1. Introduction
1.1. Cell-Free DNA
Mandel and Metais first identified cell-free DNAs (cfDNAs) in human serum in 1948 (1,2). DNA fragments known as cell-free DNAs (cfDNAs) are dispersed into extracellular surroundings. They are defined as DNA fragments that exploded via apoptosis, necrosis, and active releasing mechanisms (3,4), fueled both from the nucleus and mitochondria (5). Their length is thought to range from 40 to 200 base pairs (bp), peaking at 166 bp (6), about the size of the DNA that is wrapped around a nucleosome (7). However, larger segments up to >30 kb have also been found (8). In general, longer DNA fragments > 10 kb are thought to be the result of necrotic cell death, such as those of cells in necrotized tumor tissue. The level of cfDNA in the blood varies greatly; it ranges from 0 to 100 ng/ml in healthy individuals and from 0 to 5 to >1000 ng/ml in cancer patients (6).
This type of DNA has been discovered in a variety of bodily fluids. cfDNA fragments can result from both healthy and diseased cells and are able to reveal vital details about a person's health. Blood, urine, saliva, spinal fluid, semen, and follicular fluid have all been found to contain cfDNA, which is also in abundance in the physiological extracellular environment (8–10).
Mitochondria are found in the cytoplasm of eukaryotic cells and are responsible for energy production. They multiply independently of cell division, and contain their unique genome, which is double-stranded circular DNA, the mitochondrial DNA (or mtDNA). mtDNA differs from nuclear DNA in several ways, as is the length, the chemical structure, and the number of copies. Unlike nuclear DNA, which is inherited from both parents, mitochondrial DNA is only maternally inherited but rare exceptions may occur (11–13).
mtDNA contains a heavy (H) strand (purine-rich) and a light (L) strand (pyrimidine-rich). In human cells, the mt-DNA is a 16,569 base pair long molecule that encodes the 22 tRNAs, 2 rRNAs, and 13 polypeptides which are necessary for the synthesis of adenosine triphosphate (ATP) (14).
Mammalian cells can contain from a few hundred to thousands of mitochondria, depending on their size and energy requirements. One of the cell types harboring the largest concentrations of mitochondria and mtDNA is the mature human oocyte, with approximately 100,000-600,000, while most other cells in the human body have about 100-10,000 mitochondria (15). During fetal development, oocyte mitochondria begin to replicate; each oogonia cell contains about 200 mitochondria. An oocyte stalled at metaphase II contains roughly 100,000 mitochondria and between 50,000 and 550,000 copies of the DNA immediately before fertilization because replication proceeds in synchronization with maturation (11,16,17). This is possibly due to the large energy demands of fertilization, syngamy, cleavage, and early embryonic development (18).
1.2. Follicular Fluid
The cf-mtDNA has been found in a variety of bodily fluids (19,20), including the follicular fluid. The follicular fluid offers a rich research biological material because it represents the microenvironment required for the maturation of the oocytes. As a result, some of its biochemical properties may be crucial to the quality of the oocyte as well as to its likelihood of successful fertilization and fetal development. Oocyte quality is a significant determinant of embryo developmental ability and clinical pregnancy percentage, although, in most IVF facilities, the evaluation of oocyte quality is primarily restricted to an assessment of the morphological parameters. Promising biomarkers have been found in granulosa cells and follicular fluid according to several studies (21,22). CfDNA concentrations in human follicular fluid were found to be substantially correlated with the viability of the oocytes and embryos and may be a potential, non-invasive diagnostic tool to enhance the success of In Vitro Fertilization (IVF).
According to a previous research report, granulosa cells (GC) actively released cf-mtDNA into the medium in response to mitochondrial malfunction. This relationship between cf-nDNA and cf-mtDNA and the cfDNA concentration in FF was also demonstrated (23). Furthermore, it is still uncertain if the quantity of cf-n- and/or cf-mtDNA reflects the oocytes' capacity for development. For instance, it has been found that low cf-mtDNA concentration in FF, but not low cf-nDNA, is associated with good oocyte development potential (24).
Follicular atresia has historically been associated with GC apoptosis. Nevertheless, recent research has shown that follicular atresia can activate various types of programmed cell death (PCD), primarily in GCs, including autophagy. Although different mitophagy signaling pathways have different roles depending on the types of cells or outside factors. Several types of autophagy result in the breakdown of particular organelles, such as mitophagy for the destruction of mitochondria. Mitophagy, a mechanism by which cells selectively eliminate extra or damaged mitochondria by autophagy, is essential for maintaining mitochondrial homeostasis and cellular survival (25).
Aberrant mitochondria can be eliminated by mitophagy in the early stages of oogenesis. Despite the existence of mitophagy regulators in oocytes, after oocyte formation, mitophagy is not completely activated to clear defective mitochondria. As a result, malfunctioning mitochondria are transferred from the oocyte to the embryo. However, by increasing mitophagy, GC in the vicinity of oocytes can enhance mitochondrial activity thus improving oocyte developmental potential (26).
1.3. The Requirement for ART Procedure Indicators
In order to figure out cfDNA potential for the intended uses, researchers have recently investigated the cfDNA profile within its primary sources in reproductive medicine such as semen, follicular fluid (FF), serum, and blastocyst (27,28). Follicular fluid and granulosa cells constitute the unique environment for follicular maturation, oocyte development, and embryo development capacity and thus represent reliable oocyte and embryo biomarkers that could be used as supplementary prognostic/diagnostic tools useful for assisted reproduction techniques (29).
Several studies revealed that the amount of cfDNA in human follicular fluid correlated with embryo quality and could be used as a new biomarker, mainly in an assisted reproduction laboratory. More specifically, the amount of cf-nDNA detected in the follicular fluid has been shown to reflect granulosa cell apoptosis, while the cf-mtDNA content reflects the change in mitochondrial function and granulosa cell dynamics (30,31). Based on several studies, women who underwent unsuccessful IVF treatments had significantly higher levels of cf-mtDNA in their follicular fluid than women who were successful in getting a positive pregnancy test. Women with poor oocyte quality also had significantly higher levels of cf-mtDNA in their follicular fluid than women with good oocyte quality (9,32).
The present study is a contribution to the existing research on markers in IVF techniques. Consequently, we focused on the follicular fluid cf-mtDNA/cf-nDNA ratio levels also known as the amount of mitochondrial DNA about nuclear DNA, and its role in oocyte quality, as well as its potential involvement in the successful outcome of pregnancy resulting from IVF.
2. Materials and Methods
2.1. Patients’ Characteristics and Follicular Fluid Collection
A total of 101 women undergoing IVF/ICSI were included in our study. The samples of the follicular fluid were obtained from the IVF Unit in the Department of Obstetrics and Gynecology at the University Hospital of Ioannina. Only follicular fluid samples free of blood were used in the investigation. 31 PCOS patients and 70 without any certain reproductive disorder. The latter were divided into two categories according to their age: women of the first reproductive age up to 35 years age and women of the second reproductive age, older than 35 years of age. The informed consent was signed by each participant. Rotterdam criteria were used to identify PCOS disease (33). The Rotterdam PCOS diagnostic criteria in women include two of the following three characteristics: menstrual irregularity, hyperandrogenism, and polycystic ovarian morphology on ultrasonography (34). The control group consisted of women aged up to 35 years old, who had successful pregnancies. The patient’s age was between 23-43. Other characteristics information was collected about each participant: age, egg count, number of cumulus cells, and pathologies such as polycystic ovary syndrome (PCOS). [
Table 1]. Several hormone level estimations were performed such as follicle stimulating hormone [FSH], luteinizing hormone [LH], and estradiol [E2] at day 2 or 3 of the cycle. Anti-Mullerian hormone [AMH] was also measured in all women. All hormones were estimated using the ELISA technique [
Table 2]. All 101 patients had received antagonist treatment and ovarian stimulation with recombinant human FSH (brand name: Gonal F), or human menopausal gonadotropin (hMG) (brand name: Menopur). Ovarian stimulation response was monitored by measuring 17-β estradiol [E2] concentration.
2.2. CfDNA Extraction and Quantification
Follicular fluid was centrifuged at 5000 rpm for 5 min to get rid of granulosa cells, blood cells, and any other small cells contained in the fluid and the supernatant was collected. To maximize the performance of cfDNA-based assays, prior to DNA extraction, each pool of FF was centrifuged at 10,000 g for 15 min and the supernatant was transferred to a new Eppendorf tube. A volume of 200 μl from each FF Eppendorf was processed for cfDNA extraction by Invitrogen PureLink Genomic DNA mini kit (Thermo Fisher Scientific, USA) according to the manufacturer’s instructions. The extracted cfDNAs were eluted in 50 μl of elution buffer and quantified by NanoDrop Spectrophotometer (Thermo Fisher Scientific, USA) for measuring purity and concentrations. The purity of the extracted DNA was assessed by my measuring the A260/A80 ratio via spectroscopy for cfDNA analysis, both nuclear and mitochondrial-specific primers were used. For cf-nDNA quantification, we used the RPE primers 5’-ATAGGAAGCCAGAGAAGAGAGACT-3’ and 5’-TCTATCTCTGCGGACTTTGAGCAT-3’, (200bp), corresponding to the human retinoid isomerohydrolase gene (RPE65), as a nuclear DNA reference gene, and for the cf-mtDNA quantification, we used the pair of primers 5’-TAGAGGAGCCTGTTCTGTAATCG-3’ and 5’-TAAGGGCTATCGTAGTTTTCTGG-3’, (205bp), corresponding to a portion of the mitochondrially encoded 12S RNA, as the mitochondrial DNA reference gene, as described in [
Table 3].
