Infective endocarditis (IE) is a rare and challenging diagnosis, Q fever endocarditis being a major cause of culture-negative endocarditis
. [
1] Although there are suggestive risk factors (predisposing abnormal native valves, age over 40 years old) and accompanying clinical manifestations ( recurrent fever, vasculitis, renal and hepatic derangements, cardiac involvement), Coxiella endocarditis diagnosis is challenging mostly because valvular vegetation is usually very small and can be missed even in transoesophageal echocardiograms, but also because of its particular difficult bacteriological detection, frequently requiring repeated blood cultures (before antimicrobial initiation) or serological detection of antibodies through immunofluorescence methods.[2, 3] This particular difficult detection through blood cultures is explained by Coxiella’s intracellular affinity [
3]. Important prognostic factors for infectious endocarditis relate to the occurrences of nervous system complications, especially overt or clinically occult cerebrovascular events ( 60 % ischemic lesions, 21 % haemorrhagic strokes). [4, 5] IE can be suspected through a particular imaging pattern of acute/subacute small cerebral ischemic lesions in watershed territories and cortical cerebral microbleeds (CMBs). [
6] The presence of multiple CMB suggests a particular vascular frailty and could be correlated to potential emergence of a haemorrhagic stroke in IE. [
7] Although CMBs don’t yet have a clear mechanism, many hypotheses have been proposed such as pyogenic vasculitis, endothelial dysfunction, micro emboli and subacute microvascular inflammatory processes. [
8]
Case report. We report a challenging case of a 45 years-old patient that presented with left peripheral facial palsy, horizontal gaze palsy, gait and postural ataxia. He also declared a fever episode (up to 39°C) 3 weeks previously and a history of arterial hypertension with normal values at admission. He had no antithrombotic treatment of any type.
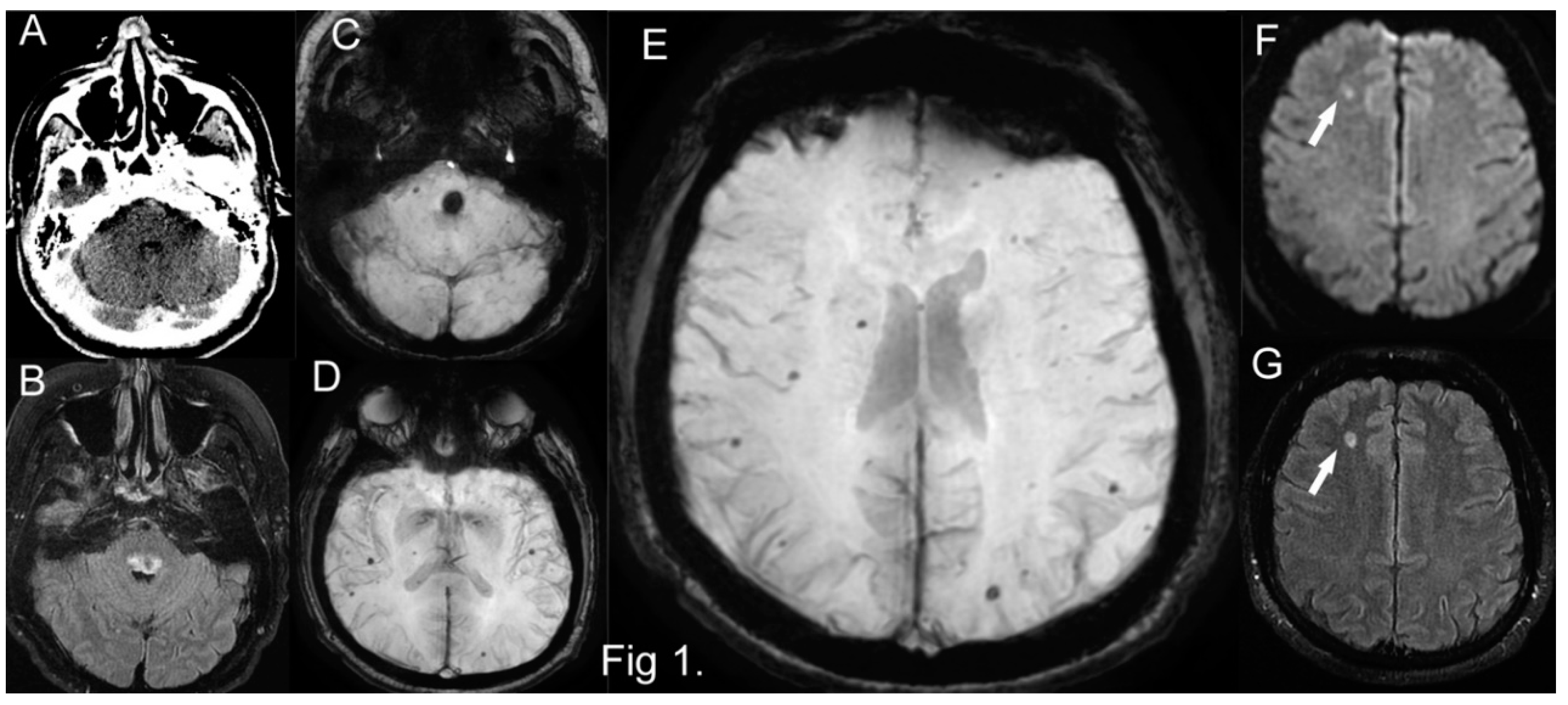
On admission cerebral CT, no acute hemorrhagic or ischemic lesions were visible (
Figure 1A). A cerebral MRI performed next day showed a dorsal medullary-pontine hemorrhage of about 14 mm, but also multiple bilateral subcortical lobar cerebral microbleeds (CMBs) and a few others in the cerebellum. A small (9mm) subacute ischemic lesion was visible in the left frontal white matter, but no other cortical ischemic lesions and no pathological changes on arterial or venous angiography (cerebral imaging on
Figure 1B,C,D,E,F,G).
