1. Introduction
Pandemic SARS-CoV-2 is a respiratory virus that can affect multiple organs, causing a wide range of symptoms in some patients [
1]. Cutaneous involvement, in which many types of skin lesions are identified [
2] has been described in the first published papers. COVID-19 skin reactions were generally higher in Western Europe than in Asia, with 6.6% reported in Europe compared with 0.2% in Asia [
3]. Initially, an Italian group described 6 types of skin lesions: maculopapular rashes, urticarial rashes, vesicular rashes, erythema multiforme, cutaneous vasculitis, and chilblain-like lesions [
4]. These lesions were considered secondary to the infection, but hypersensitivity to the treatments received could not be ruled out with absolute certainty [
5]. The lesions described in patients with SARS-CoV-2 infection were very heterogeneous and had a similar pattern to those seen in delayed drug hypersensitivity reactions (e.g., maculopapular exanthema (MPE) and fixed drug eruption (FDE)), to drug-induced liver injury (DILI) and severe cutaneous adverse reactions (SCARs) (e.g., Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP) [
6,
7,
8]. The most common drugs prescribed for COVID-19 treatment were hydroxychloroquine (18.5%), azithromycin (11.1%), lopinavir (7.4%), ritonavir (7.4%), and paracetamol (9.2%) [
3].
Late skin reactions to drugs belong to a mechanism of type IV hypersensitivity mediated by T cells. Advances in knowledge of the cells and cytokines involved in these types of reactions have allowed them to be classified into four types (IVa-IVd) [
9]. Type IVa corresponds to T-helper type 1 (Th1) cytokine-driven responses associated with high IFN- γ /TNF-α secretion. Type IVb corresponds to T helper type 2 (Th2) cytokine-driven reactions with increased secretion of IL-4, IL-5, and IL-13. Type IVc corresponds to the cytotoxic reactions mediated by cytotoxic CD8 T cells and seems to be the primary mechanism of bullous skin reactions such as SJS and TEN. Type IVd represents the T cell-induced sterile neutrophilic inflammatory response, e.g., AGEP [
10].
Diagnostic tests for delayed drug hypersensitivity are scarce. In vivo tests like epicutaneous patches are the most readily available. These patches must be prepared with the suspected drug involved in the reactions (based on a very detailed allergy clinical history) with an appropriate concentration and vehicle in order to yield accurate results. A positive result confirms the involvement of the drug, but the predictive value of a negative test is unknown. Therefore, drug challenge is still considered the gold standard for diagnosing drug allergy. In most delayed reactions, this option is not possible due to the patient's risk of reaction and lack of standardization of the challenged, a complete single dose of drugmay rule out an immediate IgE-mediated reaction, but not a delayed reaction that may occur after consecutive doses in a longer treatment.
In the last few years, the lymphocyte transformation test (LTT) has been used to diagnose delayed drug-induced hypersensitivity reactions by detecting the proliferation of drug-specific memory T cells [
11]. In addition, previous studies have shown that measurements of cytokine secretion in PBMC may be useful in diagnosing drug hypersensitivity [
10,
12,
13].
Our group conducted a prospective, observational, and descriptive study to determine whether drug hypersensitivity was the real cause of skin lesions. The results have been reported previously [
14]. The aim of the present study was to confirm the mechanism of hypersensitivity and the drugs involved in the skin lesions observed in patients with SARS-CoV-2 infection by means of an immunological study.
2. Results
2.1. Design and setting
Patients were selected from a previous study, which was a prospective, observational, and descriptive study whose main objective was to determine whether drug hypersensitivity could have been a cause of skin lesions in patients admitted to our hospital with SARS-CoV-2 infection between March and May 2020 [
14]. Of the 72 patients included in this study, 37 were classified as having a possible drug cause according to the Spanish Pharmacovigilance System (ASPS) [
15]. Of these, only 16 agreed to continue in the study. In all cases, a complete allergological study was performed with skin tests, epi-patches, and oral challenge against the drugs used during the period of infection and skin lesions [
14]. In the present study, 11 of those 16 patients agreed to finish the "in vitro" study. We also included 5 nonallergic patients (NAP) who were exposed to these drugs but did not develop lesions. The main treatments used were dolquine (hydroxychloroquine -HCQ-), azithromycin (AZT), kaletra (lopinavir/ritonavir -LOP/RIT-) and/or betalactam antibiotics such as amoxicillin/clavulanic acid (AMOX/CLA) or ceftriaxone (Table 1).
The patients included in this study presented three types of cutaneous lesions: maculopapular exanthema (MPE), urticarial exanthema (UEX), and vesicular exanthema (VEX). Accordingly, patients were classified as generalized exanthema (n=10) or cutaneous vasculitis (n=1) (Table 1). In all cases, the time from the start of treatment to the onset of lesions was between 1 and 15 days, with a mean of 7.5 days.