2.3. Real-Time Quantitative-PCR (qPCR)
The cf-mtDNA/cf-nDNA ratio was evaluated by using quantitative polymerase chain reaction (qPCR). In summary, we mixed 0.02-0.03 μg of extracted cf-DNA, 0,25 μl of 10 μM of each primer, forward and reverse, and 5 μl of SYBR Green Master Mix (PowerUp SYBR Green Master Mix, Applied Biosystems, Thermo Fisher Scientific, USA), and 2,5 μl of distilled water, in a total volume of 10 μl for each reaction. The reaction was run on a Corbett Rotor-Gene 3000 Real-Time Rotary Analyzer (Corbett Research, Sydney, Australia) with the following cycling conditions: 50oc for 2 min (melt 1), 95oc for 2 min (melt 2), followed by 40 cycles of denaturing at 95oc for 15 sec and 60oc for 1 min. Each sample was analyzed in duplicate. Subsequently, the ratio of cf-mtDNA to cf-nDNA was calculated by the ΔΔCt method (35,36)as described below, and used as a measure of the cell-free mtDNA content in each FF sample.
For the relative quantification (also known as the ΔΔCt method), we assigned a control group and used the cycle threshold Ct values as follows:
(a) ΔCt for every sample as described above, by subtracting the average nuclear Ct value from the average mitochondrial Ct value: ∆Ct= (mtDNA Ct − nDNA Ct),
(b) the mean ΔCt value for the control group, in our case, the control group consisted of reproductively younger women (≤ 35 years of age) who had a positive pregnancy outcome and without any reproductive disorders
(c) the ΔΔCt for each sample by subtracting the ΔCt of the control group from the mean ΔCt of the sample, that is ΔΔCt = ΔCt of a sample of interest − ΔCt of the control group (36).
(d) the fold difference using the formula 2-ΔΔCt
The ΔΔCt values for each sample by subtracting the ΔCt of the control group from the mean ΔCt of the sample, that is ΔΔCt = ΔCt of a sample of interest minus the ΔCt of the control group. As a control group, we considered the group of reproductively younger women (≤ 35 years of age) who had a positive pregnancy outcome and no reproductive disorders. The corresponding values (average ± SD) were as follows: for women ≤35, non-pregnant=5065.4 ± 6414.7 and pregnant=3904.6 ± 2863.4; for women >35, non-pregnant=2159.8 ± 6045.3 and pregnant=540.2 ± 440.7; for PCOS, non-pregnant= 4084.2 ± 4334.9 and pregnant: 13344.3 ± 19621.4.
2.4. Statistical Analysis
For analyzing the data between two groups we used Student's t-test, and the data from three or more groups were analyzed using analysis of variance (ANOVA). Export data, the mt-DNA copy numbers, and ΔCt rates were placed on Microsoft Excel spreadsheets for graph design. p < 0.05 was considered statistically significant.
3. Results
In age groups of women undergoing IVF and in women with PCOS cf-mtDNA in follicular fluid had a positive correlation with age. Our result is in agreement with that reported by Qasemi et al (2021), (37) who found that the clearance of cfDNA is less efficient in women with endocrine pathology such as PCOS. FSH and AMH are a couple of hormones that interact to produce follicles and oocytes in the ovary (38). In the FF of the PCOS and non-PCOS women in our study, we found a negative association between cfDNA and both FSH and AMH levels.
3.1. Assessment by Female Age
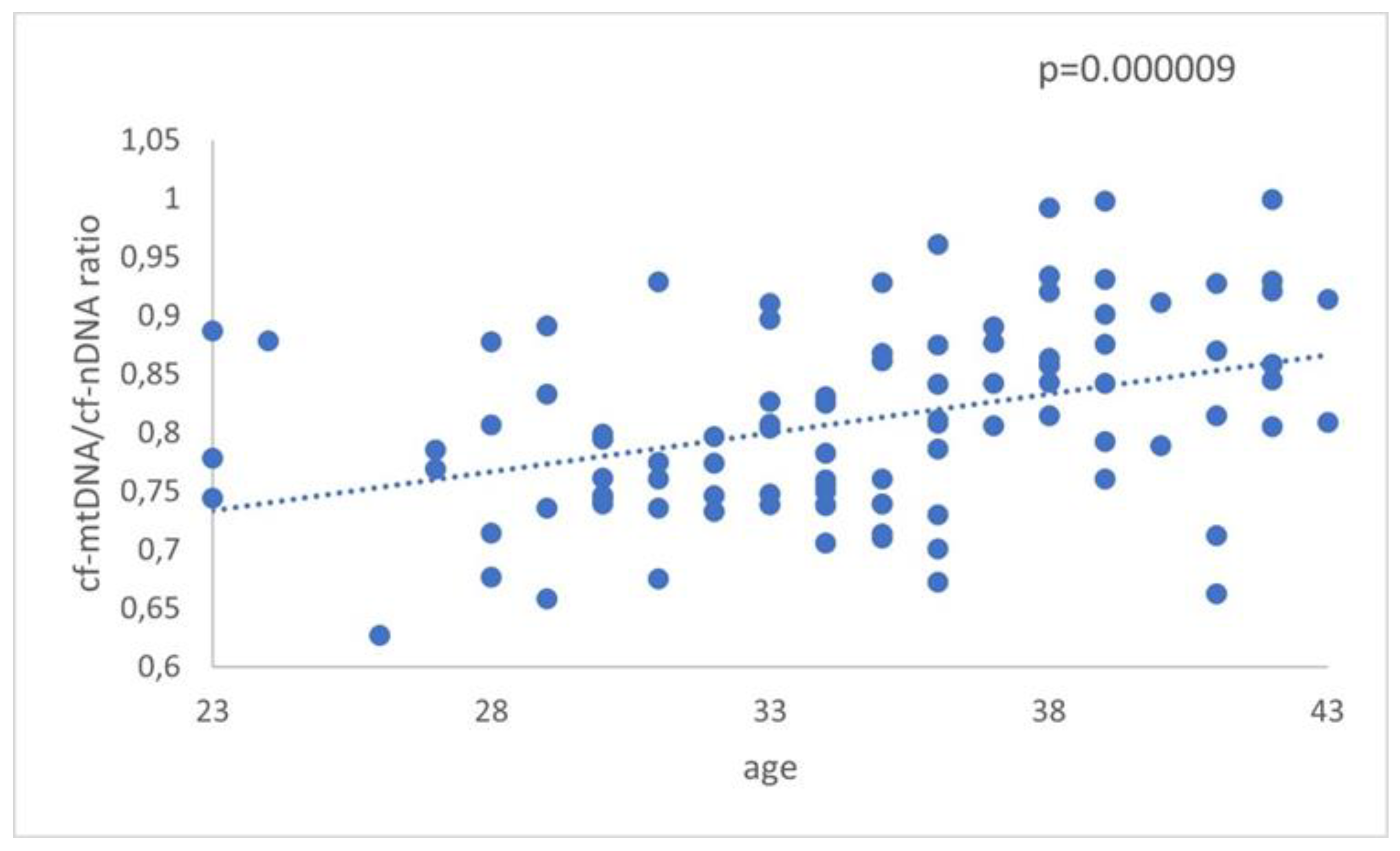
The relative ratio of cf-mtDNA to cf-nDNA was assessed in relation to female age. More specifically, a comparison of 57 FF cfDNA obtained from a group of women between 23-35 years (average age 31 years,) and 44 FF cfDNA from an older group with a range between 36–43 years (average age 38.8 years,) was analyzed with real-time PCR. Data analysis resulted in a statistically significant increase (p = 0.000009) in the amount of the ratio mtDNA/nDNA in FF samples from the older women (
Figure 1). The relative amounts of mtDNA/nDNA ratio for the female age groups under investigation are summarized in
Table 4 and illustrated in
Figure 1.
