; Pandit, P.; Dalai, A.K. Occurrence and Removal of Antiviral Drugs in Environment: A Review. Water Air Soil Poll 2013, 224. [Google Scholar] [CrossRef]
- Prasse, C.; Schlusener, M.P.; Schulz, R.; Ternes, T.A. Antiviral Drugs in Wastewater and Surface Waters: A New Pharmaceutical Class of Environmental Relevance? Environ Sci Technol 2010, 44, 1728–1735. [Google Scholar] [CrossRef]
- Boulard, L.; Dierkes, G.; Ternes, T. Utilization of large volume zwitterionic hydrophilic interaction liquid chromatography for the analysis of polar pharmaceuticals in aqueous environmental samples: Benefits and limitations. J Chromatogr A 2018, 1535, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Funke, J.; Prasse, C.; Ternes, T.A. Identification of transformation products of antiviral drugs formed during biological wastewater treatment and their occurrence in the urban water cycle. Water Res 2016, 98, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Abafe, O.A.; Spath, J.; Fick, J.; Jansson, S.; Buckley, C.; Stark, A.; Pietruschka, B.; Martincigh, B.S. LC-MS/MS determination of antiretroviral drugs in influents and effluents from wastewater treatment plants in KwaZulu-Natal, South Africa. Chemosphere 2018, 200, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Mosekiemang, T.T.; Stander, M.A.; de Villiers, A. Simultaneous quantification of commonly prescribed antiretroviral drugs and their selected metabolites in aqueous environmental samples by direct injection and solid phase extraction liquid chromatography - tandem mass spectrometry. Chemosphere 2019, 220, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Schoeman, C.M.; Mashiane, M.J.; Dlamini, M.; Okonkwo, O.J. Quantification of Selected Antiretroviral Drugs in a Wastewater Treatment Works in South Africa Using GC-TOFMS. Journal of Chromatography & Separation Techniques 2015, 6, 1–7. [Google Scholar]
- Thi, L.-A.P.; Panchangam, S.C.; Do, H.-T.; Nguyen, V.H. Prospects and challenges of photocatalysis for degradation and mineralization of antiviral drugs. Nanostructured Photocatalysts, 2021; 489–517. [Google Scholar]
- Wang, H.J.; Li, X.; Zhao, X.X.; Li, C.Y.; Song, X.H.; Zhang, P.; Huo, P.W.; Li, X. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chinese J Catal 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Mills, A.; LeHunte, S. An overview of semiconductor photocatalysis. J Photoch Photobio A 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Benjamin, S.; Vaya, D.; Punjabi, P.B.; Ameta, S.C. Enhancing photocatalytic activity of zinc oxide by coating with some natural pigments. Arab J Chem 2011, 4, 205–209. [Google Scholar] [CrossRef]