A subcapsular splenic infarction was visible on the thoraco-abdominal CT, but no pulmonary pathology. Laboratory tests revealed systemic inflammation (C reactive protein 173 mg/L, fibrinogen 1083 mg/dl), no leukocytosis, slightly increased aminotransferases values, nephritic syndrome (proteinuria 492 mg/d and hematuria 17/mL on Stansfeld-Webb), moderate anemia (hemoglobin 8.7 g/dL), negative urine culture, negative nasopharyngeal and tracheal bacteriologic culture.
The first diagnostic hypothesis was of possible bacterial IE with hemorrhagic transformation of a brainstem infarct, but transthoracic and transesophageal echocardiogram revealed only calcified bicuspid aortic valve with moderate stenosis and regurgitation, without any vegetations and the blood cultures on conventional media were negative. Markers of systemic vasculitis and autoimmune diseases, multiple myeloma, hepatitis, human immunodeficiency, Epstein Barr viruses were negative. The cerebral lobar distribution of many CMBs and a few cerebellar seen on SWI MRI and the brainstem hemorrhage could raise the hypothesis of a cerebral amyloid angiopathy (CAA).[
9] Although rare in young patients, an autopsy confirmed of a similar case with a brainstem hemorrhage in the 40’s due to CAA was described [
10] and the isolated left frontal small ischemic lesion could be also related to CAA.[
11] The inclusion of CMBs increased from 48% to 63% the sensitivity of the modified Boston criteria for probable CAA, while for symptomatic subjects only, the sensitivity was 100% [
12], the specificity being very high. However the systemic inflammatory tests, the splenic infarct and kidney pathology could not be explained by a variant of CAA, neither the normal initial cerebral CT scan when the patient had already the brainstem clinical signs without evidencing the hemorrhage. Recent studies and case series [
5] showed that MRI detected CMBs could be present in up to 63,2% of patients with IE, with microbleeds not being related to a microembolic process, but rather to a subacute inflammatory or pyogenic microvascular process, explaining why in some reports an association between CMBs and mycotic aneurysms or parenchymal hemorrhage was not significant.
Bearing in mind that an IE could explain most of the clinical and imaging features of our patient, we decided to search for blood culture-negative endocarditis, starting with the most frequent involved agent, Coxiella burnetti. Serology for Coxiella burnetii was positive (antiphase I Ig G antibody 1/16 and antiphase II Ig G antibody 1/256). C4 was normal, C3 was elevated. Coxiella endocarditis is a challenging diagnosis because valvular vegetations are usually very small (explaining the lack of cerebral ischemic lesions, since vegetations >4 mm length are associated with cerebral embolic lesions) and can be missed even by transesophageal echocardiography. Endocarditis diagnosis of this patient relied on the fulfillment of 5 minor criteria [
13]: 1. predisposing valvular heart condition; 2. fever; 3. vascular phenomena including splenic emboli and intracranial hemorrhages; 4. immunologic phenomena: glomerulonephritis; 5. serological evidence of active infection with IE consistent organism. Antiphase I IgG antibody titer was insufficient for fulfilling the corresponding major criterion. Alternatively, Coxiella endocarditis itself may be responsible for the calcified aortic valve, as the histology of Coxiella endocarditis seems to be characterized by a preponderance of chronic scarring inactive lesions (fibrosis, calcifications), a paucity of active ones (inflammation, vascularization), and few or no vegetations [
14]. Proteinuria and hematuria could be present in Coxiella endocarditis [
15]. After the patient was started on doxycycline and hydroxychloroquine treatment the inflammatory and renal function tests improved significantly.