Epicutaneous patch tests were performed 4-6 months after hospital discharge, with a negative result for the eleven patients. In the case of beta-lactam antibiotics, skin prick test with late lecture ware was also performed, and all results were negative. Afterward, drug provocation test (DPT) was performed with the implicated drugs on alternative days. DPT was performed in 9 of the 11 patients. Two patients had no exposure to DPT, one because of the severity of his initial lesions, cutaneous vasculitis, and the other refused DPT. DPT was positive in 3 patients, two for AZT (late maculopapular exanthema and vesicular exanthema, respectively) and one for AMOX/ CLA (macular exanthema). It is important to mention that patient P2 presented an immediate reaction to 12,5 mg of AZT. In all 3 cases, cutaneous were consistent with the initial ones during COVID-19 treatment (Table 1).
| |
|
|
|
Epicutaneous Patch Test |
Oral challenges |
LTT |
| Patient |
Age (years)/Sex |
COVID-19 treatments |
Reaction |
Positive |
Negative |
Positive |
Negative |
Positive |
Negative |
| P1 |
60/M |
AZT, DOL |
MPE(1)
|
|
AZT, DOL |
|
AZT, DOL |
HCQ |
AZT, AMOX, CLA, LOP, RIT |
| P2 |
61/V |
AZT, KAL, DOL, CEL |
UEX (1)
|
|
AZT, DOL, KAL, CEL |
AZT §
|
DOL, KAL |
|
HCQ, AZT, AMOX, LOP, RIT |
| P3 |
53/V |
AZT, KAL, DOL, CEL, AMOX/CLA |
MPE(1)
|
|
DOL, KAL, CEL, AMOX |
AMOX/CLA |
KAL, AZT, HCQ |
CLA |
HCQ, AZT, AMOX, LOP, RIT |
| P4 |
63/M |
AZT, KAL, DOL, CEL |
MPE(1), VEX(2)
|
|
AZT, DOL, KAL |
|
AZT, HCQ, KAL |
LOP, RIT |
HCQ, AZT, AMOX |
| P5 |
66/V |
AZT, KAL, DOL, CEL |
MPE(1), VEX(2)
|
|
AZT, DOL, KAL, CEL |
AZT |
KAL, CEL |
AZT, HCQ |
AMOX, LOP, RIT |
| P6 |
61/M |
AZT, DOL |
MPE(1) VEX(2)
|
|
AZT, DOL |
|
DOL, AZT |
AZT, LOP, RIT |
HCQ, AMOX |
| P7 |
77/M |
KAL, DOL, CEL |
MPE(1)
|
|
|
* |
* |
LOP, RIT |
HCQ, AZT, AMOX |
| P8 |
84/V |
AZT, KAL, DOL, CEL |
MPE(1), VEX(2)
|
|
AZT, DOL, KAL, CEL |
|
AZT, DOL, KAL, CEL |
LOP, RIT |
HCQ, AZT, AMOX |
| P9 |
76/V |
AZT, DOL |
VEX(2)
|
|
AZT, DOL |
|
AZT, DOL |
HCQ, RIT |
AZT, AMOX, LOP |
| P10 |
74/V |
AZT, DOL, CEL |
CVAS(3), CLL(4)
|
|
AZT, DOL, CEL |
* |
* |
AZT, RIT |
AZT, AMOX, LOP |
| P11 |
64/V |
AZT, KAL, DOL |
MPE(1)
|
|
AZT, KAL, DOL |
|
AZT, KAL, DOL |
LOP, RIT |
HCQ, AZT, AMOX |
| NAP1 |
58/V |
AZT, KAL, DOL |
|
|
|
|
|
|
HCQ, AZT, AMOX, LOP, RIT |
| NAP2 |
52/V |
AZT, KAL, DOL, CEL |
|
|
|
|
|
|
HCQ, AZT, AMOX, LOP, RIT |
| NAP3 |
59/V |
AZT, KAL, DOL, CEL |
|
|
|
|
|
|
HCQ, AZT, AMOX, LOP, RIT |
| NAP4 |
49/V |
AZT, DOL |
|
|
|
|
|
|
HCQ, AZT, AMOX, LOP, RIT |
| NAP5 |
70/V |
AZT, KAL, DOL, CEL |
|
|
|
|
|
|
HCQ, AZT, AMOX, LOP, RIT |
| (1) Generalized exanthema: MPE, maculopapular exanthem or UEX, urticarial exanthem; (2) VEX, vesicular exanthem; (3) CVAS, cutaneous vasculitis; (4) CLL, chilblain-like lesion. AZT, azitromicin; AMOX, Amoxicillin; CLA, Clavulanic Acid, DOL, Dolquine; HCQ, hydroxychloroquine; KAL, Kaletra; LOP, Lopinavir; RIT, Ritonavir; CEL, Ceftriaxona; § Immediate reaction with 12,5 mg; * Oral challege not posible because generalized severe reaction. |
2.2. Lymphocyte transformation test (LTT)
In order to identify the possible culprit drugs causing skin lesions in these patients, we performed an LTT one year after recovery from COVID-19. LTT was also performed in 5 nonallergic patients. The study was conducted one year after recovery from COVID-19. In all cases, LTT was performed with three doses of AZT, AMOX, CLA, HCQ, LOP, and RIT (
Table 2). Ceftriaxone was excluded from the LTT study due to the limited sample size and based on the results of the allergology study. The test was considered positive with a stimulation index ≥3. Except for patient 2, who had an immediate reaction to AZT, all patients had a positive LTT to at least one of the drugs tested. AZT was positive in 3 patients (P5, P6, and P10). Of these, patient 5 was positive for DPT. CLA was positive in one patient with a positive DPT (P3). HCQ was positive in 3 patients (P1, P5, and P9). LOP was positive in four patients (P4, P6, P7, and P8). RIT was positive in 7 patients (P4, P6, P7, P8, P9, P10, and P11). None of the patients responded to AMOX. Two patients responded to only one drug, and eight responded to two drugs. None of the nonallergic patients had a positive LTT for any of the drugs tested. All results are shown in
Table 2.