3.2. Assessments Based on Polycystic Ovary Syndrome and the Age of the Female
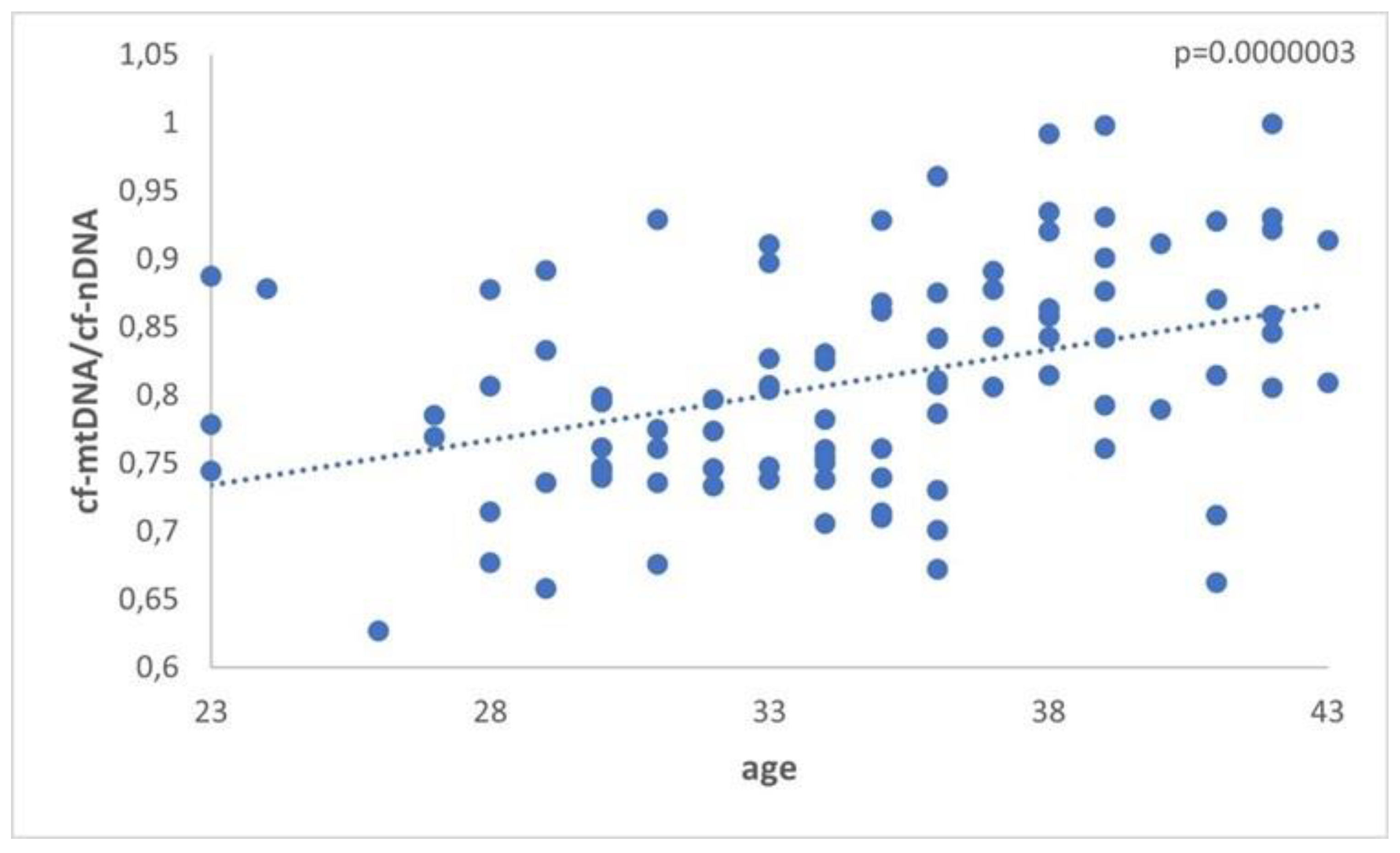
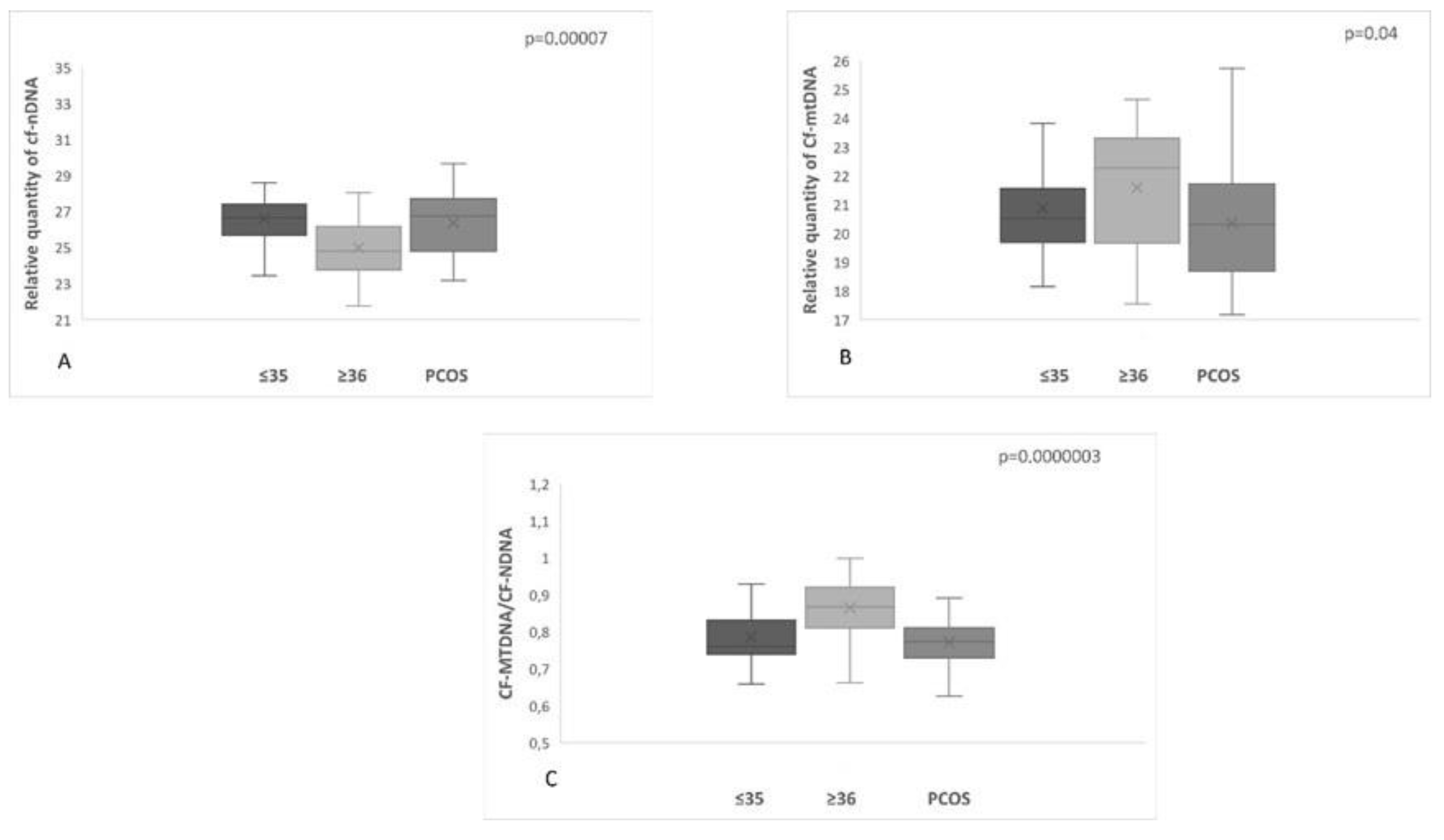
A significant difference (p = 0.0000003) in the levels of mtDNA to nDNA ratio was also observed in the 3 categories of women compared. The first two groups included the reproductively younger (n=34) and older(n=36) women, respectively, and the third group included women with PCOS (n=31). Once again, data analysis clearly showed a statistically significant increase in the ratio of FF samples from the reproductively older women. As concerns women with PCOS, no statistical difference was observed. It appeared that all samples follow the age pattern illustrated in
Figure 1. For the female groups included in the study, the relative mtDNA/nDNA ratio amounts are listed in
Table 5 and presented in
Figure 2 and
Figure 3.
3.3. Comparison between Pregnant and Not Pregnant Women
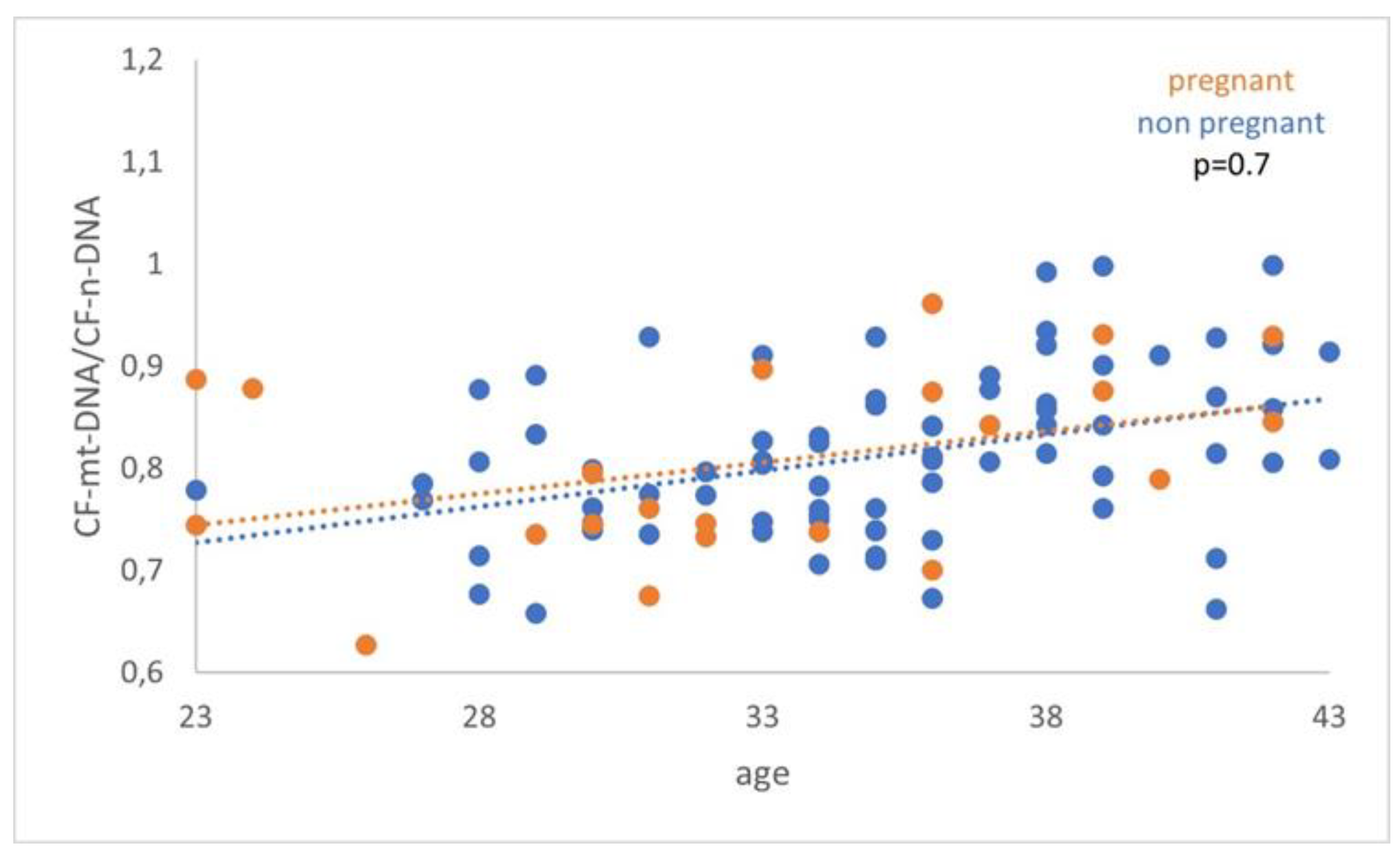
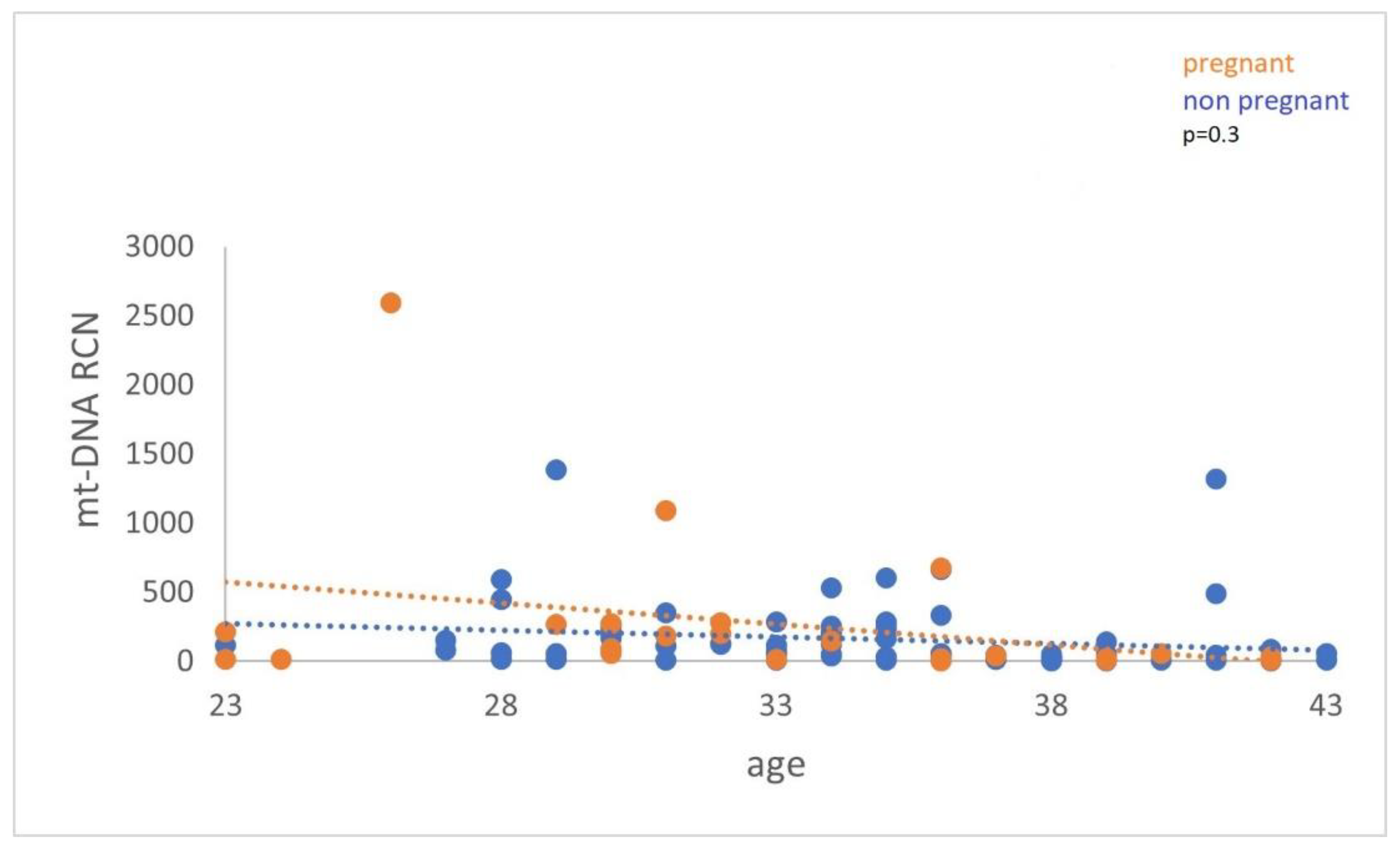
A similar comparison was made between the FF cf-mtDNA/ cf-nDNA ratio and the cf-mtDNA relative content (RCN), of women with or without successful pregnancy. However, the analysis demonstrated a non-statistically significant difference (p=0.7) (p=0.3) (
Figure 4 and
Figure 5).
3.4. Correlation of the mtDNA Relative Copy Number (RCN) in Individual Groups