- Firozjaee, T.T.; Mehrdadi, N.; Baghdadi, M.; Bidhendi, G.N. Application of Nanotechnology in Pesticides Removal from Aqueous Solutions - A review. International Journal of NanoScience and Nanotechnology 2018, 14, 43–56. [Google Scholar]
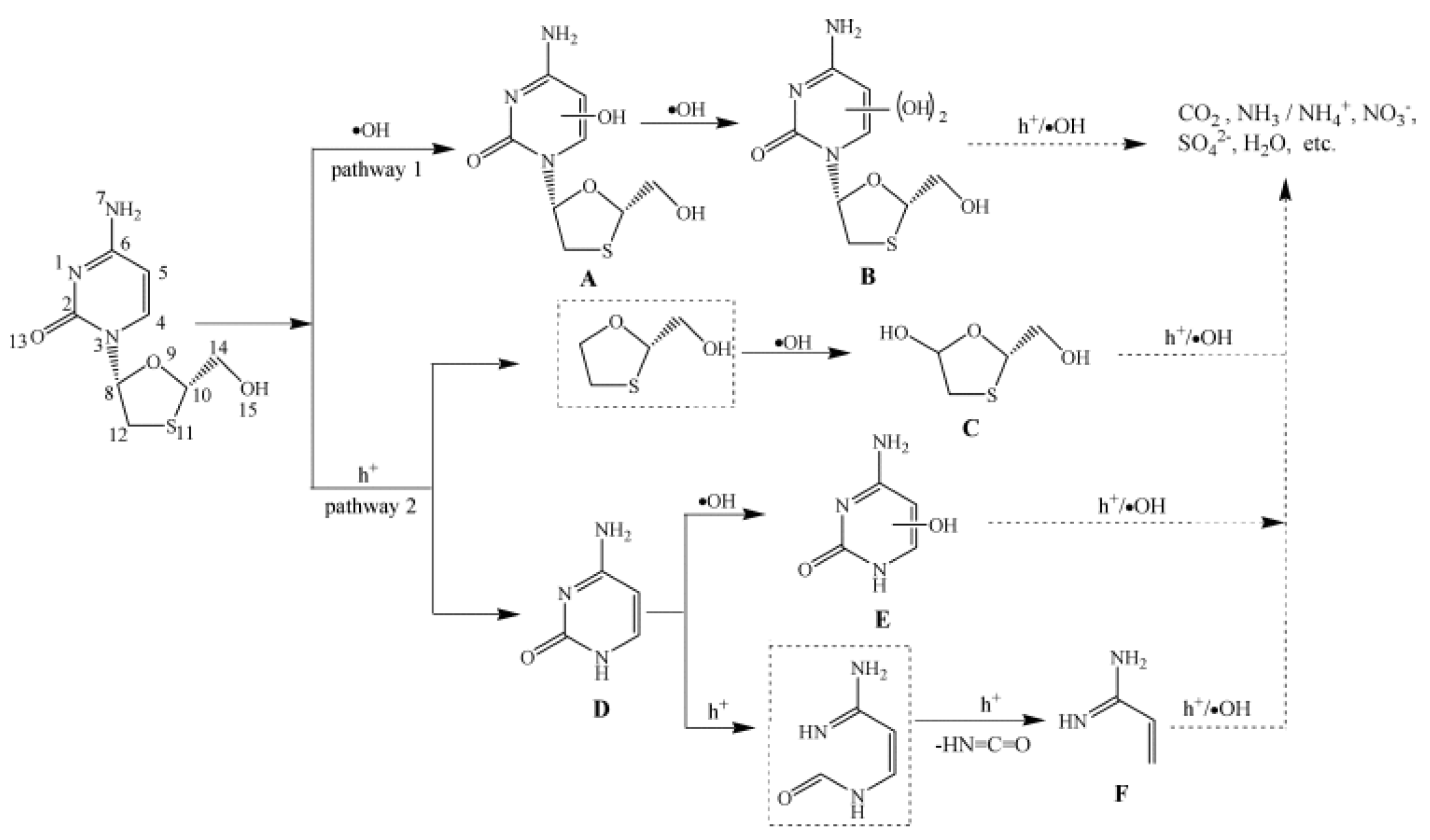
- An, T.; An, J.; Gao, Y.; Li, G.; Fang, H.; Song, W. Photocatalytic degradation and mineralization mechanism and toxicity assessment of antivirus drug acyclovir: Experimental and theoretical studies. Appl Catal B-Environ 2015, 164, 279–287. [Google Scholar] [CrossRef]
- An, T.C.; An, J.B.; Yang, H.; Li, G.Y.; Feng, H.X.; Nie, X.P. Photocatalytic degradation kinetics and mechanism of antivirus drug-lamivudine in TiO2 dispersion. J Hazard Mater 2011, 197, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Y.; Nie, X.; Gao, Y.P.; An, T.C. Can environmental pharmaceuticals be photocatalytically degraded and completely mineralized in water using g-C3N4/TiO2 under visible light irradiation?-Implications of persistent toxic intermediates. Appl Catal B-Environ 2016, 180, 726–732. [Google Scholar] [CrossRef]
- Wang, W.L.; Wu, Q.Y.; Wang, Z.M.; Hu, H.Y.; Negishi, N.; Torimura, M. Photocatalytic degradation of the antiviral drug Tamiflu by UV-A/TiO2: Kinetics and mechanisms. Chemosphere 2015, 131, 41–47. [Google Scholar] [CrossRef] [PubMed]
- An, J.B.; Li, G.Y.; An, T.C.; Song, W.H.; Feng, H.X.; Lu, Y.J. Photocatalytic degradation of three amantadine antiviral drugs as well as their eco-toxicity evolution. Catal Today 2015, 258, 602–609. [Google Scholar] [CrossRef]
- Woche, M.; Scheibe, N.; von Tumpling, W.; Schwidder, M. Degradation of the antiviral drug zanamivir in wastewater - The potential of a photocatalytic treatment process. Chem Eng J 2016, 287, 674–679. [Google Scholar] [CrossRef]
- Trawinski, J.; Wronski, M.; Skibinski, R. Efficient removal of anti-HIV drug- maraviroc from natural water by peroxymonosulfate and TiO2 photocatalytic oxidation: Kinetic studies and identification of transformation products. J Environ Manage 2022, 319. [Google Scholar] [CrossRef]
- Silveyra, R.; Saenz, L.D.T.; Flores, W.A.; Martinez, V.C.; Elguezabal, A.A. Doping of TiO2 with nitrogen to modify the interval of photocatalytic activation towards visible radiation. Catal Today 2005, 107-08, 602–605. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on Modified TiO2 Photocatalysis under UV/Visible Light: Selected Results and Related Mechanisms on Interfacial Charge Carrier Transfer Dynamics. J Phys Chem A 2011, 115, 13211–13241. [Google Scholar] [CrossRef]
- Pazoki, M.; Parsa, M.; Farhadpour, R. Removal of the hormones dexamethasone (DXM) by Ag doped on TiO2 photocatalysis. Journal of environmental chemical engineering 2016, 4, 4426–4434. [Google Scholar] [CrossRef]
- Li, Y.X.; Li, D.; Chen, Z.L. Study on preparation of Ag, Cu doped TiO2 and photocatalytic degration of acyclovir. New chem mat 2018, 46, 143–150. [Google Scholar]