2.3. Cytokine secretion
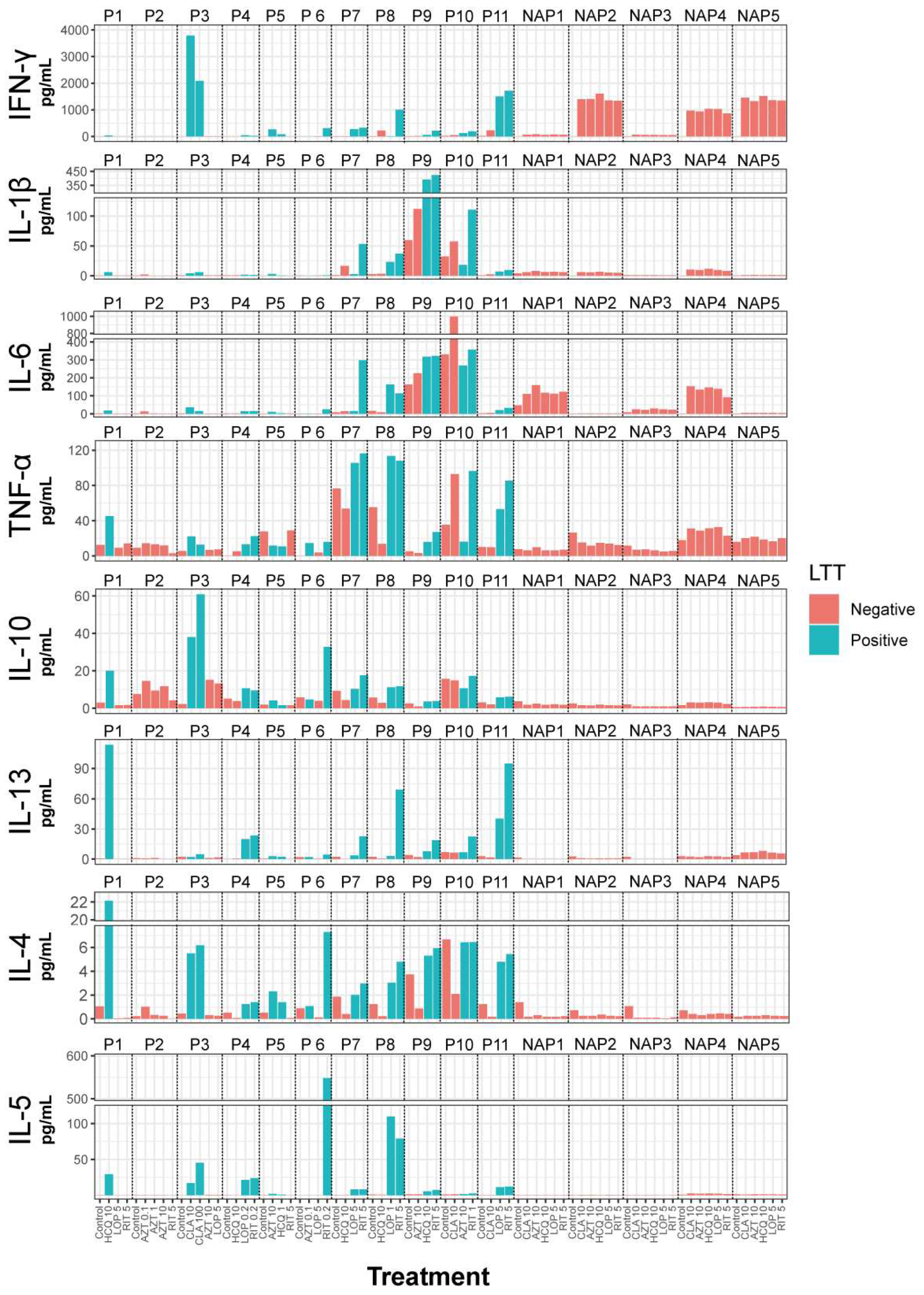
Next, we analyzed the cytokine secretion of PBMC in response to all relevant drugs four days after drug stimulation. In patients with cutaneous lesions, cytokine release was measured in all conditions with a positive LTT and at least one negative drug. In the case of patient 2, who had an immediate reaction to AZT, cytokine secretion was analyzed at all concentrations of this drug (0.1, 1, and 10 μg/μl) and RIT 5μg/μl. In the group of nonallergic patients without cutaneous lesions, cytokine secretion was analyzed at a representative concentration of each drug, except AZT, which did not give any positive LTT. In the group of patients with cutaneous lesions, the LTT-positive drugs strongly induced the secretion of IL-4, IL-5, and IL-13 (
Figure 1 and Supplementary Table 1) in most patients. The levels of these cytokines in the nonallergic patient's group were consistently low, and no increase was observed with any of the selected drugs. Although there was an apparent increase in IFN-γ with the LTT-positive drugs, it also increased in three nonallergic patients, which occurred with all the drugs tested. It is interesting to note that patient 2, who had an immediate response to AZM and a negative LTT, had very low levels of these cytokines. The response of the other cytokines studied, IL-1β, Il-6, TNF-α, and IL-10, was inconsistent, as they increased with some treatments but not others. These cytokines were also increased by some non-proliferation-stimulating drugs and in control patients.
3. Discussion
During the first wave of the COVID-19 pandemic, up to 20% of patients had skin lesions of different characteristics [
4,
16,
17]. The skin lesions of COVID-19 were classified into six categories: maculopapular exanthems, urticarial exanthems, vesicular exanthems, erythema multiforme, cutaneous vasculitis, and chilblain-like lesions [
4]. Due to the heterogeneity of the treatments, it has not been possible to clearly establish whether or not some of the skin lesions that were presented during the first wave of COVID-19 could be secondary to drug hypersensitivity. In the present study, we have analyzed the possible cause of skin lesions during SARS-CoV-2 infection in 11 patients. Through lymphocyte proliferation and cytokine secretion assays, we identified a drug candidate as the culprit, despite only three patients having positive drug provocation tests.
The fact that skin manifestations were greatly reduced in subsequent waves when COVID-19 treatments were changed supports the view that the skin manifestations observed in the first wave were mainly due to hypersensitivity reactions to the drugs used at that time [