An increased risk of age-related diseases such as cancer, cardiovascular disease, and neurological disorders is linked to the reduction in mtDNA copy number with age. This is due to the vital role of mitochondria in cellular functions such as energy production and others that are necessary for preserving good cellular function and tissue homeostasis. Overall, the loss of mtDNA copies with age can influence both a woman's ability to get pregnant and children's health. We, therefore, compared the relative mtDNA content in these samples. We observed that this number is reduced in reproductively older women, a finding consistent with the currently available research. The relative mtDNA copy number was calculated based on the ΔΔCt method as described in materials and methods. We evaluated the samples to assess the multiplicity of times they are expressed as compared to the control group, which consisted of reproductively younger women who had successful pregnancies, using the 2
-ΔΔCt formula. A value of 2
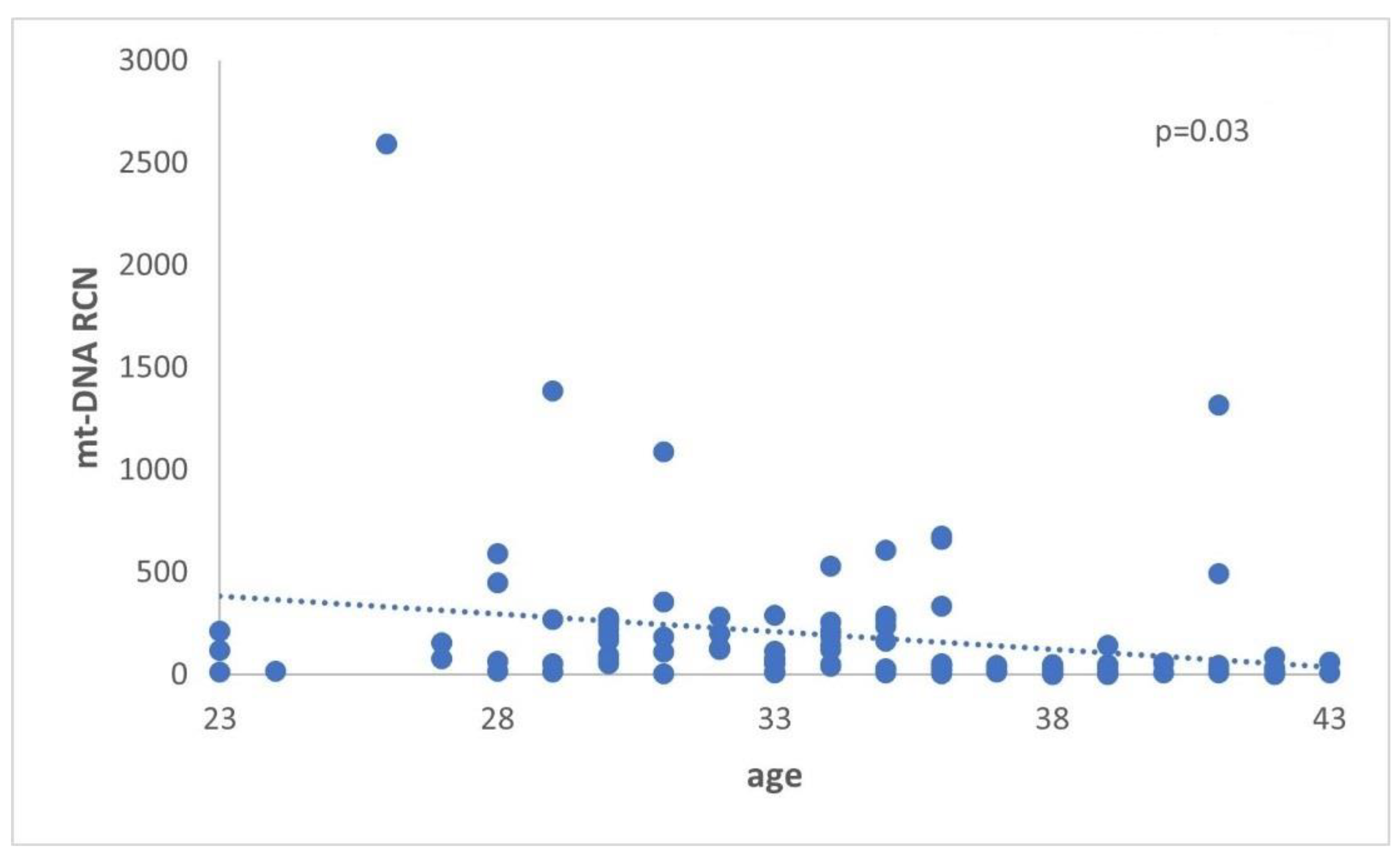
-ΔΔCt >1 indicates that the group under examination exhibits higher levels of mtDNA than the control group. It has been previously reported that FF cf-mtDNA levels are higher in PCOS than in women without this condition. According to Qasemi et al. (2021), (37) in women without a specific reproductive problem, FF cf- mtDNA can be used as a biomarker for potential ART outcomes. As shown in
Table 6 and
Figure 6, our data are in agreement with this authors, suggesting that abnormal mtDNA accumulation in these pathological situation may cause increased FF cf-mtDNA. The data are presented in
Table 6 and illustrated in
Figure 6.