In conclusion we presented a clinical case of a patient with Coxiella burnetii endocarditis, with a brainstem hemorrhage and cerebral MRI CMBs resembling those of CAA. There are proposals that inclusion of CMBs as a new minor imaging criteria for infective endocarditis could upgrade the diagnosis in almost 50% of patients, like in this case [
16].
Funding
This research received no external funding.
Informed Consent Statement
Written informed consent has been obtained from the patientto publish this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rajani R, Klein JL. Infective endocarditis: A contemporary update. Clin Med (Northfield Il). 2020 Jan;20(1):31–5. [CrossRef]
- Brouqui P, Raoult D. Endocarditis Due to Rare and Fastidious Bacteria. Clin Microbiol Rev. 2001 Jan;14(1):177–207. [CrossRef]
- Musso D, Raoult D. Coxiella burnetii blood cultures from acute and chronic Q-fever patients. J Clin Microbiol. 1995 Dec;33(12):3129–32. [CrossRef]
- Corral I, Martín-Dávila P, Fortún J, Navas E, Centella T, Moya JL, et al. Trends in neurological complications of endocarditis. J Neurol. 2007 Sep;254(9):1253–9. [CrossRef]
- Champey J, Pavese P, Bouvaist H, Kastler A, Krainik A, Francois P. Value of brain MRI in infective endocarditis: a narrative literature review. Eur J Clin Microbiol Infect Dis. 2016 Feb;35(2):159–68. [CrossRef]
- Hess A, Klein I, Iung B, Lavallée P, Ilic-Habensus E, Dornic Q, et al. Brain MRI Findings in Neurologically Asymptomatic Patients with Infective Endocarditis. Am J Neuroradiol. 2013 Aug;34(8):1579–84. [CrossRef]
- Okazaki S, Sakaguchi M, Hyun B, Nagano K, Tagaya M, Sakata Y, et al. Cerebral Microbleeds Predict Impending Intracranial Hemorrhage in Infective Endocarditis. Cerebrovasc Dis. 2011;32(5):483–8. [CrossRef]
- Haller S, Vernooij MW, Kuijer JPA, Larsson E-M, Jäger HR, Barkhof F. Cerebral Microbleeds: Imaging and Clinical Significance. Radiology. 2018 Apr;287(1):11–28. [CrossRef]
- Jung YH, Jang H, Park SB, Choe YS, Park Y, Kang SH, et al. Strictly Lobar Microbleeds Reflect Amyloid Angiopathy Regardless of Cerebral and Cerebellar Compartments. Stroke. 2020 Dec;51(12):3600–7. [CrossRef]
- Ohtani S, Shimizu K, Asari M, Maseda C, Oka K, Yamada H, et al. Brain stem hemorrhage due to cerebral amyloid angiopathy: The autopsy of a patient with Alzheimer’s disease at a young age. Leg Med. 2014 Mar;16(2):98–101. [CrossRef]
- van Veluw SJ, Lauer A, Charidimou A, Bounemia N, Xiong L, Boulouis G, et al. Evolution of DWI lesions in cerebral amyloid angiopathy. Neurology. 2017 Nov;89(21):2136–42. [CrossRef]
- van Rooden S, van der Grond J, van den Boom R, Haan J, Linn J, Greenberg SM, et al. Descriptive Analysis of the Boston Criteria Applied to a Dutch-Type Cerebral Amyloid Angiopathy Population. Stroke. 2009 Sep;40(9):3022–7.
- Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Ryan T, et al. Proposed Modifications to the Duke Criteria for the Diagnosis of Infective Endocarditis. Clin Infect Dis. 2000 Apr;30(4):633–8. [CrossRef]
- Lepidi H, Houpikian P, Liang Z, Raoult D. Cardiac Valves in Patients with Q Fever Endocarditis: Microbiological, Molecular, and Histologic Studies. J Infect Dis. 2003 Apr;187(7):1097–106. [CrossRef]
- Stein A, Raoult D. Q fever endocarditis. Eur Heart J. 1995 Apr;16(suppl B):19–23.
- Duval X. Effect of Early Cerebral Magnetic Resonance Imaging on Clinical Decisions in Infective Endocarditis. Ann Intern Med. 2010 Apr;152(8):497.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).