18].
LTT is recommended for the diagnosis of drug hypersensitivity reactions (DHR) in which the distal effector phase is mediated by T cells [
11]. After PBMC culture, activation of the lipocytes begins within minutes by a specific drug antigen presented by major histocompatibility complex (MHC) class I or II antigen-presenting cells (APCs). Following T cell receptor (TCR) activation, Ca
2+ increases, and a signalling cascade activates early antigen recognition genes. Over the next few hours, the expression of genes encoding several cytokines (IL-2, 3, 4, 5, and 6, IFN-γ, TGF-β) and early activation markers increases. One to two days after T-cell activation, IL-2 induces the proliferation of activated T-cells, and consequently, DNA synthesis starts. Approximately three to five days after activation, T cells enter the functional differentiation phase and produce different cytokine patterns: Th1, Th2, or Th3. Th1 is mainly associated with the production of IL-2, IFN-γ, and TNF-α, Th2 with the production of IL-4, IL-5, and IL-13, and Th3 with the production of IL-17A and IL-17F. The type of specific T cell produced depends on the sensitization phase, and these cytokine patterns determine the effector functions of the T lymphocytes. [
10]. Several studies have shown that cytokine secretion in the supernatant of drug-stimulated PBMC may also be useful in the diagnosis of drug hypersensitivity [
12,
13]. The production of Th1 cytokines, mainly IL-2, IFN-γ and TNF-α, in PBMCs has been associated with DHR in several studies [
10]. High IFN-γ production by drug-stimulated PBMCs has been observed during the acute allergic phase in SCARs such as SJS, TEN, DRESS, or AGEP [
19]. IL-5 increases in patients with drug-induced MPE and DRESS [
20,
21], and it has been proposed as a useful in vitro method for detecting drug sensitization. Furthermore, the combination of IL-5 measure and LTT may better indicate drug sensitization than LTT alone [
22]. Other studies have shown a mixed Th1/Th2 cytokine pattern with the production of IL-5, IL-4, and IL-13 in addition to IFN-γ. Indeed, high levels of IL-5 and IFN-γ secretion by CD4 cells are associated with maculopapular exanthema [
9] and have been proposed as promising in vitro indicators of drug hypersensitivity [
10,
23]. Lochmatter et al. [
12] extensively studied the secretion of 17 cytokines and chemokines in PBMC from patients with well-documented drug allergies. They found that the measurement of IL-5 combined with IFN- γ, IL-13, or IL-2 is the more sensitive marker for detecting T-cell sensitization to drugs.