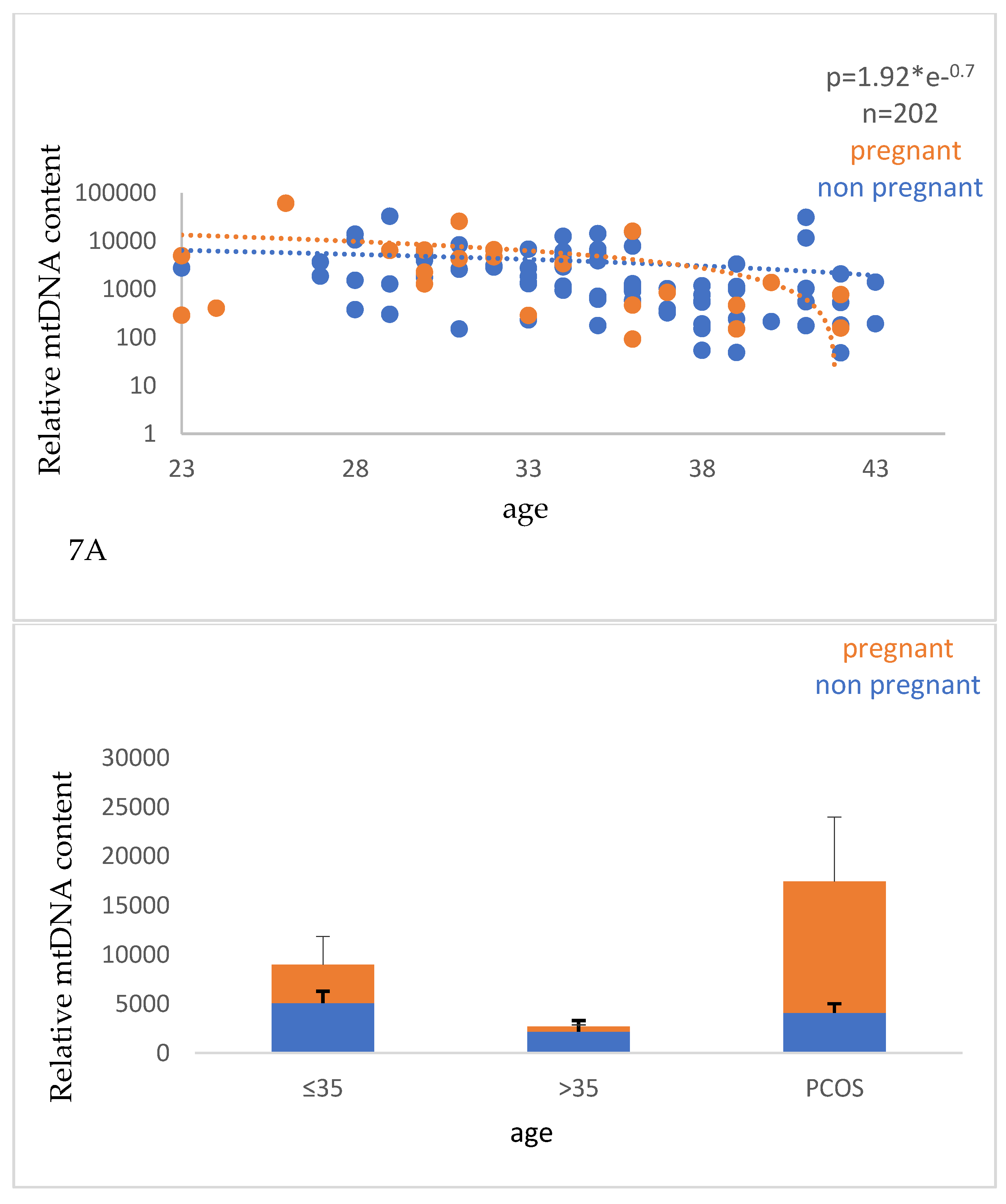
Interestingly, we observed a significant decrease (p<0.0001) in the relative mtDNA content with advancing age (
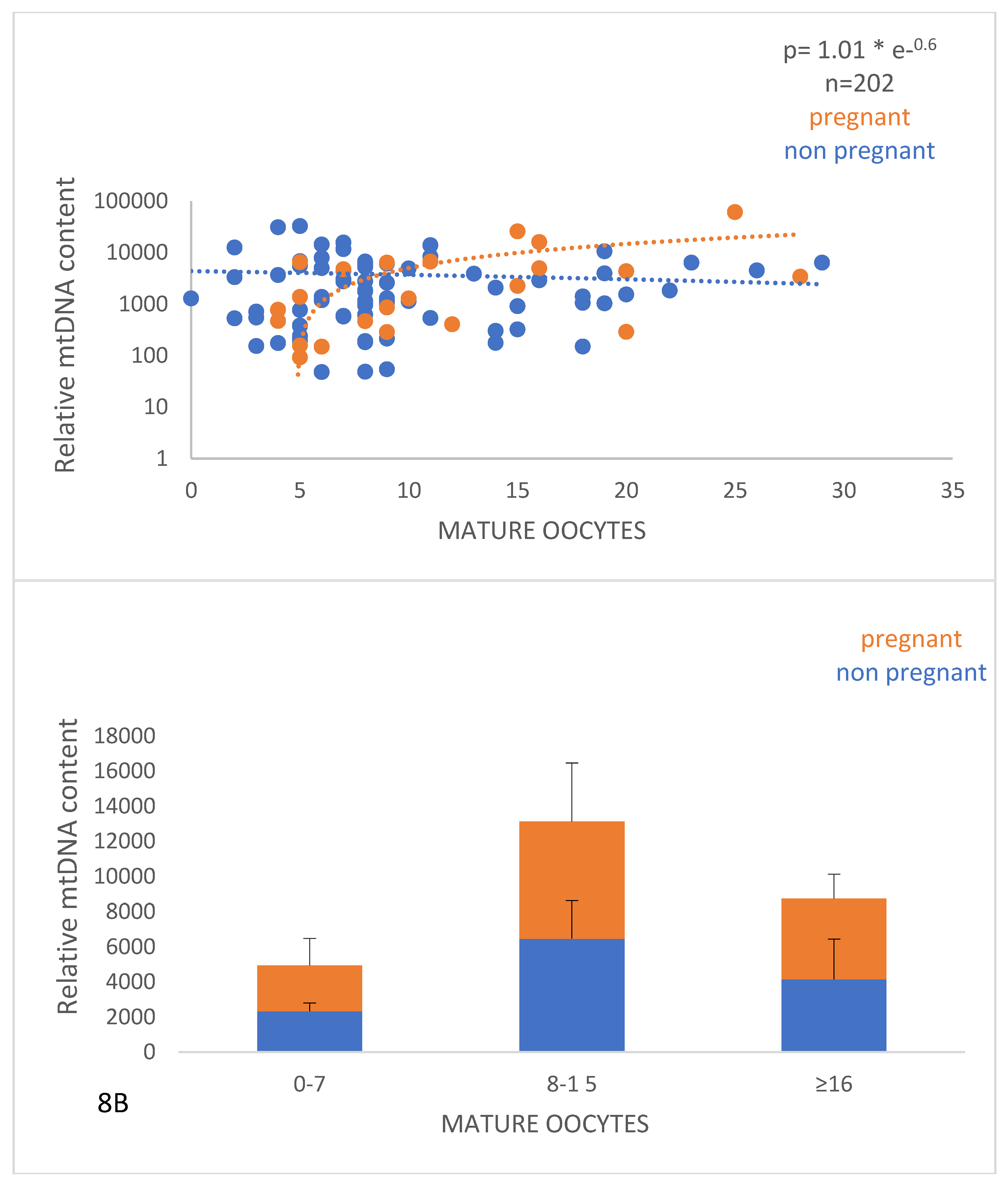
Figure 7), and a positive correlation (p<0.0001) with the number of mature oocytes (MII stage), indicating that this parameter can be a useful biomarker of oocyte quality and ovarian aging.
Figure 7.
7A.Correlation between the relative mtDNA content and the age of the women. Values corresponding to women with positive and negative pregnancy tests are shown with orange and blue circles, respectively. The data were obtained from qPCR analysis of cell-free DNA isolated from the FF samples that were taken from women undergoing IVF and analyzed by the 2-ΔΔCt method. A significant decrease (p<0.0001) in the relative mtDNA content with advancing age was observed. 7B. Graphical representation of the relative mtDNA content with the age of the women. The orange part of the bars corresponds to pregnant women and the blue part to non-pregnant women. A significant difference (p<0.05) of the relative mtDNA content between the young and older women was observed.
Figure 7.
7A.Correlation between the relative mtDNA content and the age of the women. Values corresponding to women with positive and negative pregnancy tests are shown with orange and blue circles, respectively. The data were obtained from qPCR analysis of cell-free DNA isolated from the FF samples that were taken from women undergoing IVF and analyzed by the 2-ΔΔCt method. A significant decrease (p<0.0001) in the relative mtDNA content with advancing age was observed. 7B. Graphical representation of the relative mtDNA content with the age of the women. The orange part of the bars corresponds to pregnant women and the blue part to non-pregnant women. A significant difference (p<0.05) of the relative mtDNA content between the young and older women was observed.
Figure 8.
8A.The relationship between the relative mtDNA content and the number of mature (MII) oocytes. Values corresponding to women with positive and negative pregnancy tests are shown with orange and blue circles, respectively. The data were derived as described in
Figure 7. 8B. Graphical representation of the relative mtDNA content with the number of mature (MII) oocytes. The orange part of the bars corresponds to pregnant women and the blue part to non-pregnant women. A significant difference (p<0.05) of the relative mtDNA content between women who had relatively small (0-7 and 8-15) number of mature oocytes was revealed.
Figure 8.
8A.The relationship between the relative mtDNA content and the number of mature (MII) oocytes. Values corresponding to women with positive and negative pregnancy tests are shown with orange and blue circles, respectively. The data were derived as described in
Figure 7. 8B. Graphical representation of the relative mtDNA content with the number of mature (MII) oocytes. The orange part of the bars corresponds to pregnant women and the blue part to non-pregnant women. A significant difference (p<0.05) of the relative mtDNA content between women who had relatively small (0-7 and 8-15) number of mature oocytes was revealed.