- Wang, H.L.; Zhang, L.S.; Chen, Z.G.; Hu, J.Q.; Li, S.J.; Wang, Z.H.; Liu, J.S.; Wang, X.C. Semiconductor heterojunction photocatalysts: design, construction, and photocatalytic performances. Chem Soc Rev 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Yang, H. A short review on heterojunction photocatalysts: Carrier transfer behavior and photocatalytic mechanisms. Mater Res Bull 2021, 142. [Google Scholar] [CrossRef]
- Wang, Z.P.; Lin, Z.P.; Shen, S.J.; Zhong, W.W.; Cao, S.W. Advances in designing heterojunction photocatalytic materials. Chinese J Catal 2021, 42, 710–730. [Google Scholar] [CrossRef]
- Tan, L.L.; Ong, W.J.; Chai, S.P.; Mohamed, A.R. Reduced graphene oxide-TiO2 nanocomposite as a promising visible-light-active photocatalyst for the conversion of carbon dioxide. Nanoscale Res Lett 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.B.; Zhang, H.M.; Liu, P.R.; Wang, D.; Li, Y.; Zhao, H.J. Cross-Linked g-C3N4/rGO Nanocomposites with Tunable Band Structure and Enhanced Visible Light Photocatalytic Activity. Small 2013, 9, 3336–3344. [Google Scholar] [CrossRef]
- Zhang, Z.C.; He, D.Y.; Liu, H.Y.; Ren, M.; Zhang, Y.N.; Qu, J.; Lu, N.; Guan, J.N.; Yuan, X. Synthesis of graphene/black phosphorus hybrid with highly stable P-C bond towards the enhancement of photocatalytic activity. Environ Pollut 2019, 245, 950–956. [Google Scholar] [CrossRef]
- Evgenidou, Ε.; Vasilopoulou, K.; Ioannidou, E.; Koronaiou, L.-A.; Nannou, C.; Trikkaliotis, D.G.; Bikiaris, D.; Kyzas, G.Z.; Lambropoulou, D.A. Photocatalytic Degradation of the Antiviral Drug Abacavir Using Titania-Graphene Oxide Nanocomposites in Landfill Leachate. Journal of Photochemistry and Photobiology A: Chemistry, 2023. [Google Scholar]
- Chen, J.; Luo, H.; Shi, H.; Li, G.; An, T. Anatase TiO2 nanoparticles-carbon nanotubes composite: Optimization synthesis and the relationship of photocatalytic degradation activity of acyclovir in water. Applied Catalysis A-general 2014, 485, 188–195. [Google Scholar] [CrossRef]
- Guo, R.T.; Wang, J.; Bi, Z.X.; Chen, X.; Hu, X.; Pan, W.G. Recent advances and perspectives of g-C3N4-based materials for photocatalytic dyes degradation. Chemosphere 2022, 295. [Google Scholar] [CrossRef]
- Mamba, G.; Mishra, A.K. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl Catal B-Environ 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Wu, M.; Lv, H.Y.; Wang, T.; Ao, Z.M.; Sun, H.Q.; Wang, C.Y.; An, T.C.; Wang, S.B. Ag2MoO4 nanoparticles encapsulated in g-C3N4 for sunlight photodegradation of pollutants. Catal Today 2018, 315, 205–212. [Google Scholar] [CrossRef]