Consistent with this, we found a mixed Th1/Th2 cytokine secretion (IL-4, IL-5, IL-13, and IFN-γ) in patients with skin lesions. It, therefore, allows us to classify these patients as having mixed type IVa and IVb drug hypersensitivity reactions, corresponding to T-helper type 1 (Th1) cytokine-driven responses, associated with high levels of IFN-γ secretion, and Th2 cytokine-driven responses, associated with high levels of IL-4, IL-5 and IL-13 secretion.
It is important to note that we found elevated levels of IFN-γ in some patients without skin lesions, a phenomenon that has been described previously [
24,
25].
Among the eleven patients studied, an immediate clinical response was observed in only one case, confirmed by a positive oral challenge and a negative LTT to AZT. In the remaining cases, a specific immune response to some of the drugs the patients had received during treatment could be established, which was not found in patients with SARS-CoV-2 infection but without skin lesions. Interestingly, only two patients had a positive oral challenge, one to AZT and one to CLA. The discordant results between oral challenge and LTT could be due to two situations. Firstly, the drugs were not given for a sufficient time or at a sufficient dose during the oral challenge. This may be because the drugs used are so toxic that they cannot be given for long periods without clinical necessity [
10]. Alternatively, because the patient no longer has the inflammatory state present during the viral infection, the study is carried out 6 months later and therefore does not have the cofactor necessary to trigger the cutaneous symptoms again. The 12-month period was chosen for the in vitro study because this is the recommended latency period for LTT studies in severe late drug reactions such as DRESS or exanthema multiforme.
On the other hand, it is important to emphasize the importance of performing the LTT at least 2 months after the resolution [
12,
26], with a recommendation of 6 to 12 months
[27], otherwise, the risk of false-positive results increases, as described previously. Indeed, a case of COVID-19-related cutaneous manifestations has been described in which the proliferation assay was performed 21 days after infection and reported sensitization to all drugs tested [
28]. This may be a sign of hyperreactivity caused by a viral infection and consequently false positive LTT results.
Therefore, considering all patients with late reactions had presented skin lesions and had a positive LTT with a mixed Th1/Th2 cytokine release, it would be reasonable to recommend avoiding the drug in question in all cases. If drug administration is necessary, an exhaustive study under allergological supervision with appropriate dosage and administration time. These results highlight the need for a multidisciplinary approach to the management of adverse drug reactions [
5].
4. Materials and Methods
4.1. Lymphocyte transformation test (LTT)
The LTT was performed according to Margarita et al. with minor modifications [
29]. PBMCs were freshly isolated from heparinized venous blood samples (30 mL) by Ficoll (LymphoPrep™) gradient centrifugation. The cells were resuspended in AIM-V Medium (Gibco) (2 × 106 cell/mL) and cultured in 96-well U-bottomed plates (200 µl/well) containing the following stimuli: Dynabeads Human T-Activator CD3/CD28 (1 μL/well) (Gibco) as positive control; medium AIM-V or DMSO as control negative (unstimulated condition); azitromicin (0.1 μg/μL, 1 μg/μL and 10 μg/μL), amoxicilin (100 μg/μL, 200 μg/μL and 500 μg/μL), clavulanate (1 μg/μL, 10 μg/μL, 100 μg/μL), hydroxychloroquine (1 μg/μL, 10 μg/μL, 100 μg/μL), lopinavir (0.02 μg/μL, 0.1 μg/μL, 0.5 μg/μL and 2.5 μg/μL) and ritonavir (0.04 μg/μL, 0.2 μg/μL, 1 μg/μL and 5 μg/μL). Cultures were performed in triplicate and incubated for 4 days in a humidified incubator (37°C and 5% CO2). On day 4, the culture plates were centrifuged, and 100 µL aliquots of the culture supernatant were transferred to another 96-well plate and stored at -40ºC for cytokine analysis. 100 µL of fresh AIM-V medium containing 10 μCi of
3H-thymidine (Perkin Elmer) was added to the cells and gently resuspended the cell pellet. On day 6 the cultures were transferred into Multiscreen®-HV 96-Well Filter Plate (Millipore) and cells were harvested with MultiScreen®Vacuum Manifold (Millipore). Each 96-Well Filter was punched into a scintillation vial and radioactivity incorporation into DNA was measured using a liquid scintillation counter (Wallach, Perkin Elmer). The proliferative response was expressed as a stimulation index (SI), which was calculated by the ratio of disintegrations per minute (dpm) of the drug-stimulated T cells and the mean of dpm of the unstimulated T cells. As standard criteria, SI >3 in at least one concentration was considered positive.