4. Discussion
Mitochondria are crucial for numerous biological processes and their involvement in human reproduction is particularly significant. Mitochondria are essential for all cellular functions, including the generation of ATP, embryonic development, and oocyte fertilization (39). The distinctive feature of mitochondria is that they are vertically maternally transmitted. The oocyte has more mitochondria because the egg cell supplies the developing embryo with far more cytoplasm than the sperm. The sperm contributes half of the genetic material to the embryo, but after fertilization, its mitochondria are frequently destroyed or damaged. Studies demonstrating that errors in mitochondrial activity can result in a variety of reproductive pathologies, including infertility, miscarriage, and developmental abnormalities, have raised awareness of the significance of mitochondrial function in human reproduction (40).
Although mitochondria have their DNA, nuclear DNA also plays a significant role in most of the protein production and maintenance in mitochondria (41,42). The relative abundance of mitochondrial DNA about nuclear DNA within a cell is referred to as the mtDNA/nDNA ratio. Changes in the mtDNA/nDNA ratio and mitochondrial number in aging females are influenced by several factors. One significant factor is the accumulation of mtDNA mutations over time. Mitochondrial DNA is more vulnerable to oxidative damage, and its DNA repair mechanisms are less efficient (43). As a result, mtDNA accumulated mutations may become more prevalent with age. These mutations may interfere with mitochondria's ability to replicate and maintain, which could be detrimental to fertility (44). Age-related declines in mitochondrial content can also be caused by several factors such as hormonal changes reduction in mitochondrial biogenesis, lifestyle, genetics, and environmental influences (45).
Apoptosis and mitophagy are two cellular processes that are closely related, with a complicated and unclear interaction. Nevertheless, it has been put forward that mitophagy may function as a pro-survival process by eliminating defective mitochondria that could trigger apoptosis (46). Furthermore, it has been demonstrated that inhibiting mitophagy can promote apoptosis (47). Both the effectiveness of apoptosis and mitophagy can decrease as women age. An accumulation of damaged mitochondria in the cells could result from this decline. Age-related increases in damaged or malfunctioning mitochondria lead to a higher mtDNA/nDNA ratio. Both ovarian follicular growth and atresia (follicle degeneration) depend heavily on apoptosis. Only a percentage of the follicles that form during folliculogenesis mature and release a viable oocyte. The remaining follicles deteriorate and undergo apoptosis (48). According to investigations on the cf-DNA's integrity in follicular fluid, the release of cf-nDNA into the follicular fluid is one of the several cellular and molecular pathways that are activated during apoptosis and up to 85% of the cf-DNA originate from cell apoptosis (24).
Based on the findings of our study, there is an apparent contradiction about the fact that the follicular mtDNA/nDNA ratio increases with age, although the number of mitochondria itself has indeed decreased. It is already known that the amount of cfDNA in the follicular fluid may be a good indicator of the oocyte quality inside the follicle and the level of follicular apoptosis. High amounts of cfDNA have been linked to decreased oocyte developmental capacity and higher follicular atresia (9).
To enhance the effectiveness of infertility therapies, new techniques for assessing oocyte competency and embryo viability should be developed. Studies focusing on the mitochondrial function, granulosa and cumulus cells, oocytes, and embryos as well as follicular fluid will likely lead to novel findings. Most of the research has established a link between the success of ART and the capacity of oocytes for supporting preimplantation development and pregnancy. The identification of a high-quality embryo using trustworthy molecular technology and the measurement of the success of ART are the two main goals of the biomarkers employed in human reproductive medicine. However, to make these techniques as efficient as possible, it is crucial to search for the factors that affect the quality of the follicles and, subsequently of the oocytes. There have been numerous evaluations of mtDNA in IVF clinical outcomes assessment (49). Higher trophectoderm quality, effective implantation outcomes, and successful embryonic development into blastocysts are also related to an increased cf-mtDNA/cf-nDNA ratio (50). CfDNA as a biomarker has created new opportunities for non-invasive diagnostic tool applications in biological processes (11). Both the cell-free nuclear DNA (cf-nDNA) and the cell-free mitochondrial DNA (cf-mtDNA) are considered to be quite valuable tools in the diagnosis and prognosis of cancer as well as, in prenatal diagnosis(12,13). Prenatal testing is one of the most well-known uses of cfDNA since it can be used to find genetic anomalies in a growing fetus.
The current study aims to highlight mitochondria as a potential high-sensitivity prognostic tool for assessing an embryo's viability in artificial fertilization and to ascertain whether their use can be implemented in ART. We intended to provide an alternative, non-time-consuming, non-cost-intensive, and non-invasive approach to measuring the amount of cell-free mitochondrial DNA (cf-mtDNA) by calculating the ratio cf-mtDNA/cf-nDNA in FF samples of women undergoing follicular aspiration for IVF/ICSI. We found that the FF cf-mtDNA/cf-nDNA ratio level was higher in women, who also had the highest rates of successful IVF. The relative ratio of cf-mtDNA to cf-nDNA content in human follicular fluid was positively correlated with age and the implantation potential, only for females without a specific reproductive pathology, but not among those with PCOS, demonstrating that it could be potentially an effective predictor for assessing the corresponding oocyte’s performance in IVF.
Last but not least, it should be highlighted that the majority of experts in the field of medicine agree that mitochondria are an excellent predictor. Despite the significance of mitochondria in human reproduction, their function and regulation remain poorly understood. Many studies have demonstrated the potential clinical utility of cfDNA (cf-mtDNA and cf-nDNA) analysis in follicular fluid in ART for the quality of the oocyte and the possibility of a successful pregnancy, however, more studies are required. Fertility, time to pregnancy, and aging of the oocytes appear to be influenced by cf-mtDNA copy counts. Even if, the cf-mtDNA copy numbers can be employed as a clinical tool for diagnosing, and treating reproductive pathologies, further study is required to fully understand the mechanisms underlying these correlations. Finally, due to mtDNA's significant degree of variability, the implementation of other markers, such as the cf-mtDNA and cf-nDNA ratio, hormones, and morphology can be exploited in conjunction with other biomarkers.
5. Conclusions
Biomarkers are enhancing what is already known in prognosis, diagnosis, and treatment. They are useful tools with important biological and therapeutic applications in many medical disciplines. Preserving follicular fluid milieu is crucial for producing suitable eggs, and hence, well-developed embryos. Trustworthy relevant biomarkers and non-invasive diagnostic tests are essential in the field of human reproductive medicine. In recent years, there has been growing interest in the role of cfDNA in human reproduction. Due to genomic DNA degradation, low concentration, and high fragmentation, studying cfDNA is typically challenging but it might be enhanced to serve as a useful tool in Medically Assisted Reproduction. The follicular fluid, as the immediate microenvironment of the egg, provides all the potentially necessary ingredients to nourish the oocytes before fertilization. However, until recently the selection of the embryos with the highest implantation potential during assisted reproduction procedures was taking into account the morphological criteria of the eggs. Since follicular fluid is only obtained when oocytes are aspirated from follicles, it is commonly acknowledged that this procedure is not entirely invasive. As a result, the qualitative and quantitative determination of its cf-nDNA and cf-mtDNA is rapid, exhibits great sensitivity, and is simple to carry out. Furthermore, it provides a general overview of the quality of the follicular microenvironment, enhancing the success of ART. As it is generally known that the mother's age has an adverse relationship with the likelihood that an egg will develop into a viable embryo, a fact that is strongly confirmed by the significant difference in the success rate of IVF treatment for reproductively older women compared to younger. However, the decrease in cf-mtDNA with age seen in FF samples during the current study raises the question of whether mitochondria might play a direct role in female fertility and age. Last but not least to sum up, more investigation is needed to thoroughly examine the potential applications of cell-free DNA, nDNA, mtDNA, and the mt/n ratio, in reproductive biology and to develop more effective diagnostic and therapeutic approaches based on this biomarker.
Author Contributions
Data curation, Athanasios Zikopoulos, Kyriaki Papageorgiou, Charilaos Kostoulas and Ioannis Tsigkas; Methodology, Athanasios Zikopoulos, Kyriaki Papageorgiou, Charilaos Kostoulas and Ioannis Tsigkas; Project administration, Theologos Michaelidis and Ioannis Georgiou; Resources, Theologos Michaelidis and Ioannis Georgiou; Supervision, Ioannis Georgiou; Writing – original draft, Georgia Tsirka; Writing – review & editing, Efthalia Moustakli, Aris Kaltsas and Eleftheria Sarafi.
Funding
This work was funded by the project “Understanding pathways of healthy aging (in health and disease) through the integration of high-resolution omics data - pathAGE” (MIS 5047228) which is implemented under the Action “Regional Excellence in R&D Infrastructures”, funded by the Operational Programme “Competitiveness, Entrepreneurship, and Innovation” (NSRF 2014–2020) and co-financed by Greece and the European Union (European Regional Development Fund).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Patient consent was waived due to In Vitro Fertilization (IVF).
Data Availability Statement
Data is unavailable due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mandel, P.; Metais, P. [Nuclear Acids In Human Blood Plasma]. C R Seances Soc Biol Fil. 1948, 142, 241–243. [Google Scholar] [PubMed]
- Meddeb, R.; Dache, Z.A.A.; Thezenas, S.; Otandault, A.; Tanos, R.; Pastor, B.; et al. Quantifying circulating cell-free DNA in humans. Sci Rep. 2019, 9, 5220. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Hoon, D.S.B.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011, 11, 426–437. [Google Scholar] [CrossRef]
- Schwarzenbach, H.; Müller, V.; Milde-Langosch, K.; Steinbach, B.; Pantel, K. Evaluation of cell-free tumour DNA and RNA in patients with breast cancer and benign breast disease. Mol Biosyst. 2011, 7, 2848. [Google Scholar] [CrossRef]
- Sun, Y.; An, K.; Yang, C. Circulating Cell-Free DNA. In: Strumfa I, Gardovskis J, editors. Liquid Biopsy [Internet]. IntechOpen; 2019. Available online: https://www.intechopen.com/books/liquid-biopsy/circulating-cell-free-dna (accessed on 24 May 2023).