- Jin, D.X.; Lv, Y.H.; He, D.Y.; Zhang, D.M.; Liu, Y.; Zhang, T.T.; Cheng, F.Y.; Zhang, Y.N.; Sun, J.Q.; Qu, J. Photocatalytic degradation of COVID-19 related drug arbidol hydrochloride by Ti3C2 MXene/supramolecular g-C3N4 Schottky junction photocatalyst. Chemosphere 2022, 308. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, Z.T.; Zeng, G.M.; Huang, D.L.; Xiao, R.; Zhang, C.; Zhou, C.Y.; Xiong, W.P.; Wang, W.J.; Cheng, M.; et al. Ti3C2 Mxene/porous g-C3N4 interfacial Schottky junction for boosting spatial charge separation in photocatalytic H2O2 production. Appl Catal B-Environ 2019, 258. [Google Scholar] [CrossRef]
- Masunga, N.; Mamba, B.B.; Kefeni, K.K. Magnetically separable samarium doped copper ferrite-graphitic carbon nitride nanocomposite for photodegradation of dyes and pharmaceuticals under visible light irradiation. J Water Process Eng 2022, 48. [Google Scholar] [CrossRef]
- Hu, X.Y.; Fan, J.; Zhang, K.L.; Yu, N.; Wang, J.J. Pharmaceuticals Removal by Novel Nanoscale Photocatalyst Bi4VO8Cl: Influencing Factors, Kinetics, and Mechanism. Ind Eng Chem Res 2014, 53, 14623–14632. [Google Scholar] [CrossRef]
- Ayodhya, D. Ag-SPR and semiconductor interface effect on a ternary CuO@Ag@Bi2S3 Z-scheme catalyst for enhanced removal of HIV drugs and (photo)catalytic activity. New J Chem 2022, 46, 15838–15850. [Google Scholar] [CrossRef]
- Ngumba, E.; Gachanja, A.N.; Tuhkanen, T.A. Removal of selected antibiotics and antiretroviral drugs during post-treatment of municipal wastewater with UV, UV/chlorine and UV/hydrogen peroxide. Water and Environment Journal 2020, 34. [Google Scholar] [CrossRef]
- Hojamberdiev, M.; Czech, B.; Wasilewska, A.; Boguszewska-Czubara, A.; Yubuta, K.; Wagata, H.; Daminova, S.S.; Kadirova, Z.C.; Vargas, R. Detoxifying SARS-CoV-2 antiviral drugs from model and real wastewaters by industrial waste-derived multiphase photocatalysts. J Hazard Mater 2022, 429. [Google Scholar] [CrossRef]
- Bhembe, Y.A.; Lukhele, L.P.; Hlekelele, L.; Ray, S.S.; Sharma, A.; Vo, D.V.N.; Dlamini, L.N. Photocatalytic degradation of nevirapine with a heterostructure of few-layer black phosphorus coupled with niobium (V) oxide nanoflowers (FL-BP@Nb2O5). Chemosphere 2020, 261. [Google Scholar] [CrossRef]
- Zhang, F.B.; Wang, X.M.; Liu, H.N.; Liu, C.L.; Wan, Y.; Long, Y.Z.; Cai, Z.Y. Recent Advances and Applications of Semiconductor Photocatalytic Technology. Appl Sci-Basel 2019, 9. [Google Scholar] [CrossRef]
- Li, H.J.; Tu, W.G.; Zhou, Y.; Zou, Z.G. Z-Scheme Photocatalytic Systems for Promoting Photocatalytic Performance: Recent Progress and Future Challenges. Adv Sci 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Bie, C.B.; Wang, L.X.; Yu, J.G. Challenges for photocatalytic overall water splitting. Chem-Us 2022, 8, 1567–1574. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