4.2. Secreted cytokine measurement
Four-day cell-culture supernatants were centrifuged and stored at -40ºC. Th1 (IFN-γ, IL-1β, and TNF-α) and Th2 (IL-5, IL-4, IL-6, IL-10, IL-13) cytokines were measured using the MILLIPLEX® MAP Human High Sensitivity T Cell Magnetic Beads panel (Millipore-Sigma) according to the manufacturer's instructions and acquired on the Luminex Magpix System (Luminex, Austin, Tex).
5. Conclusions
Through lymphocyte proliferation and cytokine secretion assays, we identified a drug candidate as the culprit of skin lesions during SARS-CoV-2 infection, despite only three patients having positive drug provocation tests. Therefore, considering all patients with late reactions had presented skin lesions and had a positive LTT with a mixed Th1/Th2 cytokine release, it would be reasonable to recommend avoiding the drug in question in all cases.
Author Contributions
Conceptualization, M.F.G., J.M.B, and B.H.C.; sample collection and data acquisition, C.F.L., E.S.S , I.E.S., I.P.A., and D.F.N.; data analysis, C.F.L., E.S.S., D.F.N., A.A., J.M.B., and B.H.C.; writing-original draft preparation, E.S.S., J.M.B., and B.H.C.; writing-review and editing, C.F.L., I.E.S., I.P.A., L.D.M., A.A., D.F.N., D.G.O., and M.F.G.; visualization, C.F.L., E.S.S., J.M.B., and B.H.C.; supervision, M.F.G., J.M.B, and B.H.C.; funding acquisition, M.F.G., J.M.B., and B.H.C. All authors have read and agreed to the published version of the manuscript.
Funding This research was funded by FONDO SUPERA COVID19, grant identification "Cutinmfarm" by the SANTADER FOUNDATION and associated to the Instituto Ramón y Cajal de Investigación Sanitaria (IRYCIS) and Alcala University (AU) of Madrid (Spain).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of HOSPITAL UNIERSITARIO RAMÓN Y CAJAL (protocol code 197/20, 22/06/2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
JMB is a researcher at FIBio-HRC supported by the Consejería de Sanidad (Comunidad Autónoma de Madrid).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Riggioni, C.; Comberiati, P.; Giovannini, M.; Agache, I.; Akdis, M.; Alves-Correia, M.; Anto, J. M.; Arcolaci, A.; Azkur, A. K.; Azkur, D.; Beken, B.; Boccabella, C.; Bousquet, J.; Breiteneder, H.; Carvalho, D.; De Las Vecillas, L.; Diamant, Z.; Eguiluz-Gracia, I.; Eiwegger, T.; Eyerich, S.; Fokkens, W.; Gao, Y. D.; Hannachi, F.; Johnston, S. L.; Jutel, M.; Karavelia, A.; Klimek, L.; Moya, B.; Nadeau, K. C.; O'Hehir, R.; O'Mahony, L.; Pfaar, O.; Sanak, M.; Schwarze, J.; Sokolowska, M.; Torres, M. J.; van de Veen, W.; van Zelm, M. C.; Wang, Y.; Zhang, L.; Jimenez-Saiz, R.; Akdis, C. A. , A compendium answering 150 questions on COVID-19 and SARS-CoV-2. Allergy 2020, 75, 2503–2541. [Google Scholar] [CrossRef] [PubMed]
- Galvan Casas, C.; Catala, A.; Carretero Hernandez, G.; Rodriguez-Jimenez, P.; Fernandez-Nieto, D.; Rodriguez-Villa Lario, A.; Navarro Fernandez, I.; Ruiz-Villaverde, R.; Falkenhain-Lopez, D.; Llamas Velasco, M.; Garcia-Gavin, J.; Baniandres, O.; Gonzalez-Cruz, C.; Morillas-Lahuerta, V.; Cubiro, X.; Figueras Nart, I.; Selda-Enriquez, G.; Romani, J.; Fusta-Novell, X.; Melian-Olivera, A.; Roncero Riesco, M.; Burgos-Blasco, P.; Sola Ortigosa, J.; Feito Rodriguez, M.; Garcia-Doval, I. , Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol 2020, 183, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Sameni, F.; Hajikhani, B.; Yaslianifard, S.; Goudarzi, M.; Owlia, P.; Nasiri, M. J.; Shokouhi, S.; Bakhtiyari, M.; Dadashi, M. , COVID-19 and Skin Manifestations: An Overview of Case Reports/Case Series and Meta-Analysis of Prevalence Studies. Frontiers in medicine 2020, 7, 573188. [Google Scholar] [CrossRef] [PubMed]