- Kustanovich, A.; Schwartz, R.; Peretz, T.; Grinshpun, A. Life and death of circulating cell-free DNA. Cancer Biol Ther. 2019, 20, 1057–1067. [Google Scholar] [CrossRef]
- Henikoff, S.; Church, G.M. Simultaneous Discovery of Cell-Free DNA and the Nucleosome Ladder. Genetics. 2018, 209, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Thierry, A.R.; El Messaoudi, S.; Gahan, P.B.; Anker, P.; Stroun, M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016, 35, 347–376. [Google Scholar] [CrossRef]
- Scalici, E.; Traver, S.; Molinari, N.; Mullet, T.; Monforte, M.; Vintejoux, E.; et al. Cell-free DNA in human follicular fluid as a biomarker of embryo quality. Hum Reprod. 2014, 29, 2661–2669. [Google Scholar] [CrossRef]
- Wang, Y.; Springer, S.; Zhang, M.; McMahon, K.W.; Kinde, I.; Dobbyn, L.; et al. Detection of tumor-derived DNA in cerebrospinal fluid of patients with primary tumors of the brain and spinal cord. Proc Natl Acad Sci. 2015, 112, 9704–9709. [Google Scholar] [CrossRef]
- Cummins, J.M. Fertilization and elimination of the paternal mitochondrial genome. Hum Reprod. 2000, 15 (suppl 2), 92–101. [Google Scholar] [CrossRef]
- Schatten, H.; Sun, Q.Y.; Prather, R. The impact of mitochondrial function/dysfunction on IVF and new treatment possibilities for infertility. Reprod Biol Endocrinol. 2014, 12, 111. [Google Scholar] [CrossRef] [PubMed]
- Song, W.H.; Ballard, J.W.O.; Yi, Y.J.; Sutovsky, P. Regulation of Mitochondrial Genome Inheritance by Autophagy and Ubiquitin-Proteasome System: Implications for Health, Fitness, and Fertility. BioMed Res Int. 2014, 2014, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, T.J.; Gustafsson, C.M. Separating and Segregating the Human Mitochondrial Genome. Trends Biochem Sci. 2018, 43, 869–881. [Google Scholar] [CrossRef]
- May-Panloup, P.; Chrétien, M.F.; Jacques, C.; Vasseur, C.; Malthièry, Y.; Reynier, P. Low oocyte mitochondrial DNA content in ovarian insufficiency. Hum Reprod. 2005, 20, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Motta, P.M.; Nottola, S.A.; Makabe, S.; Heyn, R. Mitochondrial morphology in human fetal and adult female germ cells. Hum Reprod. 2000, 15 (suppl 2), 129–147. [Google Scholar] [CrossRef]
- Fragouli, E.; Spath, K.; Alfarawati, S.; Kaper, F.; Craig, A.; Michel, C.E.; et al. Altered Levels of Mitochondrial DNA Are Associated with Female Age, Aneuploidy, and Provide an Independent Measure of Embryonic Implantation Potential. Kim SK, editor. PLOS Genet. 2015, 11, e1005241. [Google Scholar] [CrossRef]
- Reynier, P.; May-Panloup, P.; Chretien, M.F.; Morgan, C.J.; Jean, M.; Savagner, F.; et al. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol Hum Reprod. 2001, 7, 425–429. [Google Scholar] [CrossRef]
- Kohler, C.; Radpour, R.; Barekati, Z.; Asadollahi, R.; Bitzer, J.; Wight, E.; et al. Levels of plasma circulating cell free nuclear and mitochondrial DNA as potential biomarkers for breast tumors. Mol Cancer. 2009, 8, 105. [Google Scholar] [CrossRef]
- Ellinger, J.; Albers, P.; Müller, S.C.; Von Ruecker, A.; Bastian, P.J. Circulating mitochondrial DNA in the serum of patients with testicular germ cell cancer as a novel noninvasive diagnostic biomarker. BJU Int. 2009, 104, 48–52. [Google Scholar] [CrossRef]
- Rao, M.; Zhou, F.; Tang, L.; Zeng, Z.; Hu, S.; Wang, Y.; et al. Follicular fluid humanin concentration is related to ovarian reserve markers and clinical pregnancy after IVF–ICSI: A pilot study. Reprod Biomed Online. 2019, 38, 108–117. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Meldrum, D.R.; Katz-Jaffe, M.G.; Krisher, R.L.; Schoolcraft, W.B. Oocyte environment: Follicular fluid and cumulus cells are critical for oocyte health. Fertil Steril. 2015, 103, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Kansaku, K.; Munakata, Y.; Itami, N.; Shirasuna, K.; Kuwayama, T.; Iwata, H. Mitochondrial dysfunction in cumulus-oocyte complexes increases cell-free mitochondrial DNA. J Reprod Dev. 2018, 64, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, Q.; Zhao, X.; Zou, M.; Shao, S.; Li, J.; et al. Cell-free mitochondrial DNA in human follicular fluid: A promising bio-marker of blastocyst developmental potential in women undergoing assisted reproductive technology. Reprod Biol Endocrinol. 2019, 17, 54. [Google Scholar] [CrossRef]
- Shen, M.; Jiang, Y.; Guan, Z.; Cao, Y.; Sun, S.c.; Liu, H. FSH protects mouse granulosa cells from oxidative damage by repressing mitophagy. Sci Rep. 2016, 6, 38090. [Google Scholar] [CrossRef]
- Shen, Q.; Liu, Y.; Li, H.; Zhang, L. Effect of mitophagy in oocytes and granulosa cells on oocyte quality†. Biol Reprod. 2021, 104, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, D. DNA Methylation Analysis from Body Fluids. In: Schulz WA, Hoffmann MJ, Niegisch G, editors. Urothelial Carcinoma [Internet]. New York, NY: Springer New York; 2018. p. 239–49. (Methods in Molecular Biology; vol. 1655). Available online: http://link.springer.com/10.1007/978-1-4939-7234-0_18 (accessed on 24 May 2023).
- Nabhan, A.; Salama, M.; Elsayed, M.; Nawara, M.; Kamel, M.; Abuelnaga, Y.; et al. Indicators of infertility and fertility care: A systematic scoping review. Hum Reprod Open. 2022, 2022, hoac047. [Google Scholar] [CrossRef] [PubMed]
- Baka, S.; Malamitsi-Puchner, A. Novel follicular fluid factors influencing oocyte developmental potential in IVF: A review. Reprod Biomed Online. 2006, 12, 500–506. [Google Scholar] [CrossRef]
- Traver, S.; Assou, S.; Scalici, E.; Haouzi, D.; Al-Edani, T.; Belloc, S.; et al. Cell-free nucleic acids as non-invasive biomarkers of gynecological cancers, ovarian, endometrial and obstetric disorders and fetal aneuploidy. Hum Reprod Update. 2014, 20, 905–923. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Anifandis, G.; Messini, C.I.; Dafopoulos, K.; Kouris, S.; Sotiriou, S.; et al. Follicular Fluid Oocyte/Cumulus-Free DNA Concentrations as a Potential Biomolecular Marker of Embryo Quality and IVF Outcome. BioMed Res Int. 2014, 2014, 1–5. [Google Scholar] [CrossRef]
- Ichikawa, K.; Shibahara, H.; Shirasuna, K.; Kuwayama, T.; Iwata, H. Cell-free DNA content in follicular fluid: A marker for the developmental ability of porcine oocytes. Reprod Med Biol. 2020, 19, 95–103. [Google Scholar] [CrossRef]
- The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004, 19, 41–47.
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018, 110, 364–379. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Quiros, P.M.; Goyal, A.; Jha, P.; Auwerx, J. Analysis of mtDNA/nDNA Ratio in Mice. Curr Protoc Mouse Biol. 2017, 7, 47–54. [Google Scholar] [CrossRef]
- Qasemi, M.; Aleyasin, A.; Mahdian, R.; Ghanami Gashti, N.; Shabani Nashtaei, M.; Ashrafnezhad, Z.; et al. Cell-free mtDNA level and its biomarker potency for ART outcome are different in follicular fluid of PCOS and non-PCOS women. Mitochondrion. 2021, 59, 30–36. [Google Scholar] [CrossRef]
- Agrawal, Y.; Seth, S.; Goyal, V.; Kumar, P.; Bala, J. Correlation between anti-Müllerian and follicle-stimulating hormone in female infertility. Int J Health Allied Sci. 2014, 3, 232. [Google Scholar] [CrossRef]
- Alizadegan, A.; Dianat-Moghadam, H.; Shadman, N.; Nouri, M.; Hamdi, K.; Ghasemzadeh, A.; et al. Application of cell free DNA in ART. Placenta. 2022, 120, 18–24. [Google Scholar] [CrossRef]
- Zou, W.; Slone, J.; Cao, Y.; Huang, T. Mitochondria and Their Role in Human Reproduction. DNA Cell Biol. 2020, 39, 1370–1378. [Google Scholar] [CrossRef]
- Stojanovski, D.; Johnston, A.J.; Streimann, I.; Hoogenraad, N.J.; Ryan, M.T. Import of Nuclear-Encoded Proteins into Mitochondria. Exp Physiol. 2003, 88, 57–64. [Google Scholar] [CrossRef]
- Kummer, E.; Ban, N. Mechanisms and regulation of protein synthesis in mitochondria. Nat Rev Mol Cell Biol. 2021, 22, 307–325. [Google Scholar] [CrossRef]
- Ziada, A.S.; Smith, M.S.R.; Côté, H.C.F. Updating the Free Radical Theory of Aging. Front Cell Dev Biol. 2020, 8, 575645. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.B.; Chinnery, P.F. The dynamics of mitochondrial DNA heteroplasmy: Implications for human health and disease. Nat Rev Genet. 2015, 16, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Sobenin, I.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. Mitochondrial Aging and Age-Related Dysfunction of Mitochondria. BioMed Res Int. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wanderoy, S.; Hees, J.T.; Klesse, R.; Edlich, F.; Harbauer, A.B. Kill one or kill the many: Interplay between mitophagy and apoptosis. Biol Chem. 2020, 402, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Bloemberg, D.; Quadrilatero, J. Autophagy, apoptosis, and mitochondria: Molecular integration and physiological relevance in skeletal muscle. Am J Physiol-Cell Physiol. 2019, 317, C111–C130. [Google Scholar] [CrossRef]
- Markstrom, E.; Svensson, E.c.; Shao, R.; Svanberg, B.; Billig, H. Survival factors regulating ovarian apoptosis -- dependence on follicle differentiation. Reproduction. 2002, 123, 23–30. [Google Scholar] [CrossRef]
- Fragouli, E.; McCaffrey, C.; Ravichandran, K.; Spath, K.; Grifo, J.A.; Munné, S.; et al. Clinical implications of mitochondrial DNA quantification on pregnancy outcomes: A blinded prospective non-selection study. Hum Reprod. 2017, 32, 2340–2347. [Google Scholar] [CrossRef]
- Stigliani, S.; Persico, L.; Lagazio, C.; Anserini, P.; Venturini, P.L.; Scaruffi, P. Mitochondrial DNA in Day 3 embryo culture medium is a novel, non-invasive biomarker of blastocyst potential and implantation outcome. MHR Basic Sci Reprod Med. 2014, 20, 1238–1246. [Google Scholar] [CrossRef]
Figure 1.
The relationship between cf-mtDNA/cf-nDNA quantity and female age. Data obtained during quantitative real-time PCR analysis of FF samples taken from women undergoing IVF demonstrated a statistically significant increase (p = 0.000009) in the level of ratio about advancing female age.
Figure 1.
The relationship between cf-mtDNA/cf-nDNA quantity and female age. Data obtained during quantitative real-time PCR analysis of FF samples taken from women undergoing IVF demonstrated a statistically significant increase (p = 0.000009) in the level of ratio about advancing female age.
Figure 2.
The relationship between cf-mtDNA/cf-nDNA quantity in the 3 groups. Data obtained during quantitative real-time PCR analysis of FF samples taken from women undergoing IVF demonstrated a statistically significant increase (p = 0.0000003) in the level of ratio compared to advancing female age. Specifically, the p-value was further decreased in older women. No apparent difference was indicated for women with PCOS.
Figure 2.
The relationship between cf-mtDNA/cf-nDNA quantity in the 3 groups. Data obtained during quantitative real-time PCR analysis of FF samples taken from women undergoing IVF demonstrated a statistically significant increase (p = 0.0000003) in the level of ratio compared to advancing female age. Specifically, the p-value was further decreased in older women. No apparent difference was indicated for women with PCOS.
Figure 3.
A. The relationship between cf-nDNA quantity, female age, and PCOS demonstrated a statistically significant increase (p = 0.00007) in women with PCOS. B. The relationship between cf-mtDNA quantity, female age, and PCOS demonstrated a statistically significant increase (p=0.04) in women ≥36 years of age. C. The relationship between the ratio cf-mtDNA/cf-nDNA, female age, and PCOS demonstrated a statistically significant increase (p=0.0000003) in the reproductively older women.
Figure 3.
A. The relationship between cf-nDNA quantity, female age, and PCOS demonstrated a statistically significant increase (p = 0.00007) in women with PCOS. B. The relationship between cf-mtDNA quantity, female age, and PCOS demonstrated a statistically significant increase (p=0.04) in women ≥36 years of age. C. The relationship between the ratio cf-mtDNA/cf-nDNA, female age, and PCOS demonstrated a statistically significant increase (p=0.0000003) in the reproductively older women.
Figure 4.
The analysis did not demonstrate a statistically significant difference (p=0.7) between women with positive and negative pregnancy tests.
Figure 4.
The analysis did not demonstrate a statistically significant difference (p=0.7) between women with positive and negative pregnancy tests.
Figure 5.
cf-mtDNA relative copy number (RCN) between women with positive and negative pregnancy tests. Data analysis has not demonstrated a statistically significant difference (p = 0.3).
Figure 5.
cf-mtDNA relative copy number (RCN) between women with positive and negative pregnancy tests. Data analysis has not demonstrated a statistically significant difference (p = 0.3).
Figure 6.
Cf-mtDNA relative copy number (RCN) concerning female age. Data analysis has demonstrated a statistically significant (p=0.03) decrease in the cf-mtDNA copy number with advanced female age.
Figure 6.
Cf-mtDNA relative copy number (RCN) concerning female age. Data analysis has demonstrated a statistically significant (p=0.03) decrease in the cf-mtDNA copy number with advanced female age.
Table 1.
General characteristics of the women who participated in the study, including the number of oocytes and granulosa cells. SD, standard deviation.
Table 1.
General characteristics of the women who participated in the study, including the number of oocytes and granulosa cells. SD, standard deviation.
| Group |
No of Samples |
Mean Age
± SD |
Mean No of Oocytes
± SD |
No of Granulosa Cells |
| ≤35 |
34 |
31.4 ± 3.5 |
10.7 ± 4.1 |
112.213 |
| ≥36 |
36 |
39.3 ± 2.1 |
10.1 ± 5.7 |
135.000 |
| PCOS |
31 |
32.0 ± 3.6 |
18.0 ± 8.1 |
199.298 |
Table 2.
Hormone concentrations in each group of participants. Clinical characteristics of the women who participated in the study. AMH, FSH, and LH blood serum levels were determined in a cycle preceding IVF treatment. The estradiol blood serum levels were measured 2 days before oocyte retrieval. SD, standard deviation; AMH, anti-Mullerian hormone; FSH, follicle-stimulating hormone; LH, Luteinizing hormone; Estr, estrogen.
Table 2.
Hormone concentrations in each group of participants. Clinical characteristics of the women who participated in the study. AMH, FSH, and LH blood serum levels were determined in a cycle preceding IVF treatment. The estradiol blood serum levels were measured 2 days before oocyte retrieval. SD, standard deviation; AMH, anti-Mullerian hormone; FSH, follicle-stimulating hormone; LH, Luteinizing hormone; Estr, estrogen.
| Group |
AMH (ng/ml) ± SD |
FSH
± SD |
LH
± SD |
Estr (pg/ml) |
| ≤35 |
3.58 ± 4.2 |
6.11 ± 1.5 |
7.98 ± 7.7 |
2398.73 |
| ≥36 |
4.56 ± 6.9 |
7.16 ± 2.7 |
6.45 ± 11.6 |
2226.96 |
| PCOS |
10.58 ± 18.3 |
6.45 ± 1.1 |
14.65 ± 14.6 |
2901.20 |
Table 3.
Oligonucleotide sequences for cf-Mt/cf-N determination used for the real-time quantitative PCR, the corresponding Tm, and the size of the target area.
Table 3.
Oligonucleotide sequences for cf-Mt/cf-N determination used for the real-time quantitative PCR, the corresponding Tm, and the size of the target area.
| Primer Name |
Sequence(Forward/Reverse) |
Amplicon bp |
Tm |
Nuclear gene
(RPE) |
5’-ATAGGAAGCCAGAGAAGAGAGACT-3’
5’-TCTATCTCTGCGGACTTTGAGCAT-3’ |
200bp |
60 oc |
| Mitochondrial gene |
5’-TAGAGGAGCCTGTTCTGTAATCG-3’
5’-TAAGGGCTATCGTAGTTTTCTGG-3’, |
205bp |
59 oc |
Table 4.
The table displays the mean values for the age and the ratio of the samples.
Table 4.
The table displays the mean values for the age and the ratio of the samples.
| Group |
No of samples |
Mean age ± SD |
Mean Cf-mtDNA/Cf- n DNA ± SD |
| Reproductively younger women |
57 |
31,0 ± 3.3 |
0.77 ± 0.06 |
| Reproductively older women |
44 |
38,8 ± 2.3 |
0.85 ± 0.08 |
Table 5.
Data are represented as mean ± SD.
Table 5.
Data are represented as mean ± SD.
| Group |
No of Samples |
Age ± SD |
Cf-mtDNA/
Cf-nDNA ± SD |
| Reproductively younger women |
34 |
31.4 ± 3.5 |
0.78 ± 0.06 |
| Reproductively older women |
36 |
39.3± 2.1 |
0.86 ± 0.07 |
| PCOS women |
31 |
32.0 ±3.6 |
0.77 ± 0.06 |
Table 6.
The quantitative real-time PCR analysis resulted in the above outcomes. There are also highlight the ratio cf-mtDNA/cf-nDNA and the relative amount of mitochondria. Data are represented as mean ± SD.
Table 6.
The quantitative real-time PCR analysis resulted in the above outcomes. There are also highlight the ratio cf-mtDNA/cf-nDNA and the relative amount of mitochondria. Data are represented as mean ± SD.
| Group |
CfDNA
(ng/μl) ± SD |
Cf-nDNA
(Ct Average)
± SD |
Cf-mtDNA
(Ct Average)
± SD |
Cf-mtDNA/
Cf-n DNA
± SD |
2*2ΔCt
(RCN) |
2-ΔΔCt
|
| ≤35 |
10.38 ± 9.1 |
26.62 ± 1.2 |
20.88 ± 1.5 |
0.78 ± 0.06 |
207 |
2.28 |
| ≥36 |
7.68 ± 7.2 |
24.99 ± 1.7 |
21.59 ± 2.1 |
0.86 ± 0.07 |
76 |
9.26 |
| PCOS |
8.67 ± 5.9 |
26.37 ± 1.9 |
20.36 ± 2.3 |
0.77 ± 0.06 |
289 |
1.42 |
| Control group |
12.8 ± 6.2 |
26.3 ± 0.9 |
20.7 ± 1.4 |
0.79 ± 0.07 |
166 |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).