- Recalcati, S.; Gianotti, R.; Fantini, F. , COVID-19: The experience from Italy. Clinics in dermatology 2021, 39, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Hernandez, R.; Solano-Solares, E.; Chica-Guzman, V.; Fernandez-Guarino, M.; Fernandez-Nieto, D.; Ortega-Quijano, D.; de-Andres-Martin, A.; Moreno, C.; Carretero-Barrio, I.; Garcia-Abellas, P.; Gonzalez-de-Olano, D.; de-la-Hoz-Caballer, B. , SARS-CoV-2, skin lesions and the need of a multidisciplinary approach. Journal of the European Academy of Dermatology and Venereology : JEADV 2020, 34, e659–e662. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, G. A.; Ripa, M.; Burastero, S.; Benanti, G.; Bagnasco, D.; Nannipieri, S.; Monardo, R.; Ponta, G.; Asperti, C.; Cilona, M. B.; Castagna, A.; Dagna, L.; Yacoub, M. R. , Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): Focus on the Pathophysiological and Diagnostic Role of Viruses. Microorganisms 2023, 11. [Google Scholar] [CrossRef]
- Karimi, A.; Pourbakhtiaran, E.; Fallahi, M.; Karbasian, F.; Armin, S.; Babaie, D. , Is It Stevens-Johnson Syndrome or MIS-C with Mucocutaneous Involvement? Case reports in pediatrics 2021, 2021, 1812545. [Google Scholar] [CrossRef]
- Lootah, S.; Alshammari, E.; Alqanatish, J. , Complete Remission in a Child With Multisystem Inflammatory Syndrome and Stevens-Johnson Syndrome Treated With Infliximab. Cureus 2023, 15, e37076. [Google Scholar] [CrossRef]
- Pichler, W. J. , Delayed drug hypersensitivity reactions. Annals of internal medicine 2003, 139, 683–693. [Google Scholar] [CrossRef]
- Porebski, G.; Gschwend-Zawodniak, A.; Pichler, W. J. , In vitro diagnosis of T cell-mediated drug allergy. Clin Exp Allergy 2011, 41, 461–470. [Google Scholar] [CrossRef]
- Sachs, B.; Fatangare, A.; Sickmann, A.; Glassner, A. , Lymphocyte transformation test: History and current approaches. J Immunol Methods 2021, 493, 113036. [Google Scholar] [CrossRef] [PubMed]
- Lochmatter, P.; Beeler, A.; Kawabata, T. T.; Gerber, B. O.; Pichler, W. J. , Drug-specific in vitro release of IL-2, IL-5, IL-13 and IFN-gamma in patients with delayed-type drug hypersensitivity. Allergy 2009, 64, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Srinoulprasert, Y. , Lymphocyte transformation test and cytokine detection assays: Determination of read out parameters for delayed-type drug hypersensitivity reactions. J Immunol Methods 2021, 496, 113098. [Google Scholar] [CrossRef] [PubMed]
- Solano-Solares, E.; Chica-Guzman, V.; Perez-Allegue, I.; Cabrera-Hernandez, R.; Fernandez-Guarino, M.; Fernandez-Nieto, D.; Moreno-Garcia-Del-Real, C.; de-Andres-Martin, A.; Garcia-Bermejo, L.; Gonzalez-de-Olano, D.; de-la-Hoz-Caballer, B. , Role of Drug Hypersensitivity in the Cutaneous Manifestations of SARS-CoV-2 Infection. Journal of investigational allergology & clinical immunology 2022, 32, 218–220. [Google Scholar]
- Cabanas, R.; Ramirez, E.; Sendagorta, E.; Alamar, R.; Barranco, R.; Blanca-Lopez, N.; Dona, I.; Fernandez, J.; Garcia-Nunez, I.; Garcia-Samaniego, J.; Lopez-Rico, R.; Marin-Serrano, E.; Merida, C.; Moya, M.; Ortega-Rodriguez, N. R.; Rivas Becerra, B.; Rojas-Perez-Ezquerra, P.; Sanchez-Gonzalez, M. J.; Vega-Cabrera, C.; Vila-Albelda, C.; Bellon, T. , Spanish Guidelines for Diagnosis, Management, Treatment, and Prevention of DRESS Syndrome. Journal of investigational allergology & clinical immunology 2020, 30, 229–253. [Google Scholar]
- Mendez Maestro, I.; Pena Merino, L.; Udondo Gonzalez Del Tanago, B.; Aramburu Gonzalez, A.; Orbea Sopena, A.; Sanchez De Vicente, J.; Raton Nieto, J. A.; Acebo Marinas, E.; Gardeazabal Garcia, J. , Skin manifestations in patients hospitalized with confirmed COVID-19 disease: a cross-sectional study in a tertiary hospital. International journal of dermatology 2020, 59, 1353–1357. [Google Scholar] [CrossRef]
- Nakashima, C.; Kato, M.; Otsuka, A. , Cutaneous manifestations of COVID-19 and COVID-19 vaccination. The Journal of dermatology 2023, 50, 280–289. [Google Scholar] [CrossRef]
- Fernandez-Nieto, D.; Ortega-Quijano, D.; Suarez-Valle, A.; Jimenez-Cauhe, J.; Jaen-Olasolo, P.; Fernandez-Guarino, M. , Lack of skin manifestations in COVID-19 hospitalized patients during the second epidemic wave in Spain: a possible association with a novel SARS-CoV-2 variant - a cross-sectional study. Journal of the European Academy of Dermatology and Venereology : JEADV 2021, 35, e183–e185. [Google Scholar] [CrossRef]
- Suthumchai, N.; Srinoulprasert, Y.; Thantiworasit, P.; Rerknimitr, P.; Tuchinda, P.; Chularojanamontri, L.; Rerkpattanapipat, T.; Chanprapaph, K.; Disphanurat, W.; Chakkavittumrong, P.; Tovanabutra, N.; Srisuttiyakorn, C.; Sukasem, C.; Klaewsongkram, J. , The measurement of drug-induced interferon gamma-releasing cells and lymphocyte proliferation in severe cutaneous adverse reactions. Journal of the European Academy of Dermatology and Venereology : JEADV 2018, 32, 992–998. [Google Scholar] [CrossRef]
- Yawalkar, N.; Shrikhande, M.; Hari, Y.; Nievergelt, H.; Braathen, L. R.; Pichler, W. J. , Evidence for a role for IL-5 and eotaxin in activating and recruiting eosinophils in drug-induced cutaneous eruptions. J Allergy Clin Immunol 2000, 106, 1171–1176. [Google Scholar] [CrossRef]
- Sachs, B.; Erdmann, S.; Malte Baron, J.; Neis, M.; al Masaoudi, T.; Merk, H. F. , Determination of interleukin-5 secretion from drug-specific activated ex vivo peripheral blood mononuclear cells as a test system for the in vitro detection of drug sensitization. Clin Exp Allergy 2002, 32, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Merk, H. F. , Diagnosis of drug hypersensitivity: lymphocyte transformation test and cytokines. Toxicology 2005, 209, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Glassner, A.; Wurpts, G.; Roseler, S.; Yazdi, A. S.; Sachs, B. , In vitro detection of T cell sensitization by interferon-gamma secretion in immediate-type drug allergy. Clin Exp Allergy 2023, 53, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Halevy, S.; Cohen, A.; Livni, E. , Acute generalized exanthematous pustulosis associated with polysensitivity to paracetamol and bromhexine: the diagnostic role of in vitro interferon-gamma release test. Clinical and experimental dermatology 2000, 25, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Gaspard, I.; Guinnepain, M. T.; Laurent, J.; Bachot, N.; Kerdine, S.; Bertoglio, J.; Pallardy, M.; Lebrec, H. , Il-4 and IFN-gamma mRNA induction in human peripheral lymphocytes specific for beta-lactam antibiotics in immediate or delayed hypersensitivity reactions. Journal of clinical immunology 2000, 20, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Cabanas, R.; Calderon, O.; Ramirez, E.; Fiandor, A.; Caballero, T.; Heredia, R.; Herranz, P.; Madero, R.; Quirce, S.; Bellon, T. , Sensitivity and specificity of the lymphocyte transformation test in drug reaction with eosinophilia and systemic symptoms causality assessment. Clin Exp Allergy 2018, 48, 325–333. [Google Scholar] [CrossRef]
- Glassner, A.; Dubrall, D.; Weinhold, L.; Schmid, M.; Sachs, B. , Lymphocyte transformation test for drug allergy detection: When does it work? Ann Allergy Asthma Immunol 2022, 129, 497–506. [Google Scholar] [CrossRef]
- Hayakawa, J.; Takakura, H.; Mizukawa, Y.; Shiohara, T. , COVID-19-related cutaneous manifestations associated with multiple drug sensitization as shown by lymphocyte transformation test. Journal of the European Academy of Dermatology and Venereology : JEADV 2020, 34, e779–e781. [Google Scholar] [CrossRef]
- Giraldo-Tugores, M.; Sanmartin-Fernandez, M.; Fernandez-Lozano, C.; Martinez-Botas, J.; De-la-Hoz-Caballer, B.; Gonzalez-de-Olano, D. , Kounis Syndrome and Vanadium allergy: heed your hunch. Journal of investigational allergology & clinical immunology 2023, 0. